Monarch butterflies are an icon of nature: spectacular in form, known for their unfathomable annual migration, and frequent visitors in our backyards (Fig. 1). It is no wonder they are a darling among invertebrates. And what has now captured our attention is the striking and precipitous decline of monarch populations over the past two decades. So much of the decline in biodiversity, part of the current mass extinction, seems abstract to us—the polar bear floating on an iceberg in the arctic, or the slash and burn of tropical rain forests. But the decline of monarch butterflies has been observed like a “silent spring,” with biologists and casual observers alike noticing the missing butterflies from so many of our recent summers, especially in the northeastern and midwestern United States. Two new studies published in PNAS add fresh analyses, considerably new data, and novel approaches to tackle the monarch mystery (1, 2).
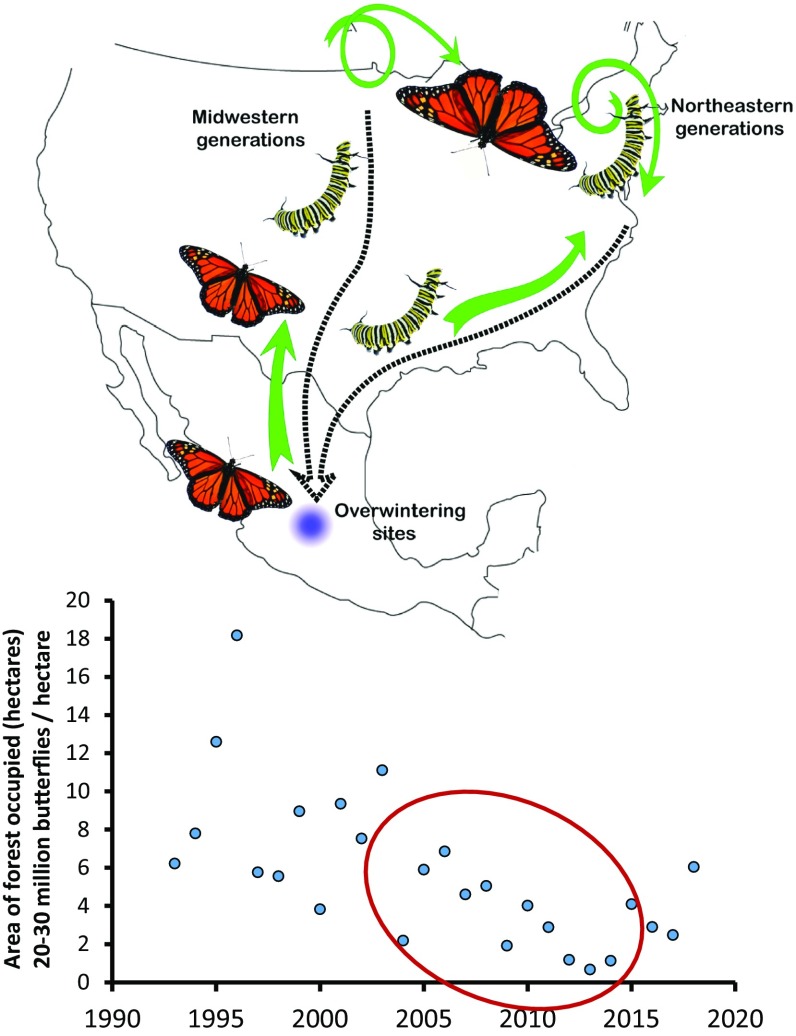
Fig. 1.
(Top) The annual migratory cycle of eastern North American monarch butterflies. Generation 1 occurs in the US Gulf Coast states centered on Texas (March–April), and generations 2 to 4 occur in the Northeast and Midwest (May–September). The fourth migratory generation lives for 8 mo, including overwintering in Mexico and returning to the Gulf Coast states, and does not rely on milkweed between September and February. (Bottom) The population decline of monarch butterflies in the highlands of Central Mexico (generation 4, estimated during overwintering) (data collected by World Wildlife Fund Mexico). The circled years are those investigated by Saunders et al. (1).
Although concerns about monarch conservation have been voiced at least since 1977 (a year after their overwintering grounds were discovered by citizen scientists, in collaboration with Canadian biologists Fred and Nora Urquhart), two key moments in the monarch’s rise to public prominence came in 1999 and 2012 (3). The former was based around a scare surrounding genetically modified crops engineered with insecticidal Bacillus thuringiensis toxins (4), and the latter quantitatively demonstrated a decline of the monarch population and implicated herbicides applied to genetically modified crops engineered to withstand herbicides (5, 6). Although many threats have been identified, there has been vigorous scientific debate as to their relative importance over the past 7 y (6–11). The continental scale of the problem and the decadal timescale on which the decline is occurring present substantial issues for identifying the drivers of this declining species. Indeed, additional data and statistical modeling are needed.
In PNAS, Saunders et al. (1) employ a hierarchical set of statistical models and variable selection to construct a predictive set of population input and environmental variables for winter colony sizes in Mexico. Due to limitations in availability of individual-colony census data, the authors focus their analysis on the years 2004 to 2015, which has two important implications (Fig. 1). First, this temporal window is after the large-scale adoption of genetically modified herbicide-tolerant crops (and corresponding herbicide use) topped out; thus, herbicide usage was no longer increasing. Second, this is the same window when the monarch population (as censused in Mexico) experienced the steepest and most persistent decline in recorded history, with the all-time low recorded in 2013 (Fig. 1). Saunders et al. report that winter abundances can be predicted by variation in the input of summer breeding monarchs, in addition to variation in an index of flower nectar availability (during the southern migration) and forest patch size (at the overwintering sites).
Monarchs use a wide variety of flowers for nectar, and milkweed is typically no longer flowering during the migration; therefore, the findings of Saunders et al. (1) support a long-standing but previously untested hypothesis of floral nectar limitation. The impact of overwintering forest cover at colony sites has also been suspected to be critically limiting to monarchs, but only recently has a quantitative link been made showing the limiting effects of degraded forests (12). From these factors, it can be concluded that during the decade of the steepest declines in monarchs, climate and its impacts on availability of floral nectar and the continued degradation of Mexican forests were critical factors. Nonetheless, why the summer breeding population of monarchs plummeted during the years 2004 to 2015 is still being debated. Was it a delayed response to the biofuel boom and enhanced usage of herbicides? Was it the 100-y drought experienced in Texas, which is a critical spring and fall bottleneck? Or was it some combination of stresses?
In the second study in PNAS, Boyle et al. (2) take the temporal long view, examining monarch and milkweed dynamics over the past 116 y. The extraordinary approach was to employ tens of thousands of museum records of both monarchs and milkweeds as indices of their population sizes. Although this approach will surely be criticized for all sorts of limitations, it importantly brings an entirely new set of data, and one of tremendous temporal depth, to the table. Boyle et al.’s key conclusion is that the monarch decline began over 60 y ago and was concordant with the abandonment of small-scale farms (which served as a reservoir for milkweeds). This result is concordant with the long-term decline of monarchs in California supported by observational data remarkably collected by a single researcher over a 45-y period (13). Such multidecadal declines have important implications, the first of which is that no single recent event, such as the advent of genetically modified crops, can be implicated in monarch declines. Indeed, a blue-ribbon panel of the National Academy of Sciences in 2016 came to this same conclusion about genetically modified crops and monarch butterflies (14).
Perhaps more importantly, the whole of land-use change, including agricultural practices, chemical inputs, habitat fragmentation, pollution, development, disturbance, and so forth, is what has been incrementally creeping up over the last century. Which of the many aspects of this long-term environmental crumbling is responsible for monarch declines is unclear. To me, one of the most disturbing findings of Boyle et al.’s study (2) is the strong and persistent declines of eight milkweed species over several decades. The common weedy milkweeds (Asclepias syriaca and A. speciosa) have enjoyed the benefits of agriculturalization through soil disturbance, fertilization, and creation of ditches resulting in larger populations. However, the nonweedy Asclepias, numbering well over 100 species in North America, appear to be in trouble.
Several missing pieces to the puzzle of monarch declines remain. First, the role of predators and parasites in monarch populations is likely to be underestimated (3). Monarchs are consumed at every life stage, and some studies have suggested increases in these natural threats. For example, the incidence of the protozoan parasite Ophryocystis elektroscirrha has increased from about 1% in the early 1990s to 10% beginning around 2010 (12). Although one model found only a modest impact of this parasite’s increase in prevalence on monarch population declines (12), further study is needed. Second, nonnative milkweeds have recently been implicated not only in disrupting the monarch migration, but also in increased levels of disease (15); if invasive species and disease synergize, there could be dire consequences. Third, we have relatively little
The decline of monarch butterflies has been observed like a “silent spring,” with biologists and casual observers alike noticing the missing butterflies from so many of our recent summers, especially in the northeastern and midwestern United States. Two new studies published in PNAS add fresh analyses, considerably new data, and novel approaches to tackle the monarch mystery.
quantitative insight as to the role of insecticides (especially neonicotinoids, which have recently come under fire) in suppressing monarch populations. Evidence to date suggests that they can have lethal and sublethal effects that may impact butterfly populations at multiple stages (16, 17). Insecticide use is also likely to be correlated with herbicide use, and thus teasing apart their relative roles may be difficult. Finally, there is much to be learned from the correlated declines of the somewhat independent populations of monarchs in Florida (18) and California (19), in addition to the many other declining migratory species, including birds and bats (20), which together may indicate independent, common, or synergizing threats.
Saving an iconic butterfly is important and would help us sustain beauty, wonder, and majesty in nature. But for me, the concern is much larger than a single species. The warning sign I see is that the health of our continent may be at risk. Monarchs are sentinels, traversing the continent and tasting their way as they move. Thus, to understand what is happening to environmental health more generally, monarchs may be a source of information for both our own population and biodiversity writ large.
In closing, last year marked the end of an era with the passing of legendary monarch biologist Lincoln Brower. Over the decades of his lifelong passion for everything monarch, from their metamorphosis and chemistry to their migration and conservation, his views on the demise of the monarchs evolved and swirled. He was frequently quoted in the New York Times, and the following is some of what he said to their journalists. In 1986: “Herbicides are a major threat to monarchs, because the butterflies need weeds and wildflowers to survive.” In 1990 on deforestation in Mexico: “But until it is clear that cutting has stopped, there is danger of a catastrophe that’s going to spell the end of monarch butterflies in eastern North America.” In 2002 on monarch mortality caused by a winter storm in Mexico: “It was really macabre, I’ve been going down there for 25 years, and I’ve never seen anything like it.” In 2006: “The biggest threat to the migration is the steady attrition of forests because of illegal logging.” In 2011 on use of herbicides in agricultural fields: “It kills everything. It’s like absolute Armageddon for biodiversity over a huge area.” And in 2017 on climate change impacts on monarch butterflies: “It’s hard to know what’s going to happen, but I don’t think it will be good.”
No single stress is causing the monarch butterfly’s population decline. Lincoln Brower saw many stressors over his career, and perhaps they conspire to make matters worse. Only through a combination of approaches, both scientific and social, can we hope to reverse this downward spiral. Monarchs are representatives of many species, and we should listen to what they are telling us.
Acknowledgments
I thank Hidetoshi Inamine for commenting on the manuscript. My research (see www.eeb.cornell.edu/agrawal/) is currently supported by the NSF Division of Integrative Organismal Systems Grant 1645256 and the US Department of Agriculture National Institute of Food and Agriculture Multistate Hatch Project 1008470.
Footnotes
References
- 1.Saunders SP, et al. Multiscale seasonal factors drive the size of winter monarch colonies. Proc Natl Acad Sci USA. 2019;116:8609–8614. doi: 10.1073/pnas.1805114116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyle JH, Dalgleish HJ, Puzey JR. Monarch butterfly and milkweed declines substantially predate the use of genetically modified crops. Proc Natl Acad Sci USA. 2019;116:3006–3011. doi: 10.1073/pnas.1811437116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal AA. Monarchs and milkweed: A migrating butterfly, a poisonous plant, and their remarkable story of coevolution. Princeton Univ Press; Princeton: 2017. [Google Scholar]
- 4.Losey JE, Rayor LS, Carter ME. Transgenic pollen harms monarch larvae. Nature. 1999;399:214. doi: 10.1038/20338. [DOI] [PubMed] [Google Scholar]
- 5.Brower LP, et al. Decline of monarch butterflies overwintering in Mexico: Is the migratory phenomenon at risk? Insect Conserv Divers. 2012;5:95–100. [Google Scholar]
- 6.Brower LP, Taylor OR, Williams EH. Response to Davis: Choosing relevant evidence to assess monarch population trends. Insect Conserv Divers. 2012;5:327–329. [Google Scholar]
- 7.Davis AK. Are migratory monarchs really declining in eastern North America? Examining evidence from two fall census programs. Insect Conserv Divers. 2012;5:101–105. [Google Scholar]
- 8.Dyer LA, Forister ML. Wherefore and whither the modeler: Understanding the population dynamics of monarchs will require integrative and quantitative techniques. Ann Entomol Soc Am. 2016;109:172–175. [Google Scholar]
- 9.Inamine H, Ellner SP, Springer JP, Agrawal AA. Linking the continental migratory cycle of the monarch butterfly to understand its population decline. Oikos. 2016;125:1081–1091. [Google Scholar]
- 10.Pleasants JM, et al. Interpreting surveys to estimate the size of the monarch butterfly population: Pitfalls and prospects. PLoS One. 2017;12:e0181245. doi: 10.1371/journal.pone.0181245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal AA, Inamine H. Mechanisms behind the monarch’s decline. Science. 2018;360:1294–1296. doi: 10.1126/science.aat5066. [DOI] [PubMed] [Google Scholar]
- 12.Thogmartin WE, et al. Monarch butterfly population decline in North America: Identifying the threatening processes. R Soc Open Sci. 2017;4:170760. doi: 10.1098/rsos.170760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espeset AE, et al. Understanding a migratory species in a changing world: Climatic effects and demographic declines in the western monarch revealed by four decades of intensive monitoring. Oecologia. 2016;181:819–830. doi: 10.1007/s00442-016-3600-y. [DOI] [PubMed] [Google Scholar]
- 14.National Academies of Sciences, Engineering, and Medicine . Genetically Engineered Crops: Experiences and Prospects. National Academies Press; Washington, DC: 2016. [PubMed] [Google Scholar]
- 15.Satterfield DA, et al. Migratory monarchs that encounter resident monarchs show life-history differences and higher rates of parasite infection. Ecol Lett. 2018;21:1670–1680. doi: 10.1111/ele.13144. [DOI] [PubMed] [Google Scholar]
- 16.Tracy JL, Kantola T, Baum KA, Coulson RN. Modeling fall migration pathways and spatially identifying potential migratory hazards for the eastern monarch butterfly. Landsc Ecol. February 18, 2019 doi: 10.1007/s10980-019-00776-0. [DOI] [Google Scholar]
- 17.Forister ML, et al. Increasing neonicotinoid use and the declining butterfly fauna of lowland California. Biol Lett. 2016;12:20160475. doi: 10.1098/rsbl.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brower LP, et al. A long-term survey of spring monarch butterflies in north-central Florida. J Nat Hist. 2018;52:2025–2046. [Google Scholar]
- 19.Schultz CB, Brown LM, Pelton E, Crone EE. Citizen science monitoring demonstrates dramatic declines of monarch butterflies in western North America. Biol Conserv. 2017;214:343–346. [Google Scholar]
- 20.Rappole JH, McDonald MV. Cause and effect in population declines of migratory birds. Auk. 1994;111:652–660. [Google Scholar]