Significance
AD is a lethal vascular disease with a high mortality. The present study reports a critical role of prostaglandin E2 receptor EP4 in the pathogenesis of AD. VSMC-specific EP4 deletion (VSMC-EP4−/−) significantly increases the incidence and severity of AngII-induced aortic aneurysm and dissection. Compared with WT mice, VSMC-EP4−/− mice exhibit increased vascular inflammation, oxidative stress, elastic fiber degradation, and VSMC dedifferentiation following chronic AngII infusion. VSMC-EP4−/− mice also have higher blood pressure under basal and AngII-infused conditions. Our findings demonstrate that VSMC-EP4 plays an important role in the maintenance of vascular integrity and blood pressure and may represent a potential therapeutic target for the treatment of AD as well as hypertension.
Keywords: PGE2, EP4, vascular remodeling, inflammation, hypertension
Abstract
Prostaglandin E2 (PGE2) plays an important role in vascular homeostasis. Its receptor, E-prostanoid receptor 4 (EP4) is essential for physiological remodeling of the ductus arteriosus (DA). However, the role of EP4 in pathological vascular remodeling remains largely unknown. We found that chronic angiotensin II (AngII) infusion of mice with vascular smooth muscle cell (VSMC)-specific EP4 gene knockout (VSMC-EP4−/−) frequently developed aortic dissection (AD) with severe elastic fiber degradation and VSMC dedifferentiation. AngII-infused VSMC-EP4−/− mice also displayed more profound vascular inflammation with increased monocyte chemoattractant protein-1 (MCP-1) expression, macrophage infiltration, matrix metalloproteinase-2 and -9 (MMP2/9) levels, NADPH oxidase 1 (NOX1) activity, and reactive oxygen species production. In addition, VSMC-EP4−/− mice exhibited higher blood pressure under basal and AngII-infused conditions. Ex vivo and in vitro studies further revealed that VSMC-specific EP4 gene deficiency significantly increased AngII-elicited vasoconstriction of the mesenteric artery, likely by stimulating intracellular calcium release in VSMCs. Furthermore, EP4 gene ablation and EP4 blockade in cultured VSMCs were associated with a significant increase in MCP-1 and NOX1 expression and a marked reduction in α-SM actin (α-SMA), SM22α, and SM differentiation marker genes myosin heavy chain (SMMHC) levels and serum response factor (SRF) transcriptional activity. To summarize, the present study demonstrates that VSMC EP4 is critical for vascular homeostasis, and its dysfunction exacerbates AngII-induced pathological vascular remodeling. EP4 may therefore represent a potential therapeutic target for the treatment of AD.
Arterial remodeling is a process of adaptive alterations of vascular wall architecture and is physiologically important for vascular homeostasis. However, pathological vascular remodeling is involved in the pathogenesis of many cardiovascular diseases including vascular restenosis, hypertension, myocardial infarction, stroke, atherosclerosis, and aortic aneurysm, which account for the greatest number of deaths in both developing and developed countries. VSMCs are major resident cells of the arteries and play a critical role in vascular remodeling in conjunction with or without inflammatory cells (1). Increasing evidence demonstrates that the change in VSMCs from contractile to synthetic phenotype and the infiltration of monocytes and macrophages promote pathological vascular remodeling, leading to the development of many vascular diseases especially vascular restenosis and aortic aneurysm (2). Therefore, maintenance of the VSMC contractile phenotype and suppression of vascular inflammation are mainstays in the prevention and treatment of vascular diseases.
PGE2 is one of most important prostanoids in the regulation of vascular structure and function. It binds and activates four distinct G protein-coupled membrane-associated receptors, i.e., EP1, EP2, EP3, and EP4, to maintain vascular homeostasis. We and others have previously reported that the EP1 and EP3 receptors are vasoconstrictors, whereas the EP2 and EP4 receptors are vasodilators, indicating the PGE2/EPs system is essential for blood pressure regulation (3–6). Increasing evidence also demonstrates that the PGE2/EPs system plays a critical role in vascular remodeling (7, 8). EP4 appears to be important for both physiological and pathological vascular remodeling. Global EP4 gene deletion results in perinatal lethality due to patent DA (PDA) (9), indicating a critical role of EP4 in the physiological closure of the DA after birth. However, the role of EP4 in pathological vascular remodeling remains uncertain. It has been reported that inhibition of EP4 attenuates aortic aneurysm formation (10–12), whereas the lack of EP4 in hematopoietic cells is associated with increased prevalence and severity of AngII-induced aortic aneurysms (13).
In the present study, we found VSMC-specific EP4 deletion did not affect the DA closure but significantly promoted the incidence and severity of AD in AngII-infused mice, potentially via increasing blood pressure, vascular inflammation, matrix metalloproteinase activity, and VSMC dedifferentiation.
Results
Generation of Mice with VSMC-Specific EP4 Gene Deficiency.
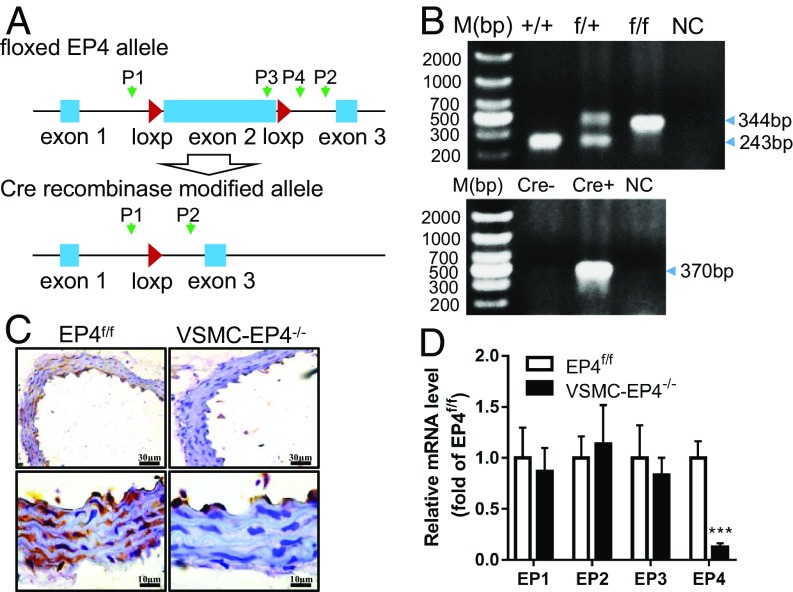
Dysfunction of VSMC proliferation and contractivity in neonatal DA has been proposed to be responsible for the development of PDA in EP4−/− mice. To determine the role of EP4 in vascular homeostasis, we generated a conditional gene knockout mouse line lacking EP4 specifically in VSMCs by crossing mice in which the exon 2 of the EP4 gene was flanked by two loxP sites (EP4f/f) (14) with the mice containing the SMMHC-Cre-ERT2 transgene (Fig. 1A). The WT, EP4f/+, and EP4f/f alleles and Cre transgene were genotyped by PCR (Fig. 1B). Near-complete excision of the EP4 gene (SI Appendix, Fig. S1A) and the loss of EP4 mRNA expression (SI Appendix, Fig. S1B) were evident in the SM layers of the aortas of VSMC-EP4−/− mice. Immunohistochemistry analysis further demonstrates that specific ablation of the EP4 protein was observed in VSMCs of the aortas of VSMC-EP4−/− mice with little change in endothelial cells (ECs) (Fig. 1C). Furthermore, VSMC-specific EP4 gene deletion resulted in a significant reduction in aortic EP4 mRNA expression, without affecting gene expression levels of the other three EP receptors (Fig. 1D).
Fig. 1.
Validation of EP4 deficiency in VSMCs. (A) Schematic showing of the floxed EP4 allele (EP4f/f) and Cre recombinase modified allele (EP4f/f_Cre+). The locations of two LoxP sites flanking the exon 2 of the EP4 gene were shown. P1 and P2 were primers designed to detect the presence or absence of the exon 2. (B) PCR validation of WT, EP4f/+, and EP4f/f alleles (Upper) and Cre recombinase transgene (Lower). The band with 243 or 344 bp represents the WT allele and floxed allele, respectively. The band with 370 bp indicates the presence of the SMMHC-Cre transgene. (C) Immunohistochemistry showing the absence of EP4 protein expression was in the aortic SM layer of the VSMC-EP4−/− mouse. (D) Expression levels of four EP receptors in the thoracic aortas of EP4f/f and VSMC-EP4−/− mice. n = 5–6; ***P < 0.001 vs. EP4f/f.
Unlike global EP4 gene-deficient mice (EP4−/−), VSMC-EP4−/− mice exhibited unimpaired DA closure with no perinatal death (SI Appendix, Fig. S2 A and B). No gender difference was found in mice with WT and VSMC-EP4−/− genotypes (SI Appendix, Fig. S2B). Similarly, no genotypic difference was observed in either male or female mice (SI Appendix, Fig. S2C). Furthermore, no developmental defect was found in VSMC-EP4−/− mice. The VSMC-EP4−/− mice exhibited normal body weight gain compared with EP4f/f mice (SI Appendix, Fig. S2D). These findings demonstrate that VSMC deficiency of EP4 in the DA very unlikely contributes to the development of PDA seen in global EP4 gene knockout mice.
EP4 Deletion in VSMCs Exacerbates AngII-Induced Aortic Dissection Without Increasing Serum Lipid Levels.
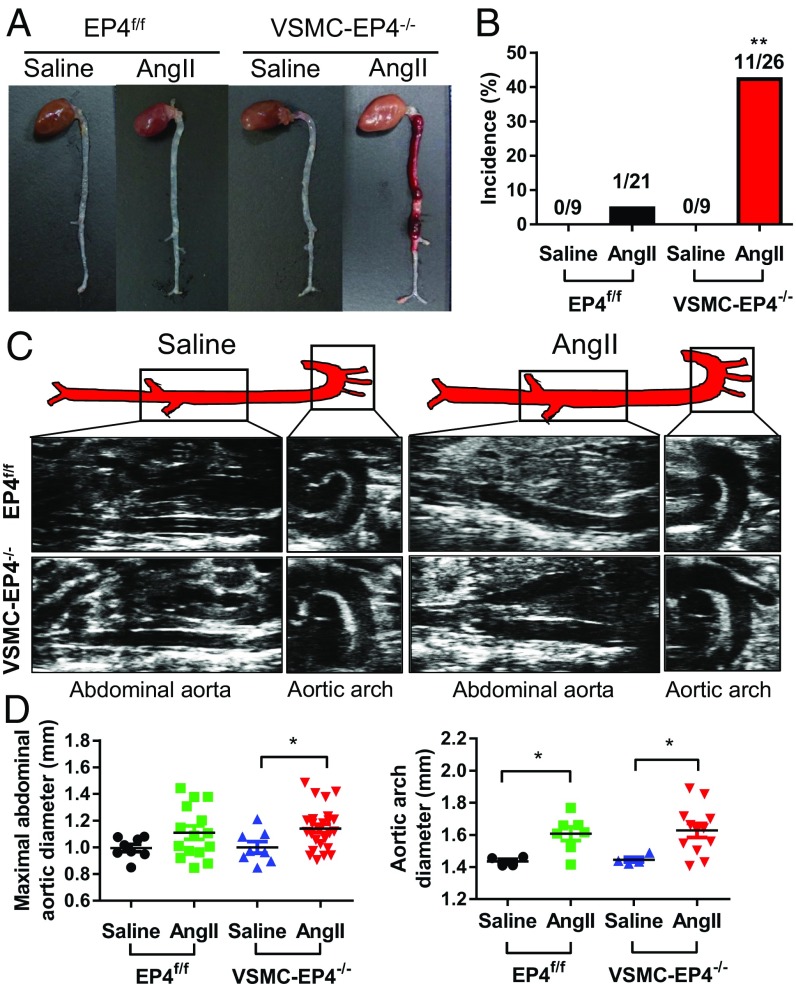
To further determine the role of EP4 in vascular homeostasis, we infused the VSMC-EP4−/− mice and their WT littermates with or without AngII for 28 d. Although AngII treatment resulted in aortic aneurysm in both genotypes, the incidence was significantly higher in VSMC-EP4−/− mice (11 out of 26 mice, 42.3%) than that in WT littermates (1 out of 21 mice, 4.76%) (P < 0.01) (Fig. 2 A and B). Aortic aneurysm exclusively developed in the thoracic and abdominal aortas in VSMC-EP4−/− mice (Fig. 2A). Furthermore, it was noticed that frequent aortic rupture occurred in VSMC-EP4−/− mice. Although 4-wk AngII treatment resulted in vascular expansion in injured aortas in both genotypes, no difference was found in maximal aortic arch and abdominal aorta diameters between WT and VSMC-EP4−/− mice (Fig. 2 C and D), suggesting VSMC deletion of EP4 exacerbates AngII-induced aortic dissection. Interestingly, AngII-induced aortic dissection in VSMC-EP4−/− mice was not associated with hyperlipidemia since AngII treatment had little effect on the serum lipid profile and serum levels of triglycerides, total cholesterol (Ch), LDL-Ch, and HDL-Ch were comparable between WT and VSMC-EP4−/− mice (SI Appendix, Fig. S3).
Fig. 2.
Mice with VSMC-specific deletion of EP4 are susceptible to AngII-induced aortic dissection. (A) Representative photographs of aortic dissecting aneurysms in male WT (EP4f/f) and VSMC-EP4−/− mice. AngII (1,000 ng/kg per min) was chronically infused for 28 d, and saline was used as the control. (B) Incidence of aortic dissection in WT and VSMC-EP4−/− animals. Difference in the incidence rate was analyzed by a χ2 test, **P < 0.01 vs. EP4f/f+AngII group. (C and D) Representative pictures from ultrasonography (C) and quantification of maximal aortic arch and abdominal aortic diameters (D) of WT and VSMC-EP4−/− mice at the end of 28-d saline or AngII infusion. n = 4–25; *P < 0.05 vs. saline group.
VSMC-EP4 Deficiency Results in Aortic Elastic Fiber Degradation in Mice.
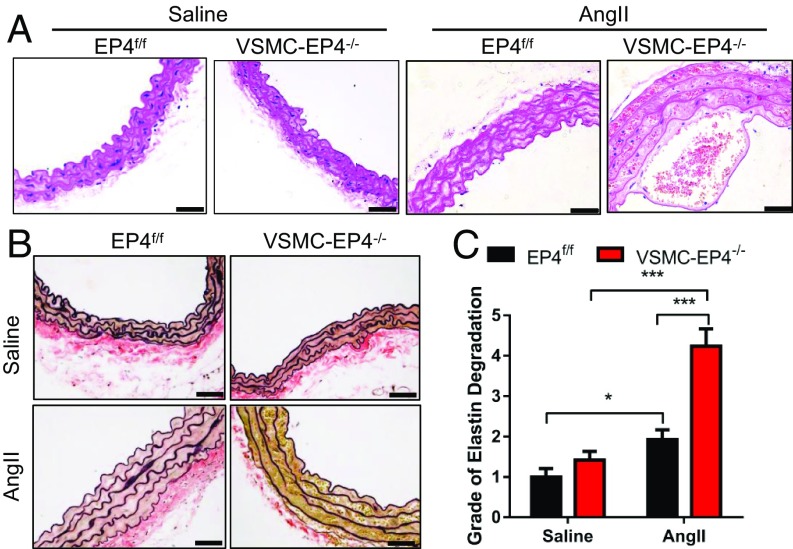
Histopathological analysis revealed typical aortic dissection lesions and identified aortic dissection as the main cause of death in VSMC-EP4−/− mice receiving chronic treatment of AngII (Fig. 3A). Elastic van Gieson (EVG) staining of aortic sections from VSMC-EP4−/− mice infused with AngII showed more severe media degeneration including elastic fiber fragmentation, stiffness, and disorganization (Fig. 3 B and C). However, Masson staining and Sirius Red staining found no difference in aortic collagen content between EP4f/f and VSMC-EP4−/− mice (SI Appendix, Fig. S4). These findings suggest a major role of VSMC EP4 in the dynamic maintenance of aortic elastic fiber.
Fig. 3.
VSMC-EP4 deficiency causes aortic elastin fiber degradation in mice. WT (EP4f/f) and VSMC-EP4−/− mice were infused with AngII (1,000 ng/kg per min) for 28 d. Saline infusion was used as the control. Aortas were collected for the examination of vascular morphology and structure. (A) Representative hematoxylin and eosin staining of the aortas of WT and VSMC-EP4−/− mice receiving saline or AngII treatment. (Scale bars: 100 μm.) (B) Representative EVG staining of aortic elastin in saline- or AngII-treated WT and VSMC-EP4−/− mice. (Scale bars: 100 μm.) (C) Quantification of aortic elastin degradation in B. n = 8–14; *P < 0.05; ***P < 0.001.
VSMC-Specific Deletion of EP4 Enhances AngII-Elicited Vascular Inflammation.
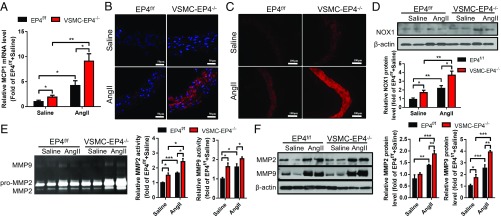
Extensive inflammation and oxidative stress are important for the development of aortic aneurysm and dissection (15). Chronic administration of AngII resulted in vascular inflammation as reflected by increased expression of MCP-1 in the aortas. Compared with WT mice, VSMC-EP4−/− mice exhibited significantly elevated MCP-1 mRNA levels under a basal condition and after AngII infusion (Fig. 4A). Consistently, VSMC-EP4−/− mice showed exaggerated F4/80 positive macrophage infiltration in the aortic media before and after AngII administration (Fig. 4B and SI Appendix, Fig. S5A). To further explore the potential role of EP4 in the regulation of MCP-1 expression in VSMCs, the EP4 agonist PGE1-OH was used to treat cultured VSMCs. Pretreatment of PGE1-OH greatly reduced AngII-evoked MCP-1 mRNA expression and protein secretion (SI Appendix, Fig. S5 B and C). In addition, Cre adenovirus-mediated EP4 gene ablation in primary cultured EP4f/f VSMCs resulted in a significant increase in MCP-1 mRNA expression. EP4 gene deficiency significantly up-regulated MCP-1 expression in VSMCs treated with AngII (SI Appendix, Fig. S5 D and E). Together, these findings indicate that VSMC-EP4 deficiency increases vascular inflammation mainly via inducing MCP-1 expression and secretion.
Fig. 4.
VSMC-specific deletion of EP4 results in increased vascular inflammation, oxidative stress, and MMP2/9 activity. (A) Real-time PCR analysis of aortic MCP-1 expression. n = 11–20; *P < 0.05; **P < 0.01. (B) Immunofluorescence staining of F4/80 (red) in the aortic walls of EP4f/f and VSMC-EP4−/− mice infused with saline or AngII. (Scale bars: 50 μm.) (C) DHE immunofluorescence staining of an aortic superoxide anion. (Scale bars: 100 μm.) (D) Representative Western blot analysis and quantification of NOX1 expression in each treatment group. n = 7–9; *P < 0.05; **P < 0.01. (E) Zymography analysis demonstrating enzymatic activity of MMP-2 and MMP-9 in the aortas of WT and VSMC-EP4−/− mice following saline or AngII treatment. n = 5 to 6; *P < 0.05. (F) Immunoblotting assay showing protein levels of MMP-2 and MMP-9 in the aortas of WT and VSMC-EP4−/− mice treated with or without AngII. n = 5–6; *P < 0.05; **P < 0.01; ***P < 0.001.
VSMC-Specific Deletion of EP4 Accelerates AngII-Induced Vascular Oxidative Stress.
AngII treatment markedly increased the numbers of dihydroethidium (DHE)-positive cells in WT mouse aortas. However, DHE-positive cells were further increased in VSMC-EP4−/− mice (Fig. 4C and SI Appendix, Fig. S6A), suggesting VSMC-specific EP4 deletion exacerbates AngII-induced vascular oxidative stress. To further determine the role of EP4 in regulating vascular oxidative stress in VSMCs, aortic NOX activity was measured in both WT and VSMC-EP4−/− mice treated with or without AngII. VSMC-specific EP4 deletion significantly increased AngII-induced vascular NAPDH activity (SI Appendix, Fig. S6B). Consistently, AngII infusion significantly up-regulated NOX1 protein expression in the aortas of both genotypes with much higher levels found in VSMC-EP4−/− mice (Fig. 4D). Similarly, VSMC-specific EP4 deletion significantly enhanced AngII-induced NOX1 mRNA expression with no effect on NOX2 and NOX4 (SI Appendix, Fig. S6C). In primary cultured VSMCs, the selective and nonselective EP4 agonist PGE1-OH and PGE2 significantly inhibited AngII-induced NOX1 expression at both mRNA and protein levels (SI Appendix, Fig. S6 D and E). In contrast, ablation of the EP4 gene in cultured VSMCs increased the basal levels of NOX1 mRNA and protein expression and further enhanced AngII-induced NOX1 expression. In addition, the adenylate cyclase activator forskolin dramatically attenuated AngII-induced NOX1 expression in both WT and EP4-deficient VSMCs (SI Appendix, Fig. S6 F and G). Collectively, these findings demonstrate that VSMC-EP4 deficiency enhances AngII-induced vascular oxidative stress by up-regulating NOX1 activity, possibly in a cAMP/PKA-dependent manner.
Specific Deletion of EP4 in VSMCs Increases Aortic MMP2/9 Activity.
One of the characteristic features of aortic aneurysm and dissection is the disruption and degradation of structural extracellular matrix proteins by MMPs, particularly MMP2 and MMP9 (16). Zymography and Western blot assays demonstrate that, although AngII treatment induced MMP2/9 protein expression and activity in both genotypes, EP4 deficiency in VSMCs resulted in significantly higher MMP2/9 levels than that in the WT group (Fig. 4 E and F). These observations indicate that VSMC-specific EP4 deletion leads to a marked increase in vascular MMP2/9 expression and activity in the aortas.
Deletion of EP4 in VSMCs Results in a Loss of SM Contractile Phenotype.
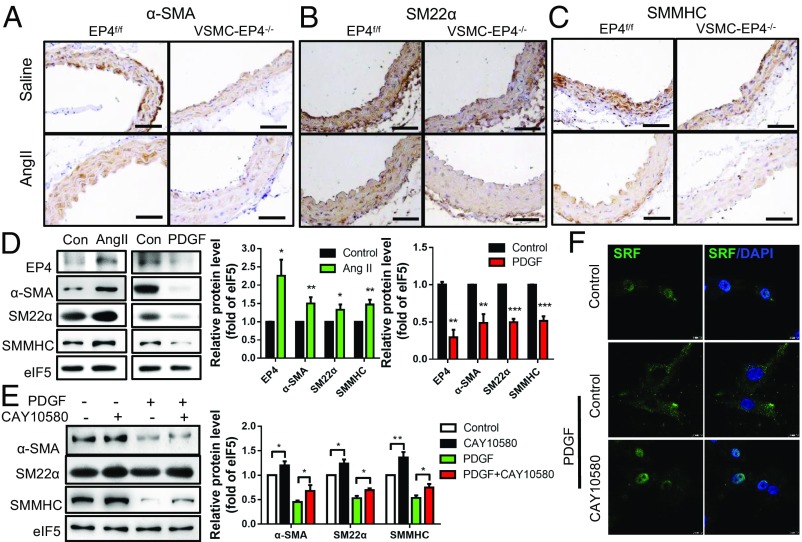
Hallmarks associated with aortic aneurysms and dissections include the loss of VSMC contractile markers, such as α-SMA, SM22α, and SMMHC (17). Immunohistochemical staining revealed that VSMC-specific EP4 deletion had little effect on α-SMA and SMMHC levels but significantly down-regulated SM22α expression at the basal level. Following chronic AngII infusion, VSMC-EP4−/− mice exhibited a significant reduction in aortic expression of all three contractile markers compared with WT mice (Fig. 5 A–C and SI Appendix, Fig. S7 A–C). These results demonstrate that EP4 is essential in the maintenance of the VSMC contractile phenotype.
Fig. 5.
EP4 is essential for maintaining VSMC contractile phenotype. (A–C) Representative immunostaining of α-SMA (A), SM22α (B), and SMMHC (C) in the suprarenal aortas of EP4f/f and VSMC-EP4−/− mice infused with saline or AngII. (D) Western blot analysis of AngII (100 nM) or platelet-derived growth factor BB (PDGF-BB) (25-ng/mL) treatment on protein expression of EP4 and VSMC markers (α-SMA, SM22α, and SMMHC) in rat aortic VSMCs. n = 3–6; *P < 0.05; **P < 0.01 vs. control. (E) Western blot analysis demonstrating the effect of EP4 activation on PDGF-BB-induced dedifferentiation of aortic VSMCs. Cells were pretreated with the EP4 agonist CAY10580 (100 nM) for 1 h and then treated with PDGF-BB (25 ng/mL) for 24 h. n = 5–6; *P < 0.05. (F) Immunofluorescence study showing that EP4 activation enhanced nuclear localization of SRF in primary cultured rat aortic VSMCs. Cells were pretreated with the EP4 agonist CAY10580 (100 nM) for 1 h and then treated with PDGF-BB (25 ng/mL) for 6 h.
EP4 Enhances Nuclear Translocation of SRF to Maintain the VSMC Contractile Phenotype.
In cultured VSMCs, EP4 expression levels are closely correlated with the phenotypic status (Fig. 5D). In an AngII-induced contractile phenotype, EP4 expression was increased as α-SMA, SM22α, and SMMHC levels were induced (Fig. 5D). In contrast, in the PDGF-BB-induced synthetic phenotype, EP4 expression was decreased as α-SMA, SM22α and SMMHC levels were reduced (Fig. 5D). Furthermore, pretreatment of VSMCs with the EP4 agonist CAY10580 prevented PDGF-BB-mediated contractile phenotype loss as well as α-SMA, SM22α, and SMMHC down-regulation (Fig. 5E and SI Appendix, Fig. S7D).
Previous studies reported that both global EP4 gene knockout and neural crest-restricted myocardin inactivation resulted in PDA in mice (18). Myocardin physically associates with the MADS-box transcription factor SRF to activate a subset of genes important for VSMC differentiation (19). Our results showed that the EP4 agonist CAY10580 significantly increased SRF nuclear translocation in the presence of PDGF-BB (Fig. 5F). Taken together, EP4 increases transcriptional activity of the SRF to maintain the contractile phenotype of VSMCs.
VSMC EP4 Disruption Increases Blood Pressure.
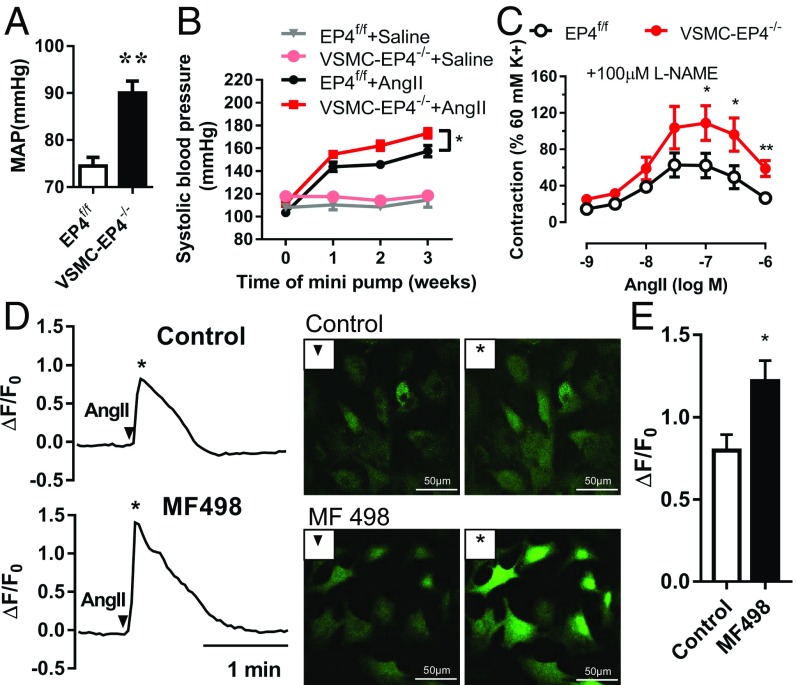
Compared with WT mice, basal blood pressure levels in VSMC-EP4−/− mice were significantly elevated (Fig. 6A). Upon AngII chronic infusion, VSMC-EP4−/− mice exhibited much higher blood pressure levels than that in WT mice (Fig. 6B). These findings demonstrate that EP4 is a vasorelaxant receptor. Its dysfunction is associated with enhanced response to AngII-induced vasoconstriction and the pathogenesis of hypertension.
Fig. 6.
VSMC-specific EP4 gene deletion causes hypertension. (A) Mean arterial blood pressure (MAP) levels in WT and VSMC-EP4−/− mice. Anesthetized EP4f/f and VSMC-EP4−/− mice were measured via the carotid arterial catheterization. n = 32–69; **P < 0.01 vs. EP4f/f. (B) Systolic blood pressure in EP4f/f and VSMC-EP4−/− mice at the baseline and in response to chronic AngII infusion. Blood pressure was monitored via tail cuff every 7 d over 3 wk. n = 9–26; *P < 0.05 vs. EP4f/f+AngII. (C) AngII-evoked vasoconstriction of mesenteric arterial rings from EP4f/f and EP4−/− mice. The rings were pretreated with N(ω)-nitro-l-arginine methyl ester (l-NAME, 100 μM) and then challenged with various doses of AngII. n = 10; *P < 0.05; **P < 0.01 vs. EP4f/f. (D) Representative images of AngII-elicited intracellular Ca2+ increase in VSMCs. VSMCs were pretreated with or without the EP4 antagonist MF498. ▼ represents Ca2+ fluorescence images at the baseline. * indicates images at the peak of induction. (E) The ratio of peak-to-basal change (ΔF/F0) in Fluo-4 acetoxymethyl fluorescence intensity in VSMCs treated with or without MF498. n = 7–12; *P < 0.05 vs. control.
VSMC-EP4−/− Mouse Mesenteric Arteries Exhibit Increased Response to AngII.
AngII-induced vasoconstriction was markedly increased in the mesenteric arteries from VSMC-EP4−/− mice compared with that from EP4f/f mice (Fig. 6C). However, no difference in phenylephrine-evoked vasoconstriction was observed between EP4f/f and VSMC-EP4−/− mice (SI Appendix, Fig. S8 A and B). In addition, both VSMC-EP4−/− mice showed a comparable decrease in EC-dependent vasodilatation induced by acetylcholine in mesenteric arteries (SI Appendix, Fig. S8C). These data support a critical role for VSMC EP4 in blood pressure homeostasis by antagonizing AngII-induced vasoconstriction.
EP4 Suppresses Ang II-Elicited Increase in Intracellular Ca2+ Levels in VSMCs.
Upon binding to its Gq-coupled receptor (AT1), AngII increases intracellular Ca2+ levels. Ca2+ then binds to calmodulin to form the Ca2+/calmodulin complex and activate the myosin light-chain (MLC) kinase to phosphorylate the MLC, leading to VSMC constriction. To determine the role of EP4 in AngII-induced intracellular Ca2+ increase, the effect of the EP4 antagonist (MF498) or agonists (PGE1-OH and CAY10580) on AngII-induced Ca2+ release was examined. Incubation of VSMCs with MF498 (1 μmol/L) for 30 min markedly potentiated AngII-induced increase in intracellular Ca2+ levels (Fig. 6 D and E). In contrast, pretreatment with the EP4 agonist PGE1-OH or CAY10580 for 30 min inhibited AngII-evoked Ca2+ signaling (SI Appendix, Fig. S9). Together, these results indicate that EP4 can attenuate AngII-elicited intracellular Ca2+ signaling and vasoconstriction.
Discussion
It has been previously reported that the PGE2/EPs system is essential for blood pressure regulation and is important for physiological and pathological vascular remodeling (3, 5, 6, 8–10, 20, 21). In the present study, we generated VSMC-specific EP4 gene knockout mice using the Cre-LoxP system and showed that EP4 gene deficiency in VSMCs greatly exacerbated AngII-induced aortic dissection. Following AngII infusion, VSMC-EP4−/− mice exhibited more severe vascular inflammation, macrophage infiltration, and oxidative stress, greater MMP2/9 activity, less contractile phenotype of SMCs, and higher blood pressure levels than that in WT mice. Mechanistic studies revealed that VSMC-EP4 gene deletion resulted in a marked increase in MCP-1 and NOX1 expression and a reduced SRF transcription activity. In addition, vasopressor response of mesenteric arteries to AngII was significantly enhanced in VSMC-specific EP4 deficient mice compared with WT littermates. Collectively, our findings demonstrate an important role for EP4 in regulating vascular remodeling and in the pathogenesis of aortic dissection.
Global EP4 gene ablation results in PDA, a clinical condition frequently occurring in preterm infants, suggesting an essential role for EP4 in physiological closure of the DA (9). Multiple mechanisms have been proposed to mediate the role of EP4 in DA closure, including promoting hyaluronic acid accumulation to form an intimal cushion at birth and inhibiting elastogenesis in the DA to permit the vascular collapse (21, 22). However, to date, it remains uncertain which cell type(s) of the DA are involved in this process. Based on our findings, VSMC-specific deletion of EP4 does not appear to cause PDA and perinatal lethality, suggesting that VSMC EP4 alone may not be sufficient for mediating DA closure. Other cell types especially the endothelium may be also required for physiological remodeling of the DA after birth.
Endothelium-specific deletion of EP4 in mice suppresses reendothelialization, increases neointimal leukocyte infiltration, and exacerbates neointimal formation after angioplasty wire injury (20), suggesting an important role for EP4 in vascular restenosis. However, the role of EP4 in the pathogenesis of aortic aneurysm has been the subject of conflicting reports. It has been previously reported that EP4 blockade attenuates abdominal aortic aneurysm in the ApoE−/− mice (10–12), whereas deletion of EP4 in bone marrow-derived cells worsens AngII-induced abdominal aortic aneurysm in low-density lipoprotein receptor−/− mice (13). In the present study, we provide clear evidence that VSMC-specific deletion of EP4 markedly sensitizes the mice to develop aortic aneurysms and dissection after chronic AngII infusion. Furthermore, it is also interesting to note that the development of aortic dissection in AngII-treated VSMC-EP4−/− mice does not require the presence of hyperlipidemia. Collectively, these observations demonstrate that VSMC EP4 may play a critical role in maintaining vascular integrity and function, and its dysfunction contributes to the pathogenesis of aortic aneurysm and dissection.
Aortic aneurysm/dissection are characterized by extensive molecular changes in the vascular wall and the synthetic phenotype of VSMCs. Multiple mechanisms have been proposed to promote the pathogenesis of aortic aneurysm/dissection, including vascular inflammation, oxidative stress, extracellular matrix (ECM) degradation, and VSMC dedifferentiation (23). In the present study, we found that VSMC-EP4−/− mice exhibit significantly higher aortic MCP-1 expression and more macrophage infiltration in comparison with WT animals. Enhanced vascular inflammation is mainly due to increased expression and secretion of MCP-1 in EP4-deficient VSMCs. VSMC-EP4−/− mice also show markedly increased vascular reactive oxygen species levels and ECM degradation, which appears to be a result of induced NOX1 expression and MMP2/9 activity in EP4−/− VSMCs and/or activated infiltrated macrophages. To date, the mechanism by which EP4 reduces vascular inflammation remains uncharacterized. Since it has been previously reported that IL-1β induces the transcription of MCP-1 via a NF-κB-dependent manner and EP4 activation can down-regulate the expression of MCP-1 in IL-1β-stimulated synovial fibroblasts (24), we anticipate that EP4 may suppress the MCP-1 expression in VSMCs and vascular inflammation by blocking NF-κB activity.
Loss of the contractile phenotype of VSMCs is associated with many vascular disorders, such as atherosclerosis, hypertension, and aortic aneurysm/dissection. In healthy vessels, VSMCs express a serial of contractile proteins, including α-SMA, SM22α, and SMMHC with a low replication rate. In response to vascular injury, VSMCs undergo a phenotypic switch from a contractile phenotype to a synthetic one with decreased expression of α-SMA, SM22α, and SMMHC and increased proliferation rate (2). Increasing evidence demonstrates that VSMC contractile gene expression and differentiation is under direct transcriptional control of the transcription factor SRF (25). EP4 has been reported to regulate cell differentiation of the preadipocyte, mesenchymal cell, and mouse embryonic fibroblast (26, 27). In the present study, we found that genetic deletion or pharmacological inhibition of EP4 leads to VSMC dedifferentiation with a significant loss of contractile marker proteins. In addition, EP4 activation can block PDGF-BB-induced SRF translocation from the nucleus to the cytoplasm. Collectively, these findings indicate that EP4 plays an essential role in the maintenance of VSMC contractile phenotype, possibly via increasing SRF transcription activity.
Aortic aneurysm/dissection is a life-threatening condition due to a tear in the intimal layer of the aorta or bleeding within the aortic wall, resulting in the separation of the different layers of the aortas. The likelihood that an aneurysm will rupture is largely influenced by persistent hypertension (28). Hypertension may contribute to the pathogenesis of aortic aneurysm/dissection via inducing VSMC dedifferentiation (29). A large body of evidence has demonstrated that the PGE2-EP receptor system plays an important role in blood pressure homeostasis. Vasopressor effect of EP1 and EP3 and vasodepressor effect of EP2 have been previously reported (3, 5, 6). In the present study, we found that VSMC-specific EP4 deficiency results in a significant increase in blood pressure level, suggesting that EP4 is a vasodilator. Consistently, VSMC-EP4−/− mice develop more severe hypertension following chronic AngII administration, and the VSMC-EP4−/− mesenteric artery shows more robust vasoconstriction in response to AngII treatment. Furthermore, pharmacological blockade of EP4 markedly enhanced AngII-induced increase in intracellular Ca2+ concentrations. Taken together, EP4 is essential in the maintenance of normal blood pressure, and its dysfunction in VSMCs elevates blood pressure to promote the pathogenesis of aortic aneurysm/dissection possibly via increasing intracellular Ca2+ signaling in VSMCs.
Hypertension plays an important role in the pathogenesis of aortic dissection. However, high incidence of aortic dissection in AngII-treated VSMC-EP4−/− mice appears to be not directly related to hypertension since blood pressure levels in the mice with aortic dissection were similar to the mice with normal aortas. In addition, regarding the role of VSMC-EP4 deficiency in vascular oxidative stress and inflammation, increased blood pressure in VSMC-EP4−/− mice may be important under both basal and AngII-treated conditions. Although it remains unknown why VSMC-EP4−/− mice exhibit increased vasopressor response to AngII, it is speculated that EP4 and the AngII receptor AT1 may have a functional cross talk in VSMCs.
To summarize, we have uncovered a critical role of the PGE2 receptor EP4 in vascular homeostasis. VSMC-specific EP4 deficiency significantly increases AngII-elicited vascular inflammation, oxidative stress, SMC dedifferentiation, and ECM degradation. VSMC-selective EP4 gene targeting also significantly increases arterial blood pressure level. These findings support an essential role of EP4 in the maintenance of vascular integrity and normal blood pressure level (SI Appendix, Fig. S10). Its dysfunction may contribute to the development of aortic aneurysm/dissection via sensitizing AngII-induced vascular inflammation and hypertension. EP4 may therefore represent an attractive therapeutic target in the treatment of aortic aneurysm and dissection.
Materials and Methods
All animal experiments were reviewed and approved by the Animal Care and Use Review Committee of Dalian Medical University. The study conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (30). VSMC-specific EP4 gene-deficient mice were generated using the Cre/LoxP system. The SMMHC-Cre transgenic mice on a 129 background were purchased from the Jackson Laboratory and backcrossed to a C57BL/6 background for four generations. EP4 flox/flox mice on a C57BL/6 background were generated as previously reported (14).
The AngII-infused hypertensive model, ultrasound imaging, chemicals and reagents, blood pressure recording, vascular tension assay of mesenteric arteries, histology, methods for primary culture of VSMCs, real-time PCR, Western blot, immunostaining, immunofluorescence staining, and statistical analyses are described in SI Appendix, Supplemental Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China Grants 91639201 and 81390351 (to Y.G.) and 81570636 and 81722010 (to X.Z.), and by Dalian High-level Talent Innovation Support Program 2016RD13. J.-Å.G. was supported by the Robert A. Welch Foundation (Grant E-0004).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1902119116/-/DCSupplemental.
References
- 1.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78:539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rzucidlo EM, Martin KA, Powell RJ. Regulation of vascular smooth muscle cell differentiation. J Vasc Surg. 2007;45(Suppl A):A25–A32. doi: 10.1016/j.jvs.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Guan Y, et al. Antihypertensive effects of selective prostaglandin E2 receptor subtype 1 targeting. J Clin Invest. 2007;117:2496–2505. doi: 10.1172/JCI29838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, et al. Characterization of murine vasopressor and vasodepressor prostaglandin E(2) receptors. Hypertension. 2000;35:1129–1134. doi: 10.1161/01.hyp.35.5.1129. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy CR, et al. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat Med. 1999;5:217–220. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, et al. Inactivation of the E-prostanoid 3 receptor attenuates the angiotensin II pressor response via decreasing arterial contractility. Arterioscler Thromb Vasc Biol. 2012;32:3024–3032. doi: 10.1161/ATVBAHA.112.254052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartlett CS, Boyd KL, Harris RC, Zent R, Breyer RM. EP1 disruption attenuates end-organ damage in a mouse model of hypertension. Hypertension. 2012;60:1184–1191. doi: 10.1161/HYPERTENSIONAHA.112.199026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu S, et al. Targeted disruption of the prostaglandin E2 E-prostanoid 2 receptor exacerbates vascular neointimal formation in mice. Arterioscler Thromb Vasc Biol. 2011;31:1739–1747. doi: 10.1161/ATVBAHA.111.226142. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen M, et al. The prostaglandin receptor EP4 triggers remodelling of the cardiovascular system at birth. Nature. 1997;390:78–81. doi: 10.1038/36342. [DOI] [PubMed] [Google Scholar]
- 10.Cao RY, et al. Prostaglandin receptor EP4 in abdominal aortic aneurysms. Am J Pathol. 2012;181:313–321. doi: 10.1016/j.ajpath.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama U, et al. Inhibition of EP4 signaling attenuates aortic aneurysm formation. PLoS One. 2012;7:e36724. doi: 10.1371/journal.pone.0036724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mamun A, et al. A selective antagonist of prostaglandin E receptor subtype 4 attenuates abdominal aortic aneurysm. Physiol Rep. 2018;6:e13878. doi: 10.14814/phy2.13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang EH, et al. Deletion of EP4 on bone marrow-derived cells enhances inflammation and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2011;31:261–269. doi: 10.1161/ATVBAHA.110.216580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao M, et al. Disruption of prostaglandin E2 receptor EP4 impairs urinary concentration via decreasing aquaporin 2 in renal collecting ducts. Proc Natl Acad Sci USA. 2015;112:8397–8402. doi: 10.1073/pnas.1509565112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuivaniemi H, Platsoucas CD, Tilson MD., 3rd Aortic aneurysms: An immune disease with a strong genetic component. Circulation. 2008;117:242–252. doi: 10.1161/CIRCULATIONAHA.107.690982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longo GM, et al. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ailawadi G, et al. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. J Thorac Cardiovasc Surg. 2009;138:1392–1399. doi: 10.1016/j.jtcvs.2009.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J, et al. Myocardin regulates expression of contractile genes in smooth muscle cells and is required for closure of the ductus arteriosus in mice. J Clin Invest. 2008;118:515–525. doi: 10.1172/JCI33304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mack CP. Signaling mechanisms that regulate smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol. 2011;31:1495–1505. doi: 10.1161/ATVBAHA.110.221135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao H, et al. Protective role of mPGES-1 (microsomal prostaglandin E synthase-1)-derived PGE2 (prostaglandin E2) and the endothelial EP4 (prostaglandin E receptor) in vascular responses to injury. Arterioscler Thromb Vasc Biol. 2018;38:1115–1124. doi: 10.1161/ATVBAHA.118.310713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokoyama U, et al. Prostaglandin E2 inhibits elastogenesis in the ductus arteriosus via EP4 signaling. Circulation. 2014;129:487–496. doi: 10.1161/CIRCULATIONAHA.113.004726. [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama U, et al. Chronic activation of the prostaglandin receptor EP4 promotes hyaluronan-mediated neointimal formation in the ductus arteriosus. J Clin Invest. 2006;116:3026–3034. doi: 10.1172/JCI28639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakalihasan N, et al. Abdominal aortic aneurysms. Nat Rev Dis Primers. 2018;4:34. doi: 10.1038/s41572-018-0030-7. [DOI] [PubMed] [Google Scholar]
- 24.Largo R, et al. EP2/EP4 signalling inhibits monocyte chemoattractant protein-1 production induced by interleukin 1beta in synovial fibroblasts. Ann Rheum Dis. 2004;63:1197–1204. doi: 10.1136/ard.2003.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horita H, et al. Nuclear PTEN functions as an essential regulator of SRF-dependent transcription to control smooth muscle differentiation. Nat Commun. 2016;7:10830. doi: 10.1038/ncomms10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noack C, Hempel U, Preissler C, Dieter P. Prostaglandin E2 impairs osteogenic and facilitates adipogenic differentiation of human bone marrow stromal cells. Prostaglandins Leukot Essent Fatty Acids. 2015;94:91–98. doi: 10.1016/j.plefa.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Inazumi T, et al. Prostaglandin E2-EP4 signaling suppresses adipocyte differentiation in mouse embryonic fibroblasts via an autocrine mechanism. J Lipid Res. 2011;52:1500–1508. doi: 10.1194/jlr.M013615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tchana-Sato V, Sakalihasan N, Defraigne JO. Aortic dissection. Rev Med Liege. 2018;73:290–295. [PubMed] [Google Scholar]
- 29.Zhang MJ, et al. TRPV1 attenuates intracranial arteriole remodeling through inhibiting VSMC phenotypic modulation in hypertension. Histochem Cell Biol. 2017;147:511–521. doi: 10.1007/s00418-016-1512-x. [DOI] [PubMed] [Google Scholar]
- 30.National Research Council . Guide for the Care and Use of Laboratory Animals. 7th Ed National Academies Press; Washington, DC: 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.