Significance
The premalignant colonic adenoma presents a long-lived target to prevent colon cancer. The levels of peptide biomarkers for four serum proteins—F5, ITIH4, LRG1, and VTN—are each elevated in sera in two adenoma-bearing animal models and in patients. The elevated levels of these serum proteins are correlated with total colonic adenoma number in the rat model. Longitudinal analysis of patients demonstrated their association with the subset of adenomas that continue to grow, but not with the total volume of the adenoma burden. This pattern of correlation with the number and growth, but not total adenoma volume, indicates that this class of marker can complement an emergent class of volume-dependent markers to detect the growing early colonic adenoma, minimizing overdiagnosis.
Keywords: murine models, quantitative mass spectrometry, longitudinal CT colonography, overdiagnosis, tumor volume
Abstract
A major challenge for the reduction of colon cancer is to detect patients carrying high-risk premalignant adenomas with minimally invasive testing. As one step, we have addressed the feasibility of detecting protein signals in the serum of patients carrying an adenoma as small as 6–9 mm in maximum linear dimension. Serum protein biomarkers, discovered in two animal models of early colonic adenomagenesis, were studied in patients using quantitative mass-spectrometric assays. One cohort included patients bearing adenomas known to be growing on the basis of longitudinal computed tomographic colonography. The other cohort, screened by optical colonoscopy, included both patients free of adenomas and patients bearing adenomas whose risk status was judged by histopathology. The markers F5, ITIH4, LRG1, and VTN were each elevated both in this patient study and in the studies of the Pirc rat model. The quantitative study in the Pirc rat model had demonstrated that the elevated level of each of these markers is correlated with the number of colonic adenomas. However, the levels of these markers in patients were not significantly correlated with the total adenoma volume. Postpolypectomy blood samples demonstrated that the elevated levels of these four conserved markers persisted after polypectomy. Two additional serum markers rapidly renormalized after polypectomy: growth-associated CRP levels were enhanced only with high-risk adenomas, while PI16 levels, not associated with growth, were reduced regardless of risk status. We discuss biological hypotheses to account for these observations, and ways for these signals to contribute to the prevention of colon cancer.
The overarching goal guiding this research is to reduce the increasing burden of colon cancer in the human population, first by identifying asymptomatic individuals at high risk of developing colorectal cancer. One route toward this goal is to detect and excise the premalignant 6- to 9-mm adenoma (1). The adenoma presents an estimated 17-y window for detection, much wider than the estimated 2-y interval from the localized frank colonic carcinoma to metastatic disease (2). The gold standard for the detection of colonic lesions is optical colonoscopy (OC). Recently, computed tomographic colonoscopy (CTC) has become an accepted alternative (3). The bowel preparation required for both OC and CTC reduces compliance in the general population. More generally, the requirement for a trained gastroenterologist or radiologist limits the range of populations that can be followed by OC or CTC. These screening methods demand resources inappropriate to the screening of large or isolated populations.
Progress is being made in developing minimally invasive methods to detect colon cancer, including tests for occult fecal blood and tumor-derived DNA (4). In contrast to their performance for frank colon cancer, the ability of these methods to detect the advanced colonic adenoma is currently unsatisfactory, ranging from 11 to 42% (4). Lutz et al. (5) have argued that that plausible levels of proteins secreted by the adenoma would be undetectable in blood. This study investigates whether significant changes in the level of serum proteins can be detected in patients carrying premalignant colonic adenomas. The study has been stimulated by the observation of altered levels of 19 serum proteins in ApcMin/+ (Min) mutant mice bearing early adenomas throughout the intestine (6). Nine of these proteins have also been successfully analyzed in sera from ApcPirc/+ (Pirc) mutant rats, whose intestinal adenomas are largely limited to the colon, as in the human (7). The precise measurement of the levels of these proteins has been made possible by the controlled genetic and environmental status of the mouse and rat models, and quantitative isotopic dilution methods involving selective reaction monitoring (SRM) mass spectrometry (6). However, a caveat to the interpretation of the observed changes in the murine models is the possibility that the mutant signal reflects a process other than intestinal adenomagenesis. The gene that is mutated in the germline of each animal model, adenomatous polyposis coli (Apc), is broadly expressed in mammals. Its mutations are known to confer extracolonic heterozygous phenotypes in mice (8–10), rats (11), and patients (12). Addressing this caveat, the 19 serum proteins were quantified in sera from patients carrying colonic adenomas that likely involve only somatic mutations in APC, not the constitutional heterozygosity for Apc of the two animal models.
Consensus evaluation of these two animal models by panels of histopathologists have reported that their colonic tumors rarely progress beyond the pedunculated adenoma—the colonic polyp (13, 14). It is plausible that the short life of a mouse or rat model will restrict cancer development unless additional mutations are introduced into the germline of the animal model (15). This early stage of the disease in humans is the optimal stage for prevention by polypectomy (16) because it is long-lived (2). Correspondingly, this report focuses on early colonic adenomas in patients. In this report, we use “tumor” in its generic sense to include adenomas as well as invasive frank adenocarcinomas. In describing the results of this study, we use “polyp,” “adenomatous polyp,” and “adenoma” interchangeably.
Longitudinal analysis of colorectal polyps in patients by CTC has shown that growing adenomas are likely to become high-risk adenomas and then develop into colorectal cancer (1, 17, 18). However, only between 22% and 33% of 6- to 9-mm polyps continue to grow; the majority of colorectal polyps in patients remain static or spontaneously regress (18, 19). Improvements in the prevention of colon cancer by early detection must be balanced by minimizing overdiagnosis. To this end, the longitudinally monitored CTC cohort provides a basis to judge the extent of association of changed levels of serum proteins with the growing adenoma. Additionally, samples from OC patients test the association of markers with colonic adenomas judged histologically to be at high risk to develop into frank cancer.
Thus, this study unifies the analysis of serum proteins from animal models for familial early adenomagenesis (14) from two genera—the mouse (Mus) and the rat (Rattus) (20, 21)—to a third genus (Homo). The same proteotypic peptide probe has been used for quantitation of each protein in the sera from all three genera. Thus, the overall strategy of discovery/validation for signals of adenomagenesis reduces biological “noise” by seeking conservation among three distinct genera. Each genus contributes a feature that enhances the signal-to-noise character of the experimental analysis. The mouse model enables differential metabolic labeling of the serum proteome, although with limited statistical significance (6). The rat model develops an informative range of numbers of early adenomas in the colon (7). The mouse and rat models each minimize genetic and environmental variation. Finally, the patient resource includes individuals who have consented to annotation of the growth trajectory of their early adenoma by CTC. In cases of observable growth in these patients, serum samples taken prepolypectomy and postpolypectomy enable a paired-sample analysis that controls for constitutional variation among patients. These paired samples additionally test for rapidly reversible dependence of the alteration in biomarker level on the presence of the intact adenoma.
Results
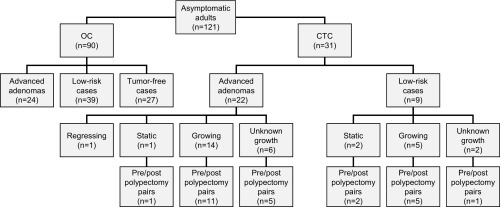
Ninety patients underwent screening by OC. In parallel, 31 patients with adenomas discovered by CTC were followed longitudinally (Fig. 1). Within the OC cohort, 27 appeared free of adenomas, while 63 had adenomatous polyps in the colon that were then resected. Based on the pathology reports of these 63 patients, 24 cases were classified as high-risk and 39 as low-risk. In the absence of longitudinal data, the tumors of the entire 63 adenoma-positive OC patients were necessarily classified as “unknown growth.” Because two independent, published CTC studies have shown that only 22–33% of adenomas 6–9 mm in maximum linear dimension continue to grow (18, 19), we assume that the majority of the adenomas classified as unknown growth in the OC cohort are nongrowing.
Fig. 1.
A summary of patient cases prospectively enrolled into this study. Some OC patients were judged to be free of colonic tumors (screening normal). Others carried polyps of unknown growth profile. These polyps were excised and classified by standard histopathologic criteria as low-risk or advanced adenomas. Polyps excised from CTC patients were also classified as advanced or low-risk adenomas. When available, their longitudinal size profiles classified them independently as growing, static, or regressing. Most, but not all, CTC patients returned for a postpolypectomy blood draw. The level of a biomarker of interest relative to its standard was compared between prepolypectomy and postpolypectomy sera.
Patients who opted for longitudinal CTC were monitored for a median time period of 5 y; the range was 1–11 y (SI Appendix, Fig. S1 and Table S1). Of 19 patients shown to carry growing adenomas, only 14 were then classified histologically as high-risk. Of the five growing adenomas that were classified histologically as low-risk, one had a maximum linear dimension of 10.4 mm. Nine of the CTC patients were histologically classified as carrying low-risk adenomas. Of these, five were paradoxically classified by longitudinal analysis as growing, two as static, and two as unknown in growth pattern. One member of the static class was histologically classified as high-risk (Fig. 1). Finally, five polyps observed longitudinally in three of the CTC patients regressed over time. When excised, three of these regressing polyps were also scored histologically as high-risk adenomas. Finally, one CTC patient carried an adenoma judged histologically as high-risk, but its growth pattern had not been ascertained. These observations indicate that the histological “high-risk” classification is correlated with, but not identical to, the longitudinal “growing” classification. In the interest of attenuating overdiagnosis, we would consider both growing and high-risk adenomas to be candidates for polypectomy. In the CTC cohort, diminutive lesions smaller than 6 mm in maximum linear dimension were ignored. In all CTC cases recorded in Fig. 1, at least one 6- to 9-mm, nondiminutive polyp was observed.
The median growth rate for all 31 polyps monitored by CTC was 3.2 mm3/y (mean, 11.4 mm3/y; range, −38.7 to +154.5 mm3/y) with a median final polyp volume of 74.0 mm3 (mean, 124.4 mm3; range, 7.0–900 mm3). The median growth rate for the 19 growing polyps was 7.7 mm3/y (mean, 19.5 mm3) with a median final polyp volume of 89.5 mm3 (mean, 159.5 mm3).
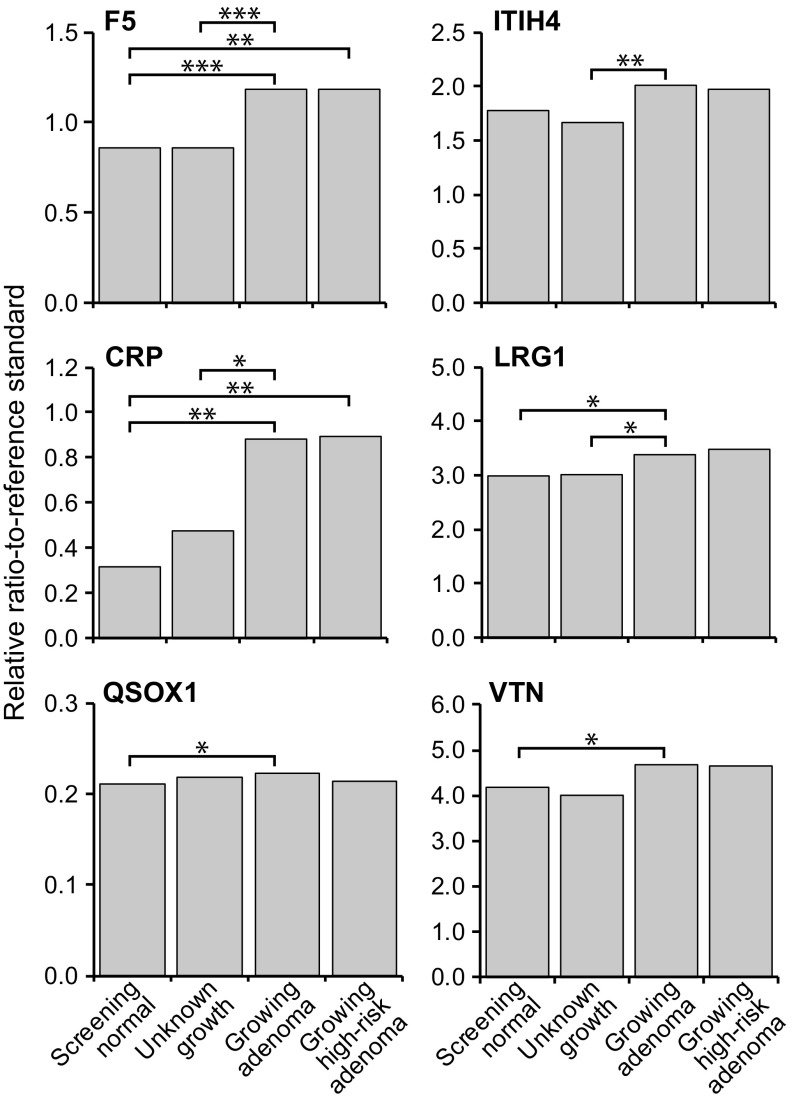
The 19 protein biomarker candidates (Table 1) from the mouse study (6) were also compared quantitatively in sera from two sets of OC patients (SI Appendix, Table S3): those classified as “screening normal” who were free of adenomas vs. those carrying adenomas of unknown growth. The cases scored as static or regressing were excluded from this analysis owing to their limited numbers. Patients with adenomas of unknown growth did not show statistically significant changes in serum protein concentration for any of the tested biomarkers, compared with screening adenoma-free cases (SI Appendix, Table S4a–c). The set of adenomas of unknown growth in the OC cohort is expected to contain only a minority of growing adenomas (18, 19). By contrast, the growing adenoma cases accrued in the CTC patient cohort gave a different result: CRP, F5, LRG1, QSOX1, and VTN each showed significantly enhanced serum levels compared with normal adenoma-free cases (Fig. 2). Consistent with this observation, in the known growing adenoma cases in the CTC cohort, CRP, F5, ITIH4, and LRG1 each also showed significant increases in serum concentration compared with the cases of unknown growth in the OC cohort. Although the observed differences in level are significant by targeted Mann–Whitney U test, they are not quantitatively strong enough to serve by themselves in detecting premalignant, growing colonic adenomas in patient populations. In Discussion, we consider the complementary roles they can play in the overall effort to detect selectively the growing premalignant colonic adenoma, reducing overdiagnosis.
Table 1.
Summary of protein biomarkers surveyed for colonic adenomagenesis from mouse to rat to human
| Animal model* | Panels: human (H); conserved (C) | ||||
| Gene symbol | Protein name | ApcMin/+ vs. +/+ | ApcPirc/+ vs. +/+ | This report† | |
| APCS | Serum amyloid P-component | 1 | ND | 0 | |
| CD44 | CD44 antigen | 3 | 0 | 0 | |
| CDH2 | Cadherin 2, type 1, N-Cadherin (neuronal) | 3 | ND | 0 | |
| CFI | Complement factor I | 2 | 0 | 0 | |
| CRP | C-reactive protein, pentraxin-related | 2 | ND | E | H |
| DPP4 | Dipeptidyl-peptidase 4 | 3 | ND | 0 | |
| EGFR | Epidermal growth factor receptor | 1 | D | 0 | |
| F5 | Coagulation factor V | 2 | E | E | H, C |
| FETUB | Fetuin B | 1 | ND | 0 | |
| HPX | Hemopexin | 2 | E | 0 | |
| ITIH3 | Inter-α trypsin inhibitor, heavy chain H3 | 1 | 0 | 0 | |
| ITIH4 | Inter-α trypsin inhibitor, heavy chain 4 | 1 | E | E | H, C |
| LRG1 | Leucine-rich α-2 glycoprotein | 1 | E | E | H, C |
| PI16 | Peptidase inhibitor 16 | 3 | ND | D | |
| QSOX1 | Quiescin Q6 sulfhydryl oxidase 1 | 2 | ND | E | |
| SOD3 | Superoxide dismutase 3, extracellular | 3 | ND | 0 | |
| THBS4 | Thrombospondin 4 | 3 | ND | 0 | |
| VITDBP | Vitamin D-binding protein | 1 | ND | 0 | |
| VTN | Vitronectin | 1 | E | E | H, C |
ApcMin/+ vs. +/+ data from Ivancic et al. (6): 1, high-replicate, high–statistical-confidence proteins were identified as being statistically differentially expressed in at least three out of four samples at the 52- and/or 66-d time points. Statistical significance was defined as having a U value less than 0.05 and a corresponding q value less than 0.05. 2, The category with high replicates but reduced statistical confidence included proteins that had statistically relevant differential expression in only one reciprocally labeled sample, or single peptide identifications where no statistical calculations could be made. 3, The proteins with low-replicate results that had little or no statistical substantiation either displayed no reciprocal sample validation, or the protein had only a single unique peptide hit changing at a log2 ratio of 1.0 or greater. ApcPirc/+ vs. +/+ data from Ivancic et al. (7): 0, no significant difference observed; D, diminished in sera from the F1 ApcPirc/+ rat vs. F1 wild-type rats; E, enhanced in sera from the F1 ApcPirc/+ rat vs. F1 wild-type rats; ND, not detected.
This report: 0, no significant difference observed; D, diminished in polyps; E, enhanced in growing adenomas.
Fig. 2.
Relative ratio-to-standard for four comparison groups in six proteins that showed statistically significant tumor-associated enhanced levels. Asterisks represent the significance level (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001) across the different adenoma growth and risk groups.
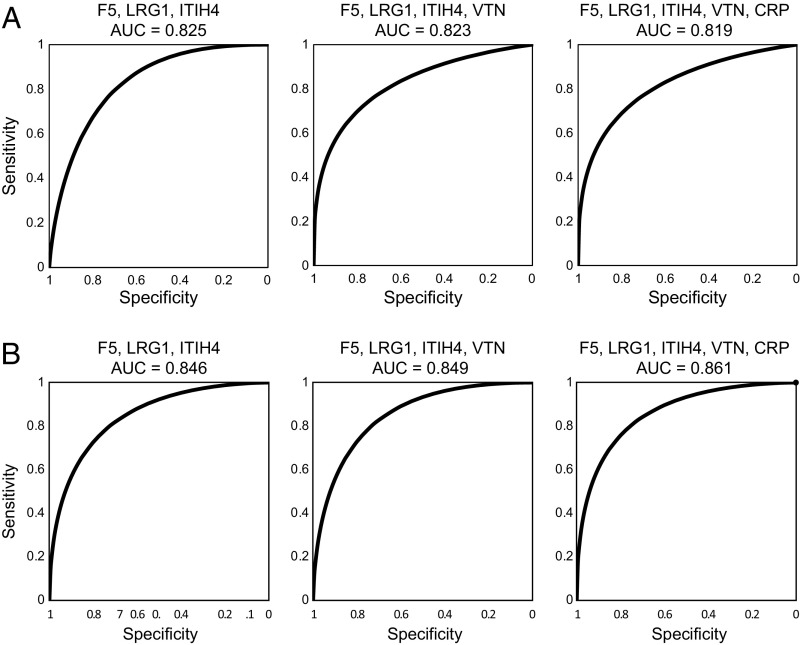
We assessed quantitatively the sensitivity and specificity for using these serum proteomic biomarkers to detect growing adenomas compared with adenomas of unknown growth. As summarized in Methods, a logistic regression analysis was carried out for each of the proteins, followed by a receiver operating characteristic (ROC) analysis for the probabilities of a positive test (SI Appendix, Table S5). The proteins that fit best in a logistic regression model were CRP, F5, ITIH4, LRG1, and VTN. The nominal area under the curve (AUC) values for these individual markers ranged from 0.632 (VTN) to 0.784 (F5) (SI Appendix, Fig. S2). Then, CRP, F5, ITIH4, LRG1, and VTN were assessed as panels of biomarkers (SI Appendix, Fig. S3). The highest AUC was 0.825 for a combination of F5, LRG1, and ITIH4 (Fig. 3). Adding VTN gave a statistically equivalent AUC of 0.823. When patients with growing adenomas were compared instead with adenoma-free screening normal patients, the performance of the panels marginally improved as measured by nominal AUC values: 0.846 for F5, ITIH4, and LRG1; 0.849 for F5, ITIH4, LRG1, and VTN; and 0.861 for CRP, F5, ITIH4, LRG1, and VTN (Fig. 3B). As outlined in Methods, these nominal AUC values can be made more precise in future studies to serve to distinguish rigorously among the several multimarker panels of elevated serum protein markers for the growing colonic adenoma in patients (SI Appendix, Fig. S2).
Fig. 3.
ROC analysis showing sensitivity and specificity of panels of biomarkers for detecting growing adenomas compared with adenomas of unknown growth status (A) or compared with normal controls (B).
These nominal AUC scores, although suggestive, are not sufficient evidence for the significance of the four marker panel F5, ITIH4, LRG1, and VTN. A strong test of significance can come from quantitative tests in an independent population, that in the Pirc rat model of early colonic adenomagenesis. This test of significance, depending on evidence for conservation between distinct mammalian genera, is addressed in Discussion.
Is the level of these biomarkers a function of the total adenoma volume in a patient? The four serum protein biomarkers F5, ITIH4, LRG1, and VTN as documented in the ApcPirc/+ rat showed levels positively correlated with the number of colonic adenomas (Fig. 2 and supplemental tables in ref. 7). In principle, this correlation could reflect a dependence on total adenoma volume, or simply on adenoma number alone. Correlation analyses of the sized adenomas in the CTC patient cohort resolved this issue. We have found no significant positive correlation between the total adenoma volume in a patient and the prepolypectomy levels of the four conserved serum markers, F5, ITIH4, LRG1, and VTN, nor of the two rapidly reversible markers, CRP and peptidase inhibitor 16 (PI16) (Table 2). The absence of a significant positive correlation with adenoma volume is not consistent with the hypothesis of secretion of the protein by the growing adenoma (5). In Discussion, we consider possible biological bases for the association of proteomic signals of interest with the number but not the final volume of the emergent colonic adenoma.
Table 2.
Test for correlation between tumor volume and differential biomarker level
| Patient | Total volume of adenomas | Prepolypectomy value for patient minus median of tumor-free controls | |||||
| F5 | ITIH4 | LRG1 | VTN | CRP | PI16 | ||
| 1 | 88 | 0.2006 | 0.1682 | −0.4543 | 0.6935 | 0.4216 | −0.2411 |
| 2 | 83 | 0.2705 | 0.2666 | −0.1245 | 0.0220 | −0.2630 | 0.2372 |
| 3 | 45 | 0.3427 | 0.6316 | 2.1361 | 1.6167 | 5.6626 | −0.1137 |
| 7 | 900 | 0.3438 | 0.6752 | −0.3025 | 0.9683 | 0.5870 | −0.2807 |
| 9 | 19 | 0.3245 | 0.4132 | 0.3231 | 0.6464 | 0.4393 | −0.1040 |
| 10 | 266 | 0.0067 | −0.3177 | −0.6473 | 0.1241 | 0.2493 | −0.2497 |
| 15 | 111 | 0.0346 | 0.1481 | 0.3792 | −0.0883 | 0.5677 | 0.4204 |
| 17 | 69 | −0.0431 | −0.0961 | 2.4822 | 1.2550 | 1.0925 | 0.0398 |
| 20 | 100 | 0.1949 | 0.3348 | −0.5072 | 0.6225 | −0.0682 | 0.0118 |
| 24 | 211 | 0.2733 | −0.1468 | 0.7395 | 0.1380 | 0.5134 | −0.0742 |
| 32 | 231 | 0.6716 | 0.7877 | 0.3385 | −0.1937 | −0.1975 | −0.1876 |
| 33 | 38 | −0.0376 | −0.2199 | −0.4052 | −0.7240 | −0.1866 | −0.0825 |
| Spearman | ρ | 0.24 | 0.06 | −0.25 | −0.15 | −0.04 | −0.38 |
| Two-sided P value | 0.42 | 0.83 | 0.40 | 0.63 | 0.89 | 0.21 | |
| Pearson | r | 0.26 | 0.32 | −0.27 | 0.13 | −0.12 | −0.41 |
| Two-sided P value | 0.42 | 0.32 | 0.40 | 0.68 | 0.70 | 0.18 | |
The total volume of the colonic adenomas carried by the patients shown in SI Appendix, Table S1 was calculated from their CTC images. The corresponding levels of each of the conserved markers (F5, ITI4, LRG1, and VTN) and the two rapidly reversible markers (CRP and PI16) were determined, relative to the labeled standard probe of each marker. A Spearman test was carried out for correlation between the monotonic rank orders of tumor volume and biomarker level. The Pearson r and Spearman ρ values and their P values are consistent with the null hypothesis—a lack of correlation between tumor volume and the level of each informative serum biomarker.
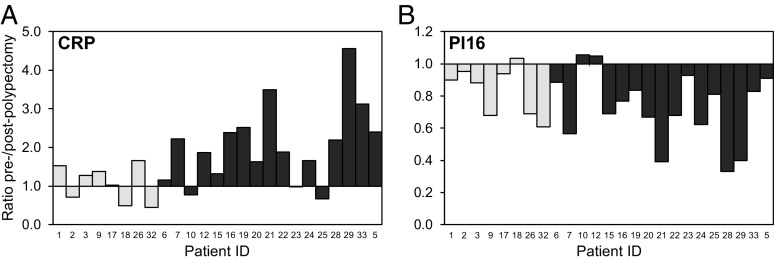
By controlling for constitutional variation among patients, the paired-sample analysis provided enhanced statistical power to associate the changes in serum level of a biomarker with a particular class of adenoma. Seventeen of the 25 CTC patients who provided a postpolypectomy blood sample (Fig. 1) were scored as high-risk and eight as low-risk, regardless of growth status. As displayed in Fig. 1, 16 of these 25 cases were classified as growing according to volumetric growth measurements, while 6 were of unknown growth status, and 3 were “static.” By Mann–Whitney U test, high-risk patients had an increase in CRP level that was significantly higher than that of low-risk patients (P = 0.013; Fig. 4A). Thus, the altered CRP signal appears to require the high-risk adenoma. The signal is rapidly reversed after polypectomy.
Fig. 4.
Paired-sample analysis of the specificity of biomarkers CRP (A) and PI16 (B). Each bar represents the ratio of prepolypectomy to postpolypectomy values for a single patient case. Patients are presented in the same left-to-right order in the two graphs. Dark gray represents high-risk cases, and light gray represents low-risk cases. CRP prepolypectomy/postpolypectomy ratios in high-risk cases were significantly different compared with low-risk cases (P = 0.013, Mann–Whitney U test). The PI16 prepolypectomy/postpolypectomy ratios did not significantly differ between high-risk and low-risk cases (P = 0.28, Mann–Whitney U test).
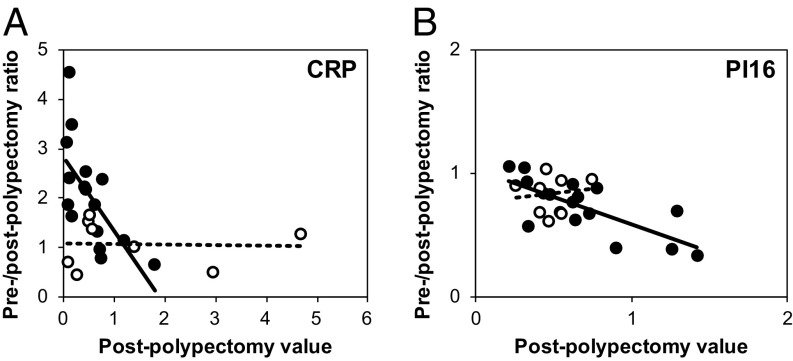
Matched pair analysis also gave evidence for association of reduced levels of the PI16 with adenomagenesis in patients. In contrast to CRP, the alteration in level of PI16 showed no significant separation between high-risk and low-risk cases (Fig. 4B; P = 0.28 by Mann–Whitney U test). This observation indicates that a reduced PI16 level may involve all polyps, independent of their risk status. Indeed, PI16 did not score among the set of markers associated with the growing adenoma in patients. For this analysis, the ambient level of the protein of interest in the adenoma-free host can play a decisive role in detection (5). As described in Methods, the prepolypectomy/postpolypectomy detection ratio was plotted against the postpolypectomy value (Fig. 5). As predicted, enhancement of the ratio for CRP was most significant at low ambient levels of the analyte. Correspondingly, significant normalization of PI16 levels was observed at higher ambient levels.
Fig. 5.
Paired-sample analysis of CRP (A) and PI16 (B) vs. ambient biomarker level. For CRP and PI16, the calculated ratios of biomarkers level are plotted against their corresponding baseline, expressed as the postpolypectomy level. Black-filled symbols represent high risk cases; white-filled symbols represent low-risk cases.
These matched pair analyses of CRP and PI16 provided added specificity that was not achieved in unpaired analyses of grouped prepolypectomy vs. postpolypectomy samples (SI Appendix, Fig. S4). CRP levels trended higher in prepolypectomy compared with postpolypectomy cases. However, this observed trend was not statistically significant over the study population at large. For PI16, the downward trend for grouped prepolypectomy cases compared with that of all postpolypectomy cases was significant (P value of 0.038). The four conserved markers found to be associated significantly with the growing adenoma—F5, ITIH4, LRG1, and VTN—did not rapidly revert to normal levels postpolypectomy. The possible biological significance of the more persistent changes in level of these markers, vs. the rapidly reversible changes in level of CRP and PI16, is considered in Discussion.
Discussion
In this study, 19 serum proteomic biomarkers, first drawn to our attention in studies in two distinct animal models for early colonic adenomagenesis, were quantitatively analyzed in sera from patients. Seven of the 19 markers showed significant differences in level in sera from patients carrying early adenomas. Four of these markers—F5, ITIH4, LRG1, and VTN—among nine that were also successfully validated in the rat, gave significantly enhanced levels in sera from the patients bearing polyps shown to be growing by CTC surveillance (Table 1). Two other markers that had not been able to validate in the rat model (CRP and QSOX1) also gave significantly enhanced levels in sera from patients bearing adenomas classified as high-risk on the basis of developing either histologically advanced or more than two adenomas (Fig. 2). This concordance of altered levels of expression of serum proteins in the murine models of familial colonic adenomatosis compared with the observations in sporadic adenomagenesis in patients rules out a caveat to the prior observations in the murine models. In particular, whereas each animal model involves a germline mutation in the broadly expressed murine Apc gene, sporadic adenomas in patients commonly involve only somatic mutations in the corresponding human gene APC (22). Thus, observing conserved enhanced expression of these serum proteins in patients bearing sporadic adenomas demonstrates that the signals observed in the mouse and rat models are not owing to extracolonic mutant phenotypes uncovered by constitutional germline heterozygosity for the mutated Apc gene in the animal models (8–10).
Two established factors in determining the clinical importance of a colorectal polyp are size and histology (23). This study considers in addition the criterion of continued growth that provides the opportunity to accumulate further mutations in the lineage. The longitudinal monitoring of early colonic adenomas in the CTC patient cohort provided evidence for the association of this conserved set of serum proteomic markers with the presence of growing early adenomas (Figs. 2 and 3). Of the 22 CTC patients with growing adenomas, 16 were classified as high-risk cases by standard histopathologic criteria (24). In 8 of these 22 cases, the terminal polyp size was large (≥10-mm maximum linear size), meeting one criterion for likelihood to develop into a frank carcinoma (17). As shown in Fig. 2, changes in the serum levels of F5, ITIH4, LRG1, and VTN are associated with growing adenomas in patients compared with the larger set of adenomas of unknown growth, where only a minority were expected to continue to grow (18, 19). The CRP protein, not yet successfully analyzed in the rat model, also shows association between elevated levels and the subset of patients with growing adenomas.
As stated in Results, the nominal ROC curves of the four conserved markers from the analysis of 19 candidates in patient sera lack rigorous confidence limits (Fig. 3). In future investigations, the precision of these ROC curves must be narrowed by bootstrapping analysis (Methods). On a qualitative level, the validity of the markers chosen by the ROC analysis must be tested in an independent population. Toward this end, 9 of the 19 candidates have been analyzed quantitatively in the independent study of sera from the Pirc rat model for familial colonic polyposis (7). At 135 d of age, when the multiplicity of colonic adenomas in the rat model is maximal, fold elevations in level of each of these four candidates were reported: F5, 1.24, P = 0.007; ITIH4, 1.37, P = 0.001; LRG1, 1.43, P < 0.001; and VTN, 1.20, P = 0.02. Confirmation across three distinct mammalian genera gives confidence in the significance of the four-protein panel of serum proteins for the detection of the growing early colonic adenoma. The primate lineage is estimated to have separated from the murid lineage ∼75 million years ago in evolution. The mouse and rat lineages then diverged from one another an estimated 12–24 million years ago (25).
The conservation of these altered signals across three distinct mammalian genera indicates that their expression is fundamental to colonic adenomagenesis (26). What biological processes are involved? Are they specific to colonic adenomagenesis? In what ways can the resources described in this report contribute to the overarching goal of reducing the incidence of colon cancer, worldwide? We shall discuss these emergent issues in light of the observations of this initial study.
A central finding of this study is the association in patients between changes in the levels of four conserved serum biomarkers, F5, ITIH4, LRG1, and VTN, with the subset of colonic adenomas observed to be growing or high risk in patients. Previous studies with the Pirc rat model for colonic adenomagenesis demonstrated that the level of these serum biomarkers is correlated with the numbers of colonic adenomas (7). However, the magnitude of elevation in patients is not correlated with the total size of the adenoma burden (Table 2). An effect associated with the growth rate of the adenoma seems unlikely, since differences in growth rate are reported also to affect the final size of the adenoma in the Min mouse model (27).
The enhanced statistical power of the paired-sample analysis, prepolypectomy vs. postpolypectomy, provided further information regarding the specificity and persistence of the association between serum biomarkers and colonic adenomagenesis. For CRP, only high-risk cases showed a statistically significant reversion of enhanced levels after polypectomy (Fig. 4A). For PI16, however, both high-risk and low-risk cases showed normalized levels in serum after polypectomy (Fig. 4B). This reversion to normal levels of the CRP and PI16 signals indicates that the presence of the polyp is necessary, directly or indirectly, for the change in level of these two proteomic signals. These adenoma-dependent changes in level were transient after polypectomy, and significantly detectable at low (CRP) or high (PI16) ambient levels (SI Appendix, Fig. S4). By contrast, the adenoma-associated enhanced levels of the four conserved serum protein markers, F5, ITIH4, LRG1, and VTN, did not normalize rapidly after polypectomy and were not dependent on their ambient levels (SI Appendix, Fig. S5). On a technical level, reversion to normal levels may require more than 3 wk after polypectomy. Alternatively, we consider testable biological hypotheses to explain changes in the level of a marker that is associated with the number but not the total volume of colonic adenomas—a slowly reversible biological basis for the persistent elevation of the F5, ITIH4, LRG1, and VTN levels, and a rapidly reversible basis for the changes in level of CRP and PI16.
One class of hypothesis would invoke a host response to the nascent adenoma. In principle, the response can be local, for example, in the formation of the stroma surrounding the tumor (28), or distal, as from the host liver (7), or systemic, as with mediators of the immune response (29) or the repair of epithelial wounding associated with the nascent adenoma. In this hypothesis, the four persistent markers, F5, ITIH4, LRG1, and VTN, would be involved in the repair of epithelial wounds that remain after polypectomy. For instance, both ITIH4 and VTN have been implicated in wound repair (30). By contrast, this class of hypotheses of a host response to the early adenoma, the two markers whose levels in serum were significantly normalized within 3 wk after polypectomy, CRP and PI16, would each be associated with an inflammatory response. Here, CRP overexpression has been associated with inflammation (31), while PI16 has been reported to be strongly down-regulated by cytokines associated with inflammation (32). Overall, in investigating these hypotheses involving host response to the adenoma, the regulatory transcription factor NF-κB is a candidate integrator of cancer, inflammation, and wound repair (29, 33, 34), worthy of experimental test.
Are the reported serum markers specific to early adenomagenesis? Are they specific to cancer only in the colon? The National Cancer Institute has developed from prostate, lung, colon, and ovary cancer patients an archive of serum, plasma, and buccal smears (35). This archive may help to address this question. The answers to these questions of the biological specificity of the elevated levels of F5, ITIH4, LRG1, and VTN observed in this study will determine the range and precision with which they can contribute to the detection and reduction in overdiagnosis of precancer and frank cancer over the entire cancer spectrum. Ahlquist (36) has outlined a global pan-cancer perspective into which the diagnostic range of quantitated blood protein markers can be incorporated. Quantitative mass spectrometry is an important feature of the analytic platform utilized in this study; its high molecular specificity lends itself to multiplexing.
Finally, how can the resources, principles, and finding underlying this study contribute to the overarching community goal of enhancing the power of detection of the premalignant colonic adenoma to reduce the incidence of colon cancer, worldwide? First, the genetically and environmentally uniform platforms of the Min mouse and Pirc rat enhance the signal/noise characteristics of discovery. The principle of seeking conservation between distinct genera sharpens the signal/noise character of the discovery and initial validation phases of the nomination of candidate biomarkers. Finally, the presentation by these animal models of the early, long-lived premalignant stage of colon cancer enhances the biological focus of the discovery process. The Pirc rat strain (F344-ApcPircUwm, RRID:RGD_1641862, RRRC_00782) is available to investigators at large through the Rat Resource and Research Center (RRRC) at the University of Missouri, an NIH-funded strain repository. The Min mouse strain used for this research project, C57BL/6JMlcr-ApcMin/Mmmh, RRID:MMRRC_043849-MU, is also available through the Mutant Mouse Resource and Research Center (MMRRC) at the University of Missouri. The biological precision of these animal models can be improved further by manipulating their genetic background, without sacrificing their genetic and environmental homogeneity. For example, in the standard Min mouse strain, adenomagenesis arises primarily in the small intestine rather than the colon (37). Clevers and coworkers (38) have developed an inducible transgenic mouse model that develops adenomas specifically in the proximal colon and cecum. The genetic background of the Pirc rat model can be manipulated to control the number of colonic adenomas (7). Overall, the principle of conservation across genera to discover the fundamental elements of a biological process can foreseeably be extended to the small prosimian mouse lemur if successfully inbred to minimize genetic and environmental noise (39).
On the molecular side, at the outset of this study in the Min mouse, the serum proteome was resolved only to a depth of 1,116 protein species (6). Note that changes in the level of CPR are detected preferentially at low ambient levels (Fig. 5). The sets of proteins identified in sera of adenoma-bearing Min mice (6), Pirc rats (7), and patients do not correlate with the major proteins detected by differential metabolic labeling in the colonic adenomas themselves of the Min mouse (40). Thus, further proteome-based discovery with animal models should proceed by a deeper exploration of the serum or plasma proteome. Recent advances have resolved plasma over nine orders of abundance (41).
This study has provided evidence (Table 2) that the enhanced levels of serum proteins associated with early colonic adenomagenesis, although associated with adenoma number, are not correlated with the total colonic adenoma volume. A quantitative method using alginate gels has been developed to follow longitudinally the growth profile of the early colonic adenoma in the Pirc rat model (42). As shown in that report, this method can document growing vs. static or regressing early colonic adenomas in the Pirc rat model.
In the end, the value of this approach to the prevention of colon cancer by the detection of the early growing or high-risk adenoma requires enhancement of the power of these serum protein markers by quantitative markers developed by other modalities (43). An immediate challenge is to determine whether it is possible to detect quantifiable markers whose levels are correlated with both the number and volume of the growing early premalignant adenoma. We expect that markers whose levels can be shown to be correlated with total adenoma volume (5) are likely to be statistically orthogonal in their scoring to the serum biomarkers observed in this study.
Research programs are being pursued for circulating tumor-specific DNA (44), circulating methylated DNA (45, 46), stool DNA (47), urinary metabolites (48, 49), and near-infrared imaging (50, 51). In the community-wide expansion of candidate markers through these distinct modalities, it is plausible that complementarity will be observed between the markers discovered from the sequencing (22) or methylation of tumor DNA, which may be idiotypic to the adenoma, compared with the markers involving quantitative levels of imaging, metabolic, or blood protein signals. We suggest that the quantitative power of these serum markers for discovering the growing early colonic adenoma will complement the power of statistically orthogonal markers discovered by other modalities. Markers whose levels are shown to be correlated with the total adenoma volume are prime candidates for such complementary signals.
Enhancing discovery must be balanced by attenuating overdiagnosis. In practice, ∼50% of all screening adults harbor at least one sub-centimeter polyp, but the prevalence of large polyps and the lifetime cancer risk are each only about 5% (1, 52, 53). Therefore, overdiagnosis with respect to cancer risk is at least 10-fold but probably even higher on a per polyp basis since many patients present with multiple 6- to 9-mm polyps. Selectively identifying the subset of early adenomas that continue to grow would constitute one step toward minimizing overdiagnosis. The Pirc rat model can effectively contribute candidates for final validation by resource-intensive longitudinally monitored patient resources.
Extending the strategies, resources, and findings of this report to the screening of populations encounters not only substantial research challenges, but also myriad economic, cultural, and logistical issues. One hopes that, if successful, the science can fruitfully intersect with these issues.
Methods
Human Subjects Protocol.
As diagrammed in Fig. 1, asymptomatic adult patients underwent colorectal cancer screening either by OC or by longitudinal CTC screening at the University Hospital and Clinics in Madison, Wisconsin. For the patients enrolled in CTC, the volume of a polyp was measured at an initial CTC scan and during at least one subsequent visit between 2 and 10 y later. Patients with growing polyps then underwent OC and polyp resection. For the patients undergoing routine OC screening, identified polyps were resected and analyzed for pathology. Patients in whom no polyps were identified during OC were considered normal screening controls free of adenomas. From all OC and CTC patients, blood was drawn and processed into serum according to procedures outlined by the Early Detection Research Network (54). For patients monitored longitudinally by CTC, a second blood draw was completed ∼3 wk postpolypectomy. The OC and CTC cohorts were each drawn from the same population of patients at the University of Wisconsin Hospital and Clinics. The age distribution of the 90 patients in the OC cohort was 58.3 ± 8.3 y and that of 24 patients in the CTC cohort was 60.5 ± 7.1 y (mean ± SD). By two-sided Wilcoxon rank sum test of the null hypothesis for difference in the age distribution between the two cohorts, the P value was 0.16. The distribution of sexes was 47 males to 43 females in 90 members of the OC cohort and 19 males to 12 females in 31 members of the CTC cohort. By χ2 test (1 df) of the null hypothesis of difference in the sex ratio of the two cohorts, the P value was 0.38. The Institutional Review Board at the University of Wisconsin–Madison approved all procedures related to this study. Patients were enrolled after providing informed consent.
Analysis of CTC and Colonoscopy Data.
The CT colonography procedure has been described in detail elsewhere (55). The majority of the patients enrolled in the longitudinal CTC cohort presented at least one polyp that was growing over time. Patients with static or regressing adenomas were generally excluded from further CTC analysis. Polyps less than 6 mm in maximum linear dimension were considered diminutive and were also not monitored further by CTC (56). Many of the 90 OC patients had large or diminutive polyps of unknown growth trajectory. These unknown-growth polyps were recorded as part of the final polyp count.
The set of data collected from screening OC and CTC patients was divided into three categories: from OC screening patients with polyps of unknown growth; from OC patients found to be adenoma-free; and from patients with polyps longitudinally monitored by CTC. A longitudinally monitored polyp was classified as growing, static, or regressing based on its volumetric growth profile: “growing” if it had increased in volume by 30% over time since first detected; “regressing” if it decreased in volume by 30%; or otherwise “static.” Volume is a much more sensitive indicator of change than maximum linear dimension. The 30% change criterion is an arbitrary threshold, chosen to enable unambiguous categorization into adenomas that are clearly progressing (figure 6 of ref. 1). Almost all frank colorectal adenocarcinomas are larger than 1 cm in maximum linear dimension. Although we cannot yet assign an actual per-polyp risk for cancer, we assert that a growing adenoma is at an enhanced risk for cancer, for instance owing to its capacity to acquire further mutations that support progression to the frank adenocarcinoma.
When a CTC patient was found to carry multiple polyps differing in growth pattern, the following classification hierarchy was used: patients carrying any polyp classified as growing were placed in the growing category; patients with polyps of unknown growth trajectory who also carried regressing or static polyps were classified as unknown growth; patients with both static and regressing polyps were grouped in the static class; and, finally, patients carrying a regressing polyp were classified as regressing only if all of their polyps were regressing.
All data for CTC patients were tabulated (SI Appendix, Table S1). The pathology for each resected tissue specimen from colonoscopy was evaluated regardless of its CTC-determined growth status. Based on standardized histopathologic criteria for assessing adenoma status (24), all polyps, regardless of growth status, were also histologically classified as either high-risk or low-risk. Here, a patient case was considered high risk if any of the following features was identified: three or more adenomas; at least one adenoma large in size (>1 cm in maximum linear dimension); presence of a villous component; a large size with a serrated histology; or high-grade dysplasia.
Protein Biomarker Selection.
As summarized in Table 1, serum protein biomarkers to be tested on patients were selected on the basis of previous serum biomarker studies performed with the pair of murine models of familial adenomatous polyposis (6, 7). Briefly, in these studies, sera had been resolved by liquid chromatography and proteins characterized by MS/MS. Candidate proteins of interest had been discovered first by their difference in level between members of pairs of differentially isotopically labeled sera from the ApcMin/+ mouse compared with Apc+/+ wild type. Nine of these 19 candidates were then successfully validated quantitatively in sera from F1 ApcPirc/+ rats compared with F1 Apc+/+ wild-type rats. The other 10 candidates could not be quantified in sera from the rat model, perhaps owing to the fact that the Pirc rat develops adenomas primarily in the colon, like the human, while the Min mouse model develops adenomas primarily in the small intestine (37). From these two animal studies, proteotypic peptides, conserved from mouse to rat to human, were selected for all 19 biomarker candidates (Table 1). These were used to analyze patient sera by SRM-MS/MS. Isotopically labeled proteotypic peptide reference standards were synthesized by the University of Wisconsin–Madison Biotechnology Center’s peptide synthesis facility, in general incorporating one 15N13C-doubly labeled amino acid into each peptide (SI Appendix, Table S2). Seven of the 19 candidates gave significant signals in sera from the patient cohorts. The two candidates of the nine that had given significant results in the Pirc rat analysis, HPX and EGFR, failed to give significant evidence for changes in level in the patient samples, plausibly owing to variation among patients that overwhelmed any signal. Of the seven candidates that gave significant changes in level in the patient samples, CRP, PI16, and QSOX1 were among those that had failed to provide significant values for changes in the Pirc rat study. CRP and PI16 are considered further in this report, on the basis of their changes in level after polypectomy. Finally, F5, ITIH4, LRG1, and VTN are considered further as conserved in providing positive evidence of adenoma-associated enhancement in level in all three genera.
Sample Preparation and Liquid Chromatography Coupled with MS/MS Data Collection.
Serum samples from patients were prepared for quantitative analysis as previously described (7). Briefly, whole blood serum (40 μL) was filtered using a 0.22-μm filter and then depleted of the top seven most abundant serum proteins using a 4.6 × 100-mm human multiple affinity removal system column (MARS; Agilent Technologies). A fixed amount of the proteotypic reference standard was spiked into each protein sample, before trypsin digestion. A 90-min liquid chromatography gradient then resolved 2 μg of purified tryptic peptides on a NanoLC Ultra 2D HPLC (Eksigent) column, equipped with a nano-flex cHiPLC set at 37 °C. Finally, the ratio of test to reference peptides was analyzed after resolution on a QTrap 5500 model triple quadrupole mass spectrometer (Sciex) with Q1 as a precursor ion mass filter, q2 to fragment, and Q3 to select the top three or four fragment ions for quantitation.
MS Data Analysis.
MS data were analyzed using Skyline Software (57). An average relative ratio-to-standard of three technical replicates was calculated by dividing the average peak area of the most intense transition by the average peak area of its corresponding reference standard peak. Protein levels were compared for each of the seven quantifiable candidate proteins across the different patient groups, using a nonparametric Mann–Whitney U test, setting significance at a P value of 0.05. Logistic regression analysis was carried out to estimate the probability of identifying, between the sets of “unknown growth” and “growing adenoma” cases, a patient with a growing adenoma, using a panel of two or more protein biomarkers (58). Probabilities calculated from this logistic regression (statpages.info/logistic.html) were then used to generate ROC curves for sensitivity and specificity [method 5 in the JROC fit calculator; www.rad.jhmi.edu/jeng/javarad/roc/JROCFITi.html (59)]. Here, the Mann–Whitney analysis of the patient samples, targeted to the candidate markers generated by the animal models, gave acceptable false-discovery rates (FDRs) (60, 61). Our confidence intervals were estimated conditional on the estimated probability of tumor growth status. For future improvements of the statistical analysis, we accept the point made by a reviewer that the generated ROC curves and AUC estimates are inherently biased since we used the same dataset to fit the model and to assess its predictive ability. Together with the fact that the dataset used to fit/train the model is small, this leads to predictive ability estimates that may be “optimistic,” by fitting the current dataset somewhat better than they would fit a new dataset. Alternative methods of obtaining predictive ability measures that adjust for this optimism need to be explored in future work with larger numbers of patient samples, including optimism-corrected AUC calculations (62) based on bootstrap replicates. Here, P values were reported without adjustment for multiple testing, as it was not our goal to control FDR across a list of initial candidates. Rather, in this first phase of this study where candidates for testing patient samples were initially identified, the risk of false negatives greatly outweighed the risk of false positives. As addressed in Discussion, we have been able to eliminate false positives through the results of independent quantitative tests using Mann–Whitney statistics of the conserved candidate markers in the Pirc rat model (7).
The analysis of Lutz et al. (5) emphasized that the detection of a signal depends on the background level of the marker in question. From the paired-sample analysis, a differential index was determined for each marker as the ratio in level between prepolypectomy and postpolypectomy samples. The numerator and denominator were each normalized by comparison with the standard for the biomarker in question. Two of the markers, CRP and PI16, changed in level within 3 wk after polypectomy. We assume that the level postpolypectomy closely represents the ambient level for these markers. For the other markers, the equivalent prepolypectomy and postpolypectomy level approximates the ambient level of the serum protein. The effect of the ambient biomarker level on detecting a signal was assessed by plotting the differential index against the estimated ambient level. Here, future studies are needed to determine the absolute levels of analytes that can detect these signals (5).
Supplementary Material
Acknowledgments
This research has depended on the community spirit of the patients involved, providing informed consent and in many cases second blood draws. We thank Christina Kendziorski and the staff of the University of Wisconsin Carbone Cancer Center (UWCCC) Biostatistics Shared Resource for continuing input on statistical issues and Shigeki Miyamoto for input on regulation by NF-κB. Alexandra Shedlovsky, Jeff Ross, and James Amos-Landgraf and the three external reviewers have provided extensive feedback on this communication. The University of Wisconsin–Madison Biotechnology Center mass spectrometry and peptide synthesis facilities have provided essential equipment and guidance in these studies. The University of Wisconsin–Madison Translational Sciences BioCore Biobank and UWCCC 3P Facility collected and processed patient blood samples. The project was supported by the Clinical and Translational Science Award Program through the NIH National Center for Advancing Translational Sciences [Grant UL1TR002373 (to W.F.D. and B.M.)]; The Wisconsin Alumni Research Foundation (to M.M.I., L.W.A., and M.R.S.); NIH Grant R37 CA063677 (to W.F.D.); NIH Grants T32 GM08349 and T32 CA157322-03 (to M.M.I.); Advanced Opportunity Fellowship through SciMed Graduate Research Scholars at University of Wisconsin–Madison (to M.M.I.); NIH Grants R01 CA169331-01, R01 CA144835-01, and R01 CA155347-01 (to P.J.P.); and NIH Grant R01 ES020900, University of Wisconsin School of Medicine and Public Health Shapiro Research Program (to B.M.). Shared research services at the UWCCC are supported by Cancer Center Support Grant P30 CA014520. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest statement: P.J.P. is cofounder of VirtuoCTC, consultant for Bracco and Check-Cap, and shareholder of SHINE, Elucent, and Cellectar. M.M.I., P.J.P., M.R., M.R.S., and W.F.D. are inventors on patent application PCTUS2015065049 submitted by The Wisconsin Alumni Research Foundation that covers the quantitative proteomic analysis, animal model, and patient resource design described in this report. The other authors declare no potential conflicts of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1813212116/-/DCSupplemental.
References
- 1.Pickhardt PJ, et al. The natural history of colorectal polyps: Overview of predictive static and dynamic features. Gastroenterol Clin North Am. 2018;47:515–536. doi: 10.1016/j.gtc.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones S, et al. Comparative lesion sequencing provides insights into tumor evolution. Proc Natl Acad Sci USA. 2008;105:4283–4288. doi: 10.1073/pnas.0712345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pickhardt PJ. CT colonography: Does it satisfy the necessary criteria for a colorectal screening test? Expert Rev Gastroenterol Hepatol. 2014;8:211–213. doi: 10.1586/17474124.2014.887436. [DOI] [PubMed] [Google Scholar]
- 4.Issa IA, Noureddine M. Colorectal cancer screening: An updated review of the available options. World J Gastroenterol. 2017;23:5086–5096. doi: 10.3748/wjg.v23.i28.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lutz AM, Willmann JK, Cochran FV, Ray P, Gambhir SS. Cancer screening: A mathematical model relating secreted blood biomarker levels to tumor sizes. PLoS Med. 2008;5:e170. doi: 10.1371/journal.pmed.0050170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivancic MM, et al. Candidate serum biomarkers for early intestinal cancer using 15N metabolic labeling and quantitative proteomics in the Apcmin/+ mouse. J Proteome Res. 2013;12:4152–4166. doi: 10.1021/pr400467c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ivancic MM, Irving AA, Jonakin KG, Dove WF, Sussman MR. The concentrations of EGFR, LRG1, ITIH4, and F5 in serum correlate with the number of colonic adenomas in ApcPirc/+ rats. Cancer Prev Res (Phila) 2014;7:1160–1169. doi: 10.1158/1940-6207.CAPR-14-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smits R, et al. Apc1638N: A mouse model for familial adenomatous polyposis-associated desmoid tumors and cutaneous cysts. Gastroenterology. 1998;114:275–283. doi: 10.1016/s0016-5085(98)70478-0. [DOI] [PubMed] [Google Scholar]
- 9.Kuo TL, et al. APC haploinsufficiency coupled with p53 loss sufficiently induces mucinous cystic neoplasms and invasive pancreatic carcinoma in mice. Oncogene. 2016;35:2223–2234. doi: 10.1038/onc.2015.284. [DOI] [PubMed] [Google Scholar]
- 10.Stoddart A, et al. Haploinsufficiency of del(5q) genes, Egr1 and Apc, cooperate with Tp53 loss to induce acute myeloid leukemia in mice. Blood. 2014;123:1069–1078. doi: 10.1182/blood-2013-07-517953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irving AA, et al. The utility of Apc-mutant rats in modeling human colon cancer. Dis Model Mech. 2014;7:1215–1225. doi: 10.1242/dmm.016980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasen HF, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP) Gut. 2008;57:704–713. doi: 10.1136/gut.2007.136127. [DOI] [PubMed] [Google Scholar]
- 13.Boivin GP, et al. Pathology of mouse models of intestinal cancer: Consensus report and recommendations. Gastroenterology. 2003;124:762–777. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 14.Washington MK, et al. Pathology of rodent models of intestinal cancer: Progress report and recommendations. Gastroenterology. 2013;144:705–717. doi: 10.1053/j.gastro.2013.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halberg RB, et al. Tumorigenesis in the multiple intestinal neoplasia mouse: Redundancy of negative regulators and specificity of modifiers. Proc Natl Acad Sci USA. 2000;97:3461–3466. doi: 10.1073/pnas.050585597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zauber AG, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lieberman D, Moravec M, Holub J, Michaels L, Eisen G. Polyp size and advanced histology in patients undergoing colonoscopy screening: Implications for CT colonography. Gastroenterology. 2008;135:1100–1105. doi: 10.1053/j.gastro.2008.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pickhardt PJ, et al. Assessment of volumetric growth rates of small colorectal polyps with CT colonography: A longitudinal study of natural history. Lancet Oncol. 2013;14:711–720. doi: 10.1016/S1470-2045(13)70216-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tutein Nolthenius CJ, et al. Evolution of screen-detected small (6–9 mm) polyps after a 3-year surveillance interval: Assessment of growth with CT colonography compared with histopathology. Am J Gastroenterol. 2015;110:1682–1690. doi: 10.1038/ajg.2015.340. [DOI] [PubMed] [Google Scholar]
- 20.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 21.Amos-Landgraf JM, et al. A target-selected Apc-mutant rat kindred enhances the modeling of familial human colon cancer. Proc Natl Acad Sci USA. 2007;104:4036–4041. doi: 10.1073/pnas.0611690104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrence MS, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levin B, et al. American Cancer Society Colorectal Cancer Advisory Group; US Multi-Society Task Force; American College of Radiology Colon Cancer Committee Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 24.Ahnen D, Macrae FA. 2016 Approach to the patient with colonic polyps. UpToDate. Available at https://www.uptodate.com/home Accessed August 14, 2017.
- 25.Mullins LJ, Mullins JJ. Insights from the rat genome sequence. Genome Biol. 2004;5:221. doi: 10.1186/gb-2004-5-5-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pleiman JK, et al. The conserved protective cyclic AMP-phosphodiesterase function PDE4B is expressed in the adenoma and adjacent normal colonic epithelium of mammals and silenced in colorectal cancer. PLoS Genet. 2018;14:e1007611. doi: 10.1371/journal.pgen.1007611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cormier RT, Dove WF. Dnmt1N/+ reduces the net growth rate and multiplicity of intestinal adenomas in C57BL/6-multiple intestinal neoplasia (Min)/+ mice independently of p53 but demonstrates strong synergy with the modifier of Min 1AKR resistance allele. Cancer Res. 2000;60:3965–3970. [PubMed] [Google Scholar]
- 28.Zheng H, Roy S, Soherwardy A, Rahman S, Kuruc M. Stroma liquid biopsyTM—a proteomic model of the systemic response to cancer. MOJ Proteomics Bioinform. 2017;6:236–241. [Google Scholar]
- 29.Maeda S, et al. Colon cancer-derived factors activate NF-κB in myeloid cells via TLR2 to link inflammation and tumorigenesis. Mol Med Rep. 2011;4:1083–1088. doi: 10.3892/mmr.2011.545. [DOI] [PubMed] [Google Scholar]
- 30.Adair JE, et al. Inter-alpha-trypsin inhibitor promotes bronchial epithelial repair after injury through vitronectin binding. J Biol Chem. 2009;284:16922–16930. doi: 10.1074/jbc.M808560200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu L, et al. Diets that promote colon inflammation associate with risk of colorectal carcinomas that contain Fusobacterium nucleatum. Clin Gastroenterol Hepatol. 2018;16:1622–1631.e3. doi: 10.1016/j.cgh.2018.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hazell GG, et al. PI16 is a shear stress and inflammation-regulated inhibitor of MMP2. Sci Rep. 2016;6:39553. doi: 10.1038/srep39553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaiopoulos AG, Papachroni KK, Papavassiliou AG. Colon carcinogenesis: Learning from NF-kappaB and AP-1. Int J Biochem Cell Biol. 2010;42:1061–1065. doi: 10.1016/j.biocel.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 34.DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 35.Carrick DM, et al. The PLCO biorepository: Creating, maintaining, and administering a unique biospecimen resource. Rev Recent Clin Trials. 2015;10:212–222. doi: 10.2174/1574887110666150730121429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahlquist DA. Universal cancer screening: Revolutionary, rational, and realizable. NPJ Precis Oncol. 2018;2:23. doi: 10.1038/s41698-018-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amos-Landgraf JM, et al. Sex disparity in colonic adenomagenesis involves promotion by male hormones, not protection by female hormones. Proc Natl Acad Sci USA. 2014;111:16514–16519. doi: 10.1073/pnas.1323064111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tetteh PW, et al. Generation of an inducible colon-specific Cre enzyme mouse line for colon cancer research. Proc Natl Acad Sci USA. 2016;113:11859–11864. doi: 10.1073/pnas.1614057113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ezran C, et al. The mouse lemur, a genetic model organism for primate biology, behavior, and health. Genetics. 2017;206:651–664. doi: 10.1534/genetics.116.199448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huttlin EL, et al. Discovery and validation of colonic tumor-associated proteins via metabolic labeling and stable isotopic dilution. Proc Natl Acad Sci USA. 2009;106:17235–17240. doi: 10.1073/pnas.0909282106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang HY, Beer LA, Speicher DW. In-depth analysis of a plasma or serum proteome using a 4D protein profiling method. Methods Mol Biol. 2011;728:47–67. doi: 10.1007/978-1-61779-068-3_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Irving AA, et al. A simple, quantitative method using alginate gel to determine rat colonic tumor volume in vivo. Comp Med. 2014;64:128–134. [PMC free article] [PubMed] [Google Scholar]
- 43.Song M, Vogelstein B, Giovannucci EL, Willett WC, Tomasetti C. Cancer prevention: Molecular and epidemiologic consensus. Science. 2018;361:1317–1318. doi: 10.1126/science.aau3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bettegowda C, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barault L, et al. Discovery of methylated circulating DNA biomarkers for comprehensive non-invasive monitoring of treatment response in metastatic colorectal cancer. Gut. 2018;67:1995–2005. doi: 10.1136/gutjnl-2016-313372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen SY, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579–583. doi: 10.1038/s41586-018-0703-0. [DOI] [PubMed] [Google Scholar]
- 47.Imperiale TF, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287–1297. doi: 10.1056/NEJMoa1311194. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Tso V, Wong C, Sadowski D, Fedorak RN. Development and validation of a highly sensitive urine-based test to identify patients with colonic adenomatous polyps. Clin Transl Gastroenterol. 2014;5:e54. [Google Scholar]
- 49.Deng L, et al. Development and validation of a high-throughput mass spectrometry based urine metabolomic test for the detection of colonic adenomatous polyps. Metabolites. 2017;7:E32. doi: 10.3390/metabo7030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim J, et al. Molecular imaging of colorectal tumors by targeting colon cancer secreted protein-2 (CCSP-2) Neoplasia. 2017;19:805–816. doi: 10.1016/j.neo.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones JE, Busi SB, Mitchem JB, Amos-Landgraf JM, Lewis MR. Evaluation of a tumor-targeting, near-infrared fluorescent peptide for early detection and endoscopic resection of polyps in a rat model of colorectal cancer. Mol Imaging. 2018;17:1536012118790065. doi: 10.1177/1536012118790065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.American Cancer Society 2017. Cancer Facts and Figures 2017 (Am Cancer Soc, Atlanta)
- 53.Pickhardt PJ, Kim DH. Colorectal cancer screening with CT colonography: Key concepts regarding polyp prevalence, size, histology, morphology, and natural history. AJR Am J Roentgenol. 2009;193:40–46. doi: 10.2214/AJR.08.1709. [DOI] [PubMed] [Google Scholar]
- 54.Tuck MK, et al. Standard operating procedures for serum and plasma collection: Early detection research network consensus statement standard operating procedure integration working group. J Proteome Res. 2009;8:113–117. doi: 10.1021/pr800545q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pickhardt PJ. Screening CT colonography: How I do it. AJR Am J Roentgenol. 2007;189:290–298. doi: 10.2214/AJR.07.2136. [DOI] [PubMed] [Google Scholar]
- 56.Pickhardt PJ, et al. Small and diminutive polyps detected at screening CT colonography: A decision analysis for referral to colonoscopy. AJR Am J Roentgenol. 2008;190:136–144. doi: 10.2214/AJR.07.2646. [DOI] [PubMed] [Google Scholar]
- 57.MacLean B, et al. Skyline: An open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26:966–968. doi: 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pezzullo JC. 2015 Logistic Regression. Available at statpages.info/logistic.html.Accessed March 28, 2019.
- 59.Eng J. 2017 ROC Analysis: Web-Based Calculator for ROC Curves. Available at www.rad.jhmi.edu/jeng/javarad/roc/JROCFITi.html. Accessed March 28, 2019.
- 60.Pascovici D, Handler DC, Wu JX, Haynes PA. Multiple testing corrections in quantitative proteomics: A useful but blunt tool. Proteomics. 2016;16:2448–2453. doi: 10.1002/pmic.201600044. [DOI] [PubMed] [Google Scholar]
- 61.Pawitan Y, Michiels S, Koscielny S, Gusnanto A, Ploner A. False discovery rate, sensitivity and sample size for microarray studies. Bioinformatics. 2005;21:3017–3024. doi: 10.1093/bioinformatics/bti448. [DOI] [PubMed] [Google Scholar]
- 62.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.