Abstract
Arginine is a conditionally essential amino acid that has been identified as an important player in a number of biologic processes, including normal function of the cardiovascular and immune systems. Countless studies have demonstrated that arginine is necessary for cellular growth and can become limiting in states of rapid growth like malignancy. Given this requirement for arginine in malignant cells, investigators have examined the potential for arginine deprivation therapy as an adjuvant treatment for cancers that are unable to synthesize arginine de novo. On the contrary, arginine has also been identified as being critically important for immune surveillance that also targets and destroys malignant cells. Thus, arginine has been paradoxically identified as being both necessary for cancer growth and normal immune function. A number of factors that include the type of cancer, expression of arginine synthetic genes, tumor microenvironment and host immune cell-cancer cell interactions can affect the progression of malignancy. In this manuscript, we review the data supporting arginine deprivation and supplementation in cancer therapies and the currently registered trials that are trying to understand which of these strategies might lead to advances in cancer therapies.
Introduction
Arginine (2-amino-5-guanidinovaleric acid) is an alpha-amino acid that is involved in a number of critical processes in human health and disease. Aside from being the nitrogen source for nitric oxide generated by endothelial and immune cells in vasodilatory and host-defense mechanisms, respectively, arginine is also used to synthesize creatine to meet muscle metabolic demands as well as urea synthesis to maintain whole-body nitrogen balance. Additionally, arginine stimulates protein translation and polyamine synthesis – anabolic and proliferative functions that become unregulated in cells after malignant transformation.
Given its vital role in cellular growth, proliferation and immune responses, arginine has been examined as a potential target for anti-cancer therapies. In the following review, we examine the in vitro and in vivo evidence supporting a role for this important amino acid as a metabolic target for anti-cancer therapy, including cellular arginine metabolism and the role of the microenvironment in metastasis and tumor growth. We also highlight the current studies that examine whether or not arginine supplementation or deprivation could provide an effective metabolic therapy for cancer patients.
Arginine Biochemistry and in vitro Tumor Cell Metabolism
Arginine is a conditionally essential amino acid, meaning that the body can synthesize sufficient amounts of arginine to meet basal metabolic demands. In times of stress or rapid growth (e.g. trauma, infection, wound healing, neonatal development), however, arginine demand is increased and availability becomes limiting. In mammals, arginine is synthesized via two major pathways. The first is referred to as the “Intestinal-Renal Axis”, in which dietary amino acids (i.e. proline, glutamate and glutamine) are converted to citrulline within the intestinal enterocytes. The newly synthesized citrulline is then released into the hepatic portal circulation. There is essentially zero hepatic clearance of citrulline, and thus all plasma citrulline recirculates from the portal to the systemic circulation. Once in the systemic circulation, the majority of citrulline is converted to arginine by the kidney, which expresses two key cytosolic urea cycle enzymes. These enzymes are argininosuccinate synthase (AS) and argininosuccinate lyase (ASL). Biochemically, cellular arginine can be synthesized depending on the expression of these arginine metabolizing enzymes or degraded if the cell expresses arginase or another arginine-degrading enzyme. The second pathway of arginine biosynthesis, which is similar to the intestinal-renal axis, is referred to as the “citrulline-nitric oxide cycle” and also relies on the expression of these same enzymes1. The citrulline-NO cycle exists in immune cells as a postulated mechanism to ensure a constant supply of arginine in lymphocytes for NO synthesis, a key player in the host immune response. In this pathway arginine is converted to NO and citrulline. Once again, AS and ASL interconvert cytosolic citrulline within the immune cell to arginine for continued NO production as needed for host defense.
Arginine Metabolism and Tumor Cells
In terms of tumor growth and proliferation, the ability to synthesize endogenous arginine from metabolic precursors is associated with expression of AS. Interestingly, no previous studies have demonstrated tumors that are null for ASL2. However, tumors can be conveniently grouped into those that express AS and those that do not express AS. The mechanism of why this dichotomization of expression occurs with AS and not ASL is unknown. AS is considered a house-keeping gene in normal cells and a rate-limiting biosynthetic enzyme in hepatocytes and endothelial cells. As mentioned, many tumor cell lines do not express AS3,4 and therefore have a critical dependence on exogenous arginine. Thus, tumor AS is a potential prognostic biomarker and predictor of sensitivity to arginine deprivation therapy5. Tumor cells that have either lost or do not have the ability to synthesize arginine de novo are considered auxotrophic for arginine6.
A number of human tumor cells, including melanoma, hepatocellular carcinoma and prostate carcinoma are frequently deficient in AS3. The mechanism by which AS is silenced in tumors is not completely understood. Previous work in lymphoma cell lines use methylation-dependent transcriptional silencing of AS, while melanoma cells repress the AS promoter via hypoxia-inducible factor-1alpha5,7,8. Additional evidence has demonstrated transcriptional silencing of AS in tumors due to gene hypermethylation of the AS promoter sequence9–11 and other silencing mechanisms12 have been described, though the complete molecular mechanisms are still needing identification. Because of the critical role of AS in tumor biology, this AS silencing event may function as a tumor suppressor. Consistent with this idea, studies in sarcoma and bladder cancer cell lines have shown that AS reduces colony forming ability, proliferation and invasion of tumor cells and abrogating growth of tumor xenografts in mouse models13–15.
The second key enzyme in arginine biosynthesis is argininosuccinate lyase (ASL) that acts immediately downstream of AS and catalyzes the conversion of argininosuccinate into arginine and fumarate. ASL is critical in channeling arginine for NO production via the NO-citrulline cycle. The function of ASL in cancer seems to be dependent on tumor type, and further work is necessary to understand the implications of ASL expression in human cancer. ASL has been found to be regulated in hepatocellular carcinoma and associated with aggressiveness mediated by NO and cyclin A2 signaling14. Studies have demonstrated that methylated ASL contributes to the arginine auxotrophy of glioblastoma multiforme, with loss of AS and ASL conferring greater sensitivity to ADI-PEG20, a compound that has been shown to significantly decrease the levels of arginine in the serum16.
There are numerous studies showing that arginine is required for in vitro tumor cell growth, consistent with the idea that many tumors are auxotrophic for arginine. Scott and colleagues17 conducted a screen of arginine starvation in twenty-six different murine or human cell lines and showed that with arginine starvation less than 10% survived more than five days under these conditions. In cells that remain viable, arginine starvation is reversible with arginine replacement18. It is important, especially from a cancer therapeutic perspective, to note that arginine starvation does not merely halt further cell growth, but it actually induces cell death in some cells lines. On a molecular level the exact mechanisms of cell death induction are not completely elucidated. However, arginine starvation has been linked to induction of autophagy19,20 as well as apoptosis. Consistent with this link to apoptosis, arginine auxotrophic cells in the absence of arginine undergo a Bcl-2-associated X protein (BAX) activation that likely effects the apoptotic cascade4.
The known induction of autophagy in response to arginine starvation is consistent with the growing link between autophagy and cancer cell apoptosis19–21. Autophagy and nutrient sensing are strongly linked to mTOR and GCN2 kinase22. For example, colorectal cancer cells in vitro quickly deplete intracellular arginine stores, which is known to trigger activation of the GCN2-mediated pathway and inhibition of mTOR signaling23. In tumor cells that have inhibited AS expression, depletion of exogenous arginine activates stress-related signaling pathways that may also result in cell death. mTOR-C1 signaling is down regulated once arginine starvation is sensed, resulting in mitochondrial and endoplasmic reticulum dysfunction, nuclear DNA breakage and chromatin/cell autophagy2. If protein synthesis is not restored by exogenous amino acid transport, cell death ensues. This phenomenon is currently the object of multiple studies to exploit it as a cancer therapy using three different approaches; nutritional deprivation of arginine, arginine transport inhibition, and enzymatic degradation of arginine.
Arginine and the Lymphocyte
Studies from the last four decades have confirmed that the immune system plays a critical role in immune surveillance of malignant cells within the body24–26. Early studies clearly established a role for arginine in immune system function, demonstrating that arginine supplementation has effects on T-cell proliferation27 and abrogation of posttraumatic T-cell suppression28,29. Arginine was also shown to enhances T-cell responses in nude mice30, although those effects have not been observed in other immune-activated states31. Regardless, it is well-accepted that arginine is necessary for optimal immune system function independent of its function in nitric oxide (NO) production for the host defenses. The exact mechanisms for the role of arginine outside of its critical role in NO production are still being elucidated.
Arginine Metabolism and the Tumor Microenvironment
Tumors cells interact with surrounding cells to create a tumor microenvironment that is permissive for growth and proliferation of the tumor itself. Tumors manipulate non-transformed host cells to create a supportive and protective microenvironment by depleting essential nutrients and accumulating immunosuppressive metabolites32. One such example is the local depletion of arginine by arginase or inducible nitric oxide synthase (iNOS), resulting in suppression of tumor-specific T cell responses33. Tumors activate arginase by expressing cyclooxygenase-2, whose concentration is elevated within the tumor microenvironment. This cyclooxygenase expression also aids with the suppression of T cell activation32. Additionally, macrophages, granulocytes and myeloid derived suppressor cells (MDSCs) that co-localize within the tumor microenvironment may also express arginase and iNOS to further exacerbate this local immunosuppressive environment. This subset of myelomonocytic cells, highly efficient at suppressing activated T cells, promotes tumor growth and metastasis. With this T cell suppression, the tumor-specific adaptive immune response is weakened as well as the immune response in general33,34. Recent studies in mouse breast cancer models have showed that supplementation with arginine prolonged survival of the host and inhibited tumor growth due to enhancement of innate and adaptive immune responses35
Arginine and Angiogenesis:
NO within the tumor microenvironment is an important mediator of tumor angiogenesis. Establishing ingrowth of vessels is necessary to provide oxygen to tumors larger than 1mm. Tumor-derived NO enhances angiogenesis and tumor invasion. Consistent with its role in angiogenesis and oxygen delivery, prior arginine administration enhances tumor sensitivity towards chemotherapy36. Moreover, in one model of murine colorectal cancer, arginine supplementation decreased tumor incidence and overall tumor burden when supplemented early in the disease course. Both of these examples demonstrate that despite arginine being critical for anabolic processes within the tumor cell itself, arginine can be viewed as tumor-promoting and tumor-inhibiting at the same time.
Aside from its role in angiogenesis and vascular relaxation, NO has also been linked directly to carcinogenesis by acting as a free radical and causing DNA damage. NO inactivates DNA repair enzymes, as well as inactivating the p53 tumor suppressor gene37,38. Local concentration of NO measured between benign and malignant tissues from breast, cervix, ovary, and stomach reveal significant correlations with increasing malignant phenotype39. Similar to the paradoxical effect on angiogenesis and chemotherapy, however, high concentrations of NO may cause p53-dependent cytostasis and apoptosis40. One mechanism that may be responsible for increased levels of NO in tumor microenvironment may be activation of iNOS and/or lactate dehydrogenase enzymes at high concentration, as opposed to eNOS and nNOS which are activated with lower concentrations of NO. Again, whether or not arginine and thus NO excess or deprivation is more beneficial in cancer biology appears highly dependent on tumor type and other factors.
Arginine metabolism and cachexia:
Even though there is not a universally accepted definition for cachexia, it is characterized by disproportional muscle mass loss to fat loss despite adequate food intake41. Glutamine is the a precursor for arginine synthesis and is mainly stored in skeletal muscle. One of the tumors’ protective mechanisms against the immune system is the recruitment of myeloid derived suppressor cells (MDSC). The MDSC are known to deplete arginine levels and disturbing NO production within the tumor environment. In an attempt to compensate for this arginine depression, glutamine and arginine are mobilized from skeletal muscle. On a molecular level, the decreased cellular arginine concentrations combined with the abnormal NO production activate several cascades known to inhibit protein synthesis and promote proteolysis, leading to cachexia42.
Arginine and the immune response:
Clearly there is evidence that arginine and its availability affect lymphocyte function, though it is not exactly clear whether arginine excess or deficiency in the microenvironment is beneficial or impairing in lymphocytic cancer surveillance. Along these lines, Sipple and colleagues conducted ex vivo studies of T-cell function in myeloid linear populations between patients with glioblastoma (GBM) and normal donors43. Patients with GBM were associated with elevated levels of circulating arginase-1 and T cell suppression; with this suppression being reversed by arginine supplementation. Similarly Rodriguez and colleagues34,44 showed that myeloid-derived suppressor cells are a subset of lymphocytes that exist in patients with renal cell carcinoma that overexpress arginase-1, theoretically limiting the availability for T-cells to use this as part of the immune response. Again these results, suggest that tumor cells may express arginase as a protective mechanism to subvert the host immune system. Overall, is not clear how suppressor cells of the immune system interact to promote or alternatively allow tumor cells to evade the immune system, however, this is an area that is receiving much attention (for reviews see45). Thus, the function or potential dysfunction of the immune system is a significant player in the development of malignant states and appears to be modulated by either presence or absence of arginine – a balance that is not yet entirely clear.
The specific role(s) of arginine in the immune response are of significant interest, as are the effects of arginine supplementation or deprivation on immune function and cancer surveillance. The role of the presence or absence of arginine in lymphoid malignancy and lymph function are still being identified. Interestingly, lymphoid malignancy cell lines10 and melanoma46 are particularly sensitive to arginine deprivation therapy in some studies, while other studies with breast cancer cell lines are inhibited by arginine supplementation35. This mechanism may be highly dependent on the tumor microenvironment effects involving lymphocytes, which is consistent with the anti-cancer functions of the immune system.
Our understanding of how arginine and its metabolism affect the immune system is complex, especially because each of the cellular components of the immune system may affect arginine availability or disposal differently. Tumor infiltrating macrophages (TIM) have a high content of arginase and may regulate the availability of arginine in the microenvironment of the tumor. Therefore, the finding that arginine supplementation inhibits the growth of immunogenic tumors may be due to the positive effects of arginine on immune system particularly macrophages, natural killer and T cell cytotoxicity39.
T cells play a very important role in cell-mediated immunity, maturing in the thymus and characteristically expressing the T cell receptor. To mount an efficient immune response, T cells require the assistance of myeloid-derived accessory cells to present antigen to the T cell receptor. Therefore, these accessory cells modulate the immune response as they decide whether an immune response should be initiated and T cells activated, and ensure that activated T cells are correctly switch off to avoid damage to host tissues. It has been shown that myeloid-derived accessory cells mobilize to specific tissues in number proportional to the antigenic insult47. These cells assist in clearance of antigen and, when necessary, mature into dendritic cells to sustain the immune response48,49. Moreover, myelomonocytic cells are capable of destroying invading pathogens, myeloid suppressor cells (MSCs), a subset of such cells, are also highly efficient at suppressing activated T cells. It has been shown that manipulation of arginine metabolism through the enzymes nitric-oxide synthase (NOS) and arginase, which, are regulated by T-helper 1 (TH1) and T-helper 2 (TH2) cytokines, respectively, is one way in which these cells regulate T cells. MSCs also seem to have an important physiological role in the regulation of T-cell activation during the contraction phase of the immune response33. MSCs generally inhibit T- and B-cell activation induced by antigen or polyclonal stimuli, using an MHC-independent mechanism that requires cell–cell contact.
Aside from altered the T-cell response, myeloid-induced lymphocyte dysfunction has been shown to occur with the release of common substances known to be released during host immune response such as cytokines, prostaglandins, reactive oxygen species, and eicosanoids50. Arginase and NOS, either independently or in conjunction, are used by MSCs to inhibit T-cell responses to antigen. Myeloid cells isolated from a mouse model of lung cancer incorporated and digested arginine from the extracellular environment, inhibiting re-expression of the ζ-chain of CD3 after its TCR-signaling-induced internalization by antigen-stimulated T cells, thereby impairing their function44. This is one of the few mechanisms that have been shown to directly inhibit T cells by means of decreasing amounts of available extracellular arginine. However, as arginine is depleted and other amino acid concentrations are depleted, this has been shown to alter transcription and translation of proteins in the tumor microenvironment. This includes T cells by inhibition of the mTOR pathway and translation initiation factors such as eukaryotic translation initiation factor 2 (EIF2-alpha)33.
On the other hand, NOS and its role in immunosuppression have been established. In T cells, NO has been shown to inhibit the activation of the IL-2-receptor cascade such as including Janus activated kinase 1 (JAK1), JAK3, STAT5, extracellular-signal-regulated kinase (ERK) and AKT51. In addition, when arginine levels are low in the microenvironment, the enzymatic reactions of arginase and NOS shift so that there is an accumulation of reactive oxygen species and reactive nitrogen oxide species (RNOS), which have been shown to negatively regulate immune response. Some experiments have shown that in the presence of ROS and/or RNOS apoptosis is triggered in activated T cells52. Overall, numerous cell types are likely differentially affected by the altered availability of arginine and the altered immune responses. Further research is necessary to understand the complex interplay between the immune system and the tumor microenvironment in order to identify an exploitable target in these multiple pathways that involved arginine and its metabolism.
Targeting Arginine as a Metabolic Therapy for Cancer Patients
As discussed, many cancers are dependent on a constant supply of arginine – either within the cell or from the organism/microenvironment. Since arginine is critical for proliferation/growth as well as the host immune response, arginine availability is at a crossroads of two potential pathways for cancer therapies. Each of these processes relies on ample arginine for optimal functioning, however, in one case ample arginine is contributing to tumor growth/proliferation, while the other case ample arginine is allowing the host immune response to function optimally. Thus, arginine deprivation or supplementation both have theoretical roles in cancer therapy.
Decreasing the availability of arginine to impair tumor growth is an idea that has been around for more than 40 years53. As mentioned above, arginine can be synthesized de novo in some human cells but, as one might expect, this endogenous synthesis can easily become overwhelmed in times of metabolic demand54. However, dietary restriction of arginine only leads to approximately 30% reduction of circulating arginine concentration55. Thus, other pharmacologic means are necessary to decrease arginine availability to have any meaningful tumor suppressive effect.
There are three major enzymes (arginase, arginine deiminase (ADI), and arginine decarboxylase (ADC)) that have been examined for effectiveness of arginine depletion in vitro (for a comprehensive review of these enzymes see56). Of note, the Km values of these enzymes for arginine tend to be exceedingly different. Thus only two enzymes, arginase and ADI, have been used for arginine deprivation therapies in human patients (reviewed in57).
Arginase is classically known as the final enzyme in the urea cycle, which converts arginine into ornithine and urea. In humans two isoforms exist, with arginase-1 being the cytoplasmic isoform and arginase-2 being expressed in the mitochondria58. Unlike arginase-1 that is expressed mostly in the liver, expression of the arginase-2 gene is nearly ubiquitous. Biochemically the Km of arginase-1 is ~5mM59, much higher than the typical arginine concentration in the plasma that is approximately 60–140μM60. Thus, arginase has a suboptimal effect for depleting circulating arginine from human plasma at physiologic concentrations55.
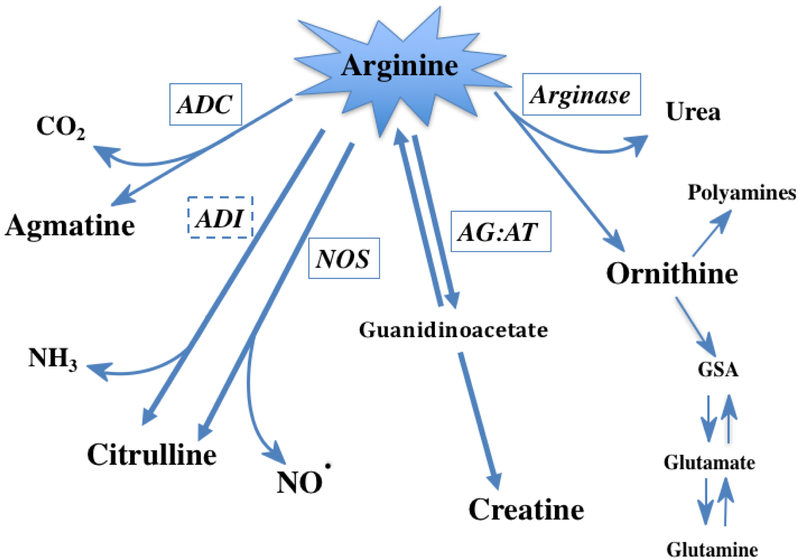
Arginine deiminase (ADI) is an arginine catabolizing enzyme that is not expressed by human tissues (Figure 1). It catalyzes the reaction that converts arginine to citrulline and ammonia. ADI has been shown to retard the growth of murine cell lines in culture61,62, an effect that is thought to be secondary to arginine deprivation. Consistent with the deprivation hypothesis, a number of in vitro studies from human cells have demonstrated that the effectiveness of ADI is dependent on deficiency of AS in the malignant cell. These tumors that are deficient in AS (reviewed in57), include: bladder15, breast21, esophageal63, glioblastoma multiforme16, head and neck64, malignant pleural mesothelioma4, myxofibrosarcoma11, nasopharyngeal carcinoma9, osteosarcoma13, ovarian65 as well as pancreatic cancer cell lines66. As a bacterially-produced enzyme, ADI does have the potential disadvantage of being immunogenic in humans. However, continued understanding of this enzyme and potential alterations to its structure and function using modern molecular biologic approaches67 may continue to improve the prospects for this enzyme in arginine-deprivation therapies.
Figure 1. Metabolic Fates of Arginine.
Arginine can be degraded by a number of enzymes within the cell. Of the five enzymes pictured, four are expressed in humans while arginine deiminase (ADI) has only been described in non-mammalian organisms/bacteria. Arginine decarboxylase (ADC) has been detected in neural tissue and degrades arginine to carbon dioxide and agmatine. ADI and nitric oxide synthase (NOS) both produce citrulline and either ammonia or nitric oxide, respectively. Arginine:glycine amidinotransferase (AG:AT) is important for synthesis of creatine. Arginase, the final enzyme of the urea cycle, produces urea as well as ornithine that can re-enter the urea cycle or be used by another set of enzymes for the production of polyamines or converted to other amino acids. Note: all of the enzymes pictured above except for ADI (dashed box) are expressed in humans (solid boxes).
As mentioned above, arginine decarboxylase (ADC) converts arginine to carbon dioxide and agmatine, and is mostly found in plants and bacteria. However, it does appear to be expressed in humans at very low amounts in the brain/CNA68. It has been shown that recombinant human ADC has good activity, however, the PEGylated version of the enzyme loses most of its activity69. Thus, whether or not ADC might be used therapeutically in the future is unclear.
Should we be depriving arginine or supplementing arginine?
The crux of the issue of whether or not to supplement or deprive arginine in cancer patients is still not entirely clear. This is due to the fact that sufficient arginine is important for immune function and cancer surveillance, although sufficient arginine also allows cancer cells to growth uninhibited with respect to arginine availability. Molecular characteristics, (e.g. AS expression) and production of endogenous arginine may lead to a more ‘precision-like’ approach to individual cancer care depending on biopsy results and tissue testing for AS or other important enzymes metabolically. A number of studies have or are currently examining the effects of either arginine-deprivation (Table 1) or arginine-supplementation (Table 2) on patient outcomes in cancer.
Table 1.
Trials Examining Arginine-Deprivation in Cancer Patients
| Trial | Status | Subjects (enrollment) |
Phase | Therapy | Findings or Primary Endpoint |
|---|---|---|---|---|---|
| Izzo et al73 | Completed | HCC (n=19) |
1/2 | PEG-ADI | PEG-ADI well-tolerated, possible efficacy |
| NCT0045037246 | Completed | Metastatic Melanoma (n=38) |
1/2 | PEG-ADI | Tumor AS expression predicted drug resistance and progression |
| NCT000569922 | Completed | HCC (n=80) |
2 | PEG-ADI | PEG-ADI antibodies developed at 4 weeks and correlated with increasing plasma arginine |
| NCT01910025 | Completed | Non-Hodgkin’s Lymphoma (n=18) |
2 | PEG-ADI | Results Pending – patients had previously failed medical therapy |
| NCT01910012 | Ongoing | Acute Myeloid Leukemia (n=43) |
2 | PEG-ADI | Response Rate |
| NCT01665183 | Ongoing | Metastatic Melanoma (n=8) |
1 | PEG-ADI + Cisplatin | Number of participants with adverse events |
| NCT01287585 | Ongoing | Advanced HCC with Prior Failed Medical Therapy (n=636) |
3 | PEG-ADI | Overall Survival vs. Placebo |
| NCT01266018 | Ongoing | Relapsed Sensitive or Refractory Small Cell Lung Cancer (n=45) |
2 | PEG-ADI | Efficacy at 4 weeks |
| NCT01528384 | Ongoing | Any AS-deficient Pediatric Cancer (n=8) |
1 | PEG-ADI | Safety Monitoring |
| NCT02029690 | Ongoing | Arginine Auxotrophic Cancers (n=88) |
1 | PEG-ADI + Pemetrexed & Cisplatin | Safety Monitoring / Estimates of Efficacy |
| NCT02285101 | Ongoing | Advanced Arginine Auxotrophic Tumors (n=36) |
1 | Arginase-1 (recombinant human Arg1) | Number of participants with adverse events |
Abbreviations: PEG, polyethylene glycol; HCC, hepatocellular carcinoma; ADI, arginine deiminase; AS, argininosuccinate synthetase
Table 2.
Trials Examining Arginine-Supplementation in Cancer Patients
| Trial | Status | Subjects (enrollment) | Phase | Arginine Dose | Endpoint / Findings |
|---|---|---|---|---|---|
| NCT02017249 | Results Pending | Glioblastoma Multiforme (n=1) |
1 | 24.15g TID x 14 days | Change in Immune Function Labs/Testing |
| NCT00006340 | Ongoing | EBV(+) Cancer or Lymphoproliferative disorders (n=20) |
1 | Escalating Arginine dose with ganciclovir therapy | Safety and Toxicity |
| NCT00917826 | Terminated | EBV(+) Lymphoid Malignancies (n=1) |
1 | - | - |
Abbreviations: EBV, Epstein Barr Virus; TID, 3 times daily.
Clinical data continue to emerge to further our understanding of arginine in cancer therapeutics, but results continue to generate more questions as the complexity of this metabolic response is increasingly appreciated. For example, in lung cancer patients, plasma levels of arginine are decreased compared to controls regardless of the patient’s body mass index, cancer stage, or patient’s weight loss70,71. This may be due in part to reduction of endogenous arginine synthesis and parallel increase in NO production72. Administration of an amino acid mixture increased plasma arginine concentration by increasing whole body arginine synthesis. However, the plasma arginine concentration after the intake of the mixture remained lower in the non-small cell lung cancer as compared with the healthy group, though the effects of cancer progression were not measured72.
Animal studies have shown that oral arginine supplementation may provide some protection against tumorigenesis in certain settings. In a colon cancer mouse model, tumor production and crypt cell hyperproliferation was decreased when arginine was given during early stages of carcinogenesis. However, when given during promotion stage, tumor growth was enhanced36. There are a few current studies registered in Clinicaltrials.gov (Table 2) that are attempting to gather evidence on the safety of arginine supplementation.
In terms of clinical therapeutics, PEGylated arginine deiminase (ADI-PEG20, Polaris Group) is furthest along the path of clinical development from combinatorial phase 1 to phase 3 trials as an arginine starvation mechanism (Table 1). Studies on hepatocellular carcinoma and melanoma have shown that after administration of ADI-PEG 20, plasma arginine levels decrease by 24 hours and remain low for at least seven days. In addition, therapy also generates a reciprocal increase in plasma citrulline and a decline in plasma nitrite and nitrate levels due to reduced NO synthesis. Phase 1 data of several studies show response rates of between 25–47% with good safety and tolerability73,74. The common adverse reactions have been self-limiting injection site reactions, skin rashes, arthralgia, and rarely neutropenia, anaphylactoid reactions and serum sickness. The latest ADI-PEG20 clinical trials recorded stable disease as the best response, however, evidence of a rebound in arginine levels in plasma at about 50 days post initiation of therapy was found, possibly due to drug neutralizing antibodies75,76. The role of combining arginine deprivation with autophagy inhibitors, glutamine and glycolytic inhibitors, modulators of the tumor microenvironment and radiotherapy, are other options to explore in the context of future clinical trials.
Summary
Our understanding of the complex intersection between arginine biochemistry, physiology and tumor cell biology has made tremendous advances over the last several decades. As tissue genetics and typing of malignancies becomes increasingly common, it is increasingly likely that focused metabolic therapies for cancer exploiting arginine and potentially other nutrients will be targeted in cancer and other diseases. The results from current trials comparing the effects of arginine supplementation and deprivation will likely lead to a better understanding of the susceptibility of particular cancers to metabolic therapies and novel approaches in cancer treatment. As the field of nutritional therapeutics continues to progress, Warburg’s seminal observation of tumor metabolism and strategies to exploit it for the benefit of human health continue to be realized.
References
- 1.Husson A, Brasse Lagnel C, Fairand A, Renouf S & Lavoinne A Argininosuccinate synthetase from the urea cycle to the citrulline–NO cycle. European Journal of Biochemistry 270, 1887–1899 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Fultang L, Vardon A, De Santo C & Mussai F Molecular basis and current strategies of therapeutic arginine depletion for cancer. International Journal of Cancer 139, 501–509 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Dillon BJ et al. Incidence and distribution of argininosuccinate synthetase deficiency in human cancers. Cancer 100, 826–833 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Szlosarek PW et al. In vivo Loss of Expression of Argininosuccinate Synthetase in Malignant Pleural Mesothelioma Is a Biomarker for Susceptibility to Arginine Depletion. Clinical Cancer Research 12, 7126–7131 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Delage B et al. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. International Journal of Cancer 126, 2762–2772 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Feun L et al. Arginine deprivation as a targeted therapy for cancer. Curr. Pharm. Des 14, 1049–1057 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long Y et al. Arginine Deiminase Resistance in Melanoma Cells Is Associated with Metabolic Reprogramming, Glucose Dependence, and Glutamine Addiction. Mol Cancer Ther 12, 2581–2590 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai W-B et al. Resistance to arginine deiminase treatment in melanoma cells is associated with induced argininosuccinate synthetase expression involving c-Myc/HIF-1 /Sp4. Mol Cancer Ther 8, 3223–3233 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lan J et al. Deficiency in expression and epigenetic DNA Methylation of ASS1. Tumor Biol. 35, 161–169 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Delage B et al. Promoter methylation of argininosuccinate synthetase-1 sensitises lymphomas to arginine deiminase treatment, autophagy and caspase-dependent apoptosis. Cell Death Dis 3, e342 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang H-Y et al. ASS1 as a Novel Tumor Suppressor Gene in Myxofibrosarcomas: Aberrant Loss via Epigenetic DNA Methylation Confers Aggressive Phenotypes, Negative Prognostic Impact, and Therapeutic Relevance. Clinical Cancer Research 19, 2861–2872 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Cheon DJ, Walts AE, Beach JA & Lester J Differential expression of argininosuccinate synthetase in serous and non-serous ovarian carcinomas. The Journal of … (2015). doi:10.1002/cjp2.4/asset/cjp24.pdf;jsessionid=B47D281F3D2008CB808B4FF1399F2544.f02t02?v=1&t=iqu843ql&s=33ed63c0196cf4bc6fea9e2d1ff330a63cb18f6d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi E et al. Reduced Argininosuccinate Synthetase Is a Predictive Biomarker for the Development of Pulmonary Metastasis in Patients with Osteosarcoma. Mol Cancer Ther 9, 535–544 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Huang HL et al. Attenuation of Argininosuccinate Lyase Inhibits Cancer Growth via Cyclin A2 and Nitric Oxide. Mol Cancer Ther 12, 2505–2516 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Allen MD et al. Prognostic and Therapeutic Impact of Argininosuccinate Synthetase 1 Control in Bladder Cancer as Monitored Longitudinally by PET Imaging. Cancer Research 74, 896–907 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Syed N et al. Epigenetic status of argininosuccinate synthetase and argininosuccinate lyase modulates autophagy and cell death in glioblastoma. Cell Death Dis 4, e458 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott L, Lamb J, Smith S & Wheatley DN Single amino acid (arginine) deprivation: rapid and selective death of cultured transformed and malignant cells. Br. J. Cancer 83, 800–810 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García-Navas R, Munder M & Mollinedo F Depletion of L-arginine induces autophagy as a cytoprotective response to endoplasmic reticulum stress in human T lymphocytes. Autophagy 8, 1557–1576 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Changou CA et al. Arginine starvation-associated atypical cellular death involves mitochondrial dysfunction, nuclear DNA leakage, and chromatin autophagy. Proc. Natl. Acad. Sci. U.S.A 111, 14147–14152 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouyang L et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Proliferation 45, 487–498 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu F et al. Arginine Starvation Impairs Mitochondrial Respiratory Function in ASS1-Deficient Breast Cancer Cells. Sci. Signal 7, ra31–ra31 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jewell JL & Guan K-L Nutrient signaling to mTOR and cell growth. Trends in Biochemical Sciences 38, 233–242 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vynnytska-Myronovska BO et al. Arginine starvation in colorectal carcinoma cells: Sensing, impact on translation control and cell cycle distribution. Experimental Cell Research 341, 67–74 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Finn OJ Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol 23 Suppl 8, viii6–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cerwenka A & Lanier LL Natural killer cell memory in infection, inflammation and cancer. Nat Rev Immunol 16, 112–123 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Callahan MK, Postow MA & Wolchok JD Targeting T Cell Co-receptors for Cancer Therapy. Immunity 44, 1069–1078 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Daly JM et al. Immune and metabolic effects of arginine in the surgical patient. Ann. Surg 208, 512–523 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbul A et al. Immunostimulatory effects of arginine in normal and injured rats. Journal of Surgical Research 29, 228–235 (1980). [DOI] [PubMed] [Google Scholar]
- 29.Barbul A, Rettura G, Levenson SM & Seifter E Arginine: a thymotropic and wound-healing promoting agent. (Surgical forum, 1977). [PubMed] [Google Scholar]
- 30.Kirk SJ, Regan MC, Wasserkrug HL, Sodeyama M & Barbul A Arginine Enhances T-Cell Responses in Athymic Nude Mice. JPEN J Parenter Enteral Nutr 16, 429–432 (1992). [DOI] [PubMed] [Google Scholar]
- 31.Torre PM, Ronnenberg AG, Hartman WJ & Prior RL Oral arginine supplementation does not affect lymphocyte proliferation during endotoxin-induced inflammation in rats. J. Nutr 123, 481–488 (1993). [DOI] [PubMed] [Google Scholar]
- 32.Becker JC, Andersen MH, Schrama D & Straten PT Immune-suppressive properties of the tumor microenvironment. Cancer Immunol Immunother 62, 1137–1148 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bronte V & Zanovello P Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol 5, 641–654 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez PC & Ochoa AC Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunological Reviews 222, 180–191 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao Y, Feng Y, Zhang Y, Zhu X & Jin F L-Arginine supplementation inhibits the growth of breast cancer by enhancing innate and adaptive immune responses mediated by suppression of MDSCs in vivo. BMC Cancer 2016 16:1 16, 1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma Q, Williamson KE, O’Rourke D & Rowlands BJ The Effects ofl-Arginine on Crypt Cell Hyperproliferation in Colorectal Cancer. Journal of Surgical Research 81, 181–188 (1999). [DOI] [PubMed] [Google Scholar]
- 37.Kirk SJ et al. Arginine stimulates wound healing and immune function in elderly human beings. Surgery 114, 155–9– discussion 160 (1993). [PubMed] [Google Scholar]
- 38.Witte MB, Thornton FJ, Tantry U & Barbul A L-Arginine supplementation enhances diabetic wound healing: involvement of the nitric oxide synthase and arginase pathways. Metab. Clin. Exp 51, 1269–1273 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Thomsen LL & Miles DW Role of nitric oxide in tumour progression: Lessons from human tumours. Cancer Metastasis Rev 17, 107–118 (1998). [DOI] [PubMed] [Google Scholar]
- 40.Ambs S et al. Relationship Between p53 Mutations and Inducible Nitric Oxide Synthase Expression in Human Colorectal Cancer. J. Natl. Cancer Inst 91, 86–88 (1999). [DOI] [PubMed] [Google Scholar]
- 41.Berk L et al. A randomized, double-blind, placebo-controlled trial of a beta-hydroxyl beta-methyl butyrate, glutamine, and arginine mixture for the treatment of cancer cachexia (RTOG 0122). Support Care Cancer 16, 1179–1188 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Buijs N, Luttikhold J, Houdijk APJ & van Leeuwen PAM The role of a disturbed arginine/NO metabolism in the onset of cancer cachexia: a working hypothesis. Curr. Med. Chem 19, 5278–5286 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Sippel TR et al. Neutrophil Degranulation and Immunosuppression in Patients with GBM: Restoration of Cellular Immune Function by Targeting Arginase I. Clinical Cancer Research 17, 6992–7002 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez PC et al. Arginase I–Producing Myeloid-Derived Suppressor Cells in Renal Cell Carcinoma Are a Subpopulation of Activated Granulocytes. Cancer Research 69, 1553–1560 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peranzoni E et al. Role of arginine metabolism in immunity and immunopathology. Immunobiology 212, 795–812 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Feun LG et al. Negative argininosuccinate synthetase expression in melanoma tumours may predict clinical benefit from arginine-depleting therapy with pegylated arginine deiminase. Br. J. Cancer 106, 1481–1485 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barreda D Regulation of myeloid development and function by colony stimulating factors. Developmental & Comparative Immunology 28, 509–554 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Gordon S Alternative activation of macrophages. Nat Rev Immunol 3, 23–35 (2003). [DOI] [PubMed] [Google Scholar]
- 49.Geissmann F, Jung S & Littman DR Blood Monocytes Consist of Two Principal Subsets with Distinct Migratory Properties. Immunity 19, 71–82 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Elgert KD, Alleva DG & Mullins DW Tumor-induced immune dysfunction: the macrophage connection. J Leukoc Biol 64, 275–290 (1998). [DOI] [PubMed] [Google Scholar]
- 51.Bingisser RM, Tilbrook PA, Holt PG & Kees UR Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J. Immunol 160, 5729–5734 (1998). [PubMed] [Google Scholar]
- 52.Aulak KS et al. Proteomic method identifies proteins nitrated in vivo during inflammatory challenge. Proceedings of the National Academy of Sciences 98, 12056–12061 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bach SJ & Swaine D The effect of arginase on the retardation of tumor growth. Br. J. Cancer 19, 379–386 (1965). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tong BC & Barbul A Cellular and physiological effects of arginine. Mini Rev Med Chem 4, 823–832 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Dillon BJ, Holtsberg FW & Ensor CM Biochemical characterization of the arginine degrading enzymes arginase and arginine deiminase and their effect on nitric oxide production. Medical Science … (2002). doi: 10.1164/ajrccm-conference.2010.181.1_MeetingAbstracts.A2699 [DOI] [PubMed] [Google Scholar]
- 56.Patil MD, Bhaumik J, Babykutty S, Banerjee UC & Fukumura D Arginine dependence of tumor cells: targeting a chink in cancer’s armor. Oncogene (2016). doi: 10.1038/onc.2016.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qiu F, Huang J & Sui M Targeting arginine metabolism pathway to treat arginine-dependent cancers. Cancer Letters 364, 1–7 (2015). [DOI] [PubMed] [Google Scholar]
- 58.Cederbaum SD et al. Arginases I and II: do their functions overlap? Molecular Genetics and Metabolism 81, 38–44 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Durante W, Johnson FK & Johnson RA Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol 34, 906–911 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwedhelm E et al. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: impact on nitric oxide metabolism. British Journal of Clinical Pharmacology 65, 51–59 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Claesson MH, Tscherning T, Nissen MH & Lind K Inhibitory Effect of Mycoplasma-Released Arginase. Scandinavian Journal of Immunology 32, 623–630 (1990). [DOI] [PubMed] [Google Scholar]
- 62.Takaku H, Takase M, Abe SI, Hayashi H & Miyazaki K In vivo anti-tumor activity of arginine deiminase purified from Mycoplasma arginini. International Journal of Cancer 51, 244–249 (1992). [DOI] [PubMed] [Google Scholar]
- 63.Lagarde SM, Van Themaat P & Moerland PD Analysis of gene expression identifies differentially expressed genes and pathways associated with lymphatic dissemination in patients with adenocarcinoma of the …. Annals of surgical … (2008). [DOI] [PubMed] [Google Scholar]
- 64.Huang C-C et al. Arginine deprivation as a new treatment strategy for head and neck cancer. Oral Oncology 48, 1227–1235 (2012). [DOI] [PubMed] [Google Scholar]
- 65.Nicholson LJ et al. Epigenetic silencing of argininosuccinate synthetase confers resistance to platinum-induced cell death but collateral sensitivity to arginine auxotrophy in ovarian cancer. International Journal of Cancer 125, 1454–1463 (2009). [DOI] [PubMed] [Google Scholar]
- 66.Bowles TL et al. Pancreatic cancer cell lines deficient in argininosuccinate synthetase are sensitive to arginine deprivation by arginine deiminase. International Journal of Cancer 123, 1950–1955 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han R-Z, Xu G-C, Dong J-J & Ni Y Arginine deiminase: recent advances in discovery, crystal structure, and protein engineering for improved properties as an anti-tumor drug. Appl Microbiol Biotechnol 100, 4747–4760 (2016). [DOI] [PubMed] [Google Scholar]
- 68.Zhu MY, Iyo A, Piletz JE & Regunathan S Expression of human arginine decarboxylase, the biosynthetic enzyme for agmatine. Biochim. Biophys. Acta (2004). doi: 10.1016/j.bbagen.2003.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wheatley DN & Campbell E Arginine catabolism, liver extracts and cancer. Pathol. Oncol. Res 8, 18–25 (2002). [DOI] [PubMed] [Google Scholar]
- 70.Naini AB, Dickerson JWT & Brown MM Preoperative and postoperative levels of plasma protein and amino acid in esophageal and lung cancer patients. Cancer 62, 355–360 (1988). [DOI] [PubMed] [Google Scholar]
- 71.Vissers YLJ et al. Plasma arginine concentrations are reduced in cancer patients: evidence for arginine deficiency? Am. J. Clin. Nutr 81, 1142–1146 (2005). [DOI] [PubMed] [Google Scholar]
- 72.Engelen MPKJ, Safar AM, Bartter T, Koeman F & Deutz NEP Reduced arginine availability and nitric oxide synthesis in cancer is related to impaired endogenous arginine synthesis. Clinical Science 130, 1185–1195 (2016). [DOI] [PubMed] [Google Scholar]
- 73.Izzo F et al. Pegylated arginine deiminase treatment of patients with unresectable hepatocellular carcinoma: results from phase I/II studies. Journal of Clinical Oncology 22, 1815–1822 (2004). [DOI] [PubMed] [Google Scholar]
- 74.Ascierto PA et al. Pegylated Arginine Deiminase Treatment of Patients With Metastatic Melanoma: Results From Phase I and II Studies. Journal of Clinical Oncology 23, 7660–7668 (2005). [DOI] [PubMed] [Google Scholar]
- 75.Glazer ES et al. Phase II Study of Pegylated Arginine Deiminase for Nonresectable and Metastatic Hepatocellular Carcinoma. Journal of Clinical Oncology 28, 2220–2226 (2010). [DOI] [PubMed] [Google Scholar]
- 76.Ott PA et al. Phase I/II study of pegylated arginine deiminase (ADI-PEG 20) in patients with advanced melanoma. Invest New Drugs 31, 425–434 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]