Summary
Bendamustine (B) with rituximab (R) is a standard frontline treatment for medically fit follicular lymphoma (FL) patients. The safety and efficacy of maintenance rituximab (MR) after BR induction has not been formally compared to observation for FL, resulting in disparate practice patterns. Prospective trials have shown benefit of MR after R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) or R-CVP (rituximab, cyclophosphamide, vincristine, prednisone), yet recent data from the GALLIUM study comparing outcomes of patients treated with chemotherapy with Ror obinutuzumab (G) showed higher than anticipated fatal adverse events with BR/BG. In order to assess the efficacy and tolerability of MR after BR, we retrospectively collected data on 640 newly diagnosed patients treated with FL. We found that patients who achieved partial remission (PR) after ≥4 cycles of BR had improved duration of response (DOR) with MR vs. no maintenance, whereas those in complete remission did not. These findings were confirmed in a validation cohort. In the entire study population, the known fatal adverse event rate after BR was 2.5% and differed in those receiving MR vs. no maintenance. Within the limitations inherent to retrospective analysis, these data suggest that FL patients with a PR to BR experience prolongation of remission with MR with an acceptable safety profile.
Keywords: Follicular lymphoma, bendamustine, rituximab, maintenance
Follicular lymphoma (FL) is the most common indolent non-Hodgkin lymphoma (NHL) and displays a variable clinical course. The initial management of asymptomatic patients with low tumour burden generally involves active surveillance until an indication for therapy develops, with a median time from diagnosis to treatment of approximately 2–3 years.(Nastoupil, et al 2016) For young FL patients without significant comorbidities, treatment has historically been chemoimmunotherapy using the anti-CD20 monoclonal antibody rituximab in combination with conventional chemotherapeutic agents, such as cyclophosphamide, vincristine and prednisone (R-CVP) or these agents with doxorubicin (R-CHOP).(Hiddemann, et al 2005, Marcus, et al 2005)
The German Study group for indolent Lymphomas (StiL) trial directly compared the safety and efficacy of front-line treatment with R-CHOP vs. bendamustine with rituximab (BR).(Rummel, et al 2013) Among 279 FL patients enrolled, median progression-free survival (PFS) was significantly longer in the BR group than in the R-CHOP group (not reached vs. 40.9 months, P = 0.0072) with lower rates of haematological toxicity associated with BR. There was no overall survival (OS) difference. These findings were validated by the BRIGHT trial that showed non-inferior response rates and PFS of BR compared to R-CHOP or R-CVP.(Flinn, et al 2014) As a result of these studies, BR has become the most commonly used frontline therapeutic regimen for medically fit FL patients in need of treatment, such as those with high tumour burden.
FL patients are at continued risk of relapse after induction therapy, prompting significant interest in the use of relatively non-toxic therapy with rituximab on a maintenance basis (MR) as a means of prolonging the duration of response (DOR) for patients who have achieved remission after treatment with chemoimmunotherapy (Hochster, et al 2009, van Oers, et al 2010) The prospective randomized PRIMA trial demonstrated that, compared to no maintenance, MR is safe and effective when responding patients are dosed with rituximab every 2 months for 2 years after induction therapy with R-CHOP or R-CVP.(Salles, et al 2011) At a recent update with >9 years of follow-up, median PFS for patients in the observation arm was 4.1 years as compared to 10.5 years in the MR arm (P<.0001).(Salles, et al 2017)
Obinituzumab (formerly GA101, G) is a second generation anti-CD20 monoclonal antibody that may have greater clinical activity than rituximab in the treatment of FL but is also associated with greater myelosuppression.(Marcus, et al 2017, Radford, et al 2013, Sehn, et al 2016, Sehn, et al 2015) The GALLIUM study compared outcomes of FL patients treated with either BR or bendamustine in combination with obinutuzumab (BG) followed by R or G maintenance every 2 months. PFS was superior for the obinutuzumab arm but there was a fatal adverse event (AE) rate of 4.7% with BR followed by RM or 5.9% with BG followed by G maintenance at a median follow-up of 41 months(Hiddemann, et al 2017).
Given the higher-than expected fatal AE rate in the GALLIUM study with MR after frontline BR and the absence of randomized data demonstrating a clear benefit of MR after front-line BR, there is a lack of consensus regarding the use of MR in this setting. In order to (1) better understand fatal events after BR with or without MR and (2) estimate the benefits of MR after induction BR in the context of a retrospective analysis, we studied individual patient data across 13 U.S. academic medical centres and compared outcomes for the patients who received MR or no maintenance after induction treatment with BR.
Materials and methods
We conducted a comprehensive multi-institutional retrospective analysis of outcomes of 640 consecutive FL patients (grade I/II & IIIA) based on local pathologist interpretation across 13 U.S. academic medical centres. Adult patients (age ≥18 years old) were initiated on induction therapy with BR based on indications as determined by their treating physician between 2011–2015 followed by either MR or no maintenance. The decision to apply MR was based on preferences of individual treating physician and/or patient and treatment paradigms were necessarily institution- and/or physician-specific. Individual patient records were queried for baseline demographic factors, including age, gender and race, as well as pre-treatment disease factors, including FL International Prognostic Index (FLIPI) score and treatment history including doses and number of treatments with bendamustine and rituximab. Outcomes included fatal adverse events during or after BR induction, response rates at the conclusion of BR induction therapy as assessed by local investigator, PFS and OS from the time of initiation of induction treatment with BR DOR calculated from the time of completion of induction therapy with BR for responding patients. The initial cohort was analysed from 410 of the 640 patients from 12 of the 13 contributing centres that provided responses to induction therapy and then restricted to those 376 patients in complete remission (CR, N = 262) or partial remission (PR, N = 114) who received ≥4 cycles of BR to mimic the study design of the PRIMA trial, which randomized patients who had responded to induction therapy. Subsequently, additional response assessments from 207 patients treated with BR and seen at M.D. Anderson Cancer Center were obtained and this dataset was used as a single centre validation cohort.
All data was collected with local Institutional Review Board (IRB) approval. Deaths were categorized as belong to one of four categories: (1) disease related (either FL or transformed lymphoma), (2) known fatal adverse events, (3) second cancers and (4) unknown causes based on review of the medical record. For patients from the University of Iowa and Mayo Clinic, data was extracted from the lymphoma Molecular Epidemiology Resource, in which all deaths undergo medical record review by a physician and cause of death is determined from all available information. For patients seen at all other sites, cause of death was assigned by local investigator after review of patient’s medical record.
Categorical variables were compared using Chi-square or Fisher’s exact tests. Continuous variables were compared with Wilcoxon rank-sum test. PFS, DOR and OS were estimated using the Kaplan-Meier method and compared with the log-rank test. Univariate prognostic factors for OS and PFS were assessed using Cox proportional hazards analysis; results were summarized as the hazard ratio (HR) and 95% confidence interval (CI). Time to death from lymphoma (FL or transformed lymphoma), time to death from fatal adverse event and time to death from other cause (unknown and solid tumour) was estimated using cumulative incidence methods and compared between MR and no maintenance groups using the Gray Test. Statistical analysis was conducted using IBM SPSS Statistics for Windows, version 24 (IBM Corp., Armonk, N.Y., USA) and R version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
As shown in Table I, the median age at diagnosis was 60 years old (range 21–88) and 52% were male. The majority of patients were Caucasian (87%) and the large majority (95%) had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1. Most patients (89%) had advanced stage disease and 43% had FLIPI scores of ≥3. The median time from diagnosis to treatment was 1 month (range (0–139 months). In the entire study population, patients who ultimately were selected to receive MR after induction with BR (N= 357) were more likely to have better ECOG performance status than those who were selected to not receive maintenance (n=283), (P = 0.01). With the exception of performance status, there were no other statistically significant differences in the baseline characteristics among patients in the entire study population selected to undergo MR or observation at the conclusion of induction treatment with BR. When restricting the analysis of baseline patient demographic and lymphoma features to 376 patients who received ≥ 4 cycles of BR and achieved a CR or PR, we similarly found similar differences in baseline performance status but no other statistically significant differences between those patients who received MR vs. no maintenance (Table SI).
Table I:
Baseline Demographic and Disease Characteristics at Diagnosis of Entire Cohort of 640 FL Patients Receiving Frontline BR Followed by either MR or no maintenance.
| Characteristic | Entire Cohort (N = 640) | MR (N = 357) | No maintenance (N = 283) | P |
|---|---|---|---|---|
| Age, years; median (range) | 60 (21–88) | 59 (26–85) | 60 (21–88) | 0.07 |
| Gender | 1.0 | |||
| Male (n, %) | 335(52) | 187 (52) | 148 (52) | |
| Female (n, %) | 305(48) | 170 (48) | 1135 (48) | |
| Race (%) (n=397) | ||||
| Caucasian | 344 (87) | 218 (87) | 126 (86) | 0.65 |
| African-American | 17 (4) | 13 (5) | 4 (3) | |
| Asian/Pacific Island | 5 (1) | 3 (1) | 2 (1) | |
| Non-white Hispanic | 19 (5) | 11 (5) | 8 (6) | |
| Other/not Specified | 12 (3) | 6 (2) | 6 (4) | |
| ECOG performance status at diagnosis (n=404) | ||||
| 0 | 265 (66) | 177 (70) | 88 (58) | 0.01 |
| 1 | 118 (29) | 69 (27) | 49 (33) | |
| 2 | 17 (4) | 6 (2) | 11 (7) | |
| 3 | 4 (1) | 1 (1) | 3 (2) | |
| LDH > ULN (n=557) | 106 (19) | 53(17) | 53(22) | 0.16 |
| Bone marrow Involvement (n=319) | 164 (51) | 109(53) | 55(49) | 0.56 |
| Ann Arbor stage at diagnosis (n=567) | ||||
| I/II | 61 (11) | 32 (10) | 29 (12) | 0.41 |
| III/IV | 506 (89) | 296 (90) | 210 (88) | |
| B Symptoms(n=422) | 88 (21) | 47 (18) | 41 (26) | 0.05 |
| Serum β2-microglobulin > ULN (n=243) | 119 (49) | 72 (46) | 47 (55) | 0.18 |
| >4 Nodal Sites (n=584) | 322 (55) | 195 (58) | 127 (51) | 0.11 |
| Largest node ≥ 7 cm (n=408) | 95 (23) | 65 (25) | 30 (20) | 0.33 |
| Haemoglobin < 120 g/l (n=578) | 110 (19) | 57 (18) | 53 (21) | 0.29 |
| FLIPI (n=491) | ||||
| Low (0–2) | 283 (58) | 171 (60) | 112 (54) | 0.40 |
| Intermediate (3) | 141 (29) | 79 (28) | 62 (30) | |
| High (4–5) | 67 (14) | 35 (12) | 32 (16) | |
| Grade (n=632) | ||||
| I/II | 576 (91) | 328 (93) | 248 (89) | 0.09 |
| IIIA | 56 (9) | 25 (7) | 31 (11) | |
| Time from diagnosis to treatment, months; median (range) | 1 (0–139) | 1.1 (0–135) | 1 (0–139) | 0.62 |
BR; bendamustine and rituximab; ECOG: Eastern Cooperative Oncology Group; FL: follicular lymphoma; FLIPI: Follicular lymphoma International Prognostic Index; LDH: lactate dehydrogenase; MR: maintenance rituximab; ULN: upper limit of normal.
Patterns of Maintenance Rituximab (MR) Use
MR was more commonly applied in responding patients. In the 357 patients selected for MR, the CR and PR rates were 71% and 29%, respectively, among patients with known responses and in the 283 patients selected for no maintenance, the CR and PR rates were 58% and 28%, respectively; the remaining 14% had either stable disease (SD) or progressive disease (PD) (P<0.001). The median number of BR cycles was 6; the median bendamustine dose was 90 mg/m2 and did not differ between MR and no maintenance patients (P=0.14 for cycle, P=0.40 for dose). MR was administered for a median of 18 months (range 3–24) on the following schedule: every 2 months (66%), every 3 months (30%) and 4 doses every 6 months (4%).
Treatment Characteristics and Response to BR Induction Therapy
Because MR was preferentially given to patients in remission and not to those patients with suboptimal response to therapy, such as SD or PD, subsequent analysis was focused on 410 patients in an initial cohort for whom response assessment was available. In this initial cohort of response-evaluable patients, 271 (66.0%) had CR, 116 (28.3%) had PR and 23 (5.6%) had SD or PD. A flowchart of the study population is shown in Figure 1. Overall, 231 out of the 410 responses (56%) were assigned by positron emission tomography (PET), 174 (42%) were assessed by computerized tomography (CT) criteria and 1% were by unknown methods. Among the 231 cases that were assigned responses by PET criteria, there were 187 CRs (80.9%) and 32 PRs (13.8%). In contrast, the CR rate assigned by CT criteria was 84 /174 (48.3%) and the PR rate by CT criteria was 79/174 (45.4%) (p<0.001).
Figure 1. Flowchart of study patients.
Number of patients are indicated including those with unknown response, stable or refractory disease or known responses after ≥4 cycles of BR (initial cohort) followed by or MR or no maintenance based on patient-physician decision. Patient data from the single centre validation cohort were subsequently analysed.
BR; bendamustine and rituximab; CR: complete remission; FL: follicular lymphoma; MR: maintenance rituximab; PR: partial remission
Clinical Outcomes Based Response to BR Induction Therapy and FLIPI Scores
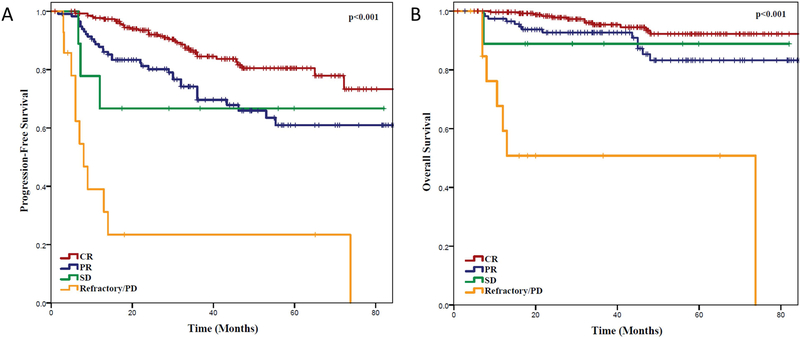
Among the entire cohort of 640 patients treated with BR, there were significant differences in clinical outcomes for patients selected for MR vs. no maintenance. The PFS at 36 months was 84.2% for MR vs 61.2% for no maintenance (P <0.001) and OS was 94.3% vs. 85.1%, respectively (P = 0.001, Figure S1A, B). Because response assessment to BR was retrospectively assigned by investigators across multiple sites, we sought to compare the outcomes of those patients in CR vs. PR vs. SD or PD as a means of validating response assessments. Kaplan Meier estimates of PFS and OS based on response to induction therapy are shown in Figure 2 A, B. The median PFS for patients with CR, PR and SD patients was not reached. The 3-year PFS for CR, PR and SD patients was 84.5%, 69.8% and 66.7%, respectively (P <0.001). Similarly, the median OS for patients with CR, PR and SD patients was not reached, while it was 73.8 months for patients with PD, respectively. The 3-year OS for CR, PR and SD patients was 95.4%, 92.8% and 88.9%, respectively (P <0.001).
Figure 2. Clinical Outcomes of Entire Patient Population Based On Response to Induction.
(A) Progression-free survival and (B) overall survival of follicular lymphoma patients treated with bendamustine and rituximab based on response to induction therapy reported from the time of initiation of induction therapy, regardless of subsequent maintenance rituximab or no maintenance.
CR: complete remission; PD: progressive disease; PR: partial remission; SD: stable disease.
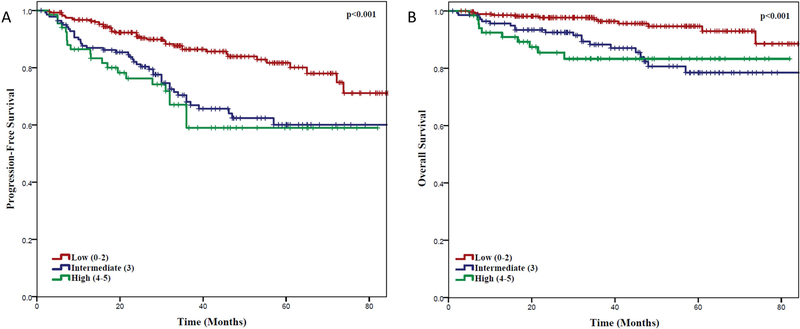
We also assessed the impact of baseline FLIPI score on PFS and OS. Patients with low (0–2), intermediate (3) or high (4–5) FLIPI scores had a 3-year PFS of 86.4%, 68.1% and 59.0%, respectively (P<0.001) and OS of 96.4%, 88.3%, and 83.3%, respectively (P <0.001) (Figure 3).
Figure 3. Clinical Outcomes Based on Baseline FLIPI Score.
(A) Progression-free survival and (B) overall survival of follicular lymphoma (FL) patients from time of initiation of induction therapy, with low (0–2), intermediate (3) or high (4–5) FL International Prognostic Index (FLIPI) score.
Duration of Remission based on MR or no maintenance
To determine the impact of MR on patients after induction therapy, DOR was analysed from the time of induction therapy completion for the 262 patients who achieved CR and 114 patients who achieved PR after at ≥ 4 cycles of BR in the initial cohort. The 3-year DOR for CR patients who underwent MR was not improved over no maintenance (85.9% vs. 80.2% respectively, P = 0.535; Figure 4A). The 3-year OS was 95.6% and 96.2%, respectively (P=0.657; Figure 4B). In contrast, the 3-year DOR for patients who achieved a PR after ≥ 4 cycles of BR was significantly longer than those who underwent MR vs. no maintenance (80% vs. 45%, P = 0.003; Figure 4C). The 3-year OS was 95.7% and 86.6% respectively (P=0.06; Figure 4D). When restricting analysis to patients in PR by CT imaging, there was a marked improvement in DOR for MR vs. no maintenance for patients in (3-year DOR 86.6% vs. 43.9%, P=0.004) as well as an OS difference (3-year OS 98.1% vs. 87.1%, P =0.02) whereas there was no difference in DOR (64.0% vs. 50.0%, P=0.248) or OS (85.9% vs. 8% P=0.99) for patients in PR by PET scan at end of treatment (Figure 5 A–D). For patients in CR by PET, DOR (85.8% vs. 85.3%, P=0.639) and OS (95.4% vs. 94.5% P=0.975) was not statistically different for MR vs. no maintenance although there was a trend (P =0.08) towards improved DOR in patients in CR by CT imaging (86.8% vs. 69.3%) with no OS difference (96.0% vs. 100%, P=0.350; Figure 5E–H).
Figure 4. Clinical Outcomes of Patients Based on Remission Status after BR.
(A) Duration of response (DOR) and (B) overall survival (OS) from the conclusion of ≥4 cycles of bendamustine and rituximab (BR) for patients who achieved complete remission after induction therapy, based on whether they subsequently received maintenance rituximab (MR) or no maintenance. (C) DOR and (D) OS from the conclusion of ≥4 cycles of BR for patients who achieved partial remission after induction therapy based on whether they subsequently received MR or no maintenance.
Figure 5. Clinical Outcomes of Patients Based on Remission Status after BR as Determined by CT vs. PET.
(A). Duration of response (DOR) for patients in partial remission by computed tomography (CT) and (B) by positron emission tomography (PET) based on whether they subsequently received maintenance rituximab (MR) or no maintenance. (C) Overall survival for patients in partial remission by CT or (D) PET based on MR or no maintenance. (E) DOR for patients in complete remission by CT and (F) by PET based on whether they subsequently received maintenance rituximab (MR) or no maintenance. (F) Overall survival for patients in partial remission by CT or (G) PET based on MR or no maintenance.
To determine the impact, if any, of MR on patients in different FLIPI risk categories, we analysed the DOR of patients treated with MR or no maintenance after BR induction based on FLIPI score. The 3-year DOR for low-intermediate FLIPI score patients in PR was 91.9% and 77.8% for those treated with MR or no maintenance, respectively (P = 0.485) and 69.5% and 25.7%, respectively (P = 0.029) for high FLIPI score patients in PR after induction (Figure S2). The 3-year DOR for low-intermediate FLIPI score patients in CR was 88.3% and 97.3% for those treated with MR or no maintenance, respectively (P = 0.199) and 81.4% and 66.7%, respectively (P = 0.270) for high FLIPI score patients in CR after induction.
Univariate analysis
Univariate analysis was performed based on baseline demographic and clinical features as well as response to treatment and use of MR or no maintenance. The features with the strongest association with PFS and OS were elevated lactate dehydrogenase, B symptoms, and FLIPI score (Table II). Grade IIIA was associated with inferior PFS and OS. PR relative to CR at the conclusion of induction treatment with BR was associated with inferior PFS and OS. Multivariable analysis was not performed because of an insufficient number of PFS and OS events.
Table II:
Univariate Analysis of Baseline Clinical and Treatment Outcomes and their Impact on PFS and OS after Frontline BR among the Entire Study Population (N = 640)
| PFS |
OS |
||||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
| LDH | |||||
| Elevated/Normal Range | 2.40 (1.64–3.26) | <0.001 | 2.47 (1.36–4.48) | 0.003 | |
| Bone Marrow | |||||
| Involved/Not-involved | 1.50 (0.91–2.48) | 0.11 | 1.43 (0.69–2.95) | 0.33 | |
| Stage | |||||
| III-IV/I-II | 1.19 (0.64–2.20) | 0.6 | 0.83 (0.36–1.96) | 0.68 | |
| B Symptoms | |||||
| Present/Absent | 2.00 (1.30–3.10) | 0.002 | 3.72 (2.00–6.93) | <0.001 | |
| B2-microglobulin | |||||
| Elevated/Normal Range | 1.78 (0.98–3.22) | 0.06 | 3.30 (1.90–9.17) | 0.02 | |
| Number of Nodal Sites | |||||
| > 4/< 4 | 1.41 (0.98–2.02) | 0.06 | 1.35 (0.76–2.32) | 0.28 | |
| Haemoglobin | |||||
| <120 g/l/ Normal | 1.90 (1.28–2.82) | 0.001 | 2.94 (1.66–5.18) | <0.001 | |
| FLIPI | |||||
| High (3–5)/Low (0–2) | 2.44 (1.64–3.61) | <0.001 | 3.48( 1.78–6.81) | <0.001 | |
| Grade | |||||
| Grade IIIa/Grade I-II | 1.61 (0.98–2.63) | 0.06 | 2.34 (1.89–4.61) | 0.01 | |
| Response | |||||
| CR/PR | 0.46 (0.29–0.73) | 0.001 | 0.43 (0.19–0.94) | 0.03 | |
| Treatment Arm | |||||
| MR/No maintenance | 0.39 (0.29–0.55) | <0.001 | 0.42 (0.25–0.69) | 0.001 | |
BR; bendamustine and rituximab; CI: confidence interval;CR: complete remission; FLIPI: Follicular lymphoma International Prognostic Index; HR: hazard ratio; LDH: lactate dehydrogenase; MR: maintenance rituximab; OS: overall survival; PFS: progression-free survival; PR: partial remission
Validation Cohort
Due to the novel observation that MR was beneficial to patients in PR, but not those in CR after induction in the initial patient cohort, we subsequently obtained detailed response assessments for an additional 207 patients at a single US site. Baseline characteristics of this validation cohort were not significantly different from the initial cohort. There were 175 patients with CR (82%) 13 with PR (6%) and 19 SD or PD (9%) with induction therapy. The majority (66%) of CR patients had response assigned by PET criteria. Among the 162 patients in this validation cohort who achieved a CR after ≥ 4 cycles of BR, the median age, gender, grade and baseline FLIPI score did not differ between patients selected to receive MR (N= 66) or no maintenance (N = 96) (Table SII). For patients in CR after induction therapy, there was no statistically significant difference in 3-year DOR for MR vs no maintenance [86.2% vs. 86%, P = 0.226, (Figure S3)]. The number of patients in PR after induction therapy was insufficient to perform detailed analysis for the impact of MR in this validation cohort.
Adverse Events
In the entire study population of 640 FL patients after a median follow-up of 36 months, 63 patients had died (9.8%). Cause of death (COD) was lymphoma in 26 cases (Table III). The known fatal AEs were due to infection or multi-organ system failure (n=12), cardiovascular events (n=2), myelodysplastic syndrome (n=1), respiratory failure (n=1) and progressive multifocal leucoencephalopathy (n=1). This represents a known fatal AE rate of 2.5% if cases of unknown COD (n=10) and solid tumour (n=10) are excluded or 4.1% if cases of unknown COD are included in determining fatal AE rate. Among patients with documented deaths, the median time to death was 22 months (range 2–74 months). When comparing COD in patients in the entire study population, the known fatal AEs (excluding solid tumours) were similar between the two groups (2.2% for MR vs. 3.2% for no maintenance) and there was no statistically significant difference in the cumulative incidence of these events between these MR vs. no maintenance (P = 0.25, Figure 6). These differences were also not significantly different if cases of solid tumour and unknown cause of death were included in the cumulative incidence analysis (Figure S4A). There was a statistically significant decrease in the cumulative incidence of death due to lymphoma for patients who received MR vs. no maintenance (P < 0.01), probably due to the higher usage of rate of MR for patients in CR after induction therapy (Figure S4B).
Table III:
Cause of death during study period of patients treated with frontline BR followed by rituximab maintenance or no maintenance
| Cause of Death | Entire Cohort (N = 640) | MR (N = 357 ) | No maintenance (N = 283) |
|---|---|---|---|
| Total (n=63) | 63 (9.8%) | 26 (7.3%) | 37 (13%) |
| Disease-related | 26 (4.1%) | 9 (2.5%) | 17 (6.0%) |
| Follicular lymphoma | 10 (1.6%) | 4 (1.1%) | 6 (2.1%) |
| Transformed DLBCL | 16 (2.5%) | 5 (1.4%) | 11 (3.9%) |
| Known fatal adverse events | 16 (2.5%) | 8 (2.2%) | 9 (3.2%) |
| Infection/multi-organ system failure | 12 (1.9%) | 4 (1.1%) | 8 (2.8%) |
| Respiratory failure | 1 (0.2%) | 1 (0.3%) | 0 (0%) |
| Cardiovascular | 2 (0.3%) | 1 (0.3%) | 1 (0.3%) |
| Progressive multifocal leucoencephalopathy | 1 (0.2%) | 1 (0.3%) | 0 (0%) |
| Myelodysplastic syndrome | 1 (0.2%) | 1 (0.3%) | 0 (0%) |
| Solid tumour (n=10) | 10 (1.6%) | 6 (1.6%) | 4 (1.4%) |
| Unknown | 10 (1.6%) | 3 (0.8%) | 7 (2.5%) |
BR; bendamustine and rituximab; DLBCL: diffuse large B-cell lymphoma; MR: maintenance rituximab
Figure 6. Cumulative Incidence of Known Fatal Adverse Events after Treatment.
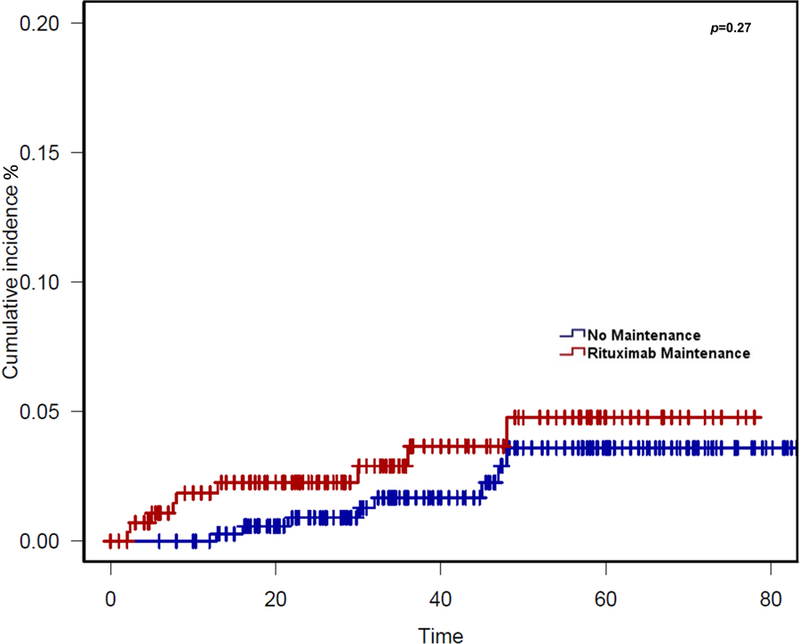
The cumulative incidence of fatal adverse events (excluding progression of lymphoma, solid tumours and unknown causes) is shown from the time of initiation of induction therapy for all 640 patients who received bendamustine and rituximab, based on whether patients received maintenance rituximab or no maintenance.
Discussion
This study represents the largest reported real-world outcomes analysis of FL patients treated with BR followed by either MR or no maintenance. We found a statistically significant DOR benefit to patients receiving MR while in PR after induction therapy with BR, but not for patients in CR. This was confirmed in univariate analysis. The DOR benefit appeared to be more robust for PR patients with high baseline FLIPI risk as compared to low FLIPI as well as those with PR as determined by CT scan (vs. PET) but these findings should be interpreted with caution due to the small numbers of patients in each subgroup. In contrast, patients in CR had no statistically significant benefit to MR vs. no maintenance, regardless of baseline FLIPI score. Although there was a trend towards improved DOR for patients in CR by CT scan at the end of treatment, there was no suggestion of benefit to MR vs. no maintenance for patients in CR by PET scan. Importantly, the lack of DOR benefit to MR in CR patients that was identified in the initial cohort was also subsequently confirmed by analysis of data from a 207-patient validation cohort from a single-centre, although there were insufficient patients with PR after induction in this validation cohort for reliable analysis. In interpreting these observations, there are several factors that should be considered.
These findings differ from those of the PRIMA study which showed a PFS benefit to patients with a PR as well as those with a CR after frontline treatment with R-CHOP/R-CVP.(Salles, et al 2011) The reasons for this discrepancy are not immediately clear but could be a reflection of the improved disease control afforded by BR vs. R-CHOP, as the rates of achieving a bone marrow with undetectable minimal residual disease (MRD) have been reported to be higher with BR than R-CHOP (87% vs. 74%, respectively) suggesting that there may be minimal benefit of MR in a deep MRD-undetectable state.(Pott, et al 2016) Our findings are consistent with recently-presented data from long-term follow-up of the BRIGHT trial, which have suggested a similar PFS benefit for patients selected for RM vs. no maintenance.(Kahl, et al 2017) Similarly, cross-trial comparison between the original German StiL Trial (NHL1) of BR induction without maintenance and the StiL NHL7 trial in which induction therapy with BR was followed by 2 or 4 years of MR showed a PFS benefit to patients treated with RM relative to historical controls treated with BR without maintenance.(Rummel, et al 2017) Future study will be needed to further explore the possibility of a differential benefit to MR based on depth of response (CR or PR) to BR.
Although the overall response rate of the initial cohort was higher than previously reported in Rummel, et al (2013), this is probably due to the use of PET rather than CT criteria in the majority of patients. In the recent GALLIUM study, the CR rate by CT criteria was 27.5% for bendamustine-containing chemotherapy vs. 72.5% by PET using Lugano 2014 criteria.(Trotman, et al 2017) Therefore, as previously shown for Hodgkin lymphoma and other NHL, these data indicate that the use of functional imaging in response assessment produces higher investigator-assigned CR rates than conventional CT imaging in FL patients treated in real-world practice.
The causes of death were primarily due to disease progression. The imbalance of deaths due to lymphoma in the patients who did not receive maintenance compared to those who received MR in the entire study population probably reflects the preferential application of MR to patients who had responded to induction therapy (Figure S4B). The known fatal AE rate was less than the rate reported in GALLIUM (Trotman, et al 2017). The deaths occurred at a median of 21.5 months after induction, with a range of 2–74 months, with no discernible increase in those undergoing MR as compared to no maintenance. In addition, there was no unexpected pattern of fatal AEs with infection or multi-organ system failure, accounting for the majority of cases, and solid tumour, accounting for the second leading cause of death.
A potential limitation of this study is that response rates were retrospectively assigned by investigators and not centrally-audited, resulting in the possibility of individual site bias. In addition, the single-site validation cohort had a higher number of CR patients but also had a higher rate of PET usage in response assessment. Despite these limitations, the clinical outcomes (PFS and OS) correlated strongly with responses at the end of induction (Figure 2) indicating that the investigator-adjudicated response assessments retained clinical significance. In addition, the lack of benefit of MR was most clearly demonstrable in patients who achieved CR by PET whereas those with who were assessed by CT may have benefited by MR. Similar trends were observed for patients with low/intermediate and high baseline FLIPI scores.
In the setting in which a randomized prospective trial is unlikely be conducted, retrospective analysis can offer important insights into real-world patient outcomes and potentially inform practice decisions as well as future clinical trial design. These data suggest that MR after BR is safe. Additionally, within the limitations of the study design, these data suggest that the benefit of MR may be limited to patients who achieve a PR after induction therapy with BR. PET imaging appears to be more reliable than CT for response determinations. Future studies should address the value of MR in patients uniformly restaged with PET imaging after bendamustine-containing induction therapy.
Supplementary Material
Acknowledgements:
The authors wish to acknowledge contribution of the Molecular Epidemiology Resource (MER) and The Lymphoma Epidemiology of Outcomes (LEO) Cohort Study lead by investigators at the Mayo Clinic and University of Iowa for providing data to this study.
Grant Number: P50 CA097274,P30 CA086862,U01 CA195568.
Footnotes
Conflict-of-interests disclosure
B.T.H., L.N., B.K., J.C., C.P.: Research funding and consultancy with Genentech;
S.S.: Research funding, Genentech;
P.M.: Consultancy Genentech.
REFERENCES
- Flinn IW, van der Jagt R, Kahl BS, Wood P, Hawkins TE, Macdonald D, Hertzberg M, Kwan YL, Simpson D, Craig M, Kolibaba K, Issa S, Clementi R, Hallman DM, Munteanu M, Chen L & Burke JM (2014) Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood, 123, 2944–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, Reiser M, Metzner B, Harder H, Hegewisch-Becker S, Fischer T, Kropff M, Reis HE, Freund M, Wormann B, Fuchs R, Planker M, Schimke J, Eimermacher H, Trumper L, Aldaoud A, Parwaresch R & Unterhalt M (2005) Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood, 106, 3725–3732. [DOI] [PubMed] [Google Scholar]
- Hiddemann W, Barbui A, Albendea M, Cannell P, Collins G, Dürig J, Forstpointner R, Herold M, Hertzberg M, Klanova M, Radford J, Tobinai K, Burciu A, Fingerle-Rowson G, Nielsen T, Wolbers M & Marcus R (2017) Immunochemotherapy with Obinutuzumab or Rituximab in Previously Untreated Follicular Lymphoma (FL) in the Randomized Phase III Gallium Study: Analysis by Chemotherapy Regimen. EHA Learning Center, June 25, 2017; 182062, S775. https://learningcenter.ehaweb.org/eha/2017/22nd/182062/wolfgang.hiddemann.immunochemotherapy.with.obinutuzumab.or.rituximab.in.html [Google Scholar]
- Hochster H, Weller E, Gascoyne RD, Habermann TM, Gordon LI, Ryan T, Zhang L, Colocci N, Frankel S & Horning SJ (2009) Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: results of the randomized phase III ECOG1496 Study. J Clin Oncol, 27, 1607–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl BS, Burke JM, van der Jagt R, Chang J, Wood P, Hawkins T, MacDonald D, Trotman J, Simpson D, Kolibaba KS, Issa S, Hallman D, Chen L & Flinn IW (2017) Assessment of Maintenance Rituximab after First-Line Bendamustine-Rituximab in Patients with Follicular Lymphoma: An Analysis from the BRIGHT Trial. Blood, 130, 484–484. [Google Scholar]
- Marcus R, Imrie K, Belch A, Cunningham D, Flores E, Catalano J, Solal-Celigny P, Offner F, Walewski J, Raposo J, Jack A & Smith P (2005) CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood, 105, 1417–1423. [DOI] [PubMed] [Google Scholar]
- Marcus R, Davies A, Ando K, Klapper W, Opat S, Owen C, Phillips E, Sangha R, Schlag R, Seymour JF, Townsend W, Trneny M, Wenger M, Fingerle-Rowson G, Rufibach K, Moore T, Herold M & Hiddemann W (2017) Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. N Engl J Med, 377, 1331–1344. [DOI] [PubMed] [Google Scholar]
- Nastoupil LJ, Sinha R, Byrtek M, Ziemiecki R, Zhou X, Taylor M, Friedberg JW, Link BK, Cerhan JR, Dawson K & Flowers CR (2016) Outcomes following watchful waiting for stage II-IV follicular lymphoma patients in the modern era. Br J Haematol, 172, 724–734. [DOI] [PubMed] [Google Scholar]
- Pott C, Hoster E, Kehden B, Unterhalt M, Herold M, van der Jagt R, Janssens A, Kneba M, Mayer J, Pocock C, Danesi N, Fingerle-Rowson G, Harbron C, Mundt K, Marcus R & Hiddemann W (2016) Minimal Residual Disease in Patients with Follicular Lymphoma Treated with Obinutuzumab or Rituximab As First-Line Induction Immunochemotherapy and Maintenance in the Phase 3 GALLIUM Study. Blood, 128, 613.27492312 [Google Scholar]
- Radford J, Davies A, Cartron G, Morschhauser F, Salles G, Marcus R, Wenger M, Lei G, Wassner-Fritsch E & Vitolo U (2013) Obinutuzumab (GA101) plus CHOP or FC in relapsed/refractory follicular lymphoma: results of the GAUDI study (BO21000). Blood, 122, 1137–1143. [DOI] [PubMed] [Google Scholar]
- Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grunhagen U, Losem C, Kofahl-Krause D, Heil G, Welslau M, Balser C, Kaiser U, Weidmann E, Durk H, Ballo H, Stauch M, Roller F, Barth J, Hoelzer D, Hinke A, Brugger W & Study group indolent L. (2013) Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet, 381, 1203–1210. [DOI] [PubMed] [Google Scholar]
- Rummel MJ, Buske C, Hertenstein B, Lerchenmüller C, Koenigsmann M, Lange E, Reeb M, Kaiser U, Balser C, Behringer D, Dürig J, Gaska T, Maschmeyer G, Schliesser G, Burchardt AC, Barth J, Kauff F, Hinke A & Greil R (2017) Four Versus Two Years of Rituximab Maintenance (R-maintenance) Following Bendamustine Plus Rituximab (B-R): Initial Results of a Prospective, Randomized Multicenter Phase 3 Study in First-Line Follicular Lymphoma (the StiL NHL7–2008 MAINTAIN study). Blood, 130, 483–483. [Google Scholar]
- Salles G, Seymour JF, Offner F, Lopez-Guillermo A, Belada D, Xerri L, Feugier P, Bouabdallah R, Catalano JV, Brice P, Caballero D, Haioun C, Pedersen LM, Delmer A, Simpson D, Leppa S, Soubeyran P, Hagenbeek A, Casasnovas O, Intragumtornchai T, Ferme C, da Silva MG, Sebban C, Lister A, Estell JA, Milone G, Sonet A, Mendila M, Coiffier B & Tilly H (2011) Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet, 377, 42–51. [DOI] [PubMed] [Google Scholar]
- Salles G, Seymour J, Feugier P, Offner F, Lopez-Guillermo A, Belada D, Xerri L, Bouabdallah R, Catalano J, Brice P, Haioun C, Martín A, Pedersen L, Delmer A, Simpson D, Leppa S, Soubeyran P, Casasnovas R-O, Intragumtornchai T, Ribraga V, Silva M, Nicolas-Virelizier E, Lister T, Estell J, Milone G, Sonet A, Assemat J, Zeuner H, Coiffier B & Tilly H (2017) Long Term Follow-up of the PRIMA Study: Half of Patients Receiving Rituximab Maintenance Remain Progression Free at 10 Years. Blood, 130, 486. [Google Scholar]
- Sehn LH, Goy A, Offner FC, Martinelli G, Caballero MD, Gadeberg O, Baetz T, Zelenetz AD, Gaidano G, Fayad LE, Buckstein R, Friedberg JW, Crump M, Jaksic B, Zinzani PL, Padmanabhan Iyer S, Sahin D, Chai A, Fingerle-Rowson G & Press OW (2015) Randomized Phase II Trial Comparing Obinutuzumab (GA101) With Rituximab in Patients With Relapsed CD20+ Indolent B-Cell Non-Hodgkin Lymphoma: Final Analysis of the GAUSS Study. J Clin Oncol, 33, 3467–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehn LH, Chua N, Mayer J, Dueck G, Trneny M, Bouabdallah K, Fowler N, Delwail V, Press O, Salles G, Gribben J, Lennard A, Lugtenburg PJ, Dimier N, Wassner-Fritsch E, Fingerle-Rowson G & Cheson BD (2016) Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol, 17, 1081–1093. [DOI] [PubMed] [Google Scholar]
- Trotman J, Barrington S, Belada D, Meignan M, MacEwan R, Owen C, Ptáčník V, Rosta A, Fingerle-Rowson G, Mattiello F, Nielsen T, Sahin D, Hiddemann W, Marcus R & Davies A (2018) Prognostic value of end-of-induction PET response after first-line immunochemotherapy for follicular lymphoma (GALLIUM): secondary analysis of a randomised, phase 3 trial. Lancet Oncol 2018 October 8. doi: 10.1016/S1470-2045(18)30618-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- van Oers MH, Van Glabbeke M, Giurgea L, Klasa R, Marcus RE, Wolf M, Kimby E, van t Veer M, Vranovsky A, Holte H & Hagenbeek A (2010) Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin’s lymphoma: long-term outcome of the EORTC 20981 phase III randomized intergroup study. J Clin Oncol, 28, 2853–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.