Abstract
Neurotensin (NT), a 13 amino-acid peptide, is predominantly released from enteroendocrine cells of the small bowel in response to fat ingestion. Free fatty acid receptors (FFARs) FFAR1 and FFAR4 regulate secretion of gut hormones and insulin. Here, we show that docosahexaenoic acid, a long-chain fatty acid, has the most dramatic effect on NT release. FFAR1 agonists slightly stimulate and FFAR4 agonists dramatically stimulate and amplify NT secretion. Double knockdown of FFAR1 and FFAR4 decreases NT release, whereas overexpression of FFAR4, but not FFAR1, increases NT release. Administration of cpdA, an FFAR4 agonist, but not TAK-875, a selective FFAR1 agonist, increases plasma NT levels and further increases olive oil–stimulated plasma NT levels. Inhibition of MAPK kinase (MEK)/ERK1/2 decreased fatty acid–stimulated NT release but increased AMP-activated protein kinase (AMPK) phosphorylation. In contrast, inhibition of AMPK further increased NT secretion and ERK1/2 phosphorylation mediated by FFAR1 or FFAR4. Our results indicate that FFAR4 plays a more critical role than FFAR1 in mediation of fat-regulated NT release and in inhibitory crosstalk between MEK/ERK1/2 and AMPK in the control of NT release downstream of FFAR1 and FFAR4.
The role of FFAs in promotion of NT secretion and the involvement of FFAR1 and FFAR4 were investigated. DHA has a dramatic effect on NT release and FFAR4 mediates FFA-stimulated NT secretion.
Neurotensin (NT), a tridecapeptide initially identified and characterized by Carraway and Leeman (1), is released from N cells of the small bowel in response to intraluminal fats (2–6). NT affects glucose homeostasis through a glucose-sensitive promotion of insulin and pancreatic polypeptide secretion (7–9). We have shown that NT-deficient mice are protected from obesity, hepatic steatosis, and insulin resistance associated with consumption of high levels of fat (10). Moreover, in human longitudinal studies among nonobese subjects, high levels of pro-NT denoted a doubling of the risk of obesity developing later in life. Importantly, our findings directly link NT with increased fat absorption and obesity, and suggest that NT may provide a prognostic marker of future obesity and a potential target for prevention and treatment. Therefore, a better understanding of the molecular mechanisms regulating NT secretion is required to delineate the effects of NT during physiologic and pathologic conditions.
Free fatty acids (FFAs) can be classified according to their chain length as short-chain fatty acids (carbon chain length, 1 to 6); medium-chain fatty acids (MCFAs; carbon chain length, 7 to 12); and long-chain fatty acids (LCFAs; carbon chain length >12) (11). Physiologic functions of FFAs include regulation of insulin secretion (12) and release of various gut hormones, such as the incretin hormones cholecystokinin from I cells, glucose-dependent insulinotropic peptide from K cells, and glucagon-like peptide-1 (GLP-1) from L cells (12), as well as NT from N cells (13–16). The LCFAs stimulate induction of intracellular Ca2+ ([Ca2+]i), MAPK activation, insulin secretion, and amplification of glucose-stimulated insulin secretion (17). FFAs can also be classified as saturated or unsaturated depending on the lack or presence of double bonds, respectively. Unsaturated LCFAs stimulate the secretion of GLP-1 in vitro and in vivo (18). In a study in rats, Ferris et al. (13) demonstrated that perfusion of the small intestine with fatty acids with four or more carbons and alcohols of two or more carbons resulted in a significant elevation of NT plasma levels. In dogs, intraduodenal or jejunal perfusion of sodium oleate stimulated NT release by mechanisms originating in the proximal small intestine (14, 15). In a human study, intraduodenal perfusion of LCFAs (C18s), but not MCFAs, increased NT release (16). However, the underlying mechanisms of how FFAs regulate NT release remain unknown.
The FFA receptors (FFARs) FFAR1 (previously named GPR40) and FFAR4 (previously named GPR120) are two G-protein–coupled receptors that are abundantly expressed in enteroendocrine cells and identified as major receptors for MCFAs and LCFAs (19). Both receptors are being evaluated as potential therapeutic targets for the control of type 2 diabetes due to the direct or indirect promotion of insulin secretion (20). Studies demonstrate that FFAs regulate insulin secretion from pancreatic β-cells through FFAR1 (17, 21–24). FFAR1-knockout mice display reduced plasma incretin levels in response to high-fat feeding or olive oil gavage (25, 26). Hirasawa et al. (18) reported abundant expression of FFAR4 mRNA in the intestinal tracts of mice and humans and colocalization with GLP-1 secreted cells. FFAR4 mRNA was also abundantly expressed in the mouse intestinal endocrine cell line STC-1 (18). Similarly, saturated and unsaturated MCFAs and LCFAs activated FFAR4, resulting in a specific rise in [Ca2+]i and activation of ERK1/2 (18). FFAR4 is expressed in adipocytes and macrophages and is involved in the development of chronic inflammation and insulin resistance. FFAR4 knockout mice fed a high-fat diet gained more body weight and developed greater insulin resistance. FFAR1 and FFAR4 are expressed in K cells and contribute to glucose-dependent insulinotropic peptide secretion after fat ingestion.
AMP-activated protein kinase (AMPK), a serine/threonine kinase comprising three subunits: α (catalytic), β, and γ (regulatory) (27–29), is a critical fuel-sensing enzyme and regulator of metabolism. AMPK plays a negative role in glucose-stimulated insulin secretion in pancreatic β-cells to maintain glucose homeostasis (30, 31). We have shown that activation of AMPK stimulates NT secretion from endocrine cells through the inhibition of mTORC1 and negative feedback activation of ERK1/2 (32, 33). In the present study, we established that docosahexaenoic acid (DHA), a polyunsaturated LCFA, has the greatest effect on stimulation of NT secretion and the involvement of FFAR1 and FFAR4 in NT release in vitro and in vivo. Furthermore, ERK1/2 is functionally involved as a downstream effector of FFAR1 and FFAR4 signaling. Importantly, we describe an inhibitory crosstalk model between MAPK and AMPK signaling cascades in which both signaling pathways can serve in an inhibitory role to control the release of NT mediated by FFAR1 and FFAR4 activation.
Materials and Methods
Reagents
Phorbol 12-myristate 13-acetate (PMA), sodium oleate, oleic acid (OA), α-linolenic acid (ALA), DHA, palmitoleic acid (POA), lauric acid (LA), palmitic acid, and butyric acid were obtained from Sigma-Aldrich (St. Louis, MO). Potent and selective FFAR1 (GW 9508) and FFAR4 (TUG 891) agonists were obtained from Tocris (Minneapolis, MN); GPR40 agonist III and cpdA from MilliporeSigma (Billerica, MA); and Fasiglifam (TAK-875), a selective FFAR1 agonist, from Selleckchem (Houston, TX). Phospho-AMPKα (Thr172) (34), AMPKα (35), phospho-ERK1/2 (Thr202/Tyr204) (36), ERK1/2 (37), phospho-acetyl-CoA carboxylase (ACC; Ser79) (38), and ACC (39) antibodies were obtained from Cell Signaling Technology (Danvers, MA); NT antibody (40) from Abcam (Cambridge, MA); β-actin antibody (41) and sodium taurodeoxycholate from Sigma-Aldrich; and Alexa Fluor 488 Secondary antibody (42) from Thermo Fisher Scientific (Grand Island, NY). ON-TARGETplus SMARTpool (FFAR1 and FFAR4) and ON-TARGETplus Nontargeting Control Pool small interfering RNA (siRNA) were purchased from GE Dharmacon (Lafayette, CO); ERK1 short hairpin RNA (ERK1sh) and the nontargeting control short hairpin RNA (NTCsh) in bacterial glycerol stock from Sigma-Aldrich; hemagglutinin (HA)-tagged FFAR4 (FFAR4-HA) and the negative control lentiviral vectors from GeneCopoeia (Rockville, MD); and compound C (CC; AMPK inhibitor) and PD 0325901 [PD; MAPK kinase (MEK) inhibitor] from Cayman (Ann Arbor, MI).
Cell culture, transfection, and treatment
The BON cell line was derived from a human pancreatic carcinoid tumor (male), which has been characterized (43, 44). BON cells were maintained in a 1:1 mixture of DMEM and nutrient mixture, F12K, supplemented with 5% fetal bovine serum (FBS) in 5% CO2 at 37°C. QGP-1 cells, derived from a human pancreatic somatostatinoma (male) and purchased from Japan Health Sciences Foundation (Osaka, Japan) (45), were maintained in American Type Culture Collection–formulated RPMI-1640 medium with 10% FBS. STC-1, a mouse intestinal neuroendocrine tumor cell line from American Type Culture Collection, was maintained in DMEM medium with 10% FBS. Cells were plated in 24-well plates at a density of 15 × 104/cm2 for drug treatment and transfection (n = 3 wells). Cells were transfected with FFAR1 and FFAR4 smart pool and nontargeting control pool siRNA using RNAiMAX (Thermo Fisher Scientific ) and treated at 24 hours after transfection. All fatty acids (FAs) were freshly made in serum-free medium by sonication with probe sonicator (18). Cells were treated with FAs or drugs in serum-free media for 1 to 3 hours. For combination treatment, cells were pretreated with drugs for 30 minutes, then with DHA plus the drugs for another 3 hours. Media were collected for NT enzyme immunoassay (EIA) as described later in “Materials and Methods” and the cells lysed for western blotting analysis and normalization of NT EIA values.
Generation of stable cell lines
For generating the stable cell lines BON/NTCsh, BON/ERK1sh, BON/FFAR4-HA, and BON negative control vector (BON/NEG), lentivirus of ERK1sh and NTCsh or FFAR4-HA and the negative control were produced in 293FT cells, as described previously (32). BON cells were infected with lentiviral particles, puromycin-resistant cell pools were collected, and effective knockdown or overexpression was monitored by western blot analysis.
Immunofluorescent staining and confocal microscopy
Immunofluorescent staining was performed as described previously (10, 33, 46, 47). Briefly, STC-1 cells were grown on glass coverslips (#1) in 24-well plates for 72 hours. Cells were fixed with 4% paraformaldehyde/PBS and permeabilized with 0.3% Triton X-100/PBS. Cells were incubated with primary antibody for 1 hour, followed by Alexa Fluor-conjugated secondary antibody from Invitrogen for 30 minutes. Images were observed under a Nikon confocal microscope with a 60× oil objective.
NT EIA
Media collected from cells after treatment or plasma from mice were stored in −80°C for NT measurements using the NT EIA kit from Phoenix Pharmaceuticals (Belmont, CA), as described previously (46, 48). Media (50 μL) from each well (n = 3) were applied in duplicate for NT EIA and data were normalized by protein concentration obtained from parallel cell lysates. Similarly, plasma samples (50 μL) were assayed for measurement of NT levels.
RNA isolation and quantitative PCR analysis
Total RNA was isolated from the BON cells or scraped mucosa using RNeasy kits according to the manufacturer’s instructions (Qiagen, Valencia, CA). Each cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Quantitative PCR (qPCR) reaction was performed using a TaqMan Gene Expression Master Mix and TaqMan probes for mouse NT and human FFAR1 and FFAR4 according to the manufacturer’s protocol (Thermo Fisher Scientific). Constitutively expressed Actin (mouse) and GAPDH (human) genes were selected as an endogenous control to correct potential variation in RNA loading. Expression levels were assessed by evaluating threshold cycle values. The relative amount of mRNA expression was calculated by the 2-ΔΔCT method.
Protein preparation and western blotting
Protein preparation and western blotting were performed as described previously (48, 49). In brief, the cells were lysed with lysis buffer (Cell Signaling Technology) and equal amounts of protein were resolved on 4% to 12% NuPAGE BisTris gels from Invitrogen and electrophoretically transferred to polyvinylidene difluoride membranes; the membranes were incubated with primary antibodies overnight at 4°C, followed by secondary antibodies conjugated with horseradish peroxidase. Membranes were developed using Amersham ECL Western Blotting Detection Reagent from GE Healthcare Life Science (Piscataway, NJ) or Immobilon Western Chemiluminescent horseradish peroxidase substrate from Thermo Fisher Scientific. Intensity of phosphorylated ERK1/2 (p-ERK1/2) and AMPK (p-AMPK) blots were quantified by ImageJ software (US National Institutes of Health, https://imagej.nih.gov/ij/) and normalized by total ERK and AMPK, as described previously (10).
In vivo studies
All procedures were carried out according to protocols approved by the Institutional Animal Care and Use Committee at the University of Kentucky. For small intestinal NT mRNA expression, 3-month-old male C57BL/6 mice were euthanized and small intestine was dissected. Duodenum (5 cm from the pylorus) was excised and the remainder divided into four equal fragments. Mucosa was scraped, total RNA purified, and qPCR performed.
For jejunal or ileal perfusion, the protocol was modified from the methods described previously (15). Male, 3-month-old, C57BL/6 mice were fasted overnight and divided into groups including jejunal vehicle control, jejunal DHA, ileal vehicle control, and ileal DHA. Mice were anesthetized with isoflurane inhalation; for jejunal perfusion, the intestine just distal to the ligament of Treitz was ligated by a suture, a 200-μm glass cannula (Living Systems Instrumentation, Burlington, VT) was inserted, and the other end was glued to a standard IV tubing with a Luer adapter (Safety Blood Collection Set and Vacuette; Greiner Bio One, Kremsmünster, Austria) and connected to a 1-mL syringe. For ileal perfusion, the intestine was ligated and the glass cannula inserted 10 cm from the ileocecal junction. Both segments were perfused at a rate of 0.1 mL/min for 10 minutes via syringe pump (total volume was 1 mL). DHA (1 mM) was freshly mixed with 2.4 mM sodium taurodeoxycholate. All perfusates were warmed in a 37°C water bath. Blood was collected from the inferior vena cava 30 minutes after a 10-minute perfusion using K2EDTA blood collection tubes (Thermo Fisher Scientific).
For FFAR1 or FFAR4 agonist experiments, TAK-875 and cdpA were freshly dissolved in 0.5% carboxymethyl cellulose plus 0.25% Tween 80 and briefly sonicated with a probe sonicator. Three-month-old male C57BL/6 mice were fasted overnight and pretreated with TAK-875 and cdpA (10 mg/kg body weight) by gavage for 1 hour. Mice were then given olive oil (10 μL/g body weight) orally for 30 minutes. Mice were anesthetized with isoflurane inhalation and blood collected from the inferior vena cava. Plasma was obtained by centrifuging the blood at 10,000 rpm for 10 minutes at 4°C and aliquots were stored at −80°C. Plasma (50 μL) was used for NT measurements using NT EIA kits, as described previously in “Materials and Methods.”
Statistical analysis
NT EIA levels normalized by parallel protein concentration for control and experimental groups including treatment [FFAR1 and FFAR4 agonists, FAs, 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), CC, and PD compounds] resulting in fold-change data were summarized by means and standard deviations. General linear models including one-way ANOVA and linear mixed models for repeated measures data were used to perform comparisons across groups along with specific pairwise comparisons. mRNA levels relative to GAPDH were summarized and analyzed similarly. Adjustments for multiple pairwise testing between groups were performed using the Holm P value adjustment method.
Results
DHA significantly increased NT secretion
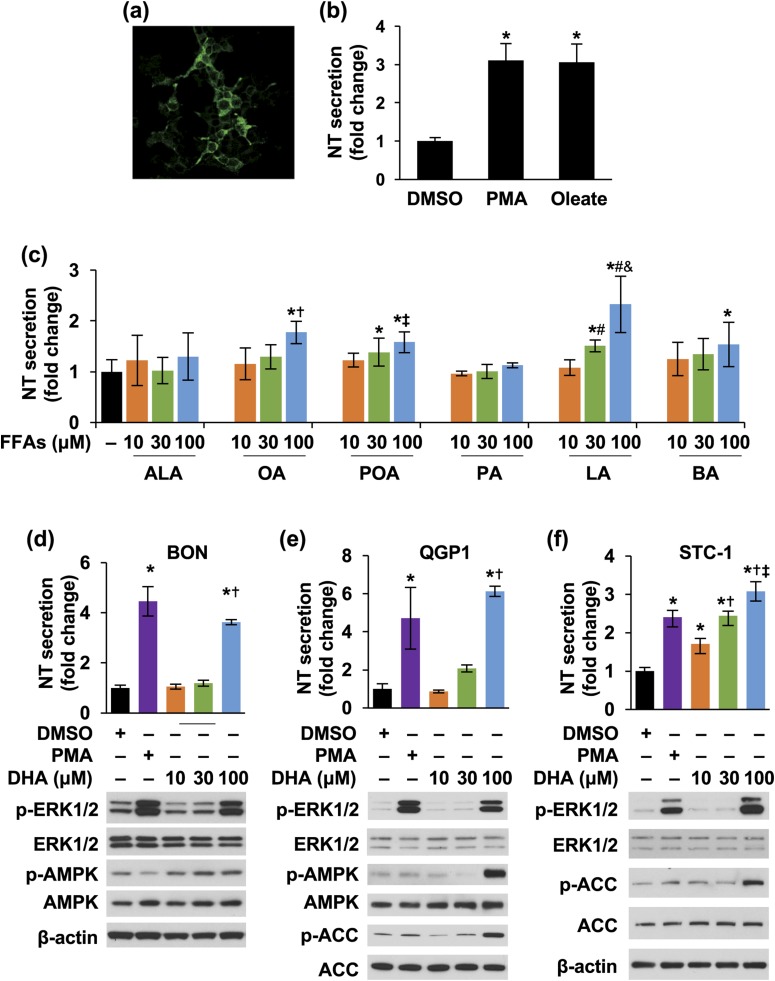
We routinely use BON and QGP-1 cell lines as our in vitro models to study the signaling pathways regulating NT secretion. In addition, the STC-1 cell line is an enteroendocrine model that has been used to study gut hormone secretion and is known to express FFAR1 and FFAR4 (18). We found that STC-1 cells also contain NT vesicles [Fig. 1(a)] and secrete NT upon PMA and oleate stimulation [Fig. 1(b)]. To identify which FFAs contribute to NT secretion, we first treated all three cell lines with different FFAs. ALA (18-carbon) and DHA (22-carbon) are polyunsaturated LCFAs, and OA (18-carbon) and POA (16-carbon) are monounsaturated LCFAs. LA (12-carbon) and palmitic acid (16-carbon) are saturated MCFAs. MCFA and LCFA are reported to be the FFAR1 and FFAR4 ligands. Butyric acid is a short-chain fatty acid and an agonist of FFAR2 and FFAR3 (50-52). As shown in Fig. 1(c), when BON cells were treated with different FFAs in various concentrations, NT release was slightly increased by OA and POA at a high dose (100 μM). LA stimulated NT release in BON cells in a dose-dependent fashion [Fig. 1(c)]. Similar results were found in QGP-1 and STC-1 cells (data not shown).
Figure 1.
FFAs stimulate NT secretion from neuroendocrine cells. (a) STC-1 cells were stained with NT antibody and observed by confocal microscope using the 60× oil objective. (b) STC-1 cells were treated with or without PMA (100 nM) or sodium oleate (0.25 mM) for 30 minutes; media were collected and NT EIA performed. *P < 0.05 vs DMSO. (c) BON cells were treated with or without different FFAs at various dosages for 3 hours; media were collected and NT EIA performed. *P < 0.05 vs control; †P < 0.05 vs 10 and 30 μM of OA; ‡P < 0.05 vs 10 μM of POA; #P > 0.05 vs 10 μM of LA; &P > 0.05 vs 30 μM of LA. (d–f) BON, QGP-1, and STC-1 cells were treated with or without different concentrations of DHA or 10 nM PMA (a positive control) for 3 hours; media (upper panels) and cells (lower panels) were analyzed by NT EIA and western blot, respectively. (d) BON cells: *P < 0.05 vs DMSO; †P < 0.05 vs 10, 30 μM DHA; (e) QGP-1 cells: *P < 0.05 vs DMSO; †P < 0.05 vs 10, 30 μM DHA; (f) STC-1 cells: *P < 0.05 vs DMSO; †P < 0.05 vs 10 μM DHA; ‡P < 0.05 vs 30 μM DHA. All data represent mean ± SD. Experiments were repeated at least three times. BA, butyric acid; DMSO, dimethyl sulfoxide; PA, palmitic acid.
DHA had the most pronounced effect on stimulation of NT secretion in BON, QGP-1, and STC-1 cells [Fig. 1(d)–1(f), upper panels]. DHA treatment increased p-ERK1/2 at 100 μM in all three cell lines [Fig. 1(d)–1(f), bottom panels]. p-AMPK was noted in BON cells treated with DHA at 10 μM and slightly increased in a dose-dependent fashion [Fig. 1(d), bottom panel]. AMPK signaling was only activated by DHA at 100 μM in QGP-1 and STC-1 cells, as demonstrated by p-AMPK or p-ACC, which is a direct target of active AMPK (53) [Fig. 1(d)–1(f), bottom panels). LA treatment did not affect p-ERK1/2 levels (data not shown). Thus, DHA, a polyunsaturated LCFA, stimulated NT secretion significantly and activated both ERK1/2 and AMPK signaling.
FFAR1 and FFAR4 agonists promoted NT release
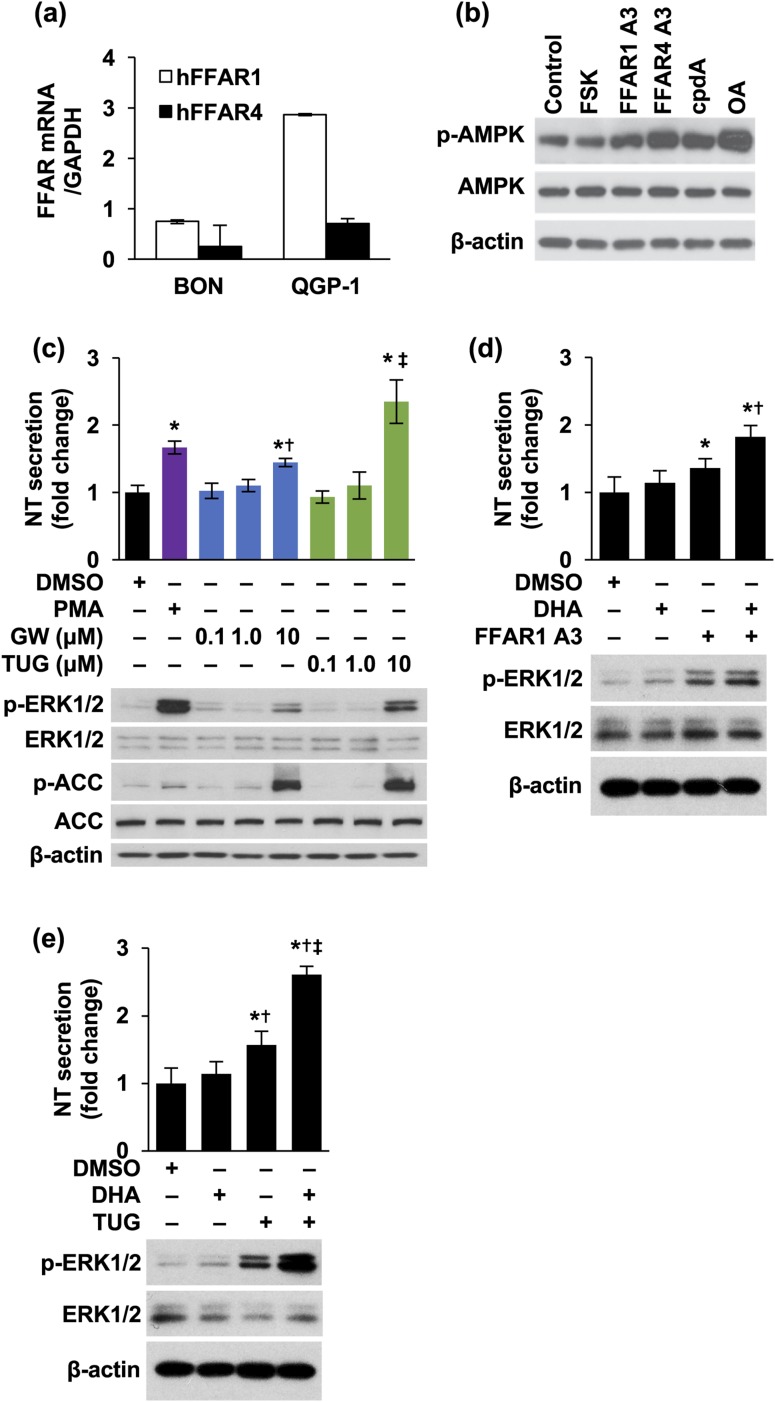
BON and QGP-1 cell lines express FFAR1 and FFAR4 mRNA [Fig. 2(a)]. We used FFAR1 or FFAR4 agonists to determine the involvement of FFAR1 and FFAR4 in NT secretion. FFAR1 agonist III is a nontoxic, potent, and selective agonist of FFAR1 (EC50, 20 nM), demonstrating 100-fold greater selectivity than FFAR4 (15). FFAR4 agonist III is a selective and potent agonist of FFAR4 (EC50 for β-arrestin 2 recruitment, 17 nM and 44 nM for mouse FFAR4 and human FFAR4, respectively; EC50 for Ca++ mobilization, 96 nM for human FFAR4) and exhibits much reduced potency toward FFAR1 (EC50 for β-arrestin 2 recruitment, 64.6 µM) (54). cpdA is a highly potent and selective activator of FFAR4 (EC50 for β-arrestin 2 recruitment, 350 nM); however, it displays very poor affinity for FFAR1 (55). As shown in Fig. 2(b), treatment of STC-1 cells with FFAR1 agonist III, FFAR4 agonist III, cpdA, and OA stimulated p-AMPK.
Figure 2.
FFAR1 and FFAR4 are involved in stimulation of NT release. (a) Total RNA was isolated from BON and QGP-1 cells and qPCR performed targeting human FFAR1 and FFAR4. GAPDH was used as the internal control. (b) STC-1 cells were treated with or without FFAR1 A3 (10 μM), FFAR4 A3 (10 μM), cpdA (10 μM), and OA (0.5 mM) for 1 hour. FSK (10 μM) was used as a negative control. (c) BON cells were treated with or without GW 9508 or TUG 891 in various dosages for 3 hours. PMA (10 nM) was used as a positive control. Media were collected and NT EIA performed (upper panel: *P < 0.05 vs DMSO; †P < 0.05 vs 0.1, 1.0 μM GW; ‡P < 0.05 vs 0.1, 1.0 μM TUG). Cells were lysed and western blotting analysis performed (lower panel). (d, e) BON cells were pretreated with (d) FFAR1 A3 (upper panels: *P < 0.05 vs DMSO; †P < 0.05 vs FFAR1 A3 alone) or (e) TUG 891 (upper panels: *P < 0.05 vs DMSO; †P < 0.05 vs DHA; ‡P < 0.05 vs TUG alone) for 30 minutes, followed by DHA (5 μM) with or without the agonists for 3 hours. Media were collected and NT EIA performed (upper panels); cells were lysed and western blotting analysis performed (lower panels). All data represent mean ± SD. Experiments were repeated at least three times. A3, agonist III; DMSO, dimethyl sulfoxide; FSK, forskolin; GW, GW 9508; TUG, TUG 891.
GW 9508 is a small molecule agonist of both FFAR1 and FFAR4, with much higher sensitivity to FFAR1 (EC50, 47 nM) vs FFAR4 (EC50, 2.2 μM) (56). TUG 891 is a potent FFAR4 agonist (EC50, 7.36 and 7.77 for human and mouse FFARF4, respectively) (54). GW 9508 slightly increased NT levels and p-ERK1/2 at 10 μM, whereas TUG 891 dramatically increased NT secretion [Fig. 2(c), upper panel] and p-ERK/1/2 [Fig. 2(c), lower panel], suggesting that FFAR4 is more important than FFAR1 in regulating NT release. Consistently, p-ACC was increased by both GW 9508 and TUG 891 at 10 μM, indicating activation of AMPK [Fig. 2(c), lower panel].
In addition, combination treatment of FFAR1 agonist III [Fig. 2(d)] or TUG 891 [Fig. 2(e)] enhanced DHA-stimulated NT release significantly and p-ERK1/2. It was also noted that activation of FFAR4 by combination treatments of TUG 891 with DHA induced more NT secretion and p-ERK1/2 compared with FFAR1 agonist III. Taken together, these results demonstrate that FFAR1 and FFAR4 function as positive factors in regulation of NT release. FFAR1 or FFAR4 agonists further amplify DHA-enhanced NT secretion. More importantly, FFAR4 appears to play a more prominent role in this process compared with FFAR1.
FFAR1 and FFAR4 double knockdown decreased NT secretion; FFAR4 overexpression increased NT secretion
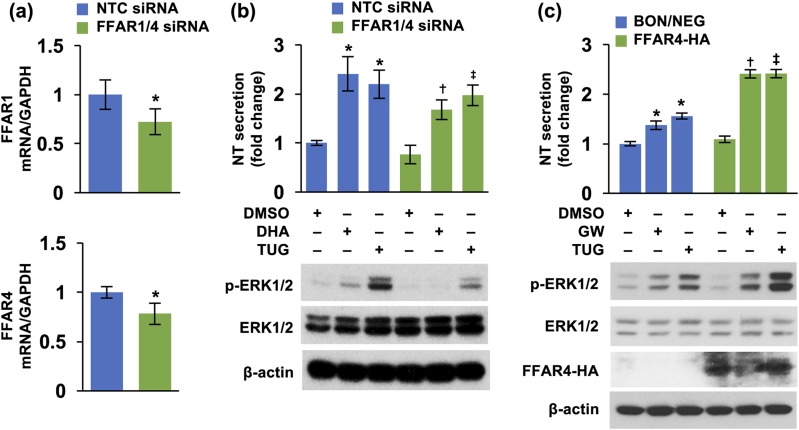
Next, we performed knockdown experiments of FFAR1 and FFAR4, identifying optimal concentrations of 30 and 5 nM for FFAR1 and FFAR4 siRNAs, respectively [Fig. 3(a)]. Consistently, double knockdown of FFAR1 and FFAR4 decreased both DHA- and TUG 891-stimulated NT secretion and p-ERK1/2 [Fig. 3(b)]. In addition, we established stable BON cell lines overexpressing either BON/NEG or HA-tagged FFAR1 or FFAR4. We did not detect the influence of FFAR1 overexpression on NT levels (data not shown); however, overexpression of FFAR4 increased both GW 9508- and TUG 891-mediated NT release and p-ERK1/2 [Fig. 3(c)]. These results further confirm that FFAR4 plays a major role in mediating NT secretion. The effects of GW 9508 on NT release in FFAR4-overexpressing cells may be due to its effects on FFAR4 because, although described as an FFAR1 selective agonist, GW 9508 has been reported to also stimulate FFAR4 (56).
Figure 3.
Double knockdown of FFAR1 and FFAR4 decreased, but overexpression of FFAR4 increased, NT release. (a) BON cells were doubly transfected with FFAR1 (30 nM) and FFAR4 (5 nM) siRNAs and grown for 24 hours. Cells were collected, total RNA extracted, and qPCR performed targeting FFAR1 and FFAR4. *P < 0.05 vs NTC siRNA. (b) BON cells, doubly transfected with FFAR1 and FFAR4 siRNAs, as in (a), were treated with DHA (100 μM) or TUG 891 (10 μM) for 3 hours. Media were collected and NT EIA performed (upper panel); cells were lysed for western blotting analysis (lower panel). Upper panels: *P < 0.05 vs DMSO in NTC siRNA; †P < 0.05 vs DHA in NTC siRNA; ‡P < 0.05 vs TUG 891 in NTC siRNA. (c) BON/NEG and BON/FFAR4-HA cell lines were treated with or without GW 9508 and TUG 891 for 3 hours. Media were collected and NT EIA performed (upper panel) and cells lysed for western blotting analysis (lower panel). Upper panels: *P < 0.05 vs DMSO in BON/NEG; †P < 0.05 vs GW 9508 in BON/NEG; ‡P < 0.05 vs TUG 891 in BON/NEG. All data are mean ± SD. Experiments were repeated at least three times. DMSO, dimethyl sulfoxide; GW, GW 9508; NTC, nontargeting control; TUG, TUG 891.
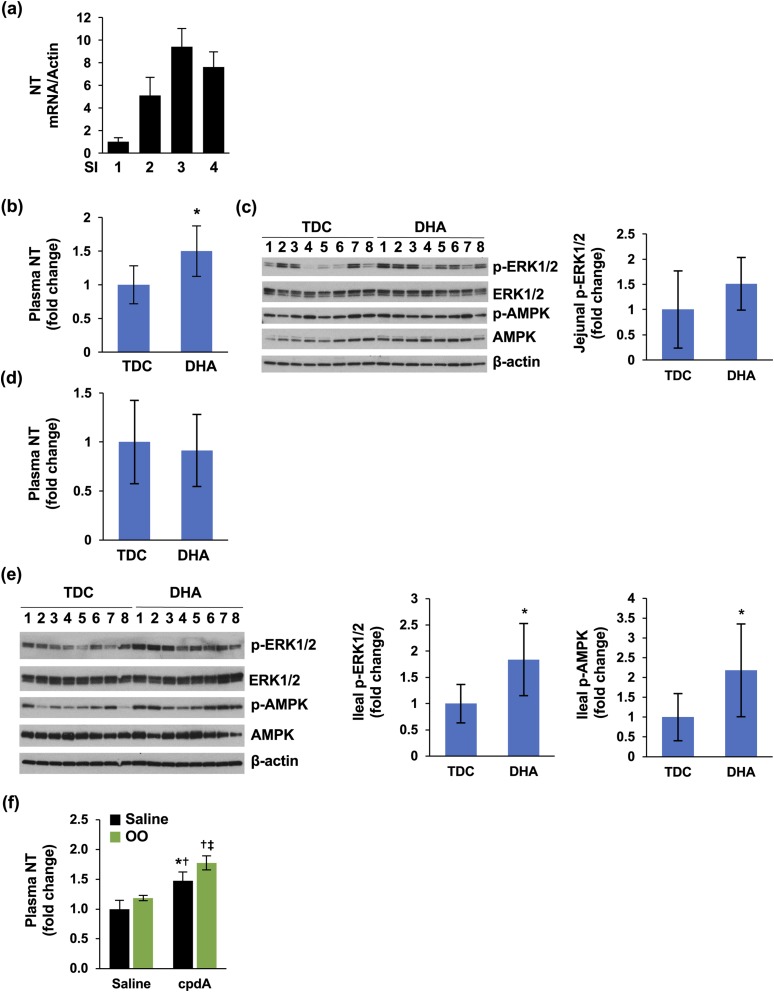
FFAR4 but not the FFAR1 agonist increased plasma NT levels in vivo
NT is highly concentrated in the ileum compared with the jejunum (57). Intraduodenal perfusion of fat increased plasma NT levels (14, 15). However, controversy exists regarding whether direct luminal administration into the ileum stimulates NT release (14, 15). Consistent with previous findings, NT mRNA was enriched in the distal intestine in the present study [i.e., fragments 3 and 4, which represent ileum; Fig. 4(a)]. In addition, only DHA perfusion of the jejunum, not the ileum, increased plasma NT levels [Fig. 4(b)]. DHA perfusion slightly increased p-ERK1/2 in mucosa of jejunum [Fig. 4(c)]. Ileal perfusion of DHA failed to stimulate plasma NT levels [Fig. 4(d)] even though p-ERK1/2 and p-AMPK were significantly elevated by DHA infusion [Fig. 4(e)].
Figure 4.
NT release in vivo. (a) SIs of C57BL/6 mice were divided into four fragments. Mucosa was scraped, total RNA isolated, and qPCR performed targeting mouse NT. (b) Jejunal perfusions were performed and plasma NT measured by NT EIA; TDC (vehicle), n = 8; DHA, n = 9. *P < 0.05 vs TDC. (c) Scraped mucosa from (b) were analyzed by western blot (left panel). Intensity of p-ERK was analyzed by ImageJ software and normalized with total ERK. (d) Ileal perfusions were performed and plasma NT measured by NT EIA. TDC (vehicle), n = 8; DHA, n = 9. (e) Scraped mucosa from (d) were analyzed by western blot (left panel). Intensity of p-ERK (middle panel) and p-AMPK (right panel) was analyzed by ImageJ software and normalized with total ERK or AMPK. (f) C57BL/6 mice were gavaged with saline or cpdA for 1 hour, followed by saline or OO for 30 minutes. Plasma NT was measured by NT EIA. N = 3 mice per group. *P < 0.05 vs saline alone; †P < 0.05 vs olive oil alone; ‡P < 0.05 vs cpdA alone. All data represent mean ± SD. OO, olive oil; SI, small intestine; TDC, taurodeoxycholate.
As an extension of these results, we treated mice with oral agonists by gavage. TAK-875, an FFAR1 agonist, has been used for in vivo studies (58), and cpdA is a highly potent, orally active and nontoxic FFAR4 agonist (55). Treatment with TAK-875 did not affect plasma NT levels (data not shown); however, cpdA alone significantly increased plasma levels of NT and further enhanced plasma NT levels in combination with olive oil gavage [Fig. 4(f)]. These findings further indicate that FFAR4 is the main FA receptor sensing FFA stimulation and NT release.
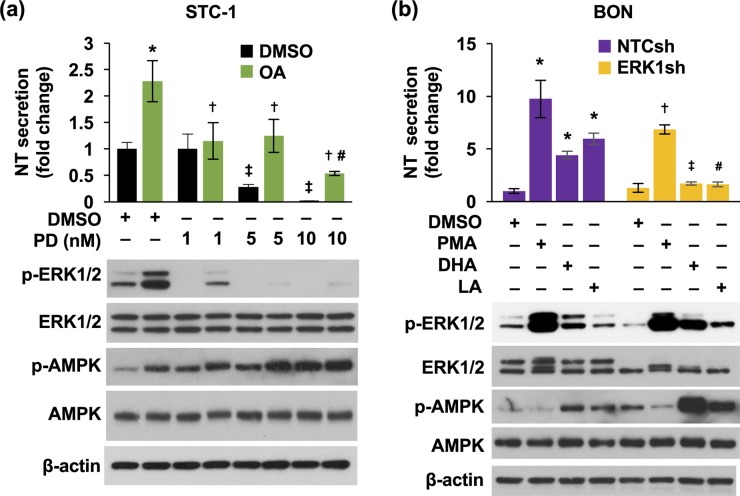
MAPK/ERK signaling positively regulated FFA-mediated NT secretion
p-ERK1/2 is often used as a convenient end point of signal generation via G-protein-coupled receptor activation and activation by FFAR1 and FFAR4 agonists (59). However, it has been demonstrated that MAPK signaling is not involved in FFAR1- or FFAR4-mediated GLP-1 or insulin secretion (18, 60). In the present study, basal and OA-stimulated NT secretion and p-ERK1/2 were inhibited by PD compound dose dependently [Fig. 5(a)]. However, p-AMPK was increased by PD treatment [Fig. 5(a), lower panel]. In addition, as shown in Fig. 5(b) (upper panel), stimulated NT secretion was significantly reduced by knockdown of ERK1. Western blot analysis showed the specific knockdown of ERK1 and reduction of basal and stimulated p-ERK1 with no effects on p-ERK2 [Fig. 5(b), lower panel). Again, p-ERK1 was not affected by LA treatment. Moreover, we found that p-AMPK was elevated by DHA and LA treatment in BON/NTCsh cells and dramatically increased in BON/ERK1sh cells [Fig. 5(b), lower panel]. These results demonstrate that the MAPK/ERK pathway is involved in DHA-stimulated NT secretion. Inhibition of MAPK/ERK signaling increased p-AMPK, suggesting a negative regulation of MAPK/ERK signaling on AMPK activity.
Figure 5.
MAPK/ERK signaling is involved in FFA-stimulated NT secretion. (a) STC-1 cells were pretreated with various dosages of PD for 30 minutes, followed by 0.5 mM OA for 1 hour. Media were collected and NT EIA performed (upper panel) and cells lysed for western blotting analysis (lower panel). Upper panel: *P < 0.05 vs DMSO alone; †P < 0.05 vs OA in DMSO; ‡P < 0.05 vs DMSO alone; #P < 0.05 vs 1, 5 nM PD. (b) Both BON/NTCsh and BON/ERK1sh cells were treated with or without DHA (100 μM) or LA (100 μM) for 1 hour. PMA (10 nM) was used as a positive control. Media were collected and NT EIA performed (upper panel) and cells lysed for western blotting analysis (lower panel). Upper panel: *P < 0.05 vs DMSO in BON/NTCsh; †P < 0.05 vs PMA in BON/NTCsh; ‡P < 0.05 vs DHA in BON/NTCsh; #P < 0.05 vs LA in BON/NTCsh. All data represent mean ± SD. Experiments were repeated at least three times. DMSO, dimethyl sulfoxide.
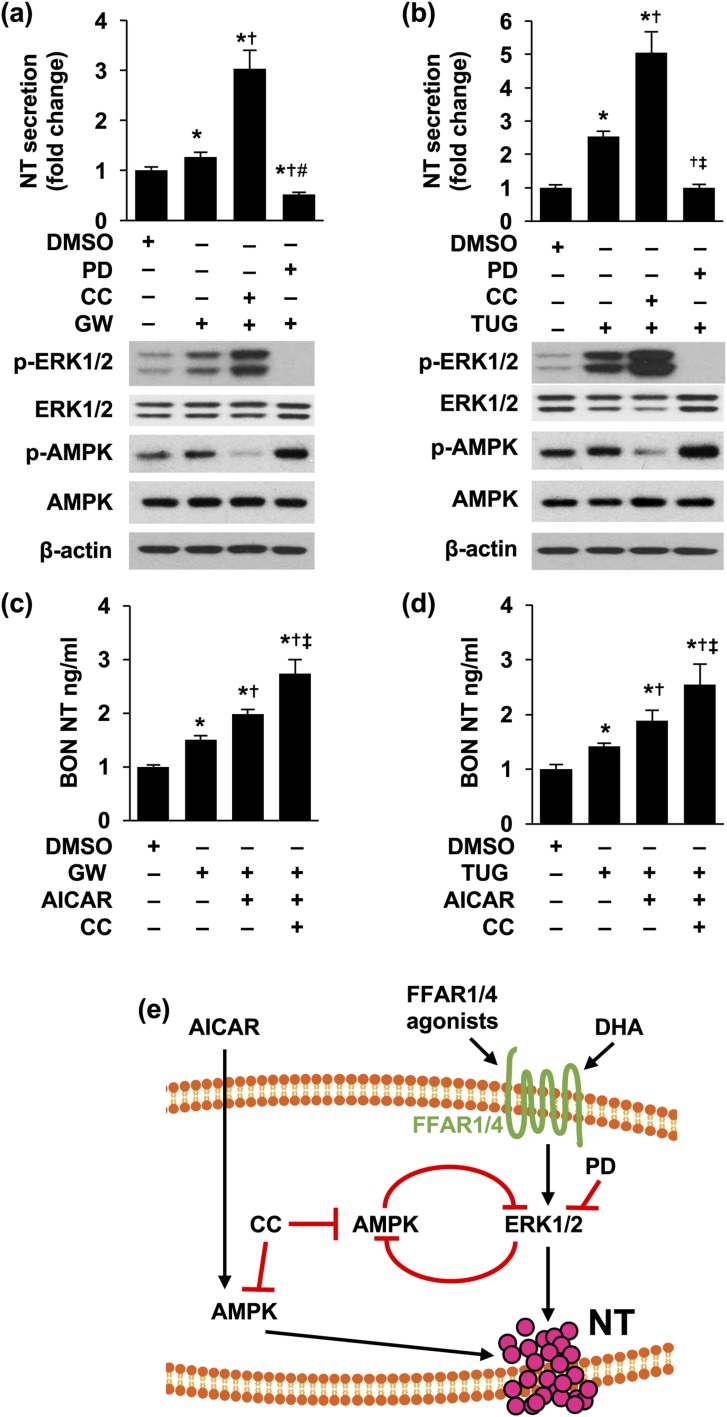
Inhibition of AMPK further increased FFAR1- and FFAR4-promoted NT secretion
We have demonstrated that activation of AMPK positively regulates NT secretion (33). However, as indicated, inhibition of MAPK signaling decreased NT release but increased p-AMPK in the present study. To determine the role of AMPK in the effects of FFAR1 and FFAR4 on NT release, we performed combination treatments of GW 9508 or TUG 891 with CC, an AMPK inhibitor. Surprisingly, we found that CC further enhanced GW 9508– or TUG 981–increased NT release [Fig. 6(a) and 6(b), upper panels]. In contrast, PD inhibited GW 9508– or TUG 981–stimulated NT release [Fig. 6(a) and 6(b), upper panels]. Treatment of CC further increased GW 9508– or TUG 981–mediated p-ERK1/2 activation, although it decreased p-AMPK [Fig. 6(a) and 6(b), lower panels]. However, p-AMPK was not inhibited by CC in combination with PD, but was further increased [Fig. 6(a) and 6(b), lower panels]. NT mRNA was not altered by the combination treatment of GW 9508 or TUG 981 with CC and PD (data not shown), suggesting these treatments are not associated with increased NT transcription.
Figure 6.
Inhibition of AMPK signaling further increased NT secretion stimulated by GW 9508 or TUG 891. (a,b) BON cells were pretreated with or without CC (10 μM) for 30 minutes, followed with or without PD (10 nM) for another 30 minutes and then by combined treatment of (a) GW 9508 (10 μM) or (b) TUG 891 (10 μM) with CC or PD for 3 hours. Media were collected and NT EIA performed (upper panels) and cells lysed for western blotting analysis (lower panels). Upper panels: *P < 0.05 vs DMSO; †P < 0.05 vs GW 9508 or TUG 891 alone; ‡P < 0.05 vs GW 9508 or TUG 891 plus CC. (c,d) BON cells were pretreated with CC (10 μM) for 30 minutes and then with or without AICAR (1 mM) for another 30 minutes, followed by combined treatment of (c) GW 9508 (10 μM) or (d) TUG 891 (10 μM) with AICAR or CC for 3 hours. Media were collected and NT EIA performed. *P < 0.05 vs DMSO; †P < 0.05 vs GW 9508 or TUG alone; ‡P < 0.05 vs GW 9508 or TUG 891 plus AICAR. Experiments were repeated at least three times. (e) Summary of signaling downstream of FFAR1 and FFAR4 in regulation of NT release. DHA and FFAR1 or FFAR4 agonists activate FFAR1 and FFAR4 and increase NT secretion through activation of ERK1/2. PD compound inhibits NT secretion by inhibiting MEK/ERK1/2 signaling. Inhibition of MEK/ERK or AMPK releases the inhibitory regulation of each other. DMSO, dimethyl sulfoxide; GW, GW 9508; TUG, TUG 891.
Last, we found GW 9508- or TUG 891-stimulated NT release was increased by combination treatment with AICAR and further enhanced by combination with CC [Fig. 6(c) and 6(d)]. Together, our results demonstrate a crosstalk between MAPK/ERK and AMPK signaling on NT secretion, mediated through FFAR1 and FFAR4, and further suggest that these two signaling cascades act in concert to regulate NT release mediated by FFAR1 or FFAR4 activation.
Discussion
In this study, we provide evidence of the involvement of FFAR1 and FFAR4 in regulation of NT secretion in vitro and in vivo. In neuroendocrine cell lines, we show that, similar to STC-1 cells, BON and QGP-1 cells express FFAR1 and FFAR4 mRNA. Although FFAR1 activation slightly increased NT release, activation of FFAR4 dramatically increased NT secretion. Activation of either FFAR1 or FFAR4 enhanced DHA-mediated NT release. Administration of the FFAR4 agonist cpdA, but not the FFAR1 agonist TAK-875, significantly increased plasma NT levels and further enhanced NT release in mice, associated with olive oil administration. Mechanistically, we demonstrated that MAPK/ERK activation plays a positive role in the promotion of NT secretion, as noted by decreased FFAR1 and FFAR4 agonist–mediated NT secretion associated with inhibition of MAPK signaling. Inhibition of MAPK increased AMPK phosphorylation, whereas AMPK inhibition increased activation of ERK1/2; however, inhibition of AMPK further enhanced FFAR1- or FFAR4-increased NT release. Therefore, our findings establish an inhibitory crosstalk in the regulation of NT secretion between MAPK and AMPK signaling pathways downstream of FFAR1 and FFAR4. MAPK plays a dominant role, whereas AMPK acts as a negative regulator in controlling NT release mediated by FFAR1/FFAR4MAPK signaling.
Although we found that both DHA (an unsaturated LCFA) and LA (a saturated MCFA) significantly stimulated NT release, only DHA-stimulated NT secretion was mediated by ERK signaling, because LA treatment had no effect on p-ERK1/2. LA-stimulated NT release was also decreased in BON/ERK1sh cells, suggesting involvement of the constitutive contribution of basal ERK to the effect of LA. Oh et al. (55) also demonstrated that only p-ERK activated by unsaturated FFAs such as DHA and ALA, but not the saturated POA, was abolished by FFAR4 knockdown. We also demonstrate that GW 9508, the selective FFAR1 agonist, slightly increased NT secretion compared with control cells. On the other hand, treatment with TUG 891, the FFAR4 agonist, significantly increased NT release. Phosphorylation of ERK1/2 was more pronounced by TUG 891 than GW 9508. Furthermore, only the administration of cpdA (an FFAR4 agonist), but not TAK-875 (an FFAR1 agonist), in mice significantly increased plasma levels of NT in vivo. These results indicate that FFAR4 is more active and positively regulates NT secretion.
A series of saturated and unsaturated MCFAs and LCFAs have been identified as ligands for FFAR1 and FFAR4 (61); however, the molecular mechanism of the ligand interaction with FFAR1 and FFAR4 is not well established. The carboxylic group of the FFAs is required for activation of both receptors (17, 18). Important residues of the binding pocket in FFAR1 have been indicated in the potential recognition and activation of FFAR1 (62, 63). FFAR1 is expressed in taste buds, the pancreatic islets, and the gut (26, 61, 64, 65). FFAR1 regulates FFA-mediated release of gastric inhibitory peptide and GLP-1 (26) and is a major contributor to the increase in insulin secretion caused by LCFAs (17, 56, 65–69). FFAR4 is expressed in the intestine (11, 55, 70, 71) and increases GLP-1 release (11, 18). Similar to our findings, although FFAR1 and FFAR4 are expressed endogenously in the enteroendocrine cell line STC-1, only FFAR4 has been shown to regulate GLP-1 release (18). LCFAs (e.g., DHA) increased p-ERK1/2 in HEK 293 cells transiently expressing mouse Ffar4 (18). FFAR4 was colocalized with ghrelin in duodenal cells; however, FFAR4 activation reduced plasma ghrelin level in vivo, indicating a negative regulation of FFAR4 on ghrelin secretion (72). Although FFAR4 is expressed in islets, FFAR4 does not appear to be involved in insulin secretion. In contrast, FFAR4 appears to promote glucagon secretion from α-cells (66). Therefore, FFAR4 may play either a positive or negative role in the regulation of hormone secretion. The findings in our present study provide evidence supporting a dominant and positive role for FFAR4 in increasing NT secretion.
When cells were treated with the PD compound, OA-stimulated NT secretion was decreased dose dependently. Attenuation of DHA-stimulated NT release was further noted in BON cells with knockdown of ERK1. Therefore, we provide direct evidence that MAPK signaling is functionally involved in FFA/FFAR1/FFAR4 signaling in the regulation of NT secretion. It has been reported that FFAR1 and FFAR4 signal via a Gαq/11-coupled pathway (20). FFAR1 and FFAR4 agonists promote the phosphorylation of ERK1/2 in cells expressing FFAR1 or FFAR4 predominantly via G proteins of the Gαq/11 family (61, 63, 73). When activated by MCFA and LCFA, FFAR1 activates G protein, which transduces the signal leading to stimulation of insulin secretion (17). Itoh et al. (17) reported that FFAs stimulate the formation of inositol 1,4,5-trisphosphate, [Ca2+]i mobilization, and ERK1/2 phosphorylation. However, inositol 1,4,5-trisphosphate signaling plays a minor role in the control of [Ca2+]i concentrations and insulin secretion in response to FFAs (20). Feng et al. (74) showed that linoleic acid reduced the voltage-gated K(+) currents in rat β-cells through FFAR1 and the cAMP-protein kinase A system, leading to an increase in [Ca2+]i and insulin secretion. FFAR4 is highly expressed in the human and mouse intestinal tract and adipocytes. Similar to the signaling mediated by FFAR1, activation of FFAR4 is also coupled to the elevation of Ca2+ and activation of the MAPK cascade in the regulation of GLP-1 secretion; however, PD098059 had no inhibitory effect on GLP-1 secretion (18). Activation of ERK1/2 was induced by palmitate and TUG-469, an FFAR1 agonist, whereas PD98059 significantly reduced phosphorylation of ERK1/2 but did not reverse the stimulation of insulin secretion (60). Therefore, our findings provide evidence for the involvement of MAPK signaling and potential new therapeutic targets to control NT release.
We also found that inhibition of MAPK signaling increased p-AMPK and, conversely, inhibition of AMPK signaling increased p-ERK1/2. Consistent with these results, a study in human mature dendritic cells demonstrated that MEK1/2/ERK1/2 signaling had an inhibitory effect on AMPK activity (75). More importantly, ERK1 directly associated with AMPK in stimulated cells as indicated by immunoprecipitation and proximity ligation assay analysis, suggesting direct interactions between these two molecules (75). These results suggest that ERK and AMPK are part of a similar protein complex and that active ERK could control p-AMPK. Hwang et al. (76, 77) also demonstrated a physical interaction of ERK-AMPK and a negative regulation of ERK1/2 on AMPK. The inhibitory regulatory loop between AMPK and ERK has also been reported in other systems. In neonatal rat cardiac fibroblasts, pretreatment with AICAR inhibited serum-stimulated p-ERK1/2 and growth (78). Shen et al. (79) reported that BRAF, an oncogenic protein kinase that stimulates cell growth and proliferation through the MEK-ERK signaling pathway, was phosphorylated by AMPK, which promoted the association of BRAF with 14-3-3 proteins and disrupted its interaction with the SKR1 scaffolding protein, leading to attenuation of the MEK-ERK signaling. Our previous findings demonstrated that activation of AMPK by AICAR or phenformin stimulated NT release, demonstrating a positive role of AMPK (33). However, in our present study, both AMPK and MEK/ERK were activated downstream of FFAR1 and FFAR4 and limited the activities of each to control release of NT, thus demonstrating a complex network for regulating NT secretion.
In summary, we have demonstrated that DHA stimulates NT secretion in neuroendocrine cell lines and in mice. The FFAR4 agonist cpdA significantly elevated plasma levels of NT and further increased olive oil–mediated NT release. Furthermore, we demonstrated an inhibitory regulation loop between MEK/ERK and AMPK [Fig. 6(e)]. These findings integrate the MEK/ERK and AMPK signaling networks, the two most important pathways in whole-body homeostasis, and further define the molecular mechanisms regulating hormone secretion from intestinal endocrine cells.
Acknowledgments
We thank the University of Kentucky Markey Cancer Center’s Research Communications Office for manuscript preparation and the Biostatistics and Bioinformatics Shared Resource Facility for statistical analyses.
Financial Support: This work was supported by National Institutes of Health Grants R01 DK112034 (to B.M.E.), R01 DK48498 (to B.M.E.), and NCI P30 CA177558 (to University of Kentucky Markey Cancer Center).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ACC
acetyl-CoA carboxylase
- AICAR
5-aminoimidazole-4-carboxamide ribonucleotide
- ALA
α-linolenic acid
- AMPK
AMP-activated protein kinase
- BON/NEG
BON negative control vector
- BON/NTCsh
BON nontargeting control small hairpin RNA
- [Ca2+]i
intracellular calcium
- CC
compound C
- DHA
docosahexaenoic acid
- EIA
enzyme immunoassay
- ERK1sh
ERK 1 small hairpin RNA
- FA
fatty acid
- FBS
fetal bovine serum
- FFA
free fatty acid
- FFAR
free fatty acid receptor
- FFAR4-HA
hemagglutinin-tagged free fatty acid receptor 4
- GLP-1
glucagon-like peptide-1
- HA
hemagglutinin
- LA
lauric acid
- LCFA
long-chain fatty acid
- MCFA
medium-chain fatty acid
- MEK
MAPK kinase
- NT
neurotensin
- NTCsh
nontargeting control small hairpin RNA
- OA
oleic acid
- p-AMPK
phosphorylated AMP–activated protein kinase
- PD
PD 0325901
- p-ERK1/2
phosphorylated ERK1/2
- PMA
phorbol 12-myristate 13-acetate
- POA
palmitoleic acid
- qPCR
quantitative PCR
- siRNA
small interfering RNA
References
- 1. Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973;248(19):6854–6861. [PubMed] [Google Scholar]
- 2. Barber DL, Cacace AM, Raucci DT, Ganz MB. Fatty acids stereospecifically stimulate neurotensin release and increase [Ca2+]i in enteric endocrine cells. Am J Physiol. 1991;261(3 Pt 1):G497–G503. [DOI] [PubMed] [Google Scholar]
- 3. Ferris CF, Carraway RE, Hammer RA, Leeman SE. Release and degradation of neurotensin during perfusion of rat small intestine with lipid. Regul Pept. 1985;12(2):101–111. [DOI] [PubMed] [Google Scholar]
- 4. Polak JM, Sullivan SN, Bloom SR, Buchan AM, Facer P, Brown MR, Pearse AG. Specific localisation of neurotensin to the N cell in human intestine by radioimmunoassay and immunocytochemistry. Nature. 1977;270(5633):183–184. [DOI] [PubMed] [Google Scholar]
- 5. Rosell S, Rökaeus A. The effect of ingestion of amino acids, glucose and fat on circulating neurotensin-like immunoreactivity (NTLI) in man. Acta Physiol Scand. 1979;107(3):263–267. [DOI] [PubMed] [Google Scholar]
- 6. Go VL, Demol P. Role of nutrients in the gastrointestinal release of immunoreactive neurotensin. Peptides. 1981;2(Suppl 2):267–269. [DOI] [PubMed] [Google Scholar]
- 7. Mazella J, Béraud-Dufour S, Devader C, Massa F, Coppola T. Neurotensin and its receptors in the control of glucose homeostasis. Front Endocrinol (Lausanne). 2012;3:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fletcher DR, Blackburn AM, Adrian TE, Chadwick VS, Bloom SR. Effect of neurotensin on pancreatic function in man. Life Sci. 1981;29(21):2157–2161. [DOI] [PubMed] [Google Scholar]
- 9. Blackburn AM, Bloom SR, Edwards AV. Pancreatic endocrine responses to exogenous neurotensin in the conscious calf. J Physiol. 1981;314(1):11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li J, Song J, Zaytseva YY, Liu Y, Rychahou P, Jiang K, Starr ME, Kim JT, Harris JW, Yiannikouris FB, Katz WS, Nilsson PM, Orho-Melander M, Chen J, Zhu H, Fahrenholz T, Higashi RM, Gao T, Morris AJ, Cassis LA, Fan TW, Weiss HL, Dobner PR, Melander O, Jia J, Evers BM. An obligatory role for neurotensin in high-fat-diet-induced obesity. Nature. 2016;533(7603):411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ichimura A, Hara T, Hirasawa A. Regulation of energy homeostasis via GPR120. Front Endocrinol (Lausanne). 2014;5:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferdaoussi M, Bergeron V, Zarrouki B, Kolic J, Cantley J, Fielitz J, Olson EN, Prentki M, Biden T, MacDonald PE, Poitout V. G protein-coupled receptor (GPR)40-dependent potentiation of insulin secretion in mouse islets is mediated by protein kinase D1. Diabetologia. 2012;55(10):2682–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferris CF, Armstrong MJ, George JK, Stevens CA, Carraway RE, Leeman SE. Alcohol and fatty acid stimulation of neurotensin release from rat small intestine. Endocrinology. 1985;116(3):1133–1138. [DOI] [PubMed] [Google Scholar]
- 14. Miyashita T, Hashimoto T, Gomez G, Townsend CM Jr, Greeley GH Jr, Thompson JC. Neurotensin secretion in response to intraduodenal and intraileal administration of fat in dogs. Biol Signals. 1992;1(5):275–281. [DOI] [PubMed] [Google Scholar]
- 15. Fujimura M, Khalil T, Sakamoto T, Greeley GH Jr, Salter MG, Townsend CM Jr, Thompson JC. Release of neurotensin by selective perfusion of the jejunum with oleic acid in dogs. Gastroenterology. 1989;96(6):1502–1505. [DOI] [PubMed] [Google Scholar]
- 16. Drewe J, Mihailovic S, D’Amato M, Beglinger C. Regulation of fat-stimulated neurotensin secretion in healthy subjects. J Clin Endocrinol Metab. 2008;93(5):1964–1970. [DOI] [PubMed] [Google Scholar]
- 17. Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422(6928):173–176. [DOI] [PubMed] [Google Scholar]
- 18. Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11(1):90–94. [DOI] [PubMed] [Google Scholar]
- 19. Milligan G, Alvarez-Curto E, Watterson KR, Ulven T, Hudson BD. Characterizing pharmacological ligands to study the long-chain fatty acid receptors GPR40/FFA1 and GPR120/FFA4. Br J Pharmacol. 2015;172(13):3254–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mancini AD, Poitout V. The fatty acid receptor FFA1/GPR40 a decade later: how much do we know? Trends Endocrinol Metab. 2013;24(8):398–407. [DOI] [PubMed] [Google Scholar]
- 21. Brownlie R, Mayers RM, Pierce JA, Marley AE, Smith DM. The long-chain fatty acid receptor, GPR40, and glucolipotoxicity: investigations using GPR40-knockout mice. Biochem Soc Trans. 2008;36(Pt 5):950–954. [DOI] [PubMed] [Google Scholar]
- 22. Fujiwara K, Maekawa F, Yada T. Oleic acid interacts with GPR40 to induce Ca2+ signaling in rat islet beta-cells: mediation by PLC and L-type Ca2+ channel and link to insulin release. Am J Physiol Endocrinol Metab. 2005;289(4):E670–E677. [DOI] [PubMed] [Google Scholar]
- 23. Itoh Y, Hinuma S. GPR40, a free fatty acid receptor on pancreatic beta cells, regulates insulin secretion. Hepatol Res. 2005;33(2):171–173. [DOI] [PubMed] [Google Scholar]
- 24. Schnell S, Schaefer M, Schöfl C. Free fatty acids increase cytosolic free calcium and stimulate insulin secretion from beta-cells through activation of GPR40. Mol Cell Endocrinol. 2007;263(1-2):173–180. [DOI] [PubMed] [Google Scholar]
- 25. Liou AP, Lu X, Sei Y, Zhao X, Pechhold S, Carrero RJ, Raybould HE, Wank S. The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology. 2011;140(3):903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57(9):2280–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carling D. AMPK. Curr Biol. 2004;14(6):R220. [DOI] [PubMed] [Google Scholar]
- 28. Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144(12):5179–5183. [DOI] [PubMed] [Google Scholar]
- 29. Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1(1):15–25. [DOI] [PubMed] [Google Scholar]
- 30. Fu A, Eberhard CE, Screaton RA. Role of AMPK in pancreatic beta cell function. Mol Cell Endocrinol. 2013;366(2):127–134. [DOI] [PubMed] [Google Scholar]
- 31. Viollet B, Lantier L, Devin-Leclerc J, Hebrard S, Amouyal C, Mounier R, Foretz M, Andreelli F. Targeting the AMPK pathway for the treatment of type 2 diabetes. Front Biosci. 2009;14:3380–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li J, Liu J, Song J, Wang X, Weiss HL, Townsend CM Jr, Gao T, Evers BM. mTORC1 inhibition increases neurotensin secretion and gene expression through activation of the MEK/ERK/c-Jun pathway in the human endocrine cell line BON. Am J Physiol Cell Physiol. 2011;301(1):C213–C226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li J, Song J, Weiss HL, Weiss T, Townsend CM Jr, Evers BM. Activation of AMPK stimulates neurotensin secretion in neuroendocrine cells. Mol Endocrinol. 2016;30(1):26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.RRID:AB_331250.
- 35.RRID:AB_330331.
- 36.RRID:AB_2315112.
- 37.RRID:AB_330744.
- 38.RRID:AB_330337.
- 39.RRID:AB_2219397.
- 40.RRID:AB_776725.
- 41.RRID:AB_476692.
- 42.RRID:AB_2576217.
- 43. Evers BM, Townsend CM Jr, Upp JR, Allen E, Hurlbut SC, Kim SW, Rajaraman S, Singh P, Reubi JC, Thompson JC. Establishment and characterization of a human carcinoid in nude mice and effect of various agents on tumor growth. Gastroenterology. 1991;101(2):303–311. [DOI] [PubMed] [Google Scholar]
- 44. Parekh D, Ishizuka J, Townsend CM Jr, Haber B, Beauchamp RD, Karp G, Kim SW, Rajaraman S, Greeley G Jr, Thompson JC. Characterization of a human pancreatic carcinoid in vitro: morphology, amine and peptide storage, and secretion. Pancreas. 1994;9(1):83–90. [DOI] [PubMed] [Google Scholar]
- 45. Doihara H, Nozawa K, Kojima R, Kawabata-Shoda E, Yokoyama T, Ito H. QGP-1 cells release 5-HT via TRPA1 activation; a model of human enterochromaffin cells. Mol Cell Biochem. 2009;331(1-2):239–245. [DOI] [PubMed] [Google Scholar]
- 46. Li J, Chen LA, Townsend CM Jr, Evers BM. PKD1, PKD2, and their substrate Kidins220 regulate neurotensin secretion in the BON human endocrine cell line. J Biol Chem. 2008;283(5):2614–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li J, Song J, Cassidy MG, Rychahou P, Starr ME, Liu J, Li X, Epperly G, Weiss HL, Townsend CM Jr, Gao T, Evers BM. PI3K p110α/Akt signaling negatively regulates secretion of the intestinal peptide neurotensin through interference of granule transport. Mol Endocrinol. 2012;26(8):1380–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li J, O’Connor KL, Cheng X, Mei FC, Uchida T, Townsend CM Jr, Evers BM. Cyclic adenosine 5′-monophosphate-stimulated neurotensin secretion is mediated through Rap1 downstream of both Epac and protein kinase A signaling pathways. Mol Endocrinol. 2007;21(1):159–171. [DOI] [PubMed] [Google Scholar]
- 49. Li J, Hellmich MR, Greeley GH Jr, Townsend CM Jr, Evers BM. Phorbol ester-mediated neurotensin secretion is dependent on the PKC-alpha and -delta isoforms. Am J Physiol Gastrointest Liver Physiol. 2002;283(5):G1197–G1206. [DOI] [PubMed] [Google Scholar]
- 50. Bourassa MW, Alim I, Bultman SJ, Ratan RR. Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7(4):2839–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hoeppli RE, Wu D, Cook L, Levings MK. The environment of regulatory T cell biology: cytokines, metabolites, and the microbiome. Front Immunol. 2015;6:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270(2 Pt 1):E299–E304. [DOI] [PubMed] [Google Scholar]
- 54. Shimpukade B, Hudson BD, Hovgaard CK, Milligan G, Ulven T. Discovery of a potent and selective GPR120 agonist. J Med Chem. 2012;55(9):4511–4515. [DOI] [PubMed] [Google Scholar]
- 55. Oh DY, Walenta E, Akiyama TE, Lagakos WS, Lackey D, Pessentheiner AR, Sasik R, Hah N, Chi TJ, Cox JM, Powels MA, Di Salvo J, Sinz C, Watkins SM, Armando AM, Chung H, Evans RM, Quehenberger O, McNelis J, Bogner-Strauss JG, Olefsky JM. A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat Med. 2014;20(8):942–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Briscoe CP, Peat AJ, McKeown SC, Corbett DF, Goetz AS, Littleton TR, McCoy DC, Kenakin TP, Andrews JL, Ammala C, Fornwald JA, Ignar DM, Jenkinson S. Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules. Br J Pharmacol. 2006;148(5):619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Evers BM, Ehrenfried JA, Wang X, Townsend CM Jr, Thompson JC. Temporal-specific and spatial-specific patterns of neurotensin gene expression in the small bowel. Am J Physiol. 1994;267(5 Pt 1):G875–G882. [DOI] [PubMed] [Google Scholar]
- 58. Tsujihata Y, Ito R, Suzuki M, Harada A, Negoro N, Yasuma T, Momose Y, Takeuchi K. TAK-875, an orally available G protein-coupled receptor 40/free fatty acid receptor 1 agonist, enhances glucose-dependent insulin secretion and improves both postprandial and fasting hyperglycemia in type 2 diabetic rats. J Pharmacol Exp Ther. 2011;339(1):228–237. [DOI] [PubMed] [Google Scholar]
- 59. Luttrell LM, Daaka Y, Lefkowitz RJ. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol. 1999;11(2):177–183. [DOI] [PubMed] [Google Scholar]
- 60. Panse M, Gerst F, Kaiser G, Teutsch CA, Dölker R, Wagner R, Häring HU, Ullrich S. Activation of extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) by free fatty acid receptor 1 (FFAR1/GPR40) protects from palmitate-induced beta cell death, but plays no role in insulin secretion. Cell Physiol Biochem. 2015;35(4):1537–1545. [DOI] [PubMed] [Google Scholar]
- 61. Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, Murdock PR, Sauls HR Jr, Shabon U, Spinage LD, Strum JC, Szekeres PG, Tan KB, Way JM, Ignar DM, Wilson S, Muir AI. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003;278(13):11303–11311. [DOI] [PubMed] [Google Scholar]
- 62. Sum CS, Tikhonova IG, Neumann S, Engel S, Raaka BM, Costanzi S, Gershengorn MC. Identification of residues important for agonist recognition and activation in GPR40. J Biol Chem. 2007;282(40):29248–29255. [DOI] [PubMed] [Google Scholar]
- 63. Smith NJ, Stoddart LA, Devine NM, Jenkins L, Milligan G. The action and mode of binding of thiazolidinedione ligands at free fatty acid receptor 1. J Biol Chem. 2009;284(26):17527–17539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N, le Coutre J, Ninomiya Y, Damak S. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci. 2010;30(25):8376–8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Del Guerra S, Bugliani M, D’Aleo V, Del Prato S, Boggi U, Mosca F, Filipponi F, Lupi R. G-protein-coupled receptor 40 (GPR40) expression and its regulation in human pancreatic islets: the role of type 2 diabetes and fatty acids. Nutr Metab Cardiovasc Dis. 2010;20(1):22–25. [DOI] [PubMed] [Google Scholar]
- 66. Suckow AT, Polidori D, Yan W, Chon S, Ma JY, Leonard J, Briscoe CP. Alteration of the glucagon axis in GPR120 (FFAR4) knockout mice: a role for GPR120 in glucagon secretion. J Biol Chem. 2014;289(22):15751–15763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Steneberg P, Rubins N, Bartoov-Shifman R, Walker MD, Edlund H. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab. 2005;1(4):245–258. [DOI] [PubMed] [Google Scholar]
- 68. Nagasumi K, Esaki R, Iwachidow K, Yasuhara Y, Ogi K, Tanaka H, Nakata M, Yano T, Shimakawa K, Taketomi S, Takeuchi K, Odaka H, Kaisho Y. Overexpression of GPR40 in pancreatic beta-cells augments glucose-stimulated insulin secretion and improves glucose tolerance in normal and diabetic mice [published correction appears in Diabetes. 2009;58(7):1721] Diabetes. 2009;58(5):1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Alquier T, Peyot ML, Latour MG, Kebede M, Sorensen CM, Gesta S, Ronald Kahn C, Smith RD, Jetton TL, Metz TO, Prentki M, Poitout V. Deletion of GPR40 impairs glucose-induced insulin secretion in vivo in mice without affecting intracellular fuel metabolism in islets. Diabetes. 2009;58(11):2607–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142(5):687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo L, Kimura I, Leloire A, Liu N, Iida K, Choquet H, Besnard P, Lecoeur C, Vivequin S, Ayukawa K, Takeuchi M, Ozawa K, Tauber M, Maffeis C, Morandi A, Buzzetti R, Elliott P, Pouta A, Jarvelin MR, Körner A, Kiess W, Pigeyre M, Caiazzo R, Van Hul W, Van Gaal L, Horber F, Balkau B, Lévy-Marchal C, Rouskas K, Kouvatsi A, Hebebrand J, Hinney A, Scherag A, Pattou F, Meyre D, Koshimizu TA, Wolowczuk I, Tsujimoto G, Froguel P. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 2012;483(7389):350–354. [DOI] [PubMed] [Google Scholar]
- 72. Gong Z, Yoshimura M, Aizawa S, Kurotani R, Zigman JM, Sakai T, Sakata I. G protein-coupled receptor 120 signaling regulates ghrelin secretion in vivo and in vitro. Am J Physiol Endocrinol Metab. 2014;306(1):E28–E35. [DOI] [PubMed] [Google Scholar]
- 73. Stoddart LA, Smith NJ, Milligan G. International Union of Pharmacology. LXXI. Free fatty acid receptors FFA1, -2, and -3: pharmacology and pathophysiological functions. Pharmacol Rev. 2008;60(4):405–417. [DOI] [PubMed] [Google Scholar]
- 74. Feng DD, Zhao YF, Luo ZQ, Keating DJ, Chen C. Linoleic acid induces Ca2+-induced inactivation of voltage-dependent Ca2+ currents in rat pancreatic beta-cells. J Endocrinol. 2008;196(2):377–384. [DOI] [PubMed] [Google Scholar]
- 75. López-Cotarelo P, Escribano-Díaz C, González-Bethencourt IL, Gómez-Moreira C, Deguiz ML, Torres-Bacete J, Gómez-Cabañas L, Fernández-Barrera J, Delgado-Martín C, Mellado M, Regueiro JR, Miranda-Carús ME, Rodríguez-Fernández JL. A novel MEK-ERK-AMPK signaling axis controls chemokine receptor CCR7-dependent survival in human mature dendritic cells. J Biol Chem. 2015;290(2):827–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hwang S-L, Lu Y, Li X, Kim YD, Cho YS, Jahng Y, Son J-K, Lee YJ, Kang W, Taketomi Y. ERK1/2 antagonize AMPK-dependent regulation of FcεRI-mediated mast cell activation and anaphylaxis. J Allergy Clin Immunol. 2014;134(3):714–721.e717 [DOI] [PubMed] [Google Scholar]
- 77. Hwang S-L, Li X, Lu Y, Jin Y, Jeong Y-T, Kim YD, Lee I-K, Taketomi Y, Sato H, Cho YS. AMP-activated protein kinase negatively regulates FcɛRI-mediated mast cell signaling and anaphylaxis in mice. J Allergy Clin Immunol. 2013;132(3):729–736.e712 [DOI] [PubMed] [Google Scholar]
- 78. Du J, Guan T, Zhang H, Xia Y, Liu F, Zhang Y. Inhibitory crosstalk between ERK and AMPK in the growth and proliferation of cardiac fibroblasts. Biochem Biophys Res Commun. 2008;368(2):402–407. [DOI] [PubMed] [Google Scholar]
- 79. Shen CH, Yuan P, Perez-Lorenzo R, Zhang Y, Lee SX, Ou Y, Asara JM, Cantley LC, Zheng B. Phosphorylation of BRAF by AMPK impairs BRAF-KSR1 association and cell proliferation. Mol Cell. 2013;52(2):161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]