Abstract
Purpose:
Meningiomas are more common in females and 70–80% express the progesterone receptor, raising the possibility that high-dose exogenous estrogen/progesterone exposure, such as occurs during fertility treatments, may increase the risk of developing a meningioma. The goal of this study was to report the incidence of prior fertility treatment in a consecutive series of female meningioma patients.
Methods:
A retrospective review (2015–2018) was performed of female patients with meningioma, and those with prior fertility treatment were compared to those without fertility treatment using standard statistical methods.
Results:
Of 206 female patients with meningioma, 26 (12.6%) had a history of fertility treatments. Patients underwent various forms of assisted reproductive technology including: in vitro fertilization (50.0%), clomiphene with or without intrauterine insemination (34.6%), and unspecified (19.2%). Median follow up was 1.8 years. Tumors were WHO grade I (78.6%) or grade II (21.4%). Patients who underwent fertility treatments presented at significantly younger mean age compared to those who had not (51.8 vs 57.3 yrs, p = 0.0135, 2-tailed T-test), and on multivariate analysis were more likely to have multiple meningiomas (OR: 4.97, 95% CI: 1.4–18.1, p = 0.0154) and convexity/falx meningiomas (OR: 4.45, 95% CI: 1.7–11.5, p = 0.0021).
Conclusions:
Patients in this cohort with a history of fertility treatment were more likely to present at a younger age and have multiple and convexity/falx meningiomas, emphasizing the importance of taking estrogen/progesterone exposure history when evaluating patients with meningioma. Future clinical studies at other centers in larger populations and laboratory investigations are needed to determine the role of fertility treatment in meningioma development.
Keywords: Fertility, Fertility Treatment, Meningioma, Estrogen, Progesterone, Risk Factor
Introduction:
Meningiomas are the most common primary brain tumor and are more common in women[1],[2]. Given the female predominance, it has been hypothesized that sexually dimorphic hormones play a role in the etiology and growth of meningiomas[3]. Several studies have looked at progesterone receptor (PR) and estrogen receptor (ER) in the setting of meningioma[4, 5]. While most meningiomas lack ER expression, PR is expressed in approximately 70–80% of meningioma[6, 7]. PR receptor expression is more frequently found in benign (WHO grade I) meningiomas[8]. Meningiomas can enlarge and become symptomatic during pregnancy, only to subsequently decrease in size post-partum[7, 9]. These observations raised the question of whether the use of high dose exogenous progesterone or estrogen agonists used during fertility treatments is associated with an increased risk of meningioma. One large case control study found no association between fertility treatment and meningioma[10]. There have been three cases of meningioma diagnosed in women with a history of fertility treatment described in the literature[11–13]. Recently, Wengel et al identified eight transgender patients who developed meningioma during hormone therapy. One of the patients experienced regression of multiple meningiomas upon discontinuation of the hormonal agent[14]. Together, these cases raise the possibility that high dose exogenous estrogen and/or progesterone could place patients at increased risk for meningioma, but there is no clear consensus on the relationship given the paucity of data. Additionally, anti-progestins (mifepristone) have demonstrated promising results as a therapy for meningioma in vitro, but have had equivocal results when used in vivo, and were stopped due to toxicity[15],[16, 17].
In the United States, the mean age of first-time mothers is increasing. According to the Center for Disease Control and Prevention (CDC), the mean age at first birth in the United States was 24.9 years in 2000, but has increased to 26.3 years old in 2014[18]. Recent surveys have shown that more women are choosing to delay having children to focus on their career and education[19]. In parallel, the use of assistive reproductive techniques (ART) has doubled over the last decade[20], giving women the reproductive flexibility to start families later in life.
Modern fertility treatment can encompass a variety of methods; however, most treatments involve a form of pharmacologic ovarian stimulation which alters the body’s hormonal environment by promoting development of multiple follicles. The majority of treatments involve either ovulation induction agents, with or without intrauterine insemination (IUI), or in vitro fertilization (IVF). Ovulation induction agents are selective estrogen receptor modulators (SERM) that increase the number of follicles that may mature and be released during ovulation and increase the levels of estradiol produced by these follicles, most commonly, clomiphene citrate. During the first boost using clomiphene, the estradiol level peaks approximately 1.5 times higher than a typical menstrual cycle[21]. IVF involves a higher level of ovarian stimulation with direct injections of follicle stimulating hormone (FSH) to stimulate supraphysiologic follicle development. An injection of human chorionic gonadotropin (hCG) is then given to trigger egg release prior to transvaginal retrieval. During IVF treatment, peak estradiol levels are approximately 9 times higher than normal menstrual cycles[22], and estradiol can upregulate progesterone receptor expression[23]. As part of treatment, women may receive supplemental progesterone for luteal phase support if embryos are implanted fresh (within the current cycle). However, due to the pulsatile secretion of progesterone from the corpus luteum, there is a wide range of progesterone levels within normal physiology, and progesterone supplementation likely sustains high-normal levels of this range[24, 25]. In contrast to fertility treatments, estradiol levels in women taking combined oral contraceptive pills are lower than levels seen during normal menstruation, usually between 73.5–294.1 pmol/L[26, 27]. Reigstad et al conducted a registry-based cohort study aiming to assess the cancer risk in women treated with fertility drugs. While they did not report on meningioma, they did find an all-site cancer risk of 1.14 (95% CI 1.03–1.26) and 1.10 (95% CI 0.98–1.23) following clomiphene citrate and assisted reproductive techniques, respectively[28].
Given the possibility of a relationship between fertility treatment and meningioma incidence, we began asking patients who were newly diagnosed with meningioma if they had undergone prior fertility treatment over a three-year time period in the senior author’s practice. Here, we present our series of meningioma patients who had undergone fertility treatments and compare them to women who had meningiomas but had not undergone fertility treatment during the same time period.
Methods:
Study design, setting, size, and participants
This is a retrospective chart review conducted at an academic medical center. All patients with a diagnosis of meningioma who present to our clinic are screened for known risk factors that contribute to meningioma development, including personal or family history of neurofibromatosis type 2, prior radiation therapy, and breast or thyroid cancer history. We began asking patients if they had undergone fertility treatments prior to meningioma diagnosis in 2015 and have continued to the present time. Data was collected from the consecutive series of female patients treated at our institution with the diagnosis of meningioma from 2015–2018. Inclusion criteria were: 1) the presence of a meningioma; 2) if fertility treatment prior to meningioma diagnosis had been taken; and 3) available clinical follow-up and documentation. No participants were excluded.
Variables
The following variables were collected. Demographic and tumor variables included patient age, gender, tumor size, and tumor grade, as defined by the WHO grading system at the time of resection. Presenting symptoms including: headache, vision, seizure, cognitive problems, facial weakness, vertigo/dizziness, and other. Surgical variables included approach and radiographic extent of resection defined as gross total, near total (> 95% resection), and subtotal (< 95%). Post-operative variables included any new post-operative deficit, complications, tumor recurrence, and any adjuvant radiotherapy.
Immunohistochemical assessment of estrogen receptor and progesterone receptor expression
Immunohistochemistry was performed on whole formalin-fixed, paraffin-embedded tissue sections from 11 cases of meningioma resected from women with history of oral contraceptive use. Three patients did not have slides available for the additional staining. Immunohistochemistry for progesterone receptor (PR) was performed using a rabbit monoclonal anti-PR antibody (clone 1E2, Ventana, cat # 790–2223) following CC1 antigen retrieval in a Ventana Benchmark Ultra automated stainer. Immunohistochemistry for estrogen receptor alpha (ERα) was performed using a rabbit monoclonal anti-ERα antibody (clone SP1, Ventana, cat # 790–4324) following CC1 antigen retrieval in a Ventana Benchmark Ultra automated stainer. Diaminobenzidine was used as the chromogen, followed by hematoxylin counterstain. Staining was scored as either negative (complete absence of labeling in tumor nuclei) or positive (with estimation of the percentage of tumor nuclei showing labeling) by an attending neuropathologist (D.A.S.).
Statistical analysis
All statistical analyses were performed in JMP (JMP®, Version 13.0. SAS Institute Inc., Cary, NC). Demographic data was assembled and analyzed in the standard fashion. For nominal data, Fisher exact tests were performed. For continuous data, two-tailed unpaired t test analysis was performed. Univariate and multi-variate logistic regression was performed and used to calculate odds ratios. The IRB at the author’s institution approved this study (IRB# 13–12587).
Results:
Demographics
From 2015–2018 we treated 180 female patients with meningioma who did not have NF2 or a history of breast cancer. We identified 26 patients who had undergone fertility treatment prior to meningioma diagnosis. Their demographics are presented in Table 1. Median follow up was 1.8 yrs. The median time from the start of fertility treatment to meningioma diagnosis was 18.0 (2.0–31.7 years) and from the end of fertility treatment to meningioma diagnosis was 13.4 (0.8–26.0). For patients with a history of fertility treatment, most discovered their meningioma as an incidental finding on workup for other reasons 57.7% (n=15). The most common presenting symptom was headache (26.9%, n=7). Other presenting complaints included visual disturbance (7.7%, n=2), cognitive problems (7.7%, n=2), seizures (3.8%, n=1), and vertigo/dizziness (3.8%, n=1). None of the patients who had received fertility treatments had a diagnosis of neurofibromatosis type 2 (NF2), prior head/neck radiation or breast cancer. Of note, 19.2% (n=5) of patients had thyroid disease including: hyperthyroidism (3.8%, n=1), hypothyroidism (7.7%, n=2), hypothyroidism with thyroid nodule (3.8%, n=1), and thyroid cancer (3.8%, n=1). All patients (100%, n=26) are currently alive. Median follow up time post diagnosis was 1.8 years and ranged from 0–18.6 years.
Table 1:
Demographics
| Patients Fertility (#) | 26 |
| Age at Diagnosis | |
| Median (yrs, range) | 48.6 (33.5–69.4) |
| Mean (yrs, std dev) | 51.8 (10.5) |
| Median follow up post diagnosis (yrs, range) | 1.8 (0–18.6) |
| Ethnicity | |
| White (Non-Hispanic) | 23 (88.5%) |
| Hispanic | 3 (11.5%) |
|
Time to Diagnosis after Fertility Treat. (yrs, range) | |
| Median time after first fertility tx | 18.0 (2.0–31.7) |
| Median time after last fertility tx | 13.4 (0.8–26.0) |
| Risk Factors for Meningioma | |
| Neurofibromatosis II | 0 |
| Prior Head/Neck Radiation | 0 |
| Breast Cancer | 0 |
| Thyroid Cancer | 1 |
| Initial presenting symptoms (#, %) | |
| Incidental | 15 (57.7%) |
| Headache | 7 (26.9%) |
| Visual disturbance | 2 (7.7%) |
| Cognitive problems | 2 (7.7%) |
| Seizures | 1 (3.8%) |
| Vertigo/Dizziness | 1 (3.8%) |
| Other | 1 (3.8%) |
| Fertility Treatment | n (%) |
| Clomiphene +/− IUI | 9 (34.6) |
| In Vitro Fertilization | 13 (50.0) |
| Unspecified/Unknown | 5 (19.2) |
| Progesterone | 1 (3.8) |
Pharmacologic Treatment
Standard of care fertility treatment typically includes an ovarian stimulation (with or without IUI) or IVF. We defined fertility treatment groups as clomiphene (with or without intrauterine insemination), IVF, and unspecified/unknown groups. Table 2 summarizes the variety of treatment regimens patients received during their fertility treatment. In this cohort, 34.6% (n=9) received clomiphene (with or without IUI), 50.0% (n=13) received IVF, and 19.2% (n=5) were unspecified/unknown treatments. Of note, one patient (3.8%) also received progesterone during her fertility treatment.
Table 2:
Tumor Characteristics/Outcomes
| n (%) | |
|---|---|
| Management (n = 26) | |
| Surgical Resection | 11 (64.7) |
| Stereotactic Radiosurgery | 3 (17.6) |
| Both Surgery and Radiation (SRS or EBRT) | 3 (17.6) |
| Observation | 9 (34.6) |
| WHO Grade (n = 14 resections) | |
| I | 11 (78.6) |
| II | 3 (21.4) |
| Histological Subtype (n = 13 avail. for review) | |
| Transitional | 8 (61.5) |
| Fibrous | 3 (23.1) |
| Meningothelial | 1 (7.7) |
| Meningothelial with focal rhabdoid features | 1 (7.7) |
| Progesterone Receptor Positive (n = 11 avail. for IHC) | |
| WHO Grade 1 (n=10) | 9 (90.0) |
| WHO Grade 2 (n = l) | 1 (100.0) |
| Estrogen Receptor Positive (n = 11 avail. for IHC) | |
| WHO Grade l (n = 10) | 1 (10) |
| WHO Grade 2 (n = l) | 0 (0) |
| Location (n = 26) | |
| Skull Base | 6 (23.1) |
| Anterior Fossa | 1 (3.8) |
| Middle Fossa | 2 (7.7) |
| Posterior Fossa | 3 (11.5) |
| Convexity | 7 (26.9) |
| Falx/Parasagittal | 8 (30.8) |
| Multiple | 5 (19.2) |
| Tumor Volume at Surgery (cm3) | |
| Mean (cm3, range) | 7.0 (3.1–28.7) |
| Largest Tumor Dimension at Treatment (cm) | |
| Mean (cm, range) | 2.2 (1.8–3.8) |
| Extent of Resection (n = 14) | |
| Gross Total | 10 (71.4) |
| Subtotal | 3 (21.4) |
| Both (for different tumors) | 1 (7.1) |
| Recurrence (n = 14) | 1 (7.1) |
Abbreviations: Avail, available; IHC, immunohistochemistry
Observation Group
Standard management strategy for patients undergoing observation is two 6-month scans. If stable, the interval between scans increases to yearly scans for five years.
Tumor Characteristics and Treatment Outcomes
Tumor characteristics and outcomes are presented in Table 2. Skull base tumors were grouped into anterior, middle or posterior fossa. Anterior cranial fossa included: tuberculum sella, olfactory groove, planum sphenoidale and parasellar tumors. Middle fossa included: cavernous sinus, clinoid, and sphenoid wing tumors. Posterior fossa included: cerebellopontine angle (CPA), clivus, petroclival, petrous face and foramen magnum tumors. Falx/parasagittal tumors included falx, falco-tentorial, tentorium, and parasagittal tumors. One patient had a peritorcular tumor that was above the tentorium, which we classified as a falx tumor. Convexity tumors included frontal, temporal, parietal, and cerebellar convexity tumors. The most common tumor location was falx/parasagittal at 30.8% (n=8). Tumor location included 26.9% (n=7) convexity, 23.1% (n=6) skull base, and 19.2% (n=5) multiple.
Tumor size for the fertility treated group was approximated by calculating the spherical volume (V=4/3πr3) using half the largest tumor diameter to approximate the radius. For multiple tumors, the largest tumor was selected for statistical analysis. The mean tumor spherical volume at surgery was 7.0 cm3 (3.1–28.7 cm3). The mean largest tumor diameter at surgery was 2.2 cm (1.8–3.8 cm).
For our series, 14 patients underwent surgical resection of their meningioma, 13 (92.9%) of which occurred at our institution and one patient (7.1%) was treated at an outside facility and referred to our institution for further management. Gross total resection was achieved in 71.4% (n=10) and a subtotal resection was achieved in 21.4% (n=3). One patient (7.1%) with more than one tumor had a subtotal resection of one tumor followed by Gamma Knife© radiosurgery for residual disease. She was subsequently taken back to the operating room for a reoperation of this tumor. Fourteen years later, she elected to have her second tumor located in the temporal convexity surgically removed at which time a gross total resection was achieved. This patient was also the only patient amongst the cohort to recur and the only patient to receive adjuvant radiation for subtotal resection. WHO grading was reported using the contemporary grade at the time of resection, with 78.6% (n=11) of tumors being WHO grade I and 21.4% (n=3) of tumors being WHO grade II. There were no WHO grade III tumors.
Characteristics of patients with or without a history of fertility treatment
We compared patients with a history of fertility treatment with the consecutive group of female patients who had not undergone fertility treatment (non-fertility group) with univariate (Table 3) and multivariate analysis (Table 4). Patients with a history of fertility treatment were diagnosed at a mean age of 51.8 years old compared to those with no prior history of fertility treatment diagnosed at a mean age of 57.3 years old (0.0135, 2-tailed T test). In patients with multiple meningiomas, we included each location separately in the analysis comparing differences between patients with or without a history of fertility treatment. On both univariate and multi-variate analysis, patients with a history of fertility treatment were more likely to have convexity/falx tumors (OR: 4.45, 95% CI: 1.7–11.5, p = 0.0021). Patients with a history of fertility treatment also were more likely to have multiple tumors (OR: 4.97, 95% CI: 1.4–18.1, p = 0.0154).
Table 3:
Univariate Analysis: Fertility vs. Non-fertility Group
| Fertility n (%) | P-Value | ||
|---|---|---|---|
| Yes | No | ||
| Sex | |||
| Female | 26 (100) | 180 (100) | |
| Mean Age at Diagnosis | 51.8 | 57.3 |
0.0135 (T test) |
| WHO Grade | |||
| I | 11 (78.6) | 133 (84.7) | |
| II | 3 (21.4) | 22 (14.0) | 0.6979 (Pearson) |
| III | 0 (0.0) | 2 (1.3) | |
| Tumor Location | |||
| Skull Base | 9 (29.0) | 112 (59.0) | 0.0019 |
| Convexity/Falx | 22 (71.0) | 78 (41.0) | (Pearson) |
| Multiple Meningiomas | |||
| Single | 21 (80.8) | 171 (95.0) | 0.0070 |
| Multiple | 5 (19.2) | 9 (5.0) | (Pearson) |
Table 4:
Multivariate analysis: Fertility vs Non-Fertility treatment
| Odds Ratio | 95% CI | P-Value | |
|---|---|---|---|
| Multiple Meningiomas | 4.97 | (1.36–18.17) | 0.0154 |
| Convexity/Falx Location | 4.45 | (1.71–11.51) | 0.0021 |
| Age at Diagnosis (OR/yr) | 1.04 | (1.00–1.08) | 0.0427 |
Histologic characteristics (ER/PR Staining)
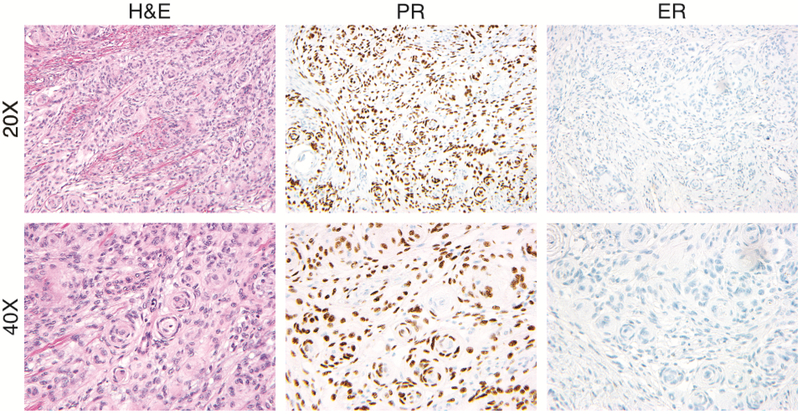
Of the 14 patients who underwent surgical resection of their meningioma, 11 tumor samples were available for immunohistochemical (IHC) assessment of progesterone receptor and estrogen receptor alpha expression. Of those, 10 were WHO grade I tumors and 9 (90%) were PR positive (Figure 1). The percentage of positive cells ranged from 10% to >90% with an average of 38.5% positivity. Only one meningioma demonstrated immunostaining for ERα, which was only positive in less than 1% of tumor cells. The one WHO grade II meningioma was positive for PR in 10% of cells and negative for ERα. Among the 13 meningiomas with diagnostic slides available for review, 61.5% (n=8) were of the transitional histologic subtype, 23.1% (n=3) fibrous, 7.7% (n=1) meningothelial, and 7.7% (n=1) meningothelial with focal rhabdoid features.
Figure 1:
Case example showing hematoxylin and eosin (H&E) staining as well as immunohistochemistry for progesterone receptor (PR) and estrogen receptor (ER) at 20x and 40x from a patient with a history of fertility treatment and a WHO grade 1 meningioma.
Discussion:
Key Results
The objective of this study was to report the incidence of prior fertility treatment among a consecutive series of female patients seen with a diagnosis of meningioma. We found that patients with a history of fertility treatment developed meningiomas presented at significantly younger at age and were more likely to have multiple meningiomas and convexity/falx meningiomas compared to patients who had not received fertility treatments. This emphasizes the importance of screening for prior fertility treatment when obtaining a history from patients diagnosed with meningioma.
Interpretation
There is a well-established relationship between female gender, progesterone and estrogen receptor expression, and the incidence of meningioma[2, 4, 5, 29], however, the impact of fertility treatment on meningioma is under-studied. Maternal age and the use of ART is increasing[19], making a relationship between ART use and meningioma incidence increasingly relevant to the general population.
While studies investigating meningioma and fertility treatment are limited to retrospective case reports and one case-control study [10–13], the association of progesterone receptor expression and meningioma has been studied more extensively[30]. There are various situations in addition to fertility treatment where patients might be exposed to high dose estrogen or progesterone. Progesterone levels are physiologically increased in pregnancy, and there are many clinical scenarios where patients receive additional exogenous progesterone for a prolonged period during their pregnancy (e.g. history of prior preterm birth or known short cervix). Patients with gender identity disorder/gender dysphoria undergoing male to female sex reassignment therapy also undergo hormone therapy, with estrogen being the basis of treatment as well as anti-androgenic therapy[31]. Long-term contraception therapy also exposes women to progesterone and Harland et al found that following resection of WHO grade I meningiomas, progesterone-only contraception was associated with increased recurrence (33.3 vs 19.6%) decreased time to recurrence (18 vs. 32 months, p=0.038)[32]. Interestingly, patients with meningioma have a stronger desire to have a child than the general population (70% vs 54%) and are more likely to intend to have a baby (27% vs 12%)[33]. Despite this desire, patients with meningioma are discouraged from using hormonally based contraceptives and cautioned that pregnancy could be a risk factor for meningioma recurrence[33].
Our finding that women with a history of fertility treatment present with meningiomas at younger age and with more convexity/falx meningiomas and multiple meningiomas raises the question of what mechanism fertility treatment or estrogen/progesterone exposure may impact meningioma tumorigenesis and growth. A recent study by Peyre et. al. compared 40 female patients who had long-term progestin therapy to a cohort that had not had progestin therapy[30]. Like our findings, patients exposed to progestin therapy were younger at tumor onset (mean 48 yrs vs 58 yrs) and had more multiple/multi-focal meningiomas (48% vs 5% p <10−12). In contrast, they had more meningiomas located at the skull base in the progestin exposed group (64% vs 50% p=0.03), whereas our patients were enriched for convexity/falx location. They performed copy number analysis and targeted sequencing for genes known to be mutated in meningioma including NF2, KLF4, TRAF7, PIK3CA, and TERT, among others[34, 35]. Interestingly, patients exposed to progestin therapy had a marked enrichment in PIK3CA mutant meningiomas, as well as an increase in the incidence of TRAF7 mutant meningiomas compared to the general population, which raises the possibility that progestin therapy selects for PIK3CA and/or TRAF7 mutant meningiomas, altering the molecular profile of the tumors. There also was a notable absence of SMO mutant meningiomas in the progestin treated group, and decreased frequency of NF2 mutations, although they were still found in 8% of cases. Finally, the observation of increased incidence of multiple meningiomas in both their progestin treated group and our cohort of patients exposed to fertility treatments suggests that these exposures may play a role in meningioma tumorigenesis, an idea that needs to be explored in the laboratory. Future large-scale clinical studies are needed to better understand the relationship between exogenous high dose estrogen/progesterone exposure and the risk this carries for the development of meningioma.
Limitations
This study is limited by its retrospective design and small size as well as our inability to quantify the details and dose that patients were exposed to from different fertility treatments. The relatively small total number of cases with a history of fertility treatment requires caution when generalizing our finding of increased incidence of multiple meningiomas to other studies and will need to be validated in additional larger cohorts. Ideally, we would have detailed information on prior fertility treatment, including: dosing, number of cycles, treatment initiation and conclusion dates, resultant pregnancy, and blood hormone levels. Unfortunately, many patients did not know these details, particularly given the median 13–18 year time gap between fertility treatment and meningioma diagnosis. This long duration between fertility treatment and meningioma diagnosis raises the question of whether the high dose hyper-estrogenic environment created at the time of fertility treatment is actually the cause of the meningioma. Given the slow growth rate of meningiomas, it is likely that the inciting event triggering tumor formation occurs years before diagnosis. However, answering whether fertility treatment could cause meningioma formation or directly promote growth is beyond the scope of this observational study and will need to be addressed in future clinical studies and the laboratory.
Conclusions:
Meningiomas present at a younger age in women who have a history fertility treatment in this cohort. They are more likely to have a convexity/falx location and multiple meningiomas. Our findings emphasize the importance of obtaining prior fertility treatments and exposure to progesterone treatments during history taking to facilitate future studies in larger cohorts and in the laboratory that will help develop our understanding of the relationship between fertility treatment and meningioma development.
Acknowledgements:
Disclosure of Funding: This work was supported by the Linda Wolfe Meningioma Research Program Project and the National Cancer Institute of the National Institutes of Health (1F32CA213944–01) to S.T.M.
Disclosure of Financial Support or Industry Affiliation: None
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interests related to this study.
Ethical Approval/Informed Consent: The study was approved by the institutional review board at the author’s institution (IRB#13–12587). After reviewing the study, the institutional review board deemed the study minimal risk and waived the need for informed consent by each subject.
References
- 1.Dolecek TA, Dressler EVM, Thakkar JP, et al. (2015) Epidemiology of meningiomas post-Public Law 107–206: The Benign Brain Tumor Cancer Registries Amendment Act. Cancer 121:2400–2410. 10.1002/cncr.29379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiemels J, Wrensch M, Claus EB (2010) Epidemiology and etiology of meningioma. J Neurooncol 99:307–314. 10.1007/s11060-010-0386-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claus EB, Calvocoressi L, Bondy ML, et al. (2013) Exogenous hormone use, reproductive factors, and risk of intracranial meningioma in females. J Neurosurg 118:649–656. 10.3171/2012.9.JNS12811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cahill D, Bashirelahi N, Solomon L, et al. (1984) Estrogen and progesterone receptors in meningiomas and gliomas. J Neurosurg 60:985–993 [DOI] [PubMed] [Google Scholar]
- 5.Horsfall DJ, Goldsmith KG, Ricciardelli C, et al. (1989) Steroid hormone and epidermal growth factor receptors in meningiomas. Aust N Z J Surg 59:881–8 [DOI] [PubMed] [Google Scholar]
- 6.Commins DL, Atkinson RD, Burnett ME (2007) Review of meningioma histopathology. Neurosurg Focus 23:1–9. 10.3171/FOC-07/10/E3 [DOI] [PubMed] [Google Scholar]
- 7.Lusis EA, Scheithauer BW, Yachnis AT, et al. (2012) Meningiomas in pregnancy: A clinicopathologic study of 17 cases. Neurosurgery 71:951–961. 10.1227/NEU.0b013e31826adf65 [DOI] [PubMed] [Google Scholar]
- 8.Buttrick S, Shah AH, Komotar RJ, Ivan ME (2016) Management of Atypical and Anaplastic Meningiomas. Neurosurg Clin N Am 27:239–247. 10.1016/j.nec.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 9.Chakravarthy V, Kaplan B, Gospodarev V, et al. (2018) Houdini Tumor: Case Report and Literature Review of Pregnancy-Associated Meningioma. World Neurosurg 114:e1261–e1265. 10.1016/j.wneu [DOI] [PubMed] [Google Scholar]
- 10.Korhonen K, Raitanen J, Isola J, et al. (2010) Exogenous sex hormone use and risk of meningioma: a population-based case–control study in Finland. Cancer Causes Control 21:2149–2156. 10.1007/s10552-010-9634-2 [DOI] [PubMed] [Google Scholar]
- 11.Motegi H, Kobayashi H, Terasaka S, et al. (2012) Hemorrhagic onset of rhabdoid meningioma after initiating treatment for infertility. Brain Tumor Pathol 29:240–244. 10.1007/s10014-012-0088-y [DOI] [PubMed] [Google Scholar]
- 12.Patterson A, Elashaal A (2016) Case Report Fast-Growing Meningioma in a Woman Undergoing Fertility Treatments. 2016:3–5. 10.1155/2016/3287381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frassanito P, de Bonis P, Mattogno PP, et al. (2012) Hormonal therapy for fertility and huge meningioma: A purely random association? Acta Neurol Belg 112:299–301. 10.1007/s13760-012-0046-9 [DOI] [PubMed] [Google Scholar]
- 14.ter Wengel PV, Martin E, Gooren L, et al. (2016) Meningiomas in three male-to-female transgender subjects using oestrogens/progestogens and review of the literature. Andrologia 48:1130–1137. 10.1111/and.12550 [DOI] [PubMed] [Google Scholar]
- 15.Chargari C, Védrine L, Bauduceau O, et al. (2008) Reapprasial of the role of endocrine therapy in meningioma management. Endocr Relat Cancer 15:931–941. 10.1677/ERC-08-0083 [DOI] [PubMed] [Google Scholar]
- 16.Grunberg SM, Weiss MH, Spitz IM, et al. (1991) Treatment of unresectable meningiomas with the antiprogesterone agent mifepristone. J Neurosurg 74:861–866. 10.3171/jns.1991.74.6.0861 [DOI] [PubMed] [Google Scholar]
- 17.Olson JJ, Beck DW, Schlechte J, Loh PM (1986) Hormonal manipulation of meningiomas in vitro. J Neurosurg 65:99–107. 10.3171/jns.1986.65.1.0099 [DOI] [PubMed] [Google Scholar]
- 18.Mathews TJ, Hamilton BE (2014) First Births to Older Women Continue to Rise. NCHS Data Brief 152: [PubMed] [Google Scholar]
- 19.Miller CC (2018) The U . S . Fertility Rate Is Down , Yet More Women Are Mothers. New York Times [Google Scholar]
- 20.(2014) CDC/ASRT 2012 Assisted Reproductive Technology Fertility Clinic Success Rates Report. Atlanta, GA [Google Scholar]
- 21.Smith DH, Picker RH, Sinosich M, Saunders DM (1980) Assessment of ovulation by ultrasound and estradiol levels during spontaneous and induced cycles. Fertil Steril 33:387–390. 10.1016/S0015-0282(16)44654-6 [DOI] [PubMed] [Google Scholar]
- 22.Suneeta M, Prerna G, Neena M, Neeta S (2014) Serum estradiol as a predictor of success of in vitro fertilization. J Obstet Gynecol India 64:124–129. 10.1007/s13224-013-0470-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nardulli AM, Greene GL, O’Malley BW, Katzenellenbogen BS (1988) Regulation of progesterone receptor messenger ribonucleic acid and protein levels in MCF-7 cells by estradiol: analysis of estrogen’s effect on progesterone receptor synthesis and degradation. Endocrinology 122:935–44. 10.1210/endo-122-3-935 [DOI] [PubMed] [Google Scholar]
- 24.Committee TP, Society A, Medicine R (2008) Progesterone supplementation during the luteal phase and in early pregnancy in the treatment of infertility: an educational bulletin. Fertil Steril 89:789–792. 10.1016/j.fertnstert.2008.02.012 [DOI] [PubMed] [Google Scholar]
- 25.Beltsos A, Robinson A, Martin-Johnston MK, et al. (2008) Serum progesterone levels with endometrin compared to progesterone in oil and associated pregnancy outcomes in a large IVF center. Fertil Steril 90:S366 10.1016/j.fertnstert.2008.07.1349 [DOI] [Google Scholar]
- 26.Gaspard UJ, Romus MA, Gillain D, et al. (1983) Plasma hormone levels in women receiving new oral contraceptives containing ethinyl estradiol plus levonorgestrel or desogestrel. Contraception 27:577–590. 10.1016/0010-7824(83)90023-9 [DOI] [PubMed] [Google Scholar]
- 27.Mishell D, Thorneycroft I, Nakamura R, et al. (1972) Serum estradiol in women ingesting combination oral contraceptive steriods. Am J Obstet Gynecol 114:923–928 [DOI] [PubMed] [Google Scholar]
- 28.Reigstad MM, Storeng R, Myklebust TA, et al. (2017) Cancer risk in women treated with fertility drugs according to parity status- A registry-based cohort study. Cancer Epidemiol Biomarkers Prev 26:953–962. 10.1158/1055-9965.EPI-16-0809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu DW, Efird JT, Hedley-Whyte ET (1997) Progesterone and estrogen receptors in meningiomas: prognostic considerations. J Neurosurg 86:113–120. 10.3171/jns.1997.86.1.0113 [DOI] [PubMed] [Google Scholar]
- 30.Peyre M, Gaillard S, de Marcellus C, et al. (2018) Progestin-associated shift of meningioma mutational landscape. Ann Oncol 29:681–686. 10.1093/annonc/mdx763 [DOI] [PubMed] [Google Scholar]
- 31.Unger CA (2016) Hormone therapy for transgender patients. Transl Androl Urol 5:877–884. 10.21037/tau.2016.09.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harland TA, Freeman JL, Davern M, et al. (2018) Progesterone-only contraception is associated with a shorter progression-free survival in premenopausal women with WHO Grade I meningioma. J Neurooncol 136:327–333. 10.1007/s11060-017-2656-9 [DOI] [PubMed] [Google Scholar]
- 33.Owens MA, Craig BM, Egan KM, Reed DR (2015) Birth desires and intentions of women diagnosed with a meningioma. J Neurosurg 122:1151–1156. 10.3171/2014.11.JNS14522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parada CA, Osbun J, Kaur S, et al. (2018) Kinome and phosphoproteome of high-grade meningiomas reveal AKAP12 as a central regulator of aggressiveness and its possible role in progression. Sci Rep 1–14. 10.1038/s41598-018-19308-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark VE, Erson-Omay EZ, Serin A, et al. (2013) Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 339:1077–80. 10.1126/science.1233009 [DOI] [PMC free article] [PubMed] [Google Scholar]