Abstract
Purpose
Breast cancer surgical techniques are evolving. Few studies have analyzed national trends for the multitude of surgical options that include partial mastectomy (PM), mastectomy without reconstruction (M), mastectomy with reconstruction (M+R), and PM with oncoplastic reconstruction (OS). We hypothesize that the use of M is declining and likely correlates with the rise of surgery with reconstructive options (M+R, OS).
Methods
A retrospective cohort analysis was conducted using the ACS-NSQIP database from 2005 to 2016 and ICD codes for IBC and DCIS. Patients were then grouped together based on current procedural terminology (CPT) codes for PM, M, M+R, and OS. In each group, categories were sorted again based on additional reconstructive procedures. Data analysis was conducted via Pearson’s chi-squared test for demographics, linear regression, and a non-parametric Mann-Kendall test to assess a temporal trend.
Results
The patient cohort consisted of 256,398 patients from the NSQIP data base; 197,387 meet inclusion criteria diagnosed with IBC or DCIS. Annual breast surgery trends changed as follows: PM 46.3–46.1% (p = 0.21), M 35.8–26.4% (p = 0.001), M+R 15.9–23.0% (p = 0.03), and OS 1.8–4.42% (p = 0.001). Analyzing the patient cohort who underwent breast conservation, categorical analysis showed a decreased use of PM alone (96–91%) with an increased use of OS (4–9%). For the patient cohort undergoing mastectomy, M alone decreased (69–53%); M+R with muscular flap decreased (9–2%); and M+R with implant placement increased (20–40%)—all three trends p < 0.0001.
Conclusion
The modern era of breast surgery is identified by the increasing use of reconstruction for patients undergoing breast conservation (in the form of OS) and mastectomy (in the form of M+R). Our study provides data showing significant trends that will impact the future of both breast cancer surgery and breast training programs.
Keywords: Breast conservation surgery, Mastectomy, Breast reconstruction, Trend analysis, Surgical incidence
Introduction
In the United States, breast cancer is the most common cancer in women with an incidence of 25.4% of all cancer diagnoses [1, 2]. One in eight women will develop invasive breast cancer in their lifetime, conversely higher than the 1-in-11 risk in 1970 [s3]. Although this associated rise is multifactorial, it can be attributed to increased life expectancy, hormone use, prevalence of obesity treatment, and screening options [s3]. Simultaneously, surgical interventions are transitioning towards a reconstructive approach that ensures aesthetic satisfaction in combination with oncologic safety [4, 5].
Surgical options for breast cancer patients can be categorized in two overall groups: Breast-conserving therapy (BCT) (including partial mastectomy (PM) and oncoplastic surgery (OS)) and MAST (including mastectomy (M) and M with breast reconstruction (M+R)). Depending on oncological guidelines, patients may choose to have partial or entire breast tissue removed with or without breast reconstruction. Breast reconstruction (OS or M+R) offers patients an improved quality of life and body image [6]. Over the years, accredited breast centers in the United States have transitioned away from only PM or M procedures and incorporated reconstructive procedures. However, previous retrospective analyses comparing PM to M showed nationwide inconsistencies between annual breast reconstruction trends [7–14].Understanding trends in breast surgery is extremely important with regards to prioritizing surgical training requirements for surgical trainees, and identifying the status quo so that improvements or adjustments could be made if these trends were not favored by the present outcomes data. Until now, no recent publication has analyzed the nationwide surgical trends in patients with invasive carcinoma (IBC) or ductal carcinoma in situ (DCIS). The purpose of our study is to use the America College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database to examine annual trends in breast surgical interventions from 2005 to 2016 in a nationwide cohort. With the rise of modern breast surgery, we hypothesize that the use of M is declining and is likely associated with the rise of more reconstructive breast cancer therapies. (M+R, OS).
Methods and materials
Data collection and participant pool
The study was deemed exempt by Tufts Medical Center Institutional Review Board given ACS NSQIP database was a de-identified data set. NSQIP is a hospital-based voluntary database with a goal of improving surgical outcomes corresponding to about 700 hospitals nationwide in 2016 with over 1 million randomly selected operations [15]. Approval was obtained from ACS NSQIP, and all participant user files (PUF) were analyzed between 2005 and 2016.
Primary surgical analysis
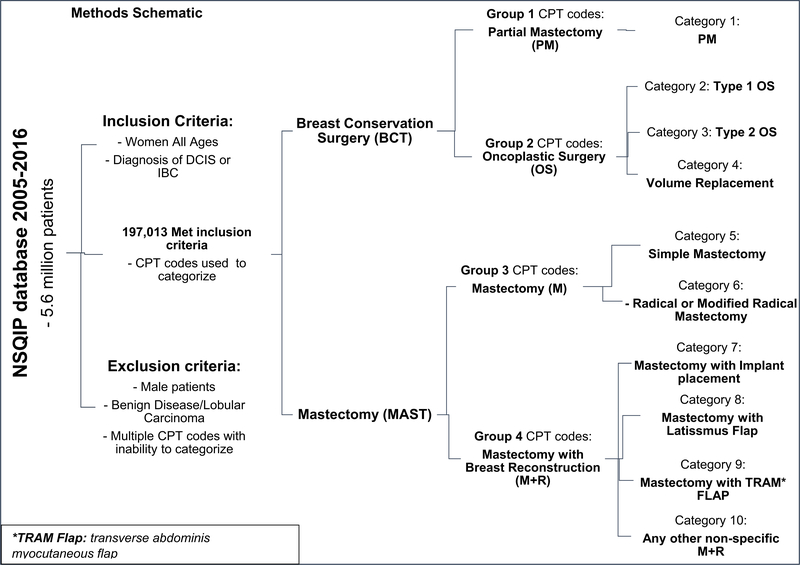
Our primary aim was to analyze temporal trends in breast cancer patients undergoing surgical interventions in four groups: Partial Mastectomy (PM), Oncoplastic Surgery (OS), Mastectomy (M), and Mastectomy with breast reconstruction (M+R). Our patient cohort included all adult women of all ages with a breast cancer diagnosis of invasive breast cancer (IBC) or ductal carcinoma in-situ (DCIS). We excluded male patients, benign breast surgery, surgeries for benign breast disease, lobular carcinoma, and patients undergoing breast cancer surgery with two CPT codes without an ability to decipher category placement. Patient demographics are shown in Table 1. Each PUF year file was separated from the participants in NSQIP via International Classification of Diseases Ninth Revision (ICD-9) code for IBC (ICD-9, 174) or DCIS (ICD-9, 233). After October 2015, ICD tenth edition replaced the previous system of classification, and patients with IBC or DCIS were classified under the appropriate ICD-10 codes: D05, D5.1-D05.99 (DCIS), and IBC (C50). All patients were then categorized into the four groups based on current procedural terminology (CPT) codes (Supplementary Material A). An overall methods schematic is shown in Fig. 1.
Table 1.
Patient demographics and characteristics
| Characteristics | Breast conservation therapy |
Mastectomy (MAST) |
|||
|---|---|---|---|---|---|
| Partial mastectomy | Oncoplastic surgery | Mastectomy | Mastectomy with reconstruction | p-value | |
| Average age (years) | 61 | 57 | 62 | 52 | < 0.00001 |
| Diagnosis: number of patients (%) | |||||
| DCIS | 17,661 (22) | 1099 (19) | 8482 (13) | 9399 (20) | < 0.00001 |
| INV | 64,254 (78) | 4695 (81) | 54,594 (87) | 37,203 (80) | |
| Race: number (%) | |||||
| White | 59,903 (73) | 4331 (77) | 43,717 (69) | 35,804 (77) | < 0.00001 |
| Black/African American | 8695(11) | 676 (12) | 705(12) | 4019 (8.6) | |
| American Indian/Alaskan Native | 414 (0.5) | 4 (0.1) | 479 (0.8) | 89 (0.2) | |
| Asian/Pacific Islander | 3006 (4) | 246 (4) | 3905 (6) | 1986 (4) | |
| Native Hawaiian | |||||
| Not reported | 9897 (12) | 437 (7) | 75,496(12) | 4678()10 | |
| Hispanic-ethnicity (%) | |||||
| Not Hispanic | 62,526 (97) | 4774 (99) | 47,507 (97) | 37,789 (97) | < 0.00001 |
| Hispanic | 1573 (2.5) | 66 (1) | 1384 (3) | 912 (2.3) | |
| Unknown | 197 (0.3) | 8 (0.2) | 229 (0.5) | 70 (0.2) | |
| Smokers | 9670 (12) | 560 (10) | 8692 (14) | 5366 (12) | < 0.00001 |
| Diabetic | |||||
| No | 71,405 (87) | 5162 (89) | 53,287 (84) | 44,031 (94) | < 0.00001 |
| Insulin | 2951 (4) | 170 (3) | 3071 (5) | 648 (1.5) | |
| Non-insulin | 7559 (9) | 462 (8) | 6744 (11) | 1897 (4) | |
| Admission status (%)a | |||||
| Inpatient | 6177 (8) | 966 (17) | 33,889 (54) | 32,638 (70) | < 0.00001 |
| Outpatient | 75,738 (92) | 4828 (83) | 29,213 (46) | 13,964 (30) | |
Percentages taken from recorded data
Fig. 1.
Methods schematic
Categorization
We further used CPT codes to stratify BCT and all MAST interventions into categories (CG) in order to examine procedural trends within four original groups. BCT consisted of patients who underwent any type of PM (CG 1) or OS (CG 2, 3, 4). OS was sub-grouped into: Level 1 adjacent tissue transfer/volume displacement (CG 2), Level 2 adjacent tissue transfer/volume displacement (CG 3), and Volume replacement with breast prosthesis, muscular flap, or other breast reconstruction technique. (CG4). Mastectomy categories consisted of CG5: simple of subcutaneous M and CG6 all radical M. M+R group was divided into four categories: CG7 (M+I) Mastectomy with breast prosthesis, delayed-insertion or tissue expander for implant placement; CG 8 and 9 (M+MF) included a mastectomy with Muscular Flap (Latissimus dorsi flap CG8, transverse abdominis myocutaneous flap CG9); and lastly, CG 10 included all other mastectomy breast reconstructions without specific involvement in other categories.
Lymph node management
After all groups were categorized, lymph node management was also quantified to determine rate of axillary management in each diagnosis. Sentinel lymph node biopsy (SLNB) and axillary lymph node dissection (ALND) in all four groups were analyzed. Unfortunately, staging and nodal involvement were not included in the NSQIP database so this was not available for inclusion.
Statistical analysis
We performed all analysis using R-Studio software. A Pearson’s chi-squared test was used to compare demographic variables between years. Linear regression analysis (R2) and a non-parametric Mann–Kendall test with Sen’s slope (SS) were used to assess a temporal trend. Results were considered significant at p values < 0.05 level. In the analysis for overall surgical interventions (4 groups), all groups summed to 100% annual operations and trend significance was calculated. In second aim, the category analysis, BCT (CG 1–4) and MAST (CG 7–9) were analyzed as separate treatment entities; each equaling 100%. CG10 was not included in the analysis for MAST as there was no specific breast reconstruction described via CPT code.
Results
Participant pool and demographics
Between 2005 and 2016, over 5 million patients were included in the NSQIP database, roughly 250 k patients were involved in breast cancer interventions: a total of 197,387 (76.8%) women met our inclusion criteria for the present analysis (Supplementary Material B). There were no significant differences between patient demographics in all four groups (Table 1). A more detailed demographics by year is shown in Supplementary Material A table.
Aim one trend analysis
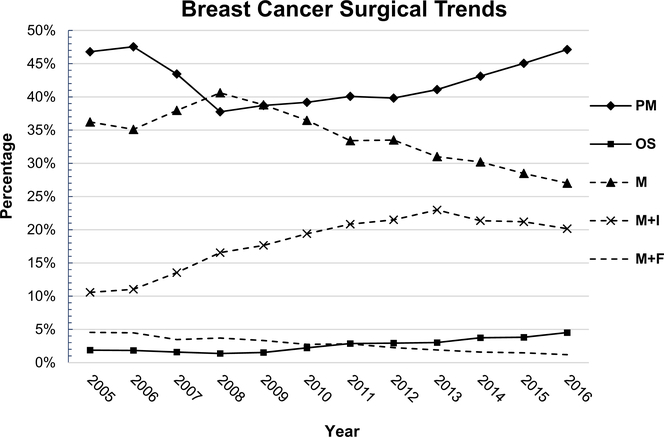
Figure 2 (Supplementary Material C) depicts the 11-year trend in nationwide breast cancer surgeries. Overall BCT and MAST groups varied, most recent data from 2016 showed 49% of patient undergo a MAST procedures and 51% had BCT. Overall, PM had no significant change in trend (p > 0.5) with no differentiation in either DCIS or IBC. M rates in both diagnostic groups dropped significantly (p = 0.001) by 10% with a negative SS; predicting 1% M decline rate per year. In M+R group, there was only a significant trend change associated with IBC, increasing from 15 to 23% (p = 0.003, R2). The overall M+R group showed a significant trend that was primarily influenced by IBC, as DCIS had no significant trend change (p = 0.54). OS had a significant trend in both DCIS ad IBC (p = 0.002, R2: 0.8). OS DCIS increased from 0.74 to 4.4% and OS IBC 2–4.4%; positive SS. All values are shown in Table 2 and plotted Fig. 2 (Categories in Supplementary Material E).
Fig. 2.
Breast Cancer Surgical Trends: Trends are based off of the surgical categorization. Each year equals 100% of breast cancer interventions done within that year; PM partial mastectomy, OS oncoplastic surgery, M mastectomy (simple + radical), M+I mastectomy and implant placement, M+F mastectomy, and flap reconstruction
Table 2.
NSQIP database surgical interventions
| Groups → | Total no. | Breast conservation therapy (BCT) |
Mastectomy (MAST) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| (1) PM (%) | (2) OS (%) | (3) M (%) | (4) M+R (%) | ||||||
| Year of study | |||||||||
| 2005 | 1776 | (46.34) | (1.86) | (35.87) | (15.93) | ||||
| DCIS | 269 | 127 | (47.21) | 2 | (0.74) | 77 | (28.62) | 63 | (23.42) |
| INV | 1507 | 696 | (46.18) | 31 | (2.06) | 560 | (37.16) | 220 | (14.60) |
| 2006 | 6090 | (47.18) | (1.82) | (34.83) | (16.17) | ||||
| DCIS | 1072 | 545 | (50.84) | 11 | (1.03) | 290 | (27.05) | 226 | (21.08) |
| INV | 5018 | 2328 | (46.39) | 100 | (1.99) | 1831 | (36.49) | 759 | (15.13) |
| 2007 | 10,677 | (43.13) | (1.57) | (37.69) | (17.61) | ||||
| DCIS | 1876 | 925 | (49.31) | 41 | (2.19) | 498 | (26.55) | 412 | (21.96) |
| INV | 8801 | 3680 | (41.81) | 127 | (1.44) | 3526 | (40.06) | 1468 | (16.68) |
| 2008 | 12,505 | (37.34) | (1.36) | (40.15) | (21.15) | ||||
| DCIS | 2099 | 967 | (46.07) | 24 | (1.14) | 566 | (26.97) | 542 | (25.82) |
| INV | 10,406 | 3702 | (35.58) | 146 | (1.40) | 4455 | (42.81) | 2103 | (20.21) |
| 2009 | 15,419 | (38.15) | (1.50) | (38.23) | (22.12) | ||||
| DCIS | 2655 | 1194 | (44.97) | 47 | (1.77) | 741 | (27.91) | 673 | (25.35) |
| INV | 12,764 | 4688 | (36.73) | 185 | (1.45) | 5154 | (40.38) | 2737 | (21.44) |
| 2010 | 15,417 | (38.63) | (2.19) | (35.95) | (23.23) | ||||
| DCIS | 2745 | 1251 | (45.57) | 82 | (2.99) | 707 | (25.76) | 705 | (25.68) |
| INV | 12,672 | 4705 | (37.13) | 255 | (2.01) | 4836 | (38.16) | 2876 | (22.70) |
| 2011 | 15,823 | (39.15) | (2.81) | (32.64) | (25.40) | ||||
| DCIS | 2976 | 1298 | (43.62) | 90 | (3.02) | 749 | (25.17) | 839 | (28.19) |
| INV | 12,847 | 4897 | (38.12) | 354 | (2.76) | 4416 | (34.37) | 3180 | (24.75) |
| 2012 | 18,795 | (38.66) | (2.85) | (32.53) | (25.96) | ||||
| DCIS | 3635 | 1605 | (44.15) | 127 | (3.49) | 945 | (26.00) | 958 | (26.35) |
| INV | 15,160 | 5661 | (37.34) | 408 | (2.69) | 5169 | (34.10) | 3922 | (25.87) |
| 2013 | 22,178 | (39.93) | (2.93) | (30.12) | (27.02) | ||||
| DCIS | 4318 | 2032 | (47.06) | 108 | (2.50) | 927 | (21.47) | 1251 | (28.97) |
| INV | 17,860 | 6824 | (38.21) | 541 | (3.03) | 5754 | (32.22) | 4741 | (26.55) |
| 2014 | 23,533 | (42.09) | (3.65) | (29.49) | (24.77) | ||||
| DCIS | 4547 | 2198 | (48.34) | 153 | (3.36) | 1005 | (22.10) | 1191 | (26.19) |
| INV | 18,986 | 7707 | (40.59) | 706 | (3.72) | 5934 | (31.25) | 4639 | (24.43) |
| 2015 | 25,987 | (43.97) | (3.72) | (27.79) | (24.52) | ||||
| DCIS | 4887 | 2567 | (52.53) | 169 | (3.46) | 954 | (19.52) | 1197 | (24.49) |
| INV | 21,100 | 8860 | (41.99) | 797 | (3.78) | 6267 | (29.70) | 5176 | (24.53) |
| 2016 | 29,187 | (46.11) | (4.42) | (26.43) | (23.04) | ||||
| DCIS | 5562 | 2952 | (53.07) | 245 | (4.40) | 1023 | (18.39) | 1342 | (24.13) |
| INV | 23,625 | 10,506 | (44.47) | 1045 | (4.42) | 6692 | (28.33) | 5382 | (22.78) |
| Overall p-value (R2) | 0.54 | (0.007) | 0.002 | (0.83) | 0.002 | (0.73) | 0.007 | (0.67) | |
| DCIS p-value (R2) | 0.63 | (0.09) | 0.002 | (0.80) | < 0.001 | (0.83) | 0.01 | (0.64) | |
| INV p-value (R2) | 0.54 | (0.03) | 0.00 | (0.77) | 0.003 | (0.68) | 0.003 | (0.70) | |
Each year equals 100% of breast cancer interventions done within that year
PM partial mastectomy, OS oncoplastic surgery, M mastectomy (simple + radical), M+I mastectomy and implant placement, M+F mastectomy, and flap reconstruction
Categorized surgical trend analysis
Significant trends were shown in both categories BCT (all p ≤ 0.001) and MAST (all p ≤ 0.005) (Fig. 2, Supplementary Material C). From 2005 to 2016, within BCT, PM decreased by ≈ 5% in DCIS and IBC (R2 0.74; 0.88 respectively); negative SS. OS increased from 1.5 to 7.6% in DCIS and 4.3–9% in IBC (R2 0.74; 0.88 respectively); positive SS. Simple M (CG5) and Radical M (CG6) both had a decreasing trends as follows CG5 from 37 to 33% (p = 0.04, R2: 0.46) and CG6 32–20% (p < 0.0001, R2: 0.96). M+I increased from 20 to 40% (p < 0.0001, R2: 0.0) and M+MF decreased from 8.6 to 2.6% (both p < 0.0001, R2: >0.93). Interestingly in the MAST group, patients undergoing reconstruction (M+I and MF) based on diagnosis, trends changed as follow, DCIS from 43 to 54% and in IDC from 27 to 42%.
Lymph node management
From 2005 to 2016, axillary lymph node management in all patients with DCIS increased from 39 to 45% (p = 0.007; R2: 0.56) and in IBC increased from 67 to 75% (p = 0.19; R2: 0.31). However, including both diagnosis, SLNB increased from 27 to 49% and ALND decreased from 36 to 20% (both p < 0.0001 and R2 > 0.9).
Discussion
Breast cancer surgery is continuously evolving to improve oncologic outcomes and quality of life in patients. In 2015, Kummerow et al. showed increasing rates of M and M+R procedures [8]. Our analysis of surgical management is the largest study showing trends of all BCT and MAST procedures using most recent available data from ACS-NSQIP database. The entire participant pool was significantly different across all demographics (< 0.00001). Patients undergoing and reconstructive procedure were younger and less diverse then PM and M groups. Inpatient admission status differed across all surgical groups as follows: PM at 8%, OS at 17%, M at 54%, and at M+R at 79%. Additionally, smokers had lowest percentage within the OS group at 10%.
Partial lumpectomy had no statistically significant overall trend change. From 2005 to 2010, there was a 7–10% decrease in partial lumpectomies per year, followed by a 4 percent increase from 2010 to 2016. The nationwide shifts in surgical management was most notable in groups involving reconstructive procedures. Between 2005 and 2016, M+R and OS increased by 146% and 241%, respectively. Patients opting for reconstructive procedures (OS or M+R) were most significant with a diagnosis of DCIS. Previous analyses from 1998 to 2011 showed that mastectomy and M+R rates increased most significantly in lower-staged breast cancers characterized by negative nodal involvement, small radius, and non-invasive pathology [8, 12]. Such results correlate with our present findings of surgical reconstruction in patients with DCIS.
Furthermore, our categorical analysis showed an increase in M+I by about 7% per year, which is even higher than previously published at 5% [13]. Although OS had a lower patient population compared to PM in the NSQIP database, it showed an 11% increase per year. This increase in OS correlates with recent breast surgeon survey results that have shown high interests in learning OS techniques amongst breast surgeons [16]. Historically, OS has evolved since its introduction in the 1980s and follows breast conservation principles that remove fairly large regions of the breast as part of the oncologic resection followed by volume displacement (mastopexy/reduction) or volume replacement (locoregional flap)techniques [4, 17, 18]. Volume replacement using a flap is a viable option in some M patients but in our study, this option has significantly decreased from 8.6 to 2.6% of reconstructions.
When compared to PM, OS has comparable complication rates, reduced re-excision rates (including reduced recurrence rates), and higher patient satisfaction from aesthetically symmetric appearing breast [19–21]. Prognostically, there is no significant difference in patient survival between PM, M, and OS procedures (16). In the past, breast cancer interventions often overlooked the psychologically burden and importance of cosmetic satisfaction. With the rise of reconstructive surgery, aesthetic satisfaction has become an important factor influencing patient decision making when considering surgical treatment choices for breast cancer removal [22]. Although both M+R and OS inherently represent reconstructive techniques, OS has higher patient satisfaction [19, 20] and has also been shown to be cost-effective [23, 24]. Collectively, these fundamental principles may influence a patient’s decision for breast reconstruction, and thereby explain the trends seen in our present analysis [6, 12].
Lastly, while mastectomy rates are decreasing, at 49%, they are still too high compared to a breast conservation goal of at least 70% [25]. Breast conservation surgery and mastectomy have equivalent survival rates (16), and with OS techniques, locoregional recurrence rates may also show no statistical difference when compared to mastectomy [26]. The results of this study show that mastectomy rates in the US are still too high when treating breast cancer and efforts should be made to educate surgeons using surgical techniques such as OS to advance breast conservation options for patients.
Limitations in this study include the interpretation of the NSQIP database based on appropriate coding and the growing rate of NSQIP being implemented in more institutions annually. The annual increase in NSQIP participants directly correlates with the proportion of breast cancer patients in our analysis. Appropriate surgical intervention offered to each patient is unique and based on oncology guidelines, such as staging, tumor size, and chemotherapy, all of which were not in NSQIP. NSQIP also did not provide information regarding patient satisfaction in regard to surgical cosmesis, treatment management, and psychological impact. Although a qualitative measure, NSQIP or future databases may take these outcome factors into consideration to understand our patient’s perspectives. Lastly, OS is a reconstructive technique with an incremental annual increase and coding may vary depending on institution. We used a coding protocol that is used in our institution as OS has no individual OS CPT code. With increasing incidence of OS, it may become necessary for oncoplastic surgery interventions to have their own CPT code to clarify surgical interventions in our future.
Conclusion
Innovations in breast reconstruction are presenting patients with treatment options that are both aesthetic and oncologically safe. This rise in reconstructive procedures is changing how patients make decisions based on their diagnosis. In this study, we successfully analyzed a nationwide change in breast surgeries from 2005 to 2016. The expanding use of breast reconstruction techniques like MAST + Recon and OS are in agreement with and greatly expound on previous analysis on breast cancer trends. Here, we demonstrate that breast cancer patients are increasingly pursuing breast reconstruction over traditional oncological procedures. The growing preference for reconstructive surgeries may foreshadow the reliance of surgical expertise in the field of oncoplastic surgery.
Supplementary Material
Acknowledgements
The ACS-NSQIP is not responsible for the statistical validity of the data analysis or the conclusions derived by the authors. The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number TL1TR002546. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- BCT
Breast conservative therapy
- IBC
Invasive breast cancer
- DCIS
Ductal carcinoma in situ
- PM
Partial mastectomy
- M
Mastectomy
- M+R
Mastectomy with reconstruction
- OS
Oncoplastic surgery
- M+I
Mastectomy with breast prosthesis, delayed-insertion or tissue expander for implant placement
- M+MF
Mastectomy with muscular flap
- SLNB
Sentinel lymph node biopsy
- ALND
Axillary lymph node dissection
- SS
Sen’s slope
- R2
Linear regression
- ACS
American College of Surgeons
- NSQIP
National Surgical Quality Improvement Program
- CPT
Current procedural terminology
- ICD-9
International classification of diseases ninth revision
- CG
Categories
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval This article does not contain any studies with animals performed by any of the authors. This article does not contain any studies with human participants performed by any of the authors.
Informed consent The study was reviewed and deemed exempt by Tufts Medical Center Institutional Review Board, given ACS NSQIP database was a de-identified data set. No individual participant consent was required.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10549-018-5018-1) contains supplementary material, which is available to authorized users.
References
- 1.Research AIfC. https://www.wcrf.org/dietandcancer/cancer-trends/worldwide-cancer-data
- 2.The L (2018) GLOBOCAN 2018: counting the toll of cancer. Lancet 392(10152):985 10.1016/S0140-6736(18)32252-9 [DOI] [PubMed] [Google Scholar]
- s3.Registries NAAoCC (2018) Online cancer database. NAACCR partners and sponsors. https://www.naaccr.org/interactive-data-on-line/. Accessed 8/15/2018 2018
- 4.Kelsall JE, McCulley SJ, Brock L, Akerlund MTE, Macmillan RD Comparing oncoplastic breast conserving surgery with mastectomy and immediate breast reconstruction: Case-matched patient reported outcomes. J Plast Reconstr Aesthet Surg. 10.1016/j.bjps.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 5.De Lorenzi F, Loschi P, Bagnardi V, Rotmensz N, Hubner G, Mazzarol G, Orecchia R, Galimberti V, Veronesi P, Colleoni MA, Toesca A, Peradze N, Mario R Oncoplastic breast-conserving surgery for tumors larger than 2 centimeters: Is it oncologically safe? A matched-cohort analysis. Ann Surg Oncol 23 (6):1852–1859. 10.1245/s10434-016-5124-4 [DOI] [PubMed] [Google Scholar]
- 6.Kimball CC, Nichols CI, Vose JG, Peled AW (2018) Trends in lumpectomy and oncoplastic breast-conserving surgery in the US, 2011–2016. Ann Surg Oncol. 10.1245/s10434-018-6760-7 [DOI] [PubMed] [Google Scholar]
- 7.Chauhan A, Sharma MM, Kumar K (2016) Evaluation of surgical outcomes of oncoplasty breast surgery in locally advanced breast cancer and comparison with conventional breast conservation surgery. Indian J Surg Oncol 7(4):413–419. 10.1007/s13193-016-0549-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA (2015) Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg 150(1):9–16. 10.1001/jamasurg.2014.2895 [DOI] [PubMed] [Google Scholar]
- 9.Wong SM, Freedman RA, Sagara Y, Aydogan F, Barry WT, Golshan M (2017) Growing use of contralateral prophylactic mastectomy despite no improvement in long-term survival for invasive breast cancer. Ann Surg 265(3):581–589. 10.1097/sla.0000000000001698 [DOI] [PubMed] [Google Scholar]
- 10.Albornoz CR, Matros E, Lee CN, Hudis CA, Pusic AL, Elkin E, Bach PB, Cordeiro PG, Morrow M (2015) Bilateral mastectomy versus breast-conserving surgery for early-stage breast cancer: the role of breast reconstruction. Plast Reconstr Surg 135(6):1518–1526. 10.1097/prs.0000000000001276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lautner M, Lin H, Shen Y, Parker C, Kuerer H, Shaitelman S, Babiera G, Bedrosian I (2015) Disparities in the use of breast-conserving therapy among patients with early-stage breast cancer. JAMA Surgery 150(8):778–786. 10.1001/jamasurg.2015.1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter SA, Lyons GR, Kuerer HM, Bassett RL Jr, Oates S, Thompson A, Caudle AS, Mittendorf EA, Bedrosian I, Lucci A, DeSnyder SM, Babiera G, Yi M, Baumann DP, Clemens MW, Garvey PB, Hunt KK, Hwang RF (2016) Operative and oncologic outcomes in 9861 patients with operable breast cancer: single-institution analysis of breast conservation with oncoplastic reconstruction. Ann Surg Oncol 23(10):3190–3198. 10.1245/s10434-016-5407-9 [DOI] [PubMed] [Google Scholar]
- 13.Albornoz CR, Bach PB, Mehrara BJ, Disa JJ, Pusic AL, McCarthy CM, Cordeiro PG, Matros E (2013) A paradigm shift in U.S. breast reconstruction: increasing implant rates. Plast Reconstr Surg 131(1):15–23. 10.1097/PRS.0b013e3182729cde [DOI] [PubMed] [Google Scholar]
- 14.Jeevan R, Mennie JC, Mohanna PN, O’Donoghue JM, Rainsbury RM, Cromwell DA (2016) National trends and regional variation in immediate breast reconstruction rates. Br J Surg 103(9):1147–1156. 10.1002/bjs.10161 [DOI] [PubMed] [Google Scholar]
- 15.Surgeons ACo (2018) ACS national surgical quality improvement program. ACS; Accessed 8/1/2018 [Google Scholar]
- 16.Chatterjee A, Gass J, Burke MB, Kopkash K, El-Tamer MB, Holmes DR, Clark P, Reiland J (2018) Results from the American Society of Breast Surgeons Oncoplastic Surgery Committee 2017 survey: current practice and future directions. Ann Surg Oncol. 10.1245/s10434-018-6586-3 [DOI] [PubMed] [Google Scholar]
- 17.Liang Y, Naber SP, Chatterjee A (2018) Anatomic and terminological description and processing of breast pathologic specimens from oncoplastic large volume displacement surgeries. Mod Pathol 31:1004–1011 [DOI] [PubMed] [Google Scholar]
- 18.Losken A, Hart AM, Broecker JS, Styblo TM, Carlson GW (2017) Oncoplastic breast reduction technique and outcomes: an evolution over 20 years. Plast Reconstr Surg 139(4):824e–833e. 10.1097/prs.0000000000003226 [DOI] [PubMed] [Google Scholar]
- 19.Bazzarelli A, Zhang J, Arnaout A (2016) Patient-reported satisfaction following oncoplastic breast-conserving therapy. In: The American Society of Breast Surgeons Annual Meeting, Dallas, Texas, 4/13/2016 [Google Scholar]
- 20.Chand ND, Browne V, Paramanathan N, Peiris LJ, Laws SA, Rainsbury RM (2017) Patient-reported outcomes are better after oncoplastic breast conservation than after mastectomy and autologous reconstruction. Plast Reconstr Surg 5(7):e1419 10.1097/gox.0000000000001419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cil TD, Cordeiro E (2016) Complications of oncoplastic breast surgery involving soft tissue transfer versus breast-conserving surgery: an analysis of the NSQIP database. Ann Surg Oncol 23(10):3266–3271. 10.1245/s10434-016-5477-8 [DOI] [PubMed] [Google Scholar]
- 22.Elston JB, Prabhakaran S, Lleshi A, Castillo B, Sun W, Kumar A, Ma Z, Smith PD, Dayicioglu D (2017) Complications and recurrence in implant-sparing oncologic breast surgery. Ann Plast Surg 78(6S Suppl 5):S269–S274. 10.1097/SAP.0000000000001039 [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee A II, Asban A, Minasian RA, Losken A, Graham R, Chen L, Czerniecki BJ, Fisher C (2018) A cost-utility analysis comparing oncoplastic breast surgery to standard lumpectomy in large breasted women. Adv Breast Cancer Res 07(02):14 10.4236/abcr.2018.72011 [DOI] [Google Scholar]
- 24.Asban A, Homsy C, Chen L, Fisher C, Losken A, Chatterjee A (2018) A cost-utility analysis comparing large volume displacement oncoplastic surgery to mastectomy with single stage implant reconstruction in the treatment of breast cancer. Breast 41:159164 10.1016/j.breast.2018.07.012 [DOI] [PubMed] [Google Scholar]
- 25.Tan MP (2016) Is there an ideal breast conservation rate for the treatment of breast cancer? Ann Surg Oncol 23(9):2825–2831. 10.1245/s10434-016-5267-3 [DOI] [PubMed] [Google Scholar]
- 26.De Lorenzi F, Hubner G, Rotmensz N, Bagnardi V, Loschi P, Maisonneuve P, Venturino M, Orecchia R, Galimberti V, Veronesi P, Rietjens M (2015) Oncological results of oncoplastic breast-conserving surgery: Long term follow-up of a large series at a single institution: a matched-cohort analysis. Eur J Surg Oncol 42(1):71–77. 10.1016/j.ejso.2015.08.160 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.