Abstract
Objective:
The presence of tumor infiltrating lymphocytes (TIL) and defects in homologous recombination (HR) are each important prognostic factors in ovarian carcinoma (OC). We characterized the association between HR deficiency (HRD) and the presence of TILs in a cohort of OC patients and the relative contribution to overall survival.
Methods:
Patients with carcinoma of the ovary, fallopian tube, or peritoneum were prospectively enrolled. Malignant neoplasm and serum samples were collected. Immunohistochemistry for CD3+ T cells and CD68+ tumor associated macrophages (TAMs) was performed on specimens collected at primary surgery. Damaging germline and somatic mutations in genes in the HR-mediated repair (HRR) pathway were identified using BROCA sequencing. HRD was defined as a damaging mutation in one of 12 genes in the HRR pathway or promoter hypermethylation in BRCA1 or RAD51C.
Results:
Ninety-eight of 250 patients included in the analysis had HRD OC (39.2%). HRD OC were enriched for CD3+ TILs and CD68+ TAMs. High CD3+ TIL was present in 65.3% of HRD OC compared to 43.4% of non-HRD OC (p=0.001). High CD68+ TAM was present in 66.3% of HRD OC compared to 50.7% of non-HRD OC (p=0.015). Patients with HRD OC and high CD3+ TILs had the longest median overall survival compared to non-HRD OC with low CD3+ TILs (70.9 vs. 35.8 months, adjusted HR 0.38, 95% CI (0.25-0.59)).
Conclusions:
Patients that have both CD3+ TILs and HRD OC are afforded the greatest improvement in overall survival. This finding may have therapeutic implications for OC patients treated with emerging immunotherapies.
Introduction
Ovarian carcinoma (OC) is the most lethal gynecologic malignant neoplasm with more than 14,000 deaths estimated to occur in the US annually (1). Identifying OC patients most likely to benefit from emerging therapies, especially those targeting the immune system, is critical. Multiple studies have demonstrated an association between the presence of tumor infiltrating lymphocytes (TIL) and survival in patients with OC (2–6). The prognostic implication of having either CD3+ or CD8+ TILs has been further demonstrated in a recent meta-analysis of over 1800 patients from 10 different studies (7). Some investigators have found tumor associated macrophages (TAMs) to be associated with more advanced stages of OC and worse survival, although this association has been inconsistent across studies (8–12).
Similarly, deleterious germline and somatic mutations of some genes in the homologous recombination mediated repair (HRR) pathway, present in more than 20% of OC, also confer a favorable prognosis (13,14). These proteins function to repair double stranded DNA breaks with high fidelity, and mutations in these genes are associated with an increased sensitivity to platinum chemotherapy and improvements in overall survival (13,15–18). Homologous recombination deficiency (HRD) may be conferred by a deleterious mutation in a key gene in the HRR pathway (such as BRCA1, BRCA2, RAD51C, RAD51D, BARD1, BRIP1, and PALB2), or by promoter hypermethylation of BRCA1 or RADS1C, which results in decreased RNA and protein expression (19–23).
OC associated with germline or somatic mutation in key genes in the HRR pathway may have differences in the tumor microenvironment including immune cell infiltration (24–27). These studies are limited by the small number of included subjects with HRD and a primary focus on BRCA1 and BRCA2 (BRCA1/2) mutations. In addition, they did not include other potential sources of HRD, such as mutations in other genes in the HRR pathway and promoter hypermethylation of BRCA1 and RAD51C (13,28,29). Nevertheless, these data raise the question of whether the favorable prognosis in HRD OC is secondary to the deficiency in HRR and/or to an improved microenvironment and immune response.
The goal of this study was to clarify the association between HRD and the presence of TILs and TAMs in a large cohort of OC patients and to determine the relative contribution of each on overall survival. We hypothesized that the improved survival observed among patients with HRD OC is conferred, in part, by the presence of higher immune cell infiltration.
Methods
Study subjects
Patients with ovarian, fallopian tube, and primary peritoneal carcinoma (collectively termed OC) who underwent primary surgery at the University of Washington were prospectively enrolled in a gynecologic oncology tumor bank between 1996 and 2011. All subjects provided informed consent for tissue banking and genetic studies. Subjects who received neoadjuvant chemotherapy were excluded. At primary debulking surgery, fresh tissue was obtained and flash frozen including primary carcinoma, metastases, and paired normal tissue. In cases where the primary site was unknown, such as primary peritoneal cancer, the primary site was arbitrarily defined as the site of the largest intraperitoneal deposit (usually omentum). Optimal surgical cytoreduction was defined as less than 1 cm of residual disease at primary surgery. Subjects were prospectively followed at the University of Washington or through correspondence with the treating oncologist.
Homologous Recombination status determination
Damaging germline and somatic mutations in genes in the HRR pathway were identified using BROCA sequencing as previously described (13,28). HRD was defined as a damaging mutation in one of 12 HRR genes (ATM, ATR, BARD1, BRCA1, BRCA2, BRIP1, CDK12, NBN, PALB2, RAD51C, RAD51D, XRCC2) or promoter methylation of BRCA1 or RAD51C (22,29).
Immunohistochemistry and scoring
Formalin-fixed, paraffin-embedded (FFPE) specimens from the primary site were cut and stained for CD3+ T cells to assess for TILs, and stained for CD68, a marker of histiocytes/macrophages. After deparaffinization and rehydration, endogenous peroxidases were blocked and antigen retrieval was performed in citrate buffer (pH 6) and incubated with the primary antibody overnight at 4°C. Primary antibodies were polyclonal rabbit anti-CD3 (Dako/Agilent #A0452) diluted 1:800, and mouse monoclonal anti-CD68 (Dako/Agilent #M0876) diluted 1:200. Slides were then counterstained, dehydrated, and mounted.
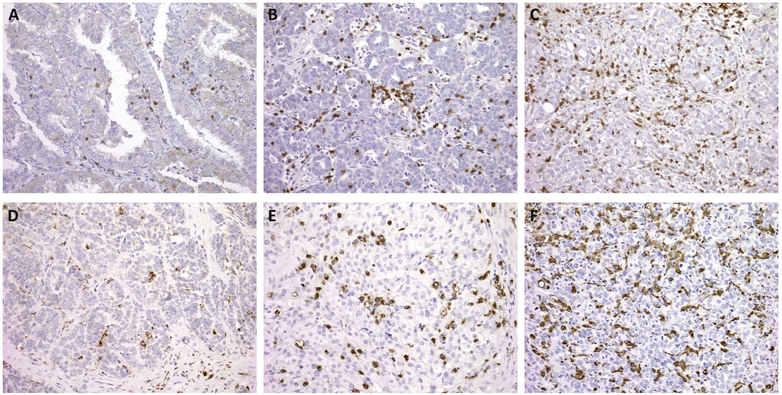
All slides were independently scored by a gynecologic pathologist and a pathology-trained gynecologic oncology fellow, both blinded to case designation. For CD3 evaluation of TILs, three high-staining regions in the tumor were identified and scored: 0 (no cells), 1+ (≤5 cells), 2+ (6-19 cells), or 3+ (≥20 cells) per 400x high-powered field (HPF) (12). For CD68 staining of TAMs, three high-staining regions in the tumor were scored: 0 (no cells), 1+ (≤20 cells), 2+ (20-49 cells), 3+ (≥50 cells) per HPF (Figure 1). TILs and TAMs in the stroma were excluded. All samples with a discrepant score were reviewed together at a multi-headed scope to determine a final score. For both CD3 and CD68, fewer than 10% of cases had discrepant scores. Interrater reliability scores were κ = 0.89 and 0.85 for CD3 and CD68, respectively, indicating very good interrater reliability.
Figure 1.
Representative immunohistochemistry (IHC) staining (200x magnification). IHC staining for CD3+ tumor infiltrating lymphocytes (TILs) are shown for 1+ (A), 2+ (B), and 3+ (C) scores. IHC staining for CD68+ tumor associated macrophages (TAMs) are shown for 1 + (D), 2+ (E), and 3+ (F) scores.
Statistical analyses
Baseline patient characteristics were compared between cohorts based on HRD status using Student’s t-test and chi-square tests, where appropriate. Survival was defined as the time from diagnosis until death and patients were censored at the date of last known follow-up. Survival analysis was performed according to the methods of Kaplan-Meier and both unadjusted and multivariable-adjusted analyses were conducted. Eighteen subjects with FIGO stage I carcinoma were excluded from survival analyses. Statistical analyses were performed using STATA (v14.0, College Station, TX).
Results
Two hundred and fifty subjects with FFPE tissues from the primary site were available for TIL evaluation and included in the present study. The HRD assessment for these OC including methylation and mutation data have been previously reported (29). Ninety-eight subjects had HRD OC (39.2%): 44 (17.6%) carcinomas had a BRCA1 mutation, 17 (6.8%) had a BRCA2 mutation, 13 (5.2%) had a deleterious mutation in a different HRR gene, and 24 (9.6%) had BRCA1/RAD51C promoter methylation. Baseline demographics and patient characteristics were similar between patients with HRD and non-HRD OC except for age at presentation and initial CA-125 (Table 1). HRD OC occurred in younger patients (P<0.001) and were associated with a higher serum CA-125 at presentation (P=0.004).
Table 1.
Baseline demographics by homologous recombination deficiency status.
| Characteristic | All (n=250) |
non-HRD (n=152) |
HRD (n=98) |
P-value |
|---|---|---|---|---|
| Age | ||||
| <50 | 56 (22.4) | 23 (15.1) | 33 (33.7) | <0.001 |
| 50-50 | 72 (28.8) | 40 (26.3) | 32 (32.7) | |
| 60-69 | 62 (24.6) | 42 (27.6) | 20 (20.4) | |
| >70 | 60 (24.0) | 47 (30.9) | 13 (13.3) | |
| Disease site | ||||
| Ovary | 216 (86.4) | 131 (86.2) | 85 (86.7) | 0.090 |
| Fallopian tube | 13 (5.2) | 5 (3.3) | 8 (8.2) | |
| Primary peritoneal | 21 (8.4) | 16 (10.5) | 5 (5.1) | |
| FIGO stage | ||||
| I | 18 (7.2) | 11 (7.2) | 7 (7.1) | 0.381 |
| II | 14 (5.6) | 10 (6.6) | 4 (4.1) | |
| III | 187 (74.8) | 115 (75.7) | 72 (73.5) | |
| IV | 29 (11.6) | 16 (10.5) | 13 (13.3) | |
| Missing | 2 (0.8) | 0 (0.0) | 2 (2.0) | |
| Histology | ||||
| Serous | 183 (73.2) | 111 (73.0) | 72 (73.5) | 0.425 |
| Endometrioid | 17 (6.8) | 10 (6.6) | 7 (7.1) | |
| Clear cell | 8 (3.2) | 6 (4.0) | 2 (2.0) | |
| Mixed | 7 (2.8) | 3 (2.0) | 4 (4.1) | |
| Undifferentiated | 23 (9.2) | 12 (7.9) | 11 (11.2) | |
| Other | 12 (4.8) | 10 (6.6) | 2 (2.0) | |
| Grade | ||||
| 1 | 6 (2.4) | 3 (2.0) | 3 (3.1) | 0.232 |
| 2 | 12 (4.8) | 10 (6.6) | 2 (2.0) | |
| 3 | 230 (92.0) | 137 (90.1) | 93 (94.9) | |
| Missing | 2 (0.8) | 2 (1.3) | 0 (0.0) | |
| Optimal cytoreduction | ||||
| Yes | 167 (66.8) | 98 (64.5) | 69 (70.4) | 0.565 |
| No | 81 (32.4) | 53 (34.9) | 28 (28.6) | |
| Missing | 2 (0.8) | 1 (0.7) | 1 (1.0) | |
| Initial CA-125 | ||||
| 0-1000 | 144 (57.6) | 98 (64.5) | 46 (46.9) | 0.004 |
| 1001-2500 | 50 (20.0) | 30 (19.7) | 20 (20.4) | |
| >2500 | 49 (19.6) | 19 (12.5) | 30 (306) | |
| Missing | 7 (2.8) | 5 (3.3) | 2 (2.0) |
Data shown are n (column %). HRD, homologous recombination deficient.
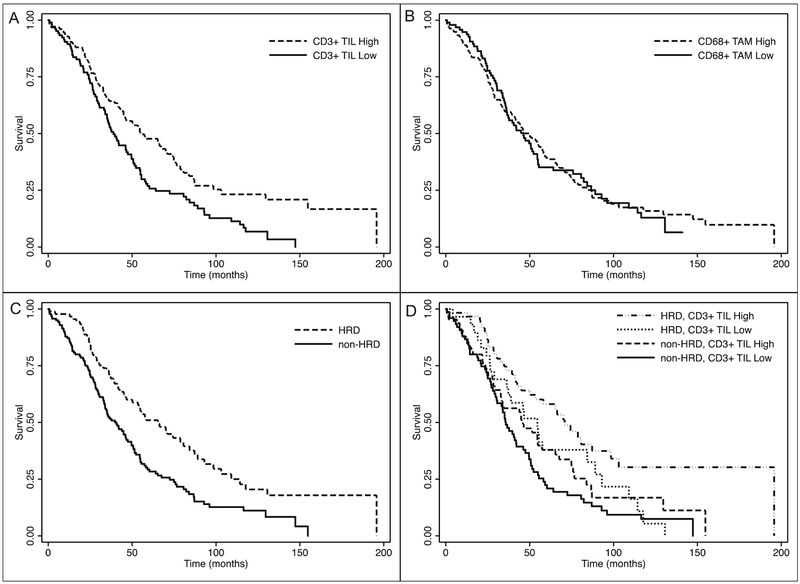
The distribution of CD3+ TIL and CD68+ TAM scores for the entire population was assessed and IHC scores were classified as either low (0 to 1+) or high (2+ to 3+) staining (Table 2 and supplementary table 1). Among all patients with OC, 52.0% had a high CD3 TIL score and 56.8% had a high CD68 TAM score. Subjects with CD3+ TIL high scores had longer median overall survival compared to patients with CD3+ TIL low scores, 54.8 months vs. 38.9 months, HR 0.62, 95% CI (0.46-0.84). In contrast, there was no survival difference among subjects with high versus low CD68+ TAM scores, 49.6 months vs. 46.2 months, HR 0.97, 95% CI (0.72-1.32) (Figure 2).
Table 2.
Association of high/low scores for CD3+ TILs and CD68+ TAMs with homologous recombination deficiency.
| HRD status | CD3+ TIL score | CD68+ TAM score | |||||
|---|---|---|---|---|---|---|---|
| Low (0-1) | High (2-3) | P value | Low (0-1) | High (2-3) | P value | Total | |
| non-HRD | 86 (56.6) | 66 (43.4) | 0.001 | 75 (49.3) | 77 (50.7) | 0.015 | 152 |
| HRD | 34 (34.7) | 64 (65.3) | 33 (33.7) | 65 (66.3) | 98 | ||
| BRCA1 mutation | 15 (34.1) | 29 (65.9) | 17 (38.6) | 27 (61.4) | 44 | ||
| BRCA2 mutation | 9 (52.9) | 8 (47.1) | 5 (29.4) | 12 (70.6) | 17 | ||
| Other HRR mutation | 4 (30.8) | 9 (69.2) | 4 (30.8) | 9 (69.2) | 13 | ||
| BRCA1/RAD51C hypermethylation | 6 (25.0) | 18 (75.0) | 7 (29.2) | 17 (70.8) | 24 | ||
| Total | 120 (48.0) | 130 (52.0) | 108 (43.2) | 142 (56.8) | 250 | ||
Data shown are n (row %). P value indicates the comparison of non-HRD to HRD ovarian carcinoma. TIL, tumor infiltrating lymphocytes; TAM, tumor-associated macrophages; HRR, homologous recombination-mediate repair, HRD homologous recombination deficient.
Figure 2.
Kaplan-Meier curves for overall survival by CD3+ tumor infiltrating lymphocyte (TIL) score (high/low) in Panel A, CD68+ tumor associated macrophage (TAM) score (high/low) in Panel B, homologous recombination deficiency (HRD) in Panel C, and by CD3+ TIL score (high/low) and HRD in Panel D.
Next, the association between CD3+ TIL and CD68+ TAM scores and HRD status was evaluated (Table 2). Both CD3+ TIL and CD68+ TAM scores were significantly higher in HRD OC. A higher proportion of HRD OC scored high for CD3+ TILs and for CD68+ TAMs. Among HRD OC, 65.3% had high CD3+ TIL scores and 66.3% had high CD68+ TAM scores. In comparison, among non-HRD OC, 43.4% had high CD3+ TIL scores and 50.7% had high CD68+ TAM scores. Median overall survival was significantly greater among subjects with HRD OC, 65.4 months vs. 40.6 months, HR 0.56, 95% CI (0.41-0.78) (Figure 2).
Patients were then categorized according to HRD status and high/low IHC score (Table 3). In both unadjusted and multivariable-adjusted survival analysis, patients with HRD OC and high CD3+ TILs had the longest overall survival when compared to patients with non-HRD OC with low CD3+ TILs. Median overall survival was 70.9 months vs. 35.8 months, adjusted HR 0.38, 95% CI (0.25-0.59). Patients with either high CD3+ TIL or HRD OC (but not both) had intermediate survival of 46.0 months and 54.6 months, respectively (Figure 2). This association was not observed when patients were similarly categorized by the presence of CD68+ TAMs.
Table 3.
Unadjusted and multivariable-adjusted association of CD3+ TILs or CD68+ TAMs and homologous recombination deficiency with OS.
| HRD status | IHC score (high/low) |
No. | Median OS (months) |
HR (95% CI) | P value | Adjusted HR (95% CI) |
P value |
|---|---|---|---|---|---|---|---|
| non-HRD | CD3 low | 76 | 35.8 | 1 (Reference) | <0.001 | 1 (Reference) | <0.001 |
| CD3 high | 65 | 46.0 | 0.75 (0.52-1.0) | 0.65 (0.44-0.95) | |||
| HRD | CD3 low | 30 | 54.6 | 0.71 (0.45-1.13) | 0.71 (0.44-1.14) | ||
| CD3 high | 61 | 70.9 | 0.40 (0.27-0.61) | 0.38 (0.25-0.59) | |||
| non-HRD | CD68 low | 67 | 40.6 | 1 (Reference) | 0.004 | 1 (Reference) | <0.001 |
| CD68 high | 74 | 35.1 | 1.04 (0.72-1.51) | 0.89 (0.61-1.30) | |||
| HRD | CD68 low | 31 | 84.3 | 0.55 (0.32-0.93) | 0.56 (0.33-0.96) | ||
| CD68 high | 60 | 65.4 | 0.59 (0.39-0.90) | 0.54 (0.35-0.83) |
Adjusted for age, stage, and optimal surgical cytoreduction. Stage 1 subjects (n=18) excluded. TIL, tumor infiltrating lymphocytes; TAM, tumor-associated macrophages; HRD homologous recombination deficient; OS, overall survival; IHC, immunohistochemistry.
Discussion
In this cohort of 250 patients with OC characterized for HRD via germline or somatic HRR mutation or promoter methylation of BRCA1/RAD51C, subjects with HRD OC had a significantly higher neoplastic infiltration of both TILs and TAMs. To our knowledge, this is the largest cohort of ovarian carcinomas characterized for TILs and TAMs and comprehensively evaluated for HRD by both methylation and mutation of key HRR genes. Our data are consistent with previous smaller studies (summarized in Table 4), showing that there are more TILs in HRD OC compared to non-HRD OC.
Table 4.
Summary of studies characterizing the tumor microenvironment in homologous recombination deficient ovarian carcinoma.
| Author (year) | No. cases |
No. HRD (%) |
IHC markers | Associations with HRD ovarian carcinoma |
|---|---|---|---|---|
| Morse (current) | 250 | 98 (39.2) | CD3, CD68 | HRD ovarian carcinoma enriched for both CD3+ TILs and CD68+ TAMs, HRD and CD3+ TILs each independently prognostic for improved OS |
| Clarke (2009) (26) | 40 | 18 (45.0) | CD3, CD4, CD8, CD20, CD43, CD 117, granzyme-B | CD8+ TILs correlated with BRCA1 mutation or loss of expression and with improved OS |
| McAlpine (2012) (24) | 131 | 52 (39.7) | CD3, CD4, CD8, CD20, FOXP3, and TIA-1 | Higher CD20+ and TIA-1 immune infiltrate in cases with BRCA1/2 mutations or BRCA1 methylation |
| Soslow (2012) (25) | 43 | 31 (72.1) | TIL assessment not specified | TILs enriched in BRCA1, but not BRCA2 mutated ovarian carcinoma |
| Strickland (2016) (27) | 53 | 37 (69.8) | CD3, CD4, CD8, CD20, PD-1, and PD-L1 | CD3+ TILs and CD8+ TILs enriched in HRD ovarian carcinoma and CD3+ TILs associated with improved OS |
HRD, homologous recombination deficient; IHC, immunohistochemistry; TIL, tumor infiltrating lymphocytes; TAM, tumor-associated macrophages; OS, overall survival.
Consistent with other publications, we confirmed the important survival advantage conferred by the presence of T cells within the tumor epithelium for patients with OC (2–6). A 2012 meta-analysis, which included 1815 patients from 10 studies, found that the presence of both CD3+ and CD8+ TILs was associated with a significant improvement in overall survival, pooled HR 2.24, 95% CI (1.71-2.92) (7). This association was recently confirmed by the Ovarian Tumor Tissue Analysis Consortium, which evaluated 5500 OC, showing a dose-response relationship between the quantity of TILs and survival benefit, which was consistent across various histologies, including endometrioid and mucinous subtypes (6).
Our study is the first to characterize the independent contribution to survival of HRD status and immune infiltration. We found that there was a survival benefit to having both CD3+ TILs and HRD carcinoma, with this subgroup having the greatest median overall survival of 70.9 months, nearly double the median overall survival of patients with non-HRD OC lacking TILs (Table 3, Figure 2). Patients with OC with either high TILs or with HRD had intermediate survival. While the improvement in survival associated with BRCA1/2 mutations is well-characterized, the independent and additive effect of having both CD3+ TILs and HRD in OC is novel (15, 16).
This finding could have important translational relevance to patients with OC. Immune checkpoint blockade (ICB) has been found to be more effective in cancers with high mutational burdens (30–34), but response to ICB has not tested relative to HRD status. We hypothesize that patients with HRD OC but low or absent TILs have cancers with more immunosuppressive microenvironments that will be less susceptible to current immunotherapies. On the other hand, HRD OC with high TILs may be more effectively treated with immunotherapies. These hypotheses will need to be tested in prospective trials and speak to the importance of classifying HRD in all OC trials, not just those that utilize PARP inhibitors.
In addition to more CD3+ TILs, HRD OC had more CD68+ TAMs than non-HRD OC. However, CD68+ TAMs were not associated with overall survival. A recent meta-analysis, which explored the prognostic effect of TAMs in OC in 794 patients, revealed that neither CD68+ TAMs nor CD163+ TAMs were associated with improved overall survival. In contrast, others report that TAMs, in particular M2 macrophages that adopt an immunosuppressive phenotype after exposure to Th2 mediators, are associated with advanced stage and poor prognosis in OC (8,11,35). A higher ratio of M1 macrophages, which suppress cancer progression, to M2 macrophages may be associated with an improved survival (10). The contribution of TAMs to survival in OC and the relationship of specific macrophage subtypes to HRD status requires further study.
The mechanism that leads to increased TILs in HRD OC is not known. Strickland et al. demonstrated that HRD OC have more predicted neoantigens, which were associated with improved survival (27). An increase in predicted neoantigens or tumor mutation burden, which is also increased in BRCA1/2 mutated ovarian carcinoma (36), may lead to lymphocyte recruitment to the tumor microenvironment. Another potential explanatory mechanism includes the interplay between HRD and accumulation of damaged free-cytosolic DNA contributing to activation of the cGAS-STING pathway (37). The relative contribution of these mechanisms, as well as other yet described mechanisms, remains to be determined.
With the increased focus on immunotherapy in the treatment of OC, it will be important to understand how patients with HRD OC respond relative to those with non-HRD OC. Initial reports of nivolumab, an anti-PD-1 antibody, in platinum resistant OC demonstrated encouraging clinical efficacy and there are numerous ongoing studies using ICB (38). We now add another consideration to that assessment. Patients with HRD OC can have varying levels of immune cell infiltration, which may also contribute to a more or less favorable response to ICB.
Strengths of this study include comprehensive assessment of both germline and somatic HRR mutation status using BROCA (28) in combination with promoter methylation of BRCA1 and RAD51C, which have been found to correlate with reduced RNA and protein expression (19–22). In addition, we manually performed immune infiltrate IHC scoring consistent with recently proposed pathology guidelines, which is critical for reproducibility in future studies (39). While we did not assess CD8+ TILs, the published literature is consistent regarding the prognostic implications of either CD3+ or CD8+ TILs (7).
In conclusion, we have demonstrated that HRD OC is associated with an increase in CD3+ TILs as well as CD68+ TAMs. Patients that have both CD3+ TILs and HRD OC are afforded the greatest improvement in overall survival. This finding may have therapeutic implications for OC patients treated with emerging immunotherapies.
Supplementary Material
Highlights.
Homologous recombination deficient (HRD) ovarian carcinoma (OC) is enriched for CD3+ tumor infiltrating lymphocytes (TIL).
Patients with both CD3+ TILs and HRD OC have the greatest overall survival, which may have therapeutic implications.
These findings highlight differences in the tumor microenvironment that could contribute to differential responses.
This is especially relevant as novel immunotherapies are being tested for the treatment of ovarian carcinoma.
Acknowledgments
Funding: This work was supported by a Stand Up To Cancer - Ovarian Cancer Research Fund Alliance - National Ovarian Cancer Coalition Dream Team Translational Research Grant (SU2C-AACR-DT16-15), the Wendy Feuer Research Fund for the Prevention and Treatment of Ovarian Cancer, and NIH T32CA009515.
Footnotes
Conflict of Interest Statement
The authors declare no potential conflicts of interest. All authors have approved the final article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams SF, Levine DA, Cadungog MG, Hammond R, Facciabene A, Olvera N, et al. Intraepithelial T cells and tumor proliferation: Impact on the benefit from surgical cytoreduction in advanced serous ovarian cancer. Cancer. 2009;115:2891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Conejo-Garcia JJR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. [DOI] [PubMed] [Google Scholar]
- 6.Ovarian Tumor Tissue Analysis (OTTA) Consortium. Dose-Response Association of CD8+ Tumor-Infiltrating Lymphocytes and Survival Time in High-Grade Serous Ovarian Cancer. JAMA Oncol. 2017;3:e173290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang W-T, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124:192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnan V, Schaar B, Tallapragada S, Dorigo O. Tumor associated macrophages in gynecologic cancers Gynecol Oncol. Elsevier Inc.; 2018;149:205–13. [DOI] [PubMed] [Google Scholar]
- 9.Yuan X, Zhang J, Li D, Mao Y, Mo F, Du W, et al. Prognostic significance of tumor- associated macrophages in ovarian cancer: A meta-analysis Gynecol Oncol. Elsevier Inc.; 2017;147:181–7. [DOI] [PubMed] [Google Scholar]
- 10.Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, et al. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res. Journal of Ovarian Research; 2014;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan C, Huang X, Lin S, Huang H, Cai Q, Wan T, et al. Expression of M2-polarized macrophages is associated with poor prognosis for advanced epithelial ovarian cancer. Technol Cancer Res Treat. 2013;12:259–67. [DOI] [PubMed] [Google Scholar]
- 12.Shah CA, Allison KH, Garcia RL, Gray HJ, Goff BA, Swisher EM. Intratumoral T cells, tumor-associated macrophages, and regulatory T cells: Association with p53 mutations, circulating tumor DNA and survival in women with ovarian cancer. Gynecol Oncol. 2008;109:215–9. [DOI] [PubMed] [Google Scholar]
- 13.Pennington KP, Walsh T, Harrell MI, Lee MK, Pennil CC, Rendi MH, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norquist BM, Brady MF, Harrell MI, Walsh T, Lee MK, Gulsuner S, et al. Mutations in homologous recombination genes and outcomes in ovarian carcinoma patients in GOG 218: An NRG oncology/Gynecologic oncology group study. Clin Cancer Res. 2018;24:777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolton KL, Chenevix-Trench G, Goh C, Sadetzki S, Ramus SJ, Karlan BY, et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. 2012;307:382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Candido-dos-Reis FJ, Song H, Goode EL, Cunningham JM, Fridley BL, Larson MC, et al. Germline mutation in BRCA1 or BRCA2 and ten-year survival for women diagnosed with epithelial ovarian cancer. Clin Cancer Res. 2015;21:652–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang D, Khan S, Sun Y, Hess K, Shmulevich I, Sood AK, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011;306:1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vencken PMLH, Kriege M, Hoogwerf D, Beugelink S, van der Burg MEL, Hooning MJ, et al. Chemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patients. Ann Oncol Off J Eur Soc Med Oncol. 2011;22:1346–52. [DOI] [PubMed] [Google Scholar]
- 19.Swisher EM, Gonzalez RM, Taniguchi T, Garcia RL, Walsh T, Goff BA, et al. Methylation and protein expression of DNA repair genes: association with chemotherapy exposure and survival in sporadic ovarian and peritoneal carcinomas. Mol Cancer. 2009;8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baldwin RL, Nemeth E, Tran H, Shvartsman H, Cass I, Narod S, et al. BRCA1 promoter region hypermethylation in ovarian carcinoma: a population-based study. Cancer Res. 2000;60:5329–33. [PubMed] [Google Scholar]
- 21.Wang C, Horiuchi A, Imai T, Ohira S, Itoh K, Nikaido T, et al. Expression of BRCA1 protein in benign, borderline, and malignant epithelial ovarian neoplasms and its relationship to methylation and allelic loss of the BRCA1 gene. J Pathol. 2004;202:215–23. [DOI] [PubMed] [Google Scholar]
- 22.Cancer Genome Atlas Research Network, Bell D, Berchuck A, Birrer M, Chien J, Cramer DW, et al. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swisher EM, Lin KK, Oza AM, Scott CL, Giordano H, Sun J, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial Lancet Oncol. Elsevier Ltd; 2017;18:75–87. [DOI] [PubMed] [Google Scholar]
- 24.McAlpine JN, Porter H, Köbel M, Nelson BH, Prentice LM, Kalloger SE, et al. BRCA1 and BRCA2 mutations correlate with TP53 abnormalities and presence of immune cell infiltrates in ovarian high-grade serous carcinoma. Mod Pathol. 2012;25:740–50. [DOI] [PubMed] [Google Scholar]
- 25.Soslow RA, Han G, Park KJ, Garg K, Olvera N, Spriggs DR, et al. Morphologic patterns associated with BRCA1 and BRCA2 genotype in ovarian carcinoma Mod Pathol. Nature Publishing Group; 2012;25:625–36. [DOI] [PubMed] [Google Scholar]
- 26.Clarke B, Tinker A V, Lee C-H, Subramanian S, van de Rijn M, Turbin D, et al. Intraepithelial T cells and prognosis in ovarian carcinoma: novel associations with stage, tumor type, and BRCA1 loss. Mod Pathol. 2009;22:393–402. [DOI] [PubMed] [Google Scholar]
- 27.Strickland KC, Howitt BE, Shukla S a, Rodig S, Ritterhouse LL, Liu JF, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7:13587–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norquist BM, Harrell MI, Brady MF, Walsh T, Lee MK, Gulsuner S, et al. Inherited Mutations in Women With Ovarian Carcinoma. JAMA Oncol. 2015;6460:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernards SS, Pennington KP, Harrell MI, Agnew KJ, Garcia RL, Norquist BM, et al. Clinical characteristics and outcomes of patients with BRCA1 or RAD51C methylated versus mutated ovarian carcinoma Gynecol Oncol. Elsevier Inc.; 2018;148:281–5. [DOI] [PubMed] [Google Scholar]
- 30.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizvi H, Sanchez-Vega F, La K, Chatila W, Jonsson P, Halpenny D, et al. Molecular Determinants of Response to Anti-Programmed Cell Death (PD)-1 and Anti-Programmed Death-Ligand 1 (PD-L1) Blockade in Patients With Non-Small-Cell Lung Cancer Profiled With Targeted Next-Generation Sequencing. J Clin Oncol. 2018;36:633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mouw KW, Goldberg MS, Konstantinopoulos PA, D’Andrea AD. DNA damage and repair biomarkers of immunotherapy response. Cancer Discov. 2017;7:675–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mhawech-Fauceglia P, Wang D, Ali L, Lele S, Huba MA, Liu S, et al. Intraepithelial T cells and tumor-associated macrophages in ovarian cancer patients. Cancer Immun. 2013;13:1. [PMC free article] [PubMed] [Google Scholar]
- 36.Birkbak NJ, Kochupurakkal B, Izarzugaza JMG, Eklund AC, Li Y, Liu J, et al. Tumor mutation burden forecasts outcome in ovarian cancer with BRCA1 or BRCA2 mutations. PLoS One. 2013;8:e80023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17:1142–9. [DOI] [PubMed] [Google Scholar]
- 38.Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. Safety and antitumor activity of Anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33:4015–22. [DOI] [PubMed] [Google Scholar]
- 39.Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, et al. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in Melanoma, Gastrointestinal Tract Carcinom. Adv Anat Pathol. 2017;24:311–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.