Abstract
Objective:
To evaluate the association between furosemide exposure and risk of bronchopulmonary dysplasia (BPD).
Study design:
This was a retrospective cohort study of infants (2004–2015) 23–29 weeks gestational age and 501–1249 g birth weight. We compared demographic and clinical characteristics of infants exposed and not exposed to furosemide between postnatal day 7 and 36 weeks postmenstrual age. We examined the association between furosemide exposure and 2 outcomes: BPD and BPD or death. We performed multivariable probit regression models that included demographic and clinical variables in addition to 2 instrumental variables furosemide exposure by discharge year; and furosemide exposure by site.
Results:
Of 37,693 included infants, 19,235 (51%) were exposed to furosemide; these infants were more premature and had higher respiratory support. Of 33,760 infants who survived to BPD evaluation, 15,954 (47%) had BPD. An increase in the proportion of furosemide exposure days by 10 percentage points was associated with a decrease in both the incidence of BPD (4.6 percentage points, P = .001), and BPD or death (3.7 percentage points, P=0.01).
Conclusion:
More days of furosemide exposure between postnatal day 7 and 36 weeks was associated with decreased risk of BPD and a combined outcome of BPD or death.
Keywords: neonate, diuretic, preterm, BPD, chronic lung disease
Bronchopulmonary dysplasia (BPD) is the most common pulmonary morbidity associated with prematurity, and premature infants with BPD are at elevated risk of death and severe developmental disability (1–4). For infants with BPD who survive, the costs of the disorder are measured in impaired childhood health and quality of life, family stress and economic hardship, and increased healthcare costs (4–6). Despite the devastating impact of BPD on premature infants, there are currently no therapies labeled by the United States Food and Drug Administration to prevent BPD. Off-label therapies shown to decrease the risk of BPD have limitations, including the need for further studies to determine optimization of timing and duration of therapy (caffeine) (7), lack of availability for widespread clinical use (vitamin A) (8), or association with neurodevelopmental impairment (postnatal steroids) (9).
Neonatologists commonly use furosemide off-label in premature infants (10). Furosemide is a loop diuretic that inhibits reabsorption of sodium and chloride in the kidney’s proximal tubules, distal tubules, and Loop of Henle. Across the United States, 34% of infants <1500 g birth weight receive furosemide, and the use of furosemide varies widely across centers (11). This practice variation likely stems from the fear of potential adverse effects of furosemide, combined with a lack of published data surrounding timing of use, appropriate dose, indication, and level of efficacy for the prevention of BPD. Previous small studies have suggested that furosemide improves lung compliance, airway resistance, and oxygenation in premature infants (12), but no randomized controlled trials of furosemide to prevent BPD have been performed. The objective of this study was to evaluate whether furosemide exposure is associated with reduced risk of BPD in a large national cohort of premature infants.
Methods
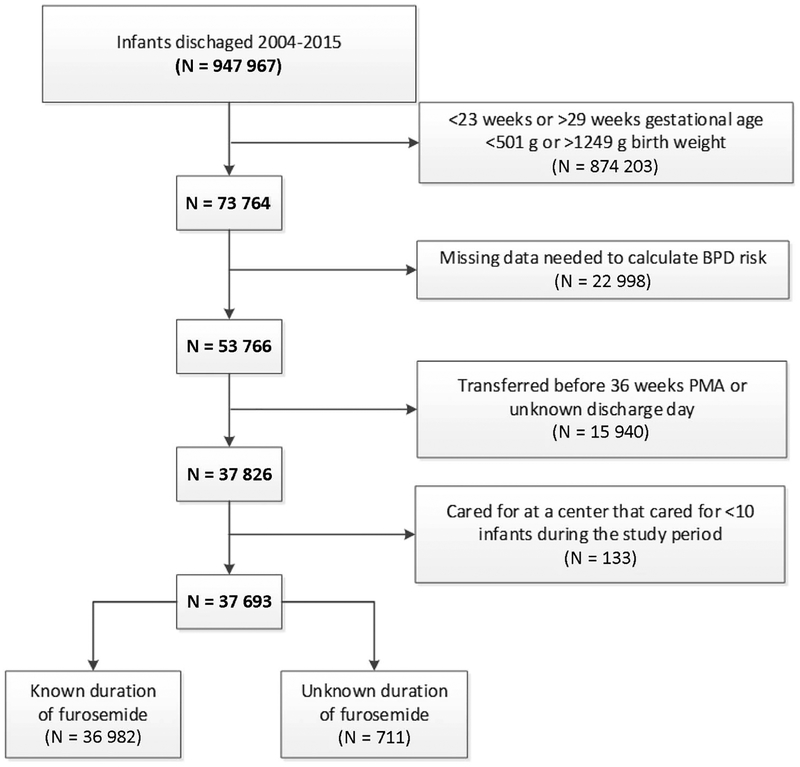
We identified hospitalized infants discharged from 190 neonatal intensive care units managed by the Pediatrix Medical Group from 2004 to 2015 (Figure 1; available at www.jpeds.com). Infants were included if they were gestational age 23–29 weeks and birth weight 501–1249 g. Infants were excluded if they were transferred between hospitals prior to 36 weeks postmenstrual age (PMA), died before 7 days postnatal age or had an unknown discharge day, were cared for in a unit with <10 infants meeting the inclusion criteria within the study period, or were missing data necessary to estimate the risk of BPD (including race or ethnicity, ventilator status, and fraction of inspired oxygen). Demographic, clinical, and maternal data were extracted from a clinical data warehouse that prospectively captures data from electronic health records, including daily progress notes and other documentation generated by clinicians using a computer-assisted tool (13). The study was approved by the Duke University Institutional Review Board as exempt research.
Figure 1.
(online only)
Furosemide exposure was defined in two distinct ways: 1) as a continuous variable, defined as the percentage of days exposed to furosemide between day-of-life 7 and 36 weeks postmenstrual age (PMA) or the date of death; and 2) as a binary variable, defined as any exposure between postnatal day 7 and 36 weeks PMA. The primary outcome, BPD, was defined by an infant receiving supplemental oxygen or respiratory support (ie, nasal cannula, continuous positive airway pressure, or mechanical ventilation) continuously from a PMA of 36 0/7 to 36 6/7 weeks (14). Secondary outcomes included: death, defined as all-cause mortality after postnatal day 7 and prior to discharge; and a combined outcome of BPD or death. The goal of the analysis was to estimate the association between furosemide exposure and these outcomes while adjusting for measured pre-treatment covariates. These other covariates included: gestational age, small for gestational age status (15), race or ethnicity, delivery type (vaginal vs cesarean delivery), sex, inborn status, antenatal steroid exposure, 5-minute Apgar score, atrial septal defect or ventricular septal defect, ligation or occlusion of a patent ductus arteriosus, receipt of dexamethasone during the study period, ventilator status on postnatal day 7, fraction of inspired oxygen on postnatal day 7, and estimated risk of BPD or death at postnatal day 7 according to a model developed by the National Institute of Child Health and Human Development Neonatal Research Network (16, 17).
Statistical Analyses
We compared demographic and clinical variables between infants exposed and not exposed to furosemide using the chi-square test for categorical variables or the Wilcoxon rank-sum test for continuous variables. Among infants who survived to 36 weeks PMA, we calculated the percentage who developed BPD each year. We evaluated furosemide exposure by discharge year and by site using 2 methods: by calculating the percentage of infants ever exposed to furosemide at each discharge year and site; and by calculating the percentage of infant-days occurring between 7 days of life and 36 weeks PMA in which an infant was exposed to furosemide on each discharge year and site.
To examine the association between furosemide exposure and outcomes of BPD in infants surviving to 36 weeks PMA and the combined outcome of BPD or death in all infants, we chose an instrumental variable approach (18). Although other methods (such as propensity scores, regression, and matching) can adjust for measured confounders, the instrumental variable approach is essential in controlling for unmeasured confounders, such as clinical disease severity. This approach has been used successfully in adult, pediatric, and perinatal settings, and it is sufficiently powerful that it is now being used to reduce bias from non-compliance in randomized controlled trials (19–22). The validity of this approach requires that the instruments used correlate with treatment and except for their influence on treatment, not otherwise be associated with the outcomes of interest.
We performed two sets of instrumental variable analyses for each outcome. In our first set of analyses, we constructed a multivariable probit regression model to predict percentage of days each infant was exposed to furosemide using the measured pre-treatment covariates listed above, and two instrumental variables, namely the percentage of infant-days occurring between 7 days of life and 36 weeks PMA in which there was exposure to furosemide in that discharge year; and the percentage of infant-days occurring between 7 days of life and 36 weeks PMA in which there was exposure at that site. Infants with uncertain duration of furosemide were excluded from these models. For a second instrumental variable analysis, we constructed a similar multivariable probit regression model, but used the following two instrumental variables to predict categorical (0, 1) furosemide exposure for the model percentage of infants exposed during that discharge year; and percentage of infants exposed at that site. Infants with an uncertain duration of furosemide were included in these models. In each of the analyses, the values of the instrumental variables were calculated for each infant individually utilizing the exposure patterns among all other infants at that site or in that year. To evaluate the validity of our instrumental variables, we examined the proportion of infants falling into categories of each covariate among quartiles of each instrumental variable. We also examined the validity of our instrumental variables using F-tests and Wald tests. As a sensitivity analysis, we repeated the above instrumental variable analyses using only the instrumental variable involving furosemide exposure by site and eliminating the instrumental variable involving furosemide exposure by discharge year. Finally, because our hypothesis was that furosemide has a physiological effect on the prevention of BPD, but that it should not otherwise be associated with death, we repeated our instrumental variable analyses using death as the dependent variable. Because death is an outcome that should be unrelated to the exposure, but is associated with the potential confounders in the study, the lack of an association between furosemide exposure and death would indicate that confounding was adequately controlled by our instrumental variable analysis. All analyses were performed using Stata (version 14.1, StataCorp, College Station, TX, USA). Pvalues <0.05 were considered to be significant.
Results
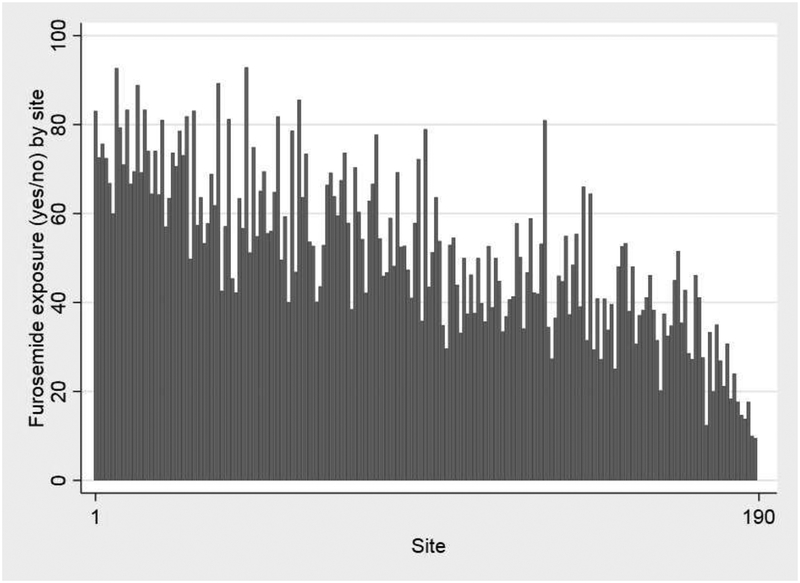
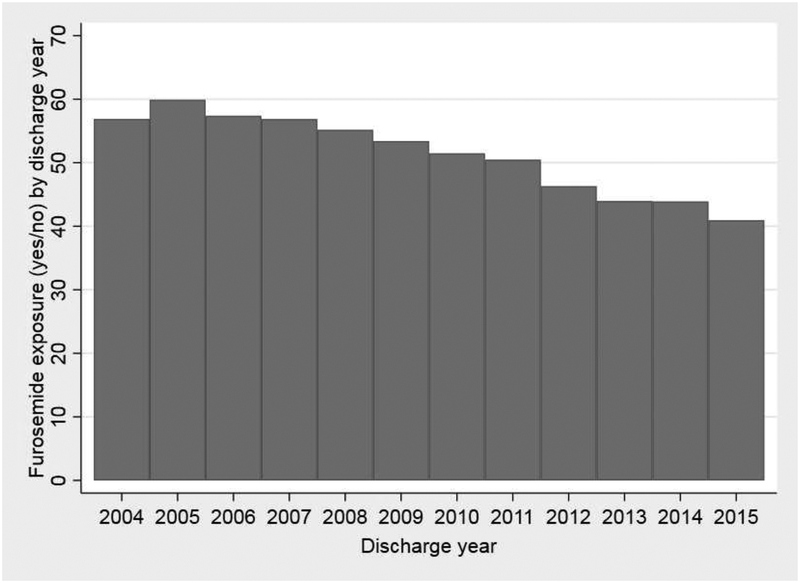
A total of 37,693 infants from 191 neonatal intensive care units (NICUs) were included in this study, with a median (25th–75th percentiles) gestational age and birth weight of 27 weeks (25–28) and 860 g (710–1028). Among all infants, 19,235/37,693 (51%) were exposed to furosemide between postnatal day 7 and 36 weeks PMA and the median percentage of days exposed to furosemide was 1.3% (0–6.7%). Among infants exposed to furosemide, the median duration of exposure was 4 days (2–10), 22% of exposed infants had a duration of 1 day, 23% of infants had a duration ≥14 days, and the longest duration was 84 days. Infants exposed to furosemide were more likely to be have lower gestational age, lower birth weight, male sex, exposure to prenatal steroids, lower Apgar scores, exposure to dexamethasone, atrial septal defect or ventricular septal defect, and patent ductus arteriosus surgery (P<0.001; Table I). Infants exposed to furosemide were also more likely to have higher respiratory support and BPD or death risk scores on postnatal day 7 (P<0.001; Table I). Infant demographics, maternal factors, and infant clinical factors were noted to be overall well-distributed among quartiles of the instrumental variables (Table II and Table III; available at www.jpeds.com). Both prevalence and duration of furosemide exposure varied substantially by site (prevalence: 0–93%; duration: 0–45% of days; Figure 2) and by discharge year (prevalence: 41–60%; duration: 4–13% of days; Figure 3), with a decreasing trend of use over time. Wald tests implied that the instrumental variables approach was appropriate (p<0.001), and F-tests suggested that the instruments we chose provide sufficient explanatory power (p<0.001).
Table I.
Demographics and clinical characteristics for infants treated with furosemide and not treated with furosemide (data are expressed as column percentages)
| Exposed to furosemide N=19,235 | Not exposed to furosemide N=18,458 | p-value | |
|---|---|---|---|
| Gestational age, weeks | <0.001 | ||
| 23–25 | 41 | 22 | |
| 26–27 | 38 | 36 | |
| 28–29 | 21 | 43 | |
| Birth weight, g | <0.001 | ||
| 500–749 | 38 | 23 | |
| 750–999 | 40 | 40 | |
| 1000–1249 | 21 | 38 | |
| Small for gestational age | 14 | 14 | 0.08 |
| Cesarean section | 73 | 74 | 0.02 |
| Prenatal steroids | 78 | 81 | <0.001 |
| Inborn | 85 | 87 | <0.001 |
| 5-minute Apgar score | <0.001 | ||
| 0–3 | 7 | 6 | |
| 4–6 | 26 | 22 | |
| 7–10 | 67 | 73 | |
| Male | 55 | 49 | <0.001 |
| Race/ethnicity | <0.001 | ||
| White | 48 | 48 | |
| Black | 31 | 29 | |
| Hispanic | 21 | 23 | |
| Percentage risk of BPD/death on postnatal day 7, median (25th-75th percentile) | 63 (38–79) | 35 (17–64) | <0.001 |
| Ventilator status on postnatal day 7 | <0.001 | ||
| High-frequency | 20 | 11 | |
| Conventional | 40 | 23 | |
| CPAP | 24 | 35 | |
| Nasal cannula/hood | 14 | 24 | |
| None | 2 | 7 | |
| Fractional inspired oxygen on postnatal day 7 | <0.001 | ||
| 21% | 39 | 63 | |
| 22–50% | 57 | 34 | |
| 51–99% | 4 | 2 | |
| 100% | 1 | 1 | |
| Received dexamethasone | 28 | 8 | <0.001 |
| Atrial septal defect or ventricular septal defect | 9 | 6 | <0.001 |
| Patent ductus arteriosus ligation | 21 | 6 | <0.001 |
BPD, bronchopulmonary dysplasia; CPAP, continuous positive airway pressure ventilation
Table II.
Distribution of infant demographics and clinical characteristics among quartiles of site instrumental variable (data are expressed as column percentages unless otherwise noted)
| Quartile of site instrumental variable | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| N=9253 | N=9293 | N=9191 | N=9245 | |
| Median percentage of infants exposed to furosemide | 2 | 5 | 7 | 14 |
| Gestational age, weeks | ||||
| 23–25 | 29 | 33 | 34 | 29 |
| 26–27 | 36 | 37 | 36 | 38 |
| 28–29 | 35 | 30 | 30 | 33 |
| Birth weight, grams | ||||
| 501–749 | 28 | 31 | 33 | 29 |
| 750–999 | 40 | 40 | 40 | 41 |
| 1000–1249 | 32 | 29 | 27 | 30 |
| Small for gestational age | 14 | 14 | 15 | 14 |
| Cesarean section | 75 | 75 | 72 | 73 |
| Prenatal steroids | 83 | 81 | 79 | 77 |
| Inborn | 89 | 85 | 84 | 86 |
| 5-minute Apgar score | ||||
| 0–3 | 6 | 6 | 7 | 6 |
| 4–6 | 24 | 23 | 25 | 23 |
| 7–10 | 70 | 71 | 68 | 71 |
| Male | 52 | 53 | 52 | 52 |
| Race/ethnicity | ||||
| White | 50 | 51 | 46 | 44 |
| Black | 30 | 26 | 32 | 33 |
| Hispanic | 20 | 23 | 21 | 23 |
| Percentage risk of BPD/death on postnatal day 7a, median | 43 | 53 | 52 | 52 |
| Ventilator status on postnatal day 7 | ||||
| High-frequency | 13 | 17 | 17 | 16 |
| Conventional | 26 | 34 | 32 | 36 |
| CPAP | 35 | 30 | 26 | 27 |
| Nasal cannula/hood | 22 | 16 | 20 | 17 |
| None | 5 | 4 | 4 | 4 |
| Fractional inspired oxygen on postnatal day 7 | ||||
| 21% | 55 | 50 | 50 | 49 |
| 22–50% | 42 | 46 | 47 | 47 |
| 51–99% | 2 | 3 | 3 | 3 |
| 100% | 0.9 | 1.1 | 0.8 | 1.4 |
| Received dexamethasone | 17 | 20 | 18 | 19 |
| Atrial septal defect or ventricular septal defect | 6 | 9 | 6 | 9 |
| Patent ductus arteriosus surgery | 9 | 17 | 14 | 13 |
| Median number of infants at site | 67 | 157 | 226 | 124 |
Table III.
Distribution of infant demographics and clinical characteristics among quartiles of year instrumental variable (data are expressed as column percentages unless otherwise noted)
| Quartile of year instrumental variable | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| N=9478 | N=9157 | N=9144 | N=9203 | |
| Median percentage of infants exposed to furosemide | 5 | 7 | 9 | 12 |
| Gestational age, weeks | ||||
| 23–25 | 29 | 31 | 33 | 31 |
| 26–27 | 38 | 34 | 37 | 38 |
| 28–29 | 33 | 34 | 30 | 31 |
| Birth weight, grams | ||||
| 500–749 | 29 | 30 | 32 | 32 |
| 750–999 | 40 | 39 | 41 | 41 |
| 1000–1249 | 31 | 31 | 28 | 28 |
| Small for gestational age | 14 | 14 | 14 | 15 |
| Cesarean section | 74 | 74 | 74 | 72 |
| Prenatal steroids | 84 | 82 | 77 | 76 |
| Inborn | 87 | 86 | 86 | 86 |
| 5-minute Apgar score | ||||
| 0–3 | 8 | 7 | 6 | 5 |
| 4–6 | 24 | 24 | 24 | 22 |
| 7–10 | 68 | 69 | 69 | 73 |
| Male | 51 | 53 | 52 | 53 |
| Race/ethnicity | ||||
| White | 46 | 49 | 47 | 49 |
| Black | 31 | 31 | 31 | 28 |
| Hispanic | 22 | 20 | 22 | 23 |
| Percentage risk of BPD/death on postnatal day 7a, median | 44 | 47 | 53 | 55 |
| Ventilator status on postnatal day 7 | ||||
| High-frequency | 15 | 16 | 17 | 14 |
| Conventional | 24 | 28 | 34 | 41 |
| CPAP | 44 | 31 | 23 | 19 |
| NC/hood | 14 | 20 | 22 | 20 |
| None | 3 | 4 | 5 | 6 |
| Fractional inspired oxygen on postnatal day 7 | ||||
| 21% | 57 | 56 | 49 | 42 |
| 22–50% | 41 | 42 | 47 | 53 |
| 51–99% | 2 | 2 | 3 | 4 |
| 100% | 0.7 | 0.9 | 1.0 | 1.6 |
| Received dexamethasone | 16 | 17 | 19 | 21 |
| Atrial septal defect or ventricular septal defect | 10 | 8 | 7 | 6 |
| Patent ductus arteriosus surgery | 8 | 12 | 16 | 18 |
>Figure 2. Furosemide exposure (percentage infant-days exposed).
Furosemide exposure (percentage infant-days exposed) by: A) site; and B) discharge year.
Figure 3. Any furosemide exposure.
Any furosemide exposure (yes/no) by: A) site; and B) discharge year.
Of the total 37,693 infants, 19,895 (53%) had BPD or died; 33,760/37,693 (90%) infants survived to 36 weeks PMA and were evaluated for BPD. Of these, 15,954/33,760 (47%) had BPD. The incidence of BPD was relatively stable over the study period (starting at 50% in 2004, hitting a low of 45% in 2013, and ending at 47% in 2015). Among infants with BPD, 7,816/15,954 (49%) were on low-flow nasal cannula at 36 weeks; 6,424/15,954 (40%) were on continuous positive airway pressure, non-invasive positive pressure ventilation, or high flow nasal cannula; and 1,567/15,954 (10%) were on the ventilator, with the remaining 147/15,954 (0.9%) receiving hood oxygen. On adjusted analysis with furosemide exposure measured as a binary variable, exposure was not significantly associated with either BPD (P=0.62) or the combined outcome of BPD or death (P=0.82; Table IV). Nevertheless, when furosemide exposure was evaluated in terms of percentage days exposed, a 10 percentage point increase in the proportion of days exposed to furosemide (e.g., from 10% to 20%) was associated with a 4.6 percentage point decrease in BPD (P=0.001) and a 3.7 percentage point decrease in BPD or death (P=0.01; Table IV). Furosemide exposure by either binary or continuous measure was not associated with death (Table IV). Our sensitivity analysis including only 1 instrumental variable showed similar results (no significant association between any furosemide exposure and BPD or the combined outcome of BPD or death, but a 4.5 percentage point decrease in BPD [P=0.004] and 3.8 percentage point decrease in BPD or death [P=0.02] in response to a 10 percentage point increase in exposure).
Table IV.
Adjusted coefficients to predict outcomes
| Change in the percentage of patients experiencing an outcome in response to a 10 percentage point increase in days exposed to furosemide (95% CI) | P-value | |
| BPD | −4.6 percentage points (−7.3, −1.8) | 0.001 |
| BPD or death | −3.7 percentage points (−6.6, −0.1) | 0.01 |
| Death | 0.04 percentage points (−0.24, 0.31) | 0.78 |
| Change in the percentage of patients experiencing an outcome associated with any exposure to furosemide (95% CI) | P-value | |
| BPD | −2.4 percentage points (−12.2, 7.3) | 0.62 |
| BPD or death | −1.2 percentage points (−11.1, 8.8) | 0.82 |
| Death | 2.2 percentage points (−1.3, 5.6) | 0.22 |
BPD, bronchopulmonary dysplasia; CI, confidence interval
Discussion
In our sample of >36,000 premature infants, increased duration of furosemide exposure was associated with a lower likelihood of developing BPD and BPD or death. Our results indicate that duration of exposure, rather than any exposure, was critical for the prevention of these outcomes. Simple exposure to furosemide, without accounting for duration, was not associated with BPD or the combined outcome of BPD or death.
Furosemide is not indicated by the Food and Drug Administration for prevention of BPD in premature infants. Despite this lack of guidance, 50% of infants in our cohort were exposed to furosemide between 7 days of life and 36 weeks PMA. There was substantial variation in the prevalence and duration of furosemide exposure by site. This practice variation in drug prescription has been observed for other drug classes within the Pediatrix Medical Group (23, 24) and for diuretics among United States hospitals in a study using the Pediatric Health Information System (10). The degree of variation in prescription results in part from a lack of Food and Drug Administration labelling for many drugs in infants, which stems from a paucity of data regarding safety and efficacy. Therefore, neonatologists are forced to prescribe drugs with promising physiological mechanisms despite limited data.
In the case of furosemide, administration is believed to promote diuresis and reduce intravascular fluid volume, thereby decreasing fluid flow to the lungs. Furosemide is also hypothesized to act by promoting peripheral or pulmonary vasodilation through prostaglandin synthesis, which decreases pulmonary congestion (25, 26). Decreased pulmonary fluid has been associated with improved respiratory outcomes in critically ill adults and in premature infants (27, 28). Although the proposed physiologic mechanism of furosemide is plausible, efficacy data are limited to small randomized controlled trials with short-term outcomes (29, 30). A meta-analysis of the existing data demonstrated that loop diuretics (including furosemide) improve lung compliance, airway resistance, and oxygenation, but no trials have explored the effect of furosemide on the development of BPD. A recent observational study of 835 extremely premature infants concluded that diuretics were not associated with short-term improvements in respiratory support (31). Although this study attempted to adjust for the multiple demographic factors that were significantly different between the exposed and unexposed groups, it is likely that infants exposed to diuretics had more severe disease. In addition, the study did not account for duration of diuretic exposure. A randomized clinical trial of furosemide safety in premature infants receiving 28 days of furosemide is currently underway (32), but the trial will not be adequately powered to address efficacy. The findings of our study support the design of a future randomized controlled trial to examine the efficacy of furosemide for the prevention of BPD in premature infants. In particular, our data suggest that a longer duration of furosemide exposure may have better effectiveness for prevention of BPD.
Although the results of our study are promising, clinicians must also consider whether the degree of benefit associated with a 10 percentage point increase in furosemide exposure days (4.6 percentage point decrease in BPD and a 3.7 percentage point decrease in BPD or death) is clinically significant. With any drug, potential benefits must be weighed against potential risks. Although currently available evidence surrounding risks of furosemide in premature infants is scarce and limited mostly to small cohort and case control studies, an ongoing trial will examine more closely the risk of adverse events such as nephrocalcinosis/nephrolithiasis and ototoxicity (32).
Our cohort of infants was at high risk for pulmonary morbidity, with 47% developing BPD. Prevalence of BPD in prior cohorts has varied according to the definition used, and the most appropriate definition remains subject to debate (33, 34). Like many of the most commonly used definitions of BPD, including the 2018 National Institutes of Health consensus definition (35), our definition is dependent on therapies administered (oxygen and respiratory support) to treat the condition, and it has been applied consistently across studies using data from the Pediatrix Medical Group (12, 36–38). The database does not include radiological data to support confirmation of parenchymal lung disease, which is a component of the National Institutes of Health definition. Compared with some definitions that require respiratory or oxygen support for a specified period (e.g., ≥28 days) for the diagnosis of BPD, our definition may have led to an overestimate of BPD prevalence. Nonetheless, the prevalence of BPD in our cohort was similar to that in a cohort of 34,636 infants cared for at National Institute of Child Health and Human Development Neonatal Research Network centers between 1993 and 2012 (39). In this study of infants 22–28 weeks gestation and 401–1500 g, the diagnosis of BPD became more prevalent over time and was >40% at the end of the study period. Consequently, our cohort appears to be representative of other large multicenter populations of infants at high risk for morbidity.
The potential effect of furosemide on the development of BPD is difficult to address correctly with observational data such as ours, due to the presence of an important confounding variable: clinical disease severity. Sicker infants are more likely to develop signs and symptoms of lung disease, which make providers more likely to prescribe furosemide. To address this problem, we chose to use an instrumental variable approach. We identified factors (exposure to furosemide at each discharge year and at each site) that we assumed to be related to furosemide exposure; not so highly correlated with potential confounders; and associated with the outcome (BPD) only through its relationship with treatment (40). We observed that furosemide exposure varied by both discharge year and site, results which were consistent with the first assumption. Most patient characteristics were fairly evenly distributed across instrumental variable quartiles. Notably, some indicators of disease severity (including risk of BPD/death and proportion of infants requiring oxygen, requiring mechanical ventilation, receiving dexamethasone, and undergoing patent ductus arteriosus surgery) appeared to be somewhat lower in the first quartile of the site instrumental variable compared with the other quartiles, suggesting that sites with lower use of furosemide may have treated a population of infants at lower risk. Similarly, disease severity appeared to be modestly greater in higher quartiles of the discharge year instrumental variable. This finding may reflect improved survival and increased lung morbidity over time, as demonstrated in the National Institute of Child Health and Human Development Neonatal Research Network cohort (39). We attempted to address these potential weaknesses by repeating our analyses using death as a falsification endpoint (41). We hypothesized that furosemide exposure should have no direct effect on death. If furosemide were observed to be associated with death, then this would suggest that its association with BPD might be spurious. In fact, there is no association between the furosemide exposure and death (Table IV), suggesting that our instrumental variables validly account for unmeasured confounders.
The strengths of our study include its focus on a clinical question critical to the field of neonatology for which previous data have been limited or nonexistent. The large sample size over a long study period at 190 centers allowed us to use an instrumental variable approach. Our analysis also benefited from the use of a BPD risk calculator to adjust for patient disease severity. We remained limited by the retrospective study design, and it is possible that our results were due to unmeasured confounders. Though we attempted to address this issue using a falsification end point, it is possible that potential confounders of the association between furosemide exposure and death may differ from those of the association between furosemide exposure and BPD. As noted above, we also found a possible association between our instrumental variables and some important disease characteristics. We attempted to mitigate this association through secondary analyses and adjustment for these characteristics in our models. Finally, we did not examine the effects of other therapies on the incidence of BPD, including diuretics less commonly used in the NICU (e.g. chlorothiazide, bumetanide), caffeine, and use of non-invasive ventilation. Genetic variation may also influence the efficacy of furosemide (42, 43); we were unable to explore such effects in our study.
In conclusion, we found that a higher percentage of days exposed to furosemide between postnatal day 7 and 36 weeks PMA in extremely premature infants was associated with a decreased risk of BPD, and a combined outcome of BPD or death. These results provide important preliminary data to support the development of prospective studies to evaluate the safety and efficacy of furosemide for the prevention of BPD.
Acknowledgments
R.G. receives salary support for research from the National Institutes of Health (NIH) (HHSN 275201000003I, HHSN 272201300017I), Centers for Disease Control (HHSN200201253663), and from the Food and Drug Administration (HHSF223201610082C). P.S. receives salary support for research from the NIH (NIH-1R21HD080606-01A1) and the NICHD (HHSN275201000003I). M.W. receives support for research from the NIH (1R01-HD076676-01A1), the National Institute of Allergy and Infectious Disease (HHSN272201500006I and HHSN272201300017I), the NICHD (HHSN275201000003I), the Biomedical Advanced Research and Development Authority (HHSO100201300009C), and industry for drug development in adults and children (www.dcri.duke.edu/research/coi.jsp). The other authors declare no conflicts of interest.
Abbreviations
- BPD
bronchopulmonary dysplasia
- PMA
postmenstrual age
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Natarajan G, Pappas A, Shankaran S, Kendrick DE, Das A, Higgins RD, et al. Outcomes of extremely low birth weight infants with bronchopulmonary dysplasia: impact of the physiologic definition. Early Hum Dev 2012;88:509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fily A, Pierrat V, Delporte V, Breart G, Truffert P. Factors associated with neurodevelopmental outcome at 2 years after very preterm birth: the population-based Nord-Pas-de-Calais EPIPAGE cohort. Pediatrics 2006;117:357–66. [DOI] [PubMed] [Google Scholar]
- 3.Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics 2000;105:1216–26. [DOI] [PubMed] [Google Scholar]
- 4.Katz-Salamon M, Gerner EM, Jonsson B, Lagercrantz H. Early motor and mental development in very preterm infants with chronic lung disease. Arch Dis Child Fetal Neonatal Ed 2000;83:F1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAleese KA, Knapp MA, Rhodes TT. Financial and emotional cost of bronchopulmonary dysplasia. Clin Pediatr (Phila) 1993;32:393–400. [DOI] [PubMed] [Google Scholar]
- 6.Gough A, Spence D, Linden M, Halliday HL, McGarvey L. General and respiratory health outcomes in adult survivors of bronchopulmonary dysplasia: a systematic review. Chest 2012;141:1554–67. [DOI] [PubMed] [Google Scholar]
- 7.Pakvasa MA, Saroha V, Patel RM. Optimizing caffeine use and risk of bronchopulmonary dysplasia in preterm infants: a systematic review, meta-analysis, and application of grading of recommendations assessment, development, and evaluation methodology. Clin Perinatol 2018;45:273–91. [DOI] [PubMed] [Google Scholar]
- 8.Couroucli XI, Placencia JL, Cates LA, Suresh GK. Should we still use vitamin A to prevent bronchopulmonary dysplasia? J Perinatol 2016;36:581–5. [DOI] [PubMed] [Google Scholar]
- 9.Doyle LW, Ehrenkranz RA, Halliday HL. Early (<8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev 2014;5:CD001146. [DOI] [PubMed] [Google Scholar]
- 10.Slaughter JL, Stenger MR, Reagan PB. Variation in the use of diuretic therapy for infants with bronchopulmonary dysplasia. Pediatrics 2013;131:716–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laughon MM, Chantala K, Aliaga S, Herring AH, Hornik CP, Hughes R, et al. Diuretic exposure in premature infants from 1997 to 2011. Am J Perinatol 2015;32:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart A, Brion LP. Intravenous or enteral loop diuretics for preterm infants with (or developing) chronic lung disease. Cochrane Database Syst Rev 2011:CD001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spitzer AR, Ellsbury DL, Handler D, Clark RH. The Pediatrix BabySteps Data Warehouse and the Pediatrix QualitySteps improvement project system--tools for “meaningful use” in continuous quality improvement. Clin Perinatol 2010;37:49–70. [DOI] [PubMed] [Google Scholar]
- 14.Trembath A, Hornik CP, Clark R, Smith PB, Daniels J, Laughon M. Comparative effectiveness of surfactant preparations in premature infants. J Pediatr 2013;163:955–60.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics 2010;125:e214–24. [DOI] [PubMed] [Google Scholar]
- 16.NICHD Neonatal Research Network. Neonatal BPD Outcome Estimator. https://neonatal.rti.org/index.cfm?fuseaction=BPDCalculator.start. Accessed June 27, 2018.
- 17.Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med 2011;183:1715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baiocchi M, Cheng J, Small DS. Instrumental variable methods for causal inference. Stat Med 2014;33:2297–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaibub Neto E Using instrumental variables to disentangle treatment and placebo effects in blinded and unblinded randomized clinical trials influenced by unmeasured confounders. Sci Rep 2016;6:37154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA 2007;297:278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meara E, Kotagal UR, Atherton HD, Lieu TA. Impact of early newborn discharge legislation and early follow-up visits on infant outcomes in a state Medicaid population. Pediatrics 2004;113:1619–27. [DOI] [PubMed] [Google Scholar]
- 22.Lorch SA, Baiocchi M, Ahlberg CE, Small DS. The differential impact of delivery hospital on outcomes of premature infants. Pediatrics 2012;130:270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu PY, Hill KD, Clark RH, Smith PB, Hornik CP. Treatment of supraventricular tachycardia in infants: analysis of a large multicenter database. Early Hum Dev 2015;91:345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ku LC, Zimmerman K, Benjamin DK, Clark RH, Hornik CP, Smith PB. Safety of Enalapril in Infants Admitted to the Neonatal Intensive Care Unit. Pediatr Cardiol 2017;38:155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dikshit K, Vyden JK, Forrester JS, Chatterjee K, Prakash R, Swan HJ. Renal and extrarenal hemodynamic effects of furosemide in congestive heart failure after acute myocardial infarction. N Engl J Med 1973;288:1087–90. [DOI] [PubMed] [Google Scholar]
- 26.Prandota J Furosemide: progress in understanding its diuretic, anti-inflammatory, and bronchodilating mechanism of action, and use in the treatment of respiratory tract diseases. Am J Ther 2002;9:317–28. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell JP, Schuller D, Calandrino FS, Schuster DP. Improved outcome based on fluid management in critically ill patients requiring pulmonary artery catheterization. Am Rev Respir Dis 1992;145:990–8. [DOI] [PubMed] [Google Scholar]
- 28.Adams EW, Harrison MC, Counsell SJ, Allsop JM, Kennea NL, Hajnal JV, et al. Increased lung water and tissue damage in bronchopulmonary dysplasia. J Pediatr 2004;145:503–7. [DOI] [PubMed] [Google Scholar]
- 29.Yeh TF, Shibli A, Leu ST, Raval D, Pildes RS. Early furosemide therapy in premature infants (less than or equal to 2000 gm) with respiratory distress syndrome: a randomized controlled trial. J Pediatr 1984;105:603–9. [DOI] [PubMed] [Google Scholar]
- 30.Rush MG, Engelhardt B, Parker RA, Hazinski TA. Double-blind, placebo-controlled trial of alternate-day furosemide therapy in infants with chronic bronchopulmonary dysplasia. J Pediatr 1990;117:112–8. [DOI] [PubMed] [Google Scholar]
- 31.Blaisdell CJ, Troendle J, Zajicek A. Acute responses to diuretic therapy in extremely low gestational age newborns: results from the Prematurity and Respiratory Outcomes Program Cohort Study. J Pediatr 2018;197:42–7.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ClinicalTrials.gov. Safety of Furosemide in Premature Infants at Risk of Bronchopulmonary Dysplasia (BPD). ClinicalTrials.gov identifier NCT02527798. https://clinicaltrials.gov/ct2/show/NCT02527798. Accessed June 27, 2018.
- 33.Bancalari E, Claure N, Sosenko IR. Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Semin Neonatol 2003;8:63–71. [DOI] [PubMed] [Google Scholar]
- 34.Hines D, Modi N, Lee SK, Isayama T, Sjors G, Gagliardi L, et al. Scoping review shows wide variation in the definitions of bronchopulmonary dysplasia in preterm infants and calls for a consensus. Acta Paediatr 2017;106:366–74. [DOI] [PubMed] [Google Scholar]
- 35.Higgins RD, Jobe AH, Koso-Thomas M, Bancalari E, Viscardi RM, Hartert TV, et al. Bronchopulmonary dysplasia: executive summary of a workshop. J Pediatr 2018;197:300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly MS, Benjamin DK, Puopolo KM, Laughon MM, Clark RH, Mukhopadhyay S, et al. Postnatal cytomegalovirus infection and the risk for bronchopulmonary dysplasia. JAMA Pediatr 2015;169:e153785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JH, Smith PB, Quek MB, Laughon MM, Clark RH, Hornik CP. Risk factors and inhospital outcomes following tracheostomy in infants. J Pediatr 2016;173:39–44.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar KR, Clark DA, Kim EM, Perry JD, Wright K, Thomas SA, et al. Association of atrial septal defects and bronchopulmonary dysplasia in premature infants. J Pediatr 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 2015;314:1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martens EP, Pestman WR, de Boer A, Belitser SV, Klungel OH. Instrumental variables: application and limitations. Epidemiology 2006;17:260–7. [DOI] [PubMed] [Google Scholar]
- 41.Prasad V, Jena AB. Prespecified falsification end points: can they validate true observational associations? JAMA 2013;309:241–2. [DOI] [PubMed] [Google Scholar]
- 42.de Denus S, Rouleau JL, Mann DL, Huggins GS, Cappola TP, Shah SH, et al. A pharmacogenetics investigation of intravenous furosemide in decompensated heart failure: a meta-analysis of three clinical trials. Pharmacogenomics 2017;17:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vormfelde SV, Sehrt D, Toliat MR, Schirmer M, Meineke I, Tzvetkov M, et al. Genetic variation in the renal sodium transporters NKCC2, NCC, and ENaC in relation to the effects of loop diuretic drugs. Clin Pharmacol Ther 2007;82:300–9. [DOI] [PubMed] [Google Scholar]