Abstract
Proper placental development is crucial to establish a successful pregnancy. Defective placentation is the major cause of several pregnancy complications, including preeclampsia (PE). We have previously demonstrated that the secreted factor Epidermal Growth Factor-like Domain 7 (EGFL7) is expressed in trophoblast cells of the human placenta and that it regulates trophoblast migration and invasion, suggesting a role in placental development. In the present study, we demonstrate that circulating levels of EGFL7 are undetectable in nonpregnant women, increase during pregnancy and decline toward term. Close to term, circulating levels of EGFL7 are significantly higher in patients affected by PE when compared to normal pregnancies. Consistent with these results, villus explant cultures obtained from placentas affected by PE display increased release of EGFL7 in the culture medium when compared to those from normal placentas. Our results suggest that increased release of placenta-derived EGFL7 and increased circulating levels of EGFL7 are associated with the clinical manifestation of PE.
INTRODUCTION
A successful pregnancy depends on a functional placenta. The placenta supports the development and survival of the mammalian embryo by providing the exchange of nutrients, oxygen, and metabolic waste, protecting the embryo against the maternal immune system, and by producing hormones. Proper placental development requires invasion of extravillous trophoblast during the first trimester and the remodeling of maternal spiral arteries, which facilitates increased supply of maternal blood to the placenta.
Impairment of uterine spiral artery remodeling leading to placental ischemia, dysfunctional syncytiotrophoblast, and concomitant release of placental antiangiogenic factors into the maternal circulation are the major determinants of preeclampsia (PE).1,2 PE, a major cause of maternal and perinatal morbidity and mortality, has been originally defined as a rise in blood pressure (>140 mm Hg/90 mm Hg) associated with proteinuria after 20 weeks of gestation. Recently, the American College of Obstetrics and Gynecology has revised the definition, excluding proteinuria from the list of required criteria.3 According to the time of clinical manifestation, PE can be divided into early (<34 weeks) and late onset (> 34 weeks). Early onset PE is classified as a fetal disorder associated with shallow placentation and inadequate remodeling of maternal spiral arteries, while late onset PE is considered a maternal disease, generally a consequence of pre-existing maternal disorders or genetic predisposition.4,5 Independent from the time of onset, syncytiotrophoblast stress and an imbalance in the release of angiogenic factors in the maternal circulation is a key element characterizing PE. Indeed, several pro- and antiangiogenic factors were recently identified to be involved in the pathogenesis of PE.6 In particular, increased levels of soluble fms-like tyrosine kinase 1 (sFLT-1) and soluble Endoglin (sENDOGLIN), and reduced levels of Vascular endothelial growth factor, Placental growth factor (PlGF), and Transforming growth factor-β have been demonstrated in the maternal serum of a subset of patients with PE.7,8
Measuring levels of these factors has been recently shown to be a useful tool in discriminating between PE and other pathologic conditions that may manifest during pregnancy, such as chronic kidney disease and chronic hypertension.9–11 However, predictive models using these angiogenic markers for PE have failed to show sufficient robustness to be acceptable for clinical practice.12 More comprehensive diagnostic tools are needed to promptly and accurately diagnose PE and to help risk stratify patients.
In a multivariable approach perspective, we hypothesized that Epidermal Growth Factor-like Domain 7 (EGFL7) is a possible additional angiogenic factor involved in PE. EGFL7 is a largely endothelial-restricted, secreted factor that is critical for embryonic vascular development in mice.13 EGFL7 is deposited into the extracellular matrix by endothelial cells, delineating the boundary of nascent vascular sprouts.14,15 During placental development in mice, Egfl7 loss-of-function results in feto-placental vascularization defects and intrauterine growth restriction.16 In the murine placenta, EGFL7 is expressed by endothelial cells in the fetal labyrinth and the maternal decidua and by trophoblast cells throughout placental development. In human placental villi, EGFL7 is present in fetal capillary endothelial cells, cytotrophoblast, and syncytiotrophoblast.17 Interestingly, placental levels of EGFL7 are downregulated in PE.17,18 Functionally, EGFL7 positively regulates cell migration and invasion of human trophoblast cells.19
In the present study, we quantified EGFL7 in both plasma and serum of women affected by PE and compared their levels with those of healthy pregnant women. Our data demonstrate that circulating levels of EGFL7 are significantly increased in the blood of women affected by PE, possibly due to increased mobilization of placental produced EGFL7, thus suggesting a positive correlation between levels of EGFL7 and the clinical symptoms of PE. We also report increased release of EGFL7 by villus explants from placentas of PE affected patients.
METHODS
Study subjects.
The study respected the principles expressed in the Declaration of Helsinki and was approved by the Bioethical Committee of the Catholic University of Sacred Heart of Rome, Italy (approval number P575(A1420)/CE/2008), and by the Institutional Review Board at Weill Cornell Medical College (IRB protocols #1011011376 and 9811003560). Informed consent was obtained from all participants. Women were considered to have PE according to the American College of Obstetrics and Gynecology.5 PE was defined according by the American College of Obstetricians and Gynecologists guidelines. In all cases, there was hypertension, defined as raised blood pressure (≥ 140/90 mm Hg) after 20 weeks gestation on 2 different occasions at least 4 hours apart, and proteinuria (>300 mg of protein in a 24-hour urine collection). All plasma samples (73 samples) were obtained from the Fondazione Policlinico A. Gemelli and all serum samples (42 samples) were obtained from New York Presbyterian Hospital/Weill Cornell Medical College. A total of 115 women were enrolled in this case-control study: 12 were nonpregnant healthy women; 39 were healthy pregnant women evaluated between 8 and 34 weeks; 26 healthy pregnant women and 38 women presenting with PE were assessed between 35 and 40 weeks of gestation, and their circulating levels of EGFL7 were compared. We excluded women with diabetes, hematological, and immunologic disorders, pre-existing renal disorders, heart disease (New York Heart Association (NYHA) 3–4), sepsis, or infection. In healthy pregnant women, analysis of EGFL7 throughout gestation was performed within intervals of 3–4 gestational weeks. For each interval, at least 5 samples were analyzed.
Placental tissue samples for explant cultures were obtained from the New York Presbyterian Hospital/Weill Cornell Medical College postdelivery from 9 nonpregnant healthy women at 37–39 weeks of gestation and 11 women presenting with PE at 35–37 weeks of gestation.
Blood sample processing.
For plasma studies, samples were collected under the protocol approved by the Bioethical Committee of the Catholic University of Sacred Heart of Rome. Briefly, peripheral venous blood was collected into ethylenediaminetetraacetic acid tubes and processed within 1 hour. Blood samples were centrifuged at 1500 × g for 10 minutes at 4°C. Plasma was aliquoted and stored at −80°C until analysis. Serum samples were obtained under an approved Weill Cornell Medicine IRB protocol. Briefly, blood was collected at the time of routine phlebotomy or at the time of evaluation for PE, allowed to coagulate at room temperature for 1 hour, centrifuged at 1500 × g for 20 minutes, and the serum was aliquoted and stored at −80°C until analysis.
Enzyme-linked immunosorbent assays.
Enzyme-linked immunosorbent assays (ELISAs) were performed using commercial kits. Human EGFL7 kits were purchased from Cusabio Biotech (College Park, Maryland). Human sFLT-1, free PlGF, and sENDOGLIN kits were purchased from R&D Systems (Minneapolis, Minnesota). Assays were performed according to the manufacturer’s instructions.
Ex vivo placental explant cultures.
Human placental explant cultures were essentially performed as described by Miller et al.20 Briefly, placental tissue samples were isolated from each placenta postdelivery, including - four 1 cm3 villous sections. Sections were extensively washed in phosphate buffered saline (PBS) to remove maternal blood and either placed directly in 4% paraformaldehyde (PFA; 0 hour control) or cultured for 24 hours in 1 mL of Dulbecco’s modified Eagle Medium (DMEM)/F12 media supplemented with 1% Penicillin-StreptomycinGlutamine in 24-well plates at 37°C and 5% CO2 (2 villi samples per placenta). Phase-contrast images were taken under a Leica DMIL inverted microscope. After 24 hours, conditioned media was collected, centrifuged, and stored at −80°C until analysis, and villi were fixed in 4% PFA. The amount of EGFL7 protein in the conditioned medium was determined by ELISAs as described above.
PFA-fixed placental samples collected at 0 and 24 hours were washed in PBS, placed in 30% sucrose in PBS at 4°C, and then embedded in OCT–30% sucrose (2:1).
Immunofluorescence staining.
Cryosections were permeabilized in 100% methanol at −20°C for 5 minutes and blocked with 10% donkey serum in PBS. Primary antibodies (CD31, BD Biosciences, 550300, 1.56 μg/mL; CYTOKERATIN, DAKO Z0622, 16.4 μg/mL; EGFL7 (hVE-Statin) R&D Systems, AF3638, 1 μg/mL) were incubated overnight at 4°C, followed by incubation with secondary antibodies (Cy3-donkey anti-mouse, Alexa647-donkey antirabbit, and Alexa488-donkey antigoat, Jackson Immunoresearch, 1.5 μg/mL), and mounted with Prolong Gold + DAPI (4′,6-diamidino-2-phenylindole). Images were acquired using a Zeiss imaging microscope.
Statistical analysis.
Continuous data were presented as median and interquartile range or as mean and standard deviation when normally distributed. The Kolmogorov-Smirnov test was used to assess the normality of the distribution of the data. Normally distributed data were compared using the 2-sample t test. A nonparametric analysis (Mann-Whitney U test) was used to compare not normally distributed data. One Way ANOVA with Tukey’s multiple comparison post-hoc test was used for multiple group comparison (Graph- Pad Prism 7). Asterisks indicate the level of statistical significance (*P = 0.05; **P < 0.05; ***P < 0.001; ****P < 0.0001). Correlation between 2 variables was analyzed using the Pearson r value (GraphPad Prism 7). To explore the degrees of freedom underlying the measured biomarker profile, all 4 biomarker values for all subjects were entered into principal component analysis (PCA—extraction limit set at smallest eigenvalue >1)—based factor analysis followed by varimax rotation with Kaiser normalization. PCA is a data transformation method which is used to convert a number of possibly correlated variables in to a set of new variables (called components or, in the context of factor analysis, factors) which are linearly uncorrelated and therefore by definition contain independent information. The original variables can then be related back to the components through linear combination coefficients which are commonly called “loadings” and can be thought of as expressing how much any particular variable has contributed to creating any particular component. In addition, in order to explore the ability of each factor to predict group membership (ie, whether a subject belongs to the healthy control group vs the PE group) independent of other factors, we employed a multivariate binary logistic regression model with group as the dependent variable and extracted factors as independent predictors. SPSS v20 was used for statistical analysis of the clinical findings. PCA and logistic regression analyses were performed in SPSS v23.
RESULTS
Maternal and perinatal findings.
Maternal and perinatal findings are reported in Tables I and II. As summarized in the tables, 38 women were diagnosed with PE and 26 healthy women were analyzed as controls. Among pregnancies with PE, 4 were twin pregnancies. Maternal age and body mass index were similar in the 2 groups. At delivery, the systolic and diastolic blood pressure was significantly different across the groups (P < 0.001). The PE group had significantly lower neonatal birth weight (P < 0.001), and neonatal birth weight percentile (P = 0.02) compared to controls.
Table I.
Maternal findings
| Variables§ | Preeclampsia (n° = 38) | Controls (n° = 26) | Significance(P value)§ |
|---|---|---|---|
| Maternal age (years) | 31.9 ± 6.7 (n° 35) | 31.14 ± 5.6 (n° 22) | 0.63 |
| Parity | 0.14 ± 0.35 (n° 35) | 0.59 ± 0.79 (n° 22) | 0.005* |
| Systolic blood pressure at delivery (mmHg) | 157.2 ± 12.4 (n° 34) | 120.2 ± 14.49 (n° 18) | <0.001* |
| Diastolic blood pressure at delivery (mmHg) | 103.00 ± 13 (n° 34) | 73.5 ± 11 (n° 18) | <0.001* |
| Protein:Creatinine Ratio | 1.72 ± 2.93 (n° 28) | 0.22 ± 0.22 (n° 7) | 0.007* |
| BMI | 22.20 ± 4.78 (n° 29) | 26.85 ± 11.69 (n° 18) | 0.189 |
| Ethnicity | 53% Caucasian | 64% Caucasian | |
| 12% African American | 18% African American | ||
| 16% Asian | 0% Asian | ||
| 0% Asian Indian | 4% Asian Indian | ||
| 19% Hispanic | 14% Hispanic |
Abbreviations: IQR, interquartile range; SD, standard deviation.
Mean ± SD when normally distributed; median ± IQR when not normally; t test when normally distributed; Mann-Whitney when not normally distributed.
Table II.
Perinatal findings
| Variables§ | Preeclampsia (n° = 42 neonates) | Controls (n° = 26 neonates) | Significance (P value)§ |
|---|---|---|---|
| Gestational age at delivery (weeks) | 38.0 ± 2.30 (n° 39) | 39.22 ± 3.05 (n° 22) | 0.045* |
| Birth weight (g) | 2914.32 ± 494.77 (n° 37) | 3438.33 ± 434.34 (n° 21) | <0.001* |
| Birth weight percentile (Fenton Calculator) | 39.17 ± 23.02 (n° 35) | 54.71 ± 25.06 (n° 21) | 0.02* |
| Baby gender | 75% female | 67% female | |
| 35% male | 33% male |
Abbreviations: IQR, interquartile range; SD, standard deviation.
Mean ± SD when normally distributed; median ± IQR when not normally; t test when normally distributed; Mann-Whitney when not normally distributed.
Levels of circulating EGFL7.
To evaluate EGFL7 expression in nonpregnant and healthy pregnant women, we performed a cross-sectional analysis of plasma samples obtained within gestational-age intervals of 3–4 weeks using an ELISA assay (Fig 1, A). EGFL7 was undetectable in nonpregnant women, and was first detected during weeks 8–12 of gestation with a concentration of 1.8 μg/mL. Levels of EGFL7 increased up to weeks 26–30, peaking at approximately 60 μg/mL. At this stage, levels of EGFL7 were significantly higher than the concentrations measured at earlier stages of gestation (Fig 1, A). High levels of EGFL7 were maintained up to 34 weeks of gestation, and then significantly declined to 25.26 μg/mL at term, a value that was not statistically different from those observed at weeks 17–25. Pearson correlation analyses showed a significant positive correlation between EGFL7 levels and gestational age (Pearson r = 0.428, P = 0.0014, Supplemental Fig 1).
Fig 1.
EGFL7 levels in nonpregnant, and healthy and PE-affected pregnant women. A, Mean (±SE) levels of Epidermal Growth Factor-like Domain 7 (EGFL7) in plasma of nonpregnant women and healthy pregnant women throughout pregnancy as measured by ELISA. EGFL7 levels are undetectable in the blood of nonpregnant women (NP). In healthy pregnancies, EGFL7 protein is first detected between 8 and 12 weeks of gestation, increases up to weeks 26–30, and declines thereafter until term. Same letters indicate that there is no statistically significant difference among the groups (P ≥ 0.05), while different letters indicate statistically significant differences (ANOVA with Tukey’s multiple comparison post-hoc test). B, ELISA assays performed on both plasma and serum samples of healthy pregnant women and pregnant women affected by preeclampsia (PE) between 35 and 40 weeks of gestation. Pregnant women affected by PE have increased levels of circulating EGFL7, compared to age-matched controls (t test, **P < 0.01). Gray dots and squares represent plasma samples from control and PE-affected pregnant women, respectively. Black dots and squares represent serum samples from control and PE-affected women, respectively. ELISAs, enzyme-linked immunosorbent assays.
To investigate if circulating levels of EGFL7 were affected in PE, we measured EGFL7 in samples from healthy pregnant patients and patients presenting with PE at the same gestational age (35–40 weeks of gestation). Our experimental samples at term included either plasma or serum. To determine if the results from the analyses of plasma and serum could be combined, we compared EGFL7 protein levels from plasma and serum from both controls and PE-affected patients and performed a student’s t test. No significant differences between plasma and serum were detected (Supplemental Fig 2). Therefore, data obtained from plasma or serum samples were combined for subsequent analyses, while identifying the 2 types of samples in the corresponding figures using gray and black symbols, respectively. When analyzing circulating levels of EGFL7 between 35 and 40 weeks of gestation, we found that samples from women affected by PE had significantly higher values when compared to control samples (mean concentration 43.59 μg/mL vs 25.26 μg/mL, respectively; P = 0.0039; Fig 1, B).
Correlation analysis between EGFL7 and known factors dysregulated in PE.
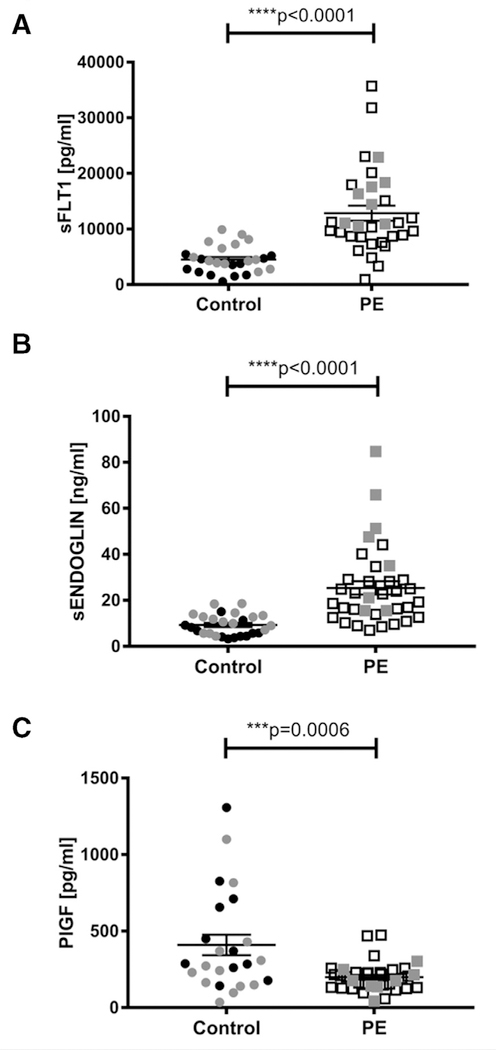
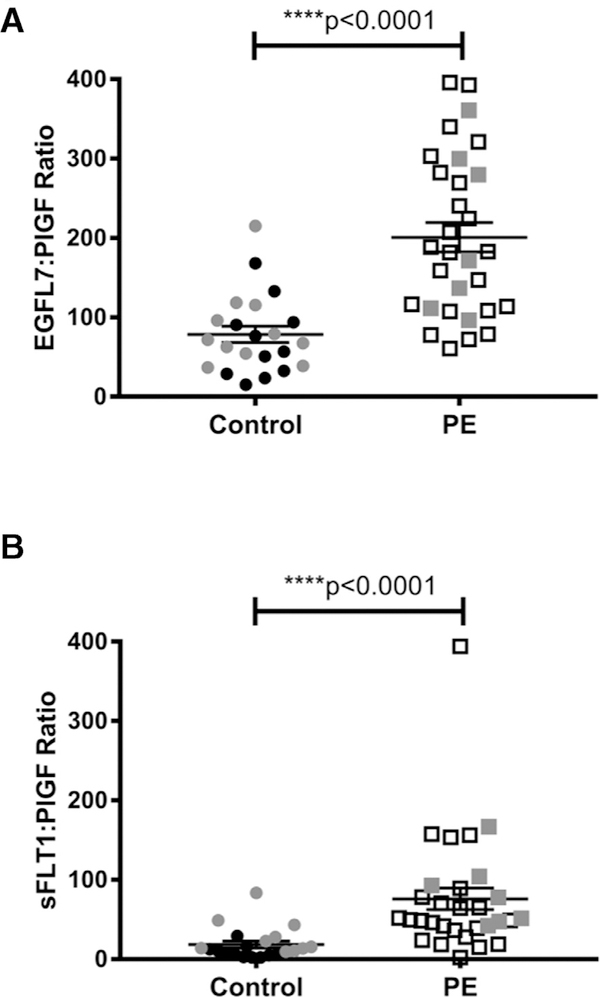
Several endothelial factors, including sFLT-1, sENDOGLIN, and PlGF, were previously shown to be dysregulated in PE.8,21 We measured the concentration of these circulating pro- and antiangiogenic factors in our controls and cases from weeks 35–40. Similar to previous studies, ELISA results demonstrated that concentrations of both sFLT-1 and sENDOGLIN were significantly increased in women with PE compared to age-matched controls (Fig 2, A and B, P < 0.0001 for both), while PlGF levels were significantly lower (Fig 2, C, P = 0.0006). We further validated these results by comparing EGFL7:PlGF ratios with sFLT-1:PlGF ratios for control and PE samples (Fig 3). EGFL7:PlGF ratios were significantly increased in PE samples (mean ratio 201 for PE and 78.50 for control samples; P < 0.0001; Fig 3, A). Similarly, sFLT-1:PlGF ratios showed a significant increase in PE vs controls (mean ratio 75.97 for PE and 18.51 for control samples; P < 0.0001; Fig 3, B), in agreement with a previous study.22
Fig 2.
sFLT-1, sENDOGLIN, and PlGF in preeclampsia-affected pregnancies. Mean (±SE) levels of soluble fms-like tyrosine kinase 1 (sFLT-1) A, soluble endoglin (sENDOGLIN) B, and placental growth factor (PlGF) C, in plasma and serum of healthy pregnant women and pregnant women affected by preeclampsia (PE) between 35 and 40 weeks of pregnancy as measured by ELISA. sFLT-1 (A) and sENDOGLIN (B) concentrations are significantly increased in women affected by PE, compared to age-matched controls (t test, ****P < 0.0001). Levels of PlGF (C) are significantly decreased in PE samples as compared to controls (t test, ***P < 0.001). Gray dots and squares represent plasma samples from control and PE-affected pregnant women, respectively. Black dots and squares represent serum samples from control and PE-affected women, respectively. ELISAs, enzyme-linked immunosorbent assays.
Fig 3.
Ratio of EGFL7 and sFLT-1 to PlGF in healthy and PE-affected pregnant women. Mean (±SE) ratios of EGFL7 to PlGF A, and of sFLT-1 to PlGF B, in plasma and serum of healthy pregnant women and pregnant women affected by PE between 35 and 40 weeks of pregnancy. Both ratios are significantly increased in women affected by PE, compared to age-matched controls (Mann-Whitney test, ****P < 0.0001). Gray dots and squares represent plasma samples from control and PE-affected pregnant women, respectively. Black dots and squares represent serum samples from control and PE-affected women, respectively.
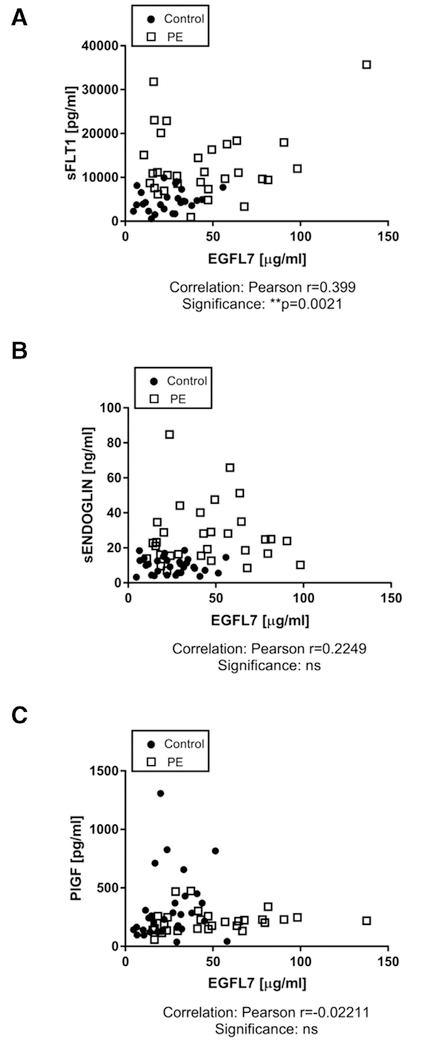
We next investigated if EGFL7 levels correlate with levels of any of these endothelial factors. Pearson correlation analyses showed a significant positive correlation between EGFL7 and sFLT-1 (Pearson r =0.399, P = 0.0021, Fig 4, A). However, there was no significant correlation between EGFL7 and sENDOGLIN, or between EGFL7 and PlGF (Fig 4, B and C).
Fig 4.
Correlation analysis between EGFL7 and known factors dysregulated in PE. ELISA measurements demonstrate that EGFL7 concentrations in healthy pregnant women (control) and women affected by PE positively correlate with levels of A, sFLT-1 and B, sENDOGLIN, reaching statistical significance for sFLT-1 (**P = 0.0021). C, EGFL7 negatively correlates with levels of PlGF, although no statistical significance is met. ELISAs, enzyme-linked immunosorbent assays.
Factor analysis and logistic regression.
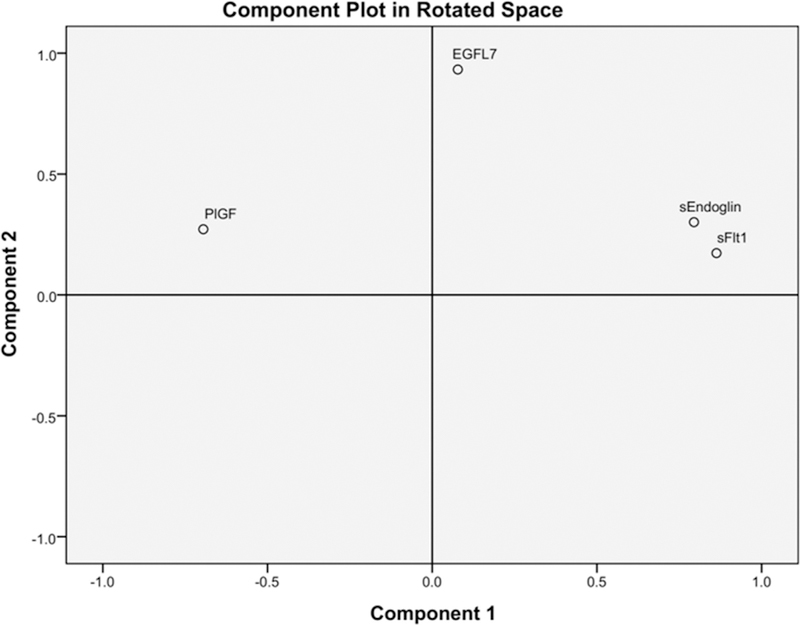
In order to simplify data and reduce the number of variables for subsequent regression analysis, we performed factor analysis. Out of 4 possible components, eigenvalue analysis showed that the first 2 components were able to explain 73% of the cumulative variance (48% and 25%, respectively). The loadings extracted for factors 1 and 2 showed that sFLT-1, sENDOGLIN, and PlGF loaded primarily on factor 1 (loadings: 0.86, 0.79, −0.69, respectively), while EGFL7 loaded primarily on factor 2 (loading: 0.93; Fig 5). These results indicate that the group with the first 3 markers contains partially redundant information, while EGFL7 represents an additional, independent degree of freedom in the data. In multivariate binary logistic regression, both factors were statistically significant (factor 1: P = 0.010, factor 2: P = 0.038) and associated with positive odds ratios, indicating that an increase in both factors would result in a significant increase in the risk of belonging to the PE group (Table III).
Fig 5. Principal component analysis between EGFL7 and known factors dysregulated in PE.
Principal component analysis (PCA), followed by varimax rotation with Kaiser normalization, for EGFL7, sFLT-1, sEN-DOGLIN, and PlGF levels in healthy pregnant women and women affected by PE. sFLT-1, sEN-DOGLIN, and PlGF loaded primarily on factor 1 (loadings: 0.86, 0.79, −0.69, respectively), while EGFL7 loaded primarily on factor 2 (loading: 0.93), indicating that EGFL7 has an independent degree of freedom in the data.
Table III.
Multivariate binary logistic regression analysis
| B | S.E. | Wald | Df | P value | Exp(B) | |
|---|---|---|---|---|---|---|
| Factor 1 | 6.809 | 2.627 | 6.720 | 1 | 0.010 | 905.729 |
| Factor 2 | 3.560 | 1.719 | 4.287 | 1 | 0.038 | 35.160 |
For each variable in the equation: coefficient (B), standard error of B (S.E.), Wald statistic, degree of freedom (Df), P value, odds ratio of the outcome (Exp(B)).
Ex vivo human placental explant cultures.
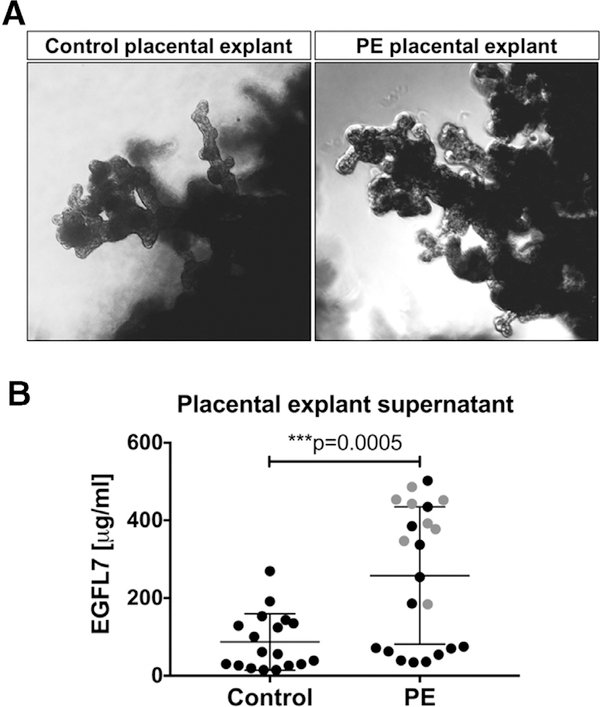
To investigate whether the placenta is a source of circulating EGFL7, we performed ex vivo explant cultures of human villous samples from placentas obtained postdelivery from healthy control women (n = 9; delivery at weeks 37–39 of gestation) or women affected by PE (n = 11, including 2 twin pregnancies; delivery at weeks 35–37 of gestation; Fig 6, A). Culture supernatant was harvested after 24 hours of culture, and EGFL7 levels were determined by ELISA. EGFL7 protein was detected in the supernatant of villi explant samples from both healthy control placentas and PE placentas. Significantly higher levels of EGFL7 were released from PE placental villi explants (mean concentration 257.99 μg/mL) than control placental villi explant samples (mean concentration 86.76 μg/mL; Fig 6, B). Our results indicate that placental villi are a source of circulating EGFL7 detected in healthy control women and, at elevated levels, in women affected by PE.
Fig 6.
Ex vivo placental villous explant cultures. A, Representative villous explant samples from control placenta (left) and PE placenta (right) at 24 hours of ex vivo culture. B, ELISA assays performed on supernatant samples from duplicates of 24-hour villous placental explant cultures of healthy pregnant women (controls: n = 9, 35–40 weeks of gestation) and pregnant women affected by preeclampsia (PE: n = 11, 37–39 weeks of gestation). PE samples have significantly increased levels of EGFL7 in placental explant culture supernatants as compared to healthy controls (t test, ***P < 0.001). Gray dots in PE samples represent values obtained from villous explant cultures from twin placentas. ELISAs, enzyme-linked immunosorbent assays.
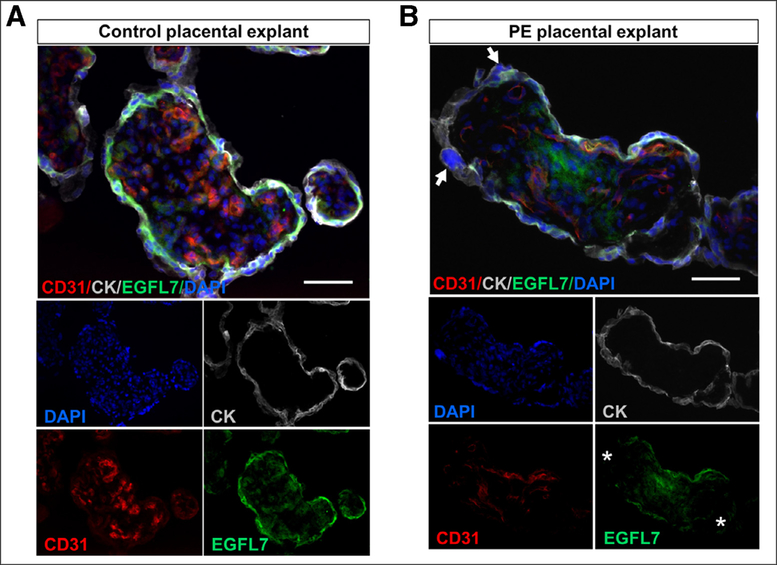
We next examined EGFL7 expression profiles on placental villi explants after 24 hours in culture by performing triple immunofluorescent staining for EGFL7, the pan-endothelial marker CD31, and the pan-trophoblast marker CYTOKERATIN (Fig 7). In cross-sections of terminal placental villi from healthy control women, EGFL7 was detected on endothelial cells in fetal capillaries, and on villous cytotrophoblast and syncytiotrophoblast (Fig 7, A). The expression profile in 24-hour explants was similar to those detected in freshly isolated samples (not shown and17). In contrast, EGFL7 expression appeared to be lower in the villous cytotrophoblast and syncytiotrophoblast layers of PE placental explants (Fig 7, B). In addition, we observed an increased incidence of syncytial knots which also presented an increase in size, and irregular deposition of cytokeratin in terminal villi from PE placentas (Fig 7, B), consistent with syncytiotrophoblast lesions and dysfunction.23–25
Fig 7.
Immunofluorescence images of sections from 24-hour villous placental samples. Representative images depicting terminal villi from control placentas A, and from PE placentas B, after 24 hours in ex vivo explant culture. Sections were triple-stained with antibodies for EGFL7, CD31 (pan-endothelial marker) and CYTOKERATIN (pan-trophoblast marker). Arrow: syncytial knot; asterisk: patchy localization of EGFL7. Scale bar = 500 μm.
DISCUSSION
In the present study, we show that levels of the secreted angiogenic factor EGFL7 are undetectable in blood of nonpregnant women, that they increase during normal pregnancy and decline again to a mean value of 25 μg/mL at 35–40 weeks of gestation, a trend similar to that observed for PlGF.8 In contrast, levels of EGFL7 are significantly increased in the circulation of pregnant women affected by PE, with a mean value of 43.59 μg/mL. Interestingly, while almost all samples from normal pregnancies had values of EGFL7 below 45 μg/mL, samples from patients affected by PE could be divided into 2 subgroups: one group of 22 samples in which EGFL7 levels remained in the same range as the controls, and a second group of 16 samples in which EGFL7 levels were between 45 and 140 μg/mL. Analysis of our data did not reveal a significant correlation between EGFL7 levels in PE patients above 45 μg/mL and clinical features, such as IUGR. Future studies will be required to investigate the possibility that higher levels of EGFL7 may identify specific subpopulations of PE patients.
Over the last decades, several groups have correlated a disruption in the balance between proangiogenic and antiangiogenic factors with the manifestation of PE8; however, their ability to reliably predict PE has not been proven.12 More recently, a study reported on a novel role for the placenta-derived hormone, ELA, in controlling cardiovascular health during pregnancy.26 The authors show that dysregulation of ELA promotes PE, and they suggest that ELA could be used for early diagnosis and therapeutic treatment of the disease. Our finding that levels of circulating EGFL7 are increased in PE patients suggests that EGFL7 may contribute to the imbalance of pro- and antiangiogenic factors characterizing PE7,27,28 and that it may represent a novel additional diagnostic factor for PE that can be detected in blood. In support of this hypothesis, we found that circulating levels for the antiangiogenic factors sFLT-1, sENDOGLIN, and the proangiogenic factor PlGF were dysregulated in our PE samples as well and followed the same trend as previously reported.6–8,27,29–31 Indeed, our PCA data demonstrated that dysregulation in EGFL7 in addition to changes in other circulating factors suggests an increased risk for PE.
Since changes in several pro- and antiangiogenic factors can be detected prior to the onset of the clinical manifestations of the disorder, the development of platforms to quantify their circulating levels in maternal blood has been proposed for an early diagnosis and management of the disease. Tests measuring circulating levels of sFLT-1 and PlGF in combination with clinical assessment have been proposed to improve the differential diagnosis ability for women with suspected PE.29 However, a large-scale prospective study failed to demonstrate that these factors, in combination with clinical biometrics, are reliable predictors for the onset of PE.12 Instead, the ratio of sFLT-1/PlGF has been demonstrated to be a better indicator of risk for PE30 and suggests that using a combination of several circulating factors is advantageous over any 1 factor alone for diagnosing PE.31 In support we demonstrate that, similar to sFLT-1/PlGF ratios, the ratios of EGFL7/PlGF are significantly higher in pregnant women affected by PE compared to healthy controls, suggesting that a combination of both ratios may help distinguishing normal from pregnancies affected by PE.
To further assess if EGFL7 is a potential prognostic biomarker of PE and explore its relationship with other PE-related factors, we performed PCA-based factor analysis. Our results demonstrate that EGFL7 distributes into a separate factor, different from sFLT-1, sEN-DOGLIN, and PlGF, indicating that EGFL7 represents a novel independent secreted factor for the diagnosis of PE. In addition, multivariate binary logistic regression analysis suggests that the factor related almost exclusively to EGFL7 is a significant predictor of PE. Taken together, these results demonstrate that, among the 4 biomarkers, EGFL7 represents an independent dimension of information, which provides additional predictive power in forecasting group membership.
Of note, EGFL7 expression is significantly elevated in several human epithelial tumors, including hepatocellular carcinoma, breast cancer, lung cancer malignant carcinoma, and cancers in the female reproductive system such as ovarian and uterine cervical cancer.32,33 Furthermore, EGFL7 may be a predictive biomarker for tumor stage, metastasis, and efficiency of chemotherapy in certain epithelial cancers.34,35 Interestingly, while EGFL7 is largely an endothelial-restricted factor, it is also expressed by tumor cells, suggesting a correlation between its expression and alterations of the physiology of epithelial cells.
Importantly, our ex vivo placental explant cultures established that the placenta is a source of circulating EGFL7, and that increased levels of EGFL7 are released from placentas of women affected by PE. Our findings that EGFL7 is prominently expressed in villous cytotrophoblast and syncytiotrophoblast and is reduced in villi from PE placentas concomitant with evidence for syncytiotrophoblast lesions, suggest an increased mobilization of EGFL7 from placental villous trophoblast. This conclusion is in agreement with placental origins of PE, where oxidative and other potential stress factors lead to villous trophoblast dysfunction followed by the release of pro- and antiangiogenic factors.1,2,36 We reason that reduced expression of EGFL7 in patients affected by PE19 and its reduced incorporation into the extra cellular matrix (ECM) of villous cytotrophoblast and syncytiotrophoblast may contribute to the instability of villous trophoblast, resulting in extensive shedding and release of ECM-bound EGFL7 into the maternal circulation. Our explant culture studies suggest that it is likely that villous trophoblast cells are the principal source of circulating EGFL7 in pregnant women and its increased levels found in patients affected by PE. This view is in agreement with previously reported damage of villous trophoblast by hypoxia and the release of syncytiotrophoblast membrane fragments into the maternal blood stream.32 However, we cannot exclude the maternal vasculature under the stress of pregnancy as an additional source.
Together, our data strongly suggest that EGFL7 is a reliable biomarker for the identification of PE and, in combination with other angiogenic factors, could be exploited to increase diagnostic specificity. Future prospective longitudinal studies will clarify its prognostic value for a risk to develop PE. Based on our observation that EGFL7 is an independent factor for predicting membership to the PE group and that it provides additional information with respect to the other known predictive angiogenic factors including sFLT-1, sENDOGLIN, and PlGF, we anticipate that introducing EGFL7 as a predictable biomarker into clinical practice will significantly increase the robustness and accuracy of PE diagnosis. This result will be of immense value to manage a major health threat for pregnant women.
CONCLUSIONS
PE is a leading cause of maternal and perinatal morbidity and mortality. The identification of prognostic biomarkers for timely diagnosis of this pregnancy complication is of major impact. In this respect, a multivariable approach appears advantageous. Our study demonstrates that EGFL7 can be measured in blood during pregnancy and that its levels are increased in PE. PCA analysis shows that EGFL7 distributes into a separate factor, different from other angiogenic factors, suggesting its role as a novel independent secreted factor for the diagnosis of PE. Altogether, our results identify EGFL7 as a unique factor that is highly correlated to PE and proposes its use in combination with the other known biomarkers for more accurate diagnosis and timely management of PE.
Supplementary Material
AT A GLANCE COMMENTARY.
Massimiani M, et al.
Background
Preeclampsia is a pregnancy disorder characterized by an imbalance of circulating pro- and antiangiogenic factors. Their dosage in blood has been proposed as a tool for PE diagnosis. We have recently identified alteration of EGFL7 expression in PE placentas, and demonstrated its role in the regulation of trophoblast migration.
Translational Significance
Here, we demonstrate that EGFL7 can be measured in blood during pregnancy and that its levels are increased in PE. PCA analysis shows that EGFL7 distributes into a separate factor, different from other angiogenic factors, suggesting a role as a novel independent secreted factor for the diagnosis of PE.
ACKNOWLEDGMENTS
Conflict of Interest: The authors have read the journal’s policy on disclosure of potential conflicts of interest and declare no conflict of interest.
We thank Nataki Douglas and Audrey Merriam for their help with providing placental samples for optimizing culture conditions of placental explants. This work was supported by the March of Dimes Foundation #6-FY14–411 (L.C. and H.S.); the National Institutes of Health grant #R01 HL082098 (H.S.) and grant #T32 HD060600 (LBA); the Dr. Robert and Veronica Atkins Foundation Curriculum in Metabolic Diseases (C.S.B.S); the ASM, Associazione Studio Malformazioni onlus (L.C. and S.S.).
All authors have read the journal’s authorship agreement and the manuscript has been reviewed by and approved by all named authors.
Abbreviations:
- EGFL7
epidermal growth like domain-7
- PE
preeclampsia
Footnotes
ETHICS STATEMENT
The study respected the principles expressed in the Declaration of Helsinki and was approved by the Bioethical Committee of the Catholic University of Sacred Heart of Rome, Italy (approval number P575(A1420)/CE/2008), and by the Institutional Review Board at Weill Cornell Medical College (IRB protocol #1011011376 and 9811003560).
SUPPLEMENTARY DATA
Supplementary data related to this article can be found at doi:10.1016/j.trsl.2018.12.004.
REFERENCES
- 1.Redman CW, Staff AC. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. Am J Obstet Gynecol 2015;213:S9–S11. [DOI] [PubMed] [Google Scholar]
- 2.Huppertz B Maternal-fetal interactions, predictive markers for preeclampsia, and programming. J Reprod Immunol 2015;108:26–32. [DOI] [PubMed] [Google Scholar]
- 3.American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–3. [DOI] [PubMed] [Google Scholar]
- 4.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy 2003;22:143–8. [DOI] [PubMed] [Google Scholar]
- 5.Raymond D, Peterson E. A critical review of early-onset and late-onset preeclampsia. Obstet Gynecol Surv 2011;66:497–506. [DOI] [PubMed] [Google Scholar]
- 6.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 2011;123:2856–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hod T, Cerdeira AS, Karumanchi SA. Molecular mechanisms of preeclampsia. Cold Spring Harb Perspect Med 2015;5:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004;350: 672–83. [DOI] [PubMed] [Google Scholar]
- 9.Rolfo A, Attini R, Nuzzo AM, et al. Chronic kidney disease may be differentially diagnosed from preeclampsia by serum biomarkers. Kidney Int 2013;83:177–81. [DOI] [PubMed] [Google Scholar]
- 10.Masuyama H, Nobumoto E, Okimoto N, et al. Superimposed preeclampsia in women with chronic kidney disease. Gynecol Obstet Invest 2012;74:274–81. [DOI] [PubMed] [Google Scholar]
- 11.Perni U, Sison C, Sharma V, et al. Angiogenic factors in superimposed preeclampsia: a longitudinal study of women with chronic hypertension during pregnancy. Hypertension 2012;59: 740–6. [DOI] [PubMed] [Google Scholar]
- 12.Kenny LC, Black MA, Poston L, et al. Early pregnancy prediction of preeclampsia in nulliparous women, combining clinical risk and biomarkers: the Screening for Pregnancy Endpoints (SCOPE) international cohort study. Hypertension 2014;64: 644–52. [DOI] [PubMed] [Google Scholar]
- 13.Nichol D, Shawber C, Fitch MJ, et al. Impaired angiogenesis and altered Notch signaling in mice overexpressing endothelial Egfl7. Blood 2010;116:6133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitch MJ, Campagnolo L, Kuhnert F, et al. Egfl7, a novel epidermal growth factor-domain gene expressed in endothelial cells. Dev Dyn 2004;230:316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campagnolo L, Leahy A, Chitnis S, et al. EGFL7 is a chemoattractant for endothelial cells and is up-regulated in angiogenesis and arterial injury. Am J Pathol 2005;167:275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacko LA, Hurtado R, Hinds S, et al. Altered feto-placental vascularization, feto-placental malperfusion and fetal growth restriction in mice with Egfl7 loss of function. Development 2017;144:2469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lacko LA, Massimiani M, Sones JL, et al. Novel expression of EGFL7 in placental trophoblast and endothelial cells and its implication in preeclampsia. Mech Dev 2014;133:163–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Junus K, Centlow M, Wikstrom AK, et al. Gene expression profiling of placentae from women with early- and late-onset preeclampsia: down-regulation of the angiogenesis-related genes ACVRL1 and EGFL7 in early-onset disease. Mol Hum Reprod 2012;18:146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massimiani M, Vecchione L, Piccirilli D, et al. Epidermal growth factor-like domain 7 promotes migration and invasion of human trophoblast cells through activation of MAPK, PI3K and NOTCH signaling pathways. Mol Hum Reprod 2015;21:435–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller RK, Genbacev O, Turner MA, et al. Human placental explants in culture: approaches and assessments. Placenta 2005;26:439–48. [DOI] [PubMed] [Google Scholar]
- 21.Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med 2006;355:992–1005. [DOI] [PubMed] [Google Scholar]
- 22.Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med 2016;374:13–22. [DOI] [PubMed] [Google Scholar]
- 23.Loukeris K, Sela R, Baergen RN. Syncytial knots as a reflection of placental maturity: reference values for 20 to 40 weeks’ gestational age. Pediatr Dev Pathol 2010;13:305–9. [DOI] [PubMed] [Google Scholar]
- 24.Weel IC, Baergen RN, Romão-Veiga M, et al. Association between placental lesions, cytokines and angiogenic factors in pregnant women with preeclampsia. PLoS One 2016;11:e0157584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Redman CW, Sargent IL, Staff AC. IFPA senior award lecture: making sense of preeclampsia—two placental causes of preeclampsia? Placenta 2014;35:S20–5. [DOI] [PubMed] [Google Scholar]
- 26.Ho L, Van Dijk M, Chye STJ, et al. ELABELA deficiency promotes preeclampsia and cardiovascular malformations in mice. Science 2017;357:707–13. [DOI] [PubMed] [Google Scholar]
- 27.Maynard SE, Karumanchi SA. Angiogenic factors and preeclampsia. SeminNephrol 2011;31:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pijnenborg R, Anthony J, Davey DA, et al. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol 1991;98:648–55. [DOI] [PubMed] [Google Scholar]
- 29.Benton SJ, Hu Y, Xie F, et al. Angiogenic factors as diagnostic tests for preeclampsia: a performance comparison between two commercial immunoassays. Am J Obstet Gynecol 2011;205:469.e1–469.e8. [DOI] [PubMed] [Google Scholar]
- 30.Verlohren S, Herraiz I, Lapaire O, et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol 2012;206:58.e1–58.e8. [DOI] [PubMed] [Google Scholar]
- 31.Hagmann H, Thadhani R, Benzing T, et al. The promise of angiogenic markers for the early diagnosis and prediction of preeclampsia. Clin Chem 2012;58:837–45. [DOI] [PubMed] [Google Scholar]
- 32.Fan C, Yang LY, Wu F, et al. The expression of Egfl7 in human normal tissues and epithelial tumors. Int J Biol Markers 2013;28:71–83. [DOI] [PubMed] [Google Scholar]
- 33.Nichol D, Stuhlmann H. EGFL7: a unique angiogenic signaling factor in vascular development and disease. Blood 2012;119:1345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamauchi M, Fukuda T, Wada T, et al. Expression of epidermal growth factor-like domain 7 may be a predictive marker of the effect of neoadjuvant chemotherapy for locally advanced uterine cervical cancer. Oncol Lett 2016;12:5183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang C, Wang YL, Sun D, Zhu XL, Li Z, Ni CF. Increased expression of epidermal growth factor-like domain-containing protein 7 is predictive of poor prognosis in patients with hepatocellular carcinoma. J Cancer Res Ther 2018;14:867–72. [DOI] [PubMed] [Google Scholar]
- 36.Huppertz B Placental origins of preeclampsia: challenging the current hypothesis. Hypertension 2008;51:970–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.