Abstract
The dynamics of central nervous system (CNS) function rely upon omnidirectional communication among CNS cell types. Extracellular vesicles (EVs) have emerged as key mediators of this communication and are actively involved in response to CNS injury, mediating inflammatory response and inflammation-related neuroprotection as they display dual beneficial and detrimental roles. Neuroimmune interactions include communication between neurons and microglia, the resident macrophages within the CNS, and these interactions are a critical mediator of healthy brain functions, mounting an inflammatory response and disease pathogenesis. This review aims to organize recent research highlighting the role of EVs in health and neurodegenerative disorders, with a specific focus on neuroimmune interactions between neurons and glia in Alzheimer’s disease.
Keywords: Alzheimer’s disease, Astrocyte, Exosome, Extracellular vesicle, Microglia, Myelin, Neurodegeneration, Neuron, Oligodendrocyte
CNS Cells and EVs in Health and Disease
Neurons and glial cells (see Glossary) orchestrate CNS homeostasis through multiple mechanisms of transcellular communication known as “neuroimmune interactions”. These interactions range from local cell-to-cell direct contact to the use of soluble factors for short and long-distance transfer of biological material. As described decades ago, extracellular vesicles (EVs) appear to be potent mediators of intercellular communication and have garnered substantial attention as a fundamental component of neuroimmune interaction. The field of EV biology is still in its nascent stage and many questions about EVs biogenesis, release and action remain to be explored. Despite these unanswered questions, there is accumulating evidence demonstrating the roles of EVs in maintaining CNS homeostasis and contributing to neurodegenerative disease pathology that will be discussed in this review.
An important aspect of EV function is their ability to facilitate local and remote communication within and outside of the CNS, as EVs originating from the brain can be found in peripheral circulation [1, 2]. This has sparked major interest in exploring the potential of circulating EVs to serve as biomarkers for CNS disease. Enrichment for neuron-derived-exosomes using antibodies against L1 cell adhesion molecule (L1CAM) or neural cell adhesion molecule 1 (NCAM1) expressed in neurons [3] allows for the isolation and enrichment of CNS-derived EVs from peripheral biospecimens which can be used to assess cerebral neuroimmune status. Thus, they can play an important role in the diagnosis of CNS disorders, including neurodegenerative diseases, for instance by allowing the assessment of amyloid-β peptide (Aβ) [4, 5] and microtubule-associated protein tau isoforms [6]. The proteomic and transcriptomic profiles of isolated CNS EVs can help to advance our understanding of the mechanisms of neuroimmune communication and shed light upon currently unknown aspect of these interactions.
EVs are commonly divided into subgroups based on size. These groups include microvesicles (MVs, 100nm to 1um in diameter) originating from the plasma membrane, exosomes (30 to 150nm in diameter) originating from intracellular multivesicular bodies (MVBs), and apoptotic bodies (0.8 to 5um in diameter) originating from apoptotic cells [7] (see Box 1). Interpreting EV-related data is difficult since current EV isolation techniques cannot make a clear distinction between exosomes, MVs, and often-contaminated lipoprotein complexes. Nevertheless, EVs have the capacity to load and transport DNA, RNA, miRNA, proteins and lipids (reviewed in [8] and [9]) with variations in cargo content according to cell type [10], the state of the cell [11–13], and also age [14], making EVs novel fine-tuning actors of neuroimmune communication pathways (see Box 1 for more details).
Box 1. Extracellular Vesicles
The three main classes of extracellular vesicles are exosomes, microvesicles, and apoptotic bodies [71, 72]. Exosomes are the smallest of these, ranging in size 30–150nm, and are generated by exocytosis of multivesicular bodies (MVBs) [71–73]. Microvesicles range in size 100–1000nm and are generated through bubbling/budding of the plasma membrane [71–73]. Apoptotic bodies are also generated through budding of the plasma membrane, although exclusively generated by cells undergoing apoptosis.
Biogenesis of EVs is controlled by several molecular machineries, the best described mechanism being the endosomal sorting complex required for transport (ESCRT) [74]. The ESCRT mechanism is composed of approximately thirty proteins which assemble into four complexes (ESCRT-0, -I, -II, -III) involved in loading and budding of MVBs. EVs can also be generated independently of ESCRT machinery mechanisms, relying instead on membrane associated proteins like tetraspanins or intracellular enzymes. Tetraspanins and other lipid raft associated proteins serve as surface markers for EVs, particularly exosomes.
Within the CNS, neurons, glia, and endothelial cells all secrete EVs, which have been shown to play significant functional roles in cellular communication, due in part to their broad range of possible contents. The contents and relative proportion of EV types secreted depends on cellular identity and physiological context, and generally reflect the origin a cell’s cytoplasmic composition. The contents ranges from cell surface receptors, mitochondrial and cytosolic proteins, and metabolic enzymes to microRNAs and mRNAs [20]. More specifically, exosomes collected from primary neuron mammalian tissue culture contain canonical EV markers such as ALIX, flotillin 2, AMPA receptor subunits and cell adhesion molecules. Oligodendrocyte derived EVs contain myelin proteins, proteins associated with protection against oxidative stress and RNA cargo. Microglia and astrocytes shed EVs enriched in inflammatory factors [75] in response to activation, or enriched in miRNAs associated with neurotrophic signaling during homeostasis. In all cases, EVs are primarily enriched with small RNAs, and are rarely found to contain DNA. A growing body of evidence suggests that uptake of EVs proceeds through fusion with the plasma membrane of the recipient cell, or through one of several forms of endocytosis [76]. This includes clathrin-mediated endocytosis, caveolin-mediated endocytosis, lipid raft-mediated endocytosis, macropinocytosis, and phagocytosis. Which mechanism is employed depends upon cell type, physiologic state and cell ligand presentation.
Outstanding question box:
Do different EV subtypes contribute equally to normal CNS function and disease?
How do EVs contribute to the normal aging process of the CNS?
What methods and assay models could improve the isolation and evaluation of EV biogenesis, secretion, transport and uptake?
How significant is the effect of peripheral EVs on CNS function and vice versa?
Can CNS EV biogenesis, secretion or uptake be targeted to treat neurodegenerative disorders?
Are there genetic risk factors associated with EV function and do these increase the risk of neurodegenerative diseases and age-related cognitive dysfunction?
While the physiological role of EVs in health, aging, and disease is still under investigation, several functions have been described in maintaining CNS health, including waste elimination[15], maintenance of myelin [16], and synaptic vesicle release [17]. Conversely, EVs have also been implicated in contributing to neurodegenerative disease spread, particularly in diseases where pathology is dictated in part by neuroimmune mechanisms, such as Alzheimer’s disease (AD) [18] and aging [19]. This review aims to synthesize recent research highlighting the role of EVs in health and neurodegenerative disorders, with a specific focus on neuroimmune interactions between neurons and glia in Alzheimer’s disease.
Neuroimmune Communication with EVs
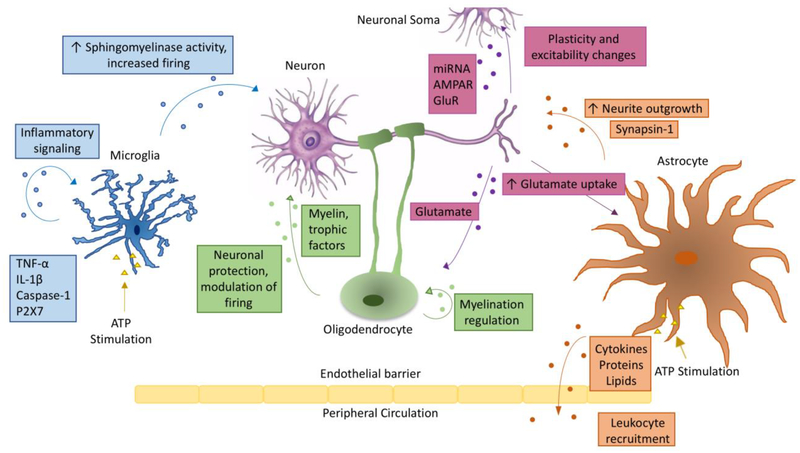
The intersection of immunology and neuroscience has been receiving increasing interest in recent years, as more research reveals multiple functional connections between the immune and nervous systems. Understanding this convergence is of particular importance in the CNS where microglia, the resident immune cells of the brain, closely interact with neurons during development and adulthood for the maintenance of brain homeostasis and innate immune response to CNS insults. Communication between neurons and microglia is bidirectional; neurons sense and respond to inflammatory signals and microglia counter-sense and respond to neuronal activity and neuropathic changes. This communication can be mediated by secreted soluble factors, contact-dependent mechanisms, as well as EVs released from neurons and microglia (Figure 1) [20], described in details in the next section.
Figure 1: Extracellular vesicle crosstalk within the CNS.
Schematic representation of extracellular vesicle transfer between different cell types within the central nervous system. Arrows indicate the direction of transfer, and are labeled with the contents of EVs commonly found for that cell-to-cell communication pathway and with some of the functional roles identified for the pathway. EVs can transfer contents between various pairs among the cell types depicted in the figure, i.e. neurons, microglia, astrocytes and oligodendrocytes. See text for complete details. ATP stimulation is included for microglia and astrocytes, whose cargo is modulated depending on activation state.
Classical neuroimmune cell-to-cell communication pathways rely on membrane contact between glia and neurons. Examples of these interactions include the neuron-specific transmembrane ligand cluster differentiation 200 (CD200) and fractalkine (CX3CL1), and their microglia-specific receptors CD200 receptor (CD200R) and fractalkine receptor (CX3CR1), respectively [21]. Both signaling pathways aim to keep microglia inactivated, in a surveying/homeostatic phenotype [22]. CD200 on the neuronal plasma membrane relies on direct cellular contact with CD200R expressed in microglia, whereas CX3CL1 in neurons is important for cellular adhesion, and its soluble form acts as a ligand for CX3CR1 in microglia. Chemokines produced by neurons and glia for similar functional communication include the neuronal chemokine (C-C motif) ligand 2 (CCL2) interacting with microglial chemokine (C-C motif) receptor 2 (CCR2), in order to trigger microglial chemotaxis. The Exocarta database, which outlines the protein content of EVs, shows the presence of CD200 in human exosomes isolated from neuroblastoma cancer cells, CCL2 in human exosomes isolated from mesenchymal stem cells, and CX3CL1 in mouse exosomes isolated from fibroblasts. EVs have the potential to express proteins on their membranes. Although intriguing, direct evidence reporting these molecules in CNS EVs is currently missing. Interestingly, apoptotic bodies express CX3CL1 and functionally attract phagocytes in the periphery [23]. The hypothesis of EV-based interaction between neurons and microglia mediated by these neuroimmune pathways (CD200/CD200R, CX3CL1/CX3Cr1, or CCL2/CCR2) needs further establishment and confirmation. It is also conceivable that neuron-derived EVs may play significant roles in the maintenance of the homeostatic phenotype of microglia through yet unrevealed canonical neuroimmune communication pathways beyond the classical cellular contact mechanism.
Recently, the homeostatic gene signature of microglia has been shown to be controlled by transforming growth factor (TGF)-β [24]. Beside the classical immune-related effect of TGF-β, it is now established that it is a master regulator of microglia identity and transcriptomic signature. Interestingly, TGF-β is present in EVs from cancer cells including gliobastoma, and has been involved in immune regulation [25, 26]. The question arises whether TGF-β in CNS EVs contributes to the control of microglial transcriptomic signature. The roles of CNS EVs in the modulation of microglial phenotype requires further investigation.
It has been established that the initiation, propagation, and resolution of an inflammatory response to CNS injury or disease relies upon soluble factors including cytokines and miRNAs, both of which have been demonstrated to be contained by EVs. Indeed, cytokines have been found encapsulated in EVs in multiple human cells and tissues, ranging from monocytes to cervical explants. Encapsulated cytokines include, but are not limited to, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-10, and CCL2 [27]. Because cytokines have classically been considered to be released into the extracellular space in large quantities, the quantity of EV cytokines appears to be small by comparison. However, this latter mechanism of cytokine transfer may be quite impactful since they remain concentrated in EVs and are directly delivered to recipient cells instead of being dissipated in a large extracellular space. One mechanism mediating cytokine release from EVs to target cells is through the activation of P2X purinoceptor 7 (P2X7R) present on microglia-derived EVs containing IL-1β [28]. When target cells release ATP in response to inflammatory or other danger signals, it will act on circulating microglia-derived EVs expressing P2X7R on their surface and induce IL-1β efflux from EVs into the extracellular space surrounding target cells [28]. More work is needed to describe other mechanisms mediating the release of EV content to target cells.
The cytokine profile in the EV cargo is strongly modulated upon immune stimulation such as lipopolysaccharide (LPS) injection [27] and ATP stimulation [29], indicating a highly plastic and adaptive system of communication through immune activation. We will mainly describe LPS and ATP signaling as the most extensively studied ligands inducing EV release. Several rodent studies have demonstrated that upon immune activation by LPS, microglia release vesicles enriched in IL-1β and miR-155 [30], or in TNF-α and IL-6 [31], presenting the capacity to activate microglia both in vitro and in vivo (Figure 1). LPS-stimulated microglia-derived EVs can propagate inflammatory signals through the activation of other microglia, as shown by the increased expression of CD68, IL-1β, IL-6, inducible nitric oxide synthase and cyclooxygenase-2 in microglia-derived EVs. These EVs can also activate astrocytes, leading to an upregulation of mRNA transcripts of Il1b, Il6, Nos2 and Ptgs2 in rodent astrocytes [32], highlighting a role for microglia-derived EVs in propagating glial inflammation in the CNS.
ATP is another powerful stimulant of EV shedding from microglia and astrocytes by the way of purinergic receptor stimulation [28]. ATP recognition by P2X7R on microglia results in the release of EVs enriched in IL-1β and gyceraldehyde-3-phosphate dehydrogenase (GAPDH), which are involved in propagating and regulating neuroinflammation [28, 33]. In addition, ATP stimulation is able to skew microglial EV content towards cellular metabolism-associated and autophagosome/lysosome-associated proteins, and strongly impact the activation state of astrocytes [29]. Microglia and astrocytes shed EVs in response to the stimulation of P2X7R, sensing the excessive ATP in the extracellular environment following tissue injury, highlighting their innate response to danger-associated pattern molecules.
In addition, EVs can transfer peripheral inflammatory conditions to the CNS. For example, serum-exosomes from LPS-injected donor mice are able to induce CNS inflammatory response after their intravenous injection into recipient mice [34]. Within the recipient mouse, the authors observed microglial activation, gliosis, increased pro-inflammatory cytokines IL-6 and TNF-α at protein and mRNA levels, and inflammatory miR-155 production. The serum-derived exosomes from the donor mice contained elevated levels of several inflammation-associated miRNA including miR-155, 15a, 15b and 21, highlighting the potential role of exosomal miRNA as mediators of inflammation. They showed that microglia absorb the labeled serum-derived exosomes from LPS-treated donor mice after intravenous injection into recipient mice, indicating that peripherally administrated exogenous exosomes can migrate into the CNS [34]. However, the purification method for exosome isolation is of an intermediate specificity according to the recently published methodological classification [35]. This only allows an intermediate recovery of the exosomes and may contain free proteins, ribonucleoproteins and lipoproteins. Validation using a more specific approach is needed to ensure that these CNS effects are derived from EV cargo molecules. EV communication between the brain and the periphery appears bidirectional since serum-derived exosomes from LPS-administered donor mice have been shown to enter the CNS of donor mice and be taken up by microglia in the brain as discussed above [34], and EVs originating from all the CNS cell types can be detected in the blood, presumably after crossing the blood brain barrier (BBB) [36]. The precise mechanisms by which EVs are transported across the BBB are in need of further exploration [37].
EV-mediated Crosstalk in CNS Homeostasis
EV-mediated crosstalk between CNS cells is mediated by EV cargo content as well as direct surface contact, and occurs in an omnidirectional manner, where all cell types influence and communicate with one another. This section describes the functional role of EVs relative to CNS cell types in a homeostatic state.
Neuron-derived EVs
In rodents, primary cultured cortical neurons release EVs from the somatodendritic compartment and synaptic terminals [38]. EVs released from neurons following depolarization are enriched in common EV markers such as ALG-2-interacting protein X (ALIX / PDCP6IP) and flotillin-2, but also contain glutamate receptor subtypes such as AMPA receptors, microtubule-associated protein 1B (MAP1B) and neurite associated miRNAs such as miR-124 and miR-1973, suggesting a role for neuron-derived EVs in miRNA export, the modulation of neuronal excitability, and neurotransmitter release [39, 40]. Interestingly, EV-mediated release of specific miRNAs from human neuroblasts following potassium chloride-induced membrane depolarization is associated with a decreased intracellular level of the same miRNAs, suggesting neuronal EV release as a mechanism of regulation of intracellular miRNA content [41]. The internalization of miRNA-containing EVs from neurons upregulated the excitatory amino acid transporter 2 (EAAT2), a modulator of glutamate uptake in synapses [42], suggesting the possibility that EV release is intimately related to the control of neuronal excitability through regulation of astrocytes. These neuron-derived EVs are taken up by other neurons and glial cells and can modulate the recipient cells’ function in multiple ways. For example, they facilitate microglial removal of degenerating neurites. This has been shown by the use of murine microglial cell line MG6 cells and exosomes derived from depolarized rat neuroblastoma cell line PC12 cells, which accelerates neurite removal from co-cultured PC12 cells. Interestingly, MG6 cells in the relevant study were found to have increased expression of complement component 3 [43]. However, these studies are conducted using immortalized cell lines from different species and further validation is necessary in a more physiologically relevant experimental design.
Oligodendrocyte-derived EVs
Several lines of evidence showed the release of EVs from oligodendrocytes. One of the roles of oligodendrocytic EVs seems to be to protect neurons. Oligodendrocytes contain MVBs at peri-axonal sites, which fuse with the plasma membrane and release EVs following glutamatergic signaling from neurons [40, 44]. Glutamate is able to induce Ca2+ influx in oligodendrocytes, followed by activation of the small GTPase Rab35, leading to EV release [40]. In addition, a transwell system in which oligodendrocytes and neurons are partitioned demonstrated that treatment of neurons with the GABAA receptor antagonist bicuculline enhances the release of EVs from oligodendrocytes. This suggests that EV secretion is enhanced by excitatory synaptic activity [38, 40]. Oligodendrocyte-derived EV uptake by neurons, as described in the transwell system above, was shown to improve neuronal viability during oxygen and glucose deprivation. Additionally, neurons exposed to oligodendrocyte-derived exosomes displayed increased firing rate as well as altered gene expression [45]. In addition to canonical exosomal markers such as Alix, tumor susceptibility gene 101 (Tsg101), and tetraspanins, oligodendrocyte-derived EVs are enriched in myelin proteolipid protein, 2’,3’-cyclic nucleotide 3’- phosphodiesterase, myelin associated glycoprotein, and myelin oligodendroglial glycoprotein. Oligodendrocyte-derived EVs have been shown to be involved in the management of oxidative stress via transfer of human superoxide dismutase and catalase coinciding with the observation that neuronal uptake of oligodendrocyte EVs is associated with enhanced stress resistance following ischemic challenge [16]. Additionally, oligodendrocyte-derived EVs seem to participate in the clearance of myelin debris mediated by microglia. Several evidences highlighted the role of microglia in myelin debris removal through CX3CR1 or IFN-β-dependent pathways [46, 47]. Interestingly, oligodendrocyte-derived EVs are taken up by microglia, followed by trafficking to lysosomes for degradation [15], suggesting a role of microglia in clearing oligodendroglial myelin membrane debris though their EV uptake.
Microglia-derived EVs
EVs can play a significant role in neuroimmune communication not only through cargo delivery but also their contact with target cells. Microglia-derived EVs have also been implicated in influencing neurite outgrowth, modulating neuronal activity, and coordinating an innate immune response with other microglia [28, 48]. Antonucci and colleagues demonstrated that microglia-derived EVs can affect neurotransmission, inducing a dose-dependent increase in miniature excitatory postsynaptic current (mEPSC), through the direct action of surface components of EVs rather than their cargo content [17]. This is suppressed by pretreatment of microglial-derived EVs with Annexin A5, suggesting the importance of phosphatidylserine present on the EV membrane. The beneficial or detrimental effect of this crosstalk remains to be assessed.
When microglia-derived EVs are taken up by neurons, they promote ceramide and sphingosine production and upregulate synaptic activity by enhancing the probability of synaptic vesicle release through the facilitation of SNARE formation. This enhancement can be suppressed by pharmacological or genetic inhibition of sphingosine synthesis [17]. Proteomic analysis of murine microglia-derived exosomes identified a number of enzymes, chaperones, tetraspanins, and membrane receptors previously reported in dendritic cell- and B cell-derived exosomes. The expression of the aminopeptidase CD13 and monocarboxylate transporter are unique to microglia and distinguish them from other hematopoietic cells [49]. Furthermore, microglia-derived EV cargo molecules, including Trombospondin-1 and 4, suppress neuronal apoptosis and promote neurite outgrowth and synaptogenesis. This suggests a neuroprotective and neuritogenic role of microglia, which requires further investigation for their roles in neural development and CNS disorders [29]. Activated microglia can shed EVs containing miR-146–5p, which can be transferred to neurons via EVs and suppress synaptotagmin-1 in presynaptic terminals and neuroligin-1 in postsynaptic terminals. Prolonged exposure to EVs reduced the number of dendritic spines in the hippocampus in vivo, and was associated with a reduced mEPSC frequency and amplitude [50]. This demonstrates that glia-derived EV-to-neuron signaling occurs through EV cargo molecule-and membrane contact-dependent mechanisms.
Astrocyte-derived EVs
Much like those of microglia, astrocyte-derived EVs have both homeostatic and pathogenic functions. EVs purported to have beneficial function carry cargo proteins including heat shock protein 70, synapsin-1, fibroblast growth factor 2, vascular endothelial growth factor, and endostatin [51, 52]. The neuritogenic role of astrocyte-derived EVs is further supported by evidence demonstrating that they are enriched in the synaptic molecule synapsin-1, which is associated with neurite outgrowth following periods of oxidative stress in vitro [53]. Interestingly, similar to microglia, astrocyte-derived EVs present the capacity to affect neurotransmission, inducing an increase in mEPSC through direct action of EV surfaces on neurons [17].
Taken together, these studies highlight the critical role of EVs in the dynamic modulation of normal homeostatic CNS functions and implicate their contribution to initiating and propagating cellular responses among neuronal cells within the CNS as well as inflammatory responses coordinated between the CNS and periphery.
EV Crosstalk in CNS Disease
Cumulative evidence over the last few decades has shed light on the role of EVs in neurodegenerative disease pathogenesis and progression. Neurodegenerative diseases are characterized by a progressive and irreversible loss of neurons, the aggregation of misfolded proteins in the brain, and widespread neuroinflammation. EVs’ contribution to disease progression has been described in AD, amyotrophic lateral sclerosis (ALS), Huntington’s disease (HD), multiple sclerosis (MS), Parkinson’s disease (PD), Prion disease, and traumatic brain injury (TBI) (Table 1) [54]. The role of EVs in neuroimmune response and disease progression taking place during neurodegenerative disorders will be discussed herein.
Table 1.
EV cargo molecules in major neurodegenerative disorders
| Neurodegenerative Disorder | Main EV-related Findings | References |
|---|---|---|
| AD |
|
[6, 20, 58, 61] |
| ALS |
|
[77–79] |
| HD |
|
[80] |
| MS |
|
[32, 81–83] |
| PD |
|
[84, 85] |
| Prion Disease |
|
[86, 87] |
| TBI |
|
[88, 89] |
EVs’ role in AD can be split into two main categories: beneficial effects through the clearance of cellular content and support of neuronal function, and detrimental effects via propagation of pathological proteins and neuroinflammation.
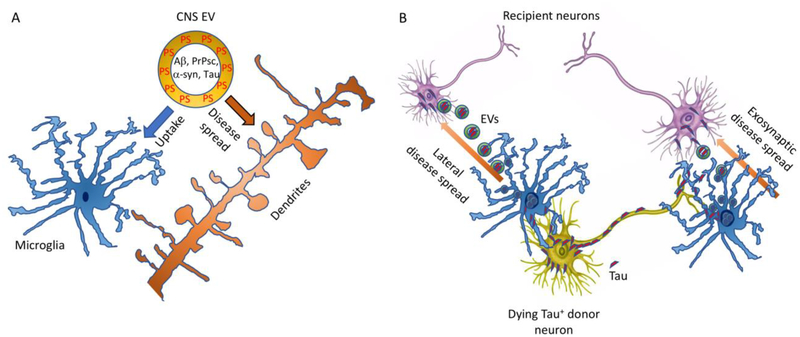
Clearance of pathological proteins has been shown to be beneficial in the context of AD. Microglia can clear Aβ by phagocytosing the Aβ-enriched exosomes (Figure 2A) [55]. Neuronal glycosphingolipids have been shown to allow Aβ sequestration in exosomes, followed by digestion by microglia to clear Aβ content in vivo [56, 57], indicating a synergistic mechanism by which neurons and microglia clear Aβ using exosomes.
Figure 2: Schematic representation of microglia-mediated tau spread via EVs.
A) The balance between the spread of pathogenic molecules and their uptake by neurons and microglia. CNS EV containing pathogenic molecules, such as Aβ, PrPsc, α-syn, and Tau are cleared by homeostatic microglia and also taken up by neurons, which may introduce seeding of pathogenic molecules resulting in protein accumulation. The balance of EV uptake by microglia and neurons is thus critical for the prevention of disease spread in the CNS. B) Microglia-mediated disease spread via EV. Microglia alter their phenotype from homeostatic to a neurodegenerative one during disease progression. Such microglia can be active for instance in phagocytosis but have impaired capacity to clear aggregated tau protein after ingestion of Tau+ donor neurons. The uncleared tau aggregates are secreted via EV, which can be taken up by recipient neurons. The aggregated tau in the EV serves as a seeding molecule for tau aggregation in the recipient neurons. Tau can be spread from the axonal terminal of donor neurons to the somatodendritic compartment of recipient neurons as exosynaptic disease spread, or from the somatodendritic compartment of donor neurons to nearby neurons as lateral disease spread via microglial EV secretion.
While EVs have a protective role by accelerating clearance of aggregated proteins, current studies have supported the idea that exosomes also contribute to the spread of pathological proteins. Aβ and tau (including pT181 and pS396 phosphorylated tau), the two hallmark proteins of AD pathology, are present in exosomes isolated from murine and human brains, human cerebrospinal fluid (CSF), and human plasma [6, 58, 59]. This concept is further supported by in vivo inhibition of exosome synthesis using neutral syphingomyelinase-2 inhibitor, which inhibits the synthesis of ceramide and decreased amyloid plaque formation in a 5XFAD mouse model expressing familial AD-linked mutations of amyloid precursor protein (APP) and presenilin-1 [60]. Similarly, in vivo inhibition of exosome synthesis by neutral syphingomyelinase-2 inhibitor also reduced tau propagation in adeno-associated virus (AAV)-based and P301S tauopathy mouse models [61]. The cellular origin of exosomes carrying abnormal proteins (Aβ and tau) is still unclear as neurons, astrocytes, and reactive microglia have all been reported to secrete them [5, 62–65]. Following engulfment of abnormally accumulated neuronal tau, microglia release tau-enriched exosomes, which are then taken up by neurons, further contributing to disease spread in vivo [61] (Figure 2B). Interestingly, recent work has revealed that tau-enriched exosomes can seed misfolded tau aggregates, and induce tau pathology once injected in wild-type mice [66]. Several studies have reported the release of neuronal tau-containing exosomes, in part, via synaptic activity [6, 67, 68]. Depolarization of primary neuronal culture was able to induce the release of tau-positive exosomes. Using a GFP-tagged-tau protein and microfluidic devices, Wang et al. showed trans-synaptic propagation of tau-containing exosomes [67]. Incubation of synthetic Aβ with astrocyte-derived exosomes enhanced its aggregation in vitro, and intracranial injection of astrocyte-derived exosomes isolated in vitro from wild-type primary cell culture accelerated Aβ aggregation in 5XFAD mouse model [60], suggesting that astrocytic exosomes can also drive AD pathology via acceleration of Aβ aggregation.
These studies beg the question of how neurons respond to the pathogenic proteins transported by EVs. Recent studies suggest that exosomes carrying Aβ or tau is taken up by local and remote cells and contributes to apoptosis and neuronal loss. Agosta and colleagues found that exosomes from myeloid origin were more abundant in the CSF of mild cognitively impaired (MCI) and AD patients compared to healthy controls [69]. This increase was associated with hippocampal atrophy, indicating neuronal loss and white matter damage as measured by magnetic resonance imaging [69]. Production of neurotoxic soluble Aβ by the interaction of extracellular Aβ aggregates with microvesicles from microglia has also been reported [5]. Moreover, cerebrospinal fluid (CSF) of patients with MCI and AD are enriched in myeloid cell marker IB4-positive microvesicles, which can transport Aβ to primary cultured neurons and have enhanced neurotoxicity. These findings reveal a novel role for microglia-derived microvesicles in AD associated Aβ-mediated neurotoxicity.
In AAV-based models of tau propagation, tau spreads from the entorhinal cortex to the dentate gyrus and reduces excitability of dentate granule cells. This was recovered by depletion of microglia, suggesting that microglia play a significant role in the transfer of tau, likely through neutral sphingomyelinase-2 sensitive exosome synthesis, and can affect neuronal functions [61]. In accord, activated microglia are a hallmark of neurodegenerative disease, and inflammatory microglia can decrease neuronal spine density and reduce excitatory neurotransmission via the release of exosomes [50]. This further indicates that there may be a potential novel mechanism by which microglial exosomes induce synaptic loss commonly seen in neurodegenerative disorders.
It is a matter of debate how to reconcile both the beneficial and detrimental effects of exosomes on progression of neuropathology, such as Aβ and tau accumulation in the brain. One possibility is that neuronal exosome synthesis is not ceramide dependent, whereas microglial exosome synthesis is. Another possibility is that exosomes can facilitate microglial clearance of aggregated proteins in homeostatic conditions (Figure 2A), whereas in disease conditions, inhibition of exosome synthesis or secretion is effective to halt propagating pathology. In the disease condition, neurodegenerative microglia show impairment in their protein clearance ability [70], which may accelerate the incorporation of aggregated proteins into exosomes, and their subsequent secretion as seeding factor for disease spread (Figure 2B). Overall, EVs seem to be crucial mediators of disease establishment and progression through the intercellular transfer of pathogenic molecules.
Concluding Remarks and Future Perspectives
The accumulating findings regarding EV biogenesis, secretion, and uptake have allowed us to better understand their role in modulating CNS function under normal and pathological conditions. EVs represent a unique mode of intercellular communication which allows proximal and distant interactions among multiple cell types in the CNS and periphery. EVs are involved in a wide variety of homeostatic processes including modulation of neuronal excitability, synaptic plasticity, myelination, microglial activation, and astrocytic gene regulation. This capacity is dictated by their similarly wide range of possible cargo molecules and outer leaflet-associated proteins and lipids. Although many interesting findings have been reported in multiple cell types and animal models, our understanding of the precise mechanisms of EV function are still in infancy and will require more sophisticated purification of specific EV subtypes, quantification methods, and robust cell type-specific EV reporter animal models. Assessment of the methodological limitations in the context of EV studies can be found in the recently published guideline: “Minimal information for studies of extracellular vesicles 2018” (MISEV2018) [35]. There are several other key questions to be answered: Do EV subtypes asymmetrically contribute to normal CNS function and disease pathogenesis? How significant is the effect of peripheral EVs on CNS function and vice versa? Are there risk factors related to EV biology which may increase susceptibility of, or protection against, neurodegenerative diseases?
In conclusion, EV biology is a growing area of investigation in the quest to understand neuroimmune communication in health and disease, and our hope is that advances in this area will support the development of effective therapeutics by targeting specific EV-mediated disease progression mechanisms.
Highlights.
CNS EVs are heterogenous vesicles with loading of specific cargo molecules depending on the cell type, cellular activity and homeostatic/disease status of the CNS.
CNS EVs cargos represent relatively small quantities of molecules compared to secreted molecules but can be highly impactful due to their concentration and targeted delivery.
CNS EVs are active mediators of neuroimmune communication via small RNAs and proteins in the healthy and diseases brain, and have both beneficial and detrimental roles.
CNS EVs participate in seeding and progression of neurodegenerative disease pathology.
Acknowledgments
We would like to thank Dr. Seiko Ikezu for the scientific guidance and critical editing for the manuscript. This work is funded in part by Nancy Lurie Marks Family Foundation (TI), Robert E. Landreth and Dona Landreth Family Foundation (TI), BrightFocus Foundation (A2016551S), Cure Alzheimer’s Fund, NIH R01AG054672 (TI), RF1AG054199 (TI), R56AG057469 (TI), 5T32GM008541 (SH). The authors have no financial conflict of interest related to this manuscript.
Glossary
- Alzheimer’s Disease
A chronic neurodegenerative disease resulting in increased decline in cognitive function over time
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor. An ionotropic transmembrane receptor for glutamate that mediates fast synaptic transmission within the central nervous system
- Amyloid Beta Peptides
Peptides of 36–43 amino acids which are found as accumulated plaques in the brains of patients with Alzheimer’s Disease. Amyloid beta proteins are fragments of the cleaved Amyloid Precursor Protein
- Blood Brain Barrier
A selective, semipermeable barrier composed of endothelial cells and glial cells that separates circulating blood from the cells and extracellular space within the brain and spinal cord
- Chemokine
Chemotactic Cytokine. Chemokines are a small family of cytokines which induce chemotaxis in recipient cells
- Cytokine
Proteins secreted by cells which signal other cells in the coordination of an immune response
- Glia
Term used to describe the group of cells consisting of microglia, astrocytes and oligodendrocytes. Glia cells are closely associated with neurons and perform a diverse array of functions within the CNS
- Gliosis
Reactive change of glial cells in response to damage, infection, or both
- Lipopolysaccharides
Large molecules found on the surface of gram-negative bacteria which are commonly used to induce an immune response
- Microglia
The primary innate immune cells within the central nervous system. Microglia play a significant role in responding to infection and injury, and maintaining brain homeostasis.miR-155: a microRNA established for its role in inflammation and oncogenesis
- miRNA
Micro RNA. Small, non-coding RNA found in nearly every form of life which serve to control gene expression and protein synthesis
- Neuroimmune System
Collection of tissue and cell types which serve the purpose of isolating the CNS from pathogens, responding to injury and coordinating an innate immune response. The neuroimmune system is composed primarily of glial cells
- Purinergic Receptor
A family of ligand gated ion channel proteins which can sense extracellular ATP
- Somatodendritic Compartment
The portion of a neuron that includes the cell body and dendrites, but not the axon
- Tau
Microtubule-associated protein tau, which stabilizes microtubules and is enriched in neurons. Abnormal accumulation of tau protein aggregates is the principal component of neurofibrillary tangles in Alzheimer’s disease
- Tetraspanins
A family of transmembrane proteins enriched in EVs and involved in many physiological processes within the cell
- MS
Multiple Sclerosis, a demyelinating disease in which the insulating sheath of neurons within the central nervous system are damaged
- TBI
Traumatic Brain Injury, an injury to the brain caused by an external force
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Graner MW et al. (2009) Proteomic and immunologic analyses of brain tumor exosomes. FASEB J 23 (5), 1541–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi M et al. (2016) CNS tau efflux via exosomes is likely increased in Parkinson’s disease but not in Alzheimer’s disease. Alzheimers Dement 12 (11), 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mustapic M et al. (2017) Plasma Extracellular Vesicles Enriched for Neuronal Origin: A Potential Window into Brain Pathologic Processes. Front Neurosci 11, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sardar Sinha M et al. (2018) Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol 136 (1), 41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joshi P et al. (2014) Microglia convert aggregated amyloid-beta into neurotoxic forms through the shedding of microvesicles. Cell Death Differ 21 (4), 582–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saman S et al. (2012) Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem 287 (6), 3842–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colombo M et al. (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30, 255–89. [DOI] [PubMed] [Google Scholar]
- 8.Thery C et al. (2002) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2 (8), 569–79. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J et al. (2015) Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics 13 (1), 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nazarenko I et al. (2010) Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res 70 (4), 1668–78. [DOI] [PubMed] [Google Scholar]
- 11.Eldh M et al. (2010) Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One 5 (12), e15353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carayon K et al. (2011) Proteolipidic composition of exosomes changes during reticulocyte maturation. J Biol Chem 286 (39), 34426–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Jong OG et al. (2012) Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pusic AD and Kraig RP (2014) Youth and environmental enrichment generate serum exosomes containing miR-219 that promote CNS myelination. Glia 62 (2), 284–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzner D et al. (2011) Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci 124 (Pt 3), 447–58. [DOI] [PubMed] [Google Scholar]
- 16.Kramer-Albers EM et al. (2007) Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteomics Clin Appl 1 (11), 1446–61. [DOI] [PubMed] [Google Scholar]
- 17.Antonucci F et al. (2012) Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J 31 (5), 1231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciregia F et al. (2017) Extracellular Vesicles in Brain Tumors and Neurodegenerative Diseases. Front Mol Neurosci 10, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robbins PD (2017) Extracellular vesicles and aging. Stem Cell Investig 4, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szepesi Z et al. (2018) Bidirectional Microglia-Neuron Communication in Health and Disease. Front Cell Neurosci 12, 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eyo UB and Wu LJ (2013) Bidirectional microglia-neuron communication in the healthy brain. Neural Plast 2013, 456857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biber K et al. (2007) Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci 30 (11), 596–602. [DOI] [PubMed] [Google Scholar]
- 23.Caruso S and Poon IKH (2018) Apoptotic Cell-Derived Extracellular Vesicles: More Than Just Debris. Front Immunol 9, 1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butovsky O et al. (2014) Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci 17 (1), 131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber CE et al. (2015) Osteopontin mediates an MZF1-TGF-beta1-dependent transformation of mesenchymal stem cells into cancer-associated fibroblasts in breast cancer. Oncogene 34 (37), 4821–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matias D et al. (2018) Microglia/Astrocytes-Glioblastoma Crosstalk: Crucial Molecular Mechanisms and Microenvironmental Factors. Front Cell Neurosci 12, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fitzgerald W et al. (2018) A System of Cytokines Encapsulated in ExtraCellular Vesicles. Sci Rep 8 (1), 8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bianco F et al. (2005) Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol 174 (11), 7268–77. [DOI] [PubMed] [Google Scholar]
- 29.Drago F et al. (2017) ATP Modifies the Proteome of Extracellular Vesicles Released by Microglia and Influences Their Action on Astrocytes. Front Pharmacol 8, 910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar A et al. (2017) Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. J Neuroinflammation 14 (1), 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y et al. (2018) Inflammation leads to distinct populations of extracellular vesicles from microglia. J Neuroinflammation 15 (1), 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verderio C et al. (2012) Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann Neurol 72 (4), 610–24. [DOI] [PubMed] [Google Scholar]
- 33.Takenouchi T et al. (2015) Extracellular ATP induces unconventional release of glyceraldehyde-3-phosphate dehydrogenase from microglial cells. Immunol Lett 167 (2), 116–24. [DOI] [PubMed] [Google Scholar]
- 34.Li JJ et al. (2018) In vivo evidence for the contribution of peripheral circulating inflammatory exosomes to neuroinflammation. J Neuroinflammation 15 (1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Théry C et al. (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles 7 (1), 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanninen KM et al. (2016) Exosomes as new diagnostic tools in CNS diseases. Biochim Biophys Acta 1862 (3), 403–10. [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto J et al. (2017) The Transport Mechanism of Extracellular Vesicles at the Blood-Brain Barrier. Curr Pharm Des 23 (40), 6206–6214. [DOI] [PubMed] [Google Scholar]
- 38.Lachenal G et al. (2011) Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci 46 (2), 409–18. [DOI] [PubMed] [Google Scholar]
- 39.Budnik V et al. (2016) Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci 17 (3), 160–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fruhbeis C et al. (2013) Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol 11 (7), e1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldie BJ et al. (2014) Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res 42 (14), 9195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morel L et al. (2013) Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J Biol Chem 288 (10), 7105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bahrini I et al. (2015) Neuronal exosomes facilitate synaptic pruning by up-regulating complement factors in microglia. Sci Rep 5, 7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fruhbeis C et al. (2012) Emerging roles of exosomes in neuron-glia communication. Front Physiol 3, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frohlich D et al. (2014) Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Philos Trans R Soc Lond B Biol Sci 369 (1652). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kocur M et al. (2015) IFNbeta secreted by microglia mediates clearance of myelin debris in CNS autoimmunity. Acta Neuropathol Commun 3, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lampron A et al. (2015) Inefficient clearance of myelin debris by microglia impairs remyelinating processes. J Exp Med 212 (4), 481–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paolicelli RC et al. (2018) Cell-to-cell Communication by Extracellular Vesicles: Focus on Microglia. Neuroscience. [DOI] [PubMed] [Google Scholar]
- 49.Potolicchio I et al. (2005) Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J Immunol 175 (4), 2237–43. [DOI] [PubMed] [Google Scholar]
- 50.Prada I et al. (2018) Glia-to-neuron transfer of miRNAs via extracellular vesicles: a new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathol 135 (4), 529–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor AR et al. (2007) Regulation of heat shock protein 70 release in astrocytes: role of signaling kinases. Dev Neurobiol 67 (13), 1815–29. [DOI] [PubMed] [Google Scholar]
- 52.Proia P et al. (2008) Astrocytes shed extracellular vesicles that contain fibroblast growth factor-2 and vascular endothelial growth factor. Int J Mol Med 21 (1), 63–7. [PubMed] [Google Scholar]
- 53.Wang S et al. (2011) Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes. J Neurosci 31 (20), 7275–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeLeo AM and Ikezu T (2018) Extracellular Vesicle Biology in Alzheimer’s Disease and Related Tauopathy. J Neuroimmune Pharmacol 13 (3), 292–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuyama K et al. (2015) A potential function for neuronal exosomes: sequestering intracerebral amyloid-beta peptide. FEBS Lett 589 (1), 84–8. [DOI] [PubMed] [Google Scholar]
- 56.Yuyama K and Igarashi Y (2016) Physiological and pathological roles of exosomes in the nervous system. Biomol Concepts 7 (1), 53–68. [DOI] [PubMed] [Google Scholar]
- 57.Yuyama K and Igarashi Y (2017) Exosomes as Carriers of Alzheimer’s Amyloid-ss. Front Neurosci 11, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rajendran L et al. (2006) Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A 103 (30), 11172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fiandaca MS et al. (2015) Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement 11 (6), 600–7 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dinkins MB et al. (2014) Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol Aging 35 (8), 1792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Asai H et al. (2015) Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci 18 (11), 1584–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuyama K et al. (2012) Sphingolipid-modulated exosome secretion promotes clearance of amyloid-beta by microglia. J Biol Chem 287 (14), 10977–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gouwens LK et al. (2018) Abeta42 Protofibrils Interact with and Are Trafficked through Microglial-Derived Microvesicles. ACS Chem Neurosci 9 (6), 1416–1425. [DOI] [PubMed] [Google Scholar]
- 64.Eitan E et al. (2016) Extracellular Vesicle-Associated Abeta Mediates Trans-Neuronal Bioenergetic and Ca(2+)-Handling Deficits in Alzheimer’s Disease Models. NPJ Aging Mech Dis 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goetzl EJ et al. (2016) Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J 30 (11), 3853–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Polanco JC et al. (2016) Extracellular Vesicles Isolated from the Brains of rTg4510 Mice Seed Tau Protein Aggregation in a Threshold-dependent Manner. J Biol Chem 291 (24), 12445–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Y et al. (2017) The release and trans-synaptic transmission of Tau via exosomes. Mol Neurodegener 12 (1), 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Medina M and Avila J (2014) The role of extracellular Tau in the spreading of neurofibrillary pathology. Front Cell Neurosci 8, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Agosta F et al. (2014) Myeloid microvesicles in cerebrospinal fluid are associated with myelin damage and neuronal loss in mild cognitive impairment and Alzheimer disease. Ann Neurol 76 (6), 813–25. [DOI] [PubMed] [Google Scholar]
- 70.Clayton KA et al. (2017) Alzheimer’s Disease: The Role of Microglia in Brain Homeostasis and Proteopathy. Front Neurosci 11, 680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.S ELA et al. (2013) Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 12 (5), 347–57. [DOI] [PubMed] [Google Scholar]
- 72.Raposo G and Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200 (4), 373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gyorgy B et al. (2011) Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci 68 (16), 2667–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andreu Z and Yanez-Mo M (2014) Tetraspanins in extracellular vesicle formation and function. Front Immunol 5, 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abels ER and Breakefield XO (2016) Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol Neurobiol 36 (3), 301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mulcahy LA et al. (2014) Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grad LI et al. (2015) From molecule to molecule and cell to cell: prion-like mechanisms in amyotrophic lateral sclerosis. Neurobiol Dis 77, 257–65. [DOI] [PubMed] [Google Scholar]
- 78.Basso M et al. (2013) Mutant copper-zinc superoxide dismutase (SOD1) induces protein secretion pathway alterations and exosome release in astrocytes: implications for disease spreading and motor neuron pathology in amyotrophic lateral sclerosis. J Biol Chem 288 (22), 15699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferrara D et al. (2018) Role of Extracellular Vesicles in Amyotrophic Lateral Sclerosis. Front Neurosci 12, 574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang X et al. (2016) Potential Transfer of Polyglutamine and CAG-Repeat RNA in Extracellular Vesicles in Huntington’s Disease: Background and Evaluation in Cell Culture. Cell Mol Neurobiol 36 (3), 459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saenz-Cuesta M et al. (2014) Extracellular Vesicles in Multiple Sclerosis: What are They Telling Us? Front Cell Neurosci 8, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Minagar A and Alexander JS (2003) Blood-brain barrier disruption in multiple sclerosis. Mult Scler 9 (6), 540–9. [DOI] [PubMed] [Google Scholar]
- 83.Prada I et al. (2013) Classical and unconventional pathways of vesicular release in microglia. Glia 61 (7), 1003–17. [DOI] [PubMed] [Google Scholar]
- 84.Shi M et al. (2014) Plasma exosomal alpha-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol 128 (5), 639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Danzer KM et al. (2012) Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener 7, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fevrier B et al. (2004) Cells release prions in association with exosomes. Proc Natl Acad Sci U S A 101 (26), 9683–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bellingham SA et al. (2012) Small RNA deep sequencing reveals a distinct miRNA signature released in exosomes from prion-infected neuronal cells. Nucleic Acids Res 40 (21), 10937–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McKee AC et al. (2013) The spectrum of disease in chronic traumatic encephalopathy. Brain 136 (Pt 1), 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stern RA et al. (2016) Preliminary Study of Plasma Exosomal Tau as a Potential Biomarker for Chronic Traumatic Encephalopathy. J Alzheimers Dis 51 (4), 1099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]