Abstract
Voltage-gated sodium channel Nav1.5 is critical for generation and conduction of cardiac action potentials. Mutations and expression level changes of Nav1.5 are associated with cardiac arrhythmias and sudden death. The ubiquitin (Ub) conjugation machinery utilizes three enzyme activities, E1, E2, and E3, to regulate protein degradation. Previous studies from us and others showed that Nedd4–2 acts as an E3 ubiquitin-protein ligase involved in ubiquitination and degradation of Nav1.5, however, more key regulators remain to be identified. In this study, we show that UBC9, a SUMO-conjugating enzyme, regulates ubiquitination and degradation of Nav1.5. Overexpression of UBC9 significantly decreased Nav1.5 expression and reduced sodium current densities, whereas knockdown of UBC9 expression significantly enhanced Nav1.5 expression and increased sodium current densities, in both HEK293 cells and primary neonatal cardiomyocytes. Overexpression of UBC9 increased ubiquitination of Nav1.5, and proteasome inhibitor MG132 blocked the effect of UBC9 overexpression on Nav1.5 degradation. Co-immunoprecipitation showed that UBC9 interacts with Nedd4–2. UBC9 with mutation C93S, which suppresses SUMO-conjugating activity of UBC9, was as active as wild type UBC9 in regulating Nav1.5 levels, suggesting that UBC9 regulates Nav1.5 expression levels in a SUMOylation-independent manner. Our findings thus identify a key structural element of the ubiquitin-conjugation machinery for Nav1.5 and provide important insights into the regulatory mechanism for ubiquitination and turnover of Nav1.5.
Keywords: UBC9, SCN5A, Nav1.5 sodium channel, ubiquitination, Nedd4-2, arrhythmia
1. Introduction
Nav1.5 is the α-subunit of the voltage-gated cardiac sodium channel and encoded by the SCN5A gene. Nav1.5 is essential for the initiation of the cardiac action potential (AP) and conduction of electrical impulses [1–4]. The important role of Nav1.5 has been exemplified by the discovery of more than 300 naturally occurring genetic mutations linked to various cardiac arrhythmias and sudden death, including Brugada syndrome (BrS) [5], long QT syndrome (LQTS) [3, 4, 6], and sick sinus syndrome [7–9]. As a plasma membrane protein, the expression level of Nav1.5 on the cell surface is critical for its function because cell electrical excitability depends not only on its own activation but also on its expression levels [10].
Nav1.5 degradation has been reported to be associated with Nedd4–2, a key component of the ubiquitin-proteasome system (UPS) [11, 12]. The UPS is an important degradation mechanism of cellular proteins including voltage-gated channels [13, 14]. Ubiquitin (Ub) is a small protein that can be covalently linked to a substrate protein [15]. The UPS contains ubiquitin, Ub-activating enzyme (E1), Ub-conjugating enzyme E2, and Ub-protein ligase E3, which together make membrane proteins mono- or poly-ubiquitinated and degraded [14, 15]. It was previously reported that Nav1.5 contains the PY-motif (xPPxY), which can interact with the WW-domain of Nedd4–2, a ubiquitin-protein ligase (E3) characterized by the presence of a C-terminal HECT catalytic domain [16]. Ubiquitination is a prerequisite for endocytosis and degradation of plasma membrane proteins [15]. The ubiquitination of Nav1.5 can be regulated by Nedd4–2, which leads to internalization and degradation of Nav1.5 [16, 17].
UBC9 is a small ubiquitin-like modifier-conjugating enzyme E2 that helps ligation of SUMO to the substrate during SUMOylation [18, 19]. Like ubiquitination, SUMOylation is a post-translational modification process involved in protein quality control [18, 19]. UBC9 has been reported to participate in protein quality control and interact with some ubiquitin E3 ligases, such as muscle-specific RING finger 1 [20, 21]. In addition to SUMOylation, UBC9 also regulates gene expression through SUMOylation-independent pathways [22].
In this study, we assessed the effects of UBC9 on the regulation of the level of Nav1.5. Surprisingly, we found that UBC9 regulates Nav1.5 degradation in a SUMOylation-independent manner. We found that UBC9 regulated the ubiquitination of Nav1.5 and cardiac sodium current densities in both a heterologous HEK293 cell expression system and neonatal cardiomyocytes. Moreover, we found that UBC9 interacted with Nedd4–2, which mediates Nav1.5 degradation through the UPS. Therefore, we identified UBC9 as a key regulator of the Ub-conjugation machinery regulating Nav1.5 ubiquitination and degradation.
2. Materials and methods
2.1. Plasmids, mutagenesis, siRNAs and t-CSM peptide
The expression construct for human cardiac sodium channel gene SCN5A in vector pcDNA3 (pcDNA3-SCN5A) was previously described [5, 6, 23–27]. The coding region of Nav1.5 was excised from pcDNA3-SCN5A by restriction enzyme digestion and subcloned into the pIRES-EGFP vector, generating pEGFP-Nav1.5. The UBC9 gene was amplified by RT-PCR analysis from RNA samples from HEK293 cells and subcloned into the pCMV-HA vector to generate the pCMV-UBC9 plasmid. The UBB gene encoding ubiquitin was amplified by RT-PCR analysis from RNA samples from HeLa cells and subcloned into the pCMV-MYC vector, generating pCMV-MYC-UBB. All expression plasmids were verified by direct DNA sequencing analysis.
The C93S mutation was created in pCMV-UBC9 by an overlapping extension PCR mutagenesis method [28, 29], generating pCMV-UBC9-C93S. The sequences of UBC9 siRNAs are UBC9-siRNA1 (Sense-GGAAUACAGGAACUUCUAA; Antisense-UUAGAAGUUCCUGUAUUCC), UBC9-siRNA2 (Sense-GCAGAGGCCUACACGAUUU; Antisense-AAAUCGUGUAGGCCUCUGC), and UBC9-siRNA3 (Sense-GGGAAGGAGGCUUGUUUAA; Antisense-UUAAACAAGCCUCCUUCCC). The t-CSM peptide (YGRKKRRQRRRGKMDENQ) was manufactured and purified by Genscript (CHINA) and was dissolved in double-distilled water.
2.2. Cell culture and transfection
A HEK293 cell line with stable expression of Nav1.5 (HEK293-Nav1.5) was described previously [23]. HEK293 and HEK293-Nav1.5 cells were cultured in a DMEM medium supplemented with 10% fetal bovine serum (FBS) (Hyclone, USA) and filtered by 0.22 μm membranes. Cells were incubated at 37°C in a humidified chamber with 5% CO2.
Transfection of plasmid DNA (varying amount) and siRNA (10 nM) was carried out using Lipofectamine™ 2000 (Invitrogen, USA) according to the manufacturer’s instructions. Cells were seeded in 6-well plates overnight, and transfection was carried out when cells approached 80% confluence.
2.3. Real-time quantitative RT-PCR analysis
Total RNA was extracted from cultured cells using Trizol regent (TakaRa, USA), and real-time RT-PCR analysis was carried out with the FastStart Universal SYBR Green Master (Roche, USA) as previously described [30–32]. The sequences of RT-PCR primers are Foward-ACCATTATTTCACCCGAATGTGT and Reverse-CTCGGACCCTTTTCTCGTACT for UBC9, and Forward-CACTGTGCCCATCTACGA and Reverse-GTAGTCAGTCGAGTCCCG for ACTB encoding β-actin.
2.4. Isolation of neonatal rat cardiomyocytes
Isolation of neonatal cardiomyocytes was carried out as previously described [33–35]. In brief, hearts were excised from eleven 0–3 day old Sprague-Dawley rats and washed in PBS to remove blood cells. Then, the heats were minced into small pieces and incubated in isolation buffer (0.05% collagenase B from Roche and 0.05% trypsin from Amresco in 1 × PBS). The isolated cardiomyocytes were cultured in the DMEM supplemented with 20% FBS for 24 h, and co-transfected with the pCMV-UBC9 expression plasmid or the pCMV-HA empty vector together with a GFP plasmid using Lipofectamine™ 3000 (Invitrogen, USA) according to the manufacture’s instruction. Transfection of siRNAs was also carried out using Lipofectamine™ 3000. The study with animals was approved by the ethics committee of Huazhong University of Science and Technology.
2.5. Western blotting and co-immunoprecipitation (Co-IP) analyses
Cells were transfected for 48 hours, and lysed in lysis buffer (50 mM Tris-HCl, pH7.5, 150 mM NaCl, 2 mM EDTA, 1% Nonidet P-40, and protease inhibitor cocktail) (Roche, USA)) to prepare cell extracts for Western blot analysis and Co-IP analysis as described previously [17, 36, 37]. A rabbit anti-Nav1.5 antibody (Alomone Labs, Jerusalem BioPark) was used at a dilution factor of 1:1000. A mouse anti-tubulin (Millipore, USA) antibody was used at a dilution factor of 1:5000. A goat anti-rabbit HRP-conjugated secondary antibody and a goat anti-mouse HRP-conjugated secondary antibody were all from Millipore (USA) and used at a dilution factor of 1:20,000. A rabbit anti-UBC9 antibody (Santa Cruz, USA) was used at a dilution factor of 1:1000. A mouse anti-Myc antibody (MBL, JAPAN) was used at a dilution factor of 1:1000. A rabbit anti-Na,K-ATPase antibody (Cell Signaling Technology, USA) was used at a dilution factor of 1:1000. A mouse anti-FLAG antibody, a rabbit anti-HA antibody and a rabbit anti-GFP antibody were all from MBL (JAPAN). The goat anti-rabbit IgG and goat anti-mouse IgG were from Santa Cruz Biotechnology. The anti-ubiquitin mouse monoclonal antibody FK2 (BMLPW8810, Enzo Life Science) was used at a dilution factor of 1:800.
2.6. Isolation of cell-surface proteins and analysis of expression level of cell surface Nav1.5
Transfected cells were washed three times with ice-cold PBS, resuspended in 5 ml Buffer I (25 mM Tris-HCl, pH7.5, 5 mM EDTA, 5 mM EGTA), sonicated on ice using a 45W sonicator with a setting of 20 cycles of 3 seconds on and 5 seconds off (JY-92-IIN SCIENTZ, CHINA), and centrifuged at 13,000 x g at 4°C for 10 minutes. Supernatant was collected and centrifuged at 100,000 x g for 1 hour at 4°C. The pellets were resolved in Buffer II (20 mM Tris-HCl, pH7.4, 137 mM NaCl, 10% v/v glycerol, 1% v/v Nonidet P-40), incubated at 4°C for 30 minutes, and then centrifuged at 13,000 x g at 4°C for 5 minutes. The supernatant contains the cell membrane proteins, and used for Western blot analysis with an anti-Nav1.5 antibody and an anti Na,K-ATPase antibody as control (Cell signaling technology, USA) as described previously [26, 27].
2.7. Proteasome inhibitor study
HEK293 cells were co-transfected with plasmids pcDNA3-SCN5A, PCMV-UBC9, or empty vector PCMV-HA for 36 hours. Cells were treated with MG132 (a potent, reversible, and cell-permeable proteasome inhibitor) for 12 hours, and then harvested for Western blot analysis as described above.
2.8. Determination of ubiquitination of Nav1.5
HEK293 cells at 70%–80% confluence in a 10 cm plate were transiently co-transfected with pcDNA3-Nav1.5 and pCMV-Myc-UBB plasmids with or without pCMV-HA-UBC9 for 48 hours, and lysed. The lysates were centrifuged at 13,000 x g at 4°C for 30 minutes. The supernatant was incubated with 2 μg of anti-Nav1.5 antibody or anti-GFP antibody on a rotator at 4°C overnight and then mixed with 30 μl of Protein A/G PLUS-agarose (Santa Cruz Biotechnology, USA). The antibody-protein A/G PLUS agarose complex was incubated on a rocker at 4°C for 2 hours, centrifuged at 1000 x g for 2 minutes, and washed three times with lysis buffer. The washed pellets were resuspended in 50 μl of 1 x SDS loading buffer containing 5% (V/V) β-mercaptoethanol and then electrophoresed through SDS-polyacrylamide gels for Western blot analysis.
2.9. Electrophysiological studies
Patch-clamping experiments were carried out as previously described [5, 26, 27, 32, 38, 39]. The pipette solution contained 20 mM NaCl, 130 mM CsCl, 10 mM EGTA, and 10 mM HEPES (pH 7.2 adjusted with CsOH). The bath solution contained 70 mM NaCl, 80 mM CsCl, 5.4 mM KCl, 2 mM CaCl2, 10 mM HEPES, 10 mM glucose, and 1 mM MgCl2 (pH 7.4 adjusted with CsOH).
2.10. Statistical analysis
All data were from at least three independent experiments and expressed as means ± SEM. Statistical analysis was performed using Student’s t-tests. A P-value of 0.05 or less was considered to be statistically significant.
3. Results
3.1. Overexpression of UBC9 downregulates the Nav1.5 protein level
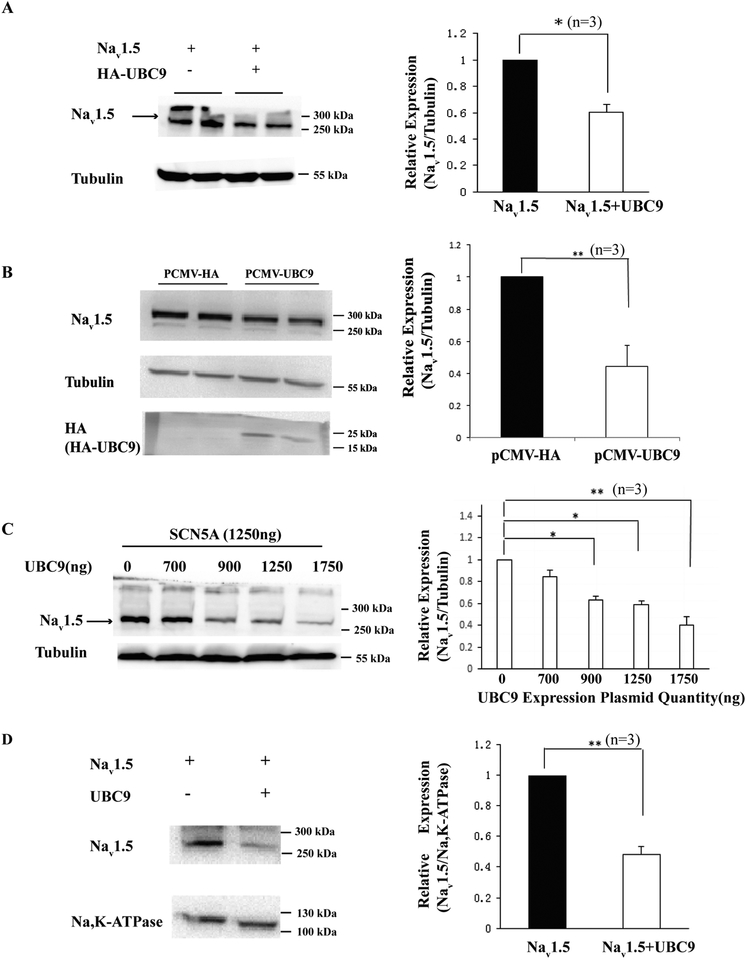
To analyze the potential role of UBC9 in Nav1.5 expression, we co-transfected pCMV-UBC9 and pcDNA3-SCN5A plasmids into HEK293 cell. Over-expression of UBC9 led to a significant decrease in Nav1.5 expression levels (Fig. 1A). Similar results were obtained from HEK293-Nav1.5 cells (with stable expression of Nav1.5) transfected with pCMV-UBC9 (Fig. 1B). To determine whether the effects of UBC9 on Nav1.5 expression was dose-dependent, we co-transfected HEK293 cells with pcDNA3-SCN5A and different amounts of pCMV-UBC9. Western blot analysis showed that the amount of Nav1.5 decreased when the amount of pCMV-UBC9 increased in a dose-dependent manner (Fig. 1C). To determine whether UBC9 affects the amount of cell surface expression of Nav1.5, we performed Western blot analysis with plasma membrane extracts. Fig. 1D showed that overexpression of UBC9 led to a significant decrease of plasma membrane Nav1.5.
Fig. 1.
Effects of overexpression of UBC9 on Nav1.5 expression levels. Western blot analysis was carried out for Nav1.5 using HEK293 cells co-transfected with pcDNA3-SCN5A and pCMV-UBC9. (A) Overexpression of UBC9 significantly reduced the expression level of Nav1.5. Tubulin served as a loading control. The nature of the band above 300 kDa is unknown, and may represent modified Nav1.5 or Nav1.5 aggregates. (B) Western blot analysis with protein extracts from HEK293-Nav1.5 cells (with stable expression of Nav1.5) transfected with pCMV-UBC9 or control plasmid pCMV-HA. (C) The effects of UBC9 overexpression on Nav1.5 expression levels were concentration-dependent. (D) Overexpression of UBC9 significantly decreased the cell surface expression of Nav1.5. Na,K-ATPase was used as a loading control. Left panel: Western blot images; Right panel: quantification of Western blot images. *P<0.05, **P<0.01, n=3/group.
3.2. Overexpression of UBC9 reduces sodium current densities in HEK293-Nav1.5 cells
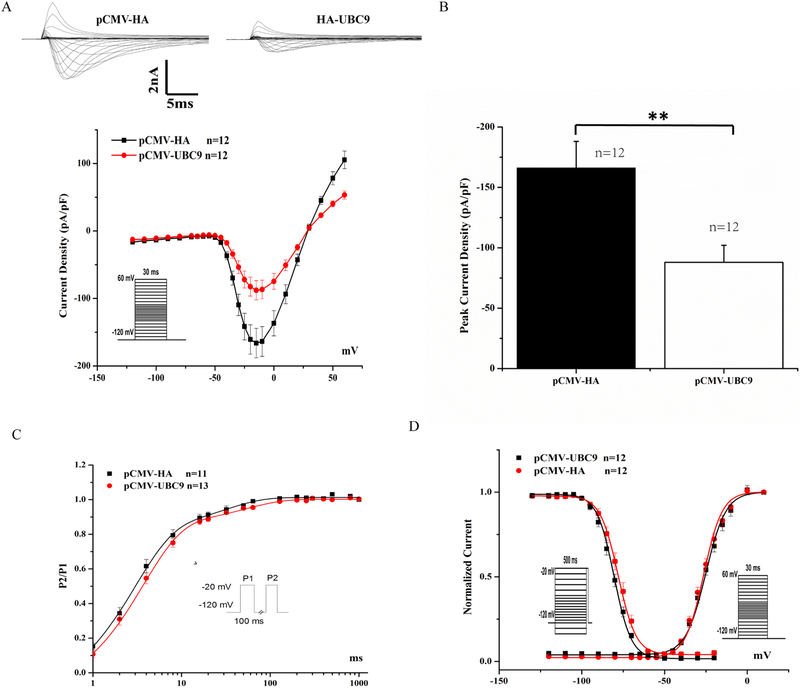
To investigate the functional role of UBC9 in Nav1.5 physiology, whole-cell sodium currents were recorded in HEK293-Nav1.5 cells transfected with pCMV-UBC9 or pCMV-HA empty vector. We found that overexpression of UBC9 significantly decreased densities of sodium currents compared to the empty vector control (n>10, p=0.0087) (Figs. 2A and 2B). However, there were no significant changes in the time-dependence curves of recovery from inactivation (Fig. 2C) or voltage-dependent kinetics of activation and inactivation of sodium currents between cells with UBC9 overexpression and those with the control plasmid (Fig. 2D).
Fig. 2.
Effects of UBC9 overexpression on sodium current densities. (A) Representative original traces of sodium currents and the current-voltage (I-V) relationship between average current densities and voltages from HEK293-Nav1.5 cells transfected with pCMV-UBC9 or a control plasmid. Overexpression of UBC9 decreased sodium current densities. (B) Peak sodium current densities at −15 mV. (C) Time course of recovery from inactivation was studied using a two-pulse protocol of −20 mV at −120 mV holding potential. (D) Steady-state activation and inactivation curves. The holding potential was −120 mV. **P<0.01, n=11–13 cells/group.
3.3. Knockdown of UBC9 upregulates Nav1.5 protein levels
To determine whether knockdown of UBC9 affects Nav1.5 expression levels, we tested the effectiveness of three different siRNAs against UBC9 in HEK293-Nav1.5 cells. After 48 hours of transfection, the gene expression level of UBC9 was quantified by real time RT-PCR analysis. Fig. 3A showed that compared to the control siRNA (NC), UBC9 siRNA2 and siRNA3, but not siRNA1, significantly decreased UBC9 mRNA levels. Western blot analysis further confirmed the knockdown of UBC9 by siRNA2 and siRNA3 (Fig. 3B). When UBC9 was knocked down by siRNA2 or siRNA3, the expression level of Nav1.5 was significantly elevated compared to control NC-siRNA (Fig. 3C). On the other hand, siRNA1 did not reduce UBC9 expression, and thus did not affect the expression level of Nav1.5 compared to control NC-siRNA (Fig. 3C).
Fig. 3.
Effects of UBC9 knockdown on Nav1.5 expression levels. (A, B) Quantitative real-time RT-PCR analysis (A) and Western blot analysis (B) of UBC9 in HEK293-Nav1.5 cells transfected with siRNAs against UBC9 (UBC9 siRNA1, siRNA2 and siRNA3) or nonspecific negative control siRNA (NC siRNA). (C) Western blot analysis of Nav1.5 in HEK293-Nav1.5 cells transfected with siRNAs against UBC9 or NC siRNA. Note that siRNA2 or siRNA3 knocked UBC9 expression down and significantly increased Nav1.5 expression, whereas siRNA1 did not knock UBC9 expression down and did not affect Nav1.5 expression. *P<0.05, n=3/group.
3.4. Knockdown of UBC9 increases sodium current densities in HEK293-Nav1.5 cells
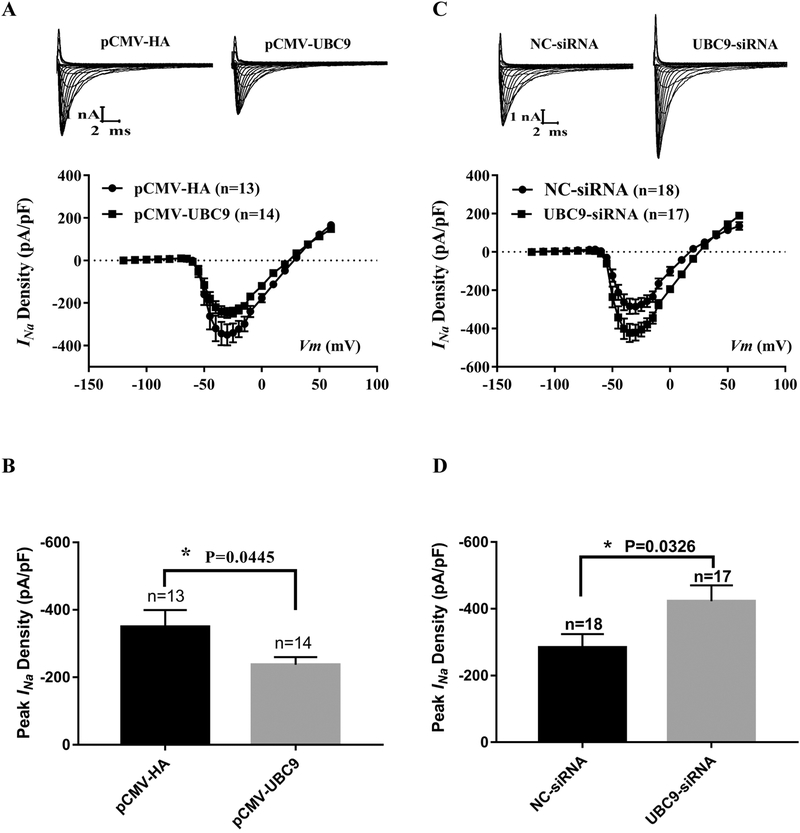
To determine the effects of knockdown of UBC9 on the function of Nav1.5, UBC9 siRNA3 was transfected into HEK293-Nav1.5 cells, and whole-cell sodium currents (INa) were recorded. Compared to NC siRNA, UBC9 siRNA led to a significant increase of INa densities (n>10, p=0.0386) (Figs. 4A and 4B). Knockdown of UBC9 did not affect the time-dependence recovery from inactivation (Fig 4C) and voltage-dependent activation and inactivation kinetics of INa (Fig 4D).
Fig. 4.
Effects of UBC9 knockdown on sodium current densities in HEK293-Nav1.5 stable cells. (A) Representative original traces of sodium currents and the current-voltage (I-V) relationship. (B) Peak sodium current densities at −15 mV. (C) Time course of recovery from inactivation. (D) Steady-state activation and inactivation curves. The holding potential was −120 mV. *P<0.05, n=11–13 cells/group.
3.5. Knockdown or overexpression of UBC9 regulates Nav1.5 sodium current densities in neonatal rat cardiomyocytes
To determine whether UBC9 regulates Nav1.5 and INa in cardiomyocytes, we isolated neonatal cardiomyocytes from rats, which were transfected with pCMV-UBC9 or control pCMV-HA empty vector. Whole-cell sodium currents were then recorded. Cardiomyocytes with overexpression of UBC9 showed significantly decreased INa densities compared to cells with the empty vector control (p=0.0445, n>10) (Fig. 5A and 5B). To determine the effects of knockdown of UBC9 on the function of Nav1.5 and INa in cardiomyocytes, UBC9-siRNA was transfected into neonatal rat cardiomyocytes, and whole-cell sodium currents were recorded. Compared to NC siRNA, knockdown of UBC9 expression by UBC9 siRNA led to a significant increase of INa densities (p=0.0326, n>10) (Fig. 5C and 5D).
Fig. 5.
Effects of overexpression or knockdown of UBC9 on INa in neonatal rat cardiomyocytes. Whole-cell sodium currents recorded from neonatal rat cardiomyocytes transfected with an expression plasmid for UBC9 or its control empty vector or (A and B), and UBC9 siRNA or its negative control NC siRNA. (A) Representative original traces of sodium currents and the relationship between the average current density (current normalized to cell capacitance) and voltage from cardiomyocytes with or without overexpression of UBC9. (B) The relative peak sodium current density at −30 mV. (C) The relationship between the average current density (current normalized to cell capacitance) and voltage from cardiomyocytes with or without knockdown of UBC9 by siRNA. (D) The relative peak sodium current density at −30 mV. Data are shown as mean ± SEM. *P < 0.05, n=13–18 cells/group.
3.6. t-CSM peptide does not affect INa densities
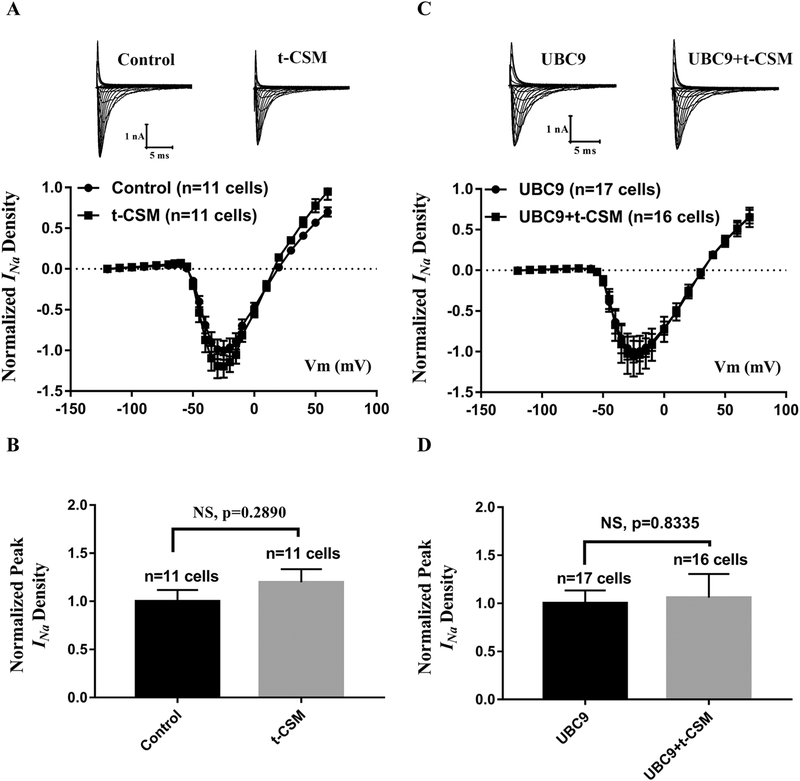
As previously reported, UBC9 can interact with CRMP2, and thus affect the Nav1.7 sodium current [40]. CRMP2 (Collapsin Response Mediator Protein 2) is a microtubule-binding protein necessary for regulating neuronal polarity, axon/dendrite fate and axonal outgrowth [41]. To determine whether the effect of UBC9 on Nav1.5 is through the UBC9-CRMP2 interaction, we used the t-CSM peptide to block the UBC9-CRMP2 interaction [40]. T-CSM is a tat-conjugated cell-penetrating peptide containing the SUMOylation consensus site of CRMP2 fused to the transduction domain of the HIV-1 tat protein [40]. HEK293-Nav1.5 cells were treated with or without 20 μM of t-CSM for 12 h, and whole-cell sodium currents were recorded. Compared to the control, the t-CSM peptide did not affect the Nav1.5 sodium current (Fig. 6A and 6B). Identical results were obtained in HEK293-Nav1.5 cells with overexpression of UBC9 (Fig. 6C and 6D). These data suggest that UBC9 does not affect the Nav1.5 function through the UBC9-CRMP2 interaction.
Fig. 6.
The effect of the UBC9-CRMP2 interaction on cardiac INa from Nav1.5. (A, B) Whole-cell sodium currents were recorded from HEK293-Nav1.5 cells treated with t-CSM that disrupts the UBC9-CRMP2 interaction. (A) Representative original traces of sodium currents and the relationship between the average current density (current normalized to cell capacitance) and voltage. (B) The relative peak sodium current density at −25 mV. NS, not significant, n=11 cells/group. (C, D) Whole-cell sodium currents were recorded from HEK293-Nav1.5 cells transfected with an expression plasmid for UBC9 and treated with t-CSM or without t-CSM. (C) The relationship between the average current density (current normalized to cell capacitance) and voltage. (D) The relative peak sodium current density at −25 mV. NS, not significant, n=16–17 cells/group.
3.7. SUMO-conjugase activity is unnecessary for regulation of Nav1.5 expression levels by UBC9
Since UBC9 is a well-characterized SUMO-conjugase, we examined if SUMOylation is involved in downregulation of Nav1.5 by UBC9. We created the mutant UBC9-C93S, which was reported to suppress the SUMO-conjugating activity of endogenous UBC9 via a mutation of a cysteine residue critical for SUMO-conjugating activity [22, 42]. Wild type UBC9 or mutant UBC9 with mutation C93S was co-expressed with GFP-tagged p53 and HA-tagged SUMO1 in HEK293 cells. GFP-tagged p53 was immunoprecipitated using an anti-GFP-antibody, and the precipitates were analyzed using immunoblotting with an anit-HA antibody recognizing HA-SUMO1. As shown in Fig. 7A (left panel), overexpression of UBC9 increased the level of SUMOylated-p53, however, the effect was blocked by the C93S mutation. A similar observation was made with Western blot analysis using cell extracts and an anti-HA antibody (Fig. 7, right panel). However, no effect was observed for mutant UBC9-C93S on downregulation of Nav1.5 levels by wild type UBC9 (Fig. 7B). These data suggest that UBC9 regulates Nav1.5 expression levels in a SUMO-independent manner.
Fig. 7.
UBC9 SUMOylation is not involved in regulation of Nav1.5 levels. (A) A positive control study showing that wild type UBC9 increases SUMO1 overexpression-induced SUMOylation of p53, but the effect was inhibited by mutant UBC9 with mutation C93S. HEK293 cells were co-transfected with multiple expression plasmids as indicated on the top, and lysed. Left panel: The cell lysates were immunoprecipitated with an anti-GFP antibody recognizing GFP-p53, and immunoblotting (IB) was carried out with an anti-HA antibody recognizing HA-SUMO1. After stripping of membranes, immunoblotting was carried out with an anti-p53 antibody. Right panel: the cell lysates were immunoblotted with an anti-HA antibody recognizing HA-SUMO1 antibody, and then with an anti-GFP antibody recognizing GFP-p53 or an anti-GAPDH antibody as loading controls after stripping of the membranes. SUMOylated p53 is indicated by a bracket around 100 kDa. (B) HEK293 cells were co-transfected with pcDNA3-SCN5A and pCMV-UBC9 or pCMV-UBC9-C93S, and used for Western blot analysis of Nav1.5. Tubulin was used as a loading control. Overexpression of UBC9 reduced the level of Nav1.5, but the Nav1.5 expression levels did not differ between wild type UBC9 and mutant UBC9-C93S lacking SUMO-conjugating activity. The nature of the band above 300 kDa is unknown, and may represent modified Nav1.5 or Nav1.5 aggregates. Data are shown as mean ± SEM. *P < 0.05, n=3/group.
3.8. UBC9 regulates the expression level of Nav1.5 through a ubiquitination-proteasome pathway
Because UBC9 does not reduce Nav1.5 expression levels via SUMOylation, we determined whether the proteasome pathway was involved in UBC9 regulation of Nav1.5 degradation using MG132, a potent and reversible proteasome inhibitor. Overexpression of UBC9 in HEK293-Nav1.5 cells reduced the level of Nav1.5 protein, however, the reduction was blocked by MG132 treatment with a 10 μM concentration (Fig. 8A). As UBC9 regulation of Nav1.5 levels was through proteasomal degradation, we analyzed the level of ubiquitination of Nav1.5 by UBC9. We used the anti-Nav1.5 and anti-GFP antibodies to immunoprecipitate Nav1.5 and GFP-Nav1.5, respectively, in HEK293 cells with co-overexpression of Nav1.5 and Myc-ubiquitin (Myc-UB) with or without UBC9 overexpression (Fig. 8B) or cells with co-overexpression of GFP-Nav1.5 and Myc-UB and with or without UBC9 overexpression (Fig. 8C). We then used an anti-Myc antibody for immunoblotting to detect ubiquitin-Nav1.5. Nav1.5 is approximately 250 kDa, however, the ubiquitinated Nav1.5 molecules were displayed in a large area of higher molecular weight than the unmodified Nav1.5. Fig. 8B and 8C showed that the amount of ubiquitinated-Nav1.5 was clearly increased in cells overexpressing UBC9. These results suggest that UBC9 can stimulate the ubiquitination of Nav1.5.
Fig. 8.
Effects of UBC9 on Nav1.5 expression involve the proteasome pathway and ubiquitination. (A) Western blot analysis of Nav1.5 and UBC9 in HEK293 cells co-transfected with pcDNA3-SCN5A together with or without pCMV-UBC9. Proteasome inhibitor MG132 increased Nav1.5 expression levels. Western blot images in the left were quantified and plotted on the right. The nature of the band above 300 kDa is unknown, and may represent modified Nav1.5 or Nav1.5 aggregates. Data are shown as mean ± SEM. *P < 0.05, n=3/group. (B) Immunoprecipitation analysis of Nav1.5 protein ubiquitination in HEK293 cells co-transfected with pcDNA3-SCN5A (overexpression of Nav1.5) and pMyc-UBB (overexpression of Myc-tagged Ub, Myc-UB) together with or without pCMV-UBC9. Cell lysates were immunoprecipitated with an anti-Nav1.5 antibody (IP: Nav1.5), and immunoblotting was carried out with anti-Myc (IB: Myc-UB) or anti-Nav1.5 (IB: Nav1.5) antibodies. Ubiquitinated-Nav1.5 (UB-Nav1.5) is indicated by a bracket above 250 kDa. (C) Immunoprecipitation analysis of Nav1.5 protein ubiquitination in HEK293 cells co-transfected with pEGFP-Nav1.5 and pMyc-UBB together with or without pCMV-UBC9. Cell lysates were immunoprecipitated with an anti-GFP antibody (IP: GFP-Nav1.5), and immunoblotting was carried out with anti-MYC (IB: Myc-Ub) or anti-Nav1.5 (IB: Nav1.5) antibodies. Overexpression of UBC9 enhanced Nav1.5 ubiquitination. Ubiquitinated-Nav1.5 (UB-Nav1.5) is indicated by a bracket above 250 kDa. (D) Confirmation of the effect of UBC9 on ubiquitination of Nav1.5 using the FK2 antibody. HEK293-Nav1.5 stable cells were transfected with pCMV-UBC9 or control pCMV-HA empty vector, and lysed. Cell lysates were immunoprecipitated with an anti-Nav1.5 antibody (IP: Nav1.5) or rabbit IgG control, and immunoblotting was carried out with FK2 recognizing ubiquitin. After stripping, the membrane was re-probed with an anti-Nav1.5 antibody (IB: Nav1.5). Ubiquitinated-Nav1.5 (UB-Nav1.5) is indicated by a bracket above 250 kDa.
To further demonstrate the effect of UBC9 on ubiquitination of Nav1.5, we performed a uniquitin assay using FK2, an anti-ubiquitin mouse monoclonal antibody. HEK293-Nav1.5 cells were transfected with an overexpression plasmid for UBC9 or an empty vector as control, and treated with MG132. The cell lysates were immunoprecipitated using an anti-Nav1.5 antibody or a control rabbit IgG, and Western blot analysis was performed with the precipitates and FK2. As shown in Fig. 8D, overexpression of UBC9 increased the level of ubiquitinated-Nav1.5. The data with FK2 further show that UBC9 can stimulate the ubiquitination of Nav1.5.
3.9. UBC9 interacts with Nedd4–2
Nedd4–2 was shown to be a candidate E3 ligase for the Ub-conjugation machinery involved in regulation of Nav1.5 degradation [16]. We tested the hypothesis that UBC9 interacts with Nedd4–2 to regulate the ubiquitination of Nav1.5. To test the hypothesis, we co-expressed HA-UBC9 and FLAG-Nedd4–2 in HEK293-Nav1.5 cells, and performed co-immunoprecipitation (Co-IP) analysis. An anti-HA antibody recognizing HA-UBC9 successfully immunoprecipitated FLAG-Nedd4–2 recognized by an anti-FLAG antibody (Fig. 9A). Reciprocal Co-IP showed that an anti-FLAG antibody recognizing FLAG-Nedd4–2 precipitated UBC9 recognized by an anti-UBC9 antibody (Fig. 9B). Overexpression of UBC9 did not affect the expression level of Nedd4–2 (Fig. 9C). These data demonstrate that UBC9 interacts with Nedd4–2.
Fig. 9.
UBC9 interacts with NEDD4–2. (A) Co-IP analysis of interaction between HA-tagged UBC9 and FLAG-tagged Nedd4–2. Immunoprecipitation was carried out with an anti-HA antibody, and immunoblotting was with an anti-FLAG antibody. Anti-HA-UBC9 successfully precipitated FLAG-Nedd4–2. (B) Co-IP analysis of interaction between FLAG-tagged Nedd4–2 and UBC9. Immunoprecipitation was carried out with an anti-FLAG antibody, and immunoblotting was with an anti-UBC9 antibody. Anti-FLAG-Nedd4–2 successfully precipitated UBC9. (C) Western blot analysis of Nedd4–2 in HEK293 cells with or without overexpression of UBC9. Overexpression of UBC9 did not affect the level of Nedd4–2 expression.
4. Discussion
Post-translational modifications by Ub regulate protein turnover and degradation involved in numerous cellular processes [15]. The Ub conjugation system is a complex machinery composed of three basic enzymatic activities, E1, E2, and E3, which mediate the transfer of Ub via an E1–E2–E3 cascade to various protein substrates to form polyUb chains [15]. The protein substrates carrying polyUb chains are targeted to the 26S proteasome for degradation [15]. In mammalian cells, there are two E1 activating enzymes, about 40 E2 Ub conjugating enzymes, and >600 E3 ubiquitin ligases [19, 43]. This generates high heterogeneity for the ubiquitin system so that the degradation of numerous protein substrates can be properly regulated. However, little is known about the specifics of ubiquitin enzymes involved in ubiquitination of Nav1.5. van Bemmelen et al [16] showed that an E3 ubiquitin ligase Nedd4–2 was able to bind to the PY-motif at C-terminus of Nav1.5, increased Nav1.5 ubiquitination and reduced cardiac sodium current densities. These data suggest that Nedd4–2 is an E3 ubiquitin ligase of the Ub conjugation machinery involved in ubiquitination of Nav1.5. Interestingly, we recently found that the activity of Nedd4–2 can be modulated by heat-shock chaperon αB-crystallin [16]. αB-crystallin showed interaction with Nedd4–2, decreased ubiquitination of Nav1.5, and reduced internalization of plasma membrane Nav1.5, resulting in an increased level of cell surface Nav1.5 and increased cardiac sodium current densities although the detailed molecular mechanism remains to be further defined [16].
In the present study, we found that UBC9 is involved in the ubiquitination of Nav1.5. UBC9 is the single E2 enzyme of the SUMOylation machinery, which is also a post-translational modification system composed of three E1, E2, and E3 enzymatic activities [18]. Different from the Ub conjugation system, SUMOylation is mediated by only one heterodimeric E1 enzyme (SAE1/SAE2), a single E2 enzyme (UBC9), and a limited number of E3 ligases [18].
To study the role of SUMOylation in regulation of Nav1.5 levels, we overexpressed UBC9 in HEK293-Nav1.5 cells and knocked its expression down, and then analyzed their impact on the level of Nav1.5 and densities of cardiac sodium currents. Our results showed that overexpression of UBC9 significantly decreased the protein levels of Nav1.5 channels (Fig. 1) and reduced cardiac sodium current densities (Fig. 2). Moreover, knockdown of UBC9 expression significantly increased the protein levels of Nav1.5 channels (Fig. 3) and increased cardiac sodium current densities (Fig. 4). Most importantly, overexpression and knockdown of UBC9 affected cardiac sodium current densities in neonatal rat cardiomyocytes (Fig. 5). UBC9 was previously shown to interact with CRMP2 to regulate the function of Nav1.7. However, our studies with the t-CSM peptide that disrupts the UBC9-CRMP2 interaction showed that the UBC9-CRMP2 interaction did not affect the Nav1.5 function with or without overexpression of UBC9 (Fig. 6).
The cysteine residue at codon 93 of UBC9 was shown to be required for the formation of a thiol ester bond with SUMO1, and a dominant negative mutation at the site, pCys93S, disrupts a thiol ester bond formation and SUMOylation of target proteins [42]. We showed that mutant UBC9 with the C93S mutation inhibited the SUMOylation of p53 (Fig. 7A), however, it did not affect the function of UBC9 regulation on the level of Nav1.5 (Fig. 7B). The data suggest that the impact of UBC9 on the level of Nav1.5 and densities of cardiac sodium currents is not associated with SUMOylation of Nav1.5. We further showed that UBC9 interacts with Nedd4–2, which supports a role of UBC9 in Nav1.5 ubiquitination (Fig. 9). Moreover, we found that UBC9 promoted Nav1.5 ubiquitination and degradation through 26S proteasomes (Fig. 8). Altogether, our data implicate UBC9 as the E2 conjugating enzyme for the Ub-conjugating system.
In conclusion, the data in this study indicate that UBC9 promotes the ubiquitination and degradation of Nav1.5 and regulates cardiac sodium current density. Because overexpression and knockdown of UBC9 significantly affect cardiac sodium current densities, agents or tools that can modulate UBC9 expression and function may help treatment or therapeutic interventions of human diseases associated with Nav1.5 mutations and altered expression.
Highlights.
UBC9 overexpression decreases Nav1.5 expression and sodium current densities.
Knockdown of UBC9 expression enhances Nav1.5 expression and sodium current densities.
UBC9 promotes Nav1.5 ubiquitination and degradation, but not SUMOylation.
UBC9 interacts with NEDD4–2, an E3 ligase for ubiquitination and degradation of Nav1.5.
UBC9 is a key structural element of the ubiquitin-conjugation machinery for Nav1.5.
UBC9 may be a target for developing treatment or therapeutic interventions of human diseases associated with Nav1.5 mutations and altered expression.
Acknowledgments
We thank Annabel Z. Wang at Harvard Medical School for careful reading and proofing of this manuscript, Drs. Jiaxiang Chen, Yuang Huang and Xia Li for technical advice and training of junior graduate students, other members of Center for Human Genome Research for discussions and assistance, Dr. Hughes Abriel for the expression plasmid for Nedd4–2 and Dr. Glenn Kirsch for HEK293/Nav1.5 cell line. This study was supported by the China National Natural Science Foundation grants (31430047, 81600263 and 81630002) (C.X. and P.W.), NIH/NHLBI grant R01 HL126729 (Q.K.W.), and Hubei Province Natural Science Program Innovative Team grant (2017CFA014) (C.X.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no conflict of interest with the contents of this article.
References
- [1].Wang Q, Li Z, Shen J, Keating MT, Genomic organization of the human SCN5A gene encoding the cardiac sodium channel, Genomics 34(1) (1996) 9–16. [DOI] [PubMed] [Google Scholar]
- [2].Wang Q, RE P, E SC, CT B, Genetic studies of myocardial and vascular disease, in: EJ T (Ed.), Textbook of Cardiovascular Medicine, Lippincott Williams & Wilkins, Philadelphia, PA, 2006, pp. 1967–1989. [Google Scholar]
- [3].Wang Q, Shen J, Li Z, Timothy K, Vincent GM, Priori SG, Schwartz PJ, Keating MT, Cardiac sodium channel mutations in patients with long QT syndrome, an inherited cardiac arrhythmia, Hum. Mol. Genet 4(9) (1995) 1603–1607. [DOI] [PubMed] [Google Scholar]
- [4].Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, Moss AJ, Towbin JA, Keating MT, SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome, Cell 80(5) (1995) 805–811. [DOI] [PubMed] [Google Scholar]
- [5].Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, Potenza D, Moya A, Borggrefe M, Breithardt G, Ortiz-Lopez R, Wang Z, Antzelevitch C, O’Brien RE, Schulze-Bahr E, Keating MT, Towbin JA, Wang Q, Genetic basis and molecular mechanism for idiopathic ventricular fibrillation, Nature 392(6673) (1998) 293–296. [DOI] [PubMed] [Google Scholar]
- [6].Wang Q, Chen S, Chen Q, Wan X, Shen J, Hoeltge GA, Timur AA, Keating MT, Kirsch GE, The common SCN5A mutation R1193Q causes LQTS-type electrophysiological alterations of the cardiac sodium channel, Journal of medical genetics 41(5) (2004) e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Benson DW, Silberbach GM, Kavanaugh-McHugh A, Cottrill C, Zhang Y, Riggs S, Smalls O, Johnson MC, Watson MS, Seidman JG, Seidman CE, Plowden J, Kugler JD, Mutations in the cardiac transcription factor NKX2.5 affect diverse cardiac developmental pathways [see comments], J. Clin. Invest 104(11) (1999) 1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Huang CL, Murine Electrophysiological Models of Cardiac Arrhythmogenesis, Physiol Rev 97(1) (2017) 283–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kapplinger JD, Tester DJ, Alders M, Benito B, Berthet M, Brugada J, Brugada P, Fressart V, Guerchicoff A, Harris-Kerr C, Kamakura S, Kyndt F, Koopmann TT, Miyamoto Y, Pfeiffer R, Pollevick GD, Probst V, Zumhagen S, Vatta M, Towbin JA, Shimizu W, Schulze-Bahr E, Antzelevitch C, Salisbury BA, Guicheney P, Wilde AA, Brugada R, Schott JJ, Ackerman MJ, An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing, Heart Rhythm 7(1) (2010) 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rook MB, Evers MM, Vos MA, Bierhuizen MF, Biology of cardiac sodium channel Nav1.5 expression, Cardiovasc. Res 93(1) (2012) 12–23. [DOI] [PubMed] [Google Scholar]
- [11].Kang L, Zheng MQ, Morishima M, Wang Y, Kaku T, Ono K, Bepridil up-regulates cardiac Na+ channels as a long-term effect by blunting proteasome signals through inhibition of calmodulin activity, Br J Pharmacol 157(3) (2009) 404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shao D, Okuse K, Djamgoz MB, Protein-protein interactions involving voltage-gated sodium channels: Post-translational regulation, intracellular trafficking and functional expression, Int. J. Biochem. Cell Biol 41(7) (2009) 1471–1481. [DOI] [PubMed] [Google Scholar]
- [13].Herrmann J, Ciechanover A, Lerman LO, Lerman A, The ubiquitin-proteasome system in cardiovascular diseases-a hypothesis extended, Cardiovascular research 61(1) (2004) 11–21. [DOI] [PubMed] [Google Scholar]
- [14].Hicke L, Gettin’ down with ubiquitin: turning off cell-surface receptors, transporters and channels, Trends Cell Biol 9(3) (1999) 107–12. [DOI] [PubMed] [Google Scholar]
- [15].Hershko A, Ciechanover A, The ubiquitin system, Annual review of biochemistry 67 (1998) 425–79. [DOI] [PubMed] [Google Scholar]
- [16].van Bemmelen MX, Rougier JS, Gavillet B, Apotheloz F, Daidie D, Tateyama M, Rivolta I, Thomas MA, Kass RS, Staub O, Abriel H, Cardiac voltage-gated sodium channel Nav1.5 is regulated by Nedd4–2 mediated ubiquitination, Circ. Res 95(3) (2004) 284–291. [DOI] [PubMed] [Google Scholar]
- [17].Huang Y, Wang Z, Liu Y, Xiong H, Zhao Y, Wu L, Yuan C, Wang L, Hou Y, Yu G, Huang Z, Xu C, Chen Q, Wang QK, alphaB-Crystallin Interacts with Nav1.5 and Regulates Ubiquitination and Internalization of Cell Surface Nav1.5, J. Biol. Chem 291(21) (2016) 11030–11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Flotho A, Melchior F, Sumoylation: a regulatory protein modification in health and disease, Annual review of biochemistry 82 (2013) 357–85. [DOI] [PubMed] [Google Scholar]
- [19].Wiechmann S, Gartner A, Kniss A, Stengl A, Behrends C, Rogov VV, Rodriguez MS, Dotsch V, Muller S, Ernst A, Site-specific inhibition of the small ubiquitin-like modifier (SUMO)-conjugating enzyme Ubc9 selectively impairs SUMO chain formation, The Journal of biological chemistry 292(37) (2017) 15340–15351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].McElhinny AS, Kakinuma K, Sorimachi H, Labeit S, Gregorio CC, Muscle-specific RING finger-1 interacts with titin to regulate sarcomeric M-line and thick filament structure and may have nuclear functions via its interaction with glucocorticoid modulatory element binding protein-1, J Cell Biol 157(1) (2002) 125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ahner A, Gong X, Frizzell RA, Cystic fibrosis transmembrane conductance regulator degradation: cross-talk between the ubiquitylation and SUMOylation pathways, The FEBS journal 280(18) (2013) 4430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Uemura A, Taniguchi M, Matsuo Y, Oku M, Wakabayashi S, Yoshida H, UBC9 regulates the stability of XBP1, a key transcription factor controlling the ER stress response, Cell Struct Funct 38(1) (2013) 67–79. [DOI] [PubMed] [Google Scholar]
- [23].Wan X, Chen S, Sadeghpour A, Wang Q, Kirsch GE, Accelerated inactivation in a mutant Na(+) channel associated with idiopathic ventricular fibrillation, Am J Physiol Heart Circ Physiol 280(1) (2001) H354–60. [DOI] [PubMed] [Google Scholar]
- [24].Wan X, Wang Q, Kirsch GE, Functional suppression of sodium channels by beta(1)-subunits as a molecular mechanism of idiopathic ventricular fibrillation, J Mol Cell Cardiol 32(10) (2000) 1873–84. [DOI] [PubMed] [Google Scholar]
- [25].Dumaine R, Wang Q, Keating MT, Hartmann HA, Schwartz PJ, Brown AM, Kirsch GE, Multiple mechanisms of Na+ channel--linked long-QT syndrome, Circ. Res 78(5) (1996) 916–924. [DOI] [PubMed] [Google Scholar]
- [26].Chakrabarti S, Wu X, Yang Z, Wu L, Yong SL, Zhang C, Hu K, Wang QK, Chen Q, MOG1 rescues defective trafficking of Na(v)1.5 mutations in Brugada syndrome and sick sinus syndrome, Circ. Arrhythm. Electrophysiol 6(2) (2013) 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wu L, Yong SL, Fan C, Ni Y, Yoo S, Zhang T, Zhang X, Obejero-Paz CA, Rho HJ, Ke T, Szafranski P, Jones SW, Chen Q, Wang QK, Identification of a new co-factor, MOG1, required for the full function of cardiac sodium channel Nav 1.5, J. Biol. Chem 283(11) (2008) 6968–6978. [DOI] [PubMed] [Google Scholar]
- [28].Li S, Xi Q, Zhang X, Yu D, Li L, Jiang Z, Chen Q, Wang QK, Traboulsi EI, Identification of a mutation in CNNM4 by whole exome sequencing in an Amish family and functional link between CNNM4 and IQCB1, Molecular genetics and genomics : MGG 293(3) (2018) 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li X, Poschmann S, Chen Q, Fazeli W, Oundjian NJ, Snoeijen-Schouwenaars FM, Fricke O, Kamsteeg EJ, Willemsen M, Wang QK, De novo BK channel variant causes epilepsy by affecting voltage gating but not Ca(2+) sensitivity, European journal of human genetics : EJHG 26(2) (2018) 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yao Y, Lu Q, Hu Z, Yu Y, Chen Q, Wang QK, A non-canonical pathway regulates ER stress signaling and blocks ER stress-induced apoptosis and heart failure, Nat. Commun 8(1) (2017) 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ye J, Yao Y, Song Q, Li S, Hu Z, Yu Y, Hu C, Da X, Li H, Chen Q, Wang QK, Up-regulation of miR-95–3p in hepatocellular carcinoma promotes tumorigenesis by targeting p21 expression, Sci. Rep 6 (2016) 34034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhao Y, Huang Y, Li W, Wang Z, Zhan S, Zhou M, Yao Y, Zeng Z, Hou Y, Chen Q, Tu X, Wang QK, Huang Z, Post-transcriptional regulation of cardiac sodium channel gene SCN5A expression and function by miR-192–5p, Biochim. Biophys. Acta 1852(10 Pt A) (2015) 2024–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chakrabarti S, Wu X, Yang Z, Wu L, Yong SL, Zhang C, Hu K, Wang QK, Chen Q, MOG1 rescues defective trafficking of Na(v)1.5 mutations in Brugada syndrome and sick sinus syndrome, Circulation. Arrhythmia and electrophysiology 6(2) (2013) 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wu L, Yong SL, Fan C, Ni Y, Yoo S, Zhang T, Zhang X, Obejero-Paz CA, Rho HJ, Ke T, Szafranski P, Jones SW, Chen Q, Wang QK, Identification of a new co-factor, MOG1, required for the full function of cardiac sodium channel Nav 1.5, The Journal of biological chemistry 283(11) (2008) 6968–78. [DOI] [PubMed] [Google Scholar]
- [35].Wang Z, Yu G, Liu Y, Liu S, Aridor M, Huang Y, Hu Y, Wang L, Li S, Xiong H, Tang B, Li X, Cheng C, Chakrabarti S, Wang F, Wu Q, Karnik SS, Xu C, Chen Q, Wang QK, Small GTPases SAR1A and SAR1B regulate the trafficking of the cardiac sodium channel Nav1.5, Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1864(11) (2018) 3672–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Luo C, Wang F, Qin S, Chen Q, Wang Q, Coronary artery disease susceptibility gene ADTRP regulates cell cycle progression, proliferation and apoptosis by global gene expression regulation, Physiol Genomics 48(8) (2016) 554–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Luo C, Wang F, Ren X, Ke T, Xu C, Tang B, Qin S, Yao Y, Chen Q, Wang QK, Identification of a molecular signaling gene-gene regulatory network between GWAS susceptibility genes ADTRP and MIA3/TANGO1 for coronary artery disease, Biochim. Biophys. Acta 1863(6) (2017) 1640–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yong SL, Ni Y, Zhang T, Tester DJ, Ackerman MJ, Wang QK, Characterization of the cardiac sodium channel SCN5A mutation, N(1325)S, in single murine ventricular myocytes, Biochem. Biophys. Res. Commun 352(2) (2007) 378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhang T, Yong SL, Tian XL, Wang QK, Cardiac-specific overexpression of SCN5A gene leads to shorter P wave duration and PR interval in transgenic mice, Biochem. Biophys. Res. Commun 355(2) (2007) 444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Francois-Moutal L, Dustrude ET, Wang Y, Brustovetsky T, Dorame A, Ju W, Moutal A, Perez-Miller S, Brustovetsky N, Gokhale V, Khanna M, Khanna R, Inhibition of the Ubc9 E2 SUMO-conjugating enzyme-CRMP2 interaction decreases NaV1.7 currents and reverses experimental neuropathic pain, Pain (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Khanna R, Wilson SM, Brittain JM, Weimer J, Sultana R, Butterfield A, Hensley K, Opening Pandora’s jar: a primer on the putative roles of CRMP2 in a panoply of neurodegenerative, sensory and motor neuron, and central disorders, Future neurology 7(6) (2012) 749–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gong L, Kamitani T, Fujise K, Caskey LS, Yeh ET, Preferential interaction of sentrin with a ubiquitin-conjugating enzyme, Ubc9, The Journal of biological chemistry 272(45) (1997) 28198–201. [DOI] [PubMed] [Google Scholar]
- [43].Claessen JH, Kundrat L, Ploegh HL, Protein quality control in the ER: balancing the ubiquitin checkbook, Trends Cell Biol 22(1) (2012) 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]