Abstract
The vast majority of patients with pancreatic ductal adenocarcinoma (PDAC) presents with symptomatic, surgically unresectable disease. While the goal of early detection of PDAC is laudable, and likely to result in significant improvement in overall survival, the relatively low prevalence of PDAC renders general population screening infeasible. The challenges of early detection include identification of at-risk individuals in the general population who would benefit from longitudinal surveillance programs, and appropriate biomarker and imaging-based modalities utilized for PDAC surveillance in such cohorts. In recent years, various subgroups at higher than average risk for PDAC have been identified, including those with familial risk due to germline mutations, a history of pancreatitis, patients with mucinous pancreatic cysts, and elderly patients with new onset diabetes (NOD). The last two categories will be discussed at length in terms of the opportunities and challenges they present for PDAC early detection. We also discuss current and emerging imaging modalities that are critical to identifying early, potentially curable, PDAC in high-risk cohorts on surveillance.
Early detection of pancreatic cancer: Overview
The overwhelming majority of patients with pancreatic ductal adenocarcinoma (PDAC) present with locally advanced or distant metastatic disease (80–85%), and only a minority of patients are surgically resectable (15–20%) 1, 2. In prior limited clinical series from the Far East, patients with incidentally discovered PDAC, especially those with sub-centimeter lesions, were documented to have prolonged survival rates 3–5. More recent data from a national registry of patients on longitudinal surveillance for PDAC incidence due to familial risk also underscores the notion that earlier diagnosis correlates with improved survival, albeit not always “cure”. As treatment options for patients with resectable cancers continue to improve, including availability of multimodality neoadjuvant therapy 6, 7, and more potent adjuvant regimens 8, a “stage shift” from the current 15% resectable proportion to 50% or greater will unequivocally lead to improved survival in this otherwise dismal disease 9. How, then, do we enable successful early detection of PDAC beyond anecdotal case reports?
While the goal of early detection in PDAC remains laudable, it is worth noting that the United States Prevention and Screening Task Force (USPSTF) has rendered a grade of “D” for screening for PDAC in the general population, suggesting that not only is it not helpful, but there is potential for significant harm. This is due, in large part, to the relatively low incidence of PDAC in the average risk population (~12 per 100,000), which substantially reduces the pre-test possibility of a laboratory test being truly positive. Thus, even biomarker assays with exceptionally high specificity of 99% will result in ~990 individuals undergoing imaging studies, and the associated anxiety that comes with the likelihood of a highly lethal cancer, without actually harboring the disease. The potential for over-diagnosis and over-treatment remains significant enough that a “PSA” (prostate specific antigen – a commonly ordered screening test for prostate cancer) assay for PDAC is impractical. Another challenge in PDAC early detection, which we will not be covering in this review due to brevity, pertains to the current lack of availability of credentialed biomarkers with performance criteria required for adoption in an asymptomatic prospective population. Nearly all classes of biomarker assays published to date in PDAC (proteins, autoantibodies, circulating DNA, microRNAs, methylated DNA, exosomes; a limited number of citations is referenced here 10–14) have been used in the context of symptomatic disease, i.e., in a “diagnostic biomarker” context, with scant data in the setting of longitudinal surveillance in asymptomatic individuals (“surveillance biomarkers”).
To avoid the perils of over-diagnosis and focus early detection efforts on individuals deemed to be at higher than average risk, we need to first define who those subsets of individuals are and quantify the degree of elevated risk. Once that has been determined, the next step is to determine when and how often to conduct surveillance in the at-risk individuals, and the modalities (biomarkers and imaging) that will be used in the surveillance versus diagnostic settings, respectively. In the context of PDAC, we are still early in deciphering this multistep paradigm, but unequivocal progress has been made, at least in the context of defining high-risk cohorts primed for surveillance. In a separate review of this special issue, Wood et al discuss one of the aforementioned high-risk groups, individuals with a familial (inherited) PDAC risk. In this article, we will focus our discussion on three remaining at-risk cohorts, patients with a history of pancreatitis, patients with mucinous cysts of the pancreas and elderly patients with new-onset diabetes (NOD) and highlight both the opportunities for leveraging these subsets as a means to achieve early detection and the pitfalls that exist today to actualize that vision. We will also discuss the current and emerging imaging modalities that are at the disposal of clinicians for localizing early primaries in individuals that are on surveillance in both cystic and non-cystic settings. Finally, we culminate this review with our vision for the future of early detection for PDAC, with an eye towards altering the trajectory of the usually lethal natural history of this cancer.
Pancreatitis associated risk factor for pancreatic cancer
There is emerging evidence that supports long-standing chronic pancreatitis as a strong risk factor for PDAC. The lag period between diagnosis of chronic pancreatitis and PDAC is usually one or two decades15. A recent meta-analysis of 13 studies showed that excluding cancer occurring in the first 2 years following a diagnosis of chronic pancreatitis, the lifetime risk of PDAC was elevated 16-fold16. Although there is a strong link between chronic pancreatitis and PDAC, < 5% of patients with chronic pancreatitis develop PDAC and it is a rare cause of PDAC17. Pancreatitis appearing a year or two before the diagnosis of PDAC is often the result of tumor-related ductal obstruction. Therefore, acute pancreatitis is considered to be a clinical manifestation of PDAC. However, the yield of PDAC after an episode of acute pancreatitis is ~1%, highest being in the first 2 years16, 18. Conversely, only a small fraction of PDAC patients (~5%) present with acute pancreatitis at the time of cancer diagnosis18. Since only a small proportion of pancreatitis, both acute and chronic, have or develop sporadic PDAC, using them as a potential high–risk screening groups for early detection of PDAC will require enrichment strategies to identify the subset with very high-risk.
Cystic precursor lesions of pancreatic cancer and the route to early detection: Overview
While a distinct minority, up to 15% of PDAC are thought to arise from mucinous pancreatic cysts that include intraductal papillary mucinous neoplasms (IPMNs) and mucinous cystic neoplasms (MCNs). Most mucinous pancreatic cysts are IPMNs and can be broadly categorized based on location and extent of involvement within the pancreas as main-duct (MD-IPMN), branch-duct (BD-IPMN) and mixed type (MT-IPMN) 19. MD-IPMNs account for 15–21% of IPMNs, often located within the pancreatic head and characterized by a segmental or diffuse dilatation of the main pancreatic duct (MPD) of >5 mm in diameter for which other causes of ductal obstruction have been excluded 20. In comparison, BD-IPMNs comprise 41–64% of IPMNs, occur throughout the pancreas with a preference for the uncinate process and are frequently multifocal. BD-IPMNs are typically described as a unilocular or grape-like/multilobulated arrangement that communicates with the MPD. MT-IPMNs meet both criteria for MD-IPMN and BD-IPMN and consist of 22–38% of IPMNs.
Not surprisingly, the incidence of PDAC with an IPMN can vary based on subtype. PDAC is reported in 11–80% of MD-IPMNs and 20–65% of MT-IPMNs 21, 22. Considering the high incidence of malignancy, patients with a MD-IPMN or MT-IPMN are often recommended to undergo surgical resection. However, malignant transformation of BD-IPMN is documented in 1–36% of surgical resections 20. Moreover, as these statistics are based on surgical series, the malignant potential of a BD-IPMN may be overestimated. Further, on the basis of preoperative clinical, radiographic and pathologic findings, the distinction between a BD-IPMN and other pancreatic cysts is not trivial. Benign neoplastic and non-neoplastic cysts, such as serous cystadenomas and lymphoepithelial cysts, can preoperatively mimic BD-IPMNs.23, 24 Consequently, key areas for early detection efforts have been the accurate diagnosis of BD-IPMNs and the identification of high-grade dysplasia and/or microscopic PDAC arising from BD-IPMNs. It is also important to note that patients with an IPMN are at an increased risk for not only developing IPMN-associated PDAC, but also PDAC independent from the IPMN (“concomitant carcinomas”). The reported incidence of concomitant carcinomas in IPMN patients ranges between 2%−11.2% 20. Hence, the detection of a concomitant carcinoma is also an important focus for early detection strategies.
Epidemiologic risk factors for cystic precursor lesions
There are several risk factors for developing an IPMN and an IPMN-associated PDAC. With increasing use and advancements in radiographic imaging (see later), the presence of a pancreatic cyst is reported in 3% of computed tomography (CT) scans and 20% of magnetic resonance imaging (MRI) scans 25, 26. This prevalence increases with age and up to 40% of patients over the age of 80 years are found to have a pancreatic cyst 27. As approximately half of all pancreatic cysts are BD-IPMNs, advanced age is a well-recognized risk factor for both BD-IPMNs and BD-IPMN-associated PDAC 28. In addition to non-IPMN associated PDAC, a causative link between diabetes and IPMNs has been described. Among BD-IPMN patients, 10–45% have a history of diabetes and, in the setting of diabetes, the incidence of detecting a BD-IPMN is higher 29–37. Capurso et al identified a strong association between insulin use and the risk of an IPMN 38. Moreover, diabetes is associated with a higher incidence of PDAC in resected IPMNs 39. New-onset or worsening diabetes is also a significant predictor of the presence of a concomitant carcinoma 40. However, NOD was not associated with an increased incidence of an IPMN in the absence of cancer and suggests that BD-IPMNs do not produce the same diabetogenic substances as PDAC (see later). Further, patients with chronic pancreatitis are at an increased risk of developing a BD-IPMN and BD-IPMN-associated PDAC 35, 38. Interestingly, BD-IPMNs can often mimic retention cysts as seen in the background of chronic pancreatitis.41 Conversely, chronic pancreatitis may be the consequence of longstanding occlusion of the pancreatic duct due to the mucin produced within an BD-IPMN itself.
Certain genetic syndromes and a family history of PDAC may also pose a risk (see review by Wood et al in this issue). IPMNs have been reported in patients with Peutz-Jeghers syndrome, McCune-Albright syndrome and in patients with familial adenomatous polyposis 42–45. Some studies have suggested that BD-IPMNs and BD-IPMN-associated cancers may be particularly common among patients with a history of a first-degree family member with PDAC 38. It is however unknown whether patients with a positive family history have a more rapid progression of developing an IPMN or associated PDAC.
Diagnostic methods of evaluating BD-IPMN patients
In most cases, BD-IPMNs are discovered incidentally on routine imaging and patients are often asymptomatic at clinical presentation. Some patients may present with abdominal discomfort, abdominal pain, malaise and nausea; however, these symptoms are typically not attributable to the IPMN even if they were the initial indication for abdominal imaging 28. Other symptoms that include back pain, weight loss, NOD, and obstructive jaundice are more often associated with malignant transformation of an IPMN, but once again are not entirely specific 29–32, 34, 35, 37, 46–52.
Considering the lack of symptoms in the majority of patients, conventional imaging modalities play a crucial role in the identification of IPMNs and IPMN-associated PDAC, as well as the detection of a concomitant carcinoma. Here we will discuss the performance of imaging modalities used in the context of cystic lesions; please see later for choice of imaging in the context of solid lesions (non-cystic PDAC).
Owing to the wide availability and rapidity of acquisition, CT is an ideal imaging method for the initial evaluation of a BD-IPMN with an accuracy of 56–85% 53. The detection of calcifications within a pancreatic cyst and surrounding pancreatic parenchyma can aid in differentiating a BD-IPMN from its mimics 54–56. MRI/magnetic resonance cholangiopancreatography (MRCP) is however considered by many as the standard modality for diagnosing a BD-IPMN with a sensitivity of up to 88% 53, 57–60. MRI/MRCP is superior to CT in its ability to identify MPD connectivity and features of malignancy. Further, complementing MRCP with secretin stimulation can elucidate pancreatic ductal anatomy 53. The lack of ionizing radiation makes MRI an ideal tool for frequent follow-up exams, especially in younger patients. But, the drawbacks of MRI include poor spatial resolution, low sensitivity for calcifications and susceptibility to motion-related artifacts 61.
Despite the quality of contemporary cross-sectional imaging, the accuracy of CT and MRI remain imperfect. Endoscopic ultrasound (EUS) has a higher resolution than cross-sectional imaging methods and can be useful for cases where a diagnosis of a BD-IPMN is uncertain, a BD-IPMN has worrisome features by CT/MRI, verification of malignancy in high-risk individuals and the identification of concomitant carcinomas 62, 63. EUS excels in evaluating for imaging features often associated with malignancy, such as thick internal septations, mural nodularity, solid masses, MPD dilatation, filling defects in the MPD and vascular invasion 64, 65. These features alone are however poor predictors of malignant transformation with an accuracy that ranges between 40–90% 66–68. The true utility of EUS is enhanced when coupled with fine-needle aspiration (FNA) of pancreatic cyst fluid that can be used for biochemical, cytological and DNA analyses.
Pancreatic cyst fluid diagnostics for early detection of progression
Pancreatic cyst fluid (PCF) from BD-IPMNs is generally thick and highly viscous. The “string sign” method is a rapid assay to evaluate fluid viscosity and is performed by placing a drop of fluid between two fingers and separating them 69. A positive “string sign” has up to 95% specificity for a mucinous pancreatic cyst 70. Similarly, high concentrations of CEA (>192 ng/mL) within PCF are reflective of a mucinous pancreatic cyst and associated with a 57–79% sensitivity 64, 71–74. However, in certain circumstances, sufficient PCF may not be available for CEA testing. Regardless, both methods do not reliably differentiate BD-IPMNs from MCNs or the presence versus absence of PDAC. Cytological examination is a highly accurate test for the detection of malignancy with a specificity that approaches 100%, but this technique is hampered by the low cellularity of PCF and, therefore, the sensitivity of cytopathology varies widely from 25–88% 67, 75, 76. The
Recently, next-generation sequencing (NGS) has emerged as an adjunct to the evaluation of PCF 71, 77–80. Although cellular content and fluid volume of PCF can be suboptimal for routine ancillary studies, such as CEA and cytological examination, DNA from lysed or exfoliated cyst epithelium shed into the PCF can be analyzed for genomic alterations. NGS studies have identified distinct mutational profiles for the major pancreatic cysts and those that have progressed to PDAC 81–84. The detection of KRAS mutations in PCF by NGS is associated with 76–89% sensitivity and 96–100% specificity for BD-IPMNs and MCNs 71, 78–80. GNAS mutations are also found in 30–45% of BD-IPMNs, but highly specific for IPMNs, and have not been reported in MCNs 71, 78, 79. Additionally, IPMN-associated cancers are reported to harbor mutations in TP53, SMAD4, PIK3CA, PTEN and/or AKT1 with sensitivities and specificities of 32–79% and 96–100%, respectively 71, 85–90. Of note, the high costs associated with NGS have impeded its widespread clinical application to PCF. However, with increasing availability of NGS, decreasing prices in reagents and the ability to batch specimens, the current cost of NGS-based PCF testing is one-third of the cost for an MRI/MRCP scan 91.
In addition to NGS-based PCF testing, there are several other genetic, epigenetic, proteomic and carbohydrate-based PCF biomarkers that are currently being validated for clinical use. For example, mucins or MUCs are a 21-member family of heavily glycosylated, high-molecular-weight glycoproteins and play a variety of roles in oncogenesis. Normal pancreatic ductal epithelium expresses low levels of mucins, such as MUC1; however, correlative histopathologic studies show that there is neo-expression and upregulation of mucins in BD-IPMNs, such as MUC2, MUC4 and MUC5AC, and more pronounced changes in expression in PDAC, such as MUC3, MUC4, MUC5AC, MUC5B, MUC6, MUC7, MUC13, MUC16 and MUC17.92–95 Moreover, carbohydrate alterations to mucins detected in PCF have demonstrated high sensitivity and high specificity in differentiating mucinous from non-mucinous pancreatic cysts, and early detection of IPMN-associated PDACs.96, 97 In fact, MUC4 and MUC16 are reported to be 100% specific for PDAC, while associated with sensitivities of 63% and 67%, respectively.98 Promising biomarker results using PCF have also been reported for differentially methylated DNA, telomerase activity, protease expression and the overexpression of Das-1.99–102 However, the majority of these biomarkers have not been rigorous validated within a diverse cohort of pancreatic cysts. Hence, the goal of the Pancreatic Cyst Biomarker Validation Study, an ongoing double blinded PCF biomarker study that is sponsored by the National Cancer Institute (NCI) Early Detection Research Network.103
Current guidelines for surveillance and management of patients with pancreatic cysts: a murky road
In the absence of a perfect assay to detect BD-IPMNs and BD-IPMN-associated PDAC, the evaluation of pancreatic cysts necessitates a multidisciplinary approach that includes clinical presentation, radiographic/endoscopic ultrasound imaging and PCF analysis. The inability to predict the malignant transformation of a BD-IPMN within the patient’s lifetime requires appropriate surveillance that accounts for epidemiologic risk factors, as well as other clinical, imaging and PCF findings. Moreover, as surgical intervention remains the preferred treatment option for mucinous pancreatic cysts, the operative mortality (2–4%) and morbidity (40–50%) of these procedures must be considered.104–107 Consequently, consensus and evidence-based guidelines for pancreatic cysts and, specifically, BD-IPMNs have been developed by several medical societies, namely the International Association of Pancreatology (Fukuoka), American Gastroenterological Association (AGA), American College of Gastroenterology (ACG), American College of Radiology (ACR) and European Study Group on Cystic Tumours of the Pancreas (ESGCTP) 22, 108–111.
While the surveillance strategy for BD-IPMNs will differ between these guidelines, they all agree that the risk of malignancy should be weighed against life expectancy and comorbidities. In addition, according to all guidelines, the presence of a mural nodule is the most predictive of malignant disease. Mural nodes are present in 36–70% of IPMN-associated cancers 31, 35, 49, 112. Further, a thickened cyst wall is present in 65% of cases with malignancy 31, 113. Studies have demonstrated a direct relationship between BD-IPMN size and the risk of malignancy; but BD-IPMN-associated cancers can occur in small cysts and larger cysts do not always harbor pancreatic cancer 34, 48, 114–116. In the absence of a more practical approach, the Fukuoka and ACG guidelines advocate for varying time intervals for surveillance based on BD-IPMN size. The growth of the BD-IPMN should also be considered as growth of >2mm/year is associated with a 45% 5-year risk of developing malignancy as compared to 1.8% in slower growing BD-IPMNs 117–119. However, it is important to note that BD-IPMN size can be discordant based on different imaging modalities and, therefore, the same imaging modality should be used for size comparison between follow-up intervals. The mean diameter of the MPD is also an important factor associated with malignancy 109, 111, 113, 120. The Fukuoka and ESGCTP guidelines use a MPD diameter of 10 mm as an absolute indication for surgery. The AGA and ACG guidelines recommend an EUS-FNA for BD-IPMNs associated with MPD dilatation.
According to the Fukuoka, ACG and ESGCTP guidelines, surveillance for a BD-IPMN should be lifelong, but the AGA recommends ending surveillance after 5 years if there is no change in cyst size or other findings. Interestingly, the ACR advocates a 9- to 10-year follow-up, terminating at the age of 80 years. Kromrey et al found no incidence of pancreatic cancer during a 5-year follow-up study of 676 patients with pancreatic cysts 121. Similarly, Moris et al identified no cases of malignancy among 112 BD-IPMNs with more than 5 years of follow-up 37. In contrast, Del Chiaro et al reported an IPMN-related mortality of 5.8% after a 10-year follow-up period in patients without high-risk features at baseline 122.
Upon resection of an BD-IPMN, the Fukuoka, ACG and ESGCTP guidelines recommend lifelong surveillance as long as the patient is a surgical candidate. However, surveillance according to the AGA guidelines is only recommended for patients with IPMNs harboring at least high-grade dysplasia. After resection of a benign BD-IPMN, He et al estimated the chances of developing a new IPMN at 1, 5 and 10 years after initial surgery were 4%, 25% and 62%, respectively, and requiring surgery due to high risk features were 1.6%, 14% and 18%, respectively 123. The authors further found the chances of developing a new IPMN-associated PDAC or a concomitant carcinoma were 0%, 7% and 38% at 1, 5 and 10 years, respectively. Interestingly, the risk of a concomitant carcinoma continues after surgical resection of a BD-IPMN. Miyasaka et al found concomitant carcinomas among 4% of patients, who underwent pancreatectomy for a BD-IPMN 124. There is however little consensus or evidence as to how to reliably survey and detect concomitant carcinomas.
Despite the development of guidelines for the management of BD-IPMNs, it is still challenging to determine which BD-IPMNs harbor PDAC, and, even more difficult, to determine which BD-IPMNs will undergo malignant transformation within the patient’s lifetime.91, 125 In addition, the quality of evidence on which these recommendations are based is admittingly poor. The aforementioned management algorithms do not address every possible clinical scenario, and, consequently, it is imperative to tailor surveillance and treatment to the individual patient. Thus, there is an urgent need for prospective, multicenter clinical trials that integrate epidemiologic risk factors, clinical presentation, radiographic findings and PCF analysis to provide evidence to guide future management decisions.
New onset diabetes (NOD) as an early detection “sieve” for PDAC surveillance: Overview
Though the association between diabetes mellitus (DM) and pancreatic ductal adenocarcinoma (PDAC) has been known since the 1800s 126, the intricate and multidirectional relationship between the two diseases is yet to be fully understood 127. While long-standing type 2 DM is a modest risk factor (1.5 to 2-fold increased risk) for PDAC, new-onset DM (NOD) is a manifestation and harbinger of PDAC 127. Increasing epidemiological, clinical and experimental evidence that NOD is a clinical manifestation of asymptomatic PDAC provides the promise for early detection of PDAC using DM.
Epidemiology of DM in PDAC
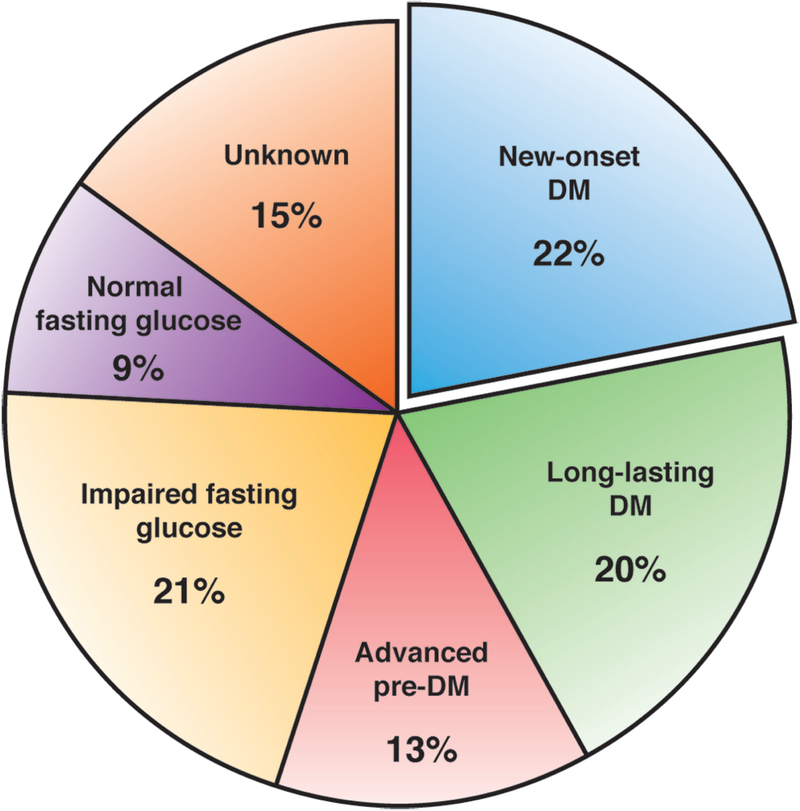
Prevalence of DM in PDAC ranges from 4% to 65%, depending on the ascertainment method of DM status 128–130. Studies relying on medical records report a prevalence of 4–20% 128, while studies screening patients using oral glucose tolerance test have a prevalence of 45–65% 129, 130. In prior studies using fasting blood glucose (FBG) levels, DM was present in nearly half the patients with PDAC at diagnosis 131. These findings were confirmed in a population-based cohort of PDAC from Olmsted County, MN in whom FBG were used levels to define the glycemic status of all PDAC patients (Figure 2). In this study it was noted that 42% met the American Diabetes Association criteria for DM (of which 52% were NOD), 13% have advanced pre-DM (defined as FBG≥120mg/dl), 21% have impaired FBG and only 9% had a normal FBG level at PDAC diagnosis. The fact that ~85% of subjects have elevated FBG and ~50% have DM at PDAC diagnosis highlights that glucose hemostasis disturbance is a near universal phenomenon in PDAC 131.
Figure 2:
Distribution of glycemic status based on fasting blood glucose levels in a population-based PDAC cohort (N = 219)
Burden of diabesity on increasing incidence of PDAC
Modifiable risk factors associated with PDAC include DM and obesity, disorders that are secondary to chronic caloric excess. There is strong evidence that obesity is associated with increased risk for PDAC and the anticipated increase in incidence of PDAC could partly be attributed to the obesity endemic132. Meta-analysis of prospective cohorts concludes that there is a positive association between body mass index (BMI) and PDAC risk, such that an overall a 5 kg/m2 increase in BMI is associated with a 12% increased risk for PDAC133. Recently, a study confirmed this association in a large cohort of obese adolescents followed for a median of 23-years, reporting a 4-fold increase risk of PDAC in adulthood134. While some epidemiologic studies have been confounded by DM contributing to the causal pathway between obesity and PDAC, larger studies indicate that obesity confers a significant cancer risk independent of the presence of diabetes135. The risk of PDAC in obesity is modestly elevated (1.12-fold increased risk/5 kg/m2 compared to normal BMI) and the cohort of obese subject’s needs enrichment to be a valid target for early detection133.
Time course of hyperglycemia in pre-diagnostic PDAC
In a recent study FBG levels were plotted in PDAC and matched controls up to 60 months prior to PDAC diagnosis and corresponding index date in controls 136. FBG levels were similar in cases and controls from −60 to −36 months. Starting 30–36 months before diagnosis glucose levels in PDAC progressively rose until diagnosis, crossing the DM threshold of 126 mg/dl around 6–12 months before diagnosis. Using clinic-based resected PDAC subjects, the same study also showed FBG levels correlates with PDAC tumor volume and FBG levels start rising when tumors are 1–2 cc in volume, crossing the DM threshold around 12cc 136. All these findings strongly suggest hyperglycemia is as biomarker of early invasive PDAC, with mostly being new-onset starting 36 months prior to cancer diagnosis.
Pancreatic cancer impairs glucose homeostasis:
PDAC is diabetogenic.
In fact, it is one of the strongest and most consistent diabetogenic forces known to humans. It destabilizes glucose homeostasis in nearly all subjects in whom it occurs, making it one of the most prevalent phenotypic traits of PDAC.
A). Clinical Evidence:
New-onset DM or worsening of long-standing DM occurs in majority of PDAC patients and proceeds by several months to few years 136–139. Further, the rise in blood glucose in PDAC occurs well before visible appearance of tumor in the pancreas, suggesting that DM in PDAC cannot be attributed merely to destruction of the gland by the tumor 3, 140. In addition, PDAC has been shown to cause insulin resistance and beta cell dysfunction, which resolve with tumor resection and glycemic status paradoxically improves despite removal of a third of the pancreas 131, 136, 141.
B). Laboratory evidence:
PDAC cell line supernatants have long been known to be metabolically active. They cause beta cell dysfunction in human islets, rat islets and isolated beta cells by producing soluble factors that impair glucose metabolism in vitro and cause hyperglycemia in vivo 127, 142, 143. They also induce insulin resistance in cultured hepatocytes and myoblasts 144, 145. PDAC-derived exosomes cause paraneoplastic dysfunction of human beta-cells and inhibit insulin secretion thereby causing hyperglycemia146. In an accompanying editorial, Dr. Murray Korc called PDAC-induced DM an exosomopathy (a disease of exosomes) 147.
C). Animal models:
There are to date no animal models of PDAC-induced DM. From published observation on KPC mice, it does not appear that they develop insulin resistance or hyperglycemia. Though a common phenomenon in humans, its lack of occurrence in animal models has hindered progress, with the entity being largely ignored. However, understanding why animal models do not develop DM and how that impacts the biology of the tumor needs further study.
Strategies for early detection of PDAC in the context of NOD
Since PDAC patients seldom exhibit disease-specific symptoms until late in the course of the disease 148, it is important to identify and develop strategies for early detection of asymptomatic PDAC. As previously stated, screening for sporadic PDAC in the “average risk” general population has been considered unrealistic because of its low incidence. In view of this, a DEF (Define, Enrich, Find) paradigm has been proposed that allows PDAC surveillance (versus screening) in a subset of higher risk asymptomatic patients where it might be most beneficial, of which NOD is currently the most promising in the elderly (≥50 years) population 149.
(i). Define:
The first step towards surveillance for asymptomatic, early PDAC is to define a patient population with a higher than average risk of PDAC. In a population-based study from Olmsted County, MN of 2,152 new-onset DM subjects (glycemically defined) over age 50 years, 18 (0.85%) developed PDAC within 3 years of DM onset, having a 6–8-fold higher risk for PDAC compared to general population 150. In a subsequent confirmatory study from another time period 0.90% of 1096 NOD subjects developed PDAC within 3 years of onset of NOD 151. These observations have not yet been confirmed outside Olmsted County using glycemic criteria for NOD. Based on these estimates, a national consortium has been set up with support of NIH/NIDDK to validate and establish NOD as a high-risk group for PDAC (see later) 152. It may be justifiably debated, however, that even with a 6–8-fold higher risk for PDAC, NOD per se does not have a high enough incidence to justify direct surveillance with imaging techniques, and therefore the need for enrichment strategies within the NOD subset.
(ii). Enrich:
The second step is to enrich the NOD population further, and one could use clinical risk prediction models or biomarkers. So far, 2 clinical models have been published that enrich the NOD population. The Health Improvement Network (THIN) database UK model included 109,385 NOD subjects identified by physician diagnosis of DM and the final prediction model was based on demographic, behavioral, and clinical variables with predicted risk threshold of 44.7% sensitivity, 94% specificity, and a positive predictive value of 2.6% 153. The other clinical model called Enriching New-onset Diabetes for Pancreatic Cancer (ENDPAC) score uses glycemic definition of NOD and includes 3 parameters; age, change in blood glucose and delta weight loss 151. The ENDPDAC model risk stratifies the NOD subjects into 3 groups based on 3-year PDAC risk: low (<0.1%), intermediate (~0.5%) and very high (~4%) with the very-high risk score cutoff having a sensitivity and specificity of 80%. While, development of these clinical models shows encouraging preliminary results in differentiating type 2-NOD from PDAC-NOD, further validation is needed before being applied in clinical practice. At present, there are no reliable biomarkers that identify early PDAC or that differentiate between “usual” Type 2 NOD and PDAC-associated NOD in asymptomatic patients.
(iii). Find:
The third step is to find a lesion in asymptomatic PDAC-NOD patient either using non-invasive imaging modalities (e.g. pancreas protocol CT) or invasive imaging (e.g. EUS). Prior pre-diagnostic imaging studies based on low quality scans suggest that PDAC is resectable as little as 6 months before clinical diagnosis when it is still asymptomatic, and that DM occurs at a resectable stage of disease 140. We further discuss the role of imaging modalities in diagnosis of early PDAC in the next section.
Current studies
The NCI and the NIDDK initiated the Consortium for the study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer (CPDPC) in 2015 with one of the key objectives being to establish an early detection approach for sporadic PDAC using NOD 152. The aim is to assemble a cohort of 10,000 subjects with NOD ≥50 years to estimate the 3-year probability of PDAC in NOD, establish a bio-bank of specimens from pre-symptomatic PDAC subjects, conduct Phase 3 validation studies of promising biomarkers for identification of incident PDAC in NOD patients and develop future interventional screening protocols for early detection of PDAC. It is expected that 85–100 incidences of PDAC will be diagnosed during the study period in this cohort of 10,000 patients, based on the prevalence first described by Chari and colleagues 150.
Imaging strategies relevant to PDAC early detection: Overview
Traditionally, the clinical indications for diagnostic imaging for PDAC include detection of the primary tumor, determination of resectability, evaluation for distant metastasis, and measurement of treatment response 154. In the context of early detection, imaging-based approaches can be grouped into traditional and non-traditional applications. Each imaging methodology has advantages and disadvantages that will be reviewed here; further, the practical implementation of imaging technologies will be discussed, with particular emphasis on areas of unmet need.
Traditional imaging techniques for pancreatic cancer detection
Over the past two decades, multiple studies have evaluated the accuracy of EUS, CT and MRI to detect a primary tumor in the pancreas, including in the context of early detection in high-risk cohorts. As noted, the following discussion is principally focused on non-cystic PDAC, with the role of imaging in cystic lesions having been discussed previously.
(i). Endoscopic ultrasound (EUS)
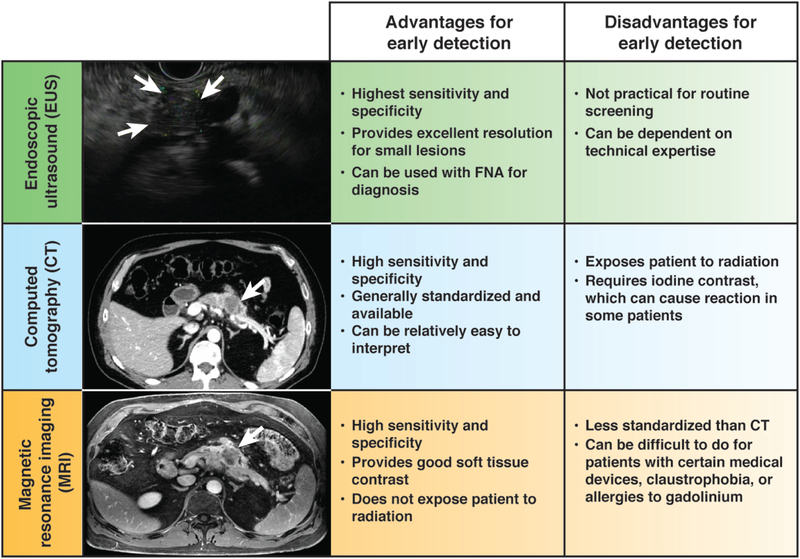
EUS is considered the most sensitive method to detect early neoplasia in the pancreas. Indeed, a direct comparison of imaging modalities in the modern era showed that EUS identified pancreatic abnormalities in individuals considered to be high risk for developing PDAC 43% of the time, compared to 33% and 11% for MRI and CT, respectively 155. A meta-analysis of 20 studies showed that the performance of EUS for PDAC varied by disease T stage. EUS had sensitivity and specificity of 72% and 90% for T1–2 cancers, respectively. For T3–4 tumors, EUS had 90% sensitivity and 72% specificity 156. This modality can detect lesions as small as 2–3 mm in the pancreas 157, which is generally the resolution of CT and MRI. Even though EUS has excellent performance with visualizing and diagnosing PDAC, it is mainly used as part of the workup to obtain fine needle aspiration or biopsy material in patients suspected of having a primary tumor. The reason is that EUS is not a readily accessible imaging modality and is highly dependent on the skill of the operator. For these reasons, EUS is considered a complementary modality to the pancreatic protocol CT in current clinical practice, and the CT is considered the gold standard.
Emerging areas for EUS include the incorporation of elastography in the characterization of lesions, as well as the use of microbubbles for contrast. Elastography has been reported to show significantly lower values of elasticity for PDAC compared to normal pancreas (0.02% [95% CI, 0.01–0.02] vs 0.53% [95% CI, 0.45–0.61]) 158. The incorporation of elastography in the evaluation of solid pancreatic lesions has resulted in sensitivities ranging from 75.9 to 100%, and specificities of 16.7 to 96.3% 159. Microbubbles are another tool that can be used in conjunction with EUS to characterize pancreatic cancer. One readout of the test is the degree of vascularity of the tumor, which has been associated with the differentiation of the tumors on histology 160. A pooled analysis of transabdominal ultrasound and EUS approaches with contrast enhancement showed a sensitivity of 89% and specificity of 84% 161. Further incorporation of advanced imaging techniques with EUS in ongoing early detection protocols may be expected to improve yield of this diagnostic test, but operator dependencies remain a challenge for this modality.
(ii). Multi-detector CT
Multi-detector CT with contrast using thin axial sections with dual-phase pancreatic protocol acquisition represents the most ubiquitous and robust method to visualize the pancreas, as its operating characteristics allow for rapid imaging with good spatial and temporal resolution 154. In general, CT has a sensitivity of 76–92% for diagnosing PDAC 162–164 and a specificity of 67% 162.
The performance of CT largely depends on the ability to administer intravenous contrast (usually at a rate of 3–5 ml/sec) and on the acquisition of the imaging at specific times relative to contrast injection. The consensus opinion 165 is that a pancreatic protocol CT scan should be done for evaluation of a suspected PDAC or when a routine CT scan was not of sufficient quality for accurate initial staging. During a pancreatic protocol CT, the arterial phase (~30 s post contrast injection) and portal-venous phase (~60–70 s post contrast injection) highlight different anatomical features of the pancreas and liver to enable visualization of primary and secondary tumors 166. The difference in physical attributes between pancreatic tumors and the pancreatic parenchyma often results in seeing the classic hypodense mass in the pancreas, which is due to the dense desmoplasia and relative hypovascularity of PDAC 167; however, there are iso-attenuating PDAC that may make detection and diagnosis more difficult. These iso-attenuating tumors with indistinct borders appear to have higher degree of stromal infiltrate and less aggressive biology compared to hypodense tumors with well-defined borders 168–170.
Recent advancements in CT technology have led to the implementation of the use of dual energy scans 171, which can simultaneously image the patient with two energies of x-rays (for example, 80 and 140 kVp). These different energies provide radiologists a wider range of images to view, and post-processing packages from vendors enable generation of images that show relative amounts of two or more materials that would be needed to obtain the imaging signal for each given voxel 172. For example, iodine/water maps have been demonstrated to result in an improvement in the conspicuity of pancreatic tumors 173. This raises the possibility that this imaging method 171, 173–177 may help increase the detection of small pancreatic tumors. Further prospective evaluation of dual energy CT in appropriate populations may be warranted.
(iii). MRI
Pancreas protocol MRI with contrast is another cross-sectional imaging modality that can be helpful in staging patients at initial presentation. Its advantages include that it does not rely on ionizing radiation for image acquisition and has better soft tissue resolution than CT. Disadvantages include the lack of standardization in the algorithms and parameters used to acquire advanced functional imaging sequences (e.g., diffusion weighted imaging [DWI], dynamic contrast enhancement [DCE]), susceptibility of the image quality to internal and external patient motion, cost relative to CT, and claustrophobia that some patients experience inside the machine. Further, a pancreatic protocol MRI with contrast is the preferred imaging alternative to a pancreatic protocol CT if a patient has an iodine contrast allergy. As mentioned above, MRI was reported to have better ability to detect pancreatic lesions than CT in a recent comparison study 155. Further, a screening protocol in Sweden for patients with a genetic risk of developing pancreatic cancer showed that MRI was able to detect pancreatic lesions in 16 of 40 patients enrolled in the prospective study 178.
Non-traditional uses and techniques for imaging of pancreatic cancer
(i). Secondary signs of pancreatic cancer
Recent work indicates that cross sectional imaging may identify secondary changes in the body that indicate an incipient PDAC due to its systemic effects. It has been well recognized that anorexia, sarcopenia, and weight loss are hallmarks of PDAC 179–181. In patients with localized PDAC, sarcopenia has been associated with survival outcomes and complications following surgery 182–184. During neoadjuvant therapy, the ability of the patient to gain lean tissue has been associated with a higher likelihood of resection 185. For example, these associations have recently been translated to patients with NOD (see above). Specifically, a change in weight was one of three factors that was developed and validated as a risk model in this population 186. Moreover, peripheral tissue wasting was found to be a common finding on pretreatment CT scans of patients with PDAC, and exocrine insufficiency was evaluated as a contributing factor 187. Although sarcopenia was not associated with patient survival in this study, the authors proposed that assessing peripheral tissue loss before overt disease presents may help identify PDAC at earlier stages. In particular, routine cross-sectional imaging may be used to measure adipose and muscle mass using validated methods 188, 189 in high-risk populations to identify early disease. These secondary signs of PDAC represent another method by which imaging may play an important role in early detection. Ongoing prospective studies in high-risk cohorts can easily integrate this assessment to potentially establish a role for anthropometric changes in the body as a method of cancer risk stratification.
(ii). Molecular imaging
The role of positron emission tomography (PET) imaging has been limited for PDAC, owing to the susceptibility of F18 fluorodeoxyglucose (FDG)-PET to both false positives (e.g., benign causes of inflammation like pancreatitis) and false negatives (e.g., non-FDG avid tumors). Several groups have investigated novel imaging agents that are coupled to 18F. These remain in early stages of development, including investigations of lactose analogues and the hepatocarcinoma-intestine-pancreas/pancreatic-associated protein (HIP/PAP) 190. Other strategies to detect pancreatic cancer with molecular imaging agents include targeting proteins that are overexpressed by the cancer (e.g., mesothelin), signaling pathways (e.g., epidermal growth factor receptor), tumor stroma (e.g., hedgehog signaling, vascular endothelial growth factor), and other targets that are associated with the disease (e.g., Plectin-1, MUC1) 191. Another molecular imaging method that is of interest for early detection is hyperpolarized MRI, which can identify metabolic aberrations in the pancreas that indicate preneoplasia 192. Further evaluation of these agents and techniques in preclinical models is warranted. Upon proper validation, translation in high-risk cohorts with pathological correlation will help bring these techniques to the forefront of early detection efforts.
Early detection of pancreatic cancer: The road ahead
In summary, we have discussed many of the opportunities in PDAC early detection that have emerged in the last decade, such as the identification of well-defined high-risk cohorts (e.g., familial kindred [discussed separately], patients with precursor cystic lesions, and those with NOD), and the improvements in imaging modalities available to clinicians. Nonetheless, vast challenges remain in terms of generalization of the lessons learned in early detection of PDAC, including (a) appropriately validated blood-based biomarkers that are poised for large-scale implementation in high-risk cohorts for diagnosing asymptomatic disease, (b) the choice of the best imaging modality for surveillance within the multitude of options discussed above, as well as (c) when and how often these imaging platforms should be used in the aforementioned cohorts for surveillance. For example, in the case of detecting circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), while both assays are reported to be highly specific as compared to an elevated serum CA19–9, they currently cannot be used for PDAC screening or early diagnosis because of their limited sensitivity.11, 193
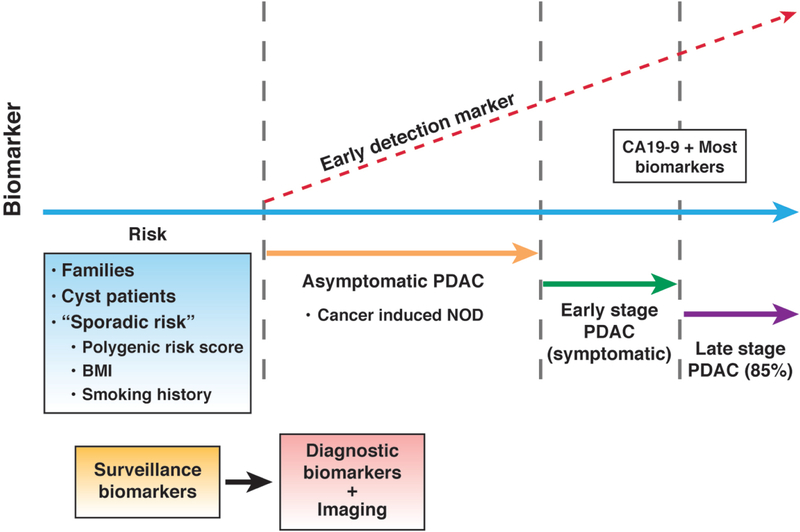
Further, individuals with a germline mutation or those with precursor cystic lesions represent only a subset of patients that develop PDAC, and challenges remain in identifying the so-called “sporadic” high-risk individuals that might need to be on surveillance programs for PDAC. As discussed, NOD represents a manifestation of occult PDAC in such “sporadic” high-risk individuals, and a rather promising one at that, but the eventual goal of early detection for a lethal disease like PDAC might transcend to an even earlier point in the natural history, where we are deciphering “risk”, and not early detection of an existing, albeit asymptomatic, cancer. This will require amalgamation of multiple genetic and environmental inputs, such as polygenic risk scores 194, BMI 195, smoking history etc. (Figure 4). Individuals that meet a defined “risk threshold” can then be placed on longitudinal surveillance programs, likely with the conduct of highly sensitive “surveillance biomarker” assays capable of identifying asymptomatic disease (the occurrence of NOD or worsening of hyperglycemia in such a surveillance population would certainly warrant additional investigation). At some point, the “surveillance biomarkers” would have to supplanted with “diagnostic biomarkers” that can predict the presence of an asymptomatic cancer with exceptionally high specificity (in order to avoid unnecessary imaging studies), culminating in diagnostic imaging of an early tumor being the final step in this multistep surveillance paradigm. There are substantial challenges to be overcome, but unequivocally, the roadmap now exists for making PDAC early detection a reality.
Figure 4:
The “future” of PDAC early detection. Currently, the majority of PDAC are diagnosed at a late stage of their natural history, when they are symptomatic, if not surgically unresectable. Individuals with a family history or with cystic lesions represent high-risk cohorts that can be entered into surveillance programs, but only comprise a subset of patients who develop PDAC. Determination of “sporadic risk” will require multiple input parameters (polygenic risk score, BMI, smoking history, other variables), but has the potential to impact the largest subset of individuals in the general population. Surveillance and diagnosis of asymptomatic PDAC in longitudinally monitored high risk cohorts will require biomarkers with exquisite sensitivity and specificity, to avoid the perils of false negatives and overdiagnosis, respectively. Imaging studies, using a bevy of localization modalities discussed in the text, represents the penultimate step before an intervention such as surgery for removing a potentially “curable” early PDAC.
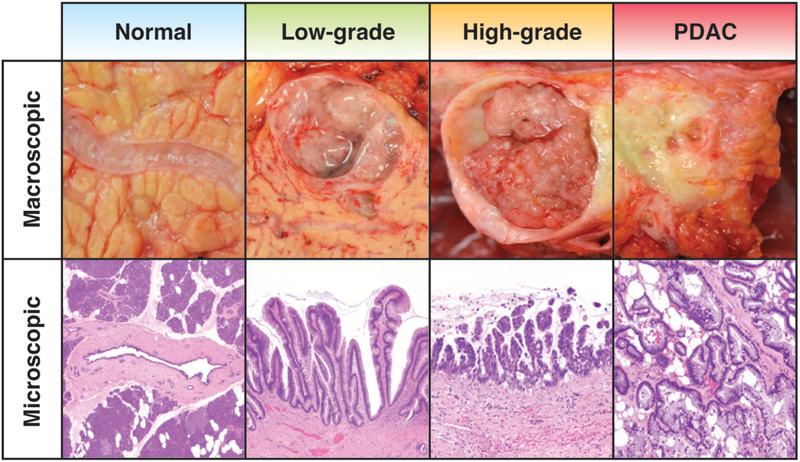
Figure 1:
The pathology of Intraductal Papillary Mucinous Neoplasms (IPMNs). The macroscopic and microscopic features of IPMNs are typically characterized by involvement of the main pancreatic duct, branch duct (shown here) or both. IPMNs are composed of mucinous epithelium that may be either flat or papillary in appearance. Based on the degree of cytoarchitectural atypia, IPMNs can be classified with low-grade or high-grade dysplasia. The most important prognosticator, however, is the absence or presence of an associated invasive pancreatic ductal adenocarcinoma (PDAC).
Figure 3:
Common imaging modalities for PDAC including endoscopic ultrasound (EUS, top), computed tomography (CT, middle), and magnetic resonance imaging (MRI, bottom). Each image shows a patient with a ~2 cm lesion in the body of the pancreas. Each modality has advantages and disadvantages for the purposes of early detection of PDAC. A few practical considerations are enumerated.
Acknowledgments
Grant support
This work was supported by NCI U01 CA196403 (AM and EJK), NCI U01 CA200468 (AM and EJK), NCI R01 CA218004 (AM and EJK), the Consortium for Study of Chronic Pancreatitis, Diabetes and Pancreatic cancer (NIH DK108288) (STC), Kenner Family Research Fund (STC), Prokopanko Gift to Mayo Foundation (STC), Pancreatic Cancer Action Network (AM, EJK and ADS), The Sky Foundation (ADS) and a Stand Up To Cancer-Lustgarten Foundation Pancreatic Cancer Interception Translational Cancer Research Grant (Grant Number: SU2C-AACR-DT25-17) (AM and EJK). Stand Up To Cancer is a program of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, the scientific partner of SU2C. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or any other funding source.
Biography




Footnotes
Conflicts of interest:
A.D.S. = Foundation Medicine (honorarium)
E.J.K = Philips Healthcare (sponsored research agreement), GE Healthcare (in-kind grant)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:1039–49. [DOI] [PubMed] [Google Scholar]
- 2.Kleeff J, Korc M, Apte M, et al. Pancreatic cancer. Nat Rev Dis Primers 2016;2:16022. [DOI] [PubMed] [Google Scholar]
- 3.Tsuchiya R, Noda T, Harada N, et al. Collective review of small carcinomas of the pancreas. Ann Surg 1986;203:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishikawa O, Ohigashi H, Imaoka S, et al. Minute carcinoma of the pancreas measuring 1 cm or less in diameter--collective review of Japanese case reports. Hepatogastroenterology 1999;46:8–15. [PubMed] [Google Scholar]
- 5.Furukawa H, Okada S, Saisho H, et al. Clinicopathologic features of small pancreatic adenocarcinoma. A collective study. Cancer 1996;78:986–90. [DOI] [PubMed] [Google Scholar]
- 6.Cloyd JM, Katz MH, Prakash L, et al. Preoperative Therapy and Pancreatoduodenectomy for Pancreatic Ductal Adenocarcinoma: a 25-Year Single-Institution Experience. J Gastrointest Surg 2017;21:164–174. [DOI] [PubMed] [Google Scholar]
- 7.Murphy JE, Wo JY, Ryan DP, et al. Total Neoadjuvant Therapy With FOLFIRINOX Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol 2018;4:963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khorana AA, Mangu PB, Berlin J, et al. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:2324–2328. [DOI] [PubMed] [Google Scholar]
- 9.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg 1996;223:273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melo SA, Luecke LB, Kahlert C, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015;523:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen JD, Li L, Wang Y, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018;359:926–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capello M, Bantis LE, Scelo G, et al. Sequential Validation of Blood-Based Protein Biomarker Candidates for Early-Stage Pancreatic Cancer. J Natl Cancer Inst 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schultz NA, Dehlendorff C, Jensen BV, et al. MicroRNA biomarkers in whole blood for detection of pancreatic cancer. JAMA 2014;311:392–404. [DOI] [PubMed] [Google Scholar]
- 14.Lennon AM, Wolfgang CL, Canto MI, et al. The early detection of pancreatic cancer: what will it take to diagnose and treat curable pancreatic neoplasia? Cancer Res 2014;74:3381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raimondi S, Lowenfels AB, Morselli-Labate AM, et al. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol 2010;24:349–58. [DOI] [PubMed] [Google Scholar]
- 16.Kirkegard J, Mortensen FV, Cronin-Fenton D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am J Gastroenterol 2017;112:1366–1372. [DOI] [PubMed] [Google Scholar]
- 17.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013;144:1252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munigala S, Kanwal F, Xian H, et al. Increased risk of pancreatic adenocarcinoma after acute pancreatitis. Clin Gastroenterol Hepatol 2014;12:1143–1150 e1. [DOI] [PubMed] [Google Scholar]
- 19.Shi C, Hruban RH. Intraductal papillary mucinous neoplasm. Hum Pathol 2012;43:1–16. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka M Intraductal Papillary Mucinous Neoplasm of the Pancreas as the Main Focus for Early Detection of Pancreatic Adenocarcinoma. Pancreas 2018;47:544–550. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012;12:183–97. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka M, Fernandez-Del Castillo C, Kamisawa T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017;17:738–753. [DOI] [PubMed] [Google Scholar]
- 23.Kimura W, Moriya T, Hirai I, et al. Multicenter study of serous cystic neoplasm of the Japan pancreas society. Pancreas 2012;41:380–7. [DOI] [PubMed] [Google Scholar]
- 24.Raval JS, Zeh HJ, Moser AJ, et al. Pancreatic lymphoepithelial cysts express CEA and can contain mucous cells: potential pitfalls in the preoperative diagnosis. Mod Pathol 2010;23:1467–76. [DOI] [PubMed] [Google Scholar]
- 25.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol 2008;191:802–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee KS, Sekhar A, Rofsky NM, et al. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol 2010;105:2079–84. [DOI] [PubMed] [Google Scholar]
- 27.de Jong K, Bruno MJ, Fockens P. Epidemiology, diagnosis, and management of cystic lesions of the pancreas. Gastroenterol Res Pract 2012;2012:147465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levink I, Bruno MJ, Cahen DL. Management of Intraductal Papillary Mucinous Neoplasms: Controversies in Guidelines and Future Perspectives. Curr Treat Options Gastroenterol 2018;16:316–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SY, Lee KT, Lee JK, et al. Long-term follow up results of intraductal papillary mucinous tumors of pancreas. J Gastroenterol Hepatol 2005;20:1379–84. [DOI] [PubMed] [Google Scholar]
- 30.Crippa S, Fernandez-Del Castillo C, Salvia R, et al. Mucin-producing neoplasms of the pancreas: an analysis of distinguishing clinical and epidemiologic characteristics. Clin Gastroenterol Hepatol 2010;8:213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang DW, Jang JY, Lee SE, et al. Clinicopathologic analysis of surgically proven intraductal papillary mucinous neoplasms of the pancreas in SNUH: a 15-year experience at a single academic institution. Langenbecks Arch Surg 2012;397:93–102. [DOI] [PubMed] [Google Scholar]
- 32.Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg 2004;239:678–85; discussion 685–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohno E, Hirooka Y, Kawashima H, et al. Natural history of pancreatic cystic lesions: A multicenter prospective observational study for evaluating the risk of pancreatic cancer. J Gastroenterol Hepatol 2018;33:320–328. [DOI] [PubMed] [Google Scholar]
- 34.Nagai K, Doi R, Kida A, et al. Intraductal papillary mucinous neoplasms of the pancreas: clinicopathologic characteristics and long-term follow-up after resection. World J Surg 2008;32:271–8; discussion 279–80. [DOI] [PubMed] [Google Scholar]
- 35.Ridtitid W, DeWitt JM, Schmidt CM, et al. Management of branch-duct intraductal papillary mucinous neoplasms: a large single-center study to assess predictors of malignancy and long-term outcomes. Gastrointest Endosc 2016;84:436–45. [DOI] [PubMed] [Google Scholar]
- 36.Marchegiani G, Malleo G, D’Haese JG, et al. Association between pancreatic intraductal papillary mucinous neoplasms and extrapancreatic malignancies. Clin Gastroenterol Hepatol 2015;13:1162–9. [DOI] [PubMed] [Google Scholar]
- 37.Moris M, Raimondo M, Woodward TA, et al. Diagnostic Accuracy of Endoscopic Ultrasound-Guided Fine-Needle Aspiration Cytology, Carcinoembryonic Antigen, and Amylase in Intraductal Papillary Mucinous Neoplasm. Pancreas 2016;45:870–5. [DOI] [PubMed] [Google Scholar]
- 38.Capurso G, Boccia S, Salvia R, et al. Risk factors for intraductal papillary mucinous neoplasm (IPMN) of the pancreas: a multicentre case-control study. Am J Gastroenterol 2013;108:1003–9. [DOI] [PubMed] [Google Scholar]
- 39.Morales-Oyarvide V, Mino-Kenudson M, Ferrone CR, et al. Diabetes mellitus in intraductal papillary mucinous neoplasm of the pancreas is associated with high-grade dysplasia and invasive carcinoma. Pancreatology 2017;17:920–926. [DOI] [PubMed] [Google Scholar]
- 40.Ingkakul T, Sadakari Y, Ienaga J, et al. Predictors of the presence of concomitant invasive ductal carcinoma in intraductal papillary mucinous neoplasm of the pancreas. Ann Surg 2010;251:70–5. [DOI] [PubMed] [Google Scholar]
- 41.Zapiach M, Yadav D, Smyrk TC, et al. Calcifying obstructive pancreatitis: a study of intraductal papillary mucinous neoplasm associated with pancreatic calcification. Clin Gastroenterol Hepatol 2004;2:57–63. [DOI] [PubMed] [Google Scholar]
- 42.Chetty R, Salahshor S, Bapat B, et al. Intraductal papillary mucinous neoplasm of the pancreas in a patient with attenuated familial adenomatous polyposis. J Clin Pathol 2005;58:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maire F, Hammel P, Terris B, et al. Intraductal papillary and mucinous pancreatic tumour: a new extracolonic tumour in familial adenomatous polyposis. Gut 2002;51:446–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sato N, Rosty C, Jansen M, et al. STK11/LKB1 Peutz-Jeghers Gene Inactivation in Intraductal Papillary-Mucinous Neoplasms of the Pancreas. Am J Pathol 2001;159:2017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parvanescu A, Cros J, Ronot M, et al. Lessons from McCune-Albright syndrome-associated intraductal papillary mucinous neoplasms: : GNAS-activating mutations in pancreatic carcinogenesis. JAMA Surg 2014;149:858–62. [DOI] [PubMed] [Google Scholar]
- 46.Schnelldorfer T, Sarr MG, Nagorney DM, et al. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch Surg 2008;143:639–46; discussion 646. [DOI] [PubMed] [Google Scholar]
- 47.Kim SC, Park KT, Lee YJ, et al. Intraductal papillary mucinous neoplasm of the pancreas: clinical characteristics and treatment outcomes of 118 consecutive patients from a single center. J Hepatobiliary Pancreat Surg 2008;15:183–8. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki Y, Atomi Y, Sugiyama M, et al. Cystic neoplasm of the pancreas: a Japanese multiinstitutional study of intraductal papillary mucinous tumor and mucinous cystic tumor. Pancreas 2004;28:241–6. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt CM, White PB, Waters JA, et al. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg 2007;246:644–51; discussion 651–4. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi G, Fujita N, Noda Y, et al. Intraductal papillary mucinous neoplasms of the pancreas showing fistula formation into other organs. J Gastroenterol 2010;45:1080–9. [DOI] [PubMed] [Google Scholar]
- 51.Yamada Y, Mori H, Hijiya N, et al. Intraductal papillary mucinous neoplasms of the pancreas complicated with intraductal hemorrhage, perforation, and fistula formation: CT and MR imaging findings with pathologic correlation. Abdom Imaging 2012;37:100–9. [DOI] [PubMed] [Google Scholar]
- 52.Kimura W, Nagai H, Kuroda A, et al. Analysis of small cystic lesions of the pancreas. Int J Pancreatol 1995;18:197–206. [DOI] [PubMed] [Google Scholar]
- 53.Sahani DV, Kambadakone A, Macari M, et al. Diagnosis and management of cystic pancreatic lesions. AJR Am J Roentgenol 2013;200:343–54. [DOI] [PubMed] [Google Scholar]
- 54.Kulzer M, Singhi AD, Furlan A, et al. Current concepts in molecular genetics and management guidelines for pancreatic cystic neoplasms: an essential update for radiologists. Abdom Radiol (NY) 2018;43:2351–2368. [DOI] [PubMed] [Google Scholar]
- 55.Procacci C, Carbognin G, Accordini S, et al. CT features of malignant mucinous cystic tumors of the pancreas. Eur Radiol 2001;11:1626–30. [DOI] [PubMed] [Google Scholar]
- 56.Sidden CR, Mortele KJ. Cystic tumors of the pancreas: ultrasound, computed tomography, and magnetic resonance imaging features. Semin Ultrasound CT MR 2007;28:339–56. [DOI] [PubMed] [Google Scholar]
- 57.Waters JA, Schmidt CM, Pinchot JW, et al. CT vs MRCP: optimal classification of IPMN type and extent. J Gastrointest Surg 2008;12:101–9. [DOI] [PubMed] [Google Scholar]
- 58.Girometti R, Pravisani R, Intini SG, et al. Evolution of incidental branch-duct intraductal papillary mucinous neoplasms of the pancreas: A study with magnetic resonance imaging cholangiopancreatography. World J Gastroenterol 2016;22:9562–9570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee HJ, Kim MJ, Choi JY, et al. Relative accuracy of CT and MRI in the differentiation of benign from malignant pancreatic cystic lesions. Clin Radiol 2011;66:315–21. [DOI] [PubMed] [Google Scholar]
- 60.Visser BC, Yeh BM, Qayyum A, et al. Characterization of cystic pancreatic masses: relative accuracy of CT and MRI. AJR Am J Roentgenol 2007;189:648–56. [DOI] [PubMed] [Google Scholar]
- 61.Tirkes T, Aisen AM, Cramer HM, et al. Cystic neoplasms of the pancreas; findings on magnetic resonance imaging with pathological, surgical, and clinical correlation. Abdom Imaging 2014;39:1088–101. [DOI] [PubMed] [Google Scholar]
- 62.Brugge WR. The use of EUS to diagnose cystic neoplasms of the pancreas. Gastrointest Endosc 2009;69:S203–9. [DOI] [PubMed] [Google Scholar]
- 63.Brugge WR. Endoscopic approach to the diagnosis and treatment of pancreatic disease. Curr Opin Gastroenterol 2013;29:559–65. [DOI] [PubMed] [Google Scholar]
- 64.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology 2004;126:1330–6. [DOI] [PubMed] [Google Scholar]
- 65.Sedlack R, Affi A, Vazquez-Sequeiros E, et al. Utility of EUS in the evaluation of cystic pancreatic lesions. Gastrointest Endosc 2002;56:543–7. [DOI] [PubMed] [Google Scholar]
- 66.Grutzmann R, Niedergethmann M, Pilarsky C, et al. Intraductal papillary mucinous tumors of the pancreas: biology, diagnosis, and treatment. Oncologist 2010;15:1294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scheiman JM, Hwang JH, Moayyedi P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015;148:824–48 e22. [DOI] [PubMed] [Google Scholar]
- 68.Ahmad NA, Kochman ML, Brensinger C, et al. Interobserver agreement among endosonographers for the diagnosis of neoplastic versus non-neoplastic pancreatic cystic lesions. Gastrointest Endosc 2003;58:59–64. [DOI] [PubMed] [Google Scholar]
- 69.Khamaysi I, Abu Ammar A, Vasilyev G, et al. Differentiation of Pancreatic Cyst Types by Analysis of Rheological Behavior of Pancreatic Cyst Fluid. Sci Rep 2017;7:45589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bick BL, Enders FT, Levy MJ, et al. The string sign for diagnosis of mucinous pancreatic cysts. Endoscopy 2015;47:626–31. [DOI] [PubMed] [Google Scholar]
- 71.Singhi AD, McGrath K, Brand RE, et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maire F, Voitot H, Aubert A, et al. Intraductal papillary mucinous neoplasms of the pancreas: performance of pancreatic fluid analysis for positive diagnosis and the prediction of malignancy. Am J Gastroenterol 2008;103:2871–7. [DOI] [PubMed] [Google Scholar]
- 73.Linder JD, Geenen JE, Catalano MF. Cyst fluid analysis obtained by EUS-guided FNA in the evaluation of discrete cystic neoplasms of the pancreas: a prospective single-center experience. Gastrointest Endosc 2006;64:697–702. [DOI] [PubMed] [Google Scholar]
- 74.Shami VM, Sundaram V, Stelow EB, et al. The level of carcinoembryonic antigen and the presence of mucin as predictors of cystic pancreatic mucinous neoplasia. Pancreas 2007;34:466–9. [DOI] [PubMed] [Google Scholar]
- 75.Maker AV, Lee LS, Raut CP, et al. Cytology from pancreatic cysts has marginal utility in surgical decision-making. Ann Surg Oncol 2008;15:3187–92. [DOI] [PubMed] [Google Scholar]
- 76.Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. Am J Gastroenterol 2007;102:2339–49. [DOI] [PubMed] [Google Scholar]
- 77.Khalid A, Zahid M, Finkelstein SD, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc 2009;69:1095–102. [DOI] [PubMed] [Google Scholar]
- 78.Springer S, Wang Y, Dal Molin M, et al. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology 2015;149:1501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singhi AD, Nikiforova MN, Fasanella KE, et al. Preoperative GNAS and KRAS testing in the diagnosis of pancreatic mucinous cysts. Clin Cancer Res 2014;20:4381–9. [DOI] [PubMed] [Google Scholar]
- 80.Nikiforova MN, Khalid A, Fasanella KE, et al. Integration of KRAS testing in the diagnosis of pancreatic cystic lesions: a clinical experience of 618 pancreatic cysts. Mod Pathol 2013;26:1478–87. [DOI] [PubMed] [Google Scholar]
- 81.Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med 2011;3:92ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu J, Jiao Y, Dal Molin M, et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A 2011;108:21188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kanda M, Sadakari Y, Borges M, et al. Mutant TP53 in duodenal samples of pancreatic juice from patients with pancreatic cancer or high-grade dysplasia. Clin Gastroenterol Hepatol 2013;11:719–30 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pea A, Yu J, Rezaee N, et al. Targeted DNA Sequencing Reveals Patterns of Local Progression in the Pancreatic Remnant Following Resection of Intraductal Papillary Mucinous Neoplasm (IPMN) of the Pancreas. Ann Surg 2017;266:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rosenbaum MW, Jones M, Dudley JC, et al. Next-generation sequencing adds value to the preoperative diagnosis of pancreatic cysts. Cancer Cytopathol 2017;125:41–47. [DOI] [PubMed] [Google Scholar]
- 86.Jones M, Zheng Z, Wang J, et al. Impact of next-generation sequencing on the clinical diagnosis of pancreatic cysts. Gastrointest Endosc 2016;83:140–8. [DOI] [PubMed] [Google Scholar]
- 87.Garcia-Carracedo D, Chen ZM, Qiu W, et al. PIK3CA mutations in mucinous cystic neoplasms of the pancreas. Pancreas 2014;43:245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schonleben F, Qiu W, Remotti HE, et al. PIK3CA, KRAS, and BRAF mutations in intraductal papillary mucinous neoplasm/carcinoma (IPMN/C) of the pancreas. Langenbecks Arch Surg 2008;393:289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schonleben F, Qiu W, Ciau NT, et al. PIK3CA mutations in intraductal papillary mucinous neoplasm/carcinoma of the pancreas. Clin Cancer Res 2006;12:3851–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garcia-Carracedo D, Turk AT, Fine SA, et al. Loss of PTEN expression is associated with poor prognosis in patients with intraductal papillary mucinous neoplasms of the pancreas. Clin Cancer Res 2013;19:6830–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singhi AD, Zeh HJ, Brand RE, et al. American Gastroenterological Association guidelines are inaccurate in detecting pancreatic cysts with advanced neoplasia: a clinicopathologic study of 225 patients with supporting molecular data. Gastrointest Endosc 2016;83:1107–1117 e2. [DOI] [PubMed] [Google Scholar]
- 92.Kaur S, Kumar S, Momi N, et al. Mucins in pancreatic cancer and its microenvironment. Nat Rev Gastroenterol Hepatol 2013;10:607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nagata K, Horinouchi M, Saitou M, et al. Mucin expression profile in pancreatic cancer and the precursor lesions. J Hepatobiliary Pancreat Surg 2007;14:243–54. [DOI] [PubMed] [Google Scholar]
- 94.Moniaux N, Chaturvedi P, Varshney GC, et al. Human MUC4 mucin induces ultra-structural changes and tumorigenicity in pancreatic cancer cells. Br J Cancer 2007;97:345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Torres MP, Chakraborty S, Souchek J, et al. Mucin-based targeted pancreatic cancer therapy. Curr Pharm Des 2012;18:2472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haab BB, Porter A, Yue T, et al. Glycosylation variants of mucins and CEACAMs as candidate biomarkers for the diagnosis of pancreatic cystic neoplasms. Ann Surg 2010;251:937–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sinha J, Cao Z, Dai J, et al. A Gastric Glycoform of MUC5AC Is a Biomarker of Mucinous Cysts of the Pancreas. PLoS One 2016;11:e0167070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Horn A, Chakraborty S, Dey P, et al. Immunocytochemistry for MUC4 and MUC16 is a useful adjunct in the diagnosis of pancreatic adenocarcinoma on fine-needle aspiration cytology. Arch Pathol Lab Med 2013;137:546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hata T, Dal Molin M, Hong SM, et al. Predicting the Grade of Dysplasia of Pancreatic Cystic Neoplasms Using Cyst Fluid DNA Methylation Markers. Clin Cancer Res 2017;23:3935–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hata T, Dal Molin M, Suenaga M, et al. Cyst Fluid Telomerase Activity Predicts the Histologic Grade of Cystic Neoplasms of the Pancreas. Clin Cancer Res 2016;22:5141–5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ivry SL, Sharib JM, Dominguez DA, et al. Global Protease Activity Profiling Provides Differential Diagnosis of Pancreatic Cysts. Clin Cancer Res 2017;23:4865–4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Das KK, Xiao H, Geng X, et al. mAb Das-1 is specific for high-risk and malignant intraductal papillary mucinous neoplasm (IPMN). Gut 2014;63:1626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Singhi AD, Feng Z, Goggins M, et al. Pancreatic Cyst Biomarker Validation Study. Early Detection Research Network: National Cancer Institute, 2017. [Google Scholar]
- 104.Kimura W, Miyata H, Gotoh M, et al. A pancreaticoduodenectomy risk model derived from 8575 cases from a national single-race population (Japanese) using a web-based data entry system: the 30-day and in-hospital mortality rates for pancreaticoduodenectomy. Ann Surg 2014;259:773–80. [DOI] [PubMed] [Google Scholar]
- 105.Hsu CC, Wolfgang CL, Laheru DA, et al. Early mortality risk score: identification of poor outcomes following upfront surgery for resectable pancreatic cancer. J Gastrointest Surg 2012;16:753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Greenblatt DY, Kelly KJ, Rajamanickam V, et al. Preoperative factors predict perioperative morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol 2011;18:2126–35. [DOI] [PubMed] [Google Scholar]
- 107.Beltrame V, Gruppo M, Pastorelli D, et al. Outcome of pancreaticoduodenectomy in octogenarians: Single institution’s experience and review of the literature. J Visc Surg 2015;152:279–84. [DOI] [PubMed] [Google Scholar]
- 108.Vege SS, Ziring B, Jain R, et al. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015;148:819–22; quize12–3. [DOI] [PubMed] [Google Scholar]
- 109.Elta GH, Enestvedt BK, Sauer BG, et al. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol 2018;113:464–479. [DOI] [PubMed] [Google Scholar]
- 110.Megibow AJ, Baker ME, Morgan DE, et al. Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol 2017;14:911–923. [DOI] [PubMed] [Google Scholar]
- 111.European Study Group on Cystic Tumours of the P. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018;67:789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marchegiani G, Andrianello S, Borin A, et al. Systematic review, meta-analysis, and a high-volume center experience supporting the new role of mural nodules proposed by the updated 2017 international guidelines on IPMN of the pancreas. Surgery 2018;163:1272–1279. [DOI] [PubMed] [Google Scholar]
- 113.Maimone S, Agrawal D, Pollack MJ, et al. Variability in measurements of pancreatic cyst size among EUS, CT, and magnetic resonance imaging modalities. Gastrointest Endosc 2010;71:945–50. [DOI] [PubMed] [Google Scholar]
- 114.Walsh RM, Vogt DP, Henderson JM, et al. Management of suspected pancreatic cystic neoplasms based on cyst size. Surgery 2008;144:677–84; discussion 684–5. [DOI] [PubMed] [Google Scholar]
- 115.Jang JY, Kim SW, Lee SE, et al. Treatment guidelines for branch duct type intraductal papillary mucinous neoplasms of the pancreas: when can we operate or observe? Ann Surg Oncol 2008;15:199–205. [DOI] [PubMed] [Google Scholar]
- 116.Weinberg BM, Spiegel BM, Tomlinson JS, et al. Asymptomatic pancreatic cystic neoplasms: maximizing survival and quality of life using Markov-based clinical nomograms. Gastroenterology 2010;138:531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kwong WT, Hunt GC, Fehmi SM, et al. Low Rates of Malignancy and Mortality in Asymptomatic Patients With Suspected Neoplastic Pancreatic Cysts Beyond 5 Years of Surveillance. Clin Gastroenterol Hepatol 2016;14:865–871. [DOI] [PubMed] [Google Scholar]
- 118.Khannoussi W, Vullierme MP, Rebours V, et al. The long term risk of malignancy in patients with branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreatology 2012;12:198–202. [DOI] [PubMed] [Google Scholar]
- 119.Tanno S, Nakano Y, Nishikawa T, et al. Natural history of branch duct intraductal papillary-mucinous neoplasms of the pancreas without mural nodules: long-term follow-up results. Gut 2008;57:339–43. [DOI] [PubMed] [Google Scholar]
- 120.Boos J, Brook A, Chingkoe CM, et al. MDCT vs. MRI for incidental pancreatic cysts: measurement variability and impact on clinical management. Abdom Radiol (NY) 2017;42:521–530. [DOI] [PubMed] [Google Scholar]
- 121.Kromrey ML, Bulow R, Hubner J, et al. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut 2018;67:138–145. [DOI] [PubMed] [Google Scholar]
- 122.Del Chiaro M, Ateeb Z, Hansson MR, et al. Survival Analysis and Risk for Progression of Intraductal Papillary Mucinous Neoplasia of the Pancreas (IPMN) Under Surveillance: A Single-Institution Experience. Ann Surg Oncol 2017;24:1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.He J, Cameron JL, Ahuja N, et al. Is it necessary to follow patients after resection of a benign pancreatic intraductal papillary mucinous neoplasm? J Am Coll Surg 2013;216:657–65; discussion 665–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Miyasaka Y, Ohtsuka T, Tamura K, et al. Predictive Factors for the Metachronous Development of High-risk Lesions in the Remnant Pancreas After Partial Pancreatectomy for Intraductal Papillary Mucinous Neoplasm. Ann Surg 2016;263:1180–7. [DOI] [PubMed] [Google Scholar]
- 125.Miller MS, Allen P, Brentnall TA, et al. Pancreatic Cancer Chemoprevention Translational Workshop: Meeting Report. Pancreas 2016;45:1080–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Green RC Jr., Baggenstoss AH, Sprague RG. Diabetes mellitus in association with primary carcinoma of the pancreas. Diabetes 1958;7:308–11. [DOI] [PubMed] [Google Scholar]
- 127.Sah RP, Nagpal SJ, Mukhopadhyay D, et al. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol 2013;10:423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Noy A, Bilezikian JP. Clinical review 63: Diabetes and pancreatic cancer: clues to the early diagnosis of pancreatic malignancy. J Clin Endocrinol Metab 1994;79:1223–31. [DOI] [PubMed] [Google Scholar]
- 129.Permert J, Ihse I, Jorfeldt L, et al. Pancreatic cancer is associated with impaired glucose metabolism. Eur J Surg 1993;159:101–7. [PubMed] [Google Scholar]
- 130.Cersosimo E, Pisters PW, Pesola G, et al. Insulin secretion and action in patients with pancreatic cancer. Cancer 1991;67:486–93. [DOI] [PubMed] [Google Scholar]
- 131.Pannala R, Leirness JB, Bamlet WR, et al. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology 2008;134:981–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer 2015;15:484–98. [DOI] [PubMed] [Google Scholar]
- 133.Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: A meta-analysis of prospective studies. Int J Cancer 2007;120:1993–8. [DOI] [PubMed] [Google Scholar]
- 134.Zohar L, Rottenberg Y, Twig G, et al. Adolescent overweight and obesity and the risk for pancreatic cancer among men and women: a nationwide study of 1.79 million Israeli adolescents. Cancer 2019;125:118–126. [DOI] [PubMed] [Google Scholar]
- 135.Jiao L, Berrington de Gonzalez A, Hartge P, et al. Body mass index, effect modifiers, and risk of pancreatic cancer: a pooled study of seven prospective cohorts. Cancer Causes Control 2010;21:1305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sharma A, Smyrk TC, Levy MJ, et al. Fasting Blood Glucose Levels Provide Estimate of Duration and Progression of Pancreatic Cancer Before Diagnosis. Gastroenterology 2018;155:490–500 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pannala R, Leibson CL, Rabe KG, et al. Temporal association of changes in fasting blood glucose and body mass index with diagnosis of pancreatic cancer. Am J Gastroenterol 2009;104:2318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Girelli CM, Reguzzoni G, Limido E, et al. Pancreatic carcinoma: differences between patients with or without diabetes mellitus. Recenti Prog Med 1995;86:143–6. [PubMed] [Google Scholar]
- 139.Mueller AM, Meier CR, Jick SS, et al. The Potential of Glycemic Control and Body Weight Change as Early Markers for Pancreatic Cancer in Patients With Long-standing Diabetes Mellitus: A Case-Control Study. Pancreas 2018;47:807–815. [DOI] [PubMed] [Google Scholar]
- 140.Pelaez-Luna M, Takahashi N, Fletcher JG, et al. Resectability of presymptomatic pancreatic cancer and its relationship to onset of diabetes: a retrospective review of CT scans and fasting glucose values prior to diagnosis. Am J Gastroenterol 2007;102:2157–63. [DOI] [PubMed] [Google Scholar]
- 141.Chari ST, Zapiach M, Yadav D, et al. Beta-cell function and insulin resistance evaluated by HOMA in pancreatic cancer subjects with varying degrees of glucose intolerance. Pancreatology 2005;5:229–33. [DOI] [PubMed] [Google Scholar]
- 142.Basso D, Brigato L, Veronesi A, et al. The pancreatic cancer cell line MIA PaCa2 produces one or more factors able to induce hyperglycemia in SCID mice. Anticancer Res 1995;15:2585–8. [PubMed] [Google Scholar]
- 143.Aggarwal G, Ramachandran V, Javeed N, et al. Adrenomedullin is up-regulated in patients with pancreatic cancer and causes insulin resistance in beta cells and mice. Gastroenterology 2012;143:1510–1517 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Basso D, Valerio A, Brigato L, et al. An unidentified pancreatic cancer cell product alters some intracellular pathways of glucose metabolism in isolated rat hepatocytes. Pancreas 1997;15:132–8. [DOI] [PubMed] [Google Scholar]
- 145.Valerio A, Basso D, Brigato L, et al. Glucose metabolic alterations in isolated and perfused rat hepatocytes induced by pancreatic cancer conditioned medium: a low molecular weight factor possibly involved. Biochem Biophys Res Commun 1999;257:622–8. [DOI] [PubMed] [Google Scholar]
- 146.Javeed N, Sagar G, Dutta SK, et al. Pancreatic Cancer-Derived Exosomes Cause Paraneoplastic beta-cell Dysfunction. Clinical cancer research : an official journal of the American Association for Cancer Research 2015;21:1722–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Korc M Pancreatic cancer-associated diabetes is an “exosomopathy”. Clin Cancer Res 2015;21:1508–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Holly EA, Chaliha I, Bracci PM, et al. Signs and symptoms of pancreatic cancer: a population-based case-control study in the San Francisco Bay area. Clin Gastroenterol Hepatol 2004;2:510–7. [DOI] [PubMed] [Google Scholar]
- 149.Chari ST. Detecting early pancreatic cancer: problems and prospects. Semin Oncol 2007;34:284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chari ST, Leibson CL, Rabe KG, et al. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology 2005;129:504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sharma A, Kandlakunta H, Nagpal SJS, et al. Model to Determine Risk of Pancreatic Cancer in Patients With New-Onset Diabetes. Gastroenterology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Maitra A, Sharma A, Brand RE, et al. A Prospective Study to Establish a New-Onset Diabetes Cohort: Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer Pancreas 2018;in press. [DOI] [PMC free article] [PubMed]
- 153.Boursi B, Finkelman B, Giantonio BJ, et al. A Clinical Prediction Model to Assess Risk for Pancreatic Cancer Among Patients With New-Onset Diabetes. Gastroenterology 2017;152:840–850 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lee ES, Lee JM. Imaging diagnosis of pancreatic cancer: a state-of-the-art review. World J Gastroenterol 2014;20:7864–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 2012;142:796804; quiz e14–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li JH, He R, Li YM, et al. Endoscopic ultrasonography for tumor node staging and vascular invasion in pancreatic cancer: a meta-analysis. Dig Surg 2014;31:297–305. [DOI] [PubMed] [Google Scholar]
- 157.Minniti S, Bruno C, Biasiutti C, et al. Sonography versus helical CT in identification and staging of pancreatic ductal adenocarcinoma. J Clin Ultrasound 2003;31:175–82. [DOI] [PubMed] [Google Scholar]
- 158.Lee TH, Cho YD, Cha SW, et al. Endoscopic ultrasound elastography for the pancreas in Korea: a preliminary single center study. Clin Endosc 2013;46:172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chantarojanasiri T, Kongkam P. Endoscopic ultrasound elastography for solid pancreatic lesions. World J Gastrointest Endosc 2017;9:506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sofuni A, Iijima H, Moriyasu F, et al. Differential diagnosis of pancreatic tumors using ultrasound contrast imaging. J Gastroenterol 2005;40:518–25. [DOI] [PubMed] [Google Scholar]
- 161.Saftoiu A, Vilmann P, Bhutani MS. The role of contrast-enhanced endoscopic ultrasound in pancreatic adenocarcinoma. Endosc Ultrasound 2016;5:368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kauhanen SP, Komar G, Seppanen MP, et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg 2009;250:957–63. [DOI] [PubMed] [Google Scholar]
- 163.Palazzo L, Roseau G, Gayet B, et al. Endoscopic ultrasonography in the diagnosis and staging of pancreatic adenocarcinoma. Results of a prospective study with comparison to ultrasonography and CT scan. Endoscopy 1993;25:143–50. [DOI] [PubMed] [Google Scholar]
- 164.Sheridan MB, Ward J, Guthrie JA, et al. Dynamic contrast-enhanced MR imaging and dual-phase helical CT in the preoperative assessment of suspected pancreatic cancer: a comparative study with receiver operating characteristic analysis. AJR Am J Roentgenol 1999;173:583–90. [DOI] [PubMed] [Google Scholar]
- 165.NCCN Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma. Volume 2018, 2018.
- 166.McNulty NJ, Francis IR, Platt JF, et al. Multi--detector row helical CT of the pancreas: effect of contrast-enhanced multiphasic imaging on enhancement of the pancreas, peripancreatic vasculature, and pancreatic adenocarcinoma. Radiology 2001;220:97–102. [DOI] [PubMed] [Google Scholar]
- 167.Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol 2008;3:157–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kim JH, Park SH, Yu ES, et al. Visually isoattenuating pancreatic adenocarcinoma at dynamic-enhanced CT: frequency, clinical and pathologic characteristics, and diagnosis at imaging examinations. Radiology 2010;257:87–96. [DOI] [PubMed] [Google Scholar]
- 169.Koay EJ, Lee Y, Cristini V, et al. A visually apparent and quantifiable CT imaging feature identifies biophysical subtypes of pancreatic ductal adenocarcinoma. Clin Cancer Res 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Koay EJ, Truty MJ, Cristini V, et al. Transport properties of pancreatic cancer describe gemcitabine delivery and response. J Clin Invest 2014;124:1525–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Macari M, Spieler B, Kim D, et al. Dual-source dual-energy MDCT of pancreatic adenocarcinoma: initial observations with data generated at 80 kVp and at simulated weighted-average 120 kVp. AJR Am J Roentgenol 2010;194:W27–32. [DOI] [PubMed] [Google Scholar]
- 172.Coursey CA, Nelson RC, Boll DT, et al. Dual-energy multidetector CT: how does it work, what can it tell us, and when can we use it in abdominopelvic imaging? Radiographics 2010;30:1037–55. [DOI] [PubMed] [Google Scholar]
- 173.Gupta S, Wagner-Bartak N, Jensen CT, et al. Dual-energy CT of pancreatic adenocarcinoma: reproducibility of primary tumor measurements and assessment of tumor conspicuity and margin sharpness. Abdom Radiol (NY) 2016;41:1317–24. [DOI] [PubMed] [Google Scholar]
- 174.Chu AJ, Lee JM, Lee YJ, et al. Dual-source, dual-energy multidetector CT for the evaluation of pancreatic tumours. Br J Radiol 2012;85:e891–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Graser A, Johnson TR, Chandarana H, et al. Dual energy CT: preliminary observations and potential clinical applications in the abdomen. Eur Radiol 2009;19:13–23. [DOI] [PubMed] [Google Scholar]
- 176.Heye T, Nelson RC, Ho LM, et al. Dual-energy CT applications in the abdomen. AJR Am J Roentgenol 2012;199:S64–70. [DOI] [PubMed] [Google Scholar]
- 177.Marin D, Nelson RC, Barnhart H, et al. Detection of pancreatic tumors, image quality, and radiation dose during the pancreatic parenchymal phase: effect of a low-tube-voltage, high-tube-current CT technique--preliminary results. Radiology 2010;256:450–9. [DOI] [PubMed] [Google Scholar]
- 178.Del Chiaro M, Verbeke CS, Kartalis N, et al. Short-term Results of a Magnetic Resonance Imaging-Based Swedish Screening Program for Individuals at Risk for Pancreatic Cancer. JAMA Surg 2015;150:512–8. [DOI] [PubMed] [Google Scholar]
- 179.Carrara G, Pecorelli N, De Cobelli F, et al. Preoperative sarcopenia determinants in pancreatic cancer patients. Clin Nutr 2017;36:1649–1653. [DOI] [PubMed] [Google Scholar]
- 180.Ozola Zalite I, Zykus R, Francisco Gonzalez M, et al. Influence of cachexia and sarcopenia on survival in pancreatic ductal adenocarcinoma: a systematic review. Pancreatology 2015;15:19–24. [DOI] [PubMed] [Google Scholar]
- 181.Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008;9:629–35. [DOI] [PubMed] [Google Scholar]
- 182.Amini N, Spolverato G, Gupta R, et al. Impact Total Psoas Volume on Short- and Long-Term Outcomes in Patients Undergoing Curative Resection for Pancreatic Adenocarcinoma: a New Tool to Assess Sarcopenia. J Gastrointest Surg 2015;19:1593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Peng P, Hyder O, Firoozmand A, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg 2012;16:1478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sandini M, Bernasconi DP, Fior D, et al. A high visceral adipose tissue-to-skeletal muscle ratio as a determinant of major complications after pancreatoduodenectomy for cancer. Nutrition 2016;32:1231–7. [DOI] [PubMed] [Google Scholar]
- 185.Sandini M, Patino M, Ferrone CR, et al. Association Between Changes in Body Composition and Neoadjuvant Treatment for Pancreatic Cancer. JAMA Surg 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sharma A, Kandlakunta H, Nagpal SJS, et al. Model to Determine Risk of Pancreatic Cancer in Patients With New-Onset Diabetes. Gastroenterology 2018;155:730–739 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Danai LV, Babic A, Rosenthal MH, et al. Altered exocrine function can drive adipose wasting in early pancreatic cancer. Nature 2018;558:600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–47. [DOI] [PubMed] [Google Scholar]
- 189.Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 190.Alauddin MM, De Palatis L. Current and Future Trends in Early Detection of Pancreatic Cancer: Molecular Targets and PET Probes. Curr Med Chem 2015;22:3370–89. [DOI] [PubMed] [Google Scholar]
- 191.England CG, Hernandez R, Eddine SB, et al. Molecular Imaging of Pancreatic Cancer with Antibodies. Mol Pharm 2016;13:8–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Serrao EM, Kettunen MI, Rodrigues TB, et al. MRI with hyperpolarised [1–13C]pyruvate detects advanced pancreatic preneoplasia prior to invasive disease in a mouse model. Gut 2016;65:465–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rhim AD, Thege FI, Santana SM, et al. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology 2014;146:647–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Khera AV, Chaffin M, Aragam KG, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet 2018;50:1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Arslan AA, Helzlsouer KJ, Kooperberg C, et al. Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Arch Intern Med 2010;170:791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]