Abstract
Increasing popularity of electronic cigarettes (e-cigs), including among women of reproductive age, is attributed to its perceived safety compared to conventional tobacco. However, there is a major knowledge gap surrounding the effects of e-cig aerosols on pregnancy and fetal development. We aimed to evaluate the effects of vaping e-cigs during gestation on offspring growth and to asses if growth deficits are accompanied by altered maternal and fetal vascular hemodynamics. Sprague-Dawley dams were assigned to Pair-Fed Control, Pair-Fed Juice, or Juice+Nicotine groups, and then underwent either a prenatal or prenatal+postnatal exposure paradigm in a custom-engineered vaping system. Mass spectrometry identified major aerosolized constituents from e-cig vaping. The Juice+Nicotine group exhibited significantly decreased fetal weight and crown-rump length (↓46.56%, and ↓23.83%, respectively). Pre- and postnatal exposure to Juice+Nicotine resulted in decreased pup weight at postnatal day (PND) 4–10. Crown-rump length was decreased by 24.71% on PND 10. Blood flow in the Juice+Nicotine group was decreased in the maternal uterine and fetal umbilical circuits by 49.50% and 65.33%, respectively. We conclude that chronic exposure to e-cig aerosols containing nicotine during early development can have deleterious health effects on the exposed offspring. Vaping e-cigs containing nicotine during pregnancy leads to a reduction in offspring weight and crown-rump length, associated with a marked decrease in blood flow in both the maternal uterine and fetal umbilical circulation (a strong indicator of growth restriction). Thus, chronic exposure to e-cig aerosols containing nicotine can lead to potentially harmful developmental effects in early life.
Keywords: Vascular, Teratogen, Growth
Introduction
Electronic nicotine delivery systems (ENDS) have gained increasing popularity within the past few years (1, 2). ENDS are more commonly referred to as electronic cigarettes (e-cig) and using such devices has been given the term vaping. E-cig liquid is comprised of propylene glycol, glycerol, nicotine, and flavorings. These chemicals are aerosolized by a heating element within the e-cig and then inhaled/exhaled during vaping. Chemical analysis of e-cig aerosols has shown that a large number of hazardous chemicals are released while vaping, leading the Surgeon General of the United States to declare e-cig use as a public health concern (3–7). Advertisement of these products is largely geared towards younger demographics, in an effort to alter the perception of e-cigs as a safer or less-harmful alternative to traditional cigarette smoking (8–11). With e-cig use among adolescents and people of reproductive age increasing from 1.8% in 2010 to 25% in 2015, a closer look at the effects of vaping is imperative (1, 2). Since approximately half of women who smoke before pregnancy continue to smoke during pregnancy and after delivery, it is crucial to assess the potential risks associated with developmental e-cig exposure during early life (12, 13).
Currently, there is a major knowledge gap in regard to the safety of e-cig use during pregnancy. To date, there are no human studies that report the health effects of gestational e-cig use. The few in vivo studies using animal models for e-cig vaping during pregnancy have found significant alterations to the pulmonary system of the mother and offspring, and to the central nervous system of offspring exposed during early life. Studies investigating the lungs of offspring exposed to e-cig vapor during pregnancy show dysregulation in gene expression associated with normal lung development (14). Neonatal e-cig exposure was reported to inhibit alveolar cell proliferation and postnatal lung development (15). In the developing fetal brain, gestational e-cig vaping was found to alter gene expression in the frontal cortex and result in localized inflammation of the hippocampus (16, 17). In many of these studies, it is suggested that constituents of the e-cig liquid other than nicotine may also play a role in the health effects associated with e-cig exposure in early life.
A common end-result of substance use in pregnancy is fetal growth restriction (18, 19). This teratogenic effect has been well documented in studies investigating the use of alcohol and traditional cigarettes during pregnancy (20–22). Further, alcohol drinking is frequently accompanied by smoking (23, 24). The growth deficits associated with substance abuse put offspring at increased risk for further health complications in later life (25, 26). Established models of intrauterine growth restriction (IUGR) demonstrate a decrease in maternal uterine artery blood flow (27–29). In normal pregnancy, the uterine artery undergoes significant adaptations to accommodate the growing nutritional requirements of the fetus, with blood flow through the uterine artery increasing by approximately 30–50 fold by the third trimester (30–33). Disruption of these normal uterine vascular adaptations may be detrimental to proper fetal growth and development. Although e-cigs produce a large number of aerosols, their effects on fetal growth and postnatal development are practically unknown. Further, nothing is known about the effects of e-cig vaping on the maternal uterine artery (which delivers oxygen and nutrients to the feto-placental compartment), or the fetal umbilical vasculature.
With very few investigations on the effects of e-cig use during pregnancy, translational animal studies are both necessary and vital to understand the implications of gestational e-cig vaping on the developing fetus. To date, much of the animal research that aims to discern the correlation between vaping and maternal/infant health employs methods such as intraperitoneal injections, orogastric gavage, and unheated vaporization. These techniques lack the heating element and delivery method that is characteristic of human e-cig vaping. Since the metabolism, and thus the effects, of nicotine differ depending on the route of delivery, we utilized a highly translational method of e-cig aerosol delivery that allowed for a vaping topography common among current e-cig users (34, 35). Using our custom engineered vaping system in combination with our well-characterized pregnant rat model, we evaluated the effects of vaping e-cigs containing nicotine during gestation and exposure to these aerosols during early neonatal development on the overall growth of the offspring, and whether growth deficits are accompanied by altered maternal and fetal reproductive vascular hemodynamics.
Methods
Treatment Groups
All experimental procedures were in accordance with National Institutes of Health guidelines (NIH Publication No. 85–23, revised 1996), with approval by the Animal Care and Use Committee at Texas A&M University. Timed-pregnant Sprague-Dawley rats, 6–7 weeks old, were purchased from Charles River (Wilmington, MA) and housed in individual cages in a temperature-controlled room (23oC) with a 12:12-hour light/dark cycle. Rats were randomly assigned to one of three treatment groups: Pair-Fed Control (Control), Pair-Fed Juice (Juice), and Juice + Nicotine (Nicotine). Prior to initiation of vaping, Control and Juice rats were yoked with a Nicotine vaping animal of similar weight, and the feed amount for these respectively yoked animals was matched daily, thus controlling for nutritional intake throughout the study. The Control group also served as a control for the vaping procedure. Control animals were maintained in vaping chambers identical to those in the Juice and Nicotine groups for an equivalent duration, but with only room air passing through the chamber at a flow rate matching the vaping groups. The Juice group controlled for any difference in the effects of vaping e-cig liquid in the absence of nicotine. The Nicotine group allowed for the testing of the effects of vaping e-cig liquid with nicotine, as a majority of e-cig consumers use devices containing e-cig liquid with nicotine.
E-cig Vaping System
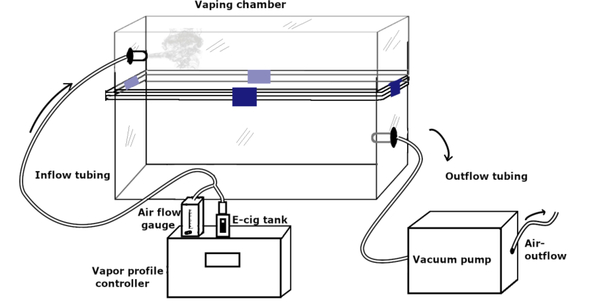
A custom-engineered vaping chamber system with precision-controlled aerosol release technology was used to establish the e-cig vaping paradigm. This setup (Figure 1) allowed for uniform and simultaneous delivery of a customized e-cig vapor profile to all vaping treatment groups. We utilized a Sense Herakles Sub-Ohm Tank (Sense Technology Co., Ltd). The tank contains a replaceable kanthal (iron-chromium-aluminum alloy) 0.6 ohm dual vertical parallel coil, 4 channel adjustable airflow control, and was matched to a 60 W output source via the programmable atomizer. The apparatus included a programmable atomizer that produced e-cig vapor plumes and regulated the volume of liquid vaporized per unit time (Figure 2A). The programmable atomizer is compatible with a wide variety of e-cig vaping media and was used for all subjects within treatment groups. The atomizer used in our system to generate the aerosols was in line with the latest generation of e-cig atomizers currently available to and used by the public. The sophisticated software interface controlled voltage delivered to the programmable atomizer, puff duration, and puff frequency within the inhalation chamber. Airflow was directed throughout the system via silicone tubing (6 mm inner diameter, 10 mm outer diameter) and one-way exhaust valves to ensure unidirectional airflow. The inhalation chambers were airtight, amber, polymer containers resembling the animal housing cages. The software interface is unique in its ability to produce puff profiles identical to those produced by commercial e-cigs. All emissions from the inhalation chambers passed through activated charcoal filters to remove any harmful particles prior to passing through facility exhaust ducts.
Figure 1.
Schematic representation of e-cig vaping chamber apparatus.
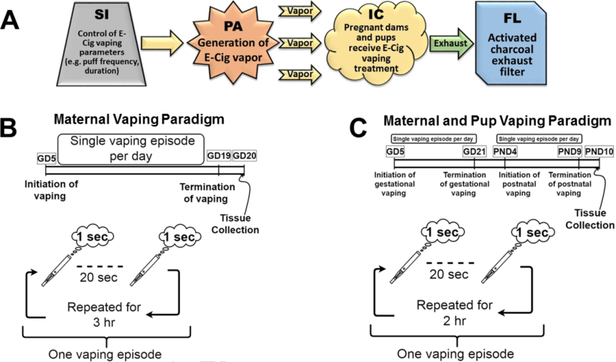
Figure 2.
Flow chart depiction of e-cig aerosol production, exposure, and elimination. (A) The software interface (SI) controlled voltage delivered to the programmable atomizer (PA), which produced the e-cig vapor plume, as well as puff duration and frequency. The puffs traveled to the custom-engineered inhalation chamber (IC) and then through an activated charcoal filter (FL), which eliminated harmful emissions from the IC exhaust. (B) Maternal Vaping Paradigm: Pregnant dams underwent vaping for three hours, five days a week, from gestational day (GD) 5–19. A one second puff was dispensed every 20 seconds. (C) Maternal and pup Vaping Paradigm: Pregnant dams underwent vaping for 2 hours, five days a week, from GD 5–21. Dams gave birth on GD 22, and dams and pups underwent vaping treatment from postnatal day (PND) 4–10, for 2 hours a day. A one second puff was dispensed every 20 seconds.
Exposure Paradigm
Airflow through the chambers was kept at a constant flow rate of 2.5 L/min, with a one second e-cig vapor puff of approximately 42 mL puff volume dispensed every 20 seconds. The duration of the puff was held at one second to ensure proper ventilation of the chambers and removal of accumulating aerosols. To accommodate for the shorter puff duration, a higher power output and nicotine concentration were used since these parameters have been shown to effectively modulate nicotine yield during vaping sessions (36). The e-cig base liquid was compounded in-house at room temperature with a composition of 80:20 propylene glycol (Fisher) to glycerol (Acros Organics), similar to e-cig liquids bought in most vaping shops (37). This e-cig liquid ratio was preferred for our paradigm to maintain adequate absorption of e-cig liquid by the cotton wick due to the relative viscosities of propylene glycol and glycerol. Recent studies on e-cig liquid composition have reported that propylene glycol based liquids provide a higher nicotine delivery ratio than glycerol based liquids (38). E-cig liquids used for the Nicotine group followed the same proportion guidelines as the e-cig base liquid with the addition of 5% (50 mg/mL) nicotine during acclimatization followed by 10% (100 mg/mL) nicotine. E-cig liquid nicotine concentration was selected with consideration for the average nicotine concentration of commercially available e-cig liquids (34, 39)
Our study utilized two different sets of animals for two different exposure periods to assess growth and cardiovascular effects of vaping during pregnancy and early development: 1. To address gestational effects, we utilized a prenatal-only exposure paradigm (Figure 2B), where dams underwent vaping treatment for three hours a day, five days a week, beginning on gestational day (GD) 5 until GD 20, two days prior to parturition (16); 2. To address maternal vaping after birth and resultant exposure to the aerosols in the environment during early postnatal life, we utilized a prenatal + postnatal paradigm (Figure 2C). Dams underwent vaping treatment for two hours a day, five days a week from GD 5 until GD 21, gave birth on GD 22, and then dams and pups resumed vaping on postnatal day (PND) 4 until PND 9, and were sacrificed on PND 10. The postnatal exposure paradigm beginning on PND 4 is an established paradigm in perinatal exposure models and was utilized to reduce imposed stress shortly after birth (16, 17, 40–42). For both prenatal and postnatal studies, the Nicotine group was first acclimatized to the vaping treatment during GD 5–8 utilizing a lower dose of 5% nicotine in the e-cig liquid. Following the acclimatization period, the Nicotine group vaped 10% nicotine for the duration of the studies.
Aerosol Analysis
Aerosol samples were collected in XAD-4 sorbent tubes (Sigma, Saint Louis, MO) at a flow rate of 1 L/min for 3 min. The aerosol was extracted through a sampling port on the side of the chamber via AirChek Touch sample pump (SKC, Houston, TX). Per operating instructions, pump airflow was calibrated prior to each collection using a chek-mate calibrator (SKC, Houston, TX). Capped tubes were stored at 4°C in UV impermeable packaging until analysis. To identify chemical constituents, each sorbent tube was disassembled according to NIOSH 2551, desorbed in 1 mL modified ethyl acetate, and analyzed by GC/MS in triplicate. Agilent 7890B gas chromatograph (Agilent, Santa Clara, CA) and Agilent 5977A mass spectrometer (Agilent, Santa Clara, CA) were utilized for analysis (Health Research Inc., Roswell Park, Buffalo, NY). Chromatograph column properties included (HP-5): 30 m length, 0.32 mm inner diameter, 0.25 μm film, and 2 mm universal liner with wool. Sample volume of 1.0 μL was injected at 250°C. Helium was used as the carrier gas, at a constant flow rate of 1.7 mL/min. Oven temperature ranged from 110°C to 250°C (held one minute) at a rate of 10°C/min. Qualitative analysis of aerosols was carried out using National Institute of Standards and Technology (NIST) 14 Mass Spectral library and Flavors and Fragrances of Natural and Synthetic Compounds (FFNSC) 3 flavoring library (Health Research Inc., Roswell Park, Buffalo, NY).
Mass Spectrometric Analysis of Blood Nicotine Levels
A separate cohort of animals was utilized to assess blood nicotine concentration and were exposed to the same vaping paradigm administered for the different dependent measures (n=5). Blood samples were collected in 0.5 mL serum tubes (BD Biosciences) following the tail-bleed procedure outlined by Omaye et al. (43). Samples were collected on GD 11. Samples were collected just prior to initiation of the vaping exposure (time point = 0 hrs) and every 3 hrs after the start of the experiment up to 12 hrs, after which a 6 hr interval was used to obtain samples at time points 18 and 24 hrs. In one dam, one sample at 18 hrs could not be collected due to sampling complications. Samples were centrifuged at 10,000 g for 5 min at 4°C. Supernatant was removed and aliquoted into 100 uL portions, flash frozen in liquid nitrogen, and stored at −80°C until further processing.
Serum nicotine concentration was measured using liquid chromatography combined with tandem mass spectrometry (LC-MS/MS). 20 μL of serum was mixed with 4 μL of 2.5 N NaOH and an extraction was performed by adding 120 μL of 50:50 methylene chloride:diethyl ether and stirring for 1.5 min. Samples were centrifuged at 4,000 rpm for 5 min and the organic phase was transferred to a 1.5 mL HPLC vial. Organic phase solvent was evaporated under a gentle stream of nitrogen gas at 35°C. Dried extract was reconstituted in 60 μL of deionized water. Liquid chromatography was performed at 30°C using a Varian diphenyl column (SN 285114); 50 mm long by 2 mm inner diameter. Particle size of stationary phase was 5 μm with an isocratic mobile phase of 5% methanol in water (0.1% formic acid). An injection volume of 20 μL was used for LC-MS/MS analysis on an Agilent HPLC 1100.
Growth Measures
To assess the developmental implications of e-cig exposure on growth, body weight and crown-rump length were measured for all offspring. For the prenatal study, animals were sacrificed on GD 20, one day after the last vaping treatment, and fetal weight (number of dams, Control n=15; Juice n=11; Nicotine n=11) was recorded. Litter size between all groups in the prenatal cohort was not significantly different (average litter size, Control=11.87; Juice=12; Nicotine=11.18). Crown-rump length was recorded in fetuses from a subset of dams (number of dams, Control n=6; Juice n=5; Nicotine n=6); litter size between all groups was not significantly different (average litter size, Control=11.17; Juice=12.4; Nicotine=11.17). In the prenatal + postnatal study, a separate cohort of animals were utilized; litter size of each dam (number of dams, Control n=8; Juice n=9; Nicotine n=8) was culled to 8 to standardize nutrition for all pups across treatment groups. Animals were sacrificed on PND 10, one day after the last postnatal vaping treatment. Individual pup weight was collected at birth (PND 1) and daily from PND 3–10. Crown-rump length was measured for each pup on PND 10.
In Vivo Hemodynamic Measurements
One day after the last vaping treatment and prior to sacrifice (GD 20), a subset of dams were imaged by Doppler/high-frequency ultrasonography to obtain heart rate and blood flow measurements (Control n=5, Juice n=5, Nicotine n=5). Ultrasound in combination with Doppler tracings is a noninvasive diagnostic tool that can be used to measure maternal and fetal heart rate as well as flow through the specific reproductive vasculature. Animals were initially sedated using 5% isoflurane in oxygen (1 L/min) in an air-tight induction box for 2–4 mins before being moved to a heated table and fitted with a nose cone that provided 2% isoflurane in oxygen (0.50 L/min). The lower abdomen was shaved and ultrasonic transmission gel (EcoGel 100) was applied prior to probing. Measurements were acquired using a 40-MHz (MX550D) probe a Vevo® 3100 ultrasonograph (VisualSonics, Toronto, Canada). The maternal uterine artery and the fetal umbilical artery were imaged in both B-mode and color pulse wave Doppler to obtain measurement parameters. Vessel identification was established primarily by the unique waveform shape of the primary uterine artery, as well as relative position and structure within the animal (44, 45). A mean maximum velocity was calculated over three continuous cardiac cycles within the Doppler tracing of uterine and umbilical arteries. Transverse vessel diameter images were acquired in B-mode and were analyzed using specialized software (Vevo LAB, Fujifilm VisualSonics) to obtain accurate measurements. Uterine artery and umbilical artery flow rates were determined from the mean peak waveform velocity and the cross-sectional area of the respective vessel.
Statistics
Fetal weight and crown-rump length, pup weight and crown-rump length (PND 10), maternal/fetal heart rate, uterine artery blood flow, and umbilical blood flow were all analyzed by one-way ANOVA with treatment group as the sole independent variable. Postnatal growth measured from PND 1 to 10 was analyzed using a mixed ANOVA model with treatment group as the “between” factor and PND as the “within” factor. Kruskal-Wallis One-Way Analysis of Variance on ranks was performed when needed (46, 47). When appropriate (when significance of a factor or of interaction was established by the initial analysis), an analysis of simple effect was performed for each postnatal day using one-way ANOVA as needed. Further, pair-wise comparisons were performed when appropriate using Tukey’s test. All data are presented as mean + SEM. The α level was established a priori at P < 0.05 for all analyses.
Results
Mass Spectrometric Analysis
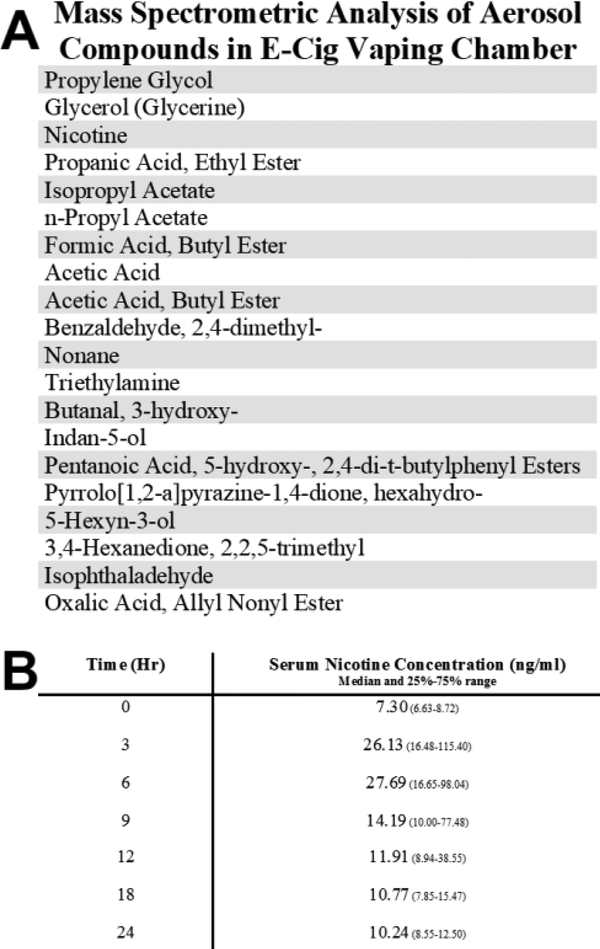
Aerosolized compounds detected within the e-cig vaping chambers were analyzed by gas chromatography/mass spectrometry (Figure 3). All compounds listed were found in the front section of the sorbent tube; the back section was used to detect blow through (overloading of sorbent tube during aerosol sampling). Compounds detected in at least two of three runs/sample were considered present in the sample. Aerosolized chemicals providing only one hit during analysis were determined to be a product of noise. In addition to propylene glycol, glycerol, and nicotine, mass spectrometry analysis showed 17 other aerosols were detected in the aerosol samples. LC-MS/MS quantification of serum nicotine levels are represented by the median for each time point with 25% and 75% range in Figure 3 (P=0.0085, Kruskal-Wallis). Median serum nicotine concentration ranged from 7.30 to 27.69 ng/mL with a peak at 6 hours after the start of the exposure.
Figure 3. Mass spectrometric analysis of aerosol compounds and serum nicotine concentration.
(A) The listed compounds met the detection criteria of being present in at least two of the three analysis runs per sample. (B) LC-MS/MS quantification of serum nicotine levels are represented by the median for each time point with 25% and 75% range.
Growth Measurements
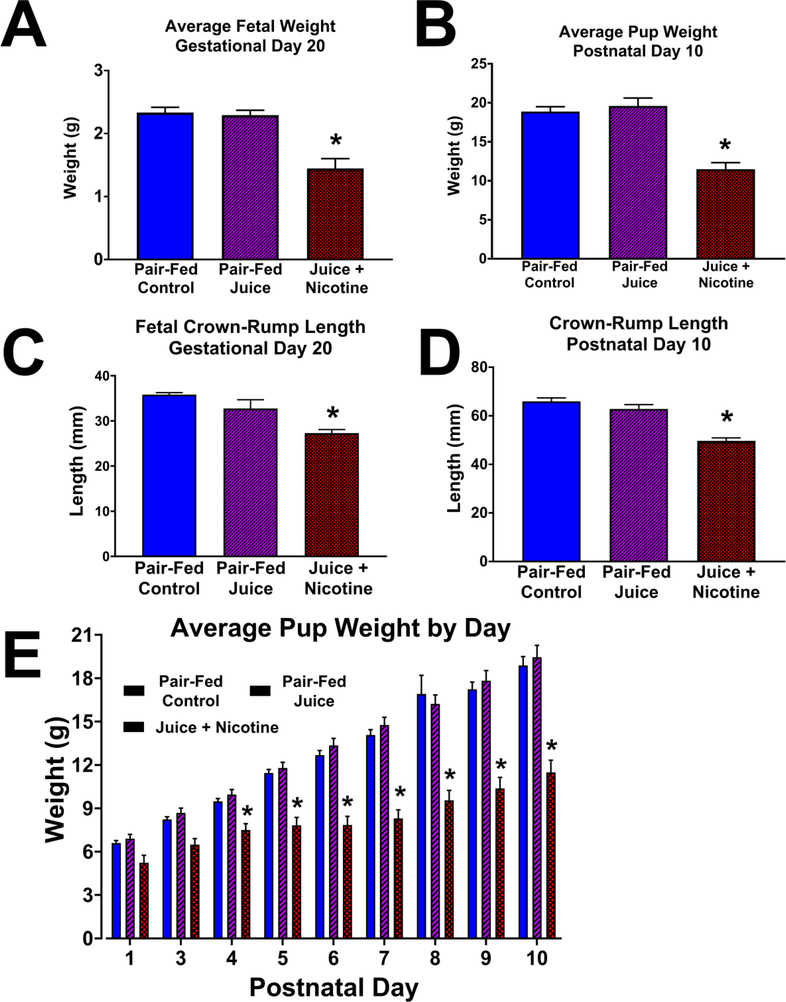
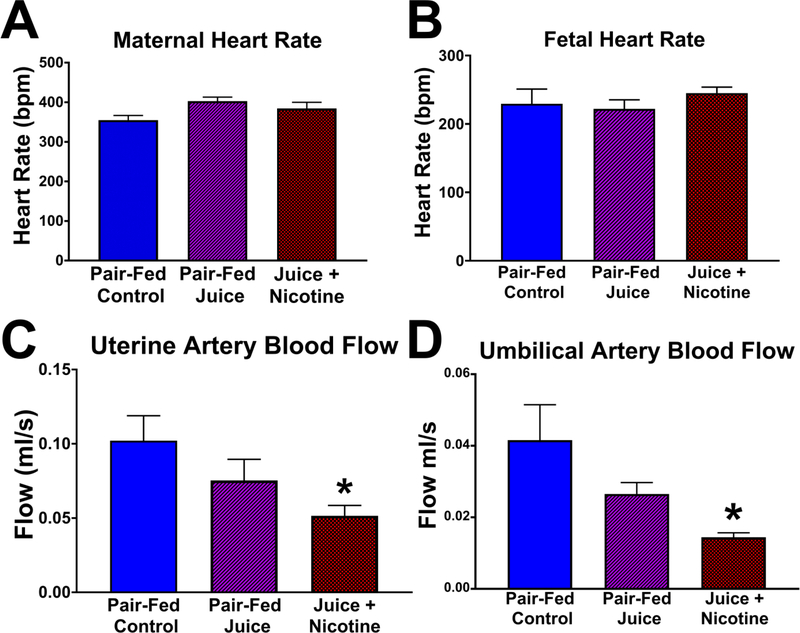
Fetal and pup growth measurements are illustrated in Figure 4. There was no statistical difference in the maternal weight among the three treatment groups prior to the start of the study on GD 5 (Control, 208.7 ± 4.80 g; Juice, 209.5 ± 5.10 g; Nicotine, 217.1 ± 4.80 g), or on GD 20 (Control, 291.9 ± 11.00 g; Juice, 300.7 ± 11.40 g; Nicotine, 300.1 ± 11.40 g). Mean fetal weight in the Control group (2.33 ± 0.084 g) was not different from those in the Juice group (2.29 ± 0.078 g). The median fetal weight with 25% and 75% range for each group were 2.31(2.09–2.47) g, 2.23(2.09–2.37) g, and 1.39(1.13–1.63) g in the Control, Juice, and Nicotine groups respectively. The Control and Juice groups were not different, and both these groups were significantly different from the Nicotine group (P<0.001, Kruskal-Wallis). Fetal weight in the Nicotine group (1.45 ± 0.16 g) was significantly decreased (P < 0.0001) by 46.56% compared to the Control group, and by 44.92% compared to the Juice group (Figure 4A). Fetal crown-rump length (Figure 4C) in the Control group (35.83 ± 0.44 mm) was not different from that in the Juice group (32.74 ± 1.96 mm). Fetal crown-rump length measured in the Nicotine group (27.29 ± 0.81 mm) was significantly decreased compared to the Control (↓23.83%; P = 0.0002) and Juice groups (↓16.65%; P = 0.0129). Average pup weight (PND 10; Figure 4B) in the Nicotine group (11.34 ± 1.06 g) was significantly decreased compared to the Control (19.04 ± 0.43 g; ↓40.44%; P = 0.0002) and Juice (19.12 ± 1.38 g; ↓40.69%; P < 0.0001) groups. Nicotine pup weights were significantly decreased (P < 0.05) on PND 4 to 10 when compared to the Control and Juice groups. There was no difference in pup weight between the Control and Juice groups during PND 1 to 10 (Figure 4E). Pup crown-rump length (PND 10; Figure 4D) in the Nicotine group (49.67 ± 1.25 mm) was significantly decreased (P < 0.0001) compared to the Control (65.97 ± 1.42 mm; ↓24.71%) and Juice (62.80 ± 1.81 mm; ↓20.91%) groups.
Figure 4. Effects of e-cig vaping during early development on offspring growth.
Following gestational and postnatal vaping, fetal and pup weight and crown-rump length were measured on gestational day (GD) 20 and postnatal day (PND) 10, respectively, one day after the last vaping episode. (A) Mean fetal weight in the Juice + Nicotine group was decreased compared with Pair-Fed Control and Pair-Fed Juice groups (P < 0.0001). (B) Pup weight in the Juice + Nicotine group was decreased compared with both Pair-Fed Control (P = 0.0002) and Pair-Fed Juice groups (P < 0.0001). (C) Fetal crown-rump length in the Juice + Nicotine group was decreased compared to the Pair-Fed Control and Pair-Fed Juice groups (P = 0.0002, P = 0.0129, respectively). (D) Following prenatal + postnatal vaping, crown-rump length in the Juice + Nicotine group was decreased compared with that in the Pair-Fed Control and Pair-Fed Juice groups. (P < 0.0001). (E) Postnatal pup weight in the Juice + Nicotine group was decreased compared with the Pair-Fed Control and the Pair-Fed Juice Groups on PND 4–10. Values are mean + SEM, * indicates statistical significance, P < 0.05.
Hemodynamic Measurements
There was no significant difference in the maternal heart rate (Figure 5A) or fetal heart rate (Figure 5B) among the three treatment groups. These values were determined using Doppler tracing by measuring the elapsed time required for three waveforms to cycle (peak-to-peak). There was no significant difference in the maternal uterine artery blood flow or the fetal umbilical artery blood flow between the Control and Juice group. Uterine artery and umbilical artery blood flow were significantly decreased in the Nicotine group compared to the Control group (uterine artery, ↓49.50%, P = 0.0489, Figure 5C; umbilical artery, ↓65.33%, P = 0.0164, Figure 5D).
Figure 5. Effect of gestational e-cig vaping on maternal uterine and fetal umbilical artery hemodynamics.
On GD 20, (A) maternal heart rate and (B) fetal heart rate were not different following chronic e-cig vaping during pregnancy. However, in the Juice + Nicotine group, both (C) maternal uterine artery blood flow (P = 0.0489) and (D) fetal umbilical artery blood flow (P = 0.0164) were reduced compared with those in the Pair-Fed Control. Values are mean + SEM, * indicates statistical significance, P < 0.05.
Discussion
To our knowledge, this is the first study to assess health effects of e-cig use during pregnancy and early development in a rat model using the latest generation e-cig atomizer technology. The vaping chamber system allowed for a vaping topography directly comparable to current e-cig users. From this study, we can glean three important conclusions: 1. Chronic exposure to e-cig aerosols containing nicotine during early development can have deleterious health effects on the exposed offspring; 2. Vaping e-cigs containing nicotine during pregnancy leads to a reduction in offspring weight and crown-rump length, consistent with an IUGR phenotype (28, 48, 49); and 3. Blood flow is markedly decreased in both the maternal uterine artery and fetal umbilical artery (a strong indicator of early-onset IUGR) after vaping e-cigs containing nicotine during pregnancy (50). Our data collectively demonstrate that chronic vaping of e-cigs containing nicotine during pregnancy can lead to potentially harmful effects in the developing fetus.
Overall, our data show that chronic exposure to e-cig aerosols containing nicotine during early development can have deleterious health effects on the exposed offspring. It is well known that smoking traditional cigarettes during pregnancy is harmful to the developing fetus, largely because the combustion process produces toxic substances, such as carbon monoxide (51). E-cigs have gained increasing popularity within the past few years, particularly among those in the reproductive age demographic (52). One reason for this is that users openly perceive these devices as a safer alternative to traditional cigarette smoking, as e-cigs aerosolize a liquid comprised of propylene glycol, glycerol, nicotine, and flavorings rather than burning tobacco (8–11). Since e-cigs are a recent development, it may take many years for research to fully delineate possible risks associated with their use. A common limitation among current e-cig related research that complicates the evaluation of health effects attributed to e-cig use is in part due to the wide selection of different e-cig products and modification packages with unique settings available to the public. Currently, little is known regarding e-cig safety, especially health effects during pregnancy. A recent survey reported that without accounting for the adverse effects of nicotine, 45% of pregnant women believed e-cigs were less harmful than traditional cigarettes (52, 53). If pregnant women use e-cigs that contain nicotine as a harm reduction alternative, our data show that this exposure could potentially result in injury to the developing fetus. Serum nicotine levels described in this study closely reflect the concentration of nicotine found in the blood of active e-cig users and traditional cigarette smokers (54–59). Experienced e-cig users (many may be ex-cigarette smokers) maintain an almost identical average blood nicotine concertation when compared to tobacco users (58, 60). Daily baseline concentration of serum nicotine in individuals who use tobacco products is known to vary from person to person depending on extent of use and rate of clearance (34, 61). Traditional cigarette smokers self-titrate nicotine by controlling the number and frequency of cigarettes smoked per day in order to achieve a baseline blood nicotine concentration (55). The daily concentration of blood nicotine in habitual smokers typically reaches a steady state of approximately 20–50 ng/mL, but can vary within the range of 5–100 ng/mL (54–57). While nicotine can accumulate in the blood throughout the day the rate of clearance is high enough that differences in nicotine absorption from one day to the next are negligible (62). However, the continual slow release of nicotine deposited in various body tissues results in serum nicotine levels above 0 ng/mL even after a brief period of abstinence (63). To date, few studies have been performed on the effects of vaping e-cigs during pregnancy and/or early development. In support of our findings, it has been shown in other animal model studies, vaping e-cigs containing nicotine during pregnancy altered gene expression in the pulmonary system and produced central nervous system (CNS) dysregulation in the offspring (14). For instance, in the lung, alveolar cell proliferation and postnatal lung development were inhibited by neonatal e-cig exposure (15). In the CNS, gestational e-cig vaping altered gene expression in the frontal cortex and resulted in localized inflammation of the hippocampus (16, 17). Collectively, we and others demonstrate that use of e-cigs containing nicotine during pregnancy is not as safe as is often perceived, with multiple organ systems being influenced by e-cig exposure.
Our data show chronic exposure to nicotine from e-cigs during early development results in a dramatic decrease in both fetal and pup weights as well as their crown-rump lengths. This is a significant finding because a smaller than average weight at birth is typically accompanied by other severe complications such as respiratory distress syndrome and necrotizing enterocolitis, making growth restriction an important clinical indicator of perinatal morbidity. (50, 64, 65). Developmental growth restriction is also associated with an increased risk for developmental delays, infant mortality, and manifestation of chronic diseases later in life (25, 26). Thus, growth restriction resulting from chronic exposure to e-cigs containing nicotine during development may increase risks for a myriad of complications at birth and for developing chronic disease in adulthood. Nutrient delivery to the fetus has been shown to be a critical factor influencing fetal growth and development (65). Substance abuse, alcohol consumption, and tobacco use during pregnancy frequently lead to IUGR as a result of poor nutrient delivery (18–20, 22). Further, vaping e-cigs containing nicotine may directly inhibit acetylcholine-facilitated transport systems responsible for moving vital amino acids across the placenta (66). To control for nutritional intake in the nicotine-exposed animals, we included pair-fed control groups, and found no difference in the maternal weights at the end of the exposure paradigm. Therefore, we conjectured that nicotine vaping-induced fetal growth restriction may still result from decreased nutrient delivery from the mother to the feto-placental compartment via reduced blood flow in the maternal uterine artery. Although we did not measure maternal and fetal oxygen and nutrient concentrations in this study, we tested if there is a decrease in blood flow in the maternal uterine artery, which directly controls the supply of oxygen and nutrients to the feto-placental compartment (33, 67).
A common theme in IUGR animal models is reduced nutrient delivery resulting from a lower than normal blood flow in the uterine artery (28–30, 33). Our study is the first to investigate the effects of e-cig exposure during pregnancy on both the uterine artery and umbilical cord hemodynamics. Utilizing ultrasonography paired with real-time Doppler tracings, we were able to determine blood flow through specific reproductive vasculature. The maternal uterine artery undergoes profound adaptations to accommodate a 30–50 fold increase in gestational blood flow to the developing fetus which are crucial for sustaining growth and normal fetal development (30–33). Our data showed that blood flow through both the maternal uterine artery and the fetal umbilical artery in animals exposed to e-cigs containing nicotine were 49.50% and 65.33% lower than those in the control group, respectively. Nicotine has been shown to possess strong vasoconstrictor properties, and acts by stimulating sympathetic outflow and impairing endothelial-dependent vasorelaxation, effectively increasing the vascular resistance in the feto-placental compartment (68, 69). A lower blood volume per unit time delivered to the feto-placental compartment will proportionally and dramatically decrease the amount of nutrients and oxygen delivered. The current study is not capable of dissecting if the decreased uterine artery blood flow leads to growth restriction, or, alternatively, if e-cig vaping-induced growth restriction leads to a decreased demand for oxygen and nutrients from the mother. As far as the fetal compartment is concerned, a decreased blood flow in the umbilical circuit may be directly due to a lower cardiac output in the growth-restricted fetuses, or an increase in nicotine-induced resistance offered by the placental blood vessels, which are in series with the umbilical artery and the umbilical vein (50, 70, 71). This study provides evidence that vaping nicotine during pregnancy can produce potentially harmful effects for the developing fetus by altering the vascular adaptations necessary for normal pregnancy. Although a cause and effect relationship cannot be established in our current study design, future studies are warranted to mechanistically test if growth restriction can be produced by directly inhibiting the uterine blood flow similar to the decreases seen in this study.
We utilized the Juice treatment group in this study to control for any difference in the effects of e-cig vaping in the absence of nicotine. Our data indicate that constituents in the e-cig aerosol other than nicotine did not produce growth restriction or impact the assessed maternal and fetal hemodynamics following gestational e-cig vaping, however, it is important to note that there may be other underlying health effects of these aerosols that have yet to be determined. Although a large body of prenatal animal research demonstrates that nicotine alone can result in significant disruptions to normal development spanning nearly all major organ systems, it remains unclear whether nicotine is the single component contributing to negative health outcomes observed in offspring exposed to e-cig aerosols during early life. The vapor created by e-cigs is a result of heating the e-cig liquid at high temperatures. This heating process can alter the chemical profile of the original liquid to produce a large number of hazardous aldehydes and numerous other chemical byproducts (4, 6, 72). Reactive aldehydes, such as those in Figure 3, have been shown to negatively impact maternal and fetal health across multiple organ systems (73, 74). Many of the chemicals produced by e-cigs have been observed to be potentially dangerous if ingested, yet the effects of these chemicals and their byproducts when inhaled is not fully understood. The analysis of aerosols described in this study will require further quantitative analysis to identify chemicals of particular investigative interest. Several studies support the idea that chemicals other than nicotine play a role in altering normal development with evidence that e-cig liquid alone can effect neurodevelopment and metabolic function of offspring (16, 27). Thus, it is imperative that further research be done to investigate the impact that such chemicals have on development. Flavorings used in commercial e-cig liquids further complicate investigations into the health effects of e-cig vaping due to their highly variable recipes and inconsistent manufacturing procedures (75, 76). Flavoring components were not included in this study, however, they may play an integral role in the health outcomes associated with e-cig vaping.
Further research is urgently warranted to fully understand the largely unknown health consequences regarding e-cig safety during pregnancy. The effects of nicotine have the potential to encompass all aspects of fetal development. To fully evaluate the effects of developmental e-cig aerosol exposure on offspring growth, a comprehensive examination of developmental parameters beyond those reported herein must be explored. Although our study shows that vaping nicotine is harmful to early development, constituents of the e-cig liquid other than nicotine may exacerbate the negative outcomes associated with e-cig exposure (77, 78). Since alcohol consumption during pregnancy is usually compounded by tobacco product use, investigating the combined effects of alcohol consumption and e-cig vaping during pregnancy is also necessary (23, 24). Additionally, it is imperative that e-cig flavorings be evaluated for safety, as recent studies show these may contain harmful additives, such as diacetyl, that can significantly influence negative health outcomes (79–81). Lastly, temporal studies are necessary to determine the various developmental windows of vulnerability for specific organ systems.
Background:
To date, much of the research that aims to investigate health effects of e-cig aerosol exposure in early development health employs delivery methods such as intraperitoneal injections, orogastric gavage, and unheated vaporization.
Translational Significance:
Our study design advances the field by implementing a state-of-the-art engineered e-cig vaping system utilizing an atomizer identical to those sold in vape shops. The study introduces a translational inhalation delivery method to generate vapor profiles directly comparable to human vaping. The current study is vital to understand the implications of e-cig vaping during early development. This will help improve policymaking regarding public health practices, and formulation of appropriate device regulation.
Acknowledgments
Dr. Rajesh Miranda (Texas A&M University). All authors have read the journal’s policy on disclosure of potential conflicts of interest. All authors have disclosed any financial or personal relationship with organizations that could potentially be perceived as influencing the described research. All authors have read the journal’s authorship statement.
Support: This work was supported by National Institutes of Health AA19446, AA23520, AA23035, and Texas A&M University Tier One Program [JR].
Abbreviations:
- ENDS
Electronic nicotine delivery systems
- e-cigs
electronic cigarettes
- IUGR
intrauterine growth restriction
- Control
Pair-Fed Control
- Juice
Pair-Fed Juice
- Nicotine
Juice + Nicotine
- GD
gestational day
- PND
postnatal day
- CNS
central nervous system
Footnotes
Conflict of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McMillen RC, Gottlieb MA, Shaefer RMW, Winickoff JP, Klein JD. Trends in Electronic Cigarette Use Among U.S. Adults: Use is Increasing in Both Smokers and Nonsmokers. Nicotine & Tobacco Research. 2015;17(10):1195–202. [DOI] [PubMed] [Google Scholar]
- 2.Kinnunen JM, Ollila H, Lindfors PL, Rimpela AH. Changes in Electronic Cigarette Use from 2013 to 2015 and Reasons for Use among Finnish Adolescents. Int J Environ Res Public Health. 2016;13(11):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Services USDoHaH. E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General—Executive Summary. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2016. [Google Scholar]
- 4.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23(2):133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden formaldehyde in e-cigarette aerosols. New England Journal of Medicine. 2015;372(4):392–4. [DOI] [PubMed] [Google Scholar]
- 6.Sleiman M, Logue JM, Montesinos VN, Russell ML, Litter MI, Gundel LA, et al. Emissions from Electronic Cigarettes: Key Parameters Affecting the Release of Harmful Chemicals. Environ Sci Technol. 2016;50(17):9644–51. [DOI] [PubMed] [Google Scholar]
- 7.Uchiyama S, Ohta K, Inaba Y, Kunugita N. Determination of carbonyl compounds generated from the E-cigarette using coupled silica cartridges impregnated with hydroquinone and 2, 4-dinitrophenylhydrazine, followed by high-performance liquid chromatography. Analytical Sciences. 2013;29(12):1219–22. [DOI] [PubMed] [Google Scholar]
- 8.Dutra LM, Glantz SA. E-cigarettes and National Adolescent Cigarette Use: 2004–2014. Pediatrics. 2017;139(2):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duke JC, Allen JA, Eggers ME, Nonnemaker J, Farrelly MC. Exploring Differences in Youth Perceptions of the Effectiveness of Electronic Cigarette Television Advertisements. Nicotine & Tobacco Research. 2016;18(5):1382–6. [DOI] [PubMed] [Google Scholar]
- 10.Padon AA, Maloney EK, Cappella JN. Youth-Targeted E-cigarette Marketing in the US. Tobacco regulatory science. 2017;3(1):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anand V, McGinty KL, O’Brien K, Guenthner G, Hahn E, Martin CA. E-cigarette Use and Beliefs Among Urban Public High School Students in North Carolina. J Adolesc Health. 2015;57(1):46–51. [DOI] [PubMed] [Google Scholar]
- 12.Wagner NJ, Camerota M, Propper C. Prevalence and Perceptions of Electronic Cigarette Use during Pregnancy. Maternal and Child Health Journal. 2017;21(8):1655–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tong VT, Dietz PM, Morrow B, D’Angelo DV, Farr SL, Rockhill KM, et al. Trends in Smoking Before, During, and After Pregnancy - Pregnancy Risk Assessment Monitoring System, United States, 40 Sites, 2000–2010. MMWR Surv Summ. 2013;62(6):1–19. [PubMed] [Google Scholar]
- 14.Chen H, Li G, Chan YL, Chapman DG, Sukjamnong S, Nguyen T, et al. Maternal E-Cigarette Exposure in Mice Alters DNA Methylation and Lung Cytokine Expression in Offspring. Am J Respir Cell Mol Biol. 2018;58(3):366–77. [DOI] [PubMed] [Google Scholar]
- 15.McGrath-Morrow SA, Hayashi M, Aherrera A, Lopez A, Malinina A, Collaco JM, et al. The Effects of Electronic Cigarette Emissions on Systemic Cotinine Levels, Weight and Postnatal Lung Growth in Neonatal Mice. Plos One. 2015;10(2):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauterstein DE, Tijerina PB, Corbett K, Oksuz BA, Shen SS, Gordon T, et al. Frontal Cortex Transcriptome Analysis of Mice Exposed to Electronic Cigarettes During Early Life Stages. Int J Environ Res Public Health. 2016;13(4):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zelikoff JT, Parmalee NL, Corbett K, Gordon T, Klein CB, Aschner M. Microglia Activation and Gene Expression Alteration of Neurotrophins in the Hippocampus Following Early-Life Exposure to E-Cigarette Aerosols in a Murine Model. Toxicol Sci. 2018;162(1):276–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anblagan D, Jones NW, Costigan C, Parker AJJ, Allcock K, Aleong R, et al. Maternal Smoking during Pregnancy and Fetal Organ Growth: A Magnetic Resonance Imaging Study. Plos One. 2013;8(7):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuczkowski KM. The effects of drug abuse on pregnancy. Curr Opin Obstet Gynecol. 2007;19(6):578–85. [DOI] [PubMed] [Google Scholar]
- 20.Naik VD, Davis-Anderson K, Subramanian K, Lunde-Young R, Nemec MJ, Ramadoss J. Mechanisms Underlying Chronic Binge Alcohol Exposure-Induced Uterine Artery Dysfunction in Pregnant Rat. Alcoholism (NY). 2018;42(4):682–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cnattingius S The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine & Tobacco Research. 2004;6:S125–S40. [DOI] [PubMed] [Google Scholar]
- 22.Services USDoHaH. The Health Consequences of Smoking: 50 Years of Progress - A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 23.Falk DE, Yi HY, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders - Findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res Health. 2006;29(3):162–71. [PMC free article] [PubMed] [Google Scholar]
- 24.Anthony JC, Echeagaray-Wagner F. Epidemiologic analysis of alcohol and tobacco use - Patterns of co-occurring consumption and dependence in the United States. Alcohol Res Health. 2000;24(4):201–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Barker DJP. Developmental origins of chronic disease. Public Health. 2012;126(3):185–9. [DOI] [PubMed] [Google Scholar]
- 26.Lunde ER, Washburn SE, Golding MC, Bake S, Miranda RC, Ramadoss J. Alcohol-Induced Developmental Origins of Adult-Onset Diseases. Alcoholism (NY). 2016;40(7):1403–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chien PFW, Arnott N, Gordon A, Owen P, Khan KS. How useful is uterine artery Doppler flow velocimetry in the prediction of pre-eclampsia, intrauterine growth retardation and perinatal death? An overview. Br J Obstet Gynaecol. 2000;107(2):196–208. [DOI] [PubMed] [Google Scholar]
- 28.Holemans K, Aerts L, Van Assche FA. Fetal growth restriction and consequences for the offspring in animal models. J Soc Gynecol Invest. 2003;10(7):392–9. [DOI] [PubMed] [Google Scholar]
- 29.Cnossen JS, Morris RK, ter Riet G, Mol BWJ, van der Post JAM, Coomarasamy A, et al. Use of uterine artery Doppler ultrasonography to predict pre-eclampsia and intrauterine growth restriction: a systematic review and bivariable meta-analysis. Can Med Assoc J. 2008;178(6):701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer SK, Zamudio S, Coffin C, Parker S, Stamm E, Moore LG. QUANTITATIVE ESTIMATION OF HUMAN UTERINE ARTERY BLOOD-FLOW AND PELVIC BLOOD-FLOW REDISTRIBUTION IN PREGNANCY. Obstet Gynecol. 1992;80(6):1000–6. [PubMed] [Google Scholar]
- 31.Caton D, Kalra PS. ENDOGENOUS HORMONES AND REGULATION OF UTERINE BLOOD-FLOW DURING PREGNANCY. Am J Physiol. 1986;250(3):R365–R9. [DOI] [PubMed] [Google Scholar]
- 32.Dowell RT, Kauer CD. Maternal hemodynamics and uteroplacental blood flow throughout gestation in conscious rats. Methods Find Exp Clin Pharmacol. 1997;19(9):613–25. [PubMed] [Google Scholar]
- 33.Lang U, Baker RS, Braems G, Zygmunt M, Kunzel W, Clark KE. Uterine blood flow--a determinant of fetal growth. Eur J Obstet Gynecol Reprod Biol. 2003;110 Suppl 1:S55–61. [DOI] [PubMed] [Google Scholar]
- 34.Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007;190(3):269–319. [DOI] [PubMed] [Google Scholar]
- 35.Robinson RJ, Hensel EC, Morabito PN, Roundtree KA. Electronic Cigarette Topography in the Natural Environment. Plos One. 2015;10(6):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Talih S, Balhas Z, Eissenberg T, Salman R, Karaoghlanian N, El Hellani A, et al. Effects of User Puff Topography, Device Voltage, and Liquid Nicotine Concentration on Electronic Cigarette Nicotine Yield: Measurements and Model Predictions. Nicotine & Tobacco Research. 2015;17(2):150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hahn J, Monakhova YB, Hengen J, Kohl-Himmelseher M, Schussler J, Hahn H, et al. Electronic cigarettes: overview of chemical composition and exposure estimation. Tob Induc Dis. 2014;12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Son Y, Wackowski O, Weisel C, Schwander S, Mainelis G, Delnevo C, et al. Evaluation of E-Vapor Nicotine and Nicotyrine Concentrations under Various E-Liquid Compositions, Device Settings, and Vaping Topographies. Chemical Research in Toxicology. 2018;31(9):861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine Levels in Electronic Cigarettes. Nicotine & Tobacco Research. 2013;15(1):158–66. [DOI] [PubMed] [Google Scholar]
- 40.Bonthius DJ, Bonthius NE, Napper RMA, West JR. Early postnatal alcohol exposure acutely and permanently reduces the number of granule cells and mitral cells in the rat olfactory bulb: A stereological study. Journal of Comparative Neurology. 1992;324(4):557–66. [DOI] [PubMed] [Google Scholar]
- 41.Diaz J, Samson H. Impaired brain growth in neonatal rats exposed to ethanol. Science. 1980;208(4445):751–3. [DOI] [PubMed] [Google Scholar]
- 42.Thomas JD, Garrison ME, Slawecki CJ, Ehlers CL, Riley EP. Nicotine exposure during the neonatal brain growth spurt produces hyperactivity in preweanling rats. Neurotoxicology and teratology. 2000;22(5):695–701. [DOI] [PubMed] [Google Scholar]
- 43.Omaye ST, Skala JH, Gretz MD, Schaus EE, Wade CE. Simple method for bleeding the unanaesthetized rat by tail venipuncture. Lab Anim. 1987;21(3):261–4. [DOI] [PubMed] [Google Scholar]
- 44.Qu D, Adamson SL, Zhou Y-Q. Method to Locate the Uterine Artery in Mice for Micro-Ultrasound Doppler Blood Velocity Examination. The Guide to Investigation of Mouse Pregnancy: Elsevier. 2014:693–7. [Google Scholar]
- 45.Arthuis CJ, Novell A, Escoffre JM, Patat F, Bouakaz A, Perrotin F. New insights into uteroplacental perfusion: quantitative analysis using Doppler and contrast-enhanced ultrasound imaging. Placenta. 2013;34(5):424–31. [DOI] [PubMed] [Google Scholar]
- 46.Jarvis M, Tunstall-Pedoe H, Feyerabend C, Vesey C, Salloojee Y. Biochemical markers of smoke absorption and self reported exposure to passive smoking. Journal of Epidemiology and Community Health. 1984;38(4):335–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milerad J, Vege Å, Opdal SH, Rognum TO. Objective measurements of nicotine exposure in victims of sudden infant death syndrome and in other unexpected child deaths. The Journal of Pediatrics. 1998;133(2):232–6. [DOI] [PubMed] [Google Scholar]
- 48.Papageorghiou AT, Ohuma EO, Altman DG, Todros T, Ismail LC, Lambert A, et al. International standards for fetal growth based on serial ultrasound measurements: the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):869–79. [DOI] [PubMed] [Google Scholar]
- 49.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–8. [DOI] [PubMed] [Google Scholar]
- 50.Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. 2000;92(1):35–43. [DOI] [PubMed] [Google Scholar]
- 51.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. Bmj. 2004;328(7455):1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhandari NR, Day KD, Payakachat N, Franks AM, McCain KR, Ragland D. Use and Risk Perception of Electronic Nicotine Delivery Systems and Tobacco in Pregnancy. Women’s Health Issues. 2018. [DOI] [PubMed] [Google Scholar]
- 53.England LJ, Tong VT, Koblitz A, Kish-Doto J, Lynch MM, Southwell BG. Perceptions of emerging tobacco products and nicotine replacement therapy among pregnant women and women planning a pregnancy. Preventive medicine reports. 2016;4:481–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benowitz NL, PJ. Daily intake of nicotine during cigarette smoking. Clinical Pharmacology & Therapeutics. 1984;35(4):499–504. [DOI] [PubMed] [Google Scholar]
- 55.Moyer TP, Charlson JR, Enger RJ, Dale LC, Ebbert JO, Schroeder DR, et al. Simultaneous Analysis of Nicotine, Nicotine Metabolites, and Tobacco Alkaloids in Serum or Urine by Tandem Mass Spectrometry, with Clinically Relevant Metabolic Profiles. Clinical Chemistry. 2002;48(9):1460–71. [PubMed] [Google Scholar]
- 56.Lawson GM, Hurt RD, Dale LC, Offord KP, Croghan IT, Schroeder DR, et al. Application of serum nicotine and plasma cotinine concentrations to assessment of nicotine replacement in light, moderate, and heavy smokers undergoing transdermal therapy. Journal of clinical pharmacology. 1998;38(6):502–9. [DOI] [PubMed] [Google Scholar]
- 57.Russell MA, Jarvis M, Iyer R, Feyerabend C. Relation of nicotine yield of cigarettes to blood nicotine concentrations in smokers. British Medical Journal. 1980;280(6219):972–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wagener TL, Floyd EL, Stepanov I, Driskill LM, Frank SG, Meier E, et al. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob Control. 2017;26(e1):e23–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benowitz NL, Kuyt F, Jacob P, Jones RT, Osman A-L. Cotinine disposition and effects. Clinical Pharmacology & Therapeutics. 1983;34(5):604–11. [DOI] [PubMed] [Google Scholar]
- 60.Etter J-F, Bullen C. A longitudinal study of electronic cigarette users. Addict Behav. 2014;39(2):491–4. [DOI] [PubMed] [Google Scholar]
- 61.Benowitz NL, Jacob P. Nicotine and cotinine elimination pharmacokinetics in smokers and nonsmokers. Clinical Pharmacology & Therapeutics. 1993;53(3):316–23. [DOI] [PubMed] [Google Scholar]
- 62.Isaac PF, Rand MJ. Cigarette Smoking and Plasma Levels of Nicotine. Nature. 1972;236:308. [DOI] [PubMed] [Google Scholar]
- 63.Urakawa N, Nagata T, Kudo K, Kimura K, Imamura T. Simultaneous determination of nicotine and cotinine in various human tissues using capillary gas chromatography/mass spectrometry. International Journal of Legal Medicine. 1994;106(5):232–6. [DOI] [PubMed] [Google Scholar]
- 64.Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan L, Vermont Oxford N. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. Am J Obstet Gynecol. 2000;182(1):198–206. [DOI] [PubMed] [Google Scholar]
- 65.Wu GY, Bazer FW, Cudd TA, Meininger CJ, Spencer TE. Maternal nutrition and fetal development. J Nutr. 2004;134(9):2169–72. [DOI] [PubMed] [Google Scholar]
- 66.Sastry BVR. PLACENTAL TOXICOLOGY - TOBACCO-SMOKE, ABUSED DRUGS, MULTIPLE CHEMICAL INTERACTIONS, AND PLACENTAL FUNCTION. Reprod Fertil Dev. 1991;3(4):355–72. [DOI] [PubMed] [Google Scholar]
- 67.Reynolds LP, Caton JS, Redmer DA, Grazul-Bilska AT, Vonnahme KA, Borowicz PP, et al. Evidence for altered placental blood flow and vascularity in compromised pregnancies. J Physiol. 2006;572(Pt 1):51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Black CE, Huang N, Neligan PC, Levine RH, Lipa JE, Lintlop S, et al. Effect of nicotine on vasoconstrictor and vasodilator responses in human skin vasculature. Am J Physiol-Regul Integr Comp Physiol. 2001;281(4):R1097–R104. [DOI] [PubMed] [Google Scholar]
- 69.Haass M, Kubler W. Nicotine and sympathetic neurotransmission. Cardiovasc Drugs Ther. 1997;10(6):657–65. [DOI] [PubMed] [Google Scholar]
- 70.Pringle PJ, Geary MPP, Rodeck CH, Kingdom JCP, Kayamba-Kay’s S, Hindmarsh PC. The influence of cigarette smoking on antenatal growth, birth size, and the insulin-like growth factor axis. J Clin Endocrinol Metab. 2005;90(5):2556–62. [DOI] [PubMed] [Google Scholar]
- 71.Machado JD, Filho PVM, Petersen GO, Chatkin JM. Quantitative effects of tobacco smoking exposure on the maternal-fetal circulation. BMC Pregnancy Childbirth. 2011;11:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine & Tobacco Research. 2014;16(10):1319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eskenazi B, Bracken MB, Holford TR, Grady J. Exposure to organic solvents and hypertensive disorders of pregnancy. American journal of industrial medicine. 1988;14(2):177–88. [DOI] [PubMed] [Google Scholar]
- 74.Henderson GI, Chen J, Schenker S. Ethanol, oxidative stress, reactive aldehydes, and the fetus. Front Biosci. 1999;4(4):541–50. [DOI] [PubMed] [Google Scholar]
- 75.Bahl V, Lin S, Xu N, Davis B, Wang YH, Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol. 2012;34(4):529–37. [DOI] [PubMed] [Google Scholar]
- 76.Zhao JY, Nelson J, Dada O, Pyrgiotakis G, Kavouras IG, Demokritou P. Assessing electronic cigarette emissions: linking physico-chemical properties to product brand, e-liquid flavoring additives, operational voltage and user puffing patterns. Inhal Toxicol. 2018;30(2):78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Farsalinos KE, Kistler KA, Gillman G, Voudris V. Evaluation of electronic cigarette liquids and aerosol for the presence of selected inhalation toxins. Nicotine & Tobacco Research. 2014;17(2):168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Varlet V, Farsalinos K, Augsburger M, Thomas A, Etter J-F. Toxicity assessment of refill liquids for electronic cigarettes. International journal of environmental research and public health. 2015;12(5):4796–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kanwal R, Kullman G, Piacitelli C, Boylstein R, Sahakian N, Martin S, et al. Evaluation of flavorings-related lung disease risk at six microwave popcorn plants. Journal of occupational and environmental medicine. 2006;48(2):149–57. [DOI] [PubMed] [Google Scholar]
- 80.Chun LF, Moazed F, Calfee CS, Matthay MA, Gotts JE. Pulmonary toxicity of e-cigarettes. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2017;313(2):L193–L206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rowell TR, Tarran R. Will chronic e-cigarette use cause lung disease? American Journal of Physiology-Lung Cellular and Molecular Physiology. 2015;309(12):L1398–L409. [DOI] [PMC free article] [PubMed] [Google Scholar]