Abstract
Objective:
To describe discordance in antenatal corticosteroid use and resuscitation following extremely preterm birth and its relationship with infant survival and neurodevelopment.
Study design:
A multicenter cohort study of 4858 infants 22–26 weeks’ gestation born 2006–2011 at 24 U.S. hospitals participating in the NICHD Neonatal Research Network, with follow-up through 2013. Survival and neurodevelopmental outcomes were available at 18–22 months’ corrected age for 4576 (94.2%) infants. We described antenatal interventions, resuscitation, and infant outcomes. We modeled the effect on infant outcomes of each hospital increasing antenatal corticosteroid exposure for resuscitated infants born at 22–24 weeks’ gestation to rates observed at 25–26 weeks’ gestation.
Results:
Discordant antenatal corticosteroid use and resuscitation, where one and not the other occurred, were more frequent for births at 22 and 23 but not 24 weeks (rate ratio [95%CI] at 22 weeks: 1.7 [1.3–2.2]; 23 weeks: 2.6 [2.2–3.2]; 24 weeks: 1.0 [0.8–1.2]) when compared to 25–26 weeks. Among infants resuscitated at 23 weeks, adjusting each hospital’s rate of antenatal corticosteroid use to the average at 25–26 weeks (89.2%) was projected to increase infant survival by 7.1% (95% CI 5.4–8.8%) and survival without severe impairment by 6.4% (95%CI 4.7–8.1%). No significant change in outcomes was projected for infants resuscitated at 22 weeks, where few (n=22) resuscitated infants received antenatal corticosteroids.
Conclusions:
Infants born at 23 weeks were more frequently resuscitated without antenatal corticosteroids than other extremely preterm infants. When resuscitation is intended, consistent provision of antenatal corticosteroids may increase infant survival and survival without impairment.
Trial registration ClinicalTrials.gov NCT00063063 (Generic Database) and NCT00009633 (Follow-Up Study)
Management of extremely preterm birth requires collaboration between obstetricians and neonatologists to take into account the balance of risks and benefits to the mother and child.1,2 Current guidelines recommend that obstetricians administer antenatal corticosteroids within 7 days prior to birth for pregnancies threatening to deliver at 24 to 34 weeks’ gestation.3–5 However, many neonates born at 22 and 23 weeks of gestation now receive intensive care following birth,6 and there is a paucity of clinical trial evidence to support antenatal corticosteroid use for births at these earlier gestations.7
Although previous observational studies have shown that exposure to antenatal corticosteroids is associated with improved infant survival and neurodevelopmental outcomes,8–10 the extent of potential benefit from a uniform approach to providing antenatal corticosteroids at extremely preterm gestations remains unknown. We undertook this study to better understand how obstetric provision of antenatal corticosteroids corresponds with resuscitation and other aspects of antenatal management of extremely preterm live births in the U.S. Based on outcomes observed in our cohort, we evaluated the potential impact of increasing hospital rates of antenatal corticosteroid provision among resuscitated infants to rates observed for births at later gestational ages.
METHODS
We studied live births at 22 0/7 through 26 6/7 weeks’ gestation in 24 U.S. hospitals participating in the NICHD Neonatal Research Network from April 1, 2006, to March 31, 2011. Live births included infants who died in the delivery room (n=740). Infants with recognized syndromes or major congenital malformations were excluded from the analyses (n=213), as factors besides prematurity may have influenced management decisions. All hospitals contributed data for the entire study period.
Data collection
Trained research personnel at each hospital obtained maternal and neonatal data and submitted them to a central data-coordinating center (RTI International). Demographic and clinical data, including for antenatal and postnatal therapies, were extracted from medical records. Gestational age at birth was determined by identifying the date of the mother’s last menstrual period and by fetal ultrasound, or if those methods were unavailable, by clinical estimation after birth.11 Birth weight for gestational age was compared with sex-specific growth curves.12
The institutional review board at each participating site approved the in-hospital and follow-up protocols. Written informed consent from a parent or guardian was obtained for the follow-up protocol at 20 hospitals and for the in-hospital protocol at 2 hospitals. For all other hospitals, the institutional review board approved a waiver of consent.
Obstetric and neonatal interventions
Obstetric intervention with antenatal corticosteroids was categorized as having occurred if the mother received at least one dose of betamethasone or dexamethasone prior to delivery, including any dose received at an outside hospital. Information on the timing of antenatal corticosteroid administration, drug dosage, and the number of doses or courses of treatment was not available in our dataset.
Additional information on obstetric intervention included data on rates of cesarean section and provision of antepartum antibiotics during the birth hospitalization. Data on the use of magnesium sulfate for fetal neuroprotection were not collected during the study period.
Infants were categorized as receiving resuscitation if they were exposed to any of the following potentially lifesaving interventions after birth: tracheal intubation, positive-pressure ventilatory support, surfactant therapy, chest compressions, epinephrine, or parenteral fluids.6 All infants not receiving one of these measures died shortly after birth.
Outcomes
Data on survival and neurodevelopmental outcomes were collected at 18 to 22 months’ corrected age by certified examiners unaware of exposure to antenatal corticosteroids or other interventions. Neurodevelopmental assessment consisted of a structured neurologic examination and developmental and behavioral tests, which have been described elsewhere.13
Severe impairment was defined as a cognitive or motor score on the Bayley Scales of Infant and Toddler Development, third edition (Bayley-III), of < 70 (ie, >2 SD below the scale mean; mean±SD, 100±15), severe cerebral palsy, a Gross Motor Function Classification System (GMFCS) level of 4 or 5 (with 0 as normal and 5 most impaired), bilateral blindness (visual acuity <20/200), or severe bilateral hearing impairment uncorrected by amplification. Moderate impairment was defined as a Bayley-III cognitive or motor score of 70 to 84, (i.e., 1 to 2 SD below the scale mean), moderate cerebral palsy, or a GMFCS level of 2 or 3. GMFCS scores were taken into account regardless of cerebral palsy diagnosis. Bayley-III motor scores were ascertained for assessments on or after January 1, 2010; data for all other criteria were ascertained throughout the study period.
Statistical analyses
We calculated rates and 95% confidence intervals of obstetric intervention and resuscitation among live births by hospital and gestational age. Demographic, clinical characteristics, and outcomes of resuscitated infants were compared by gestational age at birth and exposure to antenatal corticosteroids.
To estimate the potential impact of increasing hospital rates of antenatal corticosteroid use for resuscitated infants, we developed gestational age-specific multivariable multilevel logistic regression models with birth hospital as a random effect and infant outcome as the dependent variable. Hospital rates of antenatal corticosteroid use among resuscitated infants were included as a continuous variable in the hospital level of the models. At the individual level, models were adjusted for birth weight (grams), sex, plurality of birth (singleton versus multiple) and the mother’s age (≤19 years versus >19 years), race (white, black, or other), ethnicity (Hispanic versus non-Hispanic), enrollment in private health insurance (yes versus no), receipt of prenatal care (≥1 visit versus no visits), hypertension during pregnancy (yes versus no), insulin-dependent diabetes (yes versus no), and clinical chorioamnionitis (yes versus no). These covariates were selected on the basis of previous research in this cohort.6 Based on the actual outcomes observed in our cohort, we projected the estimated change in infant outcomes that would result from increasing each hospital’s rate of antenatal corticosteroid exposure among resuscitated infants born at 22–24 weeks to the average rate for resuscitated infants born at 25–26 weeks’ gestation, with all other factors remaining the same.
All statistical analyses were conducted at RTI International. Multilevel modeling was performed using Stata/MP software, version 14.0 (StataCorp). Other analyses were performed using SAS software, version 9.4 (SAS Institute). Two-sided P values < .05 were considered to indicate statistical significance.
RESULTS
Of 4858 live births, 3803 (78.3%) were exposed to antenatal corticosteroids and 4327 (89.1%) infants were resuscitated. In 619 (12.7%) births, resuscitation was initiated but no antenatal corticosteroids were given. In 95 (2.0%) births, antenatal corticosteroids were administered but no resuscitation was attempted.
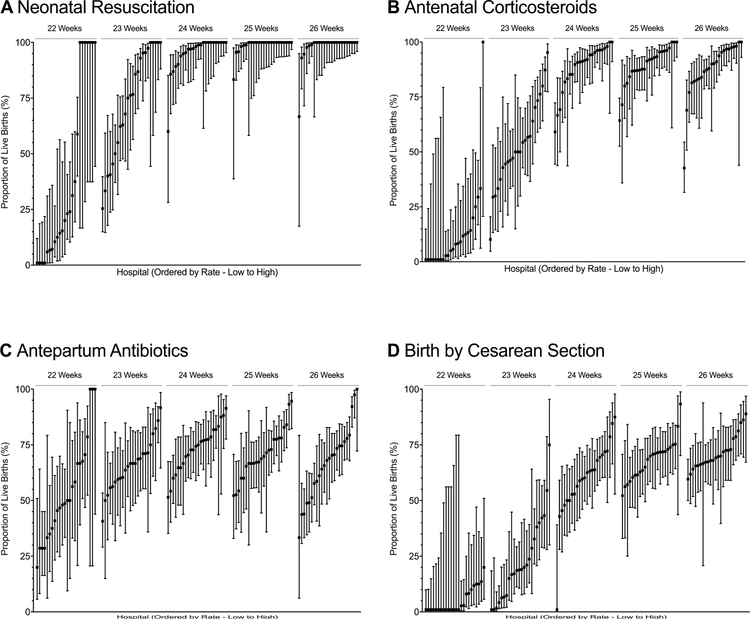
Rates of obstetric and neonatal intervention differed by hospital of birth. Hospital rates of antenatal corticosteroid exposure ranged from 0.0–100% for births at 22 weeks, and from 10.3–95.2%, 59.1–100%, 64.3–100%, and 42.6–100% for births at 23, 24, 25, and 26 weeks, respectively. Hospital rates of resuscitation ranged from 0.0–100% at 22, 25.9–100% at 23, 66.7–100% at 24, 83.3–100%, and 66.7–100% at 26 weeks.
Clinical and demographic characteristics of resuscitated infants categorized by gestational age and ANS exposure are summarized in Table I.
Table I.
Resuscitated infants by gestational age at birth and exposure to antenatal corticosteroids
| 22 weeks | 23 weeks | 24 weeks | 25 weeks | 26 weeks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exposed to ANS n=22 |
No ANS n=57 |
Exposed to ANS n=366 |
No ANS n=176 |
Exposed to ANS n=1012 |
No ANS n=107 |
Exposed to ANS n=1132 |
No ANS n=125 |
Exposed to ANS n=1176 |
No ANS n=154 |
|
| Maternal age ≤19 y | 2 (9.1) | 12 (21.1) | 45 (12.3) | 27 (15.3) | 125 (12.4) | 17 (15.9) | 117 (10.3) | 26 (20.8) | 131 (11.1) | 21 (13.6) |
| Privately insured | 8 (36.4) | 11 (19.3) | 161 (44.0) | 66 (37.5) | 425 (42.0) | 21 (19.6) | 477 (42.1) | 30 (24.0) | 491 (41.8) | 40 (26.0) |
| Prenatal care | 20 (90.9) | 51 (89.5) | 351 (95.9) | 160 (90.9) | 969 (95.8) | 91 (85.0) | 1082 (95.6) | 108 (86.4) | 1136 (96.6) | 129 (83.8) |
| Maternal race | ||||||||||
| White non-Hispanic | 7 (31.8) | 11 (19.3) | 133 (36.3) | 52 (29.5) | 405 (40.0) | 25 (23.4) | 479 (42.3) | 28 (22.4) | 455 (38.7) | 38 (24.7) |
| Black non-Hispanic | 14 (63.6) | 35 (61.4) | 170 (46.4) | 79 (44.9) | 380 (37.5) | 53 (49.5) | 408 (36.0) | 68 (54.4) | 421 (35.8) | 62 (40.3) |
| Hispanic | 1 (4.5) | 7 (12.3) | 46 (12.6) | 34 (19.3) | 164 (16.2) | 28 (26.2) | 192 (17.0) | 23 (18.4) | 221 (18.8) | 45 (29.2) |
| Maternal hypertension |
2 (9.1) | 8 (14.0) | 47 (12.8) | 13 (7.4) | 175 (17.3) | 17 (15.9) | 260 (23.0) | 28 (22.4) | 286 (24.3) | 38 (24.7) |
| Maternal diabetes | 1 (4.5) | 0 (0.0) | 8 (2.2) | 5(2.8) | 52 (5.1) | 6 (5.6) | 50 (4.4) | 7 (5.6) | 51 (4.3) | 11 (7.1) |
| Chorioamnionitis | 5 (22.7) | 21 (36.8) | 90 (24.6) | 39 (22.2) | 240 (23.7) | 12 (11.2) | 233 (20.7) | 9 (7.2) | 226 (19.2) | 13 (8.4) |
| Rupture of membranes >18 h |
5 (22.7) | 14 (26.4) | 96 (26.6) | 46 (28.2) | 303 (30.4) | 9 (8.8) | 321 (28.7) | 10 (8.3) | 362 (31.0) | 7 (4.7) |
| Antepartum antibiotics |
18 (81.8) | 29 (50.9) | 298 (81.4) | 74 (42.1) | 745 (73.6) | 26 (24.3) | 819 (72.4) | 35 (28.0) | 772 (65.6) | 45 (29.2) |
| Cesarean birth | 5 (22.7) | 4 (7.0) | 104 (28.4) | 39 (22.2) | 636 (62.9) | 70 (65.4) | 788 (69.6) | 76 (60.8) | 816 (69.4) | 119 (77.3) |
| Male infant | 9 (40.9) | 27 (47.4) | 193 (52.7) | 94 (53.4) | 518 (51.2) | 59 (55.1) | 600 (53.0) | 68 (54.4) | 592 (50.3) | 80 (51.9) |
| Singleton | 14 (63.6) | 40 (70.2) | 258 (70.5) | 134 (76.1) | 740 (73.1) | 97 (90.7) | 859 (75.9) | 102 (81.6) | 890 (75.7) | 118 (76.6) |
| 1-minute Apgar ≤3 | 16 (72.7) | 50 (87.7) | 250 (68.3) | 134 (76.1) | 566 (55.9) | 70 (65.4) | 528 (46.6) | 77 (61.6) | 473 (40.2) | 82 (53.2) |
| 5-minute Apgar ≤3 | 12 (54.5) | 35 (61.4) | 113 (30.9) | 82 (46.6) | 185 (18.3) | 34 (31.8) | 165 (14.7) | 19 (15.2) | 101 (8.6) | 24 (15.6) |
| Birth weight (g, mean (SD)) |
519.5 (94.5) | 517.5 (74.5) | 584.2 (84.4) | 591.3 (85.9) | 649.6 (106.4) | 668.8 (111.5) | 742.1 (131.5) | 791.1 (131.6) | 851.6 (162.3) | 851.9 (145.4) |
| Small for gestational age |
1 (4.5) | 2 (3.5) | 12 (3.3) | 3 (1.7) | 76 (7.5) | 6 (5.6) | 92 (8.1) | 8 (6.4) | 87 (7.4) | 12 (7.8) |
ANS=antenatal corticosteroids
Comparison of antenatal corticosteroids with other obstetric interventions
Among births at 22, 23, and 24 weeks’ gestation, 9.8%, 55.8%, and 89.8% were exposed to antenatal corticosteroids, respectively, and rates of exposure at 25 and 26 weeks’ gestation were 89.9% and 88.4%, respectively. Rates of birth by cesarean section followed a similar pattern, with rates of 4.8%, 19.8%, 61.9%, 68.5%, and 70.2% for infants at 22, 23, 24, 25 and 26 weeks, respectively. Rates of antibiotic exposure during the birth hospitalization and before the time of birth varied less by gestational age, with 47.9%, 65.3%, 72.8%, 72.7% and 67.5% of births exposed at 22, 23, 24, 25, and 26 weeks, respectively. Rates of these interventions varied by hospital and gestational age at birth (Figure 2, Figure 3, and Table IV; available at www.jpeds.com).
Figure 2. Rates of obstetric interventions and resuscitation by gestational age and hospital of birth.
Point values represent the proportion of live births at the specified gestational age that received the specified intervention at each of the 24 hospitals in the study. Bars represent 95% CIs. The x-axis represents hospital rank order by mean active treatment rate (ordered from lowest to highest). A, Hospital rates of neonatal resuscitation; B, Hospital rates of antenatal corticosteroids exposure (at least 1 dose of antenatal corticosteroids, regardless of timing, prior to birth); C, Hospital rates of antepartum antibiotics (maternal receipt of antibiotics during the current hospitalization and prior to birth); D, Hospital rates of birth by cesarean delivery. Panel A has been modified from Rysavy et al.6
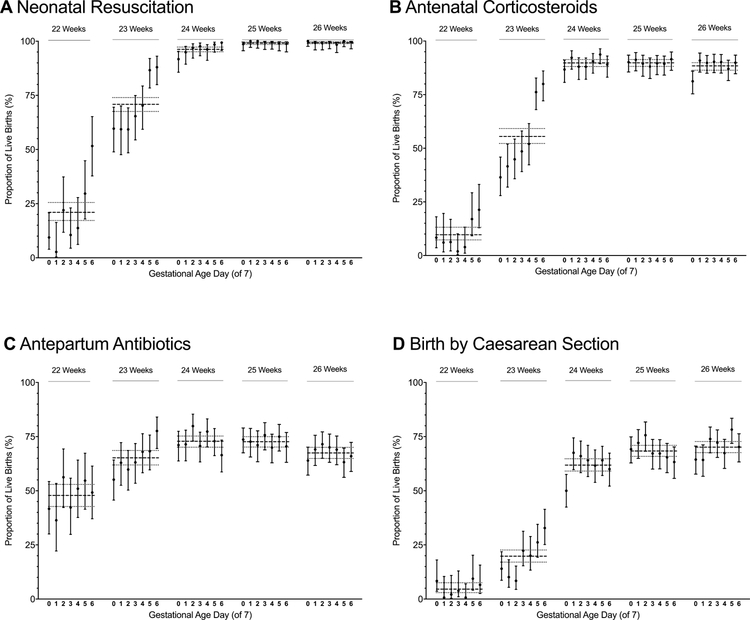
Figure 3. Rates of obstetric interventions and resuscitation by gestational age in weeks and days.
Point values represent the proportion of live births at the specified gestational age in days that received the specified intervention. Vertical bars represent 95% CIs for these estimates. Horizontal dashed lines represent the proportion of births born at a given gestational age week with the specified intervention. The dotted lines on either side of the dashed lines represent the 95% CIs for these estimates. A, Rates of neonatal resuscitation; B, Rates of antenatal corticosteroids exposure (at least 1 dose of antenatal corticosteroids, regardless of timing, prior to birth); C, Rates of antepartum antibiotics (maternal receipt of antibiotics during the current hospitalization and prior to birth); D, Rates of birth by cesarean delivery. Panel A has been modified from Rysavy et al.6
Table IV.
Median and interquartile ranges for hospital rates of obstetric interventions
| Gestational age at birth |
Antenatal Corticosteroids % (IQR) |
Antepartum antibiotics % (IQR) |
Delivery by C-section % (IQR) |
|---|---|---|---|
| 22 weeks | 6.9 (0.0–13.8) | 49.3 (36.3–67.2) | 0.0 (0.0–9.2) |
| 23 weeks | 50.0 (43.7–67.1) | 66.7 (58.9–71.2) | 19.0 (7.0–35.4) |
| 24 weeks | 91.4 (84.3–96.3) | 74.7 (65.7–80.2) | 61.4 (51.4–69.9) |
| 25 weeks | 91.7 (86.8–95.8) | 69.3 (66.0–77.8) | 70.3 (61.5–72.5) |
| 26 weeks | 90.1 (83.1–96.8) | 68.5 (54.5–75.5) | 69.5 (66.4–75.4) |
IQR=interquartile range
Among resuscitated infants born at 22 and 23 weeks’ gestation, 38.0% and 15.3%, respectively, were not exposed to antenatal corticosteroids but were exposed to antepartum antibiotics during the birth hospitalization. At 24, 25, and 26 weeks, these rates were between 2.4 and 3.4%. There were no substantial differences by gestational age in the proportion of resuscitated infants born by cesarean section and not exposed to antenatal corticosteroids, with rates between 5.1 and 8.9% (Table V; available at www.jpeds.com).
Table V.
Resuscitated infants by receipt of antenatal corticosteroids and other obstetric interventions
| Gestational age at birth |
Resuscitated infants n |
No ANS n (%) |
No ANS but Antepartum antibiotics n (%>) |
No ANS but born by C-section n (%) |
|---|---|---|---|---|
| 22 weeks | 79 | 57 (72.2) | 30 (38.0) | 4 (5.1) |
| 23 weeks | 542 | 176 (32.5) | 83 (15.3) | 39 (7.2) |
| 24 weeks | 1119 | 107 (9.6) | 27 (2.4) | 70 (6.3) |
| 25 weeks | 1257 | 124 (9.9) | 37 (2.9) | 76 (6.0) |
| 26 weeks | 1330 | 154 (11.6) | 45 (3.4) | 119 (8.9) |
ANS=antenatal corticosteroids
Discordance in antenatal corticosteroid provision and resuscitation
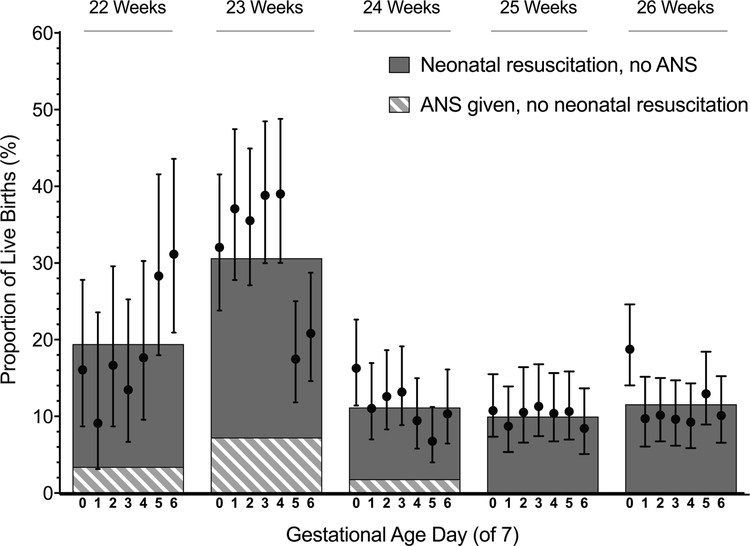
Rates of discordance between antenatal corticosteroid provision and resuscitation by gestational age week and day are shown in Figure 1. The highest rates of discordance occurred at 23 weeks; nearly 1/3 of live births at this gestation were discordant. Among live births at 23 weeks, 7.3% of births were exposed to antenatal corticosteroids but not resuscitated and 23.3% were resuscitated but were not exposed to antenatal corticosteroids.
Figure 1. Discordance in antenatal corticosteroid use and resuscitation by gestational age at birth.
Bars represent average rates of discordance between antenatal corticosteroid use and resuscitation for births at each gestational age week, as described in the legend. Points and vertical lines represent rates and 95% confidence intervals for the combined discordance for births at each gestational age day. ANS=antenatal corticosteroids.
Using the rate of discordance for births at 25 and 26 weeks (10.8%) as a reference, rates of discordance were greater for births at 22 and 23 weeks’ gestation but not at 24 weeks (rate ratio and 95% confidence interval at 22 weeks: 1.7 [1.3–2.2]; 23 weeks: 2.6 [2.2–3.2]; 24 weeks: 1.0 [0.8–1.2]). Rates of discordance were highest for births during the end of the 22nd gestational week and beginning of the 23rd week (Figure 1).
Discordance and infant outcomes
Outcomes at 18–22 months’ corrected age were known for 4575 (94.2%) infants. Outcomes at 18–22 months’ corrected age for resuscitated infants are summarized in Table II, categorized by gestational age and exposure to antenatal corticosteroids. The 531 infants who were not resuscitated died shortly after birth, regardless of gestational age or exposure to antenatal corticosteroids.
Table II.
Outcomes among resuscitated infants at 18–22 months by gestational age at birth and exposure to antenatal corticosteroids
| Gestational age at birth |
Outcome | ANS-exposed n (% [95% Cl]) |
No ANS n (% [95% CI]) |
|---|---|---|---|
| 22 weeks | N=21 | N=57 | |
| Survival | 8 (38.1 [19.1–61.7]) | 10 (17.5 [9.5–30.1]) | |
| Survival without severe impairment | 6(28.6 [12.5–52.9]) | 6 (10.5 [4.7–22.0]) | |
| Survival without moderate or severe impairment | 4 (19.0 [6.7–43.6]) | 3 (5.3 [1.6–15.6]) | |
| 23 weeks | N=347 | N=172 | |
| Survival | 131 (37.8 [32.8–43.0]) | 42 (24.4 [18.5–31.5]) | |
| Survival without severe impairment | 102 (29.4 [24.8–34.4]) | 29 (16.9 [11.9–23.3]) | |
| Survival without moderate or severe impairment | 67 (19.3 [15.5–23.8]) | 16 (9.3 [5.7–14.7]) | |
| 24 w eeks | N=955 | N=102 | |
| Survival | 552 (57.8 [54.6–60.9]) | 46 (45.1 [35.6–55.0]) | |
| Survival without severe impairment | 454 (47.5 [44.4–50.7]) | 33 (32.4 [23.9–42.2]) | |
| Survival without moderate or severe impairment | 303 (31.7 [28.8–34.8]) | 24 (23.5 [16.2–32.9]) | |
| 25 weeks | N=1058 | N=117 | |
| Survival | 769 (72.7 [69.9–75.3]) | 81 (69.2 [60.2–77.0]) | |
| Survival without severe impairment | 648 (61.2 [58.3–64.1]) | 73 (62.4 [53.2–70.8]) | |
| Survival without moderate or severe impairment | 475 (44.9 [41.9–47.9]) | 48 (41.0 [32.4–50.3]) | |
| 26 weeks | N=1072 | N=143 | |
| Survival | 879 (82.0 [79.6–84.2]) | 112 (78.3 [70.7–84.4]) | |
| Survival without severe impairment | 818 (76.3 [73.7–78.8]) | 102 (71.3 [63.3–78.2]) | |
| Survival without moderate or severe impairment | 648 (60.4 [57.5–63.3]) | 64 (44.8 [36.7–53.1]) |
Using multivariable models that adjusted for differences in clinical and demographic patient characteristics among hospitals (Table VI; available at www.jpeds.com), we projected outcomes for resuscitated infants born at 22, 23, and 24 weeks’ gestation based on the assumption that 89.2% of infants at each hospital could have been exposed to antenatal corticosteroids. The 89.2% threshold represents the average rate of antenatal corticosteroids for resuscitated infants born at 25 and 26 weeks’ gestation in our cohort; in other recent cohorts, similar rates (85–93%) were observed for preterm infants.14–16 Table III shows how the projected outcomes compare with actual observed outcomes at each gestation.
Table VI.
Model coefficients for hospital antenatal corticosteroid rate among resuscitated infants
| Gestational age at birth |
Outcome | Model coefficient β (95% CI) |
P value |
|---|---|---|---|
| 22 weeks | Survival | 0.0022 (−0.0241, 0.0286) | 0.81 |
| Survival without severe impairment | a | ||
| Survival without moderate or severe impairment | a | ||
| 23 weeks | Survival | 0.0169 (0.0012, 0.0326) | 0.04 |
| Survival without severe impairment | 0.0173 (0.0021, 0.0325) | 0.03 | |
| Survival without moderate or severe impairment | 0.0232 (0.0039, 0.0425) | 0.02 | |
| 22–23 weeks | Survival | 0.0159 (−0.0019, 0.0338) | 0.08 |
| Survival without severe impairment | 0.0165 (−0.0005, 0.0334) | 0.06 | |
| Survival without moderate or severe impairment | 0.0216 (−0.0006, 0.0439) | 0.06 | |
| 24 weeks | Survival | 0.0235 (0.0012, 0.0459) | 0.04 |
| Survival without severe impairment | 0.0213 (0.0000, 0.0426) | 0.05 | |
| Survival without moderate or severe impairment | 0.0258 (0.0045, 0.0472) | 0.02 |
Hospital rates of antenatal corticosteroid use among resuscitated infants were included as a continuous variable in the hospital level of the models. At the individual level, models were adjusted for birth weight (grams), sex, plurality of birth (singleton versus multiple) and the mother’s age (≤19 years versus >19 years), race (white, black, or other), ethnicity (Hispanic versus non-Hispanic), enrollment in private health insurance (yes versus no), receipt of prenatal care (≥1 visit versus no visits), hypertension during pregnancy (yes versus no), insulin-dependent diabetes (yes versus no), and clinical chorioamnionitis (yes versus no). CI=confidence interval
Model estimation did not converge
Table III.
Actual and model-projected outcomes among resuscitated infants
| Gestational age at birth |
Resuscitated infants n |
Mean Hospital ANS rate % |
Outcome | Actual outcome rate % |
Projected outcome rate % (95% Cl) |
|---|---|---|---|---|---|
| 22 weeksa | 78b | 28.5 | Survival | 23.1 | 25.2 (21.0– 29.5) |
| Survival without severe impairment | 15.4 | g | |||
| Survival without moderate or severe impairment |
9.0 | g | |||
| 23 weeks | 519c | 61.1 | Survival | 33.3 | 40.4 (38.7–42.1) |
| Survival without severe impairment | 25.2 | 31.6 (29.9–33.3) | |||
| Survival without moderate or severe impairment |
16.0 | 22.0 (20.3–23.8) | |||
| 22–23 weeks | 597 | 56.1 | Survival | 32.0 | 39.9 (38.6–41.2) |
| Survival without severe impairment | 24.0 | 31.4 (30.1–32.8) | |||
| Survival without moderate or severe impairment |
15.1 | 21.9 (20.4–23.5) | |||
| 24 weeks | 1057d | 88.6 | Survival | 56.6 | 56.5 (55.6–57.4) |
| Survival without severe impairment | 46.1 | 46.1 (45.3–46.9) | |||
| Survival without moderate or severe impairment |
30.9 | 29.3 (28.7–29.9) | |||
| 25 weeks | 1175e | 89.5 | Survival | 72.3 | h |
| Survival without severe impairment | 61.4 | h | |||
| Survival without moderate or severe impairment |
44.5 | h | |||
| 26 weeks | 1215f | 87.9 | Survival | 81.6 | h |
| Survival without severe impairment | 75.7 | h | |||
| Survival without moderate or severe impairment |
58.6 | h |
The actual outcome rate is calculated as the number of resuscitated infants at a given gestational age with the specified outcome as a proportion of all resuscitated infants born at that gestational age. The projected outcome rate and 95% confidence interval were estimated using models assuming that all hospitals achieved the average antenatal corticosteroid coverage rate for resuscitated infants observed at 25 and 26 weeks (89.2%).
Of the 24 hospitals, 20 contributed to the models. At 4 hospitals, no infants were resuscitated.
Excludes 1 infant lost to follow-up
Excludes 23 infants lost to follow-up
Excludes 62 infants lost to follow-up
Excludes 82 infants lost to follow-up
Excludes 115 infants lost to follow-up
Model estimation did not converge
Average rate among infants born at 25–26 weeks (89.2%) used as reference
For births at 23 weeks, if all hospitals achieved the rate of antenatal corticosteroid exposure observed among resuscitated infants at 25–26 weeks, a 7.1% (95% CI 5.4–8.8%) absolute increase in survival, from 33.3% to 40.4%, was projected. The projected effect on survival without severe impairment at 23 weeks was a 6.4% (95% CI 4.7–8.1%) absolute increase, from 25.2% to 31.6%. The precision of the effect of reducing discordance at 22 weeks was limited by the small sample at this gestational age (n=22 resuscitated infants exposed to antenatal corticosteroids). For births at 22 weeks, the projected effect of increased antenatal corticosteroid use was a 2.1% (95% CI −1.9–8.4%) increase in infant survival, which was not statistically significant; the estimated effect on survival without impairment could not be determined due to small numbers that precluded model convergence. At 24 weeks’ gestation, rates of discordance were similar to those at 25 and 26 weeks and so outcomes were not projected to change.
DISCUSSION
In a study of live births at U.S. medical centers participating in the NICHD Neonatal Research Network, we found discordance in the provision of antenatal corticosteroids and resuscitation at the earliest gestational ages that was associated with survival and survival without impairment. The discordance was most pronounced for infants born at 23 weeks’ gestation.
The provision of antenatal corticosteroids with no subsequent resuscitation, as occurred in 2.0% of live births, may represent appropriate, on-going evaluation of the goals of care. However, where postnatal care is directed at prolonging life, non-provision of antenatal corticosteroids, as occurred in 12.7% of cases, may represent a missed opportunity. It is possible that treatment with antenatal corticosteroids was intended in some cases but not provided due to a lack of time to administer the medication. We did not have information available on the length of time between when a pregnant woman arrived at the hospital and birth. However, we observed that many births at 22 and 23 weeks’ gestation that were not exposed to antenatal corticosteroids were exposed to antibiotics during the birth hospitalization and before the time of birth, and that the rate of such antibiotic use was similar across gestational ages. Other studies have suggested that antenatal corticosteroids may have physiologic benefits to the infant, such as through decreased intraventricular hemorrhage, after only several hours.17, 18 Our findings raise the question of whether rates of antenatal corticosteroid provision among resuscitated infants at these early gestations could feasibly be increased. Our study shows that, particularly for births at 23 weeks, such an increase may improve infant survival and survival without impairment.
U.S. data from Ehret et al, covering 2012–2016 (after the period of our study), suggest that an increase in antenatal corticosteroid use in patients similar to those in our cohort may be possible.10 Among infants resuscitated after birth in that study, the proportions exposed to antenatal corticosteroids by gestational age were: 52.4% at 22 weeks, 82.7% at 23 weeks, 89.3% at 24 weeks, and 90.8% at 25 weeks. This compares with 31.2% at 22 weeks, 61.1% at 23 weeks, 88.6% at 24 weeks, and 89.5% at 25 weeks in our study. Notably, rates of resuscitation for the earliest gestational ages were also higher in this more recent cohort (30.8% at 22 weeks; 87.1% at 23 weeks) compared with our cohort from 2006–2011 (22.1% at 22 weeks; 71.8% at 23 weeks).
Our study, which included nearly 5000 live births at major U.S. academic hospitals, provides important information on obstetric and neonatal management of extremely preterm birth in the U.S. However, it has several important limitations, including a lack of information on the dose, timing, and clinical decision-making surrounding antenatal corticosteroid administration. Based on the data available to us, we studied only live births; however, stillbirth is a potential outcome when decisions about administering antenatal corticosteroids and other obstetric interventions are made. Moreover, we did not have data on the pregnancies exposed to antenatal corticosteroids between 22 and 26 weeks’ gestation where birth took place after 26 weeks’ gestation; this group requires further study.19 Our study cohort included 24 hospitals providing tertiary care; however, because tertiary care is recommended for extremely preterm delivery, our cohort is relevant.1 Although our study demonstrated discordance between antenatal corticosteroid provision and resuscitation among live births, it cannot explain the causes of such discordance, such as time constraints, inadequate communication, changing goals of care, or clinician concerns about the evidence for antenatal corticosteroid efficacy and safety at extremely early gestations.
Other studies have shown differences in the perspectives of obstetricians and neonatologists regarding management of extremely preterm birth. In a survey of physicians in Nottingham, U.K., neonatologists were more likely than obstetricians to recommend antenatal corticosteroids for periviable births.20 More recently, qualitative research studying physician decision-making during simulation exercises at a U.S. medical center found that obstetricians and neonatologists often deferred questions about steroid administration to the other specialty. The authors posited that “institutional differences in antenatal corticosteroid administration may reflect variation in the quality of communication that occurs between obstetricians and neonatologists in and across their respective institutions.”21
Variation may also exist due to the paucity of clinical trial evidence to support or refute the use of antenatal corticosteroids for extremely preterm birth. A meta-analysis published in 2016 showed that, among infants resuscitated at 22 and 23 weeks, provision of antenatal corticosteroids was associated with higher rates of survival to hospital discharge.22 However, data were limited to observational studies. The most recent Cochrane review of this subject included only 49 infants born at <26 weeks’ gestation.7 The only trial of antenatal corticosteroids for extremely premature infants born at 22 and 23 weeks’ gestation that we found listed on ClinicalTrials.gov was withdrawn by the sponsor before the start of enrollment.23
Despite the paucity of trial evidence for births at <26 weeks’ gestation, guidelines published in 1994 by the National Institutes of Health (NIH) recommended antenatal corticosteroids for mothers in labor from 24 to 34 weeks’ gestation.24 At the time, few infants born at less than 24 weeks were expected to survive.25, 26 However, neonatal practices and outcomes have changed in the subsequent two decades. In 2014 (after the period of this study), an NIH workshop on periviable birth held jointly with the American Academy of Pediatrics (AAP), American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM), recommended administering antenatal corticosteroids for births at 23 weeks’ gestation.1 More recently, a 2016 consensus statement issued by ACOG and SMFM stated that clinicians should “consider” antenatal corticosteroids for births at 23 weeks but did not provide the same strength of recommendation for antenatal corticosteroid use at 23 weeks as at 24 weeks.5 These recent incongruent guidelines highlight the on-going uncertainty about antenatal corticosteroid use at the earliest gestational ages. It is unclear what impact the guidelines will have on clinical practice.
In this study of U.S. hospitals participating in the NICHD Neonatal Research Network, we observed that infants born at 23 weeks’ gestation were more likely than other extremely preterm infants to be resuscitated without prior provision of antenatal corticosteroids. We estimated that, where infant resuscitation is intended, a more consistent approach to provision of antenatal corticosteroids may improve infant survival and survival without impairment.
Acknowledgments
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
The National Institutes of Health (NIH) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) provided grant support for the Neonatal Research Network (NRN) to obtain the data used in this study. The NICHD staff had input into the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors declare no conflicts of interest.
Abbreviations:
- NICHD
Eunice Kennedy Shriver National Institute of Child Health and Human Development
- Bayley-III
Bayley Scales of Infant and Toddler Development, third edition
- GMFCS
Gross Motor Function Classification System
- SD
standard deviation
- CI
confidence interval
List of additional investigators and participating hospitals of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network
NRN Steering Committee Chairs: Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006–2011); Richard A. Polin, MD, Division of Neonatology, Columbia University College of Physicians and Surgeons, (2011-present).
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904) – Abbot R. Laptook, MD; Angelita M. Hensman, RN BSN; William Oh, MD; Martin Keszler, MD; Robert Burke, MD; Melinda Caskey, MD; Katharine Johnson, MD; Barbara Alksninis, PNP; Theresa M. Leach, MEd CAES; Bonnie E. Stephens, MD; Victoria E. Watson, MS CAS; Suzy Ventura; Kristin M. Basso, BSN MA; Elisa Vieira, RN BSN; Andrea Halbrook.
Case Western Reserve University, Rainbow Babies & Children’s Hospital (U10 HD21364, M01 RR80) – Avroy A. Fanaroff, MD; Anna Marie Hibbs, MD; Deanne E. Wilson-Costello, MD; Nancy S. Newman, BA RN; Bonnie S. Siner, RN; Monika Bhola, MD; Gulgun Yalcinkaya, MD; Harriet G. Friedman, MA.
Children’s Mercy Hospital (U10 HD68284) – William E. Truog, MD; Eugenia K. Pallotto, MD MSCE; Howard W. Kilbride MD; Cheri Gauldin, RN MSN CCRC; Anne Holmes RN MSN MBA-HCM CCRC; Kathy Johnson RN, CCRC.
Cincinnati Children’s Hospital Medical Center, University Hospital, and Good Samaritan Hospital (U10 HD27853, M01 RR8084) – Kurt Schibler, MD; Edward F. Donovan, MD; Kate Bridges, MD; Barbara Alexander, RN; Cathy Grisby, BSN CCRC; Jody Hessling, RN; Estelle E. Fischer, MHSA MBA; Lenora D. Jackson, CRC; Kristin Kirker, CRC; Holly L. Mincey, RN BSN; Greg Muthig, BS; Teresa L. Gratton, PA; Jean J. Steichen, MD; Kimberly Yolton, PhD.
Duke University School of Medicine, University Hospital, University of North Carolina, and Duke Regional Hospital (U10 HD40492, M01 RR30, UL1 TR83) – Ronald N. Goldberg, MD; Ricki F. Goldstein, MD; Kimberley A. Fisher, PhD FNP-BC IBCLC; Kathy J. Auten, MSHS; Katherine A. Foy, RN; Sandra Grimes, RN BSN; Joanne Finkle, RN JD; Kathryn E. Gustafson, PhD; Melody B. Lohmeyer, RN MSN; Matthew M. Laughon, MD MPH; Carl L. Bose, MD; Janice Bernhardt, MS RN; Gennie Bose, RN; Cindy Clark, RN.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (U10 HD27851, UL1 TR454) – David P. Carlton, MD; Ellen C. Hale, RN BS CCRC; Ira Adams-Chapman, MD; Maureen Mulligan LaRossa, RN; Sheena L. Carter, PhD.
Eunice Kennedy Shriver National Institute of Child Health and Human Development – Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Eskenazi Health (U10 HD27856, M01 RR750, UL1 TR6) – Brenda B. Poindexter, MD MS; Anna M. Dusick, MD; Ann B. Cook, MS; Dianne E. Herron, RN; Faithe Hamer, BS; Carolyn Lytle, MD MPH; Lucy C. Miller, RN BSN CCRC; Heike M. Minnich, PsyD HSPP; Leslie Dawn Wilson, BSN CCRC.
Nationwide Children’s Hospital and the Ohio State University Medical Center (U10 HD68278) – Leif D. Nelin, MD; Sudarshan R. Jadcherla, MD; Patricia Luzader, RN; Christine A. Fortney, PhD RN; Gail E. Besner; Nehal A. Parikh, MD.
RTI International (U10 HD36790) – W. Kenneth Poole, PhD; Dennis Wallace, PhD; Jamie E. Newman, PhD MPH; Jeanette O’Donnell Auman, BS; Margaret M. Crawford, BS CCRP; Carolyn M. Petrie Huitema, MS CCRP; Kristin M. Zaterka-Baxter, RN BSN CCRP.
Stanford University, Dominican Hospital, El Camino Hospital, and Lucile Packard Children’s Hospital (U10 HD27880, M01 RR70, UL1 TR93) – Krisa P. Van Meurs, MD; David K. Stevenson, MD; Marian M. Adams, MD; M. Bethany Ball, BS CCRC; Andrew W. Palmquist, RN BSN; Melinda S. Proud, RCP; Elizabeth Bruno, PhD; Maria Elena DeAnda, PhD; Anne M. DeBattista, RN PNP; Jean G. Kohn, MD MPH; Casey E. Krueger, PhD; Hali E.Weiss, MD.
Tufts Medical Center, Floating Hospital for Children (U10 HD53119, M01 RR54) – Ivan D. Frantz III, MD; John M. Fiascone, MD; Brenda L. MacKinnon, RNC; Ellen Nylen, RN BSN; Anne Furey, MPH; Elisabeth C. McGowan, MD; Cecelia E Sibley PT MHA; Ana K. Brussa, MS OTR/L.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32) – Namasivayam Ambalavanan, MD; Myriam Peralta-Carcelen, MD MPH; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN; Fred J. Biasini, PhD; Kristen C. Johnston, MSN CRNP; Kathleen G. Nelson, MD; Cryshelle S. Patterson, PhD; Vivien A. Phillips, RN BSN; Sally Whitley, MA OTR-L FAOTA; Amanda D. Soong, MD; Carin Kiser, MD; Leigh Ann Smith, CRNP; Sara Kryzwanski, MS; Richard V. Rector, PhD; Sarah Ryan, PhD; Kristy Domnanovich, PhD; Leslie Rodrigues, PhD.
University of California - Los Angeles, Mattel Children’s Hospital, Santa Monica Hospital, Los Robles Hospital and Medical Center, and Olive View Medical Center (U10 HD68270) – Uday Devaskar, MD; Meena Garg, MD; Isabell B. Purdy, PhD CPNP; Teresa Chanlaw, MPH; Rachel Geller, RN BSN.
University of California – San Diego Medical Center and Sharp Mary Birch Hospital for Women and Newborns (U10 HD40461) – Neil N. Finer, MD; Yvonne E. Vaucher, MD MPH; David Kaegi, MD; Maynard R. Rasmussen, MD; Kathy Arnell, RNC; Clarence Demetrio, RN; Martha G. Fuller, RN MSN; Chris Henderson, RCP CRTT; Wade Rich, BSHS RRT; Radmila West PhD.
University of Iowa and Mercy Medical Center (U10 HD53109, M01 RR59) – Dan L. Ellsbury, MD; John A. Widness, MD; Karen J. Johnson, RN BSN; Donia B. Campbell, RNC-NIC; Jacky R. Walker, RN; Diane L. Eastman, RN CPNP MA.
University of Miami, Holtz Children’s Hospital (U10 HD21397, M01 RR16587) – Shahnaz Duara, MD; Charles R. Bauer, MD; Ruth Everett-Thomas, RN MSN; Sylvia Hiriart-Fajardo, MD; Arielle Rigaud, MD; Maria Calejo, MS; Silvia M. Frade Eguaras, MA; Michelle Harwood Berkowits, PhD; Andrea Garcia, MA; Helina Pierre, BA; Alexandra Stoerger, BA.
University of New Mexico Health Sciences Center (U10 HD53089, UL1 TR41) – Kristi L. Watterberg, MD; Robin K. Ohls, MD; Janell F. Fuller, MD; Conra Backstrom Lacy, RN; Rebecca A. Montman, BSN RNC; Jean R. Lowe, PhD; Andrea Freeman Duncan, MD; Sandra Brown, BSN; Theresa Wussow, BSN; Carol Hartenberger, BSN MPH; Julie Rohr, MSN RNC CNS.
University of Pennsylvania, Hospital of the University of Pennsylvania, Pennsylvania Hospital, and Children’s Hospital of Philadelphia (U10 HD68244) – Barbara Schmidt, MD MSc; Haresh Kirpalani, MB MSc; Sara B. DeMauro, MD MSCE; Aasma S. Chaudhary, BS RRT; Soraya Abbasi, MD; Toni Mancini, RN BSN CCRC; Dara Cucinotta.
University of Rochester Medical Center, Golisano Children’s Hospital (U10 HD68263, U10 HD40521, UL1 RR24160, M01 RR44) – Dale L. Phelps, MD; Gary J. Myers, MD; Linda J. Reubens, RN CCRC; Erica Burnell, RN; Diane Hust, MS RN CS; Julie Babish Johnson, MSW; Rosemary L. Jensen; Emily Kushner, MA; Joan Merzbach, LMSW; Kelley Yost, PhD; Lauren Zwetsch, RN MS PNP; Satyan Lakshminrusimha, MD; Anne Marie Reynolds, MD MPH; Osman Farooq, MD; Ashley Williams, MS Ed.
University of Texas Health Science Center at Houston Medical School, Children’s Memorial Hermann Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District (U10 HD21373) – Kathleen A. Kennedy, MD MPH; Nora I. Alaniz, BS; Katrina Burson, RN BSN; Patricia W. Evans, MD; Charles Green, PHD; Beverly Foley Harris, RN BSN; Margarita Jiminez, MD MPH; Anna E. Lis, RN, BSN; Sarah Martin, RN BSN; Georgia E. McDavid, RN; Brenda H. Morris, MD; M. Layne Poundstone, RN BSN; Peggy Robichaux, RN BSN; Saba Siddiki, MD; Maegan C. Simmons, RN; Patti L. Pierce Tate, RCP; Sharon L. Wright, MT (ASCP).
University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children’s Medical Center Dallas (U10 HD40689, M01 RR633) – Pablo J. Sánchez, MD; Roy J. Heyne, MD; Walid A. Salhab, MD; Charles R. Rosenfeld, MD; Luc P. Brion, MD; LiJun Chen, RN, PhD; Alicia Guzman; Melissa H. Leps, RN; Nancy A. Miller, RN; Diana M. Vasil, RNC NIC; Lizette E. Torres, RN; Gaynelle Hensley, RN; Sally S. Adams, MS RN CPNP; Linda A. Madden, RN CPNP; Elizabeth Heyne, PsyD PA-C; Janet S. Morgan, RN; Catherine Twell Boatman, MS CIMI.
University of Utah University Hospital, Intermountain Medical Center, LDS Hospital, and Primary Children’s Medical Center (U10 HD53124, M01 RR64, UL1 TR105) – Roger G. Faix, MD; Bradley A. Yoder, MD; Anna Bodnar, MD; Karen A. Osborne, RN BSN CCRC; Shawna Baker, RN; Karie Bird, RN BSN; Jill Burnett, RNC BSN; Jennifer J. Jensen, RN BSN; Cynthia Spencer, RNC BSN; Mike Steffen, PhD; Kimberlee Weaver-Lewis, RN MS; Sarah Winter, MD; Karen Zanetti, RN.
Wake Forest University, Baptist Medical Center, Forsyth Medical Center, and Brenner Children’s Hospital (U10 HD40498, M01 RR7122) – T. Michael O’Shea, MD MPH; Robert G. Dillard, MD; Lisa K. Washburn, MD; Barbara G. Jackson, RN, BSN; Nancy Peters, RN; Korinne Chiu, MA; Deborah Evans Allred, MA LPA; Donald J. Goldstein, PhD; Raquel Halfond, MA; Carroll Peterson, MA; Ellen L. Waldrep, MS; Cherrie D. Welch, MD MPH; Melissa Whalen Morris, MA; Gail Wiley Hounshell, PhD.
Wayne State University, University of Michigan, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (U10 HD21385) – Athina Pappas, MD; John Barks, MD; Rebecca Bara, RN BSN; Laura A. Goldston, MA; Mary Johnson, RN BSN; Geraldine Muran RN BSN; Laura Sumner RN BSN; Kara Sawaya RN BSN; Kathleen Weingarden RN BSN, Mary Christensen, RT; Stephanie Wiggins, MS.
Yale University, Yale-New Haven Children’s Hospital, and Bridgeport Hospital (U10 HD27871, M01 RR125) – Richard A. Ehrenkranz, MD; Harris Jacobs, MD; Christine G. Butler, MD; Patricia Cervone, RN; Sheila Greisman, RN; Monica Konstantino, RN BSN; JoAnn Poulsen, RN; Janet Taft, RN BSN; Joanne Williams, RN BSN; Elaine Romano, MSN.
The following hospitals participated in this study:
University Hospital, Birmingham, AL
El Camino Hospital, Mountain View, CA
Lucile Salter Packard Children’s Hospital at Stanford, Palo Alto, CA
Bridgeport Hospital, Bridgeport, CT
Yale-New Haven Hospital, New Haven, CT
Crawford W. Long Hospital, Atlanta, GA
Grady Memorial Hospital, Atlanta, GA
University of Iowa Children’s Hospital, Iowa City, IA
Methodist Hospital, Indianapolis, IN
Riley Hospital for Children, Indianapolis, IN
University Hospital, Indianapolis, IN
Wishard Hospital, Indianapolis, IN
Floating Hospital for Children at Tufts-New England Medical Center, Boston, MA
Hutzel Women’s Hospital, Detroit, MI
Duke Hospital, Durham, NC
University of New Mexico Health Science Center, Albuquerque, NM
Good Samaritan Hospital, Cincinnati, OH
Rainbow Babies and Children’s Hospital, Cleveland, OH
University Hospital, Cincinnati, OH
Women and Infants Hospital of Rhode Island, Providence, RI
Memorial Hermann Children’s Hospital, Houston, TX
Parkland Memorial Hospital, Dallas, TX
LDS Hospital, Salt Lake City, UT
University of Utah Medical Center, Salt Lake City, UT
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Sharing: Data reported in this paper may be requested through a data use agreement. Further details are available at https://neonatal.rti.org/index.cfm?fuseaction=DataRequest.Home.
Portions of this study were presented at the Pediatric Academic Societies meeting, April 25–28, 2015, San Diego, California.
List of additional investigators of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network is available at www.jpeds.com (Appendix).
REFERENCES
- 1.Raju TNK, Mercer BM, Burchfield DJ, Joseph GF Jr. Periviable birth: executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists. Obstet Gynecol. 2014;123:1083–96. [DOI] [PubMed] [Google Scholar]
- 2.Cummings J, American Academy of Pediatrics Committee on Fetus and Newborn. Antenatal counseling regarding resuscitation and intensive care before 25 weeks of gestation. Pediatrics. 2015;136:588–95. [DOI] [PubMed] [Google Scholar]
- 3.Miracle X, Di Renzo GC, Stark A, Fanaroff A, Carbonell-Estrany X, Saling E. Guideline for the use of antenatal corticosteroids for fetal maturation. J Perinat Med. 2008;36:191–6. [DOI] [PubMed] [Google Scholar]
- 4.Antenatal corticosteroids to reduce neonatal morbidity and mortality Green-top Guideline No. 7. London (UK): Royal College of Obstetricians and Gynaecologists; 2010. [DOI] [PubMed] [Google Scholar]
- 5.Periviable birth. Obstetric Care Consensus No. 4. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2016;127:e157–69. [DOI] [PubMed] [Google Scholar]
- 6.Rysavy MA, Li L, Bell EF, Das A, Hintz SR, Stoll BJ, et al. Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med. 2015;372:1801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2006, Issue 3 Art. No.:CD004454. [DOI] [PubMed] [Google Scholar]
- 8.Tyson JE, Parikh NA, Langer J, Green C, Higgins RD. Intensive care for extreme prematurity—moving beyond gestational age. N Engl J Med. 2008;358:1672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlo WA, McDonald SA, Fanaroff AA, Vohr BR, Stoll BJ, Ehrenkranz RA, et al. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22 to 25 weeks’ gestation. JAMA. 2011;306:2348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehret DEY, Edwards EM, Greenberg LT, Bernstein IM, Buzas JS, Soll RF, et al. Association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks’ gestation. JAMA Network Open. 2018;1:e183235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for perinatal care. 7th ed. Elk Grove Village, IL: American Academy of Pediatrics, 2012. [Google Scholar]
- 12.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. [DOI] [PubMed] [Google Scholar]
- 13.Hintz SR, Barnes PD, Bulas D, Slovis TL, Finer NN, Wrage LA, et al. Neuroimaging and neurodevelopmental outcome in extremely preterm infants. Pediatrics. 2015;135:e32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahony R, McKeating A, Murphy T, McAuliffe F, O’Herlihy C, Foley M. Appropriate antenatal corticosteroid use in women at risk for preterm birth before 34 weeks of gestation. BJOG. 2010;117:963–7. [DOI] [PubMed] [Google Scholar]
- 15.Lee HC, Lyndon A, Blumenfeld YJ, Dudley RA, Gould JB. Antenatal steroid administration for premature neonates in California. Obstet Gynecol. 2011;117:603–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeitlin J, Manktelow BN, Piedvache A, Cuttini M, Boyle E, van Heijst A, et al. Use of evidence based practices to improve survival without severe morbidity for very preterm infants: results from the EPICE population based cohort. BMJ. 2016;354:i2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chawla S, Natarajan G, Shankaran S, Pappas A, Stoll BJ, Carlo WA, et al. Association of neurodevelopmental outcomes and neonatal morbidities of extremely premature infants with differential exposure to antenatal steroids. JAMA Pediatr .2016;170:1164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norman M, Piedvache A, Børch K, Huusom LD, Bonamy AE, Howell EA, et al. Association of short antenatal corticosteroid administration-to-birth intervals with survival and morbidity among very preterm infants: results from the EPICE cohort. JAMA Pediatr. 2017;171:678–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wapner RJ, Gyamfi-Bannerman C, Thom EA. What we have learned about antenatal corticosteroid regimens. Semin Perinatol. 2016;40:291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan KL, Kean LH, Marlow N. Staff views on the management of the extremely preterm infant. Eur J Obstet Gynecol Reprod Biol. 2006;128:142–7. [DOI] [PubMed] [Google Scholar]
- 21.Tucker Edmonds B, McKenzie F, Panoch JE, Barnato AE, Frankel RM. Comparing obstetricians’ and neonatologists’ approaches to periviable counseling. J Perinatol. 2015;35:344–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park CK, Isayama T, McDonald SD. Antenatal corticosteroid therapy before 24 weeks of gestation: a systematic review and meta-analysis. Obstet Gynecol. 2016;127:715–25. [DOI] [PubMed] [Google Scholar]
- 23.Mednax Center for Research, Education, and Quality. Effectiveness of ACS in extreme preemies. Available at: https://clinicaltrials.gov/ct2/show/NCT02351310. Retrieved December 3, 2018.
- 24.National Institute of Child Health and Human Development. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement 1994;12:1–24. [PubMed] [Google Scholar]
- 25.Bottoms SF, Paul RH, Mercer BM, MacPherson CA, Caritis SN, Moawad AH, et al. Obstetric determinants of neonatal survival: Antenatal predictors of neonatal survival and morbidity in extremely low birth weight infants. Am J Obstet Gynecol. 1999;180:665–9. [DOI] [PubMed] [Google Scholar]
- 26.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314:1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]