Abstract
Tissue nicotinamide adenine dinucleotide (NAD+) decline has been implicated in aging. We have recently identified CD38 as a central regulator involved in tissue NAD+ decline during the aging process. CD38 is an ecto-enzyme highly expressed in endothelial and inflammatory cells. To date, the mechanisms that regulate CD38 expression in aging tissues characterized by the presence of senescent cells is not completely understood. Cellular senescence has been described as a hallmark of the aging process and these cells are known to secrete several factors including cytokines and chemokines through their senescent associated secretory phenotype (SASP). Here we investigated if the cellular senescence phenotype is involved in the regulation of CD38 expression and its NADase activity. We observed that senescent cells do not have high expression of CD38. However, the SASP factors secreted by senescent cells induced CD38 mRNA and protein expression and increased CD38-NADase activity in non-senescent cells such as endothelial cells or bone marrow derived macrophages. Our data suggest a link between cellular senescence and NAD+ decline in which SASP-mediated upregulation of CD38 can disrupt cellular NAD+ homeostasis.
Keywords: CD38, NAD+, aging, senescence and inflammaging
INTRODUCTION:
It has been recently demonstrated that decreases in levels of the intracellular nucleotide nicotinamide adenine dinucleotide (NAD+) and accumulation of senescent cells play important roles in the biology of aging [1–23]. These two mechanisms have emerged as new hallmarks of the aging process, but whether there is a link between these two mechanisms is not known. Cellular senescence is a cell fate that, like replication, differentiation, or apoptosis, can occur at any stage of development, as a consequence of cell damage, or in aging [10–19, 23]. In particular, senescence may be initiated by stimuli such as repeated cell division, strong mitogenic signals, oxidative stress, inflammation, and DNA damage [10–19, 23]. Senescent cells have been shown to secrete cytokines, growth factors, extracellular matrix modifiers, and other biological compounds that promote chronic “sterile” inflammation and fibrosis through the so-called senescence-associated secretory phenotype (SASP) [13–19, 23]. Through these processes senescent cells can contribute to tissue injury [10–19, 23]. This concept has been presented in the inflammaging hypothesis [24]. As discussed above, whether there is a link between senescence/SASP and tissue NAD+ decline during aging is not known. We have previously shown that the enzyme CD38 is the main nicotinamide nucleotidase in mammalian tissues [24–25]. Furthermore, we have also demonstrated that CD38 plays a key role in the age-related NAD+ decline [2, 9]. However, to date several aspects of the biology of this enzyme remain to be elucidated [26–27]. For instance, what drives CD38 expression in the biology of aging is an open question [27–30]. Surprisingly, the possible link between CD38 expression and the cellular senescence phenotype has not been explored. Here we demonstrated that CD38 expression in cells can be induced by factors associated with the SASP, providing a possible link between these two emerging hallmarks of aging.
MATERIALS AND METHODS:
HUVECs: Preparation, Induction of Senescence, and Treatment with Conditioned Media.
Human umbilical venous endothelial cells (HUVECs-ATCC® PCS100013™) were cultured in Vascular Cell Basal Medium supplemented with Endothelial Cell Growth Kit VEGF (ATCC PCS100041). Exposure of HUVECs for 36h to MEF conditioned media was performed between passage 4 and 6 at 90–100% confluence in serum starved cells (0.5% FBS) amended at time of experiment with 2% FBS.
Senescence was induced in HUVECs which had reached approximately 100% confluence. Two different methods were used: gamma irradiation (γ-IR) or x-ray irradiation. HUVECs were sham-irradiated or irradiated in either a cesium irradiator or an X-Ray irradiator at 10 Gy. The senescence phenotype was observed at d7 post-radiation. HUVEC conditioned media was produced by culturing cells for 24 hours in medium containing 2 % FBS. Medium was steroid-free.
Patient-derived preadipocytes (PDP): Preparation and Induction of Senescence.
Abdominal subcutaneous adipose tissue for primary preadipocyte isolation [16] was obtained during intra-abdominal surgery from 2 consented clinically obese subjects (BMI 46±5.7) undergoing kidney transplant or gastric bypass surgery (female; age 51.75± 6.06 [mean ± SEM] years). Preadipocytes are also known as adipose-derived stem cells or fat cell progenitors. Cells were used in experiments between passages 4–8. The protocol was approved by the Mayo Clinic Foundation Institutional Review Board for Human Research.
Senescence was induced in PDPs which had reached approximately 60% confluence using an X-Ray irradiator at 10 Gy. Media was subsequently changed twice weekly post-irradiation. Conditioned media from treated and untreated cells was harvested at d20 post-IR following incubation of cells for 24h with serum-free media. It was centrifuged prior to use.
MEFs: Preparation and Induction of Senescence.
Primary mouse embryonic fibroblasts (MEFs) were generated from C57BL/6 as previously described [2, 9]. Briefly, embryos were harvested at d13.5 post-conception, mechanically disaggregated and digested in trypsin/EDTA (0.25%) for 1 hour. Cells were cultivated in DMEM containing FBS, sodium pyruvate, glutamine, nonessential amino acids, and antibiotics in a humidified incubator at 37°C with 5% CO2.
Senescence was induced in MEFs which had reached approximately 75% confluence by administering 10 Gy in a cesium irradiator. Media was subsequently changed at d4 and d7 post-irradiation. Conditioned media from treated and untreated cells was harvested at d10 following incubation of cells for 24h with serum-free DMEM. MEF CM was centrifuged prior to use. Senescent cells expressed increased p16-INK4a or p21Cip1 by 10 days post-irradiation.
Preparation of Bone Marrow-Derived Macrophages (BMDMs).
Mouse BMDMs were isolated as described before in Matalonga et al [31] from aged mice (18–20 months). BMDM cells were seeded at 5 million cells/60mm2 dish and cultured for 24h with conditioned media collected from senescent or non-senescent cells and amended with 2% FBS.
Western Blot Analysis.
Western immunoblotting was performed as previously described in Tarrago et al [9] using the following antibodies: mouse CD38 (R&D Systems AF4947) and β-Tubulin (Abcam Ab 15568).
Enzymatic Activity.
Measurement of CD38 hydrolase activity with nicotinamide 1,N6-ethenoadenine dinucleotide (Et-NAD) as substrate was performed as described previously [24–25].
Quantification of mRNA.
Total RNA was isolated from cells and mouse tissues using TRIzol or Qiagen RNeasy kits. cDNA was synthesized using the Qiagen QuantiTect or ABI High Capacity cDNA Reverse Transcription kit. Quantitative real-time PCR was performed using commercially available TaqMan gene expression probes (Applied Biosystems, see table below), according to the manufacturer’s instructions, on a BioRad CFX384 thermal cycler. The relative mRNA abundance of target genes was calculated by the 2(−ddCq) method. The expression changes were calculated relative to control.
TaqMan Gene Expression Assays (Mouse)
| Gene Symbol | Probe ID |
|---|---|
| Cd138 | Mm01220906_m1 |
| Cdkn2a | Mm0049449_m1 |
| Cdknla | Mm04205640_g1 |
| Il1b | Mm00434228_m1 |
| Il6 | Mm00446190_m1 |
| Nos2 | Mm00440502_m1 |
| Hprt | Mm01545399_m1 |
| Tbp | Mm00446971_m1 |
TaqMan Gene Expression Assays (Human)
| Gene Symbol | Probe ID |
|---|---|
| CD38 | Hs01120071_m1 |
| CDKN1A | Hs00355782_m1 |
| CDKN2A | Hs00923894_m1 |
| CDKN2D | Hs00176481_m1 |
| CXCL8 | Hs00174103_m1 |
| GAPDH | Hs02758991_g1 |
| IL6 | Hs00985639_m1 |
| TP53 | Hs01034249 |
Statistical analysis.
Data were analyzed by a two-tailed Student’s t test and a one-way ANOVA with a Bonferroni’s post hoc test. Analyses were performed using GraphPad Prism 7.
RESULTS AND DISCUSSION:
Senescent cells in vitro do not express CD38.
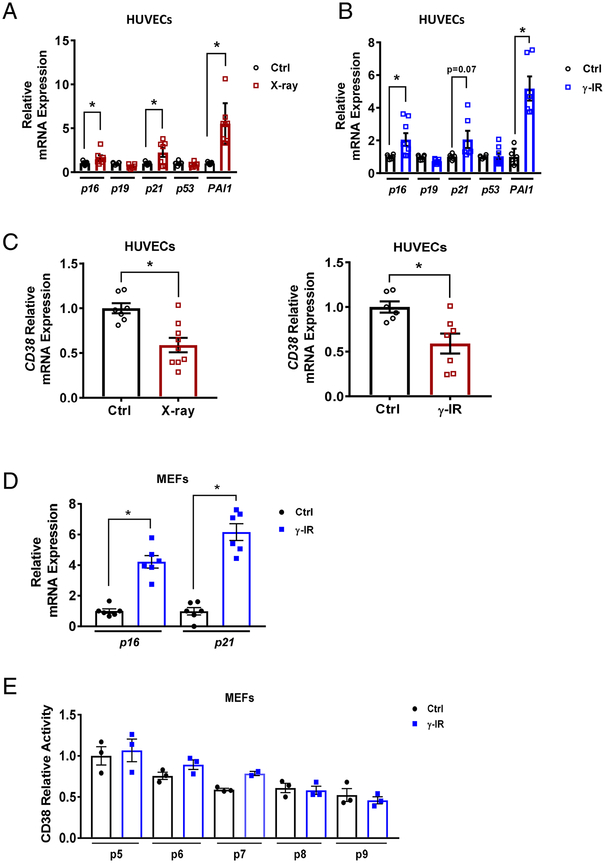
We have postulated that increased CD38 expression and accumulation of CD38+ cells in tissues with aging could be linked to the senescent cell phenotype [20]. Thus, we first investigated whether or not induction of the cellular senescence phenotype in vitro could lead to expression of CD38 in cells. We induced senescence in human umbilical vein endothelial cells (HUVECs) by DNA damage through exposure to x-ray irradiation (x-ray IR) (Figure 1A) or gamma irradiation (γ-IR) (Figure 1B), as described before by our group (12). Markers of the senescent phenotype include p21, p16Ink4a, and PAI1 (Figure 1A, B), and are induced by these treatments [10–19, 23]. We observed that, although our cells presented several markers of senescence after induction of the senescence phenotype, they showed no induction of CD38 mRNA compared to non-senescent cells (Figure 1C). Very similar results were obtained when MEFs were induced to become senescent (Figure 1D and E). In fact, although these cells expressed markers of cellular senescence such as p16 and p21, no changes in CD38 activity were observed even when several independent cell passages were induced to become senescent (Figure 1D and E).
Fig. 1. CD38 expression is not increased in senescent cells.
(A-B) Relative mRNA levels of senescence-related genes in HUVECs treated with (A) X-ray irradiation (IR) (n=7–9) or (B) γ-IR (n=4–8). (C) Relative mRNA levels of CD38 in HUVECs treated with X-ray (n=7–9) or -IR (n=6–7). (D) Relative mRNA levels of senescence-related genes in irradiated MEFs (n=6). Relative mRNA levels (A-D) were determined by qRT-PCR. (E) CD38 relative enzymatic activity in multiple independent irradiation-induced senescent MEFs (passage 5–9) (n=2–3). Data are mean ± SE, *p < 0.05 vs control.
Cytokines and chemokines expressed by senescent cells induce CD38 expression in macrophages.
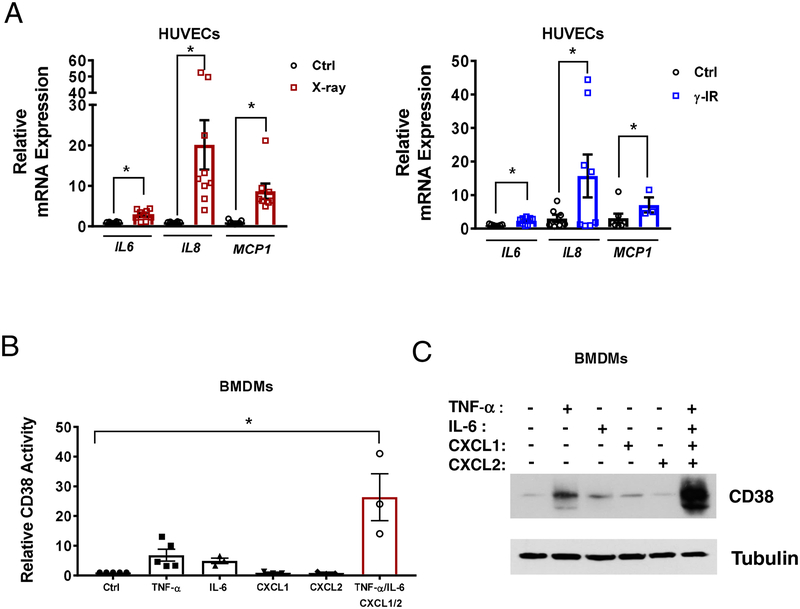
Induction of cellular senescence in cultured cells did not lead to an increase in the expression of CD38; therefore, we next tested the hypothesis that factors secreted by senescent cells could increase CD38 expression and activity in non-senescent cells. It has been previously demonstrated that senescent cells have a secretory phenotype that includes TNF-α, IL-6, IL-8, IFN-γ, MCP-1 and many other chemokines and cytokines (12). Thus, first we established the expression of some of these inflammatory genes considered SASP factors (IL-6, IL-8, MCP-1) in our HUVECs exposed to x-ray- or γ-IR (Figure 2A).
Fig. 2. Expression of SASP factors in senescent HUVECs and induction of CD38 expression by a combination of potential SASP factors.
(A) mRNA expression of selected SASP factors (IL-6, IL-8, MCP-1) in senescent HUVECs, determined by qRT-PCR (n=7–9). (B) CD38 activity (n=3–5) and (C) a representative immunoblot showing CD38 levels in BMDMs treated with various factors found in the SASP. Data are mean ± SE, *p < 0.05 vs control.
Next, we investigated whether components of the SASP were able to regulate the expression of CD38 in non-senescent cells. M1 macrophages are the prototype CD38-expressing cells, and it has been demonstrated that induction of the M1 phenotype by LPS, TNF-α, and activators of the LXR receptor leads to increased CD38 expression in these cells [20, 27–31]. In fact, CD38 is proposed to be one of the main markers of induction of the M1 phenotype in macrophages [32]. Here we show that representative SASP factors (TNFα, IL-6, CXCL1, CXCL2) had small or no significant effects on CD38 activity or protein expression when given individually, but when combined promoted a synergistic effect that led to a strong increase in CD38 activity (Figure 2B) and CD38 protein levels (Figure 2C) in murine bone marrow-derived macrophages (BMDM).
Conditioned media from senescent cells induces CD38 expression in macrophages and endothelial cells.
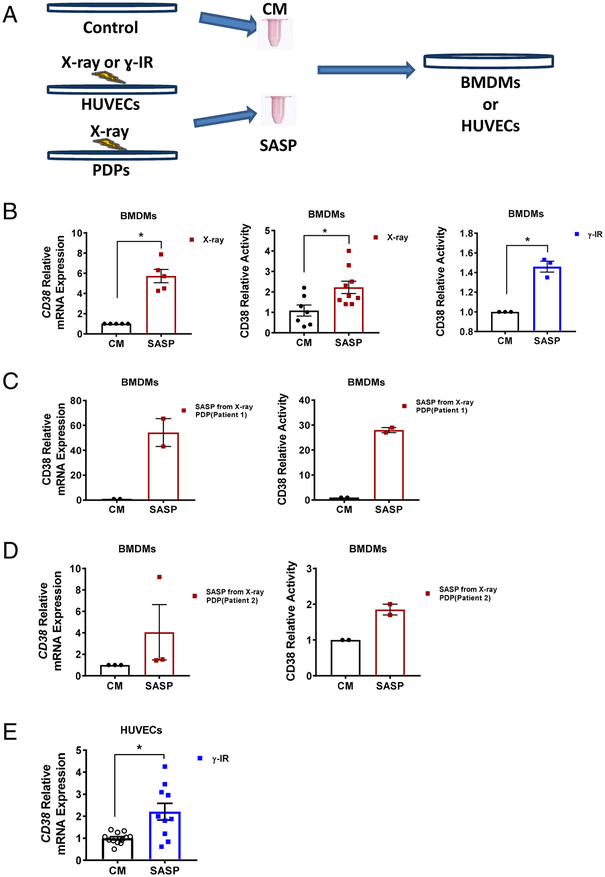
We next tested whether or not conditioned media derived from senescent cells could induce CD38 expression in both inflammatory (BMDM) and non-inflammatory (HUVEC) cells (scheme Figure 3A). We focused on BMDM and HUVECs because previous reports demonstrate that CD38 in vivo is preferentially expressed in inflammatory and endothelial cells [9, 33]. In support of our hypothesis, we observed that exposure to conditioned media from x-ray- or γ-IR-induced senescent HUVECs leads to increases in CD38 mRNA expression and NADase activity in mouse BMDMs compared to control condioned media (Figure 3B).
Fig. 3. CD38 is induced in non-senescent cells by secreted factors (SASP) from senescent cells.
(A) Scheme showing production of control conditioned media (CM) and SASP (senescent cell conditioned media) from HUVECs or PDPs for treatment of non-senescent cells. (B) Relative CD38 mRNA expression and activity in BMDMs treated with CM or SASP from x-ray- (n=7–9) or γ-IR-treated (n=3) HUVECs. (C and D) CD38 mRNA expression and activity in BMDMs treated with CM or SASP from PDP originating from (patient 1 in C) or (patient 2 in D) (n=2–3). (E) Relative CD38 expression (n=4–6) in HUVECs treated with CM or SASP from γ-IR MEFs. Data are mean ± SE, *p < 0.05 vs control.
It has been previously demonstrated that pre-adipocytes are one of the main cells that become senescent in vivo [10–12]. Thus, we next tested the possibility that conditioned medium derived from senescent human preadipocytes (PDPs) induces the expression and activity of CD38 in mouse BMDMs. In support of our hypothesis we observed that conditioned media from senescent preadipocytes derived from two independent patients induced CD38 expression and CD38 NADase activity in BMDM, although media from one of the patients caused a much stronger induction of CD38 than the other (Figure 3C and D). We also observed that the SASP from senescent cells also induced CD38 expression in endothelial cells (Figure 3E).
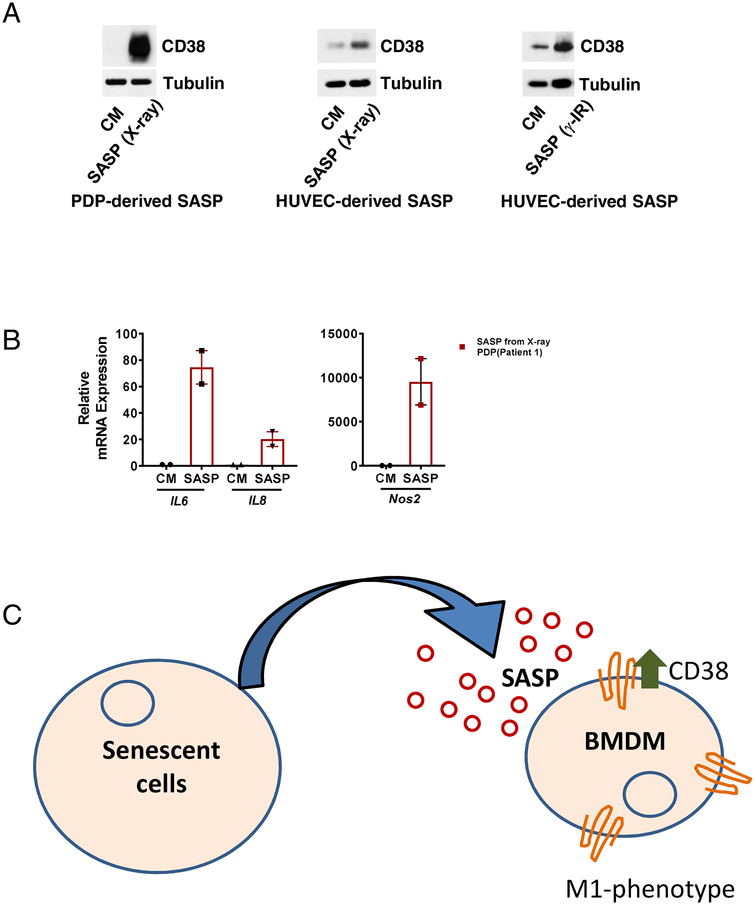
We further investigated if SASP increased protein expression of CD38 in BMDMs. Indeed, we found that SASP from different senescent cells induced a robust increase in CD38 protein levels in macrophages (Figure 4A). Compatible with the idea that the SASP could induce the M1 macrophage phenotype, we also observed that the SASP from senescent cells increased expression of other cellular markers of the M1 phenotype such as IL-6, IL-8, and iNOS (Figure 4B and C). These data together demonstrate that SASP from different cell types can induce CD38 expression in both macrophages and endothelial cells.
Fig. 4. CD38 and other M1 markers in macrophages treated with SASP.
(A) Representative immunoblots demonstrating levels of CD38 in BMDMs treated with CM (control conditional media) or SASP (x-ray- or γ-IR-induced senescent cell conditioned media) from PDPs or HUVECs. (B) mRNA expression of other M1 markers, namely IL-6, IL-8, and Nos2 (inducible nitric oxide synthase), in BMDMs treated with CM or SASP from PDPs. (C) Model of SASP-induced CD38 expression in macrophages. Data are mean ± SE, *p < 0.05 vs control.
Aging is characterized by the development of metabolic dysfunction and increased risk for age-related diseases including diabetes, loss of muscle function, and cancers. As the world population ages, the incidence of these conditions will further increase. It is not completely understood how molecular changes during aging lead to metabolic dysfunction and age-related diseases. NAD+ is a critical nucleotide involved in both catabolic and anabolic reactions, serving as an acceptor or donor of electrons, and also as a substrate for enzymatic processes involved in the control of metabolism, epigenetics, DNA repair, and cell signaling [20–21, 26]. Recently, it has been described that cellular NAD+ levels decline during chronological aging and progeroid states [1–9, 34]. Interestingly, this NAD+ decline appears to play a direct role in the development of age-related metabolic dysfunction, age-related diseases, and a decline in tissue resilience after injury in aging [1–9, 33–35]. In fact, cellular NAD+ decline is emerging as one of the hallmarks of aging [1–9, 20–21, 26]. Until recently, the prevailing hypothesis had been that activation of DNA-repair enzymes such as PARPs during the aging process would consume and deplete NAD+ [1–9, 20–21, 26]. In a recent manuscript, we challenged this paradigm and demonstrated that levels and activity of the NADase CD38 increase during aging, and that this enzyme is the main NAD-consuming enzyme responsible for the aging-related NAD+ decline [2, 9]. We showed that the activity of CD38, but not PARP, increases in several tissues during the aging process, and that genetic ablation of CD38 protects against age-related NAD+ decline and, mitochondrial dysfunction [2].
Our studies present an extremely novel connection between the NAD+ catabolizing enzyme CD38 and the senescence/SASP phenotype. Very interestingly, we observed that secreted biological compounds from senescent cells are responsible for the increased expression of CD38, at least in macrophages and endothelial cells. Although all these data indicate that senescent cells via their SASPs can lead to CD38 expression in cells, it does not exclude the possibility that other mechanisms mediated by senescent cells could also lead to the increased expression of CD38 during the aging process. It is also possible that induction of CD38 expression via senescent cells could be mediated by a different mechanism. For example, it has been previously demonstrated that senescent cells can express Major histocompatibility complex II (MHC-II) [36]. Direct interaction of immune cells with the MHC-II in senescent cells could also lead to increased CD38 expression in T cells in the liver.
CD38 expression in several cell types is induced by inflammatory cytokines such as TNF-α and endotoxins via activation of a NF-kB [28–31]. Interestingly, these pathways appear to be involved in the “sterile” inflammation observed during the aging process [17, 23], and several cytokines are secreted by senescent cells as part of the SASP [17, 23]. Furthermore, it has also been observed that clearance of senescent cells in vivo can decrease the expression of several components of the SASP including TNF-α, IFNγ, IL-1b, IL-8 and IL-6 [10–19]. This may indicate that senescent cells in vivo may modulate CD38 expression via changes in tissue cytokines and chemokines (Figure 4C). This hypothesis will be further tested in our laboratory using senolytic agents. Although at this moment we cannot exclude that senescent cells express CD38 in vivo, induction of cellular senescence by several different stimuli in mouse and human cells in vitro did not increase expression of CD38. These data support the hypothesis that senescent cells regulate CD38 expression in non-senescent cells via their SASPs.
In conclusion, we demonstrate that the factors related to the SASP induce CD38 expression and activity in inflammatory and endothelial cells. Interestingly, we have previously demonstrated that in mammalian tissues the majority of CD38+ cells are comprised of inflammatory and tissue endothelial cells, with low or undetectable expression in tissue parenchymal cells [2, 9, 33]. Thus, it is possible that SASP factors are one of the main drivers of CD38 expression and NAD+ decline mediated by CD38+ cells during age-related NAD+ decline and metabolic dysfunction (Figure 4C).
Highlights.
Senescent cells can induce via their senescent associated secretory phenotype (SASP) the expression of the main cellular NADase CD38 in non-senescent cells.
Cytokines and chemokines from SASP can induce CD38 expression in macrophages.
We provide a potential link between Cellular senescence and dysfunction of NAD homeostasis induced by CD38 expression: two emerging hallmarks of aging.
AKNOWLEDGMENTS
This work was supported in part by grants from the Glenn Foundation for Medical Research via the Paul F. Glenn Laboratories for the Biology of Aging at the Mayo Clinic, and National Institutes of Health (NIH) grants from the National Institute of Aging (NIA, grants AG-26094 and AG-58812).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: Dr. Chini holds a patent on the use of CD38 inhibitors for metabolic diseases.
REFERENCES
- 1.Gomes AP et al. [2013] Declining NAD[+] induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell= 155, 1624–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camacho-Pereira J et al. [2016] CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab. 23, 1127–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mouchiroud L et al. The NAD+/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 154, 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshino J [2011] Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 14[4], 528–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Picciotto NE et al. [2016]. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 15[3], 522–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Bonkowski MS, Moniot S, Zhang D, Hubbard BP, Ling AJY, Rajman LA, Qin B, Lou Z, Gorbunova V, Aravind L, Steegborn C, Sinclair DA (2017). A conserved NAD+ binding pocket that regulates protein-protein interactions during aging. Science 355, 1312–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams PA et al. [2017] Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science 355[6326], 756–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan Y et al. [2017] Nicotinamide Mononucleotide, an NAD+ Precursor, Rescues Age-Associated Susceptibility to AKI in a Sirtuin 1-Dependent Manner. J Am Soc Nephrol. 28[8], 2337–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarragó MG, Chini CCS, Kanamori KS, Warner GM, Caride A, de Oliveira GC, Rud M, Samani A, Hein KZ, Huang R, Jurk D, Cho DS, Boslett JJ, Miller JD, Zweier JL, Passos JF, Doles JD, Becherer DJ, Chini EN. A Potent and Specific CD38 Inhibitor Ameliorates Age-Related Metabolic Dysfunction by Reversing Tissue NAD+ Decline. Cell Metab. 2018;27(5):1081–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011; 479(7372):232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016; 530(7589):184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, Onken JL, Johnson KO, Verzosa GC, Langhi LGP, Weigl M, Giorgadze N, LeBrasseur NK, Miller JD, Jurk D, Singh RJ, Allison DB, Ejima K, Hubbard GB, Ikeno Y, Cubro H, Garovic VD, Hou X, Weroha SJ, Robbins PD, Niedernhofer LJ, Khosla S, Tchkonia T, Kirkland JL. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018. (8):1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biran A, Zada L, Abou Karam P, Vadai E, Roitman L, Ovadya Y, Porat Z, Krizhanovsky V. Quantitative identification of senescent cells in aging and disease. Aging Cell. 2017;16(4):661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodier F, Coppé JP, Patil CK, Hoeijmakers WA, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009; 11(8):973–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andriani GA, Almeida VP, Faggioli F, Mauro M, Tsai WL, Santambrogio L, Maslov A, Gadina M, Campisi J, Vijg J, Montagna C. Whole Chromosome Instability induces senescence and promotes SASP. Sci Rep. 2016; 6:35218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, White TA, Johnson KO, Stout MB, Mezera V, Giorgadze N, Jensen MD, LeBrasseur NK, Kirkland JL. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A. 2015;112(46):E6301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franceschi C and Campisi J [2014] Chronic inflammation [inflammaging] and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci 69, S4–S9 [DOI] [PubMed] [Google Scholar]
- 18.Jadeja RN, Powell FL, Jones MA, Fuller J, Joseph E, Thounaojam MC, Bartoli M, Martin PM. Loss of NAMPT in aging retinal pigment epithelium reduces NAD+ availability and promotes cellular senescence. Aging (Albany NY). 2018;10(6):1306–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, Shirakawa K, Lim HW, Davis SS, Ramanathan A, Gerencser AA, Verdin E, Campisi J.Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. 2016. February 9;23(2):303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chini CCS et al. [2017] NAD and the aging process: Role in life, death and everything in between. Mol Cell Endocrinol. 455, 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verdin E. [2015] NAD+ in aging, metabolism, and neurodegeneration. Science 350, 1208–1213 [DOI] [PubMed] [Google Scholar]
- 22.Imai S and Guarente L [2014] NAD+ and sirtuins in aging and disease. Trends Cell Biol. 24 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer AK, Tchkonia T, LeBrasseur NK, Chini EN, Xu M, Kirkland JL. Cellular Senescence in Type 2 Diabetes: A Therapeutic Opportunity. Diabetes. 2015. 64(7):2289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aksoy P et al. [2006] Regulation of SIRT 1 mediated NAD dependent deacetylation: a novel role for the multifunctional enzyme CD38. Biochem. Biophys. Res. Commun 349, 353–359. [DOI] [PubMed] [Google Scholar]
- 25.P. Aksoy P, et al. [2006] Regulation of intracellular levels of NAD: a novel role for CD38. Biochem. Biophys. Res. Commun 345, 1386–1392 [DOI] [PubMed] [Google Scholar]
- 26.Schultz MD and Sinclair DA [2016] Why NAD+ Declines during Aging: It’s Destroyed. Cell Metab. 23[6], 965–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chini EN, Chini CCS, Espindola Netto JM, de Oliveira GC, van Schooten W. The Pharmacology of CD38/NADase: An Emerging Target in Cancer and Diseases of Aging. Trends Pharmacol Sci. 2018;39(4):424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malavasi S et al. [2008] Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol. Rev 88, 841–886. [DOI] [PubMed] [Google Scholar]
- 29.Chatterjee S, et al. [2017] CD38-NAD+Axis Regulates Immunotherapeutic Anti-Tumor T Cell Response. Cell Metab. doi: 10.1016/j.cmet.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee CU, Song EK, Yoo CH, Kwak YK, Han MK. Lipopolysaccharide induces CD38 expression and solubilization in J774 macrophage cells. Mol Cells. 2012; 34(6):573–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matalonga J, Glaria E, Bresque M, Escande C, Carbó JM, Kiefer K, Vicente R, León TE, Beceiro S, Pascual-García M, Serret J, Sanjurjo L, Morón-Ros S, Riera A, Paytubi S, Juarez A, Sotillo F, Lindbom L, Caelles C, Sarrias MR, Sancho J, Castrillo A, Chini EN, Valledor AF. The Nuclear Receptor LXR Limits Bacterial Infection of Host Macrophages through a Mechanism that Impacts Cellular NAD Metabolism. Cell Rep. 2017. January 31;18(5):1241–1255 [DOI] [PubMed] [Google Scholar]
- 32.Jablonski KA, Amici SA, Webb LM, Ruiz-Rosado JdD, Popovich PG, Partida-Sanchez S, et al. (2015) Novel Markers to Delineate Murine M1 and M2 Macrophages. PLoS ONE 10(12): e0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boslett J, Helal M, Chini E, Zweier JL. Genetic deletion of CD38 confers post-ischemic myocardial protection through preserved pyridine nucleotides. J Mol Cell Cardiol. 2018. May; 118:81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheibye-Knudsen M, Mitchell SJ, Fang EF, Iyama T, Ward T, Wang J, Dunn CA, Singh N,, Veith S, Hasan-Olive MM, et al. (2014). A high-fat diet and NAD(+) activate Sirt1 to rescue premature aging in cockayne syndrome. Cell Metab. 20, 840–855.l [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y [2016] Exogenous NAD[+] administration significantly protects against myocardial ischemia/reperfusion injury in rat model. Am J Transl Res. 8[8], 3342–3350. [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshimoto S et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499, 97–101 (2013). [DOI] [PubMed] [Google Scholar]
- Kang TW et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479, 547–551 (2011). [DOI] [PubMed] [Google Scholar]