Abstract
Acute pancreatitis may be associated with both local and systemic complications. Systemic injury manifests in the form of organ failure which is seen in approximately 20% of all cases of acute pancreatitis and defines ‘severe acute pancreatitis’. Organ failure typically develops early in the course of acute pancreatitis, but may also develop later due to infected pancreatic necrosis induced sepsis. Organ failure is the most important determinant of outcome in acute pancreatitis. We review here the current understanding of the risk factors, pathophysiology, timing, impact on outcome and therapy of organ failure in acute pancreatitis. As we discuss the pathophysiology of severe systemic injury, the distinctions between markers and mediators of severity are highlighted based on evidence supporting their causality in organ failure. Emphasis is placed on clinically relevant end points of organ failure and the mechanisms underlying the pathophysiological perturbations, which offer insight into potential therapeutic targets to treat.
Keywords: Acute pancreatitis, Organ failure, Pathophysiology
Acute pancreatitis (AP) causes major morbidity and mortality. According to global estimates, the incidence of AP was shown to be 33·74 cases (95% CI 23·33-48·81) per 100 000 person-years and a mortality of 1·60 (95% CI 0·85-1·58) per 100 000 person-years due to AP.1 The severity of AP can either be mild, moderate or severe, which depends on the extent of local injury in and around the pancreas, and more importantly systemic injury to remote organs.2 Mild AP has no major local or systemic complications. More severe form of the disease seen in around 20% of all patients with AP is associated with significant local complications in the form of necrosis and often systemic injury due to systemic inflammation.3
Systemic inflammation presents initially as systemic inflammatory response syndrome (SIRS). Patients with persistent SIRS are prone to develop systemic organ dysfunction and later organ failure (OF)4, 5. OF can develop either due to involvement of a particular organ system by a primary disease process or due to systemic effects of injury/inflammation at another site. Acute respiratory failure due to severe pneumonia is an example of the former and OF due to AP is an example of the latter. The most common cause of OF in clinical practice is sepsis. However, OF can develop due to non-infectious etiologies as well: AP and trauma are prime examples. OF is a conditio sine quo non of severe acute pancreatitis (SAP). SAP is defined by the presence of persistent OF as per the revised Atlanta classification of severity of AP.2 OF largely governs the outcome and mortality in patients with AP, and therefore it is important to understand its epidemiology, risk factors, pathophysiology, potential mediators, impact on outcome and management. This review focuses on relevant pathophysiological and clinical aspects of OF in patients with AP and identifies unmet needs.
Definition of Organ Failure:
OF, as a generic term, can be defined as significant functional impairment of an organ system that is critical to sustenance of life. The severity of organ dysfunction can be quantified based on the parameter best defining the primary function of that particular organ e.g. partial pressure of arterial oxygen (PaO2) for pulmonary function or serum creatinine for renal function. In the case of AP, 3 organ systems are considered most important i.e. respiratory, renal and cardiovascular which are most commonly involved.2 The severity of organ dysfunction is graded by the modified Marshall grading in AP 2 which is preferred over the sequential organ failure assessment (SOFA) score used in sepsis. Any organ dysfunction of ≥grade 2 severity persisting for >48 hours is considered as persistent OF and defines SAP. Transient organ failure of <48 hours is considered a criterion for moderate AP.
How common is OF in AP?
The proportion of patients with AP who develop OF varies in different studies and primarily depends on the setting. Data from population-based cohort studies show a lower proportion of patients with OF while tertiary care hospital based studies have shown a much higher frequency of OF. Population based studies have shown the proportion of OF (severe AP) between 8% and 20%.6-8 On the other hand, the proportion of patients with OF in large series of patients from tertiary care hospitals may reach up to 40%.9, 10
Risk Factors for Organ Failure:
Why some patients develop OF and others do not is a matter of great importance. Host factors such as age, co-morbid conditions, obesity, triglyceride levels, etiology, extent of local pancreatic injury, and genetic predisposition have been reported to predict the development of OF in patients with AP. Older age is a risk factor for OF and worse outcome which could also be due to co-morbidities.11, 12 Co-morbidities, as measured by Charlson comorbidity index, may become worse due to AP and contribute to poor outcome but have not been directly related to another organ dysfunction.13 Obesity is an established bad prognostic marker of outcome in patients with AP.14 Visceral obesity predisposes to the development of OF.15 Lipotoxicity causes multi-organ failure and exacerbates AP in obesity.16 Peripancreatic visceral fat necrosis has been shown to worsen AP independent of pancreatic necrosis via unsaturated fatty acids resulting in OF.17 Triglyceride levels at admission have been correlated with the severity of AP and even mild to moderate hypertriglyceridemia was found to be associated with the development of persistent OF.18 Etiology has not been found to be an independent risk factor for OF although patients with alcohol induced AP may have a higher risk of early onset OF.19 Association between the extent of local pancreatic injury as measured by the extent of necrosis and development of OF has been reported but the causality is not established.3, 20-22 The mechanisms of local tissue injury leading to pancreatic necrosis and systemic injury manifesting as OF are intimately linked.23 The issue from the pathophysiology point of view is if the extent of necrosis is causally related to the development of OF or do they both signify the end result of a severe response to acute pancreatic injury. The association between extent of necrosis and OF could be bidirectional. A complex inflammatory network in which the extent of (peri)pancreatic necrosis influences the severity of OF and OF exacerbates the development of pancreatic necrosis might exist.24,25
Given the apparent similarities in the etiology and phenotype of patients with varying grades of severity of AP, differences in inter-individual inflammatory responses might explain the variability in the severity of AP. It is conceivable that the highly variable inflammatory response might be related to an underlying genetic predisposition. However, the data regarding the role of genetic polymorphisms in determining the severity of AP are scant and equivocal. TNF-α gene polymorphism was associated with severity but this finding has not been validated in other studies.26-28 MCP-1 gene polymorphism was associated with increased risk of severe AP with the G allele acting as the risk factor but the data were inconsistent.29, 30 One study has shown genetic polymorphisms of IL-6 gene to alter the level of IL-6 but did not find any association with the severity of AP.26
Clinical determinants/Characteristics of Organ Failure:
There are various characteristics of OF that affect the clinical course and outcome. The important determinants are – (i) grade of OF as per the Modified Marshall score, (ii) specific type of OF e.g. respiratory/renal, (iii) number of organs affected i.e. single or multi-organ failure, and (iv) the timing of OF from the onset of AP. A higher grade of OF naturally has a greater impact on outcome. Patients with grade 3 or 4 OF requiring organ assistance such as mechanical ventilation have a worse outcome.31 Respiratory failure is the commonest OF.10, 32, 33 Respiratory and renal failures are quite similar in their impact on the outcome but cardiovascular failure leads to the worst outcome.10 Multi-organ failure has a worse prognosis than single OF.32 The timing of onset of OF has important connotation regarding the likely cause of OF and possibly its impact on survival. Inflammation is the key pathological response both at the local and systemic levels in AP. Organ failure may develop early within a few days of onset of AP, which is termed as early severe acute pancreatitis and carries a high mortality.19, 31 This is primarily due to a sterile inflammatory response. Organ failure may also develop late during the course of AP due to sepsis as we discuss next.
Primary (Early) Sterile and Secondary (Late) Septic Organ Failure in Acute Pancreatitis:
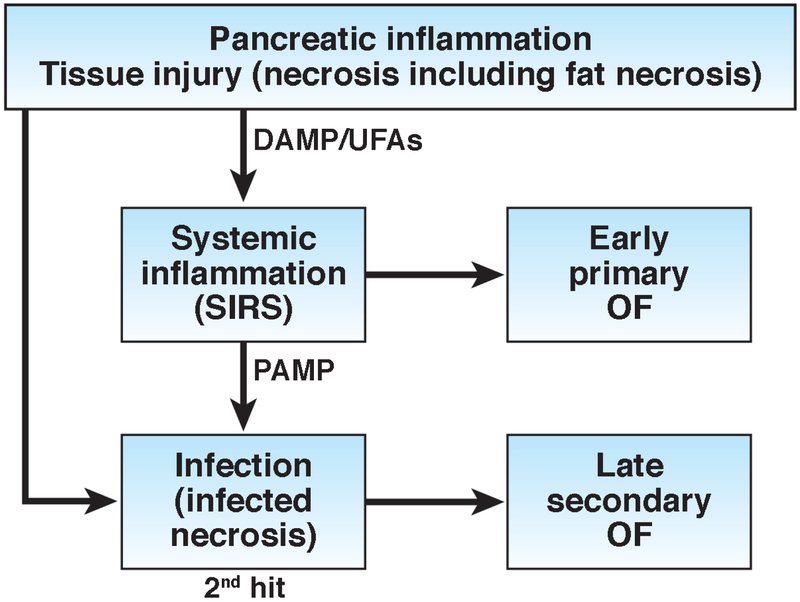
Although organ failure and its consequences are well recognized in AP, there is limited understanding about primary OF that develops early due to pancreatitis per se (sterile inflammation) and may precede necrosis, and late secondary OF due to infected pancreatic necrosis (IPN) induced sepsis (Figure 1). Infection of the necrotic pancreatic tissue is an ominous development during the course of AP. IPN is the cause of most of the late mortality during the course of AP. Although many studies have shown development of early and late OF in patients with AP, the relative contributions of primary OF and secondary OF to mortality have not been well studied. One recent study of 805 patients with AP has provided the concept of primary and secondary OF and shown several differences between the two (Table 1).9 The window of opportunity is small in case of primary OF because it leads to early mortality while temporally there is a larger window of opportunity to intervene in those with sepsis and secondary OF. The treatment is largely supportive for primary OF while control of sepsis is the goal in secondary OF. Prognosis is poorer in primary OF and somewhat better in secondary OF.
Figure 1:
A conceptual model of early sterile injury and inflammation due to damage associated molecular patterns (DAMPs)/ Unsaturated fatty acids (UFAs), and late secondary septic inflammation due to pathogen associated molecular patterns (PAMPs) that may lead to organ failure in acute pancreatitis.
Table 1:
Differences between primary and secondary organ failure
| Characteristic | Primary Organ Failure | Secondary Organ Failure |
|---|---|---|
| Cause | Sterile inflammation | Sepsis |
| Timing | Early | Late |
| Therapeutic window of opportunity | Small | Large |
| Treatment | Supportive | Control of sepsis |
| Prognosis | Poor | Relatively better |
Clinical correlates of OF: Can we predict Organ Failure?
By definition, persistent OF should last >48 hours. Therefore, it will take at least 3 days to document persistent OF even if a patient develops OF within a day of onset of pain. From triage and prognostic points of view, it is important to predict development of persistent OF in patients who present early to the hospital. Prediction of severe AP is largely based on several clinical scores, such as APACHE II, which are multifactorial and somewhat tedious to use. BISAP score has been developed and validated to predict development of severe AP and outcome.34, 35 Single prognostic markers are also used; CRP and IL-6 being sensitive markers.36, 37 Persistent SIRS is a reliable clinical marker to predict development of OF. However, despite a reasonably good sensitivity of 50-95%, SIRS has a lower specificity of 75% and suboptimal positive predictive value (PPV) of 16%-56%.38, 39 Although almost all patients with persistent OF have persistent SIRS, a significant proportion of patients with non-severe AP may also have persistent SIRS. PPV is an important attribute for a test variable as a low PPV may lead to unnecessary and avoidable referrals from primary/secondary care centers, increase monitoring and the overall cost of hospitalization. Recently, a study has shown that a combination of serum IL6 >160 pg/ml and SIRS at admission had a much higher PPV of 85% and a higher specificity of 95% for the development of severe AP.40
Clinical trajectory of Organ Failure - Impact on outcome:
OF accounts for almost all the mortality in patients with AP. Patients who develop OF early on are at risk of having a severe course of the disease. In a population based study of 1024 deaths due to AP, the median interval between the onset of AP and death was 6 days and from the onset of OF to death was 3 days.33 The concept of transient and persistent OF was introduced in 2004.4 Transient OF lasting <48 hours also has a negative impact on outcome.4 The mortality in patients with transient OF was 1.4% to 10% although the mortality was most likely due to other contributing factors such as IPN.4, 10, 13 The overall mortality in patients with persistent OF is >40% (Table 2). Patients with persistent OF have a high risk of early mortality within the first 2 weeks.9, 10, 33 particularly those with very early onset high-grade single OF or multi-organ failure termed as fulminant pancreatitis.19, 31
Table 2:
Summary of Results of Recent Studies highlighting Mortality in Patients with Persistent Organ Failure
| Study* | Number of patients with AP |
Number of patients with POF |
Mortality in patients with POF |
Mortality in patients with early onset POF$ |
Mortality in patients with late onset POF |
|---|---|---|---|---|---|
| Padhan et al 2018 (ref 9) | 805 | 365 | 156 (42.7%) | 104/225 (46%) | 52/140 (37%) |
| Schepers et al 2018 (ref 10) | 639 | 219 | 83 (38%) | 47/112 (42%) | 36/107 (33.6%) |
| Sternby et al 2018 (ref 13) | 1655 | 113 | 59 (52.2%) | 47/89 (52.8%) | 12/24 (50%) |
POF = persistent organ failure
These are recent large studies, which had categorized patients according to revised Atlanta classification and the patients were treated as per current standard of care.
Early OF defined by development of OF within the first week of onset of AP
The mortality due to OF is high even after the first 2 weeks. Patients with persistent OF who survive the first 2 weeks are prone to develop infected necrosis, which accounts for the late mortality. In a French study of 148 patients, 40 of 53 (75%) patients with persistent OF developed infected pancreatic necrosis (IPN).41 In another study, 76% of patients with persistent OF developed IPN after they survived the first 2 weeks.9 Hypotension in the first week of AP was an independent risk factor for IPN.42 There is not much difference between mortality due to early onset OF and late onset OF (Table 2) Two recent studies have focused attention on the issue of timing of onset of OF and outcome in AP. In a study of 614 patients, early onset primary OF resulted in early mortality in 15.8% of patients and a further 42.8% late mortality due to development of infected necrosis.9 In a Dutch study of 639 patients, 219 patients with persistent OF had a mortality of 38%. Mortality was not related to timing of onset of persistent OF. Mortality due to persistent OF developing within the first week, 1-2 weeks, 2-3 weeks and >3 weeks from the onset was 42%, 46%, 36% and 29% respectively in that study.10 Patients with OF and IPN have a high mortality termed ‘critical AP’ according to the Determinant based classification.43 But the data are inconsistent as to whether they have a higher mortality than those with early persistent OF without IPN. In the Dutch study, similar mortality rates were observed in patients with OF with and without IPN (28% vs. 34%, p=0.33) after excluding patients with mortality within 10 days of admission.10
In summary, the progression of an early systemic inflammatory response to organ failure defines severe AP and is associated with a high risk of mortality. Development of infected necrosis later in the clinical course exacerbates the initial injury and worsens the outcome.
Pathophysiology of systemic injury in acute pancreatitis
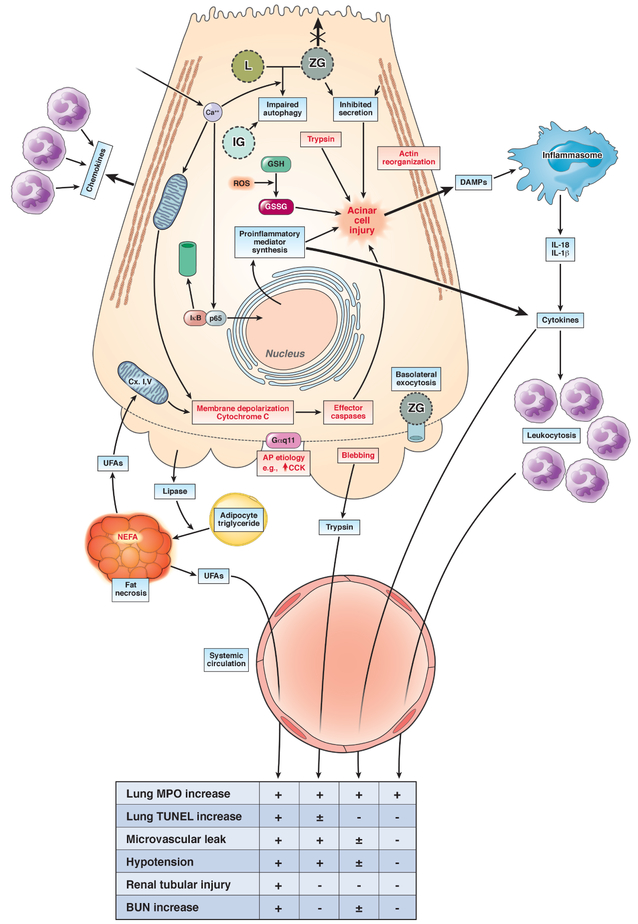
The pathophysiology of systemic injury in AP has remained enigmatic so far. The biggest hurdle has been the identification of mediators that are released locally in the pancreas and cause systemic injury. More so, as mentioned above, the systemic severity can precede local severity and the extent of pancreatic necrosis may not correlate with systemic injury, unless it is complicated by concurrent infection. It is to be noted that neither systemic involvement, nor its severity are related to the etiology of AP. Figure 2 summarizes the potential pathophysiology of systemic injury. This is based on literature in pancreatitis, as well as where individual agents have been studied in their ability to cause systemic injury irrespective of pancreatitis. The end points relevant to human disease are summarized in lower panel of Figure 2 and more than one positive study showing that the agent can incite the endpoint is taken as positive.
Figure 2: Pathophysiology of systemic injury in AP:
The upper part of the figure describes the initiation of acinar injury by an AP etiology like high dose CCK (↑CCK) during caerulein pancreatitis. The intra-acinar signaling events include the increase in cytosolic calcium (Ca2+), which has a role in mitochondrial depolarization (Memb. Depol.) and cytochrome C leakage, along with activating NF-kB via dissociation and proteasomal degradation of IkB, nuclear translocation of p65. This upregulates inflammatory mediator synthesis, which include cytokines and chemokines, and thus leads to neutrophil infiltration into the pancreas. The trypsin generated due to impaired autophagy involving lysosomes (L) and zymogen granules (ZG) and increased oxidized glutathione (GSSG) [from its reduced form (GSH), due to reactive oxygen species (ROS)], along with concurrent deleterious mechanisms, cause acinar injury. These other mechanisms include the loss of apical microvilli, inhibition of apical secretion, the reorganization of F-actin, basolateral blebbing, release of DAMPs that can activate the inflammasome, and leakage of exocrine enzymes such as lipase, trypsin. The DAMPS can worsen local injury, and may also contribute to systemic injury. Similarly, cytokines can cause the leukocytosis associated with SIRS, which can enter the systemic circulation and are a part of systemic injury. The lower part of the figure describes the types of systemic injury that may occur due to these, along with the underlying mechanisms. The mechanisms include unregulated hydrolysis of adipocyte triglyceride (Adipo. TG) by pancreatic lipase, resulting in fat necrosis, which generates UFAs, that inhibit mitochondrial complex I and V, which decrease ATP and worsen local injury. The effects of UFAs, trypsin, cytokine entry into the systemic circulation, and leukocytosis (from left to right) on end points of systemic injury are mentioned in the table below, with a + indicating 2 or more reports citing the agent in causing the end point. Unclear or weaker evidence is shown as ± or a – respectively.
It is to be noted that there are numerous and parallel steps involved in acinar cell injury induced by a single agent like caerulein, which itself does not cause systemic injury. Therefore, the role of other important factors in amplifying the signaling to become deleterious on a systemic level is essential. A clue to such factors that worsen pancreatitis lies in several reports showing hypertriglyceridemic AP to have a higher grade of severity than is usually reported. 44-46 This may be related to the fatty acids that compose the triglyceride, and is discussed in detail under the section unsaturated fatty acids.
Distinction between markers, mediators and end-points of systemic injury:
It is important to appreciate the difference between markers vs. mediators vs. end points of systemic injury. Markers of systemic injury such as SIRS, or serum cytokines are distinct from the endpoints that determine the severity of systemic injury during pancreatitis. The clinical or biochemical clues do not themselves depict end organ injury, but may be markers or mediators of it. A marker in contrast to a mediator, when administered to an organism or when inhibited would not affect the endpoint of systemic injury. However, a mediator when administered would elicit an endpoint of systemic injury or when it is neutralized or inhibited the systemic injury would be ameliorated. As discussed previously, the end points of systemic injury during AP are renal, respiratory and cardiovascular failure.
Animal models and systemic injury in AP:
Although there are several AP models, it is important to realize their limitations in measuring systemic injury. Most AP models have a strong emphasis on local pancreatic injury in the context of specific initiators or etiologies like caerulein. Recently, clinically relevant risk factors such as obesity have been shown to result in systemic injury by modifying the course of AP in animal models.47-49 These studies have used clinically relevant end points such as renal failure. Thus, it is important to analyze whether the end points used in basic/ animal models equate to human disease. For example, a common end point of lung injury used in animal models is the accumulation of myeloid inflammatory cells in the lung, shown as increase in lung myeloperoxidase (MPO) activity. Since pulmonary inflammation can be protective (please see section on neutrophils), it is important to realize the limitations of lung MPO as a parameter of lung injury. More relevant end points may be pulmonary microvascular permeability studied as the leakage into the alveolar space of an intravenously administered fluorescently tagged macromolecule such as albumin50-52, the oxygen saturation as determined by pulse oximetry47, or dead cells in the alveolar space47-49, 53 which are relevant to pulmonary edema or adult respiratory distress syndrome (ARDS) in humans.54 Similarly, clinically relevant end points for renal failure in animal models include a sustained increase in serum blood urea nitrogen (BUN)48 like in sustained renal failure in humans.48, 55
While basic models typically cannot distinguish between primary and secondary organ failure due to their short course, we cover markers and potential mediators (Table 3) increased in early human AP independent of pancreatic necrosis, and where relevant distinguish these from those associated with infection in the following discussion.
Table 3:
Markers and mediators of systemic injury in acute pancreatit
| Markers and mediators of systemic injury |
| Released from acinar cells |
| Trypsin |
| Inflammatory cells and their products |
| Neutrophils |
| NET |
| Inflammasome |
| Cytokines |
| IL-6 |
| IL-1β |
| TNF-α |
| IL-12 |
| IL-18 |
| Adipokines |
| Resistin |
| Visfatin |
| Lipid mediators: |
| Unsaturated fatty acids |
| Platelet activating factor |
| DAMPs |
| HMGB1 |
| sRAGE |
| ds-DNA |
| Histones/ nucleosomes |
| S100 proteins |
| ATP |
| Extracellular matric (e.g. hyaluronan) |
Potential mediators of systemic injury in AP:
Trypsin:
Trypsin generation is ubiquitous in AP 56-59 in both rodents and humans56, 59. Being associated with the auto-digestive hypothesis of pancreatitis for over a hundred years60, trypsin has been an attractive target to reduce pancreatitis severity. The most direct proof of trypsin’s role as a mediator of systemic injury early on came from its intravenous (IV) infusion resulting in hypotension, shock61, 62 and coagulopathy, which is consistent with the coagulation cascade being a series of proteolytic steps. In support of this observation, elevated D-dimer; a fibrin degradation products at admission has been shown to predict development of OF with a sensitivity, specificity, positive and negative predictive values of 90%, 89%, 75% and 96% respectively63. Whether this coagulation cascade plays a role in splanchnic venous thrombosis, which is rare in the absence of necrosis, but occurs in about half of the patients with pancreatic necrosis64 remains unknown. Trypsin infusion also causes lung injury65 which is dependent on neutrophils (please see next section). More recent studies have identified the protease activated receptor-2 (PAR-2)66 to be regulated by trypsin during pancreatitis. The most direct evidence comes from hypotension resulting from IV infusion of PAR-2 agonists, possibly via PAR-2 receptors on endothelial cells 67. Whether trypsin actually plays a major role in systemic injury during clinical pancreatitis remains debated, since small molecule trypsin inhibitors have not shown conclusive benefit68-77 in improving systemic injury during AP and patients with hereditary pancreatitis due to trypsinogen gene mutations that result in its activation rarely develop systemic injury compared to other AP etiologies78. The interpretation of trypsin’s role is further complicated by circulating anti-proteases such as alpha-2 macroglobulin 79, 80 which can inactivate it, and trypsin’s inherent tendency to auto-inactivate. Whether trypsin is indeed an initiator/mediator of systemic severity during pancreatitis, therefore still remains inconclusive.
Neutrophils:
Leukocytosis (>12000/mm3) is part of the SIRS criteria81, and is an early predictor of severity of AP2. The leukocytosis in early pancreatitis is predominantly neutrophilic, and several studies have shown that depletion of neutrophils reduces inflammatory cell infiltration (e.g. reduced MPO) into the lungs and improves microvascular permeability52, 65, 82, 83. Neutrophil infiltration into an organ is dependent on their adhesion to the endothelium, mediated by P- and E-selectin on the surface of endothelial cells. These bind adhesion molecules like L-selectins and integrins on the surface of neutrophils. Blood levels of P- and E-selectin were elevated in rodent84 and in human85 AP. These respectively correlated with severity of AP and lung injury. Interestingly, trypsin generation in the late phase of experimental pancreatitis has been shown to be neutrophil dependent65, and trypsin can also stimulate neutrophils to secrete matrix metalloproteinase-9. Based on these observations, trypsin mediated lung injury has been hypothesized to be neutrophil dependent. Other studies have shown that neutrophil infiltration into the lungs is due to the chemokines CXCL2 and CXCL4, and that their neutralization reduces lung inflammation86. However, neutrophils also have physiologic roles and their accumulation in the lungs, such as by increasing KC/CXCL187-89 expression does not cause damage, but conversely protects from fungal and bacterial infections. Moreover, neutropenia predisposes to infections90-92. Therefore, it remains to be seen whether interference in neutrophil recruitment or their depletion during AP will improve systemic injury in human AP.
Neutrophil extracellular traps (NETs):
NETs are web like structures containing neutrophil granule proteins (e.g. myeloperoxidase, elastase) and chromatin. These are released by neutrophils and are increased in the sera of patients with severe pancreatitis93. In addition, they occlude pancreatic ducts in human AP and may perpetuate pancreatitis94. NET formation in pancreatitis is catalyzed by the enzyme Protein Arginine Deiminase 4 (PAD4)95, which causes histone modification of arginine residues to citrulline96. This modification weakens DNA- histone interactions and allows the neutrophils to expel the de-condensed chromatin. PAD4 inhibition reduces NET formation in humans97 and rodents. Whether inhibition of NET formation will prove to be beneficial in reducing systemic injury in pancreatitis remains to be seen, more so since recent studies also show NET formation to wall off pancreatic necrosis from viable tissue in humans, and thus may play a role as a protective barrier to the progression of pancreatitis98.
Damage associated Molecular patterns (DAMPs):
DAMPs, which are released from dying cells during necrosis correlate with human AP severity 99-102, and are potential mediators of severity based on animal studies103,104 DAMPs include small molecules such ATP, proteins- including S100 proteins, the soluble receptor for advanced glycation end products (sRAGE), and high-mobility group box 1 (HMGB1), nuclear components (e.g. histones, DNA, nucleosomes), and molecules released from the extracellular matrix such as hyaluronic acid. Serum HMGB1 significantly correlated with AP severity in humans in a meta-analysis105. DAMPs can worsen inflammation by causing activation of the inflammasome103, 106, as shown for sRAGE and HMGB1, and also directly via inducing a sterile inflammatory response107 by disrupting the plasmalemma108, and further increasing DAMP release (e.g. HMGB1104). However, it remains to be confirmed if DAMPs alone can induce the clinically relevant end points of OF or their inhibition during pancreatitis models, which induce OF, averts these end points
Inflammasome:
The inflammasome109 is expressed in myeloid cells and has been implicated in systemic inflammation during pancreatitis 103, 110 Inflammasome activation results in production of IL-1β111, IL-18112-114 and HMGB-1104, 115, all of which are associated with systemic injury during pancreatitis. Activation of the inflammasome is thought to be downstream of various receptors, some of which may be relevant to pancreatitis. These include nucleosomes (i.e. DNA-histone complexes), dsDNA and RAGE activating the AIM2 (absent in melanoma 2) inflammasome110, 116 and extracellular ATP or NAD released from injured acinar cells activating the P2X7 receptor103. Additionally, cell surface pattern recognition receptors including TLR 4 and TLR9 may be activated by these DAMPs103. Interfering with nucleosomes activating the inflammasome or inhibiting RAGE signaling has been shown to reduce the severity of L-arginine and caerulein pancreatitis104, 116 including lung inflammation. Interestingly, antagonism of TLR9 reduced lung inflammation, but not edema103. Similarly while IL-1β increased lung inflammation, it did not induce lung injury47. Additionally while IL-18 (in combination with IL-12) induces pancreatitis117, the systemic severity of pancreatitis these agents induce and the associated mortality are largely dependent on an increase in visceral fat118 and its lipolysis to fatty acids53. In the absence of known genetic polymorphisms that govern inflammasome activation in pancreatitis, it is unclear how inflammasome activation variably affects the severity of pancreatitis in different individuals. This is further highlighted by early OF in pancreatitis occurring in the absence of extensive pancreatic necrosis and thus having a smaller source of inflammasome activators such as dsDNA or nucleosomes than later in AP, when necrosis is progressing. Future studies are needed to clarify the role of the inflammasome in systemic injury during pancreatitis.
Adipokines and Cytokines:
Previous clinical studies have shown that severe AP is associated with elevated serum levels of adipokines including resistin and visfatin 119, 120, and cytokines including IL-6 121-124, IL-β111, IL-8121, 124, 125, MCP-1126, TNF-α,122. Serum cytokines are part of the criteria for severity stratification 111, 121-124, 126-128. In a prospective study including 108 patients, IL-6 was one of the best discriminators between mild and severe AP129. Another study showed that blood levels of IL-6 correlated with OF and mortality. At a cut-off value of 122 pg/mL on day 3, IL-6 predicted OF and severe pancreatitis with a sensitivity and specificity of 81.8% and 77.7%, respectively37. While the increase in their levels is associated with worse local and systemic complications, the evidence to support them as potential mediators of clinically relevant end points of OF in pancreatitis is so far lacking. For example, while IL-1β induces fever and myeloid infiltration into the lungs, it does not induce respiratory failure47. Neutrophil infiltration mediated by cytokines such as IL-8, CXCL1 has been shown to protect from lung infections 88, 89,130 Similarly, IL-6 infusion in humans inhibited endotoxin induced TNF-α increase131 and its long term infusion led to hypoferremia and anemia132, 133, but not systemic injury. IL-6134 and TNF-α135 have also been shown to have a protective role in AP, and neither these or other cytokines have been shown to induce OF136-140. Therefore, targeting these cytokine alone may not improve outcomes in severe AP.
Coagulation pathway:
Vascular injury is an integral part of pancreatic inflammation and systemic injury. Endothelial activation, injury, increased vascular permeability, activation of coagulation, and increased leukocyte rolling, sticking and transmigration to pancreatic tissue have been demonstrated in AP. The inflammatory process and proteases like trypsin may activate the coagulation system leading to microvascular thrombosis. Whether this coagulation cascade contributes to systemic injury is unknown. As mentioned before, portal or splenic vein thrombosis can occur in about half of the patients with pancreatic necrosis64. Similarly while both anti-thrombin III and heparin have been shown to reduce the severity of AP in animal models, the clinical implications of this on systemic severity are unknown141.
Lipid mediators:
Platelet activating factor (PAF):
Apart from trypsin, PAF has been extensively targeted in pancreatitis. PAF is a phospholipid (acetyl-glyceryl-ether-phosphorylcholine) produced by myeloid cells, platelets and endothelial cells. Intra-arterial delivery of PAFs into the pancreas causes AP142. PAF production in inflammation is mediated by phospholipase A2, and it is degraded by PAF acetylhydrolase 143. Its receptor is a G-protein receptor144 . PAF has a broad range of effects including increasing vascular permeability, worsening inflammation and initiating cell death145. Its levels are increased in severe biliary pancreatitis146 in which it was thought to mediate shock and acute lung injury147, and the protective effect of antagonizing it was shown in multiple models including biliary, choline deficient ethionine supplemented diet (CDE diet)148, caerulein model in rats, and severe biliary pancreatitis in opossums51. While initial clinical trials using lexipafant, a PAF antagonist were promising149, the large definitive clinical trial did not show benefit in OF or mortality, even though local complications and sepsis were reduced150. Whether this was due to a limitation of targeting PAF or the high prevalence of early OF remains to be studied (please see below).
Unsaturated fatty acids (UFAs):
The pancreas by its location is proximal to visceral fat in humans. Several studies report an increased risk of severe pancreatitis to be associated with an increase in visceral fat 151-154 which ranges from 1-10% of body weight 155. This fat is composed of adipocytes, the mass of which is predominantly (>80%) triglyceride156-158. This triglyceride is predominantly composed of UFAs159, 160, covalently linked to a glycerol backbone, which when released by unregulated lipolysis affect severity of AP. Interestingly, adipocyte triglyceride has become enriched in UFAs like linoleic acid over the last few decades161, which mirrors the 15-25% linoleic acid composition of necrosectomy samples from severe AP patients47, 53. Previous studies have shown pancreatic lipases to be present in the adipocytes, damaged during AP162. This results in a morphology known as fat necrosis, which can worsen pancreatic parenchymal necrosis49, 53, but can also occur independently 163, 164. This lipolytic fatty acid generation can increase systemic injury during pancreatitis, in parallel with an increase in serum UFAs such as linoleic and arachidonic acid165, 166, which like visceral fat159,47, 48, 53, 160are unsaturated. These UFAs when liberated in excess, inhibit mitochondrial complexes I and V53, and increase apoptotic cells in the lungs17, 48, 49, 53 (similar to patients with acute respiratory distress syndrome54, 167, 168), elevate serum BUN47, 49, 53, 169 due to renal tubular injury , and result in mortality. Interestingly, elevated serum levels of TNF-α, IL-1β, MCP-1 and IL-18 can all be induced during this fatty acid toxicity, perhaps due to the widespread release of DAMPs. Importantly, inhibition of this excessive lipolysis can result in prevention of systemic injury, hypercytokinemia and mortality47-49, 53. While it remains to be seen if lipase inhibition will reduce systemic injury during human AP, it is encouraging to note that the cyclooxygenase inhibitor indomethacin, which is known to affect the metabolism of UFA products, may reduce progression of moderate-severe AP.170.
Role of Intestine in systemic injury:
The small intestine and colon may contribute to bacterial translocation and infected necrosis due to its close proximity to the pancreas, and thus to the pathophysiology of systemic inflammation. Another role may be played by mesenteric lymph from the GI tract. In an experimental study, intravenous administration of mesenteric lymph from rats with intestinal ischemia exacerbated pancreatic microcirculatory disturbances and worsened the severity of pancreatitis in the recipient rats.171 Similarly, profound vascular and coagulation changes can lead to ischemia of the pancreas172-174 and bowel175, 176. A lower gastric pH which correlated with a higher mortality in AP also supported the hypothesis of splanchnic ischemia177. Additionally, Ischemia-reperfusion can cause oxidative stress178. The major effect of such perturbations is gut barrier dysfunction with increased intestinal permeability179, as supported by human studies180 including a meta-analysis of 18 studies, which showed a pooled prevalence of gut barrier dysfunction of 59%181. However, parallel clinical observations question the gut as being the sole player. These include patients with severe ulcerative colitis having a very low prevalence of sepsis182 despite severe colonic ulcers being exposed to fecal matter for long periods. Similarly, while patients with fistulizing Crohn’s disease do develop abdominal abscesses, the prevalence of sepsis remains low182. The translocation hypothesis as being the sole reason for developing infected necrosis is further challenged by the fact that bacterial translocation is common after dental183 and endoscopic184 procedures but is transient. Thus, whether translocation of bacteria is the sole reason for infections of (peri) pancreatic necrotic tissue and collections, and the sepsis that ensues remains to be determined.
Mechanisms of Organ Dysfunction due to systemic perturbations – Lessons from Sepsis induced Organ Failure:
Mitochondria are at the center of cellular perturbations from tissue hypo-perfusion, which may result from hypovolemia, hypotension, and microvascular thrombi causing cellular hypoxia. Since mitochondrial energy production is oxygen dependent, their ability to generate ATP is severely compromised in such states, and can result in cellular dysfunction. OF may primarily be due to such mitochondrial dysfunction rather than cell death.185 The following observations support this hypothesis: (i) Cellular injury and death is minimal in post-mortem examination of failed organs,186 (ii) functional recovery of the organ is swift once the underlying pathophysiological perturbations reverse, and (iii) mitochondrial structural and functional changes have been documented in such patients. These observations are important from the point of prognosis and identifying targets for therapy.
Treatment of Organ Failure:
Treatment for OF is largely supportive. Patients with predicted severe AP should be referred to a tertiary care center with intensive care unit (ICU) facility. Patients with OF must be managed in an ICU and often require organ support such as dialysis, mechanical ventilation, and vasopressors.
Fluid and electrolyte balance is critical in the beginning of the illness. Ringer’s lactate has been shown to be better than normal saline in reducing systemic inflammation.187, 188 Optimal amount of fluid administration remains a challenge.189 Both under-and aggressive hydration can be detrimental. A randomized trial showed worsening of OF in patients given aggressive fluid therapy190. On the contrary, relative hypovolemia due to under correction of fluid deficit can lead to increased risk of necrosis. A recent trial showed benefit of aggressive fluid administration in mild AP.190 But the results cannot be extrapolated to patients with severe AP because systemic events leading to OF develop rapidly and overzealous fluid therapy may exacerbate the clinical condition in those with impending respiratory and renal failure. One of the important points to consider is that normal homeostatic mechanisms are disturbed in patients with systemic injury due to abnormalities such as increased vascular permeability and thus the capability to deal with extra fluid being infused is compromised unlike in patients with mild AP.
Enteral nutrition should be instituted as soon as possible and has been shown to reduce the length of hospital stay and possibly the risk of infected necrosis.191 A multidisciplinary team comprising of a critical care expert, gastroenterologist, intervention radiologist and surgeon should look after the patient.
Specific therapy for Organ Failure:
Since the systemic injury is a result of dysregulated and out of proportion systemic inflammation in response to the local injury, specific treatment aimed at putative critical pathways has been tried. As mentioned above, PAF antagonist, Lexipafant, failed in a randomized controlled trial (RCT) involving 290 patients with predicted severe AP having an APACHE II score of >6. However, the treatment failed to provide any therapeutic benefit mainly because the primary hypothesis was invalidated by the unexpected finding that 44% of patients had OF on entry and only 14% patients developed new OF.150 Intravenous antioxidants too failed in a RCT of 43 patients, the primary endpoint i.e. OF developed in 32% and 17% in antioxidant and placebo groups respectively.192 Probiotics were tried with an aim to prevent gut derived infection, but unexpectedly increased mortality leading to premature termination of the trial.193 TNF-α has been implicated as an important cytokine mediating systemic inflammation. In a proof of concept small study involving 28 patients with predicted severe AP, Pentoxifyllin, an oral TNF-α antagonist, resulted in significantly fewer ICU admissions and shorter hospital stay.194 A larger trial is currently in progress. Infliximab, a TNF-α antagonist, is being tested in a RCT involving patients with AP of all grades of severity (www.isrctn.com/ISRCTN16935761).
Unmet needs and potential areas for future research:
Organ failure remains the proverbial Achilles heel of managing patients with severe AP. There is no specific therapy available either to treat or prevent the development of OF. The success of anticytokine therapy in chronic diseases such as inflammatory bowel disease, psoriasis and rheumatoid arthritis195, 196 may not translate to AP. Future approaches include targeting intermediary signaling such as the increase in cytosolic calcium using ORAI1 inhibitors197. The benefits of this approach remain to be seen in human AP, since ORAI1 is involved in innate immunity as well198. Similarly, NF-kB has been shown to play a critical role in AP 199-201. Pancreas-specific truncation of its trans-activating unit (RelA/p65) worsens pancreatic injury and lung inflammation. It would, therefore be important to know whether clinically approved agents such as Bortezomib 202 that interfere with NF-kB signaling affect the course of human AP. Some other exciting potential targets include UFAs, lipase, DAMPs, inflammasome, and kynurenine203, which remain to be tested in human studies.
Acknowledgments
Funding: VPS was supported by DK092460, DK100358 (NIH) and the PR151612 by the department of Army (DOA). PKG received funding from Indian Council of Medical Research for pancreatitis related research projects.
Biography


Footnotes
Conflict of interest: None of the authors have any conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Pramod K. Garg, Department of Gastroenterology, All India Institute of Medical Sciences, New Delhi, India..
Vijay P. Singh, Department of Medicine, Mayo Clinic, Scottsdale, Arizona, USA.
References:
- 1.Xiao AY, Tan ML, Wu LM, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol 2016;1:45–55. [DOI] [PubMed] [Google Scholar]
- 2.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102–11. [DOI] [PubMed] [Google Scholar]
- 3.Garg PK, Madan K, Pande GK, et al. Association of extent and infection of pancreatic necrosis with organ failure and death in acute necrotizing pancreatitis. Clin Gastroenterol Hepatol 2005;3:159–66. [DOI] [PubMed] [Google Scholar]
- 4.Johnson CD, Abu-Hilal M. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut 2004;53:1340–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mofidi R, Duff MD, Wigmore SJ, et al. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. The British journal of surgery 2006;93:738–44. [DOI] [PubMed] [Google Scholar]
- 6.Shen HN, Lu CL. Incidence, resource use, and outcome of acute pancreatitis with/without intensive care: a nationwide population-based study in Taiwan. Pancreas 2011;40:10–5. [DOI] [PubMed] [Google Scholar]
- 7.Hamada S, Masamune A, Kikuta K, et al. Nationwide epidemiological survey of acute pancreatitis in Japan. Pancreas 2014;43:1244–8. [DOI] [PubMed] [Google Scholar]
- 8.Kamal A, Sinha A, Hutfless SM, et al. Hospital admission volume does not impact the in-hospital mortality of acute pancreatitis. HPB (Oxford) 2017;19:21–28. [DOI] [PubMed] [Google Scholar]
- 9.Padhan RK, Jain S, Agarwal S, et al. Primary and Secondary Organ Failures Cause Mortality Differentially in Acute Pancreatitis and Should be Distinguished. Pancreas 2018;47:302–307. [DOI] [PubMed] [Google Scholar]
- 10.Schepers NJ, Bakker OJ, Besselink MG, et al. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut 2018. [DOI] [PubMed] [Google Scholar]
- 11.Lowenfels AB, Maisonneuve P, Sullivan T. The changing character of acute pancreatitis: epidemiology, etiology, and prognosis. Curr Gastroenterol Rep 2009;11:97–103. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho JR, Fernandes SR, Santos P, et al. Acute pancreatitis in the elderly: a cause for increased concern? Eur J Gastroenterol Hepatol 2018;30:337–341. [DOI] [PubMed] [Google Scholar]
- 13.Sternby H, Bolado F, Canaval-Zuleta HJ, et al. Determinants of Severity in Acute Pancreatitis: A Nation-wide Multicenter Prospective Cohort Study. Ann Surg 2018. [DOI] [PubMed] [Google Scholar]
- 14.Krishna SG, Hinton A, Oza V, et al. Morbid Obesity Is Associated With Adverse Clinical Outcomes in Acute Pancreatitis: A Propensity-Matched Study. Am J Gastroenterol 2015;110:1608–19. [DOI] [PubMed] [Google Scholar]
- 15.Yoon SB, Choi MH, Lee IS, et al. Impact of body fat and muscle distribution on severity of acute pancreatitis. Pancreatology 2017;17:188–193. [DOI] [PubMed] [Google Scholar]
- 16.Navina S, Acharya C, DeLany JP, et al. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med 2011;3:107ra110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noel P, Patel K, Durgampudi C, et al. Peripancreatic fat necrosis worsens acute pancreatitis independent of pancreatic necrosis via unsaturated fatty acids increased in human pancreatic necrosis collections. Gut 2016;65:100–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nawaz H, Koutroumpakis E, Easler J, et al. Elevated serum triglycerides are independently associated with persistent organ failure in acute pancreatitis. Am J Gastroenterol 2015;110:1497–503. [DOI] [PubMed] [Google Scholar]
- 19.Isenmann R, Rau B, Beger HG. Early severe acute pancreatitis: characteristics of a new subgroup. Pancreas 2001;22:274–8. [DOI] [PubMed] [Google Scholar]
- 20.Tenner S, Sica G, Hughes M, et al. Relationship of necrosis to organ failure in severe acute pancreatitis. Gastroenterology 1997;113:899–903. [DOI] [PubMed] [Google Scholar]
- 21.Lankisch PG, Pflichthofer D, Lehnick D. No strict correlation between necrosis and organ failure in acute pancreatitis. Pancreas 2000;20:319–22. [DOI] [PubMed] [Google Scholar]
- 22.Isenmann R, Rau B, Beger HG. Bacterial infection and extent of necrosis are determinants of organ failure in patients with acute necrotizing pancreatitis. Br J Surg 1999;86:1020–4. [DOI] [PubMed] [Google Scholar]
- 23.Singh P, Garg PK. Pathophysiological mechanisms in acute pancreatitis: Current understanding. Indian J Gastroenterol 2016;35:153–66. [DOI] [PubMed] [Google Scholar]
- 24.Mole DJ, McClymont KL, Lau S, et al. Discrepancy between the extent of pancreatic necrosis and multiple organ failure score in severe acute pancreatitis. World J Surg 2009;33:2427–32. [DOI] [PubMed] [Google Scholar]
- 25.Sah RP, Garg P, Saluja AK. Pathogenic mechanisms of acute pancreatitis. Curr Opin Gastroenterol 2012;28:507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de-Madaria E, Martinez J, Sempere L, et al. Cytokine genotypes in acute pancreatitis: association with etiology, severity, and cytokine levels in blood. Pancreas 2008;37:295–301. [DOI] [PubMed] [Google Scholar]
- 27.Bishehsari F, Sharma A, Stello K, et al. TNF-alpha gene (TNFA) variants increase risk for multi-organ dysfunction syndrome (MODS) in acute pancreatitis. Pancreatology 2012;12:113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Z, Qi X, Wu Q, et al. Lack of association between TNF-alpha gene promoter polymorphisms and pancreatitis: a meta-analysis. Gene 2012;503:229–34. [DOI] [PubMed] [Google Scholar]
- 29.Chen WC, Nie JS. Genetic polymorphism of MCP-1-2518, IL-8-251 and susceptibility to acute pancreatitis: a pilot study in population of Suzhou, China. World J Gastroenterol 2008;14:5744–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papachristou GI, Sass DA, Avula H, et al. Is the monocyte chemotactic protein-1 −2518 G allele a risk factor for severe acute pancreatitis? Clin Gastroenterol Hepatol 2005;3:475–81. [DOI] [PubMed] [Google Scholar]
- 31.Sharma M, Banerjee D, Garg PK. Characterization of newer subgroups of fulminant and subfulminant pancreatitis associated with a high early mortality. Am J Gastroenterol 2007;102:2688–95. [DOI] [PubMed] [Google Scholar]
- 32.Wig JD, Bharathy KG, Kochhar R, et al. Correlates of organ failure in severe acute pancreatitis. JOP 2009;10:271–5. [PubMed] [Google Scholar]
- 33.Mole DJ, Olabi B, Robinson V, et al. Incidence of individual organ dysfunction in fatal acute pancreatitis: analysis of 1024 death records. HPB (Oxford) 2009;11:166–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chandra S, Murali A, Bansal R, et al. The Bedside Index for Severity in Acute Pancreatitis: a systematic review of prospective studies to determine predictive performance. J Community Hosp Intern Med Perspect 2017;7:208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu BU, Johannes RS, Sun X, et al. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008;57:1698–703. [DOI] [PubMed] [Google Scholar]
- 36.Parniczky A, Kui B, Szentesi A, et al. Prospective, Multicentre, Nationwide Clinical Data from 600 Cases of Acute Pancreatitis. PLoS One 2016;11:e0165309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sathyanarayan G, Garg PK, Prasad H, et al. Elevated level of interleukin-6 predicts organ failure and severe disease in patients with acute pancreatitis. J Gastroenterol Hepatol 2007;22:550–4. [DOI] [PubMed] [Google Scholar]
- 38.Mofidi R, Duff MD, Wigmore SJ, et al. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg 2006;93:738–44. [DOI] [PubMed] [Google Scholar]
- 39.Singh VK, Wu BU, Bollen TL, et al. Early systemic inflammatory response syndrome is associated with severe acute pancreatitis. Clin Gastroenterol Hepatol 2009;7:1247–51. [DOI] [PubMed] [Google Scholar]
- 40.Jain S, Midha S, Mahapatra SJ, et al. Interleukin-6 significantly improves predictive value of systemic inflammatory response syndrome for predicting severe acute pancreatitis. Pancreatology 2018. [DOI] [PubMed] [Google Scholar]
- 41.Garret C, Peron M, Reignier J, et al. Risk factors and outcomes of infected pancreatic necrosis: Retrospective cohort of 148 patients admitted to the ICU for acute pancreatitis. United European Gastroenterol J 2018;6:910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thandassery RB, Yadav TD, Dutta U, et al. Hypotension in the first week of acute pancreatitis and APACHE II score predict development of infected pancreatic necrosis. Dig Dis Sci 2015;60:537–42. [DOI] [PubMed] [Google Scholar]
- 43.Petrov MS, Shanbhag S, Chakraborty M, et al. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010;139:813–20. [DOI] [PubMed] [Google Scholar]
- 44.Deng LH, Xue P, Xia Q, et al. Effect of admission hypertriglyceridemia on the episodes of severe acute pancreatitis. World J Gastroenterol 2008;14:4558–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lloret Linares C, Pelletier AL, Czernichow S, et al. Acute pancreatitis in a cohort of 129 patients referred for severe hypertriglyceridemia. Pancreas 2008;37:13–2. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Sternfeld L, Yang F, et al. Enhanced susceptibility to pancreatitis in severe hypertriglyceridaemic lipoprotein lipase-deficient mice and agonist-like function of pancreatic lipase in pancreatic cells. Gut 2009;58:422–30. [DOI] [PubMed] [Google Scholar]
- 47.Noel P, Patel K, Durgampudi C, et al. Peripancreatic fat necrosis worsens acute pancreatitis independent of pancreatic necrosis via unsaturated fatty acids increased in human pancreatic necrosis collections. Gut 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel K, Trivedi RN, Durgampudi C, et al. Lipolysis of visceral adipocyte triglyceride by pancreatic lipases converts mild acute pancreatitis to severe pancreatitis independent of necrosis and inflammation. The American journal of pathology 2015;185:808–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Durgampudi C, Noel P, Patel K, et al. Acute Lipotoxicity Regulates Severity of Biliary Acute Pancreatitis without Affecting Its Initiation. The American journal of pathology 2014;184:1773–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song AM, Bhagat L, Singh VP, et al. Inhibition of cyclooxygenase-2 ameliorates the severity of pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol 2002;283:G1166–74. [DOI] [PubMed] [Google Scholar]
- 51.Hofbauer B, Saluja AK, Bhatia M, et al. Effect of recombinant platelet-activating factor acetylhydrolase on two models of experimental acute pancreatitis. Gastroenterology 1998;115:1238–47. [DOI] [PubMed] [Google Scholar]
- 52.Bhatia M, Saluja AK, Hofbauer B, et al. The effects of neutrophil depletion on a completely noninvasive model of acute pancreatitis-associated lung injury. Int J Pancreatol 1998;24:77–83. [DOI] [PubMed] [Google Scholar]
- 53.Navina S, Acharya C, DeLany JP, et al. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Science translational medicine 2011;3:107ra110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol 2011;6:147–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu BU, Johannes RS, Sun X, et al. Early changes in blood urea nitrogen predict mortality in acute pancreatitis. Gastroenterology 2009;137:129–35. [DOI] [PubMed] [Google Scholar]
- 56.Geokas MC, Rinderknecht H, Brodrick JW, et al. Studies on the ascites fluid of acute pancreatitis in man. Am J Dig Dis 1978;23:182–8. [DOI] [PubMed] [Google Scholar]
- 57.Buchler M, Malfertheiner P, Uhl W, et al. [Gabexate mesilate in the therapy of acute pancreatitis. Multicenter study of tolerance of a high intravenous dose (4 g/day)]. Medizinische Klinik 1988;83:320–4, 352. [PubMed] [Google Scholar]
- 58.Berling R, Borgstrom A, Ohlsson K. Peritoneal lavage with aprotinin in patients with severe acute pancreatitis. Effects on plasma and peritoneal levels of trypsin and leukocyte proteases and their major inhibitors. Int J Pancreatol 1998;24:9–17. [DOI] [PubMed] [Google Scholar]
- 59.Renner IG, Rinderknecht H, Douglas AP. Profiles of pure pancreatic secretions in patients with acute pancreatitis: the possible role of proteolytic enzymes in pathogenesis. Gastroenterology 1978;75:1090–8. [PubMed] [Google Scholar]
- 60.Chiari H Ueber Selbstverdauung des menschlichen Pankreas. Zeitschrift für Heilkunde 1896;17:69–96. [Google Scholar]
- 61.Jobling JW, Petersen W, Eggstein AA. Serum Ferments and Antiferment during Trypsin Shock : Studies on Ferment Action. Xxii. J Exp Med 1915;22:141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tagnon HJ. The Nature of the Mechanism of the Shock Produced by the Injection of Trypsin and Thrombin. J Clin Invest 1945;24:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Radenkovic D, Bajec D, Ivancevic N, et al. D-dimer in acute pancreatitis: a new approach for an early assessment of organ failure. Pancreas 2009;38:655–60. [DOI] [PubMed] [Google Scholar]
- 64.Easler J, Muddana V, Furlan A, et al. Portosplenomesenteric venous thrombosis in patients with acute pancreatitis is associated with pancreatic necrosis and usually has a benign course. Clin Gastroenterol Hepatol 2014;12:854–62. [DOI] [PubMed] [Google Scholar]
- 65.Hartwig W, Werner J, Jimenez RE, et al. Trypsin and activation of circulating trypsinogen contribute to pancreatitis-associated lung injury. Am J Physiol 1999;277:G1008–16. [DOI] [PubMed] [Google Scholar]
- 66.Singh VP, Bhagat L, Navina S, et al. Protease-activated receptor-2 protects against pancreatitis by stimulating exocrine secretion. Gut 2007;56:958–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Namkung W, Han W, Luo X, et al. Protease-activated receptor 2 exerts local protection and mediates some systemic complications in acute pancreatitis. Gastroenterology 2004;126:1844–59. [DOI] [PubMed] [Google Scholar]
- 68.Andriulli A, Caruso N, Quitadamo M, et al. Antisecretory vs. antiproteasic drugs in the prevention of post-ERCP pancreatitis: the evidence-based medicine derived from a meta-analysis study. JOP : Journal of the pancreas 2003;4:41–8. [PubMed] [Google Scholar]
- 69.Andriulli A, Leandro G, Clemente R, et al. Meta-analysis of somatostatin, octreotide and gabexate mesilate in the therapy of acute pancreatitis. Alimentary pharmacology & therapeutics 1998;12:237–45. [DOI] [PubMed] [Google Scholar]
- 70.Asang E [Changes in the therapy of inflammatory diseases of the pancreas. A report on 1 year of therapy and prophylaxis with the kallikrein- and trypsin inactivator trasylol (Bayer)]. Langenbecks Arch Klin Chir Ver Dtsch Z Chir 1960;293:645–70. [PubMed] [Google Scholar]
- 71.Buchler M, Malfertheiner P, Uhl W, et al. Gabexate mesilate in human acute pancreatitis. German Pancreatitis Study Group. Gastroenterology 1993;104:1165–70. [DOI] [PubMed] [Google Scholar]
- 72.Chen HM, Chen JC, Hwang TL, et al. Prospective and randomized study of gabexate mesilate for the treatment of severe acute pancreatitis with organ dysfunction. Hepato-gastroenterology 2000;47:1147–50. [PubMed] [Google Scholar]
- 73.Park KT, Kang DH, Choi CW, et al. Is high-dose nafamostat mesilate effective for the prevention of post-ERCP pancreatitis, especially in high-risk patients? Pancreas 2011;40:1215–9. [DOI] [PubMed] [Google Scholar]
- 74.Seta T, Noguchi Y, Shimada T, et al. Treatment of acute pancreatitis with protease inhibitors: a meta-analysis. Eur J Gastroenterol Hepatol 2004;16:1287–93. [DOI] [PubMed] [Google Scholar]
- 75.Trapnell JE, Rigby CC, Talbot CH, et al. Proceedings: Aprotinin in the treatment of acute pancreatitis. Gut 1973;14:828. [PubMed] [Google Scholar]
- 76.Trapnell JE, Rigby CC, Talbot CH, et al. A controlled trial of Trasylol in the treatment of acute pancreatitis. The British journal of surgery 1974;61:177–82. [DOI] [PubMed] [Google Scholar]
- 77.Trapnell JE, Talbot CH, Capper WM. Trasylol in acute pancreatitis. The American journal of digestive diseases 1967;12:409–12. [DOI] [PubMed] [Google Scholar]
- 78.Rebours V, Boutron-Ruault MC, Jooste V, et al. Mortality rate and risk factors in patients with hereditary pancreatitis: uni- and multidimensional analyses. The American journal of gastroenterology 2009;104:2312–7. [DOI] [PubMed] [Google Scholar]
- 79.Nakae Y, Hayakawa T, Kondo T, et al. Serum alpha 2-macroglobulin-trypsin complex and early recognition of severe acute pancreatitis after endoscopic retrograde pancreatography. J Gastroenterol Hepatol 1994;9:272–6. [DOI] [PubMed] [Google Scholar]
- 80.McMahon MJ, Bowen M, Mayer AD, et al. Relation of alpha 2-macroglobulin and other antiproteases to the clinical features of acute pancreatitis. Am J Surg 1984;147:164–70. [DOI] [PubMed] [Google Scholar]
- 81.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101:1644–55. [DOI] [PubMed] [Google Scholar]
- 82.Keck T, Balcom JHt, Fernandez-del Castillo C, et al. Matrix metalloproteinase-9 promotes neutrophil migration and alveolar capillary leakage in pancreatitis-associated lung injury in the rat. Gastroenterology 2002;122:188–201. [DOI] [PubMed] [Google Scholar]
- 83.Inoue S, Nakao A, Kishimoto W, et al. Anti-neutrophil antibody attenuates the severity of acute lung injury in rats with experimental acute pancreatitis. Arch Surg 1995;130:93–8. [DOI] [PubMed] [Google Scholar]
- 84.Telek G, Ducroc R, Scoazec JY, et al. Differential upregulation of cellular adhesion molecules at the sites of oxidative stress in experimental acute pancreatitis. J Surg Res 2001;96:56–67. [DOI] [PubMed] [Google Scholar]
- 85.Powell JJ, Siriwardena AK, Fearon KC, et al. Endothelial-derived selectins in the development of organ dysfunction in acute pancreatitis. Crit Care Med 2001;29:567–72. [DOI] [PubMed] [Google Scholar]
- 86.Wetterholm E, Linders J, Merza M, et al. Platelet-derived CXCL4 regulates neutrophil infiltration and tissue damage in severe acute pancreatitis. Transl Res 2016;176:105–18. [DOI] [PubMed] [Google Scholar]
- 87.Lira SA. Genetic approaches to study chemokine function. Journal of leukocyte biology 1996;59:45–52. [DOI] [PubMed] [Google Scholar]
- 88.Mehrad B, Wiekowski M, Morrison BE, et al. Transient lung-specific expression of the chemokine KC improves outcome in invasive aspergillosis. American journal of respiratory and critical care medicine 2002;166:1263–8. [DOI] [PubMed] [Google Scholar]
- 89.Tsai WC, Strieter RM, Wilkowski JM, et al. Lung-specific transgenic expression of KC enhances resistance to Klebsiella pneumoniae in mice. Journal of immunology 1998;161:2435–40. [PubMed] [Google Scholar]
- 90.Cryz SJ Jr., Furer E, Germanier R. Simple model for the study of Pseudomonas aeruginosa infections in leukopenic mice. Infect Immun 1983;39:1067–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Uchida K, Yamamoto Y, Klein TW, et al. Granulocyte-colony stimulating factor facilitates the restoration of resistance to opportunistic fungi in leukopenic mice. J Med Vet Mycol 1992;30:293–300. [DOI] [PubMed] [Google Scholar]
- 92.Wang E, Simard M, Ouellet N, et al. Pathogenesis of pneumococcal pneumonia in cyclophosphamide-induced leukopenia in mice. Infect Immun 2002;70:4226–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Merza M, Hartman H, Rahman M, et al. Neutrophil Extracellular Traps Induce Trypsin Activation, Inflammation, and Tissue Damage in Mice With Severe Acute Pancreatitis. Gastroenterology 2015;149:1920–1931 e8. [DOI] [PubMed] [Google Scholar]
- 94.Leppkes M, Maueroder C, Hirth S, et al. Externalized decondensed neutrophil chromatin occludes pancreatic ducts and drives pancreatitis. Nat Commun 2016;7:10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bicker KL, Thompson PR. The protein arginine deiminases: Structure, function, inhibition, and disease. Biopolymers 2013;99:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Y, Li M, Stadler S, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 2009;184:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lewis HD, Liddle J, Coote JE, et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol 2015;11:189–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bilyy R, Fedorov V, Vovk V, et al. Neutrophil Extracellular Traps Form a Barrier between Necrotic and Viable Areas in Acute Abdominal Inflammation. Front Immunol 2016;7:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Penttila AK, Rouhiainen A, Kylanpaa L, et al. Circulating nucleosomes as predictive markers of severe acute pancreatitis. J Intensive Care 2016;4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yasuda T, Ueda T, Takeyama Y, et al. Significant increase of serum high-mobility group box chromosomal protein 1 levels in patients with severe acute pancreatitis. Pancreas 2006;33:359–63. [DOI] [PubMed] [Google Scholar]
- 101.Lindstrom O, Tukiainen E, Kylanpaa L, et al. Circulating levels of a soluble form of receptor for advanced glycation end products and high-mobility group box chromosomal protein 1 in patients with acute pancreatitis. Pancreas 2009;38:e215–20. [DOI] [PubMed] [Google Scholar]
- 102.Liu T, Huang W, Szatmary P, et al. Accuracy of circulating histones in predicting persistent organ failure and mortality in patients with acute pancreatitis. Br J Surg 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hoque R, Sohail M, Malik A, et al. TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis. Gastroenterology 2011;141:358–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kang R, Zhang Q, Hou W, et al. Intracellular Hmgb1 inhibits inflammatory nucleosome release and limits acute pancreatitis in mice. Gastroenterology 2014;146:1097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lin Y, Lin LJ, Jin Y, et al. Correlation between serum levels of high mobility group box-1 protein and pancreatitis: a meta-analysis. Biomed Res Int 2015;2015:430185. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 106.Hoque R, Mehal WZ. Inflammasomes in pancreatic physiology and disease. Am J Physiol Gastrointest Liver Physiol 2015;308:G643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hoque R, Malik AF, Gorelick F, et al. Sterile inflammatory response in acute pancreatitis. Pancreas 2012;41:353–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Szatmary P, Liu T, Abrams ST, et al. Systemic histone release disrupts plasmalemma and contributes to necrosis in acute pancreatitis. Pancreatology 2017;17:884–892. [DOI] [PubMed] [Google Scholar]
- 109.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 2002;10:417–26. [DOI] [PubMed] [Google Scholar]
- 110.Algaba-Chueca F, de-Madaria E, Lozano-Ruiz B, et al. The expression and activation of the AIM2 inflammasome correlates with inflammation and disease severity in patients with acute pancreatitis. Pancreatology 2017;17:364–371. [DOI] [PubMed] [Google Scholar]
- 111.Hirota M, Nozawa F, Okabe A, et al. Relationship between plasma cytokine concentration and multiple organ failure in patients with acute pancreatitis. Pancreas 2000;21:141–6. [DOI] [PubMed] [Google Scholar]
- 112.Martin MA, Saracibar E, Santamaria A, et al. [Interleukin 18 (IL-18) and other immunological parameters as markers of severity in acute pancreatitis]. Rev Esp Enferm Dig 2008;100:768–73. [DOI] [PubMed] [Google Scholar]
- 113.Zhang XH, Li ML, Wang B, et al. Caspase-1 inhibition alleviates acute renal injury in rats with severe acute pancreatitis. World J Gastroenterol 2014;20:10457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Janiak A, Lesniowski B, Jasinska A, et al. Interleukin 18 as an early marker or prognostic factor in acute pancreatitis. Prz Gastroenterol 2015;10:203–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yuan H, Jin X, Sun J, et al. Protective effect of HMGB1 a box on organ injury of acute pancreatitis in mice. Pancreas 2009;38:143–8. [DOI] [PubMed] [Google Scholar]
- 116.Kang R, Chen R, Xie M, et al. The Receptor for Advanced Glycation End Products Activates the AIM2 Inflammasome in Acute Pancreatitis. J Immunol 2016;196:4331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sennello JA, Fayad R, Pini M, et al. Interleukin-18, together with interleukin-12, induces severe acute pancreatitis in obese but not in nonobese leptin-deficient mice. Proc Natl Acad Sci U S A 2008;105:8085–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pini M, Sennello JA, Cabay RJ, et al. Effect of diet-induced obesity on acute pancreatitis induced by administration of interleukin-12 plus interleukin-18 in mice. Obesity 2010;18:476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schaffler A, Hamer O, Dickopf J, et al. Admission resistin levels predict peripancreatic necrosis and clinical severity in acute pancreatitis. The American journal of gastroenterology 2010;105:2474–84. [DOI] [PubMed] [Google Scholar]
- 120.Schaffler A, Hamer OW, Dickopf J, et al. Admission visfatin levels predict pancreatic and peripancreatic necrosis in acute pancreatitis and correlate with clinical severity. Am J Gastroenterol;106:957–67. [DOI] [PubMed] [Google Scholar]
- 121.Messmann H, Vogt W, Falk W, et al. Interleukins and their antagonists but not TNF and its receptors are released in post-ERP pancreatitis. Eur J Gastroenterol Hepatol 1998;10:611–7. [DOI] [PubMed] [Google Scholar]
- 122.Brivet FG, Emilie D, Galanaud P. Pro- and anti-inflammatory cytokines during acute severe pancreatitis: an early and sustained response, although unpredictable of death. Parisian Study Group on Acute Pancreatitis. Crit Care Med 1999;27:749–55. [DOI] [PubMed] [Google Scholar]
- 123.Dambrauskas Z, Giese N, Gulbinas A, et al. Different profiles of cytokine expression during mild and severe acute pancreatitis. World J Gastroenterol;16:1845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Aoun E, Chen J, Reighard D, et al. Diagnostic accuracy of interleukin-6 and interleukin-8 in predicting severe acute pancreatitis: a meta-analysis. Pancreatology 2009;9:777–85. [DOI] [PubMed] [Google Scholar]
- 125.Amin M, Simerman A, Cho M, et al. 21-Hydroxylase-derived steroids in follicles of nonobese women undergoing ovarian stimulation for in vitro fertilization (IVF) positively correlate with lipid content of luteinized granulosa cells (LGCs) as a source of cholesterol for steroid synthesis. The Journal of clinical endocrinology and metabolism 2014;99:1299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Regner S, Appelros S, Hjalmarsson C, et al. Monocyte chemoattractant protein 1, active carboxypeptidase B and CAPAP at hospital admission are predictive markers for severe acute pancreatitis. Pancreatology 2008;8:42–9. [DOI] [PubMed] [Google Scholar]
- 127.Daniel P, Lesniowski B, Mokrowiecka A, et al. Circulating levels of visfatin, resistin and pro-inflammatory cytokine interleukin-8 in acute pancreatitis. Pancreatology : official journal of the International Association of Pancreatology 2010;10:477–82. [DOI] [PubMed] [Google Scholar]
- 128.Ueda T, Takeyama Y, Yasuda T, et al. Significant elevation of serum interleukin-18 levels in patients with acute pancreatitis. J Gastroenterol 2006;41:158–65. [DOI] [PubMed] [Google Scholar]
- 129.Dambrauskas Z, Giese N, Gulbinas A, et al. Different profiles of cytokine expression during mild and severe acute pancreatitis. World J Gastroenterol 2010;16:1845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Batra S, Cai S, Balamayooran G, et al. Intrapulmonary administration of leukotriene B(4) augments neutrophil accumulation and responses in the lung to Klebsiella infection in CXCL1 knockout mice. Journal of immunology 2012;188:3458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Starkie R, Ostrowski SR, Jauffred S, et al. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J 2003;17:884–6. [DOI] [PubMed] [Google Scholar]
- 132.Nieken J, Mulder NH, Buter J, et al. Recombinant human interleukin-6 induces a rapid and reversible anemia in cancer patients. Blood 1995;86:900–5. [PubMed] [Google Scholar]
- 133.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 2004;113:1271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cuzzocrea S, Mazzon E, Dugo L, et al. Absence of endogenous interleukin-6 enhances the inflammatory response during acute pancreatitis induced by cerulein in mice. Cytokine 2002;18:274–85. [DOI] [PubMed] [Google Scholar]
- 135.Guice KS, Oldham KT, Remick DG, et al. Anti-tumor necrosis factor antibody augments edema formation in caerulein-induced acute pancreatitis. The Journal of surgical research 1991;51:495–9. [DOI] [PubMed] [Google Scholar]
- 136.Wang LZ, Su JY, Lu CY, et al. Effects of recombinant human endothelial-derived interleukin-8 on hemorrhagic shock in rats. Zhongguo Yao Li Xue Bao 1997;18:434–6. [PubMed] [Google Scholar]
- 137.Morimoto K, Morimoto A, Nakamori T, et al. Cardiovascular responses induced in free-moving rats by immune cytokines. J Physiol 1992;448:307–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wogensen L, Jensen M, Svensson P, et al. Pancreatic beta-cell function and interleukin-1 beta in plasma during the acute phase response in patients with major burn injuries. Eur J Clin Invest 1993;23:311–9. [DOI] [PubMed] [Google Scholar]
- 139.Li S, Ballou LR, Morham SG, et al. Cyclooxygenase-2 mediates the febrile response of mice to interleukin-1beta. Brain Res 2001;910:163–73. [DOI] [PubMed] [Google Scholar]
- 140.Bhargava R, Janssen W, Altmann C, et al. Intratracheal IL-6 protects against lung inflammation in direct, but not indirect, causes of acute lung injury in mice. PloS one 2013;8:e61405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hackert T, Werner J, Gebhard MM, et al. Effects of heparin in experimental models of acute pancreatitis and post-ERCP pancreatitis. Surgery 2004;135:131–8. [DOI] [PubMed] [Google Scholar]
- 142.Emanuelli G, Montrucchio G, Gaia E, et al. Experimental acute pancreatitis induced by platelet-activating factor in rabbits. Am J Pathol 1989;134:315–26. [PMC free article] [PubMed] [Google Scholar]
- 143.Prescott SM, Zimmerman GA, Stafforini DM, et al. Platelet-activating factor and related lipid mediators. Annu Rev Biochem 2000;69:419–45. [DOI] [PubMed] [Google Scholar]
- 144.Seyfried CE, Schweickart VL, Godiska R, et al. The human platelet-activating factor receptor gene (PTAFR) contains no introns and maps to chromosome 1. Genomics 1992;13:832–4. [DOI] [PubMed] [Google Scholar]
- 145.Liu LR, Xia SH. Role of platelet-activating factor in the pathogenesis of acute pancreatitis. World J Gastroenterol 2006;12:539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ais G, Lopez-Farre A, Gomez-Garre DN, et al. Role of platelet-activating factor in hemodynamic derangements in an acute rodent pancreatic model. Gastroenterology 1992;102:181–7. [DOI] [PubMed] [Google Scholar]
- 147.Zhou W, McCollum MO, Levine BA, et al. Role of platelet-activating factor in pancreatitis-associated acute lung injury in the rat. Am J Pathol 1992;140:971–9. [PMC free article] [PubMed] [Google Scholar]
- 148.Leonhardt U, Fayyazzi A, Seidensticker F, et al. Influence of a platelet activating factor antagonist on severe pancreatitis in two experimental models. Int J Pancreatol 1992;12:161–6. [DOI] [PubMed] [Google Scholar]
- 149.McKay CJ, Curran F, Sharples C, et al. Prospective placebo-controlled randomized trial of lexipafant in predicted severe acute pancreatitis. Br J Surg 1997;84:1239–43. [PubMed] [Google Scholar]
- 150.Johnson CD, Kingsnorth AN, Imrie CW, et al. Double blind, randomised, placebo controlled study of a platelet activating factor antagonist, lexipafant, in the treatment and prevention of organ failure in predicted severe acute pancreatitis. Gut 2001;48:62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sadr-Azodi O, Orsini N, Andren-Sandberg A, et al. Abdominal and total adiposity and the risk of acute pancreatitis: a population-based prospective cohort study. The American journal of gastroenterology 2013;108:133–9. [DOI] [PubMed] [Google Scholar]
- 152.O'Leary DP, O'Neill D, McLaughlin P, et al. Effects of abdominal fat distribution parameters on severity of acute pancreatitis. World journal of surgery 2012;36:1679–85. [DOI] [PubMed] [Google Scholar]
- 153.Yashima Y, Isayama H, Tsujino T, et al. A large volume of visceral adipose tissue leads to severe acute pancreatitis. Journal of gastroenterology 2011;46:1213–8. [DOI] [PubMed] [Google Scholar]
- 154.Funnell IC, Bornman PC, Weakley SP, et al. Obesity: an important prognostic factor in acute pancreatitis. The British journal of surgery 1993;80:484–6. [DOI] [PubMed] [Google Scholar]
- 155.Choh AC, Demerath EW, Lee M, et al. Genetic analysis of self-reported physical activity and adiposity: the Southwest Ohio Family Study. Public health nutrition 2009;12:1052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ren J, Dimitrov I, Sherry AD, et al. Composition of adipose tissue and marrow fat in humans by 1H NMR at 7 Tesla. Journal of lipid research 2008;49:2055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Thomas LW. The chemical composition of adipose tissue of man and mice. Quarterly journal of experimental physiology and cognate medical sciences 1962;47:179–88. [DOI] [PubMed] [Google Scholar]
- 158.Garaulet M, Hernandez-Morante JJ, Lujan J, et al. Relationship between fat cell size and number and fatty acid composition in adipose tissue from different fat depots in overweight/obese humans. International journal of obesity 2006;30:899–905. [DOI] [PubMed] [Google Scholar]
- 159.Pinnick KE, Collins SC, Londos C, et al. Pancreatic ectopic fat is characterized by adipocyte infiltration and altered lipid composition. Obesity (Silver Spring) 2008;16:522–30. [DOI] [PubMed] [Google Scholar]
- 160.Panek J, Sztefko K, Drozdz W. Composition of free fatty acid and triglyceride fractions in human necrotic pancreatic tissue. Med Sci Monit 2001;7:894–8. [PubMed] [Google Scholar]
- 161.Guyenet SJ, Carlson SE. Increase in adipose tissue linoleic acid of US adults in the last half century. Adv Nutr 2015;6:660–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Aho HJ, Sternby B, Nevalainen TJ. Fat necrosis in human acute pancreatitis. An immunohistological study. Acta pathologica, microbiologica, et immunologica Scandinavica. Section A, Pathology 1986;94:101–5. [DOI] [PubMed] [Google Scholar]
- 163.Koutroumpakis E, Dasyam AK, Furlan A, et al. Isolated Peripancreatic Necrosis in Acute Pancreatitis Is Infrequent and Leads to Severe Clinical Course Only When Extensive: A Prospective Study From a US Tertiary Center. J Clin Gastroenterol 2016;50:589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bakker OJ, van Santvoort H, Besselink MG, et al. Extrapancreatic necrosis without pancreatic parenchymal necrosis: a separate entity in necrotising pancreatitis? Gut 2013;62:1475–80. [DOI] [PubMed] [Google Scholar]
- 165.Sztefko K, Panek J. Serum free fatty acid concentration in patients with acute pancreatitis. Pancreatology 2001;1:230–6. [DOI] [PubMed] [Google Scholar]
- 166.Domschke S, Malfertheiner P, Uhl W, et al. Free fatty acids in serum of patients with acute necrotizing or edematous pancreatitis. Int J Pancreatol 1993;13:105–10. [DOI] [PubMed] [Google Scholar]
- 167.Goncalves-de-Albuquerque CF, Burth P, Silva AR, et al. Oleic acid inhibits lung Na/K-ATPase in mice and induces injury with lipid body formation in leukocytes and eicosanoid production. Journal of inflammation 2013;10:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hussain N, Wu F, Zhu L, et al. Neutrophil apoptosis during the development and resolution of oleic acid-induced acute lung injury in the rat. Am J Respir Cell Mol Biol 1998;19:867–74. [DOI] [PubMed] [Google Scholar]
- 169.Patel K, Durgampudi C, Noel P, et al. Fatty Acid Ethyl Esters Are Less Toxic Than Their Parent Fatty Acids Generated during Acute Pancreatitis. Am J Pathol 2016;186:874–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Thiruvengadam NR, Forde KA, Ma GK, et al. Rectal Indomethacin Reduces Pancreatitis in High- and Low-Risk Patients Undergoing Endoscopic Retrograde Cholangiopancreatography. Gastroenterology 2016;151:288–297 e4. [DOI] [PubMed] [Google Scholar]
- 171.Flint RS, Phillips AR, Power SE, et al. Acute pancreatitis severity is exacerbated by intestinal ischemia-reperfusion conditioned mesenteric lymph. Surgery 2008;143:404–13. [DOI] [PubMed] [Google Scholar]
- 172.Fish RE, Lang CH, Spitzer JA. Regional blood flow during continuous low-dose endotoxin infusion. Circ Shock 1986;18:267–75. [PubMed] [Google Scholar]
- 173.Hiltebrand LB, Krejci V, Banic A, et al. Dynamic study of the distribution of microcirculatory blood flow in multiple splanchnic organs in septic shock. Crit Care Med 2000;28:3233–41. [DOI] [PubMed] [Google Scholar]
- 174.Krejci V, Hiltebrand L, Banic A, et al. Continuous measurements of microcirculatory blood flow in gastrointestinal organs during acute haemorrhage. Br J Anaesth 2000;84:468–75. [DOI] [PubMed] [Google Scholar]
- 175.Juvonen PO, Tenhunen JJ, Heino AA, et al. Splanchnic tissue perfusion in acute experimental pancreatitis. Scand J Gastroenterol 1999;34:308–14. [DOI] [PubMed] [Google Scholar]
- 176.Farrant GJ, Abu-Zidan FM, Liu X, et al. The impact of intestinal ischaemia-reperfusion on caerulein-induced oedematous experimental pancreatitis. Eur Surg Res 2003;35:395–400. [DOI] [PubMed] [Google Scholar]
- 177.Bonham MJ, Abu-Zidan FM, Simovic MO, et al. Gastric intramucosal pH predicts death in severe acute pancreatitis. Br J Surg 1997;84:1670–4. [PubMed] [Google Scholar]
- 178.Tian R, Tan JT, Wang RL, et al. The role of intestinal mucosa oxidative stress in gut barrier dysfunction of severe acute pancreatitis. Eur Rev Med Pharmacol Sci 2013;17:349–55. [PubMed] [Google Scholar]
- 179.Flint RS, Windsor JA. The role of the intestine in the pathophysiology and management of severe acute pancreatitis. HPB (Oxford) 2003;5:69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Capurso G, Zerboni G, Signoretti M, et al. Role of the gut barrier in acute pancreatitis. J Clin Gastroenterol 2012;46 Suppl:S46–51. [DOI] [PubMed] [Google Scholar]
- 181.Wu LM, Sankaran SJ, Plank LD, et al. Meta-analysis of gut barrier dysfunction in patients with acute pancreatitis. Br J Surg 2014;101:1644–56. [DOI] [PubMed] [Google Scholar]
- 182.Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn's disease. Gut 2017;66:839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lockhart PB, Brennan MT, Sasser HC, et al. Bacteremia associated with toothbrushing and dental extraction. Circulation 2008;117:3118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Shorvon PJ, Eykyn SJ, Cotton PB. Gastrointestinal instrumentation, bacteraemia, and endocarditis. Gut 1983;24:1078–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Singer M The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 2014;5:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Takasu O, Gaut JP, Watanabe E, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med 2013;187:509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.de-Madaria E, Herrera-Marante I, Gonzalez-Camacho V, et al. Fluid resuscitation with lactated Ringer's solution vs normal saline in acute pancreatitis: A triple-blind, randomized, controlled trial. United European Gastroenterol J 2018;6:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer's solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011;9:710–717.e1. [DOI] [PubMed] [Google Scholar]
- 189.de-Madaria E, Garg PK. Fluid therapy in acute pancreatitis - aggressive or adequate? Time for reappraisal. Pancreatology 2014;14:433–5. [DOI] [PubMed] [Google Scholar]
- 190.Mao EQ, Fei J, Peng YB, et al. Rapid hemodilution is associated with increased sepsis and mortality among patients with severe acute pancreatitis. Chin Med J (Engl) 2010;123:1639–44. [PubMed] [Google Scholar]
- 191.Vaughn VM, Shuster D, Rogers MAM, et al. Early Versus Delayed Feeding in Patients With Acute Pancreatitis: A Systematic Review. Ann Intern Med 2017;166:883–892. [DOI] [PubMed] [Google Scholar]
- 192.Siriwardena AK, Mason JM, Balachandra S, et al. Randomised, double blind, placebo controlled trial of intravenous antioxidant (n-acetylcysteine, selenium, vitamin C) therapy in severe acute pancreatitis. Gut 2007;56:1439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Besselink MG, van Santvoort HC, Buskens E, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 2008;371:651–659. [DOI] [PubMed] [Google Scholar]
- 194.Vege SS, Atwal T, Bi Y, et al. Pentoxifylline Treatment in Severe Acute Pancreatitis: A Pilot, Double-Blind, Placebo-Controlled, Randomized Trial. Gastroenterology 2015;149:318–20.e3. [DOI] [PubMed] [Google Scholar]
- 195.van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012;367:508–19. [DOI] [PubMed] [Google Scholar]
- 196.Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as Induction and Maintenance Therapy for Crohn's Disease. N Engl J Med 2016;375:1946–1960. [DOI] [PubMed] [Google Scholar]
- 197.Wen L, Voronina S, Javed MA, et al. Inhibitors of ORAI1 Prevent Cytosolic Calcium-Associated Injury of Human Pancreatic Acinar Cells and Acute Pancreatitis in 3 Mouse Models. Gastroenterology 2015;149:481–92 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ahuja M, Schwartz DM, Tandon M, et al. Orai1-Mediated Antimicrobial Secretion from Pancreatic Acini Shapes the Gut Microbiome and Regulates Gut Innate Immunity. Cell Metab 2017;25:635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gukovsky I, Gukovskaya AS, Blinman TA, et al. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol 1998;275:G1402–14. [DOI] [PubMed] [Google Scholar]
- 200.Vaquero E, Gukovsky I, Zaninovic V, et al. Localized pancreatic NF-kappaB activation and inflammatory response in taurocholate-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol 2001;280:G1197–208. [DOI] [PubMed] [Google Scholar]
- 201.Neuhofer P, Liang S, Einwachter H, et al. Deletion of IkappaBalpha activates RelA to reduce acute pancreatitis in mice through up-regulation of Spi2A. Gastroenterology 2013;144:192–201. [DOI] [PubMed] [Google Scholar]
- 202.Szabolcs A, Biczo G, Rakonczay Z, et al. Simultaneous proteosome inhibition and heat shock protein induction by bortezomib is beneficial in experimental pancreatitis. Eur J Pharmacol 2009;616:270–4. [DOI] [PubMed] [Google Scholar]
- 203.Mole DJ, Webster SP, Uings I, et al. Kynurenine-3-monooxygenase inhibition prevents multiple organ failure in rodent models of acute pancreatitis. Nat Med 2016;22:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]