Abstract
Social interactions are fundamental to survival and overall health. The mechanisms underlying social behavior are complex, but we now know that immune signaling plays a fundamental role in the regulation of social interactions. Prolonged or exaggerated alterations in social behavior often accompany altered immune signaling and function in pathological states. Thus, unraveling the link between social behavior and immune signaling is a fundamental challenge, not only to advance our understanding of human health and development, but for the design of comprehensive therapeutic approaches for neural disorders. In this review, we synthesize literature demonstrating the bidirectional relationship between social behavior and immune signaling and highlight recent work linking social behavior, immune function, and dopaminergic signaling in adolescent neural and behavioral development.
Keywords: social, immune, dopamine, microglia, adolescence, complement
Social Behavior in health and disease
Social behavior is fundamental to the survival and well-being of many species. Social interactions influence access to food, territory, and potential mates. Moreover, rank within a social hierarchy often mediates stress levels, and thereby health outcomes, in species ranging from cichlid fish and mice, to primates [1–3]. Notably, engagement in positive social interactions is inherently motivating and rewarding, and virtually all disease, neural or not, can be impacted by the quality of social relationships. Inclusion in social networks and positive social interactions are a protective factor in Alzheimer’s disease prognosis [4], decrease the likelihood of substance abuse relapse [5], and improve recovery in a model of cardiac arrest and resuscitation [6]. Conversely, negative social interactions, such as social defeat models in animals or bullying in humans, can result in anhedonia, depression, and anxiety [7, 8]. Indeed, perceived social isolation (i.e. loneliness) has been associated with negative physical and mental health outcomes and increases mortality by upwards of a staggering 50% [9–11].
The neural circuitry underlying social behavior has been increasingly well defined in recent years and involves multiple neuromodulatory systems [12], including a critical role for the immune system. Interactions between the immune system and social behavior have been well defined mechanistically, as discussed later, within the context of “sickness behavior,” in which sick animals exhibit social withdrawal. Sickness behavior is widely considered to be an adaptive, recuperative response rather than a pathological side effect of the infectious organism. Nonetheless, prolonged or exaggerated social behavior alterations often accompany altered immune signaling and function in many pathological states. While this could simply be coincidental and convergent symptomology, it is now clear that immune signaling is intimately tied to the neural processes governing social behaviors.
Importantly, the expression, frequency, and neural regulation of social behavior dramatically changes over the lifespan. Now, research has broadened to include a critical role for immune processes in the normal organization and development of social behavior in healthy individuals [13, 14]. Adolescence in particular is defined by striking changes in neural processing and behavior, including social behavior. However, the immune mechanisms that contribute to, or are affected by, developmental changes in social behavior during this critical period have received relatively little attention.
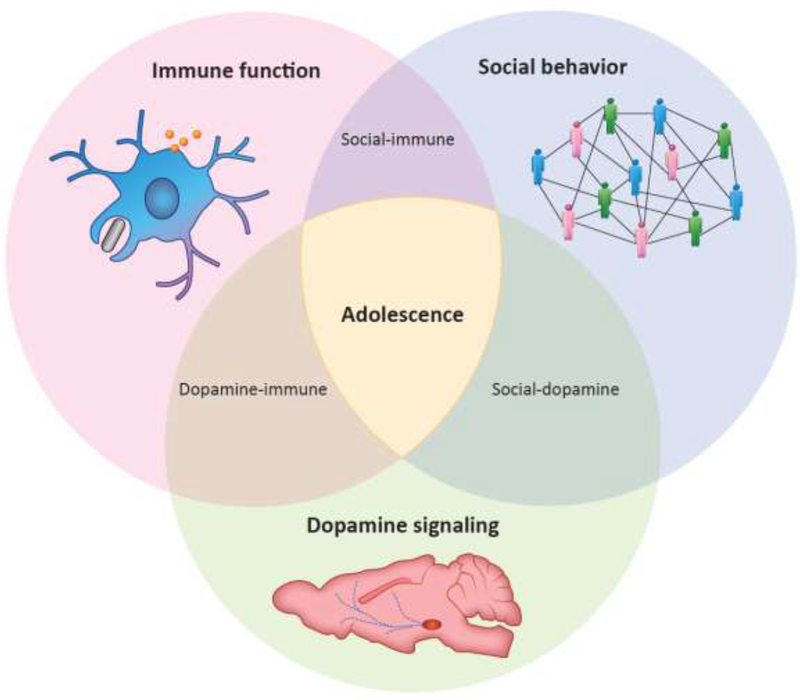
In this review, we synthesize literature demonstrating the bidirectional relationship between social behavior and immune signaling, and we propose that dopaminergic signaling may act as a key intermediary in executing these bidirectional effects, particularly at distinct phases of the lifespan. In this light, we discuss recent work linking social behavior, immune function, and dopaminergic signaling in adolescent brain and behavioral development. Using adolescent development as a model may permit the assessment of novel hypotheses generated by integrating these seemingly disparate areas of research (Fig. 1, Key Figure).
Key Figure 1. Multifaceted interactions between social behavior, immune function, and dopamine signaling.
There exist many complex interactions between social behavior, immune function, and dopamine signaling. Adolescent brain and behavioral development represent a nexus in which all three areas converge and are readily available for experimental manipulation.
The “Behavioral” Immune System
The first clue that immune signaling can modulate social behavior was likely the social withdrawal observed in the context of “sickness behavior,” [15] often modeled via exposure to infectious mimics like lipopolysaccharide (LPS), a structural motif present on gram negative bacteria. LPS is a specific agonist for the innate immune receptor, toll-like receptor 4 (TLR4), that induces cytokine and chemokine release, which in turn dampen, modify, or amplify ongoing immune responses, recruit and activate new immune cells, and alter neural communication [16]. These models have revealed that immune challenges change social behavior, and social environment in turn changes immune signaling [17].
Immune signaling changes social behavior
LPS activation of TLR4 increases pro-inflammatory cytokines, including interleukins −1β and −6 (IL-1β and IL-6), both peripherally and centrally in select brain regions. Interestingly, inhibiting IL-1β signaling in the brain with an IL-1β receptor antagonist (IL-1Ra) ameliorates social withdrawal in a variety of sickness models [18–20]. IL-1RA infusion in the brain, even 4 hours after peripheral LPS injection, increased social interaction and decreased c-fos immunoreactivity (indicative of neural activity) in limbic emotion- and motivation-processing regions, including the central amygdala, without decreasing c-fos in the hypothalamus and brainstem [20]. Reminiscent of animal studies, neural activity, as assessed (indirectly) by blood oxygen level dependent (BOLD) signal during functional magnetic resonance imaging (fMRI), was also increased in the amygdala in LPS-injected humans when they viewed socially ‘threatening’ fearful faces as opposed to nonsocial threats [21, 22]. Moreover, LPS injection increased self-reported feelings of social disconnection and loneliness in humans; this change was sex specific, with the increase more notable in females than males [23, 24]. Whereas a full survey of sex differences in social-immune interactions is beyond the scope of this review, it is a critically important question to address (Box 1).
Box 1. Sex differences in neuroimmunity across development.
The prevalence of many neuropsychiatric disorders differs between males and females. For instance, neurodevelopmental disorders such as autism spectrum disorder are more common in males than in females [73, 74]. In contrast, depression and anxiety disorders are more common in females [75]. Importantly, sex-biased prevalence in disease can be in part due to misdiagnosis, unreported symptoms, and sex-specific presentations of, nominally, the same disorder. A major limitation of basic research conducted so far to clarify the underlying pathophysiology of these disorders is that the vast majority of the studies have been conducted only in males [76].
In addition to sex differences in prevalence, these neuropsychiatric disorders share other important commonalities. First, all are characterized by social dysfunction. Second, neuroimmune alterations have been implicated in the pathophysiology of each [77]. Therefore, we argue that the neuroimmune regulation of social behavior is an area of research in which elucidating sex differences may be particularly critical.
Across the animal kingdom, the expression of social behavior often differs between males and females. In adulthood, sex differences exist in the display of sexual, aggressive, and parental behaviors. Even before the onset of puberty, sex differences can be found in the expression of behaviors such as social play [14]. Importantly, when the expression of a given behavior is the same in males and females, the underlying neural mechanisms that support that behavior can still be sex-specific. Indeed, it has been hypothesized that in some instances sex differences in the brain may serve to prevent, rather than create, sex differences in behavior [78].
An emerging body of literature suggests that neuroimmune interactions may organize the neural circuitry supporting social behavior in sexually dimorphic ways [13]. In particular, sex differences have long been described in the development, morphology, and immune-responsivity of microglia [79]. More recently, we have shown that microglial phagocytosis of dopamine D1 receptors (D1Rs) in the nucleus accumbens is critical to the developmental pattern of social play expression in adolescent male rats [14]. Critically, however, while microglial pruning within the NAc does appear to regulate social play behavior in female adolescent rats, it is not via a D1R-dependent mechanism – suggesting sex-specific patterns of microglial refinement in the adolescent reward circuitry. Future studies should aim to further characterize the neuroimmune processes by which sex differences in social behavior are established in the healthy brain, and, therefore, potentially disrupted in disease.
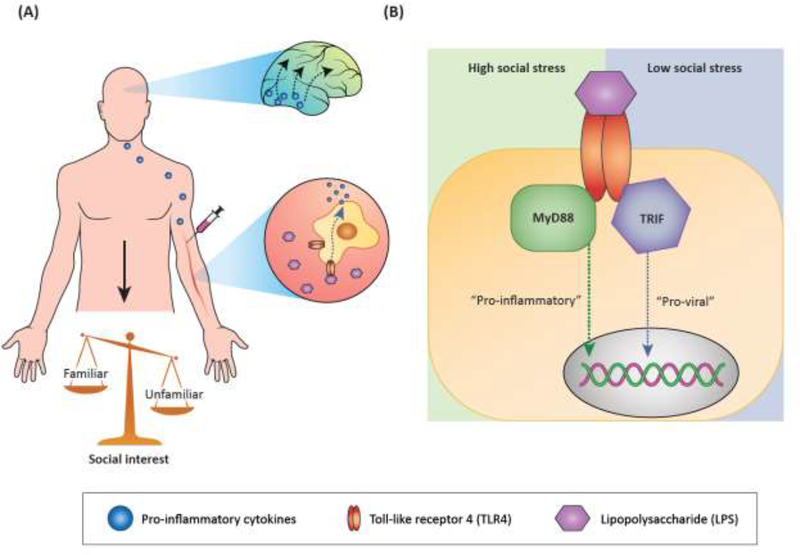
It should be noted, however, that LPS treatment is not invariably associated with social withdrawal. LPS-injected humans presented with either pictures of familiar, supportive people in their life, or strangers, reported increased desire to be near the familiar stimulus relative to placebo-injected controls [25]. Greater desire to be around familiar figures, but not strangers, was associated with increased BOLD signal in the dopaminergic striatum, important for motivation and reward processing. In fact, the larger the IL-6 cytokine response to LPS, the higher the striatal BOLD signal to familiar social stimuli [25]. In rhesus monkey, LPS exposure was also reported to increase contact with familiar social stimuli (a cage mate) [26], and similar findings were reported in rats [27]. In female prairie voles, LPS exposure was reported to facilitate pair-bond formation [28]. Together, these data suggest that activation of TLR4 on peripheral immune cells induces pro-inflammatory cytokine release in the periphery, and possibly in brain regions directly accessible by the peripheral milieu. This in turn activates central immune processes in brain regions important for emotion and motivation, thereby increasing social behavior towards familiar stimuli and decreasing social behavior towards unfamiliar stimuli (Fig. 2A).
Figure 2. Models of the bidirectional relationships between social behavior and immune function.
(A) Induction of an inflammatory response (and sickness behavior if dose is high enough) with LPS, a structural motif on gram negative bacteria, activates TLR4 on peripheral immune cells, causing release of pro-inflammatory cytokines such as IL-1β and IL-6. Peripheral immune signaling is hypothesized to activate brain regions that can be directly influenced by the periphery, such as brainstem and hypothalamic regions. Through mechanisms that are not yet clear, activated brain regions may induce immune signaling (particularly IL-1β signaling) in brain regions important for motivation, reward, and emotion processing. In turn, social interest shifts from novel/unfamiliar social investigation to increased interaction with familiar social stimuli. (B) Intracellular signaling by TLR4 may be influenced by social context or status. In organisms experiencing high social stress, activation of TLR4 may preferentially induce “pro-inflammatory,” or MyD88-dependent, signaling and subsequent transcriptional signatures. Conversely, in organisms experiencing low social stress, TLR4 activation may preferentially induce “pro-viral,” or TRIF-dependent, signaling and subsequent transcriptional signatures.
Interesting subtleties in immune-mediated social behaviors are also present in rodent models of deficient immune signaling, rather than overt sickness. Mice lacking the immune-specific fractalkine receptor (CX3CR1) have reduced sociability, but, surprisingly, only if the social stimulus is available for active, reciprocal interactions [29]. Typically, in a three-chamber sociability task, wildtype mice prefer to explore a social stimulus over an empty cage. In contrast, juvenile CX3CR1 knockout (KO) mice do not exhibit a preference for their mother over an empty cage; however, if the dam was sedated, the preference was reinstated. Similarly, adult KO mice did not display a preference to explore a same-sex juvenile over an empty cage, as in wildtype mice, but if bedding previously soiled by a same-sex juvenile was present (rather than the mouse itself), a preference for the social stimulus was evident. How elimination of CX3CR1-mediated immune signaling causes these behavioral outcomes is not fully clear, but there was evidence of impaired communication between the hippocampus and prefrontal cortex (PFC) [29]. Coincidently, CX3CR1 KO mice have significantly worse LPS-induced sickness behavior, including prolonged decreases in social interaction relative to wildtypes, which was associated with increased inflammatory gene expression in microglia, the resident immune cells of the brain [30]. It is unknown whether prolonged social withdrawal after LPS treatment in transgenic mice (relative to LPS-injected wildtype mice) would still be prolonged if reciprocal social interactions were controlled for. Importantly, it’s unclear if CX3CR1 elimination in peripheral immune cells, central immune cells (microglia), or both, contribute to neural and behavioral deficits. Microglia have specialized roles in neural circuit development and plasticity through their ability to regulate various form of synaptic remodeling and structural plasticity, including synaptogenesis, synaptic refinement, synaptic pruning, and synaptic scaling [31], as discussed in more detail in later sections. While models with full transgene elimination from birth need to be interpreted with caution, these interesting data support the hypothesis that immune signaling modulates select aspects of social behavior.
A recent report has begun to dissect the interplay between peripheral and central processes underlying social behavior [32]. The study examined severe combined immune deficiency (SCID) mice, which lack B and T cell-mediated adaptive immunity, and showed that these mice have impaired sociability, as well as hyper-connectivity between frontal and insular brain regions. In healthy mice, peripheral immune cells are recruited to the brain and secrete the cytokine interferon gamma (IFNγ), activating (among other downstream effects) inhibitory interneurons in the PFC. This peripheral to central signaling is absent in SCID mice, resulting in behavioral abnormalities and hyper-connectivity, and can be rescued by either bone marrow transfer to establish a functional immune system, or via IFNγ treatment. While microglia also express IFNγ receptors, they did not seem to contribute to social behavior deficits in this model. A cross-species assessment indicated that IFNγ transcriptional signatures are upregulated in rodents, flies, and zebrafish in response to social experiences/contexts, suggesting it may be an evolutionarily conserved mechanism underlying social behavior.
Social context changes immune function
Intriguingly, social context can also modulate immune function. Social stressors profoundly impact immune function, as recently reviewed elsewhere [33]. The immune consequences of acute social manipulations are less well understood. A study in rhesus macaques, using a sophisticated within-animal design for manipulating social status, demonstrated that immune changes in response to social context are highly malleable [34]. Specifically, female subjects with stable social hierarchies were introduced to new social groups, permitting experimental control of social rank, with more senior group members having higher social rank. Regardless of rank prior to or after this manipulation, low social status members had increased gene expression for immune signaling mediators in peripheral immune cells, including cytokines and proteins associated with the TLR4 pathway. Consistent with a higher basal capacity for inflammatory response, there was overall a much stronger LPS-induced immune response in low social status subjects. Thus, there are immediate effects of current social status on immune function both basally and after an immune challenge, independent of recent social history. Interestingly, transcriptomic data suggested differential routing of immune activation by social status. After LPS exposure, high social status was correlated with interferon-related transcriptional enrichment, whereas low social status was correlated with NFкB-dependent transcriptional enrichment, which may result from differential intracellular routing of TLR4 activation via different scaffolding proteins, MyD88 and TRIF, during low and high social stress situations, respectively. In fact, low social status, poor social integration, and social stress are so reliably accompanied by decreased interferon-related ‘anti-viral’ gene expression and increased pro-inflammatory ‘bacterial’ gene expression, that this gene expression pattern has been collectively referred to as the conserved transcriptional response to adversity (CTRA; Fig. 2B) [35]. To our knowledge, whether and how social context can directly alter the intracellular routing of an otherwise identical immune stimulus (e.g. LPS injection) has not been determined.
Thus far, we have highlighted evidence demonstrating intricate, bidirectional regulation between the immune system and social behavior. Understanding how these inter-dependent systems are disrupted, as seen in various neuropsychiatric, neurodevelopmental, and neurodegenerative disorders, we must first have a firm grasp of their normal, healthy regulation. Immune regulation of neural activity in brain regions associated with reward processing and motivation, including the PFC, amygdala, and striatum, as mentioned earlier, points to an intriguing candidate mediator between immune function and social behavior: the neurotransmitter dopamine.
Dopaminergic signaling modifies both social behavior and immune function
The mesolimbic dopaminergic ‘reward’ circuitry is an interconnected set of brain regions, including the ventral tegmental area (VTA), nucleus accumbens (NAc), and PFC, that process rewarding stimuli and direct future behaviors based on reward history or expectation [36]. Like other rewarding/motivating stimuli, social interactions engage the reward circuitry and rely on dopaminergic signaling. Interestingly, immune function is also highly dependent on central dopaminergic processes.
Dopaminergic regulation of social behaviors
The contribution of dopaminergic signaling to social behavior has been particularly well characterized in social play during adolescence [37]. Adolescent phenomena in rodents are explored at different ages in different reports, but generally speaking adolescence is thought to occur between Postnatal day (P) 25 and 55 in mice and rats, with some variation by species and sex [38, 39]. Manipulations to increase dopamine signaling in the NAc (e.g., via dopamine reuptake inhibitors or dopamine receptor agonists) increase social play behavior in adolescent rats, whereas antagonism of either D1 or D2 dopamine receptors decreases social play [40]. When rats respond on a progressive ratio schedule of reinforcement for social play, dopamine reuptake inhibitors increased responding without affecting the behavioral expression of play [41], suggesting that dopaminergic signaling may also be important for establishing the motivational salience of social play.
Dopamine is also important for social behavior in mature adult animals. In adult female mice, brief interaction with a novel social stimulus, but not with a novel non-social one, increased calcium transients, indicative of neural activity, in the VTA [42]. Indeed, optical activation or inhibition of VTA neurons elicited or inhibited social interaction, respectively. After VTA activation, neural activity (c-fos immunoreactivity) increased in both the PFC and NAc; however, only activation of VTA→NAc circuits, and not VTA→PFC circuits, increased social interaction via activation of D1, but not D2, dopamine receptors in the NAc (Fig. 3A). Moreover, in a large study of humans with gene variants in receptors important for social behaviors, genetic variations in dopaminergic and endorphin receptors were the only ones associated with social behaviors that span multiple domains, despite oxytocin being the most comprehensively researched modulator of social behavior [43]. These data collectively establish the critical, and perhaps underappreciated, importance of dopamine signaling in social behaviors.
Figure 3. Central dopaminergic signaling underlying social behavior and immune function.
(A) Optogenetic stimulation of VTA cells induces social interaction via dopamine release and D1 dopamine receptor signaling in the NAc. (B) Chemogenetic stimulation of the VTA induces increased immune function in blood and spleen, measured by cytokine release and phagocytosis, after an immune (E. coli) challenge.
Central dopaminergic regulation of immune function
Dopaminergic signaling is also a well-characterized modulator of immune function [44]. The relationship between central dopaminergic signaling and immune function (both peripherally and centrally) has been examined in rodent models of Parkinson’s disease, in which selective degradation of the brain’s dopaminergic circuitry via toxins like 6-hydroxydopamine (6-OHDA) result in a characteristic loss of motor control. Unilateral 6-OHDA lesion of the striatum, which depletes dopamine terminals locally in the targeted hemisphere, impairs immune function in the periphery [45–47], and chemogenetic activation of dopaminergic cells in the VTA increased phagocytosis and cytokine immune responses to subsequent infection in blood and spleen (Fig. 3B) [48]. Interestingly, dopaminergic lesion of the right, but not left, striatum impaired immune cell proliferation in the spleen, and lesion of the left, but not right, NAc decreased natural killer cell activity in the spleen [47]. These data suggest that, just as in the nuanced regulation of social behavior by immune signaling, there is far more order and subtlety in central dopamine-peripheral immune relationships than we currently understand.
Surprisingly, reducing dopamine synthesis by blocking tyrosine hydroxylase minimizes dopaminergic degeneration and supplying the dopamine precursor L-DOPA, thus bypassing the need for dopamine synthesis, reinstates degeneration [49]. Interestingly, reducing dopamine levels in the brain reduces the number of infiltrating immune cells (monocytes) after 6-OHDA lesion [50], suggesting that central dopamine can act as an immune recruitment signal. Dopamine, itself, may therefore make dopaminergic regions vulnerable to pro-inflammatory immune signaling and subsequent neural damage. Beyond recruitment of peripheral immune cells, immune signaling within the brain itself is also implicated in Parkinsonian-like dopaminergic degeneration. In mice, four days of daily peripheral LPS injection (1μg) causes dopaminergic degeneration 19 and 36 days later, but not 5 days later [51], and increases complement-associated gene expression in the brain. Repeated peripheral LPS exposure does not cause dopaminergic degeneration if complement signaling is disrupted via the elimination of complement protein C3 in transgenic mice [51]. In the brain, the complement system has been demonstrated to be critical for microglia-mediated phagocytosis and elimination of synapses during development [52–54], raising the possibility that dopaminergic degeneration in this model is ultimately due to aberrant immune activity within the brain itself.
Taken together, the strong associations between social behavior and dopaminergic signaling, and between dopaminergic signaling and immune function, provide a tempting platform for linking social-immune interactions. Are these relationships specific to disease states, or does dopamine act as an intermediary between immune function and social behavior in healthy individuals? In a recent study we examined this question during adolescent neural development, a time period in which social behavior, immune function, and dopaminergic signaling are dynamically changing.
Putting the pieces together: Adolescent brain, behavioral, and neuroimmune development
Adolescents have a high propensity for peer social interactions, and display increased susceptibility to social influence [38, 55, 56]. Exploratory social behaviors like social novelty-seeking [57] and social play behavior, as we briefly reviewed above, are also heightened during adolescence. Importantly, the normal behavioral changes associated with adolescent development are critical to healthy brain and behavioral maturation. In fact, an extreme case of impoverished access to social experience in which rodents are socially isolated throughout adolescence, is a model for the induction of schizophrenia-like behaviors [58].
These social behavior changes are in large part due to ongoing developmental plasticity within the dopaminergic reward circuitry of the brain [59]. These changes include both axon outgrowth – indeed, DA neurons into NAc and then onward to the PFC are the only known case of long-distance axon growth during adolescence [60] – as well as synaptic changes. During neural development, there is an overproduction of synapses, followed by activity- and experience-dependent refinement, or pruning, which ultimately produces a mature neural circuit. In humans, MRI-based measurements of grey matter density decrease throughout adolescence and into young adulthood [61]. While not a perfect correlate, these data suggest that developmental pruning may be occurring well into adulthood. Dopamine receptor density has indeed been observed to decrease in the striatum, PFC, and NAc during adolescence in rodents [62–65]. Synaptic pruning is in some cases regulated by the complement family of immune proteins and their interactions with microglia [31]. Complement “tagging” with C3b/iC3b is recognized by the C3 receptor (CR3) on macrophages [66]; the macrophage then engulfs and lysosomally digests the C3-tagged target. This process is important for clearance of infectious molecules, but also for tagging synapses for elimination during brain development. Mice lacking complement protein C3 or C1q, an upstream activator of the C3 tagging process, have synaptic pruning deficits in the developing visual thalamus [52–54], resulting from failure of microglia, the only cell type in the brain to express C3 receptor (CR3/CD11b), to prune C3-tagged retinal inputs [52, 53]. Thus, during adolescence, (i) developmental plasticity in the dopaminergic circuitry is important for normal, developmental changes in social behavior, and (ii) dopamine receptors are ‘pruned,’ or at least downregulated. Moreover, more generally, immune signaling is important for synaptic pruning during developmental critical periods, at least in some brain regions. We have recently reported data which link these areas of research (Fig. 4; [14]).
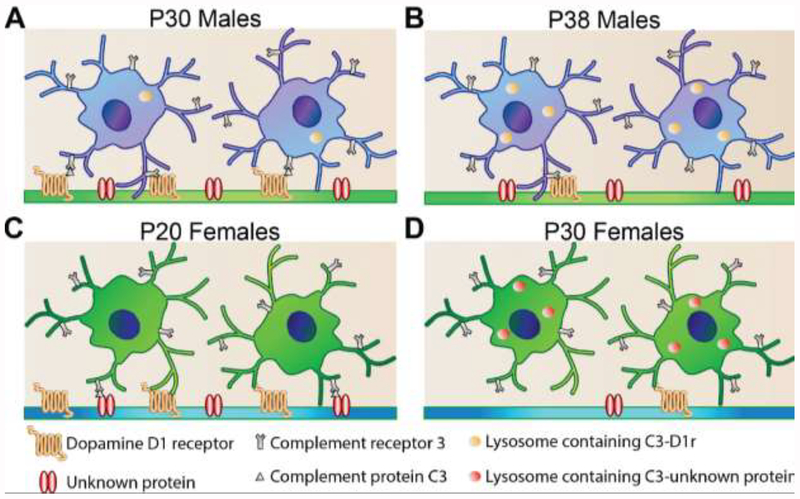
Figure 4. Immune processes regulating sex-specific adolescent development in the nucleus accumbens.
(A) In early adolescent male rats (P30), dopamine D1 receptors (D1rs) associate with the “tagging” protein, complement C3. Microglia, the only intra-parenchymal brain cell expressing the receptor for C3 (Complement receptor 3, often referred to as CD11b), recognize the C3 tag and phagocytose the C3-D1r complex. (B) By mid-adolescence (P38), D1r levels have decreased and C3-D1r content can be detected inside microglial lysosomes. This collective process results in a decrease in social play behavior in males. (C) D1r levels decline between pre- and early-adolescence (P20-P30) in female rats, but D1r downregulation is not associated with microglia and C3 immune signaling. (D) Rather, there is an as yet unknown protein that is regulated by C3-microglial interactions, and this process regulates basal levels of social play in females.
Dopamine D1 receptor (D1r) levels in the NAc and social play behavior peak at about the same age in adolescent male rats (P30, early adolescence). And, when D1r levels decline, social play declines (by P38, mid-adolescence). D1r downregulation occurs in part via C3 and microglia-mediated engulfment and lysosomal degradation, thus implicating C3-microglia signaling in the normal developmental decline of social play behavior (Fig. 4A,B). Indeed, interfering with the ability of microglial CR3 to bind C3 in the NAc using neutrophil inhibitory factor (NIF) augments both D1r levels and social play behavior. If NIF is co-injected with siRNA against D1r, such that D1r fails to be engulfed by microglia (via NIF) but is downregulated through alternative mechanisms (via siRNA), social play behavior returns to control levels. Adolescent development in female rats is slightly different. D1r downregulation in females occurs between P20 and P30 (pre- and early adolescence) and is not correlated with changes in social play behavior. There was no evidence suggesting D1r downregulation was associated with C3 or microglia; indeed, injection of NIF into the NAc did not alter D1r levels in females. However, NIF injection did slightly augment the low levels of social play behavior in females, in a D1r-independent manner. We hypothesize that in females, C3-microglia interactions are modifying a different set of undetermined receptors/proteins important for social behavior during adolescent development (Fig. 4C,D). Many other neuromodulatory systems are important for adolescent social behavior, including opioids, endocannabinoids, serotonin, oxytocin, and vasopressin [67–71], and there is evidence that receptor expression for these various systems also changes over the course of development [68, 72]. However, the neural mechanisms by which developmental changes in receptor density arise have yet to be explored. The role for microglia in the developmental elimination of D1rs during adolescence raises the possibility that microglial pruning is also involved in the organization of other neural systems that regulate social behavior.
Our data suggest that immune signaling has an organizing effect on the maturation of both dopaminergic circuitry (or at least D1r levels) and social behaviors, and that this regulation happens in a sex-specific manner. This picture is generally consistent with recent work from McCarthy and colleagues, showing that immune signaling in other brain regions, prominently the sexually dimorphic nucleus (SDN), organizes sex-specific social and sexual behaviors [13]. Of note, however, converse regulation, in which dopamine signaling and social behavior impact immune signaling, seems to exist as well, as reviewed earlier, suggesting a regulatory feedback loop where dopamine may play a pivotal role. It is possible that during adolescence, immune cells in the brain and/or periphery also experience a “critical period” of developmental plasticity that permanently organizes their function. If so, many interesting questions emerge. For instance, what are the long-term consequences of impaired immune development? And importantly, if immune signaling is influenced during adolescence by stress, drugs, sickness, etc., how is social behavior and dopaminergic circuitry altered? From a clinical perspective, these concepts raise the possibility that neuroimmune mechanisms may be therapeutically targeted during adolescence to foster resilience.
Concluding Remarks
Herein, we outlined some of the complex interactions between social behavior and immune function, and proposed dopamine signaling as a key node between the two, with the goal of emphasizing that the interdependencies between these seemingly disparate domains could have profound implications for human health. Adolescent brain and behavioral development are characterized by changes in dopaminergic and social behavior development, and new data now implicate immune signaling in organizing this development. Using the framework of adolescent development to explore how social-immune interactions develop and mature will be a novel and important means of testing hypotheses derived from models incorporating stress, sickness, or disease (Fig. 1; see Outstanding Questions). In closing, unraveling the link between social behavior and immune signaling is a fundamental challenge, not only to advancing our understanding of human health and development, but to designing comprehensive pharmaco-behavioral therapeutic approaches to combat neural disorders and disease.
Highlights.
Immune challenges impair social behavior by altering neuro-immune signaling in brain regions important for reward/motivation.
Social context influences immune function.
The dopaminergic reward circuitry is engaged during social behaviors and impacts immune function.
Microglia and immune signaling play a critical role in the developmental organization of dopaminergic neural circuitry and social behavior development during adolescence.
Adolescent neural and behavioral development may represent an ideal model in which to ask questions regarding social-immune interactions in health and disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Fernald RD and Maruska KP (2012) Social information changes the brain. Proc Natl Acad Sci U S A 109 Suppl 2, 17194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.So N et al. (2015) A Social Network Approach Reveals Associations between Mouse Social Dominance and Brain Gene Expression. PLoS One 10 (7), e0134509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sapolsky RM (2005) The influence of social hierarchy on primate health. Science 308 (5722), 648–52. [DOI] [PubMed] [Google Scholar]
- 4.Bennett DA et al. (2006) The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol 5 (5), 406–12. [DOI] [PubMed] [Google Scholar]
- 5.Atadokht A et al. (2015) The role of family expressed emotion and perceived social support in predicting addiction relapse. Int J High Risk Behav Addict 4 (1), e21250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaudier-Diaz MM et al. (2018) Social influences on microglial reactivity and neuronal damage after cardiac arrest/cardiopulmonary resuscitation. Physiol Behav 194, 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjorkqvist K (2001) Social defeat as a stressor in humans. Physiol Behav 73 (3), 435–42. [DOI] [PubMed] [Google Scholar]
- 8.Huhman KL (2006) Social conflict models: can they inform us about human psychopathology? Horm Behav 50 (4), 640–6. [DOI] [PubMed] [Google Scholar]
- 9.Holt-Lunstad J et al. (2010) Social relationships and mortality risk: a meta-analytic review. PLoS Med 7 (7), e1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cacioppo JT and Cacioppo S (2014) Social Relationships and Health: The Toxic Effects of Perceived Social Isolation. Soc Personal Psychol Compass 8 (2), 58–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo Y et al. (2012) Loneliness, health, and mortality in old age: a national longitudinal study. Soc Sci Med 74 (6), 907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modi ME and Sahin M (2018) A unified circuit for social behavior. Neurobiol Learn Mem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarthy MM et al. (2015) Surprising origins of sex differences in the brain. Horm Behav 76, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopec AM et al. (2018) Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats. Nat Commun 9 (1), 3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dantzer R and Kelley KW (2007) Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun 21 (2), 153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein SL and Flanagan KL (2016) Sex differences in immune responses. Nat Rev Immunol 16 (10), 626–38. [DOI] [PubMed] [Google Scholar]
- 17.Eisenberger NI et al. (2017) In Sickness and in Health: The Co-Regulation of Inflammation and Social Behavior. Neuropsychopharmacology 42 (1), 242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bluthe RM et al. (1994) Synergy between tumor necrosis factor alpha and interleukin-1 in the induction of sickness behavior in mice. Psychoneuroendocrinology 19 (2), 197–207. [DOI] [PubMed] [Google Scholar]
- 19.Kent S et al. (1992) Different receptor mechanisms mediate the pyrogenic and behavioral effects of interleukin 1. Proc Natl Acad Sci U S A 89 (19), 9117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konsman JP et al. (2008) Central nervous action of interleukin-1 mediates activation of limbic structures and behavioural depression in response to peripheral administration of bacterial lipopolysaccharide. Eur J Neurosci 28 (12), 2499–510. [DOI] [PubMed] [Google Scholar]
- 21.Inagaki TK et al. (2012) Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage 59 (4), 3222–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hariri AR et al. (2002) The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage 17 (1), 317–23. [DOI] [PubMed] [Google Scholar]
- 23.Moieni M et al. (2015) Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacology 40 (7), 1709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenberger NI et al. (2010) Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun 24 (4), 558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inagaki TK et al. (2015) The role of the ventral striatum in inflammatory-induced approach toward support figures. Brain Behav Immun 44, 247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willette AA et al. (2007) Environmental context differentially affects behavioral, leukocyte, cortisol, and interleukin-6 responses to low doses of endotoxin in the rhesus monkey. Brain Behav Immun 21 (6), 807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yee JR and Prendergast BJ (2010) Sex-specific social regulation of inflammatory responses and sickness behaviors. Brain Behav Immun 24 (6), 942–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bilbo SD et al. (1999) Lipopolysaccharide facilitates partner preference behaviors in female prairie voles. Physiol Behav 68 (1–2), 151–6. [DOI] [PubMed] [Google Scholar]
- 29.Zhan Y et al. (2014) Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci 17 (3), 400–6. [DOI] [PubMed] [Google Scholar]
- 30.Corona AW et al. (2010) Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. J Neuroinflammation 7, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schafer DP and Stevens B (2015) Microglia Function in Central Nervous System Development and Plasticity. Cold Spring Harb Perspect Biol 7 (10), a020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filiano AJ et al. (2016) Unexpected role of interferon-gamma in regulating neuronal connectivity and social behaviour. Nature 535 (7612), 425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber MD et al. (2017) Repeated Social Defeat, Neuroinflammation, and Behavior: Monocytes Carry the Signal. Neuropsychopharmacology 42 (1), 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snyder-Mackler N et al. (2016) Social status alters immune regulation and response to infection in macaques. Science 354 (6315), 1041–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole SW (2013) Social regulation of human gene expression: mechanisms and implications for public health. Am J Public Health 103 Suppl 1, S84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keiflin R and Janak PH (2015) Dopamine Prediction Errors in Reward Learning and Addiction: From Theory to Neural Circuitry. Neuron 88 (2), 247–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanderschuren LJ et al. (2016) The neurobiology of social play and its rewarding value in rats. Neurosci Biobehav Rev 70, 86–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spear LP (2000) The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24 (4), 417–63. [DOI] [PubMed] [Google Scholar]
- 39.Schneider M (2013) Adolescence as a vulnerable period to alter rodent behavior. Cell Tissue Res 354 (1), 99–106. [DOI] [PubMed] [Google Scholar]
- 40.Manduca A et al. (2016) Dopaminergic Neurotransmission in the Nucleus Accumbens Modulates Social Play Behavior in Rats. Neuropsychopharmacology 41 (9), 2215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Achterberg EJ et al. (2016) Contrasting Roles of Dopamine and Noradrenaline in the Motivational Properties of Social Play Behavior in Rats. Neuropsychopharmacology 41 (3), 858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gunaydin LA et al. (2014) Natural neural projection dynamics underlying social behavior. Cell 157 (7), 1535–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearce E et al. (2017) Variation in the beta-endorphin, oxytocin, and dopamine receptor genes is associated with different dimensions of human sociality. Proc Natl Acad Sci U S A 114 (20), 5300–5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arreola R et al. (2016) Immunomodulatory Effects Mediated by Dopamine. J Immunol Res 2016, 3160486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Filipov NM et al. (2002) Compromised peripheral immunity of mice injected intrastriatally with six-hydroxydopamine. J Neuroimmunol 132 (1–2), 129–39. [DOI] [PubMed] [Google Scholar]
- 46.Engler H et al. (2009) Time-dependent alterations of peripheral immune parameters after nigrostriatal dopamine depletion in a rat model of Parkinson’s disease. Brain Behav Immun 23 (4), 518–26. [DOI] [PubMed] [Google Scholar]
- 47.Deleplanque B et al. (1994) Modulation of immune reactivity by unilateral striatal and mesolimbic dopaminergic lesions. Neurosci Lett 166 (2), 216–20. [DOI] [PubMed] [Google Scholar]
- 48.Ben-Shaanan TL et al. (2016) Activation of the reward system boosts innate and adaptive immunity. Nat Med 22 (8), 940–4. [DOI] [PubMed] [Google Scholar]
- 49.De Pablos RM et al. (2005) Dopamine-dependent neurotoxicity of lipopolysaccharide in substantia nigra. FASEB J 19 (3), 407–9. [DOI] [PubMed] [Google Scholar]
- 50.Espinosa-Oliva AM et al. (2014) Role of dopamine in the recruitment of immune cells to the nigrostriatal dopaminergic structures. Neurotoxicology 41, 89–101. [DOI] [PubMed] [Google Scholar]
- 51.Bodea LG et al. (2014) Neurodegeneration by activation of the microglial complement-phagosome pathway. J Neurosci 34 (25), 8546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bialas AR and Stevens B (2013) TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci 16 (12), 1773–82. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Schafer DP et al. (2012) Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74 (4), 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stevens B et al. (2007) The classical complement cascade mediates CNS synapse elimination. Cell 131 (6), 1164–78. [DOI] [PubMed] [Google Scholar]
- 55.Casey BJ et al. (2008) The adolescent brain. Dev Rev 28 (1), 62–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Somerville LH (2013) Special issue on the teenage brain: Sensitivity to social evaluation. Curr Dir Psychol Sci 22 (2), 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doremus-Fitzwater TL et al. (2010) Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn 72 (1), 114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fone KC and Porkess MV (2008) Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev 32 (6), 1087–102. [DOI] [PubMed] [Google Scholar]
- 59.Fuhrmann D et al. (2015) Adolescence as a Sensitive Period of Brain Development. Trends Cogn Sci 19 (10), 558–566. [DOI] [PubMed] [Google Scholar]
- 60.Hoops D and Flores C (2017) Making Dopamine Connections in Adolescence. Trends Neurosci 40 (12), 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gogtay N et al. (2004) Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A 101 (21), 8174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andersen SL et al. (2000) Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse 37 (2), 167–9. [DOI] [PubMed] [Google Scholar]
- 63.Teicher MH et al. (1995) Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res 89 (2), 167–72. [DOI] [PubMed] [Google Scholar]
- 64.Tarazi FI and Baldessarini RJ (2000) Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain. Int J Dev Neurosci 18 (1), 29–37. [DOI] [PubMed] [Google Scholar]
- 65.Tarazi FI et al. (1999) Postnatal development of dopamine D1-like receptors in rat cortical and striatolimbic brain regions: An autoradiographic study. Dev Neurosci 21 (1), 43–9. [DOI] [PubMed] [Google Scholar]
- 66.Walport MJ (2001) Complement. First of two parts. N Engl J Med 344 (14), 1058–66. [DOI] [PubMed] [Google Scholar]
- 67.Smith CJ et al. (2015) Social Novelty Investigation in the Juvenile Rat: Modulation by the mu-Opioid System. J Neuroendocrinol 27 (10), 752–64. [DOI] [PubMed] [Google Scholar]
- 68.Smith CJW et al. (2018) Nucleus accumbens mu opioid receptors regulate context-specific social preferences in the juvenile rat. Psychoneuroendocrinology 89, 59–68. [DOI] [PubMed] [Google Scholar]
- 69.Argue KJ et al. (2017) Activation of Both CB1 and CB2 Endocannabinoid Receptors Is Critical for Masculinization of the Developing Medial Amygdala and Juvenile Social Play Behavior. eNeuro 4 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dolen G et al. (2013) Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501 (7466), 179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Veenema AH et al. (2012) Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Horm Behav 61 (1), 50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith CJW et al. (2018) Robust age, but limited sex, differences in mu-opioid receptors in the rat brain: relevance for reward and drug-seeking behaviors in juveniles. Brain Struct Funct 223 (1), 475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Christensen DL et al. (2018) Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill Summ 65 (13), 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kopec AM et al. (2018) Gut-immune-brain dysfunction in Autism: Importance of sex. Brain Res 1693 (Pt B), 214–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whiteford HA et al. (2013) Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 382 (9904), 1575–86. [DOI] [PubMed] [Google Scholar]
- 76.Zucker I and Beery AK (2010) Males still dominate animal studies. Nature 465 (7299), 690. [DOI] [PubMed] [Google Scholar]
- 77.McCarthy MM (2019) Sex differences in neuroimmunity as an inherent risk factor. Neuropsychopharmacology 44 (1), 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Vries GJ (2004) Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology 145 (3), 1063–8. [DOI] [PubMed] [Google Scholar]
- 79.Schwarz JM and Bilbo SD (2012) Sex, glia, and development: interactions in health and disease. Horm Behav 62 (3), 243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]