Abstract
The desmoplastic reaction of pancreas cancer may begin as a wound healing response to the nascent neoplasm, but it soon creates an insidious shelter that can sustain the growing tumor and rebuff therapy. Among the many cell types subverted by transformed epithelial cells, fibroblasts are recruited and activated to lay a foundation of extracellular matrix proteins and glycosaminoglycans that alter tumor biophysics and signaling. Their near-universal presence in pancreas cancer and ostensible support of disease progression make fibroblasts attractive therapeutic targets. More recently, however, it has also become apparent that diverse subpopulations of fibroblasts with distinct phenotypes and secretomes inhabit the stroma, and that targeted depletion of particular fibroblast subsets could either provide substantial therapeutic benefit or accelerate disease progression. An improved characterization of these fibroblast subtypes, along with their potential relationships to tumor subtypes and mutational repertoires, is needed in order to make anti-fibroblast therapies clinically viable.
Keywords: PDA, fibroblast, desmoplasia, GEMM
Introduction
Pancreatic ductal adenocarcinomas (PDA) consist of a minority of malignant tumor cells embedded in a complex environment of extracellular matrix (ECM), suppressive immune cells and fibroblasts1. Although recent improvements to chemotherapeutic regimens against tumor epithelia have increased patient survival2, 3, much of the current research and development of novel treatments for PDA have increasingly focused on targeting the various stromal constituents because of their important roles in facilitating disease progression and resistance. One example involves targeted degradation of the ECM component hyaluronan, or hyaluronic acid (HA), which has been shown to fundamentally alter the biophysical properties of the tumor by increasing interstitial gel-fluid pressures, contributing to the collapse of blood vessels and preventing drugs from efficiently entering the tumor bed4–6. A pegylated form of hyaluronidase, PEGPH20, may enhance the efficacy of standard-of-care chemotherapy by increasing tumor penetration and drug exposure4, 5, 7. In addition, agents aimed at relieving immunosuppression and adoptive immune cell strategies are also being rigorously pursued with some encouraging results8–12.
Fibroblasts have long been known to be a fundamental and near-universal component of PDA13, but their role in tumor progression and maintenance has proven to be unexpectedly nuanced, with conflicting reports suggesting that they can provide both pro-tumorigenic support and anti-tumorigenic restraint14–17. Recent advances in genetically engineered mouse models (GEMM) of PDA along with improved characterization of fibroblast subpopulations are providing a clearer understanding of their diverse roles and suggest new therapeutic approaches that may be effective alone or in combination with other therapies.
Fibroblasts accumulate in the pancreas during acute and chronic pancreatitis18, as well as in early pre-invasive lesions such as pancreatic intraepithelial neoplasms (PanIN), mucinous cystic neoplasms (MCN) and intraductal papillary mucinous neoplasms (IPMN)19–25, but increased numbers are seen in PDA. The fibrotic responses likely originate from the activation and subsequent expansion of local, so-called quiescent pancreatic stellate cells (PSC), but infiltration of fibroblasts derived from mesenchymal precursors in the circulation may also occur26. What begins as essentially a wound response to microscopic lesions evolves into a comprehensive transformation of the nascent microenvironment, involving complex paracrine signaling to and from fibroblasts, activated deposition of diverse ECM components and, ultimately, establishment of an immune- and drug-privileged sanctuary for the tumor epithelia. While tumor epithelial cells orchestrate these changes, fibroblasts are in many ways complicit in the remodeling of the tumor microenvironment (TME) as direct mediators of paracrine signaling and matrix deposition. However, lumping cancer-associated fibroblasts (CAF) into a single group is insufficient to describe the many diverse, and sometimes conflicting, roles they play. Fibroblasts can be categorized by expression of specific marker proteins into subpopulations with distinct phenotypes, gene expression patterns and functions, not unlike the hierarchical stratification of immune cell populations27, 28. Understanding the diversity of fibroblast subtypes, including their engagement in various paracrine signaling pathways, distinct programs of TME remodeling and differences in relative abundance within tumor subtypes, will be critical to the design of safe and effective therapies targeting this facet of the PDA neo-organ.
Delineation of PDA-associated fibroblast subtypes or activation states
At the most fundamental level, fibroblasts can be considered to be either quiescent or activated. Quiescent fibroblasts are primarily thought of as “normal”, tissue-resident fibroblasts or PSC that inhabit the pancreas prior to the development of preinvasive or invasive disease. Protein markers characteristic of this population include desmin and glial fibrillary acidic protein (GFAP), among others, and they are also usually non-proliferative (i.e. Ki67-negative)29–31. Quiescent PSC also exhibit cytoplasmic lipid droplets that are reservoirs for vitamin A30, 32, but the primary role of quiescent PSC is to monitor the integrity of the surrounding pancreatic tissues and, due to the precarious nature of an organ filled with digestive enzymes, respond quickly and robustly to injury to contain and heal tissue damage. Indeed, PSC can become activated by mechanical injury, chemical insult (e.g. alcohol), toxins (e.g spider venom) or inflammatory injury (e.g. autoimmune pancreatitis) to produce a fibrotic wound-healing response, which is dramatically visualized in cases of acute or chronic pancreatitis33–37. However, more detailed analyses of fibroblast subtypes and functions have not been performed in these settings. Interestingly, while fibrosis in acute pancreatitis may be resolve after removal of the inciting insult, fibroblast activation may also paradoxically decrease in more advanced stages of CP, perhaps due to complete resorption of necrotic debris, and/or depletion of inciting pancreatic enzymes and subsequent loss of paracrine signaling from nearby macrophages34. Pre-invasive lesions (i.e. PanIN38, MCN39 or IPMN40) in humans and mice19–25 also induce PSC activation, either as a result of microscopic structural deformations and ductal obstruction (inducing injury and obstructive lobular atrophy)41 or by direct paracrine signaling from transformed epithelia.
Activated fibroblasts constitute a significant fraction of the PDA bulk and may represent an organizing feature of the overall tumor architecture since they arrange themselves concentrically around epithelial clusters and deposit aligned structural components of the ECM42. As a whole, activated CAF are equipped to play many structural, biophysical and signaling roles in PDA, but some markers, including alpha smooth muscle actin (αSMA), fibroblast activation protein alpha (FAPα) and platelet-derived growth factor receptor (PDGFR), have been proposed to help delineate subpopulations of activated fibroblasts with more circumscribed features and functions. While αSMA positivity has long been the gold standard for identifying an activated fibroblast, FAPα43 has more recently risen to prominence as a marker that defines a more pro-tumorigenic fibroblast population (see below). Differing reports suggest that these two markers may describe distinct but overlapping populations or, alternatively, be mutually exclusive14, 16. There is the additional, intriguing suggestion that such populations inhabit distinct strata of the fibrotic response surrounding tumor epithelial structures, with αSMA+ myofibroblasts tightly surrounding ductal structures and FAPα+ fibroblasts more distal, indicating that spatial cues direct their activation or vice versa44, 45. PDGFR expression appears to further define a subset of αSMA+ fibroblasts, though FAPα+ fibroblasts may also express PDGFR46. Taken together, even just these three markers can potentially describe up to eight distinct populations, and although they provide a starting point to define distinct CAF populations, further discrimination with additional markers such as those found on mesenchymal stem cells47 may prove necessary.
Further complicating these attempts at classification, fibroblasts in the pancreas appear to be remarkably plastic and dynamic48. The observation of activated fibroblasts in the settings of acute and chronic pancreatitis, as well as with PDA precursors suggest a stepwise genesis of CAF, from quiescent PSC to injury-activated fibroblasts to CAF. However, it remains unclear whether these fibroblast subpopulations are wholly distinct, organized into a hierarchy or represent reversible activation states. Regardless, that both the accumulation and the activation of fibroblasts resolve after extinguishing mutant Kras expression in reversibly inducible GEMM of PDA49, 50 hints that CAF can potentially be normalized and cleared once invasive disease is eradicated. Targeted depletion of αSMA+ fibroblasts has no effect on FAPα+ populations16, whereas depletion of FAPα+ fibroblasts simultaneously reduced αSMA+ fibroblasts by 70%14. These findings, too, could result from sequential activation through hierarchical states or by asymmetric crosstalk between distinct fibroblast populations.
Paracrine signaling from and to PDA-associated fibroblast populations
CAF participate in a coordinated signaling network that is shared by tumor cells, the immune infiltrate and other cell populations. Certain critical cytokines and other signaling molecules have been identified that have principal roles in defining cell behavior and modulating the TME. At early stages of tumor development, tissue-resident PSC become activated after receiving stimuli from the nascent lesion. These signals include diverse growth factors51, inflammatory cytokines52, oxidative stress, autophagy53 and/or paracrine Hedgehog (Hh) signaling from transformed epithelial cells (see below). Ultimately, PSC activation leads to a change in phenotype wherein these quiescent fibroblasts lose their lipid droplets, express markers of activation such as αSMA and FAPα, and ramp up secretion of their own signaling molecules and ECM constituents. One model suggests that a gradient of cytokines, perhaps primarily Tgfβ and Il-1, radiating outward from epithelial lesions influences fibroblast intracellular signaling to drive the distinct subtypes as defined by αSMA or FAPα expression54.
Global analyses of the PSC secretome have identified hundreds of signaling proteins that are further enriched after PSC activation46, 55. Distinct activated fibroblast subtypes have been primarily defined by the presence or absence of αSMA expression (and in at least one study, with the αSMA- population expressed more than an order of magnitude greater FAPα at an adjusted p-value of <10−14). These subtypes express highly distinct secretomes45. Of the many notable differences, the upregulation of Il6, Lif, Csf3, Cxcl1 and Cxcl2 in αSMA-/FAPα+ versus αSMA+/FAPα- CAF undoubtedly elicit widespread effects: 1) Il6 may bolster chemoresistance of PDA through inflammatory monocytes and STAT3 signaling56, 57; 2) Lif mediates remodeling of intratumoral nerves in PDA58 and may be a key enforcer of the αSMA-/FAPα+ phenotype through autocrine JAK/STAT signaling54; 3) Csf3 may sustain granulocytic myeloid-derived suppressor cells (Gr-MDSC)10 and thereby enhance tumor growth59; and 4) the Cxcr2 ligands, Cxcl1 and Cxcl2, mediate the influx of immunosuppressive cells in PDA and support metastatic outgrowth9, 60. FAPα+ CAF in other cancer types similarly promote immunosuppression through increased expression of Il6, Cxcl2, Cxcl12 and Ccl2, suggesting the conservation of this distinction between αSMA-/FAPα+ and αSMA+/FAPα- CAF subpopulations61. Yet another example of subtype-specific paracrine signaling occurs through GM-CSF, a well-known modulator of immunosuppressive cells, and which is specifically expressed by a population of stem-like fibroblasts marked by CD90, CD49α, CD44 and CD7347. Fibroblast signaling can also affect tumor epithelial gene expression: IGF1 and HGF were highly expressed by stromal cells (although not necessarily fibroblasts or specific fibroblast subtypes) but their cognate receptors IGF1R and MET, respectively, were preferentially expressed on tumor epithelial cells in patient-derived xenograft (PDX) models of PDA62. Finally, in addition to cytokines and chemokines, secretion of metabolites such as alanine from activated, αSMA+ PSC can bolster tumor growth metabolically by supplying a carbon source for the TCA cycle and supporting biosynthesis of non-essential amino acids in tumor epithelia63, 64.
While NF-κB and JAK/STAT signaling have been shown to help define CAF subtypes45, 54, Hh signaling from tumor epithelial cells to fibroblasts is also likely among the most critical pathways in differentiating fibroblast subtypes. Sonic hedgehog (Shh) and Indian hedgehog (Ihh) are highly expressed by tumor epithelia (as opposed to stromal cells)62 and the effects of Hh signaling in stromal fibroblasts (i.e. myofibroblasts) have been extensively studied, mostly those defined by αSMA positivity65–68. Whereas short-term, pharmacological inhibition of Hh signaling in GEMM of PDA increased tumor perfusion and chemotherapeutic effect by arrest of myofibroblast proliferation15, depletion of desmoplastic stroma and stimulation of angiogenesis, long-term inhibition of Hh signaling or pancreas-specific genetic ablation of Shh in GEMM shortens survival and increases disease aggressiveness17, 69. Deletion of the surface receptor, Smoothened (Smo), that mediates Hh signaling in fibroblasts had a similar effect67. These results suggest that Hh signaling from tumor epithelia to fibroblasts may also support tumor-suppressive activities, though the exact mechanism is unknown. One intriguing possibility is that inhibition of Hh signaling selectively depletes a tumor-suppressing population of fibroblasts, with lesser effect on tumor-promoting fibroblasts. This hypothesis is supported by the observation that the expression of Hh signaling regulators, Ptch1/2 and Smo, are significantly different between αSMA+/FAPα- (putatively tumor-suppressing) and αSMA-/FAPα+ (putatively tumor-promoting) fibroblast populations45: αSMA+/FAPα- fibroblasts are also physically closer to tumor epithelia, the source of Hh ligand, and they express less Ptch1/2 and more Smo (negative and positive regulators of Hh signaling, respectively) than the more distal αSMA-/FAPα+ fibroblasts, suggesting higher overall Hh pathway activity in the former (and therefore greater sensitivity to inhibition) than in the latter population (Figure 1).
Figure 1. Differential Hh signaling in αSMA+ and FAPα+ fibroblast subpopulations.
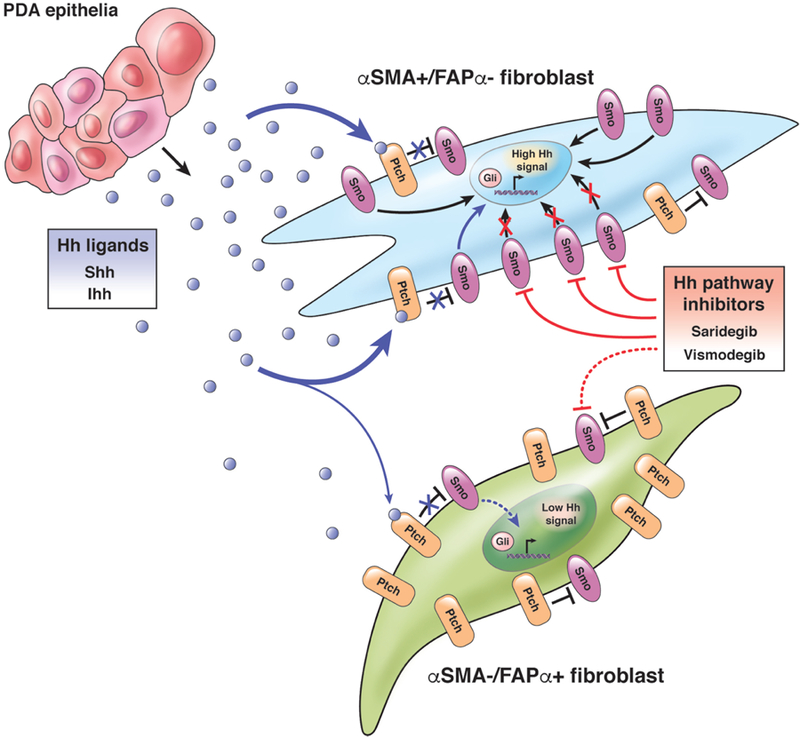
αSMA+/FAPα-fibroblasts are physically closer to PDA epithelia and likely have higher overall Hh signaling than αSMA-/FAPα+ fibroblasts due to greater exposure to epithelial-derived Hh ligand, lower expression of Ptch1/2 and higher expression of Smo. For these reasons, inhibitors of Smo, such as saridegib and vismodegib, may preferentially target this αSMA+/FAPα- fibroblast subpopulation.
Remodeling TEM structure and function by CAF subtypes
Although fibroblasts and immune cells comprise much of the cellular mass in PDA, ECM proteins and glycosaminoglycans (GAGs) secreted by activated fibroblasts also contribute substantially to the tumor bulk. Co-culture experiments have shown that fibroblasts can also stimulate tumor epithelia to contribute to ECM remodeling (e.g. by upregulated expression of hyaluronan synthase 2 (Has2), an enzyme that catalyzes the extension of HA polymers)70. Collagens, fibronectin, versican and many other proteins are secreted by activated fibroblasts62, 71, in addition to GAGs such as HA and chondroitin sulfate4. Fibroblast-mediated secretion of these structural components of the ECM can greatly influence tumor biology by altering the biophysical properties of the TME and activating additional signaling pathways in both tumor epithelia and stromal cells. The ECM proteins can be assembled and aligned in tethered networks, assigning polarity to the ECM42. The GAGs can bind water and swell against these restraints, combining to induce high interstitial pressures that collapse blood vessels and isolate the tumor from circulating therapeutics and anti-tumor immune cells4. Moreover, the aligned protein network can also provide tracks along which tumor cells can migrate42, and the degree of collagen alignment has been shown to be a negative prognostic factor for patients with resected PDA72. These matrix proteins and GAGs also engage diverse intracellular signaling pathways through cell surface receptors, such as integrins, discoidin domain receptors and CD44, that can further influence tumor phenotypes73, 74.
Many of the above described protein factors, including collagens 1a1, 1a2, 2a1, 3a1, 5a1, 11a1, 18a1, fibronectin and versican are differentially expressed by distinct fibroblast populations45, which may have important prognostic implications (see below). Furthermore, expression levels of hyaluronan synthases, Has1 and Has2, are significantly lower in αSMA+ fibroblasts45, suggesting that fibroblast subpopulations may also differentially contribute to GAG deposition. These comparative gene expression analyses are supported by experiments with targeted depletion of a single fibroblast subtype. When αSMA+ fibroblasts are depleted, collagen levels are decreased along with the elastic modulus and tissue stiffness, but HA is unchanged16; depletion of FAPα+ fibroblasts similarly reduces collagen content but also results in loss of HA deposition14 and additionally reduces versican and fibronectin levels. Thus, the distinct fibroblast subpopulations may also disparately influence distinct contributors to intratumoral biophysics, including free and gel-fluid pressures as well as solid stress.
In addition to the differences in secretion of collagens and HA described above, fibroblast subtypes exhibit different states of tension. αSMA+ fibroblasts, often called “myofibroblasts” because of their resemblance to smooth muscle cells, anchor to ECM components (i.e. collagen) via integrin receptors and propagate tension through contraction of intracellular smooth muscle actin75, 76; conversely αSMA- (FAPα+?) fibroblasts appear to be less rigid and may not contribute substantively to tissue tension. Solid stress and fluid pressures contribute to the overall forces exerted on cells in the tumor, but there are indications that tension itself can affect tumor progression, drug response and patient survival77, 78. Abrogation of these forces either by PEGPH20-mediated degradation of HA and removal of the swelling pressure that loads tethered collagen fibers or by reducing solid stress through inhibition of collagen synthesis79 or depletion of αSMA+ fibroblasts through Hh pathway inhibition, can open blood vessels in PDA GEMM and increase perfusion4, 15. Ultimately, in this respect, it will be important to determine whether targeted depletion of specific fibroblast subpopulations differentially affects free fluid pressures, gel-fluid pressures, tension or solid stress, as well as measuring the impact on drug perfusion.
Do fibroblast subtypes correlate to PDA subtypes?
In addition to the subpopulations of activated fibroblasts described above, subtypes of PDA have been identified based on the genomic analyses of the tumor epithelium80–82. Profiles of each of these compartments from the same tumor specimen have even been dichotomized virtually to test the hypothesis that epithelial and stromal subtypes evolve coordinately71. The genes used to delineate subtypes in these large-scale genomic studies often code for secreted factors, implicating the secretome as a defining feature. For example, LGALS4 and TFF1/2/3 are upregulated in the “Classical” PDA subtype across multiple studies71, 81, 82; in the stromal compartment, SPARC, COL1A2, MMP11 and POSTN contribute to the “Activated stroma” signature, while DES, IGF1 and OGN are expressed by “Normal stroma”71. The tumor (epithelial) subtype and the stromal subtype can independently influence patient prognosis. However, specific tumor epithelial subtypes did not correspond to stromal subtypes in these studies, suggesting somewhat counterintuitively that the epithelial and stromal compartments progress independently. The four cardinal genetic abnormalities found in PDA epithelial cells (KRAS mutation, P53 mutation, SMAD4 loss and CDKN2A loss) for the most part do not significantly associate with particular subtypes as defined by such global gene expression analyses, although SMAD4 loss and TP53 mutation trend with basal-like71 and squamous80 subtypes, respectively.
Despite this apparent disconnect between epithelial and stromal subtypes, discrete mutations or epigenetic alterations (such as the cardinal events in PDA progression described above) can alter intracellular signaling to modify the cancer cell secretome and subsequently influence fibroblast phenotype; while this hypothesis has not been directly tested, several studies support its merit. First, each genomic alteration is associated with distinct clinicopathologic features and patient prognosis83, 84, demonstrating not surprisingly that tumor phenotypes are influenced by their mutational repertoire. Oncogenic activation of KRAS, the common initiating event in pancreatic carcinogenesis, stimulates Il6 and Shh expression and secretion to activate fibroblasts49, 85. Since KRAS activation occurs in >95% of PDA, oncogenic KRAS-dependent secreted factors can be considered foundational influences on fibroblast activation, whereas subsequent acquired mutations may shape the diversity and phenotypes of fibroblast subpopulations (Figure 2). For example, compared to the background of GEMM with pancreas-specific Kras activation (KC), the additional heterozygous deletion of Tgfbr2 in the epithelium resulted in increased collagen deposition, tissue stiffness, fibronectin and galectin-4 in the tumor stroma77, 86. Mutation of Trp53 in the same KC model77 or in culture systems87 also altered the epithelial secretome and paracrine signaling to influence ECM composition. Loss of Cdkn2a expression similarly induced widespread changes to gene expression88, including upregulation of chondroitin sulfate synthase 3 (CHSY3). Thus, as PDA progress and evolve, successive genomic alterations in these cardinal and other passenger genes may dynamically perturb the cancer cell secretome to influence fibroblast heterogeneity and activity across time and space86, 89.
Figure 2. Effects of cardinal genomic alterations in PDA may disparately influence fibroblast context, function and heterogeneity.
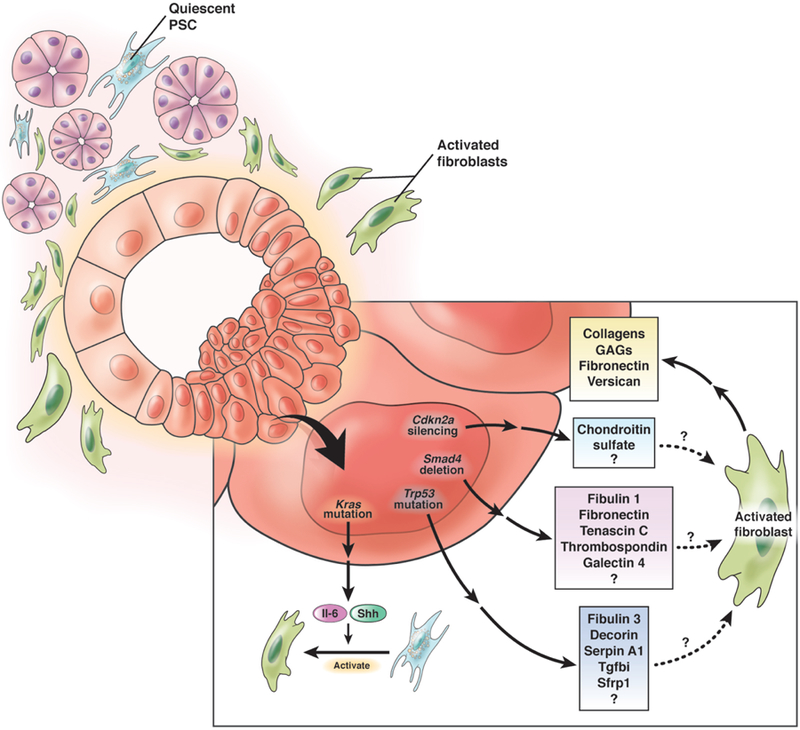
Activating Kras mutation in pancreatic epithelial cells induces production of Il-6 and Shh, which help convert quiescent PSC to activated fibroblasts. Subsequent mutation of Trp53, loss of Smad4 and/or silencing of Cdkn2a in the neoplastic epithelium may differentially shape the ECM through distinct mechanisms of paracrine signaling to fibroblasts.
Fibroblasts in MCN-PDA
One particularly notable example of PDA genotype driving fibroblast phenotype (or perhaps the reverse) is the clinically and histopathologically distinctive MCN. These PDA precursors, which are commonly discovered incidentally and often do not progress to invasive or metastatic disease90, exhibit a distinct fibroblast phenotype wherein estrogen receptor (ER) and/or progesterone receptor (PR) are expressed in peri-cystic, “ovarian-type” fibroblasts91. In fact, it is the hormone receptor positivity in these fibroblasts along with the characteristic wavy nuclei of an “ovarian stroma”, and not any molecular or phenotypic feature of the tumor epithelia, that provide the conclusive and requisite diagnostic criteria by which MCN are defined (Figure 3). Together with the >20:1 female:male incidence ratio and the frequent presentation at peri-menopausal age, these features strongly suggest that circulating hormones influence the development of the tumor and stroma in MCN. Studies in GEMM and human patients further suggest that MCN can be directed to arise after specific sequelae of genomic alterations: for example, when pancreatic epithelial cells undergo loss of Smad4 after Kras activation but before Trp53 mutation or Cdkn2a silencing MCNs are favored over PanINs20, 92. Immunohistochemical assessment of SMAD4 expression in human MCN shows that nearly 90% of invasive MCN lack SMAD4 (i.e. have lost expression from both alleles) obscuring the possibility that an initial heterozygous mutation in SMAD4 represents a (relatively) early event92. This can occur either by stepwise deletion of one allele and subsequent loss of heterozygosity or epigenetic silencing or some combination, which may or may not be detected by selective genomic techniques93. In other GEMM, virally-induced overexpression of Wnt1 or targeted expression of Hif2α in the context of pancreatic Kras activation enhanced the development of MCN lesions24, 25, indicating potential roles for Wnt signaling and hypoxia in MCN evolution.
Figure 3. MCN are characterized by unique fibroblast subpopulations.
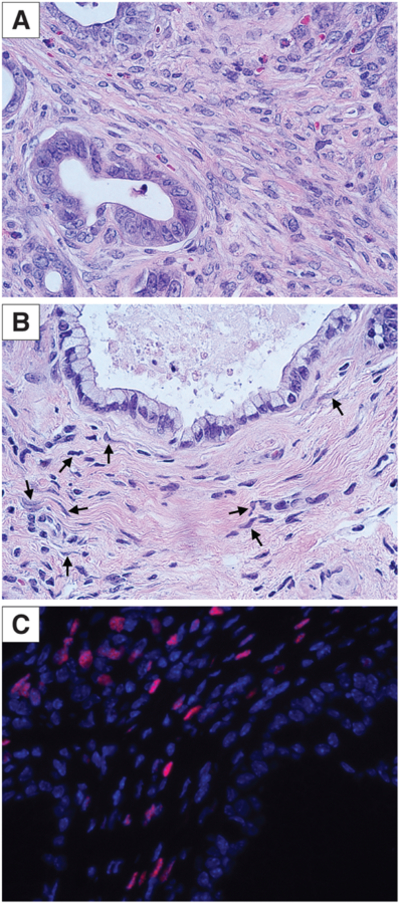
A) Stromal fibroblasts in murine PanIN. B) Ovarian-type stromal fibroblasts with characteristic wavy nuclei (arrows) in murine MCN. C) Nuclear ER positivity (red) in fibroblasts of murine MCN.
The prognosis for patients with PDA derived from MCN is substantially better than for those with conventional PDA derived from PanIN94, 95, despite a highly conserved mutational repertoire between these two forms of invasive disease96. Since the unique fibroblast features are a defining property of MCN, it is not unreasonable to suggest that these fibroblasts may be, at least partially, responsible for this difference in prognosis. Detailed comparisons of fibroblasts from conventional and MCN-derived PDA have not been performed, although αSMA expression has been shown in one study to be roughly comparable97. Thus, many important questions remain regarding this hormone receptor-positive fibroblast phenotype, including: 1) how tumoral SMAD4 loss (or other canonical genomic alterations), Wnt signaling, hypoxia and hormonal factors integrate to create a ER/PR+ ovarian stroma; 2) whether the ER/PR positive fibroblasts overlap with any of the previously defined fibroblast subtypes; 3) how the expression of secreted factors or other genes differs in the ER/PR+ population of fibroblasts; and 4) whether targeting ER/PR+ stromal fibroblasts would influence progression of MCN to PDA.
Is targeting fibroblasts a viable strategy for PDA therapy?
Fibroblasts may support tumor growth by several mechanisms, including: 1) secretion of ECM components that contribute to elevated force generation and transmission76, interstitial pressures, vascular collapse and drug exclusion4, 2) recruitment and programming of immune populations to sustain an immunosuppressive environment16, 18, 3) sequestration of intratumoral gemcitabine98 and/or 4) radioprotection of tumor cells99. Not surprisingly, given the complexity of fibroblast populations and functions in the tumor stroma, experiments addressing the question of whether to target fibroblasts in PDA have given mixed results. Several studies have arrived at divergent conclusions regarding whether a highly dense tumor stroma (or low cellularity) is a positive or negative prognostic factor for patient survival and metastasis100–103. When focusing on fibroblasts specifically, retrospective analyses have shown that high stromal levels of either αSMA or FAPα correlate with poorer survival44, 104–107. Nevertheless, targeted depletion of specific fibroblast subpopulations in PDA indicates that the distinctions between fibroblast populations are important (Table 1). In studies where depletion of fibroblasts was detrimental, αSMA was considered the primary fibroblast marker, suggesting that αSMA+ fibroblasts are anti-tumorigenic16, 17. In contrast, in experiments where fibroblast depletion improved outcomes, FAPα was generally the targeted marker and consequently FAPα+ fibroblasts may be predominantly protumorigenic14, 108.
Table 1.
Representative examples of fibroblast subtype-specific depletion strategies
| Fibroblast marker | Depletion strategies | Effect of depletion | Therapeutic potential | ||||
|---|---|---|---|---|---|---|---|
| Fibroblasts | ECM | Epithelial cells | Immune cells | Outcomes | |||
| αSMA | αSMA-directed TK/Ganciclovir suicide gene (Özdemir, et al. 2014) |
80% depletion of αSMA+ fibroblasts Unchanged FAPα+ fibroblasts |
Loss of collagen content and organization Decreased elastic modulus and tissue stiffness Unchanged HA content |
Decreased differentiation Increased expression of epithelial-to-mesynchymal transition markers |
Reduced CD45+ immune infiltrate Increased immunosuppressive T-regulatory cells Decreased T-effector cells |
Increased hypoxia Increased metastasis Decreased survival Does not improve gemcitabine therapy |
Unlikely1 |
| Hh pathway inhibition (Rhim, et al. 2014) |
80% depletion of αSMA+ fibroblasts | N/A | Decreased differentiation Increased expression of epithelial-to-mesynchymal transition markers Increased proliferation |
Reduced CD45+ immune infiltrate Decreased F4/80+ monocytes |
Increased vascular density and perfusion Increased metastasis Decreased survival Does not improve gemcitabine therapy |
Acute treatment: Perhaps Chronic treatment: No | |
| FAPα | CAR-T (Lo, et al. 2015) |
91% depletion of FAPα+ fibroblasts 70% depletion of αSMA+ fibroblasts |
Decreased collagen content Decreased HA content Decreased versican and fibronectin |
Decreased proliferation Increased apoptosis |
Decreased Gr-MDSC | Decreased vascular density Reduced tumor growth Improves gemcitabine therapy |
Yes |
There was an apparent benefit when this approach was used with anti-TReg cell agent suggesting a potential for combinatorial strategies.
In the current state of the art, the standard treatments for PDA generally include some combination of surgery, radiation and/or chemotherapy depending on clinical parameters such as disease stage, location of the tumor and its relationship to critical vessels, and performance status of the patient. No targeted therapies that account for mutational status or fibroblast heterogeneity are currently in use, though some clinical trials have incorporated the former and more global inhibition/modulation of fibroblasts has been tested. As we integrate the amassing data regarding fibroblast subpopulations and their effects on the ECM and tumor biology, novel targets such as FAPα+ fibroblasts or ER/PR+ fibroblasts may emerge more clearly. Undoubtedly, if fibroblasts are to be targeted in PDA, the treatment must precisely target specific fibroblast populations, since depletion of the anti-tumorigenic fibroblast subpopulation could (and has109) shortened patient survival. Accurate targeting could theoretically be accomplished through cutting-edge immunologic strategies such as designer CAR-T cells14, 110, but much more preclinical work is needed to characterize fibroblast subpopulations and the fates of other subpopulation(s) when one is specifically depleted.
To add to the complexity, inter- and intra-tumoral heterogeneity with respect to fibroblast subpopulations is completely unexplored; it may be that patients need to be screened by biopsy and assessed for the presence of certain fibroblast subpopulations to identify patient groups most likely to benefit from specific therapies. The ability to retrospectively stratify patients based on fibroblast markers and observe differences in survival supports the notion that, as with many other stromal targeted therapies5, anti-fibroblast therapies may have very select utility, with some patients receiving little to no benefit. Fibroblast-targeted therapies may also be less effective in metastases, eliminating the majority of patients, who present with Stage IV disease and are in most dire need of novel therapies. Conflicting reports propose that PDA metastases are either relatively astromal71 or similarly desmoplastic111 compared to the primary tumor, possibly depending on the size of metastatic lesion112. Certainly, there are at least some activated fibroblasts that associate with metastatic deposits and they may originate from local reservoirs113 or traverse tissues along with metastasizing tumor cells114. Although the inception of liver metastases seems to be independent of activated fibroblasts, metastatic outgrowth is slowed when recruitment/activation of αSMA+ fibroblasts is prevented113, which would not be predicted by the studies mentioned above describing the impact on the primary tumor of targeting αSMA+ cells. Since most PDA patients present with metastatic disease and nearly all patients eventually succumb with if not because of metastatic disease burden, a deeper comparison of fibroblast subtypes and secretomes between primary and metastatic sites may, therefore, have large ramifications for the deployment and success of fibroblast-targeted therapies in the clinic.
Overall, we should not be discouraged by the complexity of fibroblast heterogeneity in PDA. Rather, the complexity likely reflects both critical importance and therapeutic opportunity. Within the diverse ecosystem of the TME, fibroblasts are an important, influential and heterogeneous population of cells that have outsized role(s) in defining ECM composition and tumor biophysics. Anti-fibroblast therapies, even those targeting very specific subpopulations of pro-tumorigenic fibroblasts, may not be the proverbial silver bullet against PDA, but curative therapies will absolutely require a multi-faceted, multi-compartment approach, and consideration of fibroblast functions and effects will likely be of great benefit in this pursuit.
Acknowledgments
Grant Support: Work in the Hingorani Laboratory on stromal biology is supported in part by NIH/NCI R01 CA161112, U01 CA224193, R50 CA211425, Cancer Center Support Grant P30 CA015704 and the Pancreatic Cancer Action Network Precision Medicine Targeted Grant 17-85-HING.
Biography


Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: Sunil R. Hingorani is a consultant for Halozyme Therapeutics.
REFERENCES
- 1.Stromnes IM, DelGiorno KE, Greenberg PD, et al. Stromal reengineering to treat pancreas cancer. Carcinogenesis 2014;35:1451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–25. [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012;21:418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hingorani SR, Zheng L, Bullock AJ, et al. HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in Patients With Untreated, Metastatic Pancreatic Ductal Adenocarcinoma. J Clin Oncol 2018;36:359–366. [DOI] [PubMed] [Google Scholar]
- 6.DuFort CC, DelGiorno KE, Carlson MA, et al. Interstitial Pressure in Pancreatic Ductal Adenocarcinoma Is Dominated by a Gel-Fluid Phase. Biophys J 2016;110:2106–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hingorani SR, Harris WP, Beck JT, et al. Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer. Clin Cancer Res 2016;22:2848–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Byrne KT, Yan F, et al. Tumor Cell-Intrinsic Factors Underlie Heterogeneity of Immune Cell Infiltration and Response to Immunotherapy. Immunity 2018;49:178–193 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steele CW, Karim SA, Leach JDG, et al. CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer Cell 2016;29:832–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stromnes IM, Brockenbrough JS, Izeradjene K, et al. Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut 2014;63:1769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stromnes IM, Schmitt TM, Hulbert A, et al. T Cells Engineered against a Native Antigen Can Surmount Immunologic and Physical Barriers to Treat Pancreatic Ductal Adenocarcinoma. Cancer Cell 2015;28:638–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011;331:1612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yen TW, Aardal NP, Bronner MP, et al. Myofibroblasts are responsible for the desmoplastic reaction surrounding human pancreatic carcinomas. Surgery 2002;131:129–34. [DOI] [PubMed] [Google Scholar]
- 14.Lo A, Wang LS, Scholler J, et al. Tumor-Promoting Desmoplasia Is Disrupted by Depleting FAP-Expressing Stromal Cells. Cancer Res 2015;75:2800–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009;324:1457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014;25:719–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014;25:735–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helm O, Mennrich R, Petrick D, et al. Comparative characterization of stroma cells and ductal epithelium in chronic pancreatitis and pancreatic ductal adenocarcinoma. PLoS One 2014;9:e94357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003;4:437–50. [DOI] [PubMed] [Google Scholar]
- 20.Izeradjene K, Combs C, Best M, et al. Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell 2007;11:229–43. [DOI] [PubMed] [Google Scholar]
- 21.Taki K, Ohmuraya M, Tanji E, et al. GNAS(R201H) and Kras(G12D) cooperate to promote murine pancreatic tumorigenesis recapitulating human intraductal papillary mucinous neoplasm. Oncogene 2016;35:2407–12. [DOI] [PubMed] [Google Scholar]
- 22.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005;7:469–83. [DOI] [PubMed] [Google Scholar]
- 23.Ideno N, Yamaguchi H, Ghosh B, et al. GNAS(R201C) Induces Pancreatic Cystic Neoplasms in Mice That Express Activated KRAS by Inhibiting YAP1 Signaling. Gastroenterology 2018. [DOI] [PMC free article] [PubMed]
- 24.Sano M, Driscoll DR, De Jesus-Monge WE, et al. Activated wnt signaling in stroma contributes to development of pancreatic mucinous cystic neoplasms. Gastroenterology 2014;146:257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schofield HK, Tandon M, Park MJ, et al. Pancreatic HIF2alpha Stabilization Leads to Chronic Pancreatitis and Predisposes to Mucinous Cystic Neoplasm. Cell Mol Gastroenterol Hepatol 2018;5:169–185 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quante M, Tu SP, Tomita H, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 2011;19:257–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Busch S, Andersson D, Bom E, et al. Cellular organization and molecular differentiation model of breast cancer-associated fibroblasts. Mol Cancer 2017;16:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambrechts D, Wauters E, Boeckx B, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med 2018;24:1277–1289. [DOI] [PubMed] [Google Scholar]
- 29.Takase S, Leo MA, Nouchi T, et al. Desmin distinguishes cultured fat-storing cells from myofibroblasts, smooth muscle cells and fibroblasts in the rat. J Hepatol 1988;6:267–76. [DOI] [PubMed] [Google Scholar]
- 30.Apte MV, Haber PS, Applegate TL, et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut 1998;43:128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen MFB, Mortensen MB, Detlefsen S. Identification of markers for quiescent pancreatic stellate cells in the normal human pancreas. Histochem Cell Biol 2017;148:359–380. [DOI] [PubMed] [Google Scholar]
- 32.Kim N, Yoo W, Lee J, et al. Formation of vitamin A lipid droplets in pancreatic stellate cells requires albumin. Gut 2009;58:1382–90. [DOI] [PubMed] [Google Scholar]
- 33.Haber PS, Keogh GW, Apte MV, et al. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am J Pathol 1999;155:1087–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Detlefsen S, Sipos B, Feyerabend B, et al. Fibrogenesis in alcoholic chronic pancreatitis: the role of tissue necrosis, macrophages, myofibroblasts and cytokines. Mod Pathol 2006;19:1019–26. [DOI] [PubMed] [Google Scholar]
- 35.Elsasser HP, Adler G, Kern HF. Fibroblast structure and function during regeneration from hormone-induced acute pancreatitis in the rat. Pancreas 1989;4:169–78. [DOI] [PubMed] [Google Scholar]
- 36.Kloppel G, Detlefsen S, Feyerabend B. Fibrosis of the pancreas: the initial tissue damage and the resulting pattern. Virchows Arch 2004;445:1–8. [DOI] [PubMed] [Google Scholar]
- 37.Odaira C, Berger Z, Iovanna JL, et al. Localized necrohemorrhagic pancreatitis in the rat after pancreatic interstitial trypsin injection. Regressive pseudochronic lesions. Digestion 1986;34:68–77. [DOI] [PubMed] [Google Scholar]
- 38.Detlefsen S, Sipos B, Feyerabend B, et al. Pancreatic fibrosis associated with age and ductal papillary hyperplasia. Virchows Arch 2005;447:800–5. [DOI] [PubMed] [Google Scholar]
- 39.Zamboni G, Scarpa A, Bogina G, et al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol 1999;23:410–22. [DOI] [PubMed] [Google Scholar]
- 40.Kakizaki Y, Makino N, Tozawa T, et al. Stromal Fibrosis and Expression of Matricellular Proteins Correlate With Histological Grade of Intraductal Papillary Mucinous Neoplasm of the Pancreas. Pancreas 2016;45:1145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brune K, Abe T, Canto M, et al. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Pathol 2006;30:1067–76. [PMC free article] [PubMed] [Google Scholar]
- 42.Erdogan B, Ao M, White LM, et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J Cell Biol 2017;216:3799–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garin-Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci U S A 1990;87:7235–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen SJ, Alpaugh RK, Palazzo I, et al. Fibroblast activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas 2008;37:154–8. [DOI] [PubMed] [Google Scholar]
- 45.Ohlund D, Handly-Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 2017;214:579–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Djurec M, Grana O, Lee A, et al. Saa3 is a key mediator of the protumorigenic properties of cancer-associated fibroblasts in pancreatic tumors. Proc Natl Acad Sci U S A 2018;115:E1147–E1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waghray M, Yalamanchili M, Dziubinski M, et al. GM-CSF Mediates Mesenchymal-Epithelial Cross-talk in Pancreatic Cancer. Cancer Discov 2016;6:886–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalluri R The biology and function of fibroblasts in cancer. Nat Rev Cancer 2016;16:582–98. [DOI] [PubMed] [Google Scholar]
- 49.Collins MA, Bednar F, Zhang Y, et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest 2012;122:639–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ying H, Kimmelman AC, Lyssiotis CA, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012;149:656–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneider E, Schmid-Kotsas A, Zhao J, et al. Identification of mediators stimulating proliferation and matrix synthesis of rat pancreatic stellate cells. Am J Physiol Cell Physiol 2001;281:C532–43. [DOI] [PubMed] [Google Scholar]
- 52.Mews P, Phillips P, Fahmy R, et al. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut 2002;50:535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Endo S, Nakata K, Ohuchida K, et al. Autophagy Is Required for Activation of Pancreatic Stellate Cells, Associated With Pancreatic Cancer Progression and Promotes Growth of Pancreatic Tumors in Mice. Gastroenterology 2017;152:1492–1506 e24. [DOI] [PubMed] [Google Scholar]
- 54.Biffi G, Oni TE, Spielman B, et al. IL-1-induced JAK/STAT signaling is antagonized by TGF-beta to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov 2018. [DOI] [PMC free article] [PubMed]
- 55.Wehr AY, Furth EE, Sangar V, et al. Analysis of the human pancreatic stellate cell secreted proteome. Pancreas 2011;40:557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Long KB, Tooker G, Tooker E, et al. IL6 Receptor Blockade Enhances Chemotherapy Efficacy in Pancreatic Ductal Adenocarcinoma. Mol Cancer Ther 2017;16:1898–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mace TA, Ameen Z, Collins A, et al. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res 2013;73:3007–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bressy C, Lac S, Nigri J, et al. LIF Drives Neural Remodeling in Pancreatic Cancer and Offers a New Candidate Biomarker. Cancer Res 2018;78:909–921. [DOI] [PubMed] [Google Scholar]
- 59.Pickup MW, Owens P, Gorska AE, et al. Development of Aggressive Pancreatic Ductal Adenocarcinomas Depends on Granulocyte Colony Stimulating Factor Secretion in Carcinoma Cells. Cancer Immunol Res 2017;5:718–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steele CW, Karim SA, Foth M, et al. CXCR2 inhibition suppresses acute and chronic pancreatic inflammation. J Pathol 2015;237:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang X, Lin Y, Shi Y, et al. FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the Tumor Microenvironment via STAT3-CCL2 Signaling. Cancer Res 2016;76:4124–35. [DOI] [PubMed] [Google Scholar]
- 62.Nicolle R, Blum Y, Marisa L, et al. Pancreatic Adenocarcinoma Therapeutic Targets Revealed by Tumor-Stroma Cross-Talk Analyses in Patient-Derived Xenografts. Cell Rep 2017;21:2458–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sousa CM, Biancur DE, Wang X, et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 2016;536:479–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sherman MH, Yu RT, Tseng TW, et al. Stromal cues regulate the pancreatic cancer epigenome and metabolome. Proc Natl Acad Sci U S A 2017;114:1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Horn A, Palumbo K, Cordazzo C, et al. Hedgehog signaling controls fibroblast activation and tissue fibrosis in systemic sclerosis. Arthritis Rheum 2012;64:2724–33. [DOI] [PubMed] [Google Scholar]
- 66.Jung IH, Jung DE, Park YN, et al. Aberrant Hedgehog ligands induce progressive pancreatic fibrosis by paracrine activation of myofibroblasts and ductular cells in transgenic zebrafish. PLoS One 2011;6:e27941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu X, Pitarresi JR, Cuitino MC, et al. Genetic ablation of Smoothened in pancreatic fibroblasts increases acinar-ductal metaplasia. Genes Dev 2016;30:1943–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bailey JM, Swanson BJ, Hamada T, et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res 2008;14:5995–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee JJ, Perera RM, Wang H, et al. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proc Natl Acad Sci U S A 2014;111:E3091–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sato N, Maehara N, Goggins M. Gene expression profiling of tumor-stromal interactions between pancreatic cancer cells and stromal fibroblasts. Cancer Res 2004;64:6950–6. [DOI] [PubMed] [Google Scholar]
- 71.Moffitt RA, Marayati R, Flate EL, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet 2015;47:1168–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Drifka CR, Loeffler AG, Mathewson K, et al. Highly aligned stromal collagen is a negative prognostic factor following pancreatic ductal adenocarcinoma resection. Oncotarget 2016;7:76197–76213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aguilera KY, Huang H, Du W, et al. Inhibition of Discoidin Domain Receptor 1 Reduces Collagen-mediated Tumorigenicity in Pancreatic Ductal Adenocarcinoma. Mol Cancer Ther 2017;16:2473–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yan Y, Li Z, Kong X, et al. KLF4-Mediated Suppression of CD44 Signaling Negatively Impacts Pancreatic Cancer Stemness and Metastasis. Cancer Res 2016;76:2419–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gabbiani G, Hirschel BJ, Ryan GB, et al. Granulation tissue as a contractile organ. A study of structure and function. J Exp Med 1972;135:719–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paszek MJ, Zahir N, Johnson KR, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005;8:241–54. [DOI] [PubMed] [Google Scholar]
- 77.Laklai H, Miroshnikova YA, Pickup MW, et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat Med 2016;22:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vennin C, Chin VT, Warren SC, et al. Transient tissue priming via ROCK inhibition uncouples pancreatic cancer progression, sensitivity to chemotherapy, and metastasis. Sci Transl Med 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Diop-Frimpong B, Chauhan VP, Krane S, et al. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci U S A 2011;108:2909–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016;531:47–52. [DOI] [PubMed] [Google Scholar]
- 81.Collisson EA, Sadanandam A, Olson P, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med 2011;17:500–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao L, Zhao H, Yan H. Gene expression profiling of 1200 pancreatic ductal adenocarcinoma reveals novel subtypes. BMC Cancer 2018;18:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oshima M, Okano K, Muraki S, et al. Immunohistochemically detected expression of 3 major genes (CDKN2A/p16, TP53, and SMAD4/DPC4) strongly predicts survival in patients with resectable pancreatic cancer. Ann Surg 2013;258:336–46. [DOI] [PubMed] [Google Scholar]
- 84.Bournet B, Muscari F, Buscail C, et al. KRAS G12D Mutation Subtype Is A Prognostic Factor for Advanced Pancreatic Adenocarcinoma. Clin Transl Gastroenterol 2016;7:e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tape CJ, Ling S, Dimitriadi M, et al. Oncogenic KRAS Regulates Tumor Cell Signaling via Stromal Reciprocation. Cell 2016;165:910–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhong Y, Macgregor-Das A, Saunders T, et al. Mutant p53 Together with TGFbeta Signaling Influence Organ-Specific Hematogenous Colonization Patterns of Pancreatic Cancer. Clin Cancer Res 2017;23:1607–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shakya R, Tarulli GA, Sheng L, et al. Mutant p53 upregulates alpha-1 antitrypsin expression and promotes invasion in lung cancer. Oncogene 2017;36:4469–4480. [DOI] [PubMed] [Google Scholar]
- 88.Ju HQ, Ying H, Tian T, et al. Mutant Kras- and p16-regulated NOX4 activation overcomes metabolic checkpoints in development of pancreatic ductal adenocarcinoma. Nat Commun 2017;8:14437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Whittle MC, Izeradjene K, Rani PG, et al. RUNX3 Controls a Metastatic Switch in Pancreatic Ductal Adenocarcinoma. Cell 2015;161:1345–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crippa S, Salvia R, Warshaw AL, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg 2008;247:571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hruban RH, Fukushima N. Cystic lesions of the pancreas. Diagn Histopathol (Oxf) 2008;14:260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Iacobuzio-Donahue CA, Wilentz RE, Argani P, et al. Dpc4 protein in mucinous cystic neoplasms of the pancreas: frequent loss of expression in invasive carcinomas suggests a role in genetic progression. Am J Surg Pathol 2000;24:1544–8. [DOI] [PubMed] [Google Scholar]
- 93.Springer S, Wang Y, Dal Molin M, et al. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology 2015;149:1501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Griffin JF, Page AJ, Samaha GJ, et al. Patients with a resected pancreatic mucinous cystic neoplasm have a better prognosis than patients with an intraductal papillary mucinous neoplasm: A large single institution series. Pancreatology 2017;17:490–496. [DOI] [PubMed] [Google Scholar]
- 95.Postlewait LM, Ethun CG, McInnis MR, et al. Association of Preoperative Risk Factors With Malignancy in Pancreatic Mucinous Cystic Neoplasms: A Multicenter Study. JAMA Surg 2017;152:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Conner JR, Marino-Enriquez A, Mino-Kenudson M, et al. Genomic Characterization of Low- and High-Grade Pancreatic Mucinous Cystic Neoplasms Reveals Recurrent KRAS Alterations in “High-Risk” Lesions. Pancreas 2017;46:665–671. [DOI] [PubMed] [Google Scholar]
- 97.Kuroda N, Toi M, Nakayama H, et al. The distribution and role of myofibroblasts and CD34-positive stromal cells in normal pancreas and various pancreatic lesions. Histol Histopathol 2004;19:59–67. [DOI] [PubMed] [Google Scholar]
- 98.Hessmann E, Patzak MS, Klein L, et al. Fibroblast drug scavenging increases intratumoural gemcitabine accumulation in murine pancreas cancer. Gut 2018;67:497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mantoni TS, Lunardi S, Al-Assar O, et al. Pancreatic stellate cells radioprotect pancreatic cancer cells through beta1-integrin signaling. Cancer Res 2011;71:3453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heid I, Steiger K, Trajkovic-Arsic M, et al. Co-clinical Assessment of Tumor Cellularity in Pancreatic Cancer. Clin Cancer Res 2017;23:1461–1470. [DOI] [PubMed] [Google Scholar]
- 101.Torphy RJ, Wang Z, True-Yasaki A, et al. Stromal Content Is Correlated With Tissue Site, Contrast Retention, and Survival in Pancreatic Adenocarcinoma. JCO Precision Oncology 2018:1–12. [DOI] [PMC free article] [PubMed]
- 102.Wang LM, Silva MA, D’Costa Z, et al. The prognostic role of desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget 2016;7:4183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Knudsen ES, Vail P, Balaji U, et al. Stratification of Pancreatic Ductal Adenocarcinoma: Combinatorial Genetic, Stromal, and Immunologic Markers. Clin Cancer Res 2017;23:4429–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kawase T, Yasui Y, Nishina S, et al. Fibroblast activation protein-alpha-expressing fibroblasts promote the progression of pancreatic ductal adenocarcinoma. BMC Gastroenterol 2015;15:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shi M, Yu DH, Chen Y, et al. Expression of fibroblast activation protein in human pancreatic adenocarcinoma and its clinicopathological significance. World J Gastroenterol 2012;18:840–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fujita H, Ohuchida K, Mizumoto K, et al. alpha-Smooth Muscle Actin Expressing Stroma Promotes an Aggressive Tumor Biology in Pancreatic Ductal Adenocarcinoma. Pancreas 2010;39:1254–1262. [DOI] [PubMed] [Google Scholar]
- 107.Sinn M, Denkert C, Striefler JK, et al. alpha-Smooth muscle actin expression and desmoplastic stromal reaction in pancreatic cancer: results from the CONKO-001 study. Br J Cancer 2014;111:1917–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kraman M, Bambrough PJ, Arnold JN, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 2010;330:827–30. [DOI] [PubMed] [Google Scholar]
- 109.Pharmaceuticals Infinity. Infinity Reports Update from Phase 2 Study of Saridegib Plus Gemcitabine in Patients with Metastatic Pancreatic Cancer, 2012:http://phx.corporate-ir.net/phoenix.zhtml?c=121941&p=irol-newsArticle&ID=1653550.
- 110.Wang LC, Lo A, Scholler J, et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol Res 2014;2:154–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Whatcott CJ, Diep CH, Jiang P, et al. Desmoplasia in Primary Tumors and Metastatic Lesions of Pancreatic Cancer. Clin Cancer Res 2015;21:3561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aiello NM, Bajor DL, Norgard RJ, et al. Metastatic progression is associated with dynamic changes in the local microenvironment. Nat Commun 2016;7:12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nielsen SR, Quaranta V, Linford A, et al. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol 2016;18:549–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu Z, Vonlaufen A, Phillips PA, et al. Role of pancreatic stellate cells in pancreatic cancer metastasis. Am J Pathol 2010;177:2585–96. [DOI] [PMC free article] [PubMed] [Google Scholar]


