Abstract
Objective:
To determine patterns of respiratory medications used in neonatal intensive care unit (NICU) graduates.
Study design:
The Prematurity Respiratory Outcomes Program enrolled 835 babies <29 weeks gestation in the first week. Of 751 survivors, 738 (98%) completed at least 1, and 85% completed all 4, post-discharge medication usage in-person/telephone parental questionnaires requested at 3, 6, 9, and 12m corrected age. Respiratory drug usage over the first year of life after NICU discharge was analyzed.
Results:
During any given quarter, 66–75% of the babies received no respiratory medication and 45% of the infants received no respiratory drug over the first year. The most common post-discharge medication was the inhaled bronchodilator albuterol; its use increased significantly from 13% to 31%. Diuretic usage decreased significantly from 11% to 2% over the first year. Systemic steroids (prednisone, most commonly) were used in approximately 5% of subjects, in any one quarter. Inhaled steroids significantly increased over the first year from 9% to 14% at 12m. Drug exposure changed significantly based on gestational age with 72% of babies born at 23–24w receiving at least one respiratory medication, but only 40% of babies born at 28w. Overall, at some time in the first year, 55% of infants received at least one drug including an inhaled bronchodilator (45%), an inhaled steroid (22%), a systemic steroid (15%), or diuretic (12%).
Conclusion:
Many babies born at <29w have no respiratory medication exposure post-discharge during the first year of life. Inhaled medications, including bronchodilators and steroids, increase over the first year.
Despite improvements in survival among extremely premature infants, a significant number continue to experience the complication of bronchopulmonary dysplasia (BPD). Several large North American series in the last few years report between 41% and 46% of infants born at <29 weeks’ (w) gestation receive supplemental oxygen (and/or other respiratory support) at 36w postmenstrual age (PMA), the most common definition of BPD.1–4 BPD is associated with significant long-term morbidity and contributes to considerable costs, both in the neonatal intensive care unit (NICU) and beyond.5–11
A wide variety of medications, ranging from diuretics to bronchodilators to anti-inflammatory agents, are used to prevent or treat BPD among infants at risk.12–14 Multicenter and single-center observational studies have reported that caffeine citrate and furosemide are among the 10 most commonly-used medications overall in the NICU, with other diuretics and albuterol also appearing on some lists.15, 16 Analysis of data from a collaborative of freestanding children’s hospitals showed that, among infants born at <29w gestation with BPD (defined as oxygen administration at 28 days), 89% received diuretics, 25% received inhaled steroids and 33% received bronchodilators during hospitalization.17–19 However, the frequency and patterns of post-discharge medication use have not been well characterized.
As data on medication use following hospital discharge in premature infants are limited, we pursued the hypothesis that respiratory medication use would be common after discharge in extremely premature infants and use would correlate with degree of prematurity and a diagnosis of BPD. We report a comprehensive assessment of respiratory medication use from discharge to 12 months (m) corrected age in a multicenter cohort of premature infants born at <29w gestation. In-hospital/NICU respiratory medication use is being reported separately.
METHODS
The NHLBI Prematurity and Respiratory Outcomes Program (PROP) is an observational prospective cohort study performed by a consortium of 6 clinical centers incorporating 13 tertiary neonatal intensive care units and a data-coordinating center (NCT01435187). A key scientific aim of PROP is to identify early clinical, physiologic, and biochemical biomarkers during the initial NICU hospitalization that can predict respiratory morbidity through 1 year of age. With funding from the Best Pharmaceuticals for Children Act (BPCA), another aim of PROP was to evaluate dosing, safety, and efficacy of therapeutics surrounding BPD. Individual centers enrolled between 105 and 184 participants in the cohort for a total of 835 subjects. Detailed descriptions of the PROP study design, and the status of the 765 infants surviving at 36w PMA, have been published.4, 20, 21
Study Infants
Infants between 230/7 and 286/7 weeks gestation were eligible for enrollment within the first 7 days after birth. Infants not considered viable, those with congenital heart disease or structural abnormalities of the upper airway, lungs, or chest wall or other congenital malformations that adversely affect cardiopulmonary development, or those whose families were unlikely to be available for long-term follow up were excluded. The study was approved by the institutional review board at each participating clinical site and by the data-coordinating center at the University of Pennsylvania with written informed consent from a parent or guardian for each baby enrolled.
Measurements and Procedures
Trained research personnel collected detailed anthropometric and medication data on a daily basis until discharge home, transfer, or 40 weeks PMA. Follow-up data was collected from the parents at 3, 6, 9, and 12 months corrected age (± 1 month) through a focused questionnaire adminstered via telephone or at a clinic visit. At the time of each questionnaire, respiratory medication use during the previous 3 months was reported by parents and was immediately recorded on the clinical research form by research staff.
Outcomes
The diagnosis of BPD was assigned by the need for supplemental oxygen at exactly 360/7 weeks PMA. Using this definition, those on respiratory support with FiO2 21% at 36 weeks PMA are assigned “no BPD” status, regardless of type or level of respiratory support.3 This definition was modified by assigning the outcome of “no BPD” to infants who were discharged home off respiratory support prior to 36 weeks’ PMA (modified Shennan” definition).3, 4
Statistical Analyses
We report the demographic characteristics of patients who are included in the follow-up cohort. These summaries are presented for the following populations: patients alive at discharge (n=751); patients who completed at least one follow-up assessment (n=738); and patients who completed all four follow-up assessments (n=641). Each factor is summarized by frequencies with percentages, means with standard deviations, or medians with interquartile ranges, as appropriate. Similarly, we summarize and compare medication use at each follow-up time point (eg, months 3, 6, 9, and 12). Because babies are assessed at multiple time points, a logistic generalized estimating equations (GEE) approach, with an exchangeable correlation structure, was used to determine differences in medication usage over time. In summarizing medication usage by baseline gestational age and overall differences across gestational age groups, P values from chi-square tests or Fisher exact tests are presented, as appropriate. Additionally, p-values from Cochran-Armitage trend tests are presented. Finally, we examined medication usage as a function of time and BPD status (yes/no) using a logistic-GEE approach as described previously in which models included the main effect time (categorical), BPD and their interaction. P-values are presented for the odds of medication usage comparing babies with and without BPD at each time-point from the logistic-GEE model. All analyses were performed using SAS 9.4 software (SAS Institute, Cary, North Carolina) by the Data Coordinating Center.
Results
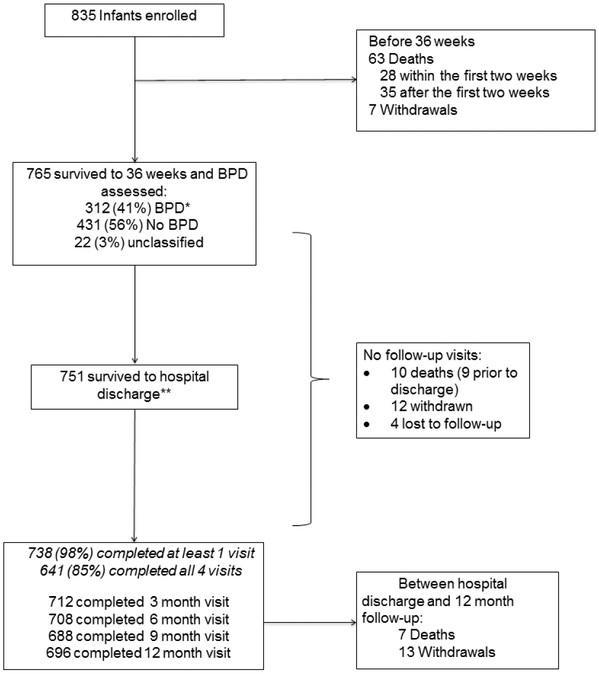
Of 751 infants discharged to home, 738 infants (98.3%) had at least one follow-up survey completed after discharge and 641 (85.4%) completed all four follow-up visits (Figure 1; available at www.jpeds.com). Of the 751 discharged, 696 (92.7%) completed the 12m follow-up at an average chronological age of 15.36 ± 1.45 months and a corrected gestational age 12.31 +/− 1.41 months. The 738 infants with at least one visit (Table 1), were similar to the 765 infants who survived to 36 weeks’ PMA as were the smaller cohorts with more complete data (data not shown). This was an extremely premature infant cohort, at a median of 27w gestation and just over 900 grams at birth, with approximately half male infants and one fourth products of multiple gestation. The cohort had 90% survival from birth to discharge.
Figure 1,
online – Participant flow diagram
Table 1:
Demographic characteristics of PROP cohort for infants: (1) alive at hospital discharge, (2) who participated in at least one visit over 12 month follow-up; and (3) who participated in all 4 follow-up visits.
| Alive at discharge n=751 | Participated in at least one follow-up visit n=738 | Participated in all 4 follow-up visits n=641 | |
|---|---|---|---|
| Gestational age, median (IQR), wks | 27.0 (25.7, 27.9) | 27.0 (25.7, 27.9) | 27.0 (25.7, 27.9) |
| Birth weight, mean (SD), g Race, n (%) | 919.0 (231.1) | 918.7 (231.8) | 922.9 (234.0) |
| Caucasian | 441 / 752 (58.6%) | 433 / 738 (58.7%) | 386 / 639 (60.4%) |
| African American | 274 / 752 (36.4%) | 269 / 738 (36.4%) | 222 / 639 (34.7%) |
| Maternal age, mean (SD), years | 28.1 (6.3) | 28.1 (6.3) | 28.1 (6.3) |
| Finished high school, n (%) | 568 / 684 (83.0%) | 555 / 670 (82.8%) | 494 / 589 (83.9%) |
| Exposed to second-hand smoke - CRFS: discharge, n (%)a | 71 / 741 (9.6%) | 71 / 729 (9.7%) | 63 / 636 (9.9%) |
| Exposed to second-hand smoke - CRFS: discharge, M6, n (%)b | 292 / 745 (39.2%) | 292 / 733 (39.8%) | 264 / 638 (41.4%) |
| Exposed to second-hand smoke - CRFS:M12, n (%)b | 343 / 745 (46.0%) | 343 / 733 (46.8%) | 300 / 638 (47.0%) |
| BPD (modified Shennan), n (%) | 302 / 729 (41.4%) | 300 / 718 (41.8%) | 257 / 622 (41.3%) |
| Days on mechanical ventilation, median (IQR) (See note.) | 7.0 (1.0,24.5) | 7.0 (1.0,25.0) | 7.0 (1.0,25.0) |
| Days on oxygen, median (IQR) (See note.) | 51.0 (22.0,83.0) | 51.0 (22.0,84.0) | 51.0 (22.0,83.0) |
| Days on respiratory supportc, median (IQR) (See note.) | 66.0 (43.0,91.0) | 66.5 (43.0,91.0) | 66.0 (44.0,91.0) |
| d Post-discharge respiratory hospitalizations, mean (SD) | 0.4 (1.0) | 0.4 (1.0) | 0.4 (1.1) |
Measured at hospital discharge; is there smoking in the home or in your vehicle?
Measured at the 6m or 12m visit; positive response to any of the following: (1) is there smoking in the home or in your vehicle? (2) Is your child exposed to smoke in the home? (3) Does the mother or primary caregiver smoke in the home? Or (4) is there at least one smoker in the home?
Respiratory support is defined as those infants who reported receiving supplemental oxygen or other respiratory support.
If 12m follow-up missing, post-discharge hospitalizations assigned a value of zero for calculating respiratory hospitalization mean and standard deviation.
Detailed respiratory medication exposure in each quarter for the most common drugs used is detailed in Table II. Beclomethasone, caffeine, furosemide, hydrochlorothiazide, hydrocortisone, ipratroprium, and racemic epinephrine were used in <5% of infants, and amiloride, aminophylline, bumetanide, formoterol, methylprednisolone, montelukast, sildenafil, and theophylline in <1% of subjects. Despite the high use of caffeine described in the neonatal period (95% in this cohort), post-discharge use remained low. In any given quarter, 66–75% of infants received no respiratory medications, with the lowest medication use in the first quarter after discharge.
Table 2:
Quarterly drug use over the first year of life.
| Drug | Month 3 (n = 712*) | Month 6 (n = 708*) | Month 9 (n = 688*) | Month 12 (n = 696*) | P-value** |
|---|---|---|---|---|---|
| No respiratory drug | 533 (74.9%) | 481 (67.9%) | 466 (67.7%) | 462 (66.4%) | 0.0005 |
| At least one | 179 (25.1%) | 227 (32.1%) | 222 (32.3%) | 234 (33.6%) | 0.0005 |
| Inhaled Bronchodilator | 93 (13.1%) | 184 (26.0%) | 198 (28.8%) | 218 (31.3%) | <.0001 |
| albuterol | 92 (12.9%) | 184 (26.0%) | 197 (28.6%) | 216 (31.0%) | <.0001 |
| formoterol | 0 | 1 (0.1%) | 0 | 0 | -- |
| ipratropium | 3 (0.4%) | 1 (0.1%) | 4 (0.6%) | 6 (0.9%) | 0.1294 |
| racemic epinephrine | 7 (1.0%) | 3 (0.4%) | 2 (0.3%) | 3 (0.4%) | 0.4182 |
| Diuretic | 78 (11.0%) | 41 (5.8%) | 11 (1.6%) | 11 (1.6%) | <.0001 |
| amiloride | 1 (0.1%) | 0 | 0 | 0 | -- |
| bumetanide | 1 (0.1%) | 1 (0.1%) | 0 | 2 (0.3%) | -- |
| chlorothiazide | 36 (5.1%) | 22 (3.1%) | 4 (0.6%) | 3 (0.4%) | <.0001 |
| furosemide | 30 (4.2%) | 13 (1.8%) | 6 (0.9%) | 5 (0.7%) | 0.0001 |
| hydrochlorothiazide | 23 (3.2%) | 10 (1.4%) | 4 (0.6%) | 3 (0.4%) | 0.0002 |
| spironolactone | 42 (5.9%) | 22 (3.1%) | 4 (0.6%) | 3 (0.4%) | <.0001 |
| Methylxanthine | 18 (2.5%) | 11 (1.6%) | 0 | 0 | -- |
| aminophylline | 1 (0.1%) | 0 | 0 | 0 | -- |
| caffeine | 17 (2.4%) | 11 (1.6%) | 0 | 0 | -- |
| theophylline | 1 (0.1%) | 0 | 0 | 0 | -- |
| Systemic Corticosteroid | 29 (4.1%) | 30 (4.2%) | 40 (5.8%) | 39 (5.6%) | 0.2294 |
| dexamethasone | 12 (1.7%) | 5 (0.7%) | 10 (1.5%) | 7 (1.0%) | 0.2535 |
| hydrocortisone | 10 (1.4%) | 4 (0.6%) | 2 (0.3%) | 2 (0.3%) | 0.0842 |
| methylprednisolone | 2 (0.3%) | 2 (0.3%) | 0 | 2 (0.3%) | -- |
| prednisone/prednisolone | 14 (2.0%) | 23 (3.2%) | 29 (4.2%) | 29 (4.2%) | 0.0185 |
| Inhaled Steroid | 61 (8.6%) | 70 (9.9%) | 86 (12.5%) | 99 (14.2%) | 0.0008 |
| beclomethasone | 2 (0.3%) | 5 (0.7%) | 7 (1.0%) | 10 (1.4%) | 0.0416 |
| budesonide | 51 (7.2%) | 53 (7.5%) | 57 (8.3%) | 61 (8.8%) | 0.6050 |
| fluticasone | 8 (1.1%) | 15 (2.1%) | 22 (3.2%) | 30 (4.3%) | 0.0016 |
| Leukotriene receptor antagonist | 0 | 0 | 2 (0.3%) | 3 (0.4%) | -- |
| montelukast | 0 | 0 | 2 (0.3%) | 3 (0.4%) | -- |
| Pulmonary Vasodilator | 2 (0.3%) | 7 (1.0%) | 6 (0.9%) | 6 (0.9%) | 0.1611 |
| sildenafil | 2 (0.3%) | 7 (1.0%) | 6 (0.9%) | 6 (0.9%) | 0.1611 |
Number of babies completing the specified follow-up visit.
Reported p-value for overall ‘time’ effect (df=3) for logistic generalized estimating equations model for drug use (yes/no), with working exchangeable correlation structure.
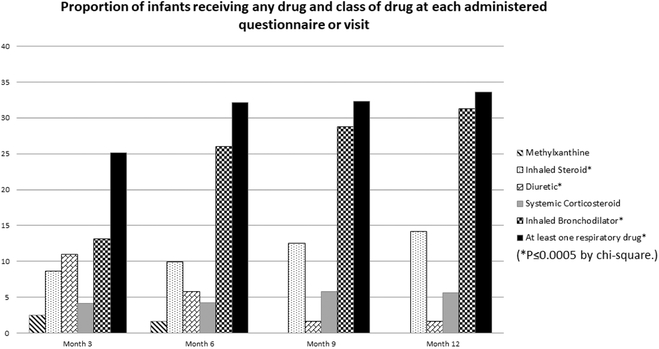
There were significant changes in respiratory medication use over the first year of life (Figure 2). The percentage of patients receiving any of the drugs in a class, and those receiving any drug at all, are reported by quarter (Figure 2). After the 3-month questionnaire, approximately one-third of infants were exposed to any respiratory drug each quarter. Reported exposure to diuretics decreased significantly (P<0.0001 by generalized estimating equations model) over the year, whereas systemic corticosteroid use increased slightly, from 4.1% to 5.7%, p=0.23, inhaled corticosteroid use increased modestly, from 8.6% to 14.2% (P=0.0008), and inhaled bronchodilator use increased substantially, from 13% to 31.0% (P<0.0001).
Figure 2 –
Each bar represents the proportion of infants whose caregiver reported use of a medication in that class during the prior three-month period at the corrected age in months as noted. The proportion of those who were on at least one respiratory drug increased significantly over time, as did the use of inhaled steroids and inhaled bronchodilators. Conversely, diuretic use decreased significantly over the first year of life. (*P≤0.0005 by chi-square.) The denominators (n) for each time period are 712 (month 3), 708 (month 6), 688 (month 9), and 696 (month 12).
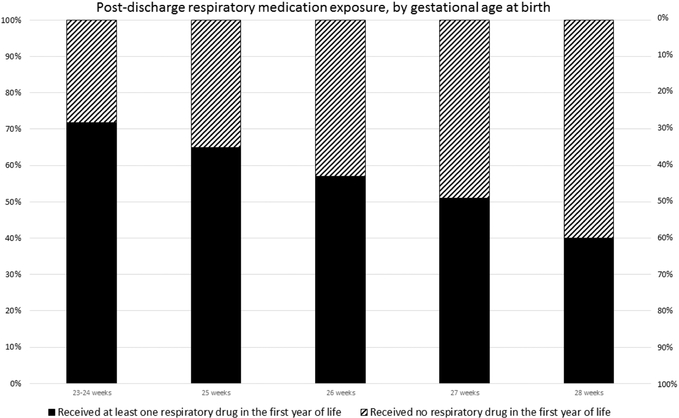
We examined the effect of gestational age at birth on post NICU-discharge drug usage (Table 3). The proportion of infants with any exposure significantly decreased with increasing gestational age group (Figure 3; available at www.jpeds.com). Data are shown by one-week gestational age at birth categories, except for the least mature infants, in which 23w and 24w gestation infants are combined. Respiratory medication use was more likely in less mature infants. For example, 28.9% of babies born at 23–24 weeks received diuretics after discharge sometime in the first year, in contrast to 3.2% of babies born at 28 weeks. The effect of gestational age was highly significant (P<0.0001). Overall, 45.5% of babies did not report exposure to any of the medications of interest at any time during the year of follow-up. Only 3.0%, 0.9% and 0.6% of infants were exposed to methylxanthines, pulmonary vasodilators and leukotriene receptor antagonists, respectively. Nearly 90% (89.7%) of patients had at least one quarter without respiratory medication exposure.
Table 3.
Drug use by gestational age for babies completing all 4 visits follow-up visits (n=641).
| Drug | All babies (n = 641*) | 23 0/7–24 6/7 (n = 83*) | 25 0/7–25 6/7 (n = 98*) | 26 0/7–26 6/7 (n = 135*) | 27 0/7–27 6/7 (n=169*) | 28 0/7–28 6/7 (n = 156*) | P-value (Fisher**) | P-value (Trend***) |
|---|---|---|---|---|---|---|---|---|
| No respiratory drug at at least one visit | 579 (90.3%) | 70 (84.3%) | 86 (87.8%) | 120 (88.9%) | 157 (92.9%) | 146 (93.6%) | 0.1029 | 0.0071 |
| At least one drug | 350 (54.6%) | 60 (72.3%) | 63 (64.3%) | 77 (57.0%) | 87 (51.5%) | 63 (40.4%) | <.0001 | <.0001 |
| No exposure to any drug | 291 (45,4%) | 23 (27.7%) | 35 (35.7%) | 58 (43.0%) | 82 (48.5%) | 93 (59.6%) | <.0001 | <.0001 |
| Diuretic | 79 (12.3%) | 24 (28.9%) | 21 (21.4%) | 14 (10.4%) | 15 (8.9%) | 5 (3.2%) | <.0001 | <.0001 |
| Amiloride | 1 (0.2%) | 0 | 1 (1.0%) | 0 | 0 | 0 | ||
| Bumetanide | 2 (0.3%) | 1 (1.2%) | 0 | 0 | 1 (0.6%) | 0 | ||
| Chlorothiazide | 37 (5.8%) | 14 (16.9%) | 9 (9.2%) | 8 (5.9%) | 5 (3.0%) | 1 (0.6%) | ||
| Furosemide | 31 (4.8%) | 7 (8.4%) | 9 (9.2%) | 6 (4.4%) | 7 (4.1%) | 2 (1.3%) | ||
| Hydrochlorothiazide | 24 (3.7%) | 7 (8.4%) | 9 (9.2%) | 3 (2.2%) | 3 (1.8%) | 2 (1.3%) | ||
| Spironolactone | 43 (6.7%) | 16 (19.3%) | 13 (13.3%) | 5 (3.7%) | 7 (4.1%) | 2 (1.3%) | ||
| Inhaled Bronchodilator | 292 (45.6%) | 46 (55.4%) | 50 (51.0%) | 67 (49.6%) | 71 (42.0%) | 58 (37.2%) | 0.0311 | 0.0014 |
| Albuterol | 290 (45.2%) | 45 (54.2%) | 50 (51.0%) | 67 (49.6%) | 70 (41.4%) | 58 (37.2%) | ||
| Formoterol | 1 (0.2%) | 0 | 0 | 1 (0.7%) | 0 | 0 | ||
| Ipratropium | 11 (1.7%) | 1 (1.2%) | 3 (3.1%) | 4 (3.0%) | 2 (1.2%) | 1 (0.6%) | ||
| Racemic epinephrine | 12 (1.9%) | 2 (2.4%) | 3 (3.1%) | 0 | 6 (3.6%) | 1 (0.6%) | ||
| Inhaled Steroid | 140 (21.8%) | 25 (30.1%) | 28 (28.6%) | 30 (22.2%) | 36 (21.3%) | 21 (13.5%) | 0.0121 | 0.0007 |
| Beclomethasone | 15 (2.3%) | 3 (3.6%) | 2 (2.0%) | 4 (3.0%) | 5 (3.0%) | 1 (0.6%) | ||
| Budesonide | 102 (15.9%) | 15 (18.1%) | 21 (21.4%) | 21 (15.6%) | 28 (16.6%) | 17 (10.9%) | ||
| Fluticasone | 40 (6.2%) | 10 (12.0%) | 11 (11.2%) | 7 (5.2%) | 7 (4.1%) | 5 (3.2%) | ||
| Leukotriene receptor antagonist | 3 (0.5%) | 1 (1.2%) | 1 (1.0%) | 1 (0.7%) | 0 | 0 | 0.2352 | 0.0827 |
| Montelukast | 3 (0.5%) | 1 (1.2%) | 1 (1.0%) | 1 (0.7%) | 0 | 0 | ||
| Methylxanthine | 19 (3.0%) | 6 (7.2%) | 5 (5.1%) | 3 (2.2%) | 5 (3.0%) | 0 | 0.0066 | 0.0013 |
| Aminophylline | 1 (0.2%) | 0 | 0 | 0 | 1 (0.6%) | 0 | ||
| Caffeine | 18 (2.8%) | 5 (6.0%) | 5 (5.1%) | 3 (2.2%) | 5 (3.0%) | 0 | ||
| Theophylline | 1 (0.2%) | 1 (1.2%) | 0 | 0 | 0 | 0 | ||
| Pulmonary Vasodilator | 5 (0.8%) | 2 (2.4%) | 0 | 1 (0.7%) | 0 | 2 (1.3%) | 0.1710 | 0.5704 |
| Sildenafil | 5 (0.8%) | 2 (2.4%) | 0 | 1 (0.7%) | 0 | 2 (1.3%) | ||
| Systemic Corticosteroid | 96 (15.0%) | 14 (16.9%) | 17 (17.3%) | 21 (15.6%) | 25 (14.8%) | 19 (12.2%) | 0.7756 | 0.2308 |
| Dexamethasone | 29 (4.5%) | 4 (4.8%) | 7 (7.1%) | 5 (3.7%) | 9 (5.3%) | 4 (2.6%) | ||
| Hydrocortisone | 9 (1.4%) | 3 (3.6%) | 2 (2.0%) | 2 (1.5%) | 1 (0.6%) | 1 (0.6%) | ||
| Methylprednisolone | 5 (0.8%) | 1 (1.2%) | 1 (1.0%) | 0 | 2 (1.2%) | 1 (0.6%) | ||
| Prednisone/prednisolone | 70 (10.9%) | 10 (12.0%) | 12 (12.2%) | 16 (11.9%) | 17 (10.1%) | 15 (9.6%) |
Includes only babies who completed all four visits.
Fisher’s exact test of association between drug use and gestational age; and
Cochran-Armitage test of trend between drug use and gestational age.
Figure 3,
online - Each bar represents the proportion of infants receiving at least one respiratory medication post-NICU discharge during the first year of life, vs. the proportion who received no respiratory medication, grouped by gestational age at birth. This was statistically significant (P<0.0001 by chi-square). The denominators (n) for each gestational age group are 83 (23–24 weeks), 98 (25 weeks), 135 (26 weeks), 169 (27 weeks), 156 (28 weeks), for a total of 641 babies who completed all four post-discharge visits.
Infants with a diagnosis of BPD (modified Shennan)4 were more likely to have any respiratory medication exposure at three of the four survey time points (Table 4), in unadjusted analyses and after adjustment for race and sex (model a). In the models that adjusted for gestational age, BPD significantly increased the odds of medication use only at Month 3. BPD had an odds ratio of 1.65 (1.11,2.45) at 3 months in the fully adjusted model (model d), compared with 2.06 (1.46,2.91) in the unadjusted model. For inhaled medications (inhaled bronchodilators and inhaled corticosteroids), BPD was a significant predictor only in the second half of the year (9 and 12 month surveys) for the unadjusted model and after adjustment for race and sex. Using a modification of the NIH workshop definition of BPD4, the pattern of significant differences in medication exposure was similar.
Table 4.
Medication usage in infants with and without Bronchopulmonary dysplasia (N = 719)*
| Drug | Visit | BPDa (n=301) | No BPD (n=418) | Unadjusted P-value | Adjusted P-valueb | Adjusted P-valuec | Adjusted P-valued | Adjusted P-valuee |
|---|---|---|---|---|---|---|---|---|
| Any respiratory medication | 3 months | 94 (31.2) | 80 (19.1) | <0.01* | <.01* | <0.01* | <0.01* | 0.01* |
| 6 months | 100 (33.2) | 117 (28.0) | 0.14 | 0.18 | 0.60 | 0.69 | 0.64 | |
| 9 months | 108 (35.9) | 107 (25.6) | <0.01* | <0.01* | <0.06 | 0.06 | 0.59 | |
| 12 months | 110 (36.5) | 118 (28.2) | 0.02* | 0.03* | 0.20 | 0.23 | 0.99 | |
| Any inhaled respiratory medicationf | 3 months | 60 (19.9) | 65 (15.6) | 0.06 | 0.08 | 0.23 | 0.25 | 0.47 |
| 6 months | 82 (27.2) | 110 (26.3) | 0.74 | 0.87 | 0.67 | 0.59 | 0.15 | |
| 9 months | 101 (33.6) | 105 (25.1) | 0.01* | <0.02* | 0.10 | 0.11 | 0.84 | |
| 12 months | 109 (36.2) | 116 (27.8) | <0.02* | <0.03* | 0.14 | 0.16 | 0.94 |
Modified Shennan definition4
Adjusted for race and gender, only.
Adjusted for gestational age, only.
Adjusted for gestational age, race and gender.
Adjusted for gestational age, race, gender, mother’s education, family asthma, maternal smoking during pregnancy and second-hand smoke exposure during follow-up (coded as a time-varying binary exposure variable for second hand smoke exposure between discharge and current visit. See Table 1 foonote for details).
Inhaled medications include inhaled bronchodilators and inhaled corticosteroids.
P<0.05
Discussion
This comprehensive assessment of respiratory medication use from discharge to 12m corrected age in a multicenter cohort of premature infants born at <29w gestation provides important insights into respiratory morbidity in the first year of life. In any given quarter, 66–75% of infants received no respiratory medications, with the lowest medication use in the first quarter after discharge. Medications were more likely to be prescribed in the infants born most prematurely and in infants with a diagnosis of BPD, also confounded by gestational age. Reported use of diuretics decreased significantly over the four quarters, whereas the use of inhaled bronchodilators and inhaled steroids increased significantly.
Prior studies demonstrate that the frequency and patterns of post-discharge medication use are not well characterized.9, 22–26 In one cohort of infants ≤32w gestation at birth followed for a year after NICU discharge, at least one medication prescription was filled for 43% of the infants; infants who filled at least one prescription filled an average of 5.5 prescriptions per year.24 Of these, 49% were for respiratory medications, including inhaled bronchodilators, 29% were for antibiotics, and 4% were for diuretics. A long-term follow-up study of a premature infant cohort in Quebec found that, among subjects 5–25 years of age followed during an 11-year period, over half received inhaled bronchodilators and/or inhaled corticosteroids.9 Infants diagnosed with BPD had approximately double the medication use of those diagnosed only with respiratory distress syndrome. Stevens et al conducted a secondary analysis of long-term respiratory outcomes in the SUPPORT trial cohort27. They included a summary of medication use defined by general categories of diuretics, systemic steroids, inhaled steroids and home oxygen, but did not document specific medication use (eg, loop diuretics vs thiazides) or longitudinal data across the first year of life. More detailed reports of patterns of use for individual medications have been restricted to single-center experiences.26, 28–30
Inhaled bronchodilators were the most frequent class of respiratory medication prescribed post-discharge in the PROP cohort, with 30% of infants receiving inhaled bronchodilators and an increase in inhaled bronchodilator use from 3m of age (12%) to 12m of age (30%). Although this analysis did not compare symptomatology to medication use, bronchodilator use may represent increased cough or wheezing after respiratory viral exposure. Inhaled bronchodilator use was more commonly seen among patients born at lower gestational age and those with a diagnosis of BPD at 36w, which may be related to the fact that infants with lower baseline lung function are more likely to have symptomatic lower respiratory tract infection31. The β2-agonist albuterol is the most common inhaled bronchodilator used. Ipratroprium and inhaled steroids have been recommended for management of tracheomalacia32 but is not supported by well-designed studies33. Ipratropium was used by only seven patients in the study population.
Systemic and inhaled steroids and diuretics were used in 12%−20% of the population. Inhaled corticosteroid use increased from 3m of age to 12m of age, but not to the same degree as inhaled bronchodilator use. The use of the corticosteroid budesonside, delivered by nebulization, remained relatively flat from 3m to 12m corrected age and may represent medication started in the NICU in those patients with a more severe BPD phenotype, and then continued at home in the year after discharge. The corticosteroids fluticasone and beclomethasone, delivered by a meter dose inhaler and mask, account for the increase in inhaled corticosteroids given over this time period. These medications are included in NHLBI guidelines [NHLBI report 2007] for the management of persistent asthma, but their effectiveness has not been established in younger children with recurrent wheezing. Although our analysis of the PROP medication database does not provide direct evidence for why these medications were initiated, or by which type of clinician, the frequency of inhaled bronchodilator use likely reflects the initiation of a medication to treat symptoms of cough or wheezing.
Variable diuretic use in the NICU is well-documented through analysis of national administrative data sets17 and in the PROP cohort (J Pediatr. 2018 Jun;197:42–47). There was no significant preferential use in our post-discharge cohort by diuretic class (loop, thiazide spironolactone). Although there is no clinical consensus regarding how and when to wean diuretics, we found minimal use by 12 months corrected age, accompanied by increased prevalence of other respiratory medications. Given the absence of studies on efficacy, this shift may reflect provider preference or other unappreciated factors.
Although there is increased recognition of pulmonary hypertension as a co-morbid condition associated with severe BPD34, the use of any pulmonary vasodilator medication was <2% in the PROP cohort. A recent analysis of administrative data for extremely premature infants during the neonatal hospitalization described variable rates of sildenafil use in infants with a diagnosis of BPD, ranging from 0–25%, however, rates of sildenafil use after discharge were not available35. A number of factors likely contribute to the low use of pulmonary vasodilators post-discharge in our cohort. Although there have been case reports describing the use of sildenafil and other pulmonary vasodilators in this patient population, there has only recently been a consensus statement to guide evaluation and therapy in infants with BPD complicated by pulmonary hypertension.36 Routine screening echocardiography was not standard of care prior to hospital discharge during our study period, and some cases of PH may not have been clinically recognized. Several PROP centers screened all babies with supplemental oxygen at 36w with an echocardiogram to assess right heart function and pulmonary hypertension. However, even with a universal surveillance protocol, only 15% of extremely low gestational infants had abnormalities concerning for pulmonary vascular disease by echocardiography at 36w PMA.34
At any given time, ~70% of the post discharge PROP cohort were on no respiratory medications, and only half were prescribed any respiratory medication over the course of the first year of life. Infants born at lower gestational age and those assigned the diagnosis of BPD were more likely to receive a respiratory medication in the first year of life, suggesting that respiratory medication may serve as a proxy for respiratory morbidity. Conversely, extremely premature infants who do not require respiratory medications during the first year of life may be relatively healthy, reflecting lower risk for future respiratory compromise.
There are a number of limitations in this study. Medication use was based on provider recall at 3-month intervals rather than from pharmacy or billing data. Medication adherence was also not collected or reported, and reliance on prescribing data conveyed by parents may have over-estimated the reported use of medications. Reasons for the use of inhaled bronchodilators and corticosteroids were not explicitly stated and may have varied by provider. Variations in socioeconomic background and ethnicity may limit the generalizability of the PROP cohort to the general population. We also did not collect any information as to the type of prescribing clinician. Prescribing practices may differ among generalists, as well as neonatologists, pediatric pulmonologists, and other subspecialists involved in post-discharge care of preterm infants.
Our findings on the pattern and timing of respiratory medication usage in former premature infants may inform the design of future clinical trials to assess drug efficacy and safety. Specifically, there may be phenotypes of premature infants who are more responsive to bronchodilators or corticosteroids. Although the use of these medications has likely been limited to symptomatic infants, there may be a role for early use of inhaled corticosteroids to alter the degree of respiratory morbidity in certain phenotypes.
ACKNOWLEDGEMENTS
In addition to the Principal Investigators, we acknowledge the critical work of all PROP Site Investigators and research staff at each participating study center as well as the lead coordinator, Julia Hoffmann, RN, at Washington University, and the lead respiratory therapy coordinator, Charles Clem, RRT, at Indiana University. The PROP logo was designed by Dr Rita Dadiz (Rochester). We also acknowledge Carol J. Blaisdell, MD, Division of Lung Diseases, National Heart, Lung, and Blood Institute, NIH, Bethesda, MD, USA of NHLBI for her guidance and review of the manuscript as well as all the PROP Investigators for their contributions to the design of individual and multicenter components (list of additional PROP Investigators is available at www.jpeds.com [Appendix]).
We acknowledge all research staff by site:
Cincinnati Children’s Hospital Medical Center site: Barbara Alexander, RN, Tari Gratton, PA, Cathy Grigsby, BSN, CCRC, Beth Koch, BHS, RRT, RPFT, Kelly Thornton BS.
Washington University School of Medicine site: Pamela Bates, CRT, RPFT, RPSGT, Claudia Cleveland, RRT, Julie Hoffmann, RN, Laura Linneman, RN, Jayne Sicard-Su, RN, Gina Simpson, RRT, CPFT,
University of California San Francisco site: Jeanette M. Asselin MS RRT-NPS1, Samantha Balan, Katrina Burson RN, BSN 4, Cheryl Chapin, Erna Josiah-Davis RN, NP2, Carmen Garcia RN, CCRP 4, Hart Horneman, Rick Hinojosa BSRT, RRT, CPFT-NPS3, Christopher Johnson MBA, RRT3, Susan Kelley RRT, Karin L. Knowles, M. Layne Lillie, RN, BSN3, Karen Martin RN3, Sarah Martin RN, BSN; Julie Arldt-McAlister RN, BSN3, Georgia E. McDavid RN3, Lori Pacello RCP1, Shawna Rodgers RN, BSN3, Daniel K. Sperry RN3
1Children’s Hospital and Research Center Oakland, Oakland CA; 2Alta Bates Summit Medical Center, Berkeley CA; 3University of Texas Health Science Center- Houston, Houston TX
Vanderbilt University Medical Center site: Amy B Beller BSN, Mark O’ Hunt, Theresa J. Rogers, RN, Odessa L. Settles, RN, MSN, CM, Steven Steele, RN, Sharon Wadley, BSN, RN, CLS1; 1Jackson-Madison County General Hospital, Jackson, TN
University of Rochester Medical Center/University at Buffalo NY site: Shannon Castiglione, RN, Aimee Horan, LPN, Deanna Maffet, RN, Jane O’Donnell, PNP, Michael Sacilowski, MAT, Tanya Scalise, RN, BSN, Elizabeth Werner, MPH, Jason Zayac, BS, Heidie Huyck, BS, Valerie Lunger, MS, Kim Bordeaux, RRT, Pam Brown, RRT, Julia Epping, AAS, RT, Lisa Flattery-Walsh, RRT, Donna Germuga, RRT, CPFT, Nancy Jenks, RN, Mary Platt, RN, Eileen Popplewell, RRT, Sandra Prentice, CRT.
Duke University site: Kim Ciccio, RN
Indiana University site: Charles Clem, RRT, Susan Gunn, NNP, CCRC, Lauren Jewett, RN, CCRC,
University of Pennsylvania, Perelman School of Medicine, DCC site: Maria Blanco, BS, Denise Cifelli, MS, Sara DeMauro, MD, Melissa Fernando, MPH, Ann Tierney, BA, MS.
University of Denver, Steering Committee Chair: Lynn M. Taussig, MD
NHLBI program officer: Carol J. Blaisdell, MD
Supported by National Institutes of Health, NHLBI and NICHD through U01 HL101794 to University of Pennsylvania, B Schmidt; U01 HL101456 to Vanderbilt University, JL Aschner; U01 HL101798 to University of California San Francisco, PL Ballard and RL Keller; U01 HL101813 to University of Rochester and University at Buffalo, GS Pryhuber, R Ryan and T Mariani; U01 HL101465 to Washington University, A Hamvas and T Ferkol; U01 HL101800 to Cincinnati Children’s Hospital Medical Center, AH Jobe and CA Chougnet; and 5R01HL105702 to Indiana University and Duke University, CM Cotton, SD Davis and JA Voynow. AH Jobe serves on the Editorial Board for The Journal of Pediatrics. The authors declare no conflicts of interest.
Abbreviations:
- BPD
bronchopulmonary dysplasia
- BPCA
Best Pharmaceuticals for Children Act
- ELGAN
extremely low gestational age neonate
- GEE
generalized estimating equations
- m
months
- NICU
neonatal intensive care unit
- PMA
postmenstrual age
- PROP
Prematurity and Respiratory Outcomes Program
- w
weeks
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this study were presented at the American Thoracic Society meeting, << >>, 2015, << >>, and the Pediatric Academic Societies annual meeting, << >>, 2015, << >>.
References
- 1.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah PS, Sankaran K, Aziz K, Allen AC, Seshia M, Ohlsson A, et al. Outcomes of preterm infants <29 weeks gestation over 10-year period in Canada: a cause for concern? J Perinatol. 2012;32:132–138. [DOI] [PubMed] [Google Scholar]
- 3.Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. 1988;82:527–532. [PubMed] [Google Scholar]
- 4.Poindexter BB, Feng R, Schmidt B, Aschner JL, Ballard RA, Hamvas A, et al. Comparisons and Limitations of Current Definitions of Bronchopulmonary Dysplasia for the Prematurity and Respiratory Outcomes Program. Ann Am Thorac Soc. 2015;12:1822–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson TJ, Patel AL, Jegier BJ, Engstrom JL, Meier PP. Cost of morbidities in very low birth weight infants. J Pediatr. 2013;162:243–249 e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaudoin S, Tremblay GM, Croitoru D, Benedetti A, Landry JS. Healthcare utilization and health-related quality of life of adult survivors of preterm birth complicated by bronchopulmonary dysplasia. Acta Paediatr. 2013;102:607–612. [DOI] [PubMed] [Google Scholar]
- 7.Bhandari A, McGrath-Morrow S. Long-term pulmonary outcomes of patients with bronchopulmonary dysplasia. Semin Perinatol. 2013;37:132–137. [DOI] [PubMed] [Google Scholar]
- 8.Landry JS, Chan T, Lands L, Menzies D. Long-term impact of bronchopulmonary dysplasia on pulmonary function. Can Respir J. 2011;18:265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landry JS, Croitoru D, Jin Y, Schwartzman K, Benedetti A, Menzies D. Health care utilization by preterm infants with respiratory complications in Quebec. Can Respir J. 2012;19:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer LT, Fulton S, Kirchner HL, Eisengart S, Lewis B, Short E, et al. Longitudinal predictors of maternal stress and coping after very low-birth-weight birth. Arch Pediatr Adolesc Med. 2010;164:518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyle LW, Anderson PJ. Long-term outcomes of bronchopulmonary dysplasia. Semin Fetal Neonatal Med. 2009;14:391–395. [DOI] [PubMed] [Google Scholar]
- 12.Ghanta S, Leeman KT, Christou H. An update on pharmacologic approaches to bronchopulmonary dysplasia. Semin Perinatol. 2013;37:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Angio CT, Maniscalco WM. Bronchopulmonary dysplasia in preterm infants: pathophysiology and management strategies. Paediatr Drugs. 2004;6:303–330. [DOI] [PubMed] [Google Scholar]
- 14.Iyengar A, Davis JM. Drug therapy for the prevention and treatment of bronchopulmonary dysplasia. Front Pharmacol. 2015;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117:1979–1987. [DOI] [PubMed] [Google Scholar]
- 16.Kumar P, Walker JK, Hurt KM, Bennett KM, Grosshans N, Fotis MA. Medication use in the neonatal intensive care unit: current patterns and off-label use of parenteral medications. J Pediatr. 2008;152:412–415. [DOI] [PubMed] [Google Scholar]
- 17.Slaughter JL, Stenger MR, Reagan PB. Variation in the use of diuretic therapy for infants with bronchopulmonary dysplasia. Pediatrics. 2013;131:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slaughter JL, Stenger MR, Reagan PB, Jadcherla SR. Utilization of inhaled corticosteroids for infants with bronchopulmonary dysplasia. PLoS One. 2014;9:e106838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slaughter JL, Stenger MR, Reagan PB, Jadcherla SR. Inhaled bronchodilator use for infants with bronchopulmonary dysplasia. J Perinatol. 2015;35:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maitre NL, Ballard RA, Ellenberg JH, Davis SD, Greenberg JM, Hamvas A, et al. Respiratory consequences of prematurity: evolution of a diagnosis and development of a comprehensive approach. J Perinatol. 2015;35:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pryhuber GS, Maitre NL, Ballard RA, Cifelli D, Davis SD, Ellenberg JH, et al. Prematurity and respiratory outcomes program (PROP): study protocol of a prospective multicenter study of respiratory outcomes of preterm infants in the United States. BMC Pediatr. 2015;15:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groothuis JR, Makari D. Definition and outpatient management of the very low-birth-weight infant with bronchopulmonary dysplasia. Adv Ther. 2012;29:297–311. [DOI] [PubMed] [Google Scholar]
- 23.Allen J, Zwerdling R, Ehrenkranz R, Gaultier C, Geggel R, Greenough A, et al. Statement on the care of the child with chronic lung disease of infancy and childhood. Am J Respir Crit Care Med. 2003;168:356–396. [DOI] [PubMed] [Google Scholar]
- 24.Wade KC, Lorch SA, Bakewell-Sachs S, Medoff-Cooper B, Silber JH, Escobar GJ Pediatric care for preterm infants after NICU discharge: high number of office visits and prescription medications. J Perinatol. 2008;28:696–701. [DOI] [PubMed] [Google Scholar]
- 25.Lorch SA, Wade KC, Bakewell-Sachs S, Medoff-Cooper B, Escobar GJ, Silber JH. Racial differences in the use of respiratory medications in premature infants after discharge from the neonatal intensive care unit. J Pediatr. 2007;151:604–610, 610 e601. [DOI] [PubMed] [Google Scholar]
- 26.Bhandari A, Chow U, Hagadorn JI. Variability in duration of outpatient diuretic therapy in bronchopulmonary dysplasia: a clinical experience. Am J Perinatol. 2010;27:529–535. [DOI] [PubMed] [Google Scholar]
- 27.Stevens TP, Finer NN, Carlo WA, Szilagyi PG, Phelps DL, Walsh MC, et al. Respiratory outcomes of the surfactant positive pressure and oximetry randomized trial (SUPPORT). J Pediatr. 2014;165:240–249> e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vrijlandt EJ, Boezen HM, Gerritsen J, Stremmelaar EF, Duiverman EJ. Respiratory health in prematurely born preschool children with and without bronchopulmonary dysplasia. J Pediatr. 2007;150:256–261. [DOI] [PubMed] [Google Scholar]
- 29.Collaco JM, Kole AJ, Riekert KA, Eakin MN, Okelo SO, McGrath-Morrow SA. Respiratory medication adherence in chronic lung disease of prematurity. Pediatr Pulmonol. 2012;47:283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collaco JM, Aherrera AD, Ryan T, McGrath-Morrow SA. Secondhand smoke exposure in preterm infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 2014;49:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez FD, Morgan WJ, Wright AL, Holberg CJ, Taussig LM. Diminished lung function as a predisposing factor for wheezing respiratory illness in infants. N Engl J Med. 1988;319:1112–1117. [DOI] [PubMed] [Google Scholar]
- 32.Fraga JC, Jennings RW, Kim PC. Pediatric tracheomalacia. Semin Pediatr Surg. 2016;25:156–164. [DOI] [PubMed] [Google Scholar]
- 33.Goyal V, Masters IB, Chang AB. Interventions for primary (intrinsic) tracheomalacia in children. Cochrane Database Syst Rev. 2012;10:CD005304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mourani PM, Abman SH. Pulmonary vascular disease in bronchopulmonary dysplasia: pulmonary hypertension and beyond. Curr Opin Pediatr. 2013;25:329–337. [DOI] [PubMed] [Google Scholar]
- 35.Backes CH, Reagan PB, Smith CV, Jadcherla SR, Slaughter JL. Sildenafil Treatment of Infants With Bronchopulmonary Dysplasia-Associated Pulmonary Hypertension. Hosp Pediatr. 2016;6:27–33. [DOI] [PubMed] [Google Scholar]
- 36.Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, et al. Pediatric Pulmonary Hypertension: Guidelines From the American Heart Association and American Thoracic Society. Circulation. 2015;132:2037–2099. [DOI] [PubMed] [Google Scholar]