Abstract
Background:
Transcranial magnetic stimulation (TMS) is a non-invasive method to stimulate localized brain regions. Despite widespread use in motor cortex, TMS is seldom performed in sensory areas due to variable, qualitative metrics.
Objective:
Assess the reliability and validity of tracing phosphenes, and to investigate the stimulation parameters necessary to elicit decreased visual cortex excitability with paired-pulse TMS at short inter-stimulus intervals.
Methods:
Across two sessions, single and paired-pulse recruitment curves were derived by having participants outline elicited phosphenes and calculating resulting average phosphene sizes.
Results:
Phosphene size scaled with stimulus intensity, similar to motor cortex. Paired-pulse recruitment curves demonstrated inhibition at lower conditioning stimulus intensities than observed in motor cortex. Reliability was high across sessions.
Conclusions:
TMS-induced phosphenes are a valid and reliable tool for measuring cortical excitability and inhibition in early visual areas. Our results also provide appropriate stimulation parameters for measuring short-latency intracortical inhibition in visual cortex.
Keywords: Phosphenes, Gamma aminobutyric acid, Recruitment curve, Short-interval cortical inhibition, Transcranial Magnetic Stimulation, Paired-Pulse
INTRODUCTION
Transcranial magnetic stimulation (TMS) is commonly used to assess cortical excitability and intracortical function. In the motor cortex, the motor evoked potential (MEP) provides an overt, graded response which is easily quantified with surface electromyography. In contrast, stimulating visual cortex often evokes the experience of seeing light (a phosphene), but that response is covert and difficult to quantify objectively. Recently, phosphene tracing was used to quantify the induced phosphene (1). However, doing so required a complicated laser tracking system. Further, the reliability of tracing has yet to be established, a critical step in the expanded use of visual TMS in healthy and clinical populations.
Paired-pulse TMS (ppTMS) has also been used to assess local inhibitory function in healthy and clinical populations in motor cortex (2) and adjacent somatosensory cortex (3). However, the validity of ppTMS as a marker of short-interval intracortical inhibition in visual cortex is questionable. The relatively few studies in visual cortex have failed to reduce phosphene perception at interstimulus intervals and conditioning stimulus (CS) intensities that elicit short-interval cortical inhibition (SICI) in sensorimotor cortex (4–6). Whether the absence of comparable inhibition in visual cortex is methodological or reflects distinct functional mechanisms is unknown. The probabilistic phosphene perception used in previous studies may be a less sensitive measure of inhibition compared to the graded magnitude of the motor evoked response. Further, unique intracortical dynamics in visual cortex could alter the relative recruitment of inhibitory and facilitatory mechanisms at CS intensities optimized to induce SICI in motor cortex (60-90% of phosphene threshold). For example, ppTMS studies in cat visual cortex show inhibition is restricted to CS intensities <60% across a range of inter-stimulus intervals associated with SICI and intracortical facilitation in motor cortex (7).
The current brief communication reports on the reliability and validity of a simple phosphene tracing approach in the assessment of stimulus input-output curves. We also demonstrate that short-latency intracortical inhibition can be observed in visual cortex, provided that parameters optimized for this brain region are used.
METHODS AND MATERIALS
Thirty healthy adults (10 male, mean age: 20.4±2.7, age range: 18-29) provided informed consent. The experimental protocol was approved by the Institutional Review Board of the University of Michigan Medical School (IRBMED). Seven subjects who did not report phosphenes were excluded. The remaining 23 subjects completed two 2-hour identical sessions at least one week apart.
Each session involved tracing phosphenes elicited by occipital TMS. The optimal occipital stimulation site was determined using a 3×3 cm grid (see Fig1A for procedure). The site eliciting the most intense and consistent phosphenes was defined as the occipital “hotspot”. Hotspot phosphene threshold (PT) was defined as the intensity that induced phosphenes on 5/10 stimuli.
Figure 1:
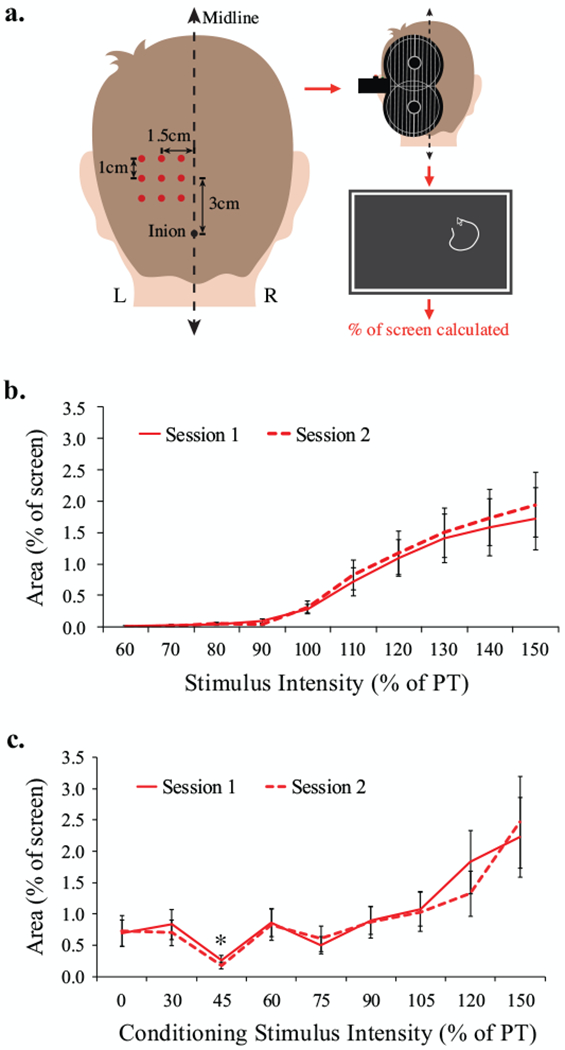
a. Grid placement over the visual cortex to elicit phosphenes through transcranial magnetic stimulation (TMS). Stimulation targets were spaced 1cm apart, with the center of the grid 3cm dorsal and 1.5 cm left-lateral of the inion. Stimulator output was initially set to 60% of maximum stimulator output and each target was stimulated three times (monophasic, lateral-medial induced current, MagPro X100 with MagOption stimulator and MC-B70 butterfly coil). If no phosphenes were induced, intensity was increased in increments of 10% until phosphenes were reported. If phosphenes were reported at one or more locations, the site reported to generate the brightest most consistent phosphenes was selected as the target “hotspot”. At the hotspot, stimulation intensity was decreased by 2% of stimulator output until no longer reported, then incremented by 1% until the phosphene threshold (PT) was reached. PT was defined as the lowest stimulator output at which at least 5 out of 10 stimuli induced a phosphene. If no phosphenes were reported at any of the 9 locations with 100% of stimulator output, the participant was excluded from further participation (n=7). The TMS coil was held at 90° to the midline. The BrainSight™ frameless stereotactic neuronavigation system was used for accuracy of targeting and trajectory. Subjects traced phosphenes on screen after each trial and area was calculated. b. Phosphene area as a function of stimulator intensity during single-pulse TMS. Error bars indicate standard error of the mean. c. Phosphene size as a function of conditioning stimulus intensity during paired-pulse TMS. Error bars indicate standard error of the mean. Asterisk denotes significant inhibition.
Occipital recruitment curves were generated from 90 randomized single pulses ranging from 60-150% PT (10% increments; 9 repetitions at each intensity). Intracortical inhibition was assessed using 10 randomized paired stimuli across a range of CS intensities (30, 45, 60, 75, 90, 105, 120, and 150% of PT) at an inter-stimulus interval of 2ms. Test stimulus (TS) intensity was fixed at 120% of PT to elicit a stable phosphene so as to be sensitive to any relative change induced by the CS (2,8).
For both the single-pulse and paired-pulse TMS protocols, participants used a mouse to draw the shape of the perceived phosphene on a 46” computer screen in a darkened room. The size of each phosphene was calculated as a percentage of the computer screen. Recruitment curves were generated by plotting phosphene area as a function of stimulus intensity. For paired-pulses, we tested whether a preceding CS reduced the response to the TS relative to when no CS was presented. The CS effect was expressed as a percentage of unconditioned phosphene area.
RESULTS
PT:
PT was 67.1±11.5% and 64.7±9.5% of maximum stimulator output for Session 1 and 2 respectively (t22 =1.03, p=0.32). PT was significantly positively correlated across sessions (r21=.44, p=.03).
Recruitment Curves:
Phosphene input-output curves exhibited a sigmoidal shape (Fig1B). The data from the three lowest intensities (60-80% of phosphene threshold) were omitted from subsequent analysis. These intensities were all clearly subthreshold and rarely elicited a phosphene. A two-way repeated-measures ANOVA with a Greenhouse-Geisser correction indicated a main effect of Intensity on phosphene size (F6,132=12.04, ε=0.21, p=.001). There were no differences in phosphene size across Session (F1,22=.73, p=.403) and Session did not interact with Intensity (F6,132=.53, ε=0.59, p=.692). Paired t-tests indicated significant increases in phosphene area with each increment across the linear portion of the curve (90-130% of PT; all p’s <.05). The slopes (r2=.82, n=23, p<.001) and intercepts (r2=.80, n=23, p<.001) across the two sessions were highly correlated indicating high relative reliability. There was no significant difference in standard error of the estimates across session (t22=.69, p=.50) indicative of high absolute reliability.
Paired-Pulse:
CS intensities above 110% PT increase, rather than decrease, phosphene size in motor cortex (2) and were omitted from the analysis of inhibition. A two-way repeated-measures ANOVA with a Greenhouse-Geisser correction revealed a main effect of CS Intensity on phosphene size (F6,132=9.23, ε=0.28, p=.001). There were no differences across Session (F1,22=.06, p=.81) and Session did not interact with CS Intensity (F6,132=.80, ε=0.41, p=.477) (Fig1C). Modified Bonferroni (9) corrected paired t-tests indicated significant suppression of phosphene area at the 45% CS intensity (p=.017). No other CS intensity caused significant suppression of phosphene area.
DISCUSSION
This study demonstrates the absolute and relative reliability of tracing TMS-induced phosphenes to assess graded change in visual cortex excitability. Additionally, the sigmoidal shape and graded increase in phosphene size observed from 100% PT to ~130% PT is evidence of a lawful relationship between stimulation energy and elicited response, similar to the input-output properties of corticospinal neurons in motor cortex.
The current study also demonstrates the validity of using phosphene tracing in assessing visual cortex inhibition. Phosphene area was significantly inhibited when the TS was preceded by a CS with an intensity of 45% PT. This CS intensity is lower than the intensity that typically elicits SICI in sensorimotor cortex (60-90% PT), but is consistent with ppTMS studies in cat visual cortex (7). Furthermore, the emergence of inhibition at low intensities that dissipate with increasing CS intensity is consistent with sensorimotor models of SICI that include a low-threshold inhibitory pathway and a high-threshold excitatory pathway (8).
Overall, our results suggest that our phosphene tracing paradigm is an effective way to quantify graded changes in visual cortical excitability. Further, we demonstrate that intracortical inhibition can be observed in visual cortex, at relatively low CS intensity.
HIGHLIGHTS.
Phosphene tracing is a reliable and valid method to measure occipital TMS response
Phosphene size scales with stimulus intensity, similar to motor cortex
TMS-induced phosphenes can be used to study short-latency inhibition in visual cortex
Conditioning intensities yielding inhibition are lower in visual than motor cortex
ACKNOWLEDGEMENTS
Funding: This work was supported by the University of Michigan; the Claude D. Pepper Older Americans Independence Center, Ann Arbor, MI [grant number P30AG024824]; the Boledovich Schizophrenia Research Fund, and the National Institutes of Health (grant number R01AG050523).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
None of the authors have any potential conflicts of interest, relevant to the content of this manuscript to disclose, except Dr. Taylor reports having received grant support from Neuronetics.
REFERENCES
- 1.Elkin-Frankston S, Fried PJ, Pascual-Leone A, Rushmore RJ, Valero-Cabr A. A novel approach for documenting phosphenes induced by transcranial magnetic stimulation. J Vis Exp JoVE. 2010. April 1;(38). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993. November;471:501–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliveri M, Caltagirone C, Filippi MM, Traversa R, Cicinelli P, Pasqualetti P, et al. Paired transcranial magnetic stimulation protocols reveal a pattern of inhibition and facilitation in the human parietal cortex. J Physiol. 2000. December 1;529 Pt 2:461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sparing R, Dambeck N, Stock K, Meister IG, Huetter D, Boroojerdi B. Investigation of the primary visual cortex using short-interval paired-pulse transcranial magnetic stimulation (TMS). Neurosci Lett. 2005. July 15;382(3):312–6. [DOI] [PubMed] [Google Scholar]
- 5.Kammer T, Baumann LW. Phosphene thresholds evoked with single and double TMS pulses. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2010. March;121(3):376–9. [DOI] [PubMed] [Google Scholar]
- 6.Ray PG, Meador KJ, Epstein CM, Loring DW, Day LJ. Magnetic stimulation of visual cortex: factors influencing the perception of phosphenes. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. 1998. July;15(4):351–7. [DOI] [PubMed] [Google Scholar]
- 7.Moliadze V, Giannikopoulos D, Eysel UT, Funke K. Paired-pulse transcranial magnetic stimulation protocol applied to visual cortex of anaesthetized cat: effects on visually evoked single-unit activity. J Physiol. 2005. August 1;566(Pt 3):955–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilić TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002. November;545(1):153–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keppel G Design and analysis: a researcher’s handbook. 3rd ed. Englewood Cliffs, N.J: Prentice Hall; 1991. p.169 [Google Scholar]


