Abstract
Background.
Medical treatment for acute heart failure (AHF) has not changed substantially over the last four decades. Emergency department (ED)-based evidence for treatment is limited. Outcomes remain poor, with a 25% mortality or re-admission rate within 30 days post-discharge. Targeting pulmonary congestion, which can be objectively assessed using lung ultrasound (LUS), may be associated with improved outcomes.
Methods.
BLUSHED-AHF is a multicenter, randomized, pilot trial designed to test whether a strategy of care that utilizes a LUS-driven treatment protocol outperforms usual care for reducing pulmonary congestion in the ED. We will randomize 130 ED patients with AHF across 5 sites to: a) a structured treatment strategy guided by LUS vs. b) a structured treatment strategy guided by usual care. LUS-guided care will continue until there are ≤ 15 B-lines on LUS or 6 hours post enrollment. The primary outcome is the proportion of patients with B-lines ≤ 15 at the conclusion of 6 hours of management. Patients will continue to undergo serial LUS exams during hospitalization, to better understand the time course of pulmonary congestion. Follow up will occur through 90 days, exploring days-alive-and-out-of-hospital between the two arms. The study is registered on ClinicalTrials.gov (NCT03136198).
In conclusion, if successful, this pilot study will inform future, larger trial design on LUS driven therapy aimed at guiding treatment and improving outcomes in patients with AHF.
Keywords: Heart failure, Ultrasound, Treatment, Quality, Outcomes
Introduction
Acute heart failure (AHF) is a major public health burden 1-4. Approximately 6 million Americans have chronic HF, and over 870,000 people are newly diagnosed annually 1. In 2013, over 30 billion dollars were spent on HF alone, with the majority of these costs due to AHF hospitalizations 5. For patients aged 65 years and older, HF is the most common reason for hospitalization 6. Within 30 days of hospital discharge, 25% of patients will be dead or re-hospitalized 7, 8.
Pulmonary congestion is the primary reason that patients with HF seek emergency care1, 9,10. Decongestion is associated with improved outcomes 11,12. Despite this, many patients remain congested at time of discharge. 10, 11, 13, 14. This may be due to continued reliance on traditional approaches to congestion assessment (i.e. signs and symptoms of HF), which lack sensitivity and have poor inter-rater reliability 10, 13,15, 16
Because pulmonary decongestion is a vital treatment goal, a more reliable method of assessment, able to be utilized by a broad range of practitioners, is needed. B-line assessment on lung ultrasound (LUS) is an objective, easy-to-learn, quantitative measure of pulmonary congestion. 16-20 Assessment for B-lines outperforms physical examination, chest radiography, and brain natriuretic peptide (BNP) in the diagnosis of AHF 21. B-lines are a dynamic marker of pulmonary congestion that clear in response to treatment, though studies have been small 22-25.
Persistence of B-lines after hospital discharge in patients with AHF is associated with a worse prognosis, including a greater than five-fold risk of hospital re-admission and mortality 26-28.
The B-lines Lung Ultrasound Guided Emergency Department Management of Acute Heart Failure (BLUSHED-AHF) pilot trial is an NHLBI funded study designed to test whether a LUS-guided protocol, compared to structured usual care, will lead to more rapid resolution of pulmonary congestion. We hypothesize that a LUS-driven protocol for ED AHF management will be feasible and will lead to a clinically significant reduction in pulmonary congestion (as measured by B-lines) during the first 6 hours of management. We chose 6 hours to demonstrate this proof-of-concept study of targeting B-lines. In addition, at the time of hospital discharge, we hypothesize patients with persistent B-lines will have worse outcomes. This pilot trial will inform a definitive outcomes study targeting B-lines both in the ED and during hospitalization.
Methods
Study Design and Population
BLUSHED-AHF is multi-center, prospective, randomized control trial. One hundred and thirty patients will be enrolled from 5 EDs, in the United States. Eligibility criteria are listed in Table 1.
Table 1:
Eligibility Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| 1) Age ≥21 years | 1) Chronic renal dysfunction, including ESRD or eGFR < 45ml//min/1.73m2 |
| 2) Presents with shortness of breath at rest or with minimal exertion | 2) Shock of any kind. Any requirement for vasopressors or inotropes |
| 3) Clinical diagnosis of AHF and presence of > 15 total bilateral B-lines on initial LUS | 3) SBP < 100 or > 175mmHg |
| 4) History of chronic HF and any one of the following: i. Chest radiograph consistent with AHF ii. Jugular venous distension iii. Pulmonary rales on auscultation iv. Lower extremity edema |
4) Need for immediate intubation |
| 5) Acute Coronary Syndrome (ACS) OR new ST-segment elevation/depression on EKG. (troponin elevation outside of ACS is allowed) | |
| 6) Fever >101.5°F | |
| 7) End stage HF: transplant list, ventricular assist device | |
| 8) Anemia requiring transfusion | |
| 9) Known interstitial lung disease | |
| 10) Suspected acute lung injury or acute respiratory distress syndrome (ARDS) | |
| 11) Pregnant or recently pregnant within the last 6 months |
ESRD – end stage renal disease; eGFR – estimated glomerular filtration rate, SBP – systolic blood pressure; HF – heart
Patients fulfilling enrollment criteria will be included after written informed consent. This study has been approved by the Institutional Review Board at all study sites and registered on ClinicalTrials.gov (NCT03136198)
Study Treatment
Enrolled patients will be randomized in a 1:1 fashion to LUS-guided strategy-of-care or structured usual care. Randomization will occur using the REDCap randomization module. Randomization block sizes of 2, 4, and 6 will be used, and stratified by site. The data coordinating center will continuously monitor the recruitment until the targeted sample size is reached.
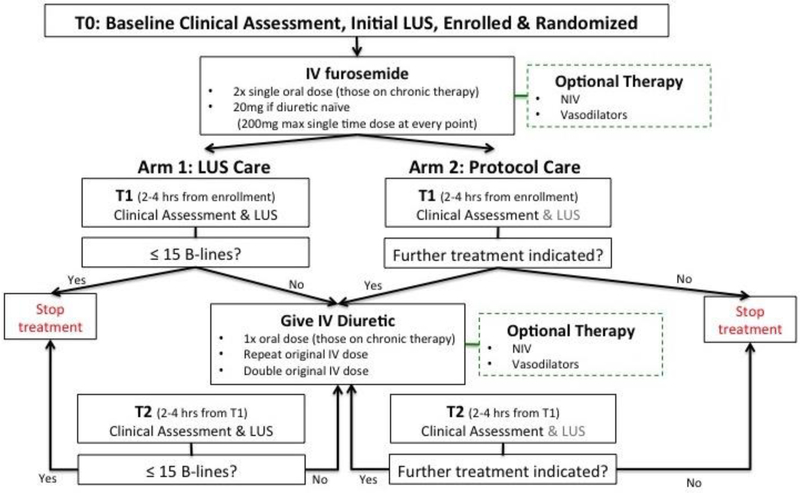
After initial ED evaluation and randomization, which includes a baseline screening LUS exam and a baseline clinical assessment, patients will have two additional assessments during the initial 6 hours of the protocol (Figure 1).
Figure 1.
Study treatment algorithm.
The first assessment will occur 2-4 hours after enrollment (T1). The second assessment will occur 2-4 hours after the first assessment (T2), or prior to ED disposition for patients discharged from the ED. If a patient is admitted to the hospital or an observation unit the second assessment (T2) will occur at this location. These additional assessments will include both a LUS performed by the study team and a clinical assessment performed by the treating physician.
Clinical Assessment
Treating clinicians in both arms will be asked a series of standardized questions, listed in Table 2, to determine whether their patient’s congestion has improved, and what, if any, methods of assessment were used to derive their determination.
Table 2:
Clinical Assessment Form
| 1. In your clinical opinion, is the patient still volume overloaded? |
| 2. If yes, do you think the patient warrants additional treatment now? |
| 3. The following questions will be asked of the physician: |
| a. Did you assess jugular venous pressure (JVP)? |
| i. If yes, did you measure it? |
| 1. If yes, record height in centimeters |
| b. Did you auscultate the lungs? |
| i. If yes, did you hear wheezing, rales, other breath sounds |
| 1. If yes for rales, did you assess how high up the lungs? |
| a. If yes, then record how high up |
| c. Did you listen to the heart? |
| i. If yes, did you hear any extra heart sounds? |
| 1. If yes, ask what did you hear? |
| d. Did you assess for peripheral edema? |
| i. If yes, did you grade severity |
Structured usual care
For patients randomized to structured usual care, the treating team will be blinded to LUS assessments. Treatment decisions in the usual care arm will be guided solely by clinical re-assessment. If the treating clinician feels that further treatment is indicated, then care will continue based on the treatment protocol, Figure 1. If the treating clinician deems that the patient has achieved adequate decongestion and no further treatment is indicated, then the treatment algorithm will be halted; however, LUS assessments will continue per protocol.
Patients randomized to the LUS-guided strategy-of-care arm will have the aforementioned clinical assessment and LUS exam performed. Clinicians in the LUS arm will be instructed to administer further treatment as outlined in Figure 1, until there is a decrease in B-lines on LUS to ≤ 15, 6 hours of care has been delivered, or the patient has been discharged.
Safety guidelines, such as significant drop in blood pressure or very brisk diuresis, are highlighted for the investigators to consider when re-dosing medications per protocol. While we will collect treating clinicians’ clinical assessments, the LUS arm treatment protocol is based solely on the persistence of B-lines on LUS. Therefore, if the LUS shows ≤15 B-lines the treatment algorithm will be stopped. In contrast, if the LUS shows >15 B-lines, algorithm guided treatment continues based on Figure 1.
During Hospitalization
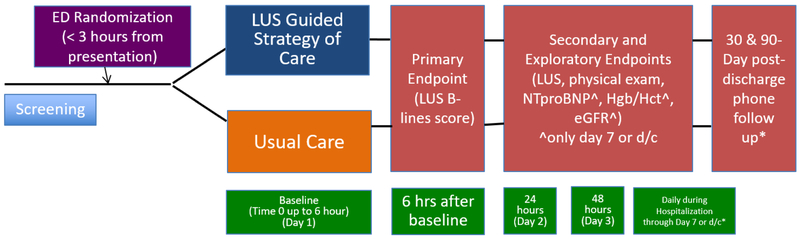
Throughout hospitalization patients will have serial LUS and physical exam assessments (taken from the medical record), (see Figure 2) regardless of treatment arm. Treating clinicians will be blinded to LUS assessments performed. These follow-up assessments will inform future studies and help determine if ongoing LUS monitoring throughout hospitalization provides meaningful clinical information regarding pulmonary congestion.
Figure 2.
Trial Schematic and patient flow through study.
Patients will be followed throughout their ED stay, hospital admission, and for 90-days after hospital discharge (Figure 2). We will call patients at both 30 and 90 days post-discharge to assess vital status, unscheduled healthcare visits and re-hospitalization.
LUS Protocol
Machine settings
All enrolled patients will have serial LUS examinations performed using Zonare ZS3 or Z One Pro(Mindray, Mountain View, CA) or Sonosite MTurbo (FUJIFilm Sonosite, Bothell, WA) ultrasound machines with the curvilinear transducer. Ultrasound machine settings will be standardized: depth of 18 centimeters, clip length 6 seconds, and tissue harmonics and multi-beam former turned off. The gain will be adjusted to the individual patient so that the rib shadows appear black and the pleural line with lung sliding are distinct.
Image Acquisition
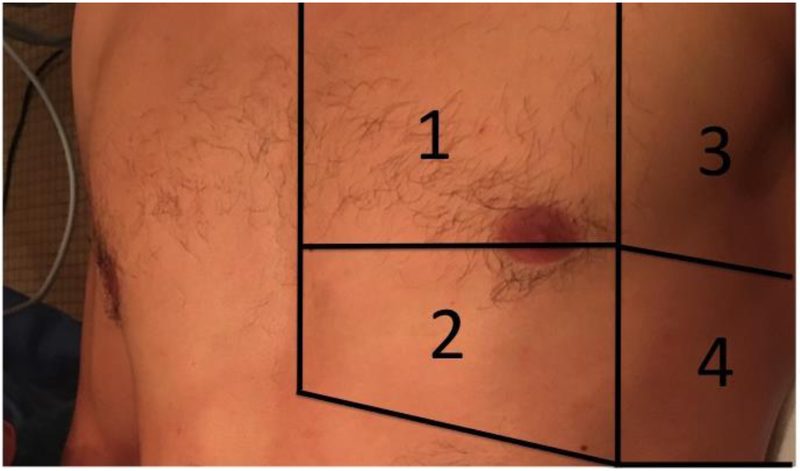
As patient positioning can affect B-line counts 29, all patients will be scanned in a semi-upright position, with the head of the bed at 45 degrees. We will follow previously published LUS scanning protocols 16 utilizing an 8-zone approach, see Figure 3. Videos will be acquired with the probe in a transverse orientation, with the probe indicator facing the patient’s right side and the probe face parallel to the adjacent ribs. Two additional videos, one on each side of the chest, will be acquired in the mid-axillary line at the caudal portion of the chest to assess for the presence and size of a pleural effusion.
Figure 3.
Pictorial representation of the 8-zone scanning protocol.
In addition to the initial LUS examination, up to two additional LUS studies will be performed within 6 hours of enrollment, if the patient remains in the ED. Repeat LUS examinations will be performed daily until discharge or hospital day 7, whichever comes first.
Sonographers and Pre-enrollment Training
Sonographers will range in experience level from novice to expert and will include research associates, postgraduate year (PGY) 1-3 emergency medicine residents, emergency ultrasound fellowship trained faculty, and non-ultrasound trained emergency medicine faculty.
Research associates will be included in those that perform and interpret LUS exams because LUS images are easy to acquire and interpret 16,30, and a tool non-physicians are able to utilize31. To ensure uniformity and reliability of LUS examinations, all sonographers will complete a standardized ultrasound training course. This will include: 1) a 2-hour training session led by the ultrasound site principal investigator consisting of didactics and image review to practice counting B-lines; and 2) proctored hands-on scanning of patients with pulmonary congestion. To be deemed proficient, sonographers must obtain ≥25 LUS videos that have been reviewed by the ultrasound site principal investigator and have achieved an intraclass correlation >0.7. Over 90% of the LUS videos will have to have B-lines. Twenty percent of these pre-study images will then be reviewed by the LUS Core Lab.
Quantifying B-lines
B-lines are vertical echogenic artifacts that originate from the pleural line, move with respiration and extend to the bottom of the ultrasound screen 16, 17 In patients with more severe pulmonary edema, B-lines may fuse together.
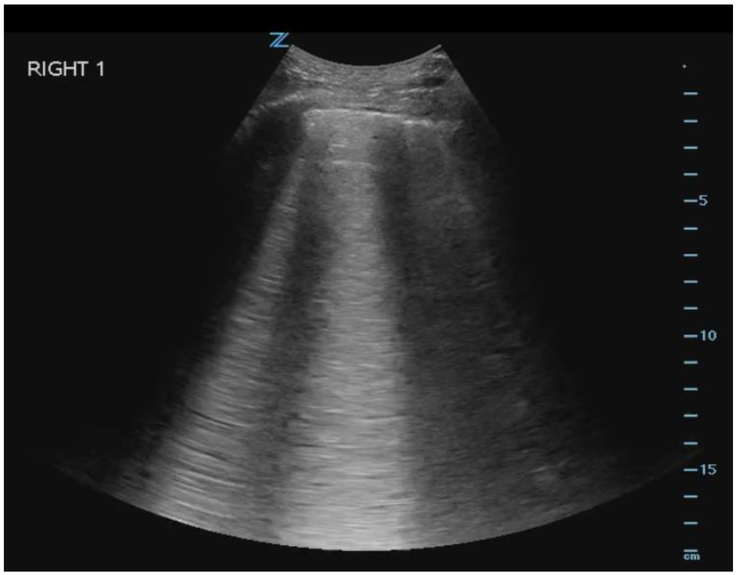
The total B-line count will be determined by summing the number of B-lines in each of the 8 zones, while the probe is placed in a transverse orientation, to maximize the amount of examined pleura. Each zone is given a B-line score of 0-20 based on the number of B-lines counted in one respiratory cycle across the entire visualized scanning field. To quantify the number of B-lines visualized in each zone, the intercostal space with the greatest number of B-lines within each zone will be used for scoring. Discrete, narrow B-lines will be counted individually. For those that are wide or fused together, the score will be determined by multiplying the percentage of the intercostal space filled with confluent B-lines by 20, thereby giving a maximum total count of 20 B-lines per individual zone (i.e. if 50% of the screen is filled with confluent B-lines, that will yield a score of 0.5 × 20 = 10 B-lines for that zone), see Figure 4.
Figure 4.
LUS image of B-lines taken from Right Zone 1. B-line score for this image is 10.
If, within a single zone, only a pleural effusion is seen but no lung is visualized, a B-line count of 0 will be assigned. If both lung and a pleural effusion are seen in the same intercostal space, then sonographers will count the number of B-lines visualized, as described above. The presence of pleural effusions will be assessed in each hemithorax in zone 4, with the probe held in a coronal plane with the indicator pointed towards the patient’s axilla. Pleural effusions will be graded as small, moderate or large.
B-lines will be counted upon completion of LUS exam by the sonographer who obtained the images. Findings will be recorded on a standardized data collection form. Individual zones and a composite B-line score will be recorded.
Core Lab
A Core Lab, consisting of two independent physicians with expertise in LUS, but not associated with one of the study sites, will individually review all images to assess for inter-observer agreement. They will be blinded to study arm, patient information, sonographer interpretation, study site, and the interpretation of the other expert reviewer. Only de-identified images from all study sites will be sent to the Core Lab. Core Lab interpretation will be recorded on a standardized data collection form.
Laboratory Testing
Patients will have labs collected at baseline (while the patient is in the ED), and on hospital day 7 or day of discharge, whichever comes first. Standard venipuncture techniques or other standard blood collection methods will be used in accordance with institutional standards.
Laboratory testing will be analyzed by the clinical lab at each respective institution for chemistry and hemoglobin/hematocrit values. Amino-terminal pro B-type natriuretic peptide (NTproBNP) and high-sensitivity (5th generation) troponin T (hsTnT) (Roche Diagnostics, Indianapolis, IN) will be drawn within 6 hours of randomization as well as prior to discharge for study purposes and will be analyzed centrally.
Endpoints
The primary endpoint is the number of patients with ≤15 B-lines on LUS at 6 hours after enrollment. Additionally, we will assess the exploratory endpoints listed in Table 3. Using these endpoints we will be able to collect vital data on the ability of LUS to guide AHF management through assessment of dynamic changes, and compare LUS to clinical assessment alone. In addition, we will further examine the prognostic value of LUS B-lines, in comparison to traditional assessments, including a preliminary determination of what B-line count warrants de-escalation of care, and determining when patients are appropriate for discharge. Importantly, assessment of B-lines during hospitalization combined with treatment will inform future study design. As a pilot trial, we have focused on the ED and early phase of management. Future studies may require LUS guidance throughout hospitalization.
Table 3:
Exploratory Endpoints
| Total DAOOH through 30 and 90 days post-discharge | Association of B-lines at discharge and 30 and 90 day outcomes |
| Change in biomarkers from presentation to pre-discharge | Association of baseline, discharge, and change with 30 and 90 day outcomes |
| Time to reach B-lines <15 | B lines < 15 at 24 hours and at discharge |
| Composite of 30-day all-cause mortality, cardiovascular (CV) re-hospitalizations, and CV emergency department (ED) revisits. CV endpoints are defined according to the 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events.32 Also for same endpoint, but through 90 days. |
All Cause readmissions, All cause ED revisits |
| Change in physical exam findings and body weight from presentation to pre-discharge | Description of ED pharmacologic treatment |
| Description of hospital based AHF treatment | Inter and intra-observer agreement |
| Trajectory of B-line clearance | Assess B-line clearance by sub-group/HF phenotype |
DAOOH - Days alive and out of hospital
Safety Measures
Mortality, unscheduled healthcare visits and re-hospitalization through 90 days will be assessed for safety as well as efficacy signals. Hypotension and acute kidney injury within the first 12 hours of therapy will also be assessed as safety endpoints. A systolic blood pressure that drops below 100 mm Hg at any time or if a patient develops evidence of clinical hypoperfusion (i.e. weakness, dizziness, etc) despite a systolic blood pressure >100 mm Hg will be immediately assessed and treated as needed. An independent data safety and monitoring board will meet throughout the duration of the study and will oversee patient safety.
Statistical Considerations
The primary hypothesis is that a higher proportion of LUS guided patients will be decongested, defined as LUS B-lines ≤15, than usual care patients at 6 hours after enrollment.
Our preliminary data suggest that 25% of patients in the usual care arm will have ≤ 15 B lines at the conclusion of ED AHF management. With 59 patients in each of the two arms, we will have 81% power to detect an effect size of 2 (i.e. 25% in the usual care versus 50% in the LUS-guided strategy), where the type I error rate is set at 0.05 (two-sided). Considering a conservative 10% drop-out rate, we will need a total of 130 subjects. We will perform two types of analysis, intent-to-treat and per-protocol. The Full Analysis Set (FAS) will include all randomized patients, which will be used in the intent-to-treat analysis where patients will be analyzed by the group to which they were randomized. Analyses in the FAS will constitute the main efficacy results for the primary and secondary study efficacy endpoints. The per-protocol analysis will be performed using the Per-Protocol Set (PPS), a subset of the FAS excluding patients with major protocol violations. The major protocol violations that will result in exclusion from the FAS will be identified prior to unblinding the treatment assignments for final analysis. Patients will be analyzed in the treatment group to which they were randomized. Such results will complement the primary efficacy analyses in the FAS.
Unless stated otherwise, two-sided p values < 0.05 will be considered statistically significant, without adjustment of multiple comparisons. Statistical tables and listings and analyses will be produced using SAS® release 9.4 or later (SAS Institute, Inc, Cary, NC, USA) or other validated statistical software.
Analysis of the Primary Efficacy Endpoint:
The comparison of binary endpoints (B-lines ≤ 15) will be performed using Chi-square or Fishers exact test, as appropriate. Potential covariates will also be considered in a logistic regression setting to improve precision, which includes baseline co-morbidities, baseline medications (in particular, guideline recommended therapies), in-hospital medications, baseline renal function, serum sodium, natriuretic peptide levels, troponin levels, renal function, baseline blood pressure, and discharge medications. Variables such as physical exam, other vital signs, and hemoconcentration may also be included. For NT-proBNP, a percent change greater than 30% and its association with the primary endpoint will be analyzed. This is based on previous work suggesting a 30% change was a key discriminatory threshold for mortality 33-35. For hemoconcentration, any increase in either hematocrit and hemoglobin during hospitalization will be considered positive 36. These covariates are known markers of risk and are standard of care assessments for the vast majority of AHF admissions. Covariates with univariate significance will be included together with the treatment indicator in a logistic regression model. We will limit the number of covariates (including treatment indicator) such that there are at least 10 events per covariate.
Analysis of the Exploratory Endpoints
Days alive and out of hospital (DAOOH) will be compared using t-test or Wilcoxon rank-sum test, as appropriate. Alternatively, we will treat DAOOH as an ordinal outcome and use the proportional odds (PO) regression model to compare the two arms. The PO regression allows for adjustment of baseline covariates to enhance power.
We will examine the distribution of B-lines measurements stratified by pre-specified outcomes. Both absolute number and relative change will be evaluated. Receiver operating characteristic (ROC) curves will be plotted together and area under the curve (AUC) will be calculated to understand the prediction performance of B-line measurement. Sensitivity, specificity, positive and negative predictive values will be computed at a number of thresholds of B-line measurements to understand the trade-off between false positive and false negative.
Confidence intervals of statistical measures will be constructed using the bootstrap method.37 Although 15 B-lines have been previously identified as a valid threshold, an alternative number may be more useful in the ED setting.
For reproducibility analysis, generalized linear mixed-effects models will be fitted to estimate the interand intra-observer variability, where both patients and observers are treated as random effects.
We will compare parameters used to identify congestion, including B-line measurements and other markers, such as physical exam, NTproBNP, eGFR, and hemoglobin/hematocrit. Bootstrap method will be used for the comparison to account for correlations between the markers and the B-line measurements. We will consider two strategies, logistic regressions and a tree-based method, to explore potential multivariate models for the prediction of 30 or 90-day outcomes.
Models will be compared using the net reclassification rate 38,39. Statistical inference of the comparison will be performed using the bootstrap method.
Discussion
Decongestion is a fundamental goal of AHF management. Failure to adequately decongest is associated with worse outcomes. Despite its importance, a universal, robust, well-validated method to assess and grade congestion with high inter-rater reliability does not exist.13
Traditional methods, such as body weight measurement, fluid balance, and physical exam continue to form the foundation of congestion assessment. Determination of whether alternative methods of congestion assessment, such as LUS, perform better than accurately performed traditional assessment is of critical importance.
The B-lines Lung Ultrasound Guided Emergency Department Management of Acute Heart Failure (BLUSHED-AHF) Pilot Trial is designed to answer whether targeting B-lines –a marker of pulmonary congestion – leads to more rapid resolution of pulmonary congestion compared to usual care during the ED phase of management. Importantly, both arms will follow the same treatment protocol. One limitation of this study design is the absence of a true ‘usual care’ arm, where there is no standard treatment protocol. However, if LUS proved superior to usual care, it could be fairly argued that LUS is less important than a standard treatment protocol.
As this is a pilot-trial, should targeting B-lines prove successful, a larger 3-arm study will be considered in future studies.
Another limitation is that ultrasound is highly operator-dependent, which could alter the sonographers acquisition and interpretation of LUS B-lines. Nevertheless, ultrasound assessment of B-lines is one of the easier ultrasound examinations to perform, and we designed a rigorous pre enrollment training program where each sonographer needs to achieve an intraclass correlation >0.7 with an expert. This is an effort to decrease variation in B-line quantification between different sonographers.
Additionally, there is no way to blind the clinical status of the patient to the study team performing LUS assessments. Despite this, all of the LUS performed for the study will be reviewed by a Core Lab of two expert sonographers, blinded to study arm, to assess for agreement.
A recent systematic review on the value of LUS B-lines in assessment of pulmonary congestion in patients with HF highlighted several gaps in the current literature 40. First, there are no objective, qualitative data on what represents adequate B-line reduction in response to standardized AHF treatment. Similarly, the time course of B-line resolution, based on treatment of different HF phenotypes, is unclear. The current body of literature in this area is limited, and lacks standardization with heterogeneity in imaging protocols, HF treatment and quantification of B-lines 40. The BLUSHED-AHF pilot trial will provide further insight into each of these questions. Other methods of decongestion assessment may also be valuable, such as hemoconcentration or changes in natriuretic peptide levels, which we will analyze these as well.
These data will help inform future studies considering LUS as a standalone tool or as part of a congestion score.
Conclusions
Pulmonary decongestion is a crucial therapeutic goal in AHF. BLUSHED-AHF will test a novel use of LUS to guide AHF management in the ED. This study will assess the incremental value of LUS compared to clinical assessment alone. If successful, this pilot study will inform future trials on LUS-driven therapy aimed at guiding acute treatment, and informing disposition decisions in patients with AHF.
Supplementary Material
Acknowledgements
This work is supported through a National Institutes of Health (NIH) grant (1R34HL136986-01). In-kind support for the biomarker assays were generously provided by Roche Diagnostics.
Footnotes
Data Supplements
Supplemental video showing dynamic B-lines during patient inspiration and expiration.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Frances M Russell, Department of Emergency Medicine, Indiana University School of Medicine, Indianapolis, IN.
Robert R Ehrman, Department of Emergency Medicine, Wayne State University School of Medicine, Detroit, MI.
Robinson Ferre, Department of Emergency Medicine, Vanderbilt University Medical Center, Nashville, TN.
Luna Gargani, Institute of Clinical Physiology, National Research Council, Pisa, Italy.
Vicki Noble, Department of Emergency Medicine, University Hospitals Cleveland Medical Center, Cleveland, OH.
Jordan Rupp, Department of Emergency Medicine, Vanderbilt University Medical Center, Nashville, TN.
Sean P. Collins, Department of Emergency Medicine, Vanderbilt University Medical Center, Nashville, TN.
Benton Hunter, Department of Emergency Medicine, Indiana University School of Medicine, Indianapolis, IN.
Kathleen A. Lane, Department of Biostatistics, Indiana University School of Medicine, Indianapolis, IN.
Phillip Levy, Department of Emergency Medicine and Integrative Biosciences Center, Wayne State University, Detroit, MI.
Xiaochun Li, Department of Biostatistics, Indiana University School of Medicine, Indianapolis, IN.
Christopher O’Connor, Division of Cardiology, INOVA Heart and Vascular Institute, Falls Church, VA.
Peter S. Pang, Department of Emergency Medicine, Indiana University School of Medicine, Indianapolis EMS, Indianapolis, IN.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW and Turner MB. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2015. [DOI] [PubMed] [Google Scholar]
- 2.Blehar DJ, Dickman E and Gaspari R. Identification of congestive heart failure via respiratory variation of inferior vena cava diameter. Am J Emerg Med. 2009;27:71–5. [DOI] [PubMed] [Google Scholar]
- 3.Adams KF Jr., Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M and Horton DP. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). American heart journal. 2005;149:209–16. [DOI] [PubMed] [Google Scholar]
- 4.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJand Wilkoff BL. 2013 ACCF/AHA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG, on behalf of the American Heart Association Advocacy Coordinating Committee CoAT, Vascular Biology CoCR, Intervention CoCCCoE and Pr. Forecasting the Impact of Heart Failure in the United States: A Policy Statement From the American Heart Association. Circulation Heart failure. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jencks SF, Williams MV and Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. The New England journal of medicine. 2009;360:1418–28. [DOI] [PubMed] [Google Scholar]
- 7.Eapen ZJ, Liang L, Fonarow GC, Heidenreich PA, Curtis LH, Peterson ED and Hernandez AF. Validated, electronic health record deployable prediction models for assessing patient risk of 30-day rehospitalization and mortality in older heart failure patients. JACC Heart failure. 2013;1:245–51. [DOI] [PubMed] [Google Scholar]
- 8.Joynt KE, Orav EJ and Jha AK. Thirty-day readmission rates for Medicare beneficiaries by race and site of care. JAMA : the journal of the American Medical Association. 2011;305:675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ and Wilkoff BL. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013. [DOI] [PubMed] [Google Scholar]
- 10.Gheorghiade M, Filippatos G, De Luca L and Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. The American journal of medicine. 2006;119:S3–S10. [DOI] [PubMed] [Google Scholar]
- 11.Lucas C, Johnson W, Hamilton MA, Fonarow GC, Woo MA, Flavell CM, Creaser JA and Stevenson LW. Freedom from congestion predicts good survival despite previous class IV symptoms of heart failure. Am Heart J. 2000;140:840–7. [DOI] [PubMed] [Google Scholar]
- 12.Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, Maggioni AP, Cook T, Swedberg K, Burnett JC Jr., Grinfeld L, Udelson JE, Zannad F, Gheorghiade M and Investigators ET. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835–43. [DOI] [PubMed] [Google Scholar]
- 13.Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Sendon JL, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, van Veldhuisen DJ, Zannad F, Anker SD, Rhodes A, McMurray JJ, Filippatos G, European Society of C and European Society of Intensive Care M. Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail. 2010;12:423–33. [DOI] [PubMed] [Google Scholar]
- 14.Testani JM, Chen J, McCauley BD, Kimmel SE and Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang CS, FitzGerald JM, Schulzer M, Mak E and Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA : the journal of the American Medical Association. 2005;294:1944–56. [DOI] [PubMed] [Google Scholar]
- 16.Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E and Petrovic T. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–91. [DOI] [PubMed] [Google Scholar]
- 17.Picano E, Frassi F, Agricola E, Gligorova S, Gargani L and Mottola G. Ultrasound lung comets: a clinically useful sign of extravascular lung water. J Am Soc Echocardiogr. 2006;19:356–63. [DOI] [PubMed] [Google Scholar]
- 18.Agricola E, Bove T, Oppizzi M, Marino G, Zangrillo A, Margonato A and Picano E. "Ultrasound comet-tail images": a marker of pulmonary edema: a comparative study with wedge pressure and extravascular lung water. Chest. 2005;127:1690–5. [DOI] [PubMed] [Google Scholar]
- 19.Gargani L, Frassi F, Soldati G, Tesorio P, Gheorghiade M and Picano E. Ultrasound lung comets for the differential diagnosis of acute cardiogenic dyspnoea: a comparison with natriuretic peptides. Eur J Heart Fail. 2008;10:70–7. [DOI] [PubMed] [Google Scholar]
- 20.Jambrik Z, Monti S, Coppola V, Agricola E, Mottola G, Miniati M and Picano E. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am J Cardiol. 2004;93:1265–70. [DOI] [PubMed] [Google Scholar]
- 21.Martindale JL. Diagnosing Acute Heart Failure in the Emergency Department. Acad Emerg Med. 2016. [Google Scholar]
- 22.Noble VE, Murray AF, Capp R, Sylvia-Reardon MH, Steele DJ and Liteplo A. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis. Time course for resolution. Chest. 2009; 135:1433–9. [DOI] [PubMed] [Google Scholar]
- 23.Volpicelli G, Caramello V, Cardinale L, Mussa A, Bar F and Frascisco MF. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med. 2008;26:585–91. [DOI] [PubMed] [Google Scholar]
- 24.Frassi F, Gargani L, Gligorova S, Ciampi Q, Mottola G and Picano E. Clinical and echocardiographic determinants of ultrasound lung comets. Eur J Echocardiogr. 2007;8:474–9. [DOI] [PubMed] [Google Scholar]
- 25.Ohman J, Harjola VP, Karjalainen P and Lassus J. Assessment of early treatment response by rapid cardiothoracic ultrasound in acute heart failure: Cardiac filling pressures, pulmonary congestion and mortality. Eur Heart J Acute Cardiovasc Care. 2017:2048872617708974. [DOI] [PubMed] [Google Scholar]
- 26.Gargani L, Pang PS, Frassi F, Miglioranza MH, Dini FL, Landi P and Picano E. Persistent pulmonary congestion before discharge predicts rehospitalization in heart failure: a lung ultrasound study. Cardiovasc Ultrasound. 2015;13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coiro S, Rossignol P, Ambrosio G, Carluccio E, Alunni G, Murrone A, Tritto I, Zannad F and Girerd N. Prognostic value of residual pulmonary congestion at discharge assessed by lung ultrasound imaging in heart failure. Eur J Heart Fail. 2015;17:1172–81. [DOI] [PubMed] [Google Scholar]
- 28.Cogliati C, Casazza G, Ceriani E, Torzillo D, Furlotti S, Bossi I, Vago T, Costantino G and Montano N. Lung ultrasound and short-term prognosis in heart failure patients. International journal of cardiology. 2016;218:104–108. [DOI] [PubMed] [Google Scholar]
- 29.Frasure SE, Matilsky DK, Siadecki SD, Platz E, Saul T and Lewiss RE. Impact of patient positioning on lung ultrasound findings in acute heart failure. Eur Heart J Acute Cardiovasc Care. 2014. [DOI] [PubMed] [Google Scholar]
- 30.Martindale JL, Noble VE and Liteplo A. Diagnosing pulmonary edema: lung ultrasound versus chest radiography. European journal of emergency medicine: official journal of the European Society for Emergency Medicine. 2013;20:356–60. [DOI] [PubMed] [Google Scholar]
- 31.See KC, Ong V, Wong SH, Leanda R, Santos J, Taculod J, Phua J and Teoh CM. Lung ultrasound training: curriculum implementation and learning trajectory among respiratory therapists. Intensive care medicine. 2016;42:63–71. [DOI] [PubMed] [Google Scholar]
- 32.Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, Limacher MC, Mahaffey KW, Mehran R, Nissen SE, Smith EE and Targum SL. 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol. 2015;66:403–69. [DOI] [PubMed] [Google Scholar]
- 33.Bettencourt P, Azevedo A, Pimenta J, Frioes F, Ferreira S and Ferreira A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004; 110:2168–74. [DOI] [PubMed] [Google Scholar]
- 34.Stienen S, Salah K, Dickhoff C, Carubelli V, Metra M, Magrini L, Di Somma S, Tijssen JP, Pinto YM and Kok WE. N-Terminal Pro-B-Type Natriuretic Peptide (NT-proBNP) Measurements Until a 30% Reduction Is Attained During Acute Decompensated Heart Failure Admissions and Comparison With Discharge NT-proBNP Levels: Implications for In-Hospital Guidance of Treatment. J Card Fail. 2015. [DOI] [PubMed] [Google Scholar]
- 35.Stienen S, Salah K, Moons AH, Bakx AL, van Pol PE, Schroeder-Tanka JM, Voogel AJ, Keijer JT, Kortz RA, Dickhoff C, Meregalli PG, Tijssen JG, Pinto YM and Kok WE. Rationale and design of PRIMA II: A multicenter, randomized clinical trial to study the impact of in-hospital guidance for acute decompensated heart failure treatment by a predefined NT-PRoBNP target on the reduction of readmIssion and Mortality rAtes. Am Heart J. 2014;168:30–6. [DOI] [PubMed] [Google Scholar]
- 36.Testani JM, Brisco MA, Chen J, McCauley BD, Parikh CR and Tang WH. Timing of hemoconcentration during treatment of acute decompensated heart failure and subsequent survival: importance of sustained decongestion. J Am Coll Cardiol. 2013;62:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Efron B and Tibshirani R. An Introduction to the Bootstrap. Boca Raton: Chapman & Hall/CRC; 1993. [Google Scholar]
- 38.Pencina MJ, D'Agostino RB Sr., D'Agostino RB Jr. and Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in medicine. 2008;27:157–72; discussion 207-12. [DOI] [PubMed] [Google Scholar]
- 39.Pencina MJ, D'Agostino RB Sr. and Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Statistics in medicine. 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Platz E, Merz AA, Jhund PS, Vazir A, Campbell R and McMurray JJ. Dynamic changes and prognostic value of pulmonary congestion by lung ultrasound in acute and chronic heart failure: a systematic review. Eur J Heart Fail.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.