Abstract
Background
Obstructive sleep apnea (OSA) is a disease seriously threatening individual health, which results in serious complications such as hypertension and stroke. These complications are associated with oxidative stress triggered by intermittent hypoxia in OSA. Sestrin2 is a crucial factor involved in oxidative stress. The goal of this study was to investigate if a relationship exists between OSA and Sestrin2.
Methods
We prospectively enrolled 71 subjects, and 16 patients of them with severe OSA completed 4 weeks of nasal continuous positive airway pressure (nCPAP) therapy. We measured and compared the concentration of Sestrin2 in the urine of all subjects, as well as the changes between before and after nCPAP treatment. Additionally, the correlation between Sestrin2 and sleep parameters was analyzed, and the multiple linear regression analysis with stepwise selection was performed to explore the relationship between Sestrin2 and various factors.
Results
A total of 71 subjects were enrolled and divided into two groups: OSA group (n = 41), control group (n = 30). The level of urinary Sestrin2 in OSA patients was significantly higher than that of the control group, and increased with the severity of OSA, while it reduced after nCPAP treatment. Additionally, Sestrin2 was positively correlated with apnea/hypopnea index (AHI), oxygen desaturation index, oxygen saturation < 90% percentage of recording time spent (PRTS) and high-density lipoprotein (HDL), while negatively correlated with the lowest oxygen saturation. Importantly, Sestrin2 was independently associated with AHI, oxygen saturation < 90% PRTS and HDL.
Conclusions
Urinary Sestrin2 is involved in OSA, and is a paramount marker of OSA severity.
Keywords: Sestrin2, Obstructive sleep apnea, Apnea/hypopnea index, High-density lipoprotein
Introduction
Obstructive sleep apnea (OSA) is a shared sleep-related disorder. According to the survey, the estimated prevalence of OSA is 0.7–3.3% [1–3]. It is characterized by periodic recurrent episodes of upper airway obstruction during sleep, resulting in apnea [4], and the occurrence of OSA is capable of disrupting sleep integrity, impairing ventilation, and resulting in intermittent hypoxia [5], which seriously lead to coronary artery disease, high blood pressure, cerebrovascular accident, stroke, etc. [6]. There is increasing evidence that these complications are closely related to oxidative stress triggered by intermittent hypoxia [6–9].
Sestrin, originally discovered as p53-inducible proteins, is a highly conserved family of proteins that are thought to protect cells from various damages [10]. The Sestrin family has three distinct members in mammals, which include Sestrin1, Sestrin2, and Sestrin3 [11]. Sestrin2, among the three members, also known as hypoxia-inducible gene (Hi95), is an acute response protein that plays a paramount role in various conditions such as excessive oxidative stress, hypoxia, DNA damage, etc. [12]. Moreover, Sestrin2 expression is obviously up-regulated in oxidative stress-related diseases, exerting anti-oxidation, preventing the production of reactive oxygen species (ROS), reducing the accumulation of ROS as an inducible factor of oxidation, and thus protecting cells from oxidative stress [13, 14]. Chronic intermittent hypoxia (CIH) occurs in patients with OSA during sleeping [15], leading to a strong oxidative stress response [16]. Accordingly, we speculated that Sestrin2 might be involved in the OSA. To study the relationship between Sestrin2 and OSA may be beneficial in discovering the reasons of oxidative stress-related complications in OSA patients. However, so far, we have not found any relevant reports. The goal of this study was to investigate the changes in urinary Sestrin2 concentration in OSA patients.
Method
Research Population
We prospectively enrolled 71 subjects who were entirely performed by polysomnography (PSG) tests because of snoring, including 41 newly diagnosed OSA patients and 30 healthy controls at the First Affiliated Hospital of Kunming Medical University, from June 2016 to June 2018. The inclusion criteria were patients with 18–65 years old, and the exclusion criteria were patients with heart failure, lung disease, cerebrovascular disease, kidney disease, metabolic diseases like diabetes, and the OSA patients who have been treated before this study.
All patients underwent a systematic physical examination prior to PSG testing to measure the height, weight, waist-to-hip ratio, and neck circumference (NC). Their body mass index (BMI) was calculated. The Epworth Sleepiness Score (ESS) was used to assess the degree of daytime sleepiness. This study was approved by the Ethics Committee of the First Affiliated Hospital of Kunming Medical University, and the patient’s informed consents were obtained.
PSG
All subjects were examined by overnight PSG (Alice 5, American) and recorded parameters including EEG, ECG, oral and nasal airflow, oxygen saturation, and chest and abdomen movements. Apnea was defined as a respiratory amplitude reduction ≥ 90% for more than 10 s. Hypopnea was defined as a decrease in respiratory airflow amplitude reduction ≥ 30% with a decrease in oxygen saturation ≥ 3% or arousal [17]. All PSG records were analyzed by two experts to obtain apnea/hypopnea index (AHI). Normal subject was defined with AHI < 5, OSA was defined with AHI ≥ 5. The hypoxemia index was indicated by oxygen saturation < 90% percentage of recording time spent (PRTS).
Pressure Titration Under PSG
An overnight pressure titration was performed under PSG testing using an auto-continuous positive airway pressure (Auto-CPAP) machine (Philips-Respironics REMstarAuto). The airflow signal was collected by the mask pressure signal. The humidifier was routinely used during titration. If the PSG result demonstrated AHI ≤ 5/h, 90th percentile recording pressure was defined as the titration pressure.
nCPAP Treatment
Patients enrolled in the treatment response analysis were treated with a nasal CPAP (nCPAP) machine (Resmed, S9 Escape, Australia). There was a data card on each machine to record the application time. We read the data card and downloaded the data after 4 weeks of treatment. Patients who were treated for more than 4 h/day were included in the treatment analysis.
Urine Test
5–7 mL urine specimens were collected from the patients on an empty stomach in the morning after the PSG test and 4 weeks nCPAP treatment, then centrifugated 20 min by 3000 rpm at 4 °C. Urine supernatant was extracted and stored in − 80 °C refrigerator for testing. The urinary Sestrin2 concentration was measured with an ELISA kit (Mlbio, Shanghai, China). Other tests including fasting blood glucose, cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and urine creatinine were tested by the First Affiliated Hospital of Kunming Medical University. Sestrin2 was adjusted with creatinine: adjust Sestrin2 = (Sestrin2/creatinine) × 104.
Statistical Analysis
Data were presented as mean ± standard derivation. In addition, the single-sample Kolmogorov–Smirnov method was used to detect whether the data were normally distributed. Normally distributed data were compared by an unpaired t-test, and the data between before and after treatment were compared with paired t-test. Rank sum test was used for non-normally distributed data. Pearson’s correlation analysis was applied for correlation analysis. Linear stepwise regression analysis was used to detect the relationship between Sestrin2 and other factors (age, gender, waist-to-hip, BMI, NC, AHI, mean oxygen saturation, lowest oxygen saturation, oxygen saturation < 90% PRTS, ESS, TG, TC, LDL, HDL, fasting blood glucose).
Result
71 Subjects were prospectively enrolled in this study, and were divided into two groups: OSA group (39.29 ± 7.73 years old, n = 41) and control group (38.70 ± 8.86 years old, n = 30). All patient characteristics and the results of urinary Sestrin2 were listed in Table 1. OSA patients were divided into three subgroups according to AHI: mild group 5 ≤ AHI < 15, moderate group 15 ≤ AHI < 30, and severe group AHI ≥ 30. Sixteen patients with severe OSA completed 4 weeks nCPAP treatment, and the treatment time was greater than 4 h/day, and then the levels of urinary Sestrin2 after treatment were measured.
Table 1.
Demographics, sleep profiles, blood and urinary measurements of the OSA and control groups
| Control group (n = 30) | OSA group (n = 41) | P values | |
|---|---|---|---|
| Age (years) | 38.70 ± 8.86 | 39.29 ± 7.73 | 0.765 |
| Gender (male/female)a | 20/10 | 32/9 | 0.416 |
| BMI (kg/m2) | 27.09 ± 4.22 | 28.55 ± 4.62 | 0.176 |
| Neck circumference (cm) | 36.06 ± 5.47 | 38.56 ± 6.90 | 0.093 |
| Waist-to-hip ratio | 0.88 ± 0.07 | 0.92 ± 0.08 | 0.022* |
| AHI (events/h) | 2.32 ± 1.20 | 35.00 ± 20.86 | < 0.001* |
| ODI (events/h) | 1.59 ± 0.90 | 31.17 ± 19.31 | < 0.001* |
| Lowest oxygen saturation (%) | 90.48 ± 0.99 | 79.93 ± 8.28 | < 0.001* |
| Mean oxygen saturation (%) | 93.50 ± 0.94 | 90.40 ± 2.91 | < 0.001* |
| Oxygen saturation < 90% PRTS (%) | 0.46 ± 1.42 | 19.35 ± 8.92 | < 0.001* |
| Total sleep time (min) | 316.8 ± 38.71 | 329.83 ± 40.71 | 0.179 |
| Arousal index (events/h) | 13.17 ± 7.32 | 35.86 ± 20.63 | < 0.001* |
| Stage of REM (%) | 13.15 ± 3.79 | 11.36 ± 3.25 | 0.035* |
| Stage of NREM (%) | 86.85 ± 3.79 | 88.64 ± 3.25 | 0.035* |
| Triglycerides (mmol/L) | 1.51 ± 0.61 | 1.77 ± 1.12 | 0.224 |
| Sestrin2 (ng/mL creatinine) | 2.69 ± 1.11 | 3.39 ± 1.65 | 0.035* |
| Total cholesterol (mmol/L) | 4.08 ± 0.80 | 4.31 ± 0.64 | 0.180 |
| Low-density lipoprotein (mmol/L) | 2.31 ± 0.63 | 2.51 ± 0.58 | 0.160 |
| High-density lipoprotein (mmol/L) | 1.03 ± 0.29 | 0.99 ± 0.24 | 0.532 |
| Fasting blood glucose (mmol/L) | 4.31 ± 0.53 | 4.56 ± 0.56 | 0.064 |
| ESS (scores) | 6.73 ± 2.46 | 11.92 ± 4.30 | < 0.001* |
Values are indicated as the mean ± standard deviation
BMI body mass index, AHI apnea/hypopnea index, ODI oxygen desaturation index, PRTS percentage of recording time spent, REM rapid eye movement, NREM non-rapid eye movement, ESS Epworth Sleepiness Score
*P < 0.05, the results are statistically significant
aValues are indicated as numbers
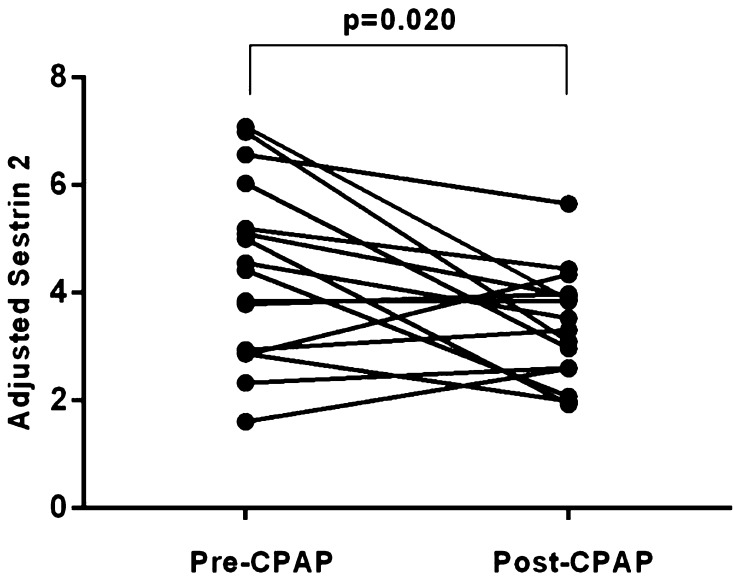
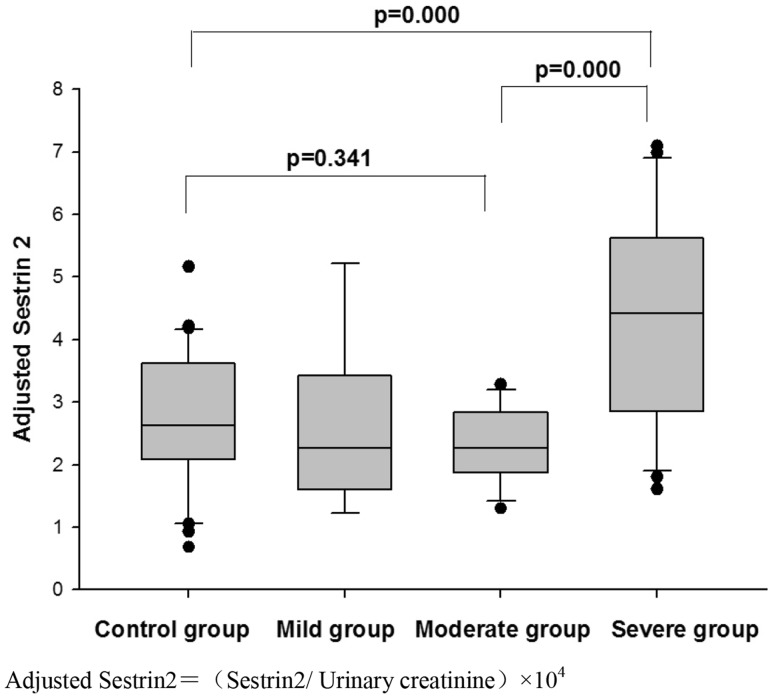
The level of urinary Sestrin2 in the OSA group was significantly higher than that of the control group (P = 0.035), and as the severity of OSA increased, Sestrin2 level also increased. In addition, Sestrin2 was significantly reduced after nCPAP treatment (Fig. 1). Subgroup analysis demonstrated that the concentration of Sestrin2 in the severe OSA group was significantly higher than that of the other subgroups and control group (Fig. 2).
Fig. 1.
Results of adjusted Sestrin2 level of 16 severe OSA before and after CPAP treatment
Fig. 2.
Adjusted Sestrin2 levels of control group and OSA subgroups. Adjusted Sestrin2 = (Sestrin2/urinary creatinine) × 104
Sestrin2 was positively correlated with AHI, oxygen desaturation index (ODI), oxygen saturation < 90% PRTS, HDL, fasting blood glucose, and OSA severity, but negatively correlated with lowest oxygen saturation. Importantly, these relationships still existed after adjusting for age, gender, waist-to-hip, NC, and BMI (Table 2).
Table 2.
Spearman’s correlations between adjusted Sestrin2 and the other factors
| r | P values | r a | P values | |
|---|---|---|---|---|
| Age | 0.170 | 0.156 | ||
| BMI | 0.075 | 0.535 | ||
| Neck circumference | −0.043 | 0.725 | ||
| Waist-to-hip ratio | 0.073 | 0.545 | ||
| AHI | 0.376 | 0.001* | 0.566 | < 0.001* |
| ODI | 0.314 | 0.008* | 0.559 | < 0.001* |
| Lowest oxygen saturation | −0.358 | 0.002* | −0.350 | 0.004* |
| Mean oxygen saturation | −0.160 | 0.183 | −0.138 | 0.268 |
| Oxygen saturation < 90% PRTS | 0.378 | 0.001* | 0.562 | < 0.001* |
| Total sleep time | −0.047 | 0.698 | 0.044 | 0.726 |
| Arousal index | −0.003 | 0.979 | 0.215 | 0.083 |
| Stage of REM | 0.070 | 0.564 | −0.016 | 0.901 |
| Stage of NREM | −0.070 | 0.564 | 0.016 | 0.901 |
| Triglycerides | −0.054 | 0.654 | −0.030 | 0.814 |
| Total cholesterol | 0.029 | 0.811 | 0.084 | 0.505 |
| Low-density lipoprotein | 0.001 | 0.996 | −0.012 | 0.922 |
| High-density lipoprotein | 0.344 | 0.003* | 0.325 | 0.008* |
| Fasting blood glucose | 0.305 | 0.010* | 0.274 | 0.026* |
| Epworth Sleepiness Score | 0.298 | 0.011* | 0.259 | 0.036* |
| Severity of OSA | 0.405 | 0.000* | 0.388 | 0.001* |
BMI body mass index, AHI apnea/hypopnea index, ODI oxygen desaturation index, PRTS percentage of recording time spent, REM rapid eye movement, NREM non-rapid eye movement, OSA obstructive sleep apnea
*P < 0.05, the results are statistically significant
aAdjusting for age, BMI, gender, waist-to-hip ratio, neck circumference
The multiple linear regression analysis with stepwise selection showed that Sestrin2 was associated with AHI, oxygen saturation < 90% PRTS, and HDL (Table 3). However, there was no association between Sestrin2 and waist-to-hip ratio.
Table 3.
Stepwise multiple regression model of adjusted urinary Sestrin2 levels in the OSA group (adjusted R2 = 0.745)
| B (SE) | β | P values | |
|---|---|---|---|
| Constant | −1.078 (0.525) | 0.047* | |
| AHI | 0.033 (0.007) | 0.420 | < 0.001* |
| Oxygen saturation < 90% PRTS | 0.094 (0.016) | 0.509 | < 0.001* |
| HDL | 1.435 (0.444) | 0.262 | 0.003* |
Independent variables considered: age, gender, BMI, waist-to-hip ratio, neck circumference, AHI, lowest oxygen saturation, oxygen saturation < 90% PRTS, total sleep time, stage of rapid eye movement sleep, Epworth Sleepiness Score, triglycerides, total cholesterol, low-density lipoprotein, HDL, fasting blood glucose
AHI apnea/hypopnea index, PRTS percentage of recording time spent, HDL high-density lipoprotein
*P < 0.05, the results are statistically significant
Discussion
This study was the first report on investigation of Sestrin2 in OSA, and the results showed that the Sestrin2 level elevated in OSA and decreased after nCPAP treatment. In addition, Sestrin2 was positively correlated with AHI, ODI, and oxygen saturation < 90% PRTS. Most importantly, the multiple regression analysis demonstrated that Sestrin2 was associated with AHI and oxygen saturation < 90% PRTS. It suggested that Sestrin2 played an important role in OSA.
Several authors [12, 13, 18–20] suggested that hypoxia, stress, or inflammation led to a growth of directed autophagy of Kelch-like ECH-associated protein 1 (Keap1), thereby decomposed the key regulator of oxidative genes, nuclear erythroid-related factor 2 (Nrf2). The production and accumulation of ROS inhibited by Nrf2 will lead to an increase in Sestrin2 [21, 22]. Moreover, hypoxia, stress, or inflammation will activate the mTORC1. Sestrin2, as a leucine sensor, is significantly increased in mTORC1 activation [23–25]. It was reported that hypoxia, oxidative stress, and inflammation recurred in OSA [26–28]. In present study, Sestrin2 was positively correlated with ODI and oxygen saturation < 90% PRTS. In addition, the multiple regression analysis showed that Sestrin2 was associated with oxygen saturation < 90% PRTS. Therefore, the increasing of urinary Sestrin2 might be triggered by intermittent hypoxia in OSA.
It was reported that Sestrin2 was a conserved antioxidant protein, and could be activated under pressure to protect cells from oxidative stress [29]. Essler et al. [30] suggested that Sestrin2 upregulation mediated by hypoxia was beneficial to counteracting the production of ROS, and ROS frequently accumulated and increased due to CIH in OSA [4]. Consequently, Sestrin2 might play an advantageous role in anti-oxidative stress in OSA.
It was reported that nCPAP could significantly reduce ODI and oxygen saturation < 90% PRTS in patients with OSA, and improve the hypoxia of OSA during sleep [31, 32]. In addition, the nCPAP could improve the oxidative stress and inflammatory response [32, 33]. In this study, the Sestrin2 reduced with the nCPAP treatment. Hypoxia, which might result in the elevation of Sestrin2, relieved after nCPAP treatment, thus the Sestrin2 decreased.
It was reported that central adiposity was associated with OSA [34], and it could predict the onset of OSA [35]. In present study, there was no relationship between waist-to-hip ratio and Sestrin2, which meant that the central adiposity had no effect on the change of Sestrin2.
Moreover, sleep architecture impairment of OSA might affect some factors, such as TNF-α [36]. However, in this study, Sestrin2 was not related to REM, NREM sleep, arousal index, and total sleep time, which meant that sleep architecture impairment of OSA did not contribute to increasing Sestrin2 level.
It was reported that there was a significant positive correlation between Sestrin2 and HDL [37]. The HDL played an antioxidant role [38], and the HDL levels were positively correlated with obesity status [39, 40]. Moreover, Sestrin2 regulated the PERK-c/EBPβ pathway by mediating mTORCI [41, 42], and inhibited the ROS-mediated p38 MAPK activation and interfered the expression of uncoupling protein 1 (Ucp1), subsequently resulting in the decrease of heat production and the increase of adipose tissue, which led to obesity [43, 44]. In addition, HDL was reduced in inflammatory conditions [45], while Sestrin2 could inhibit TLR-mediated inflammatory responses in macrophages [46]. Accordingly, it might be the obesity and inflammation that caused the association between Sestrin2 and HDL in this study.
However, there was a main limitation that the present study was not randomized; Small sample size was also a limitation. In addition, another limitation was that we used Auto-CPAP titration under PSG for pressure titration instead of the standard CPAP titration, though it was suggested that Auto-CPAP titration under PSG was also effective.
Conclusion
Urinary Sestrin2 is involved in OSA, and is closely related to the severity of OSA. It decreases with nCPAP treatment. Accordingly, urinary Sestrin2 is a paramount marker of OSA severity.
Acknowledgements
None.
Funding
This study was funded by Yunnan Provincial Department of Education (No. 2017zzx201) and Teaching and Reform Program of Kunming Medical University (Nos. 2016-JY-Y-43, 2017-JY-Y-040).
Conflict of interest
None.
Ethical Approval
All procedures performed in studies involving human participants were consistent with the Ethical Standards of the Institutional and/or Nation Research Committee, as well as the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lavie P. Incidence of sleep apnea in a presumably healthy working population: a significant relationship with excessive daytime sleepiness. Sleep. 2002;25:312–318. doi: 10.1093/sleep/25.4.380. [DOI] [PubMed] [Google Scholar]
- 2.Gislason T, Almqvist M, Eriksson G, et al. Prevalence of sleep apnea syndrome among Swedish men—an epidemiological study. J Clin Epidemiol. 1988;41:571–576. doi: 10.1016/0895-4356(88)90061-3. [DOI] [PubMed] [Google Scholar]
- 3.Bixler EO, Vgontzas AN, Ten Have T, et al. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 4.Bouloukaki I, Mermigkis C, Kallergis EM, et al. Obstructive sleep apnea syndrome and cardiovascular disease: the influence of C-reactive protein. World J Exp Med. 2015;5:77–83. doi: 10.5493/wjem.v5.i2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elsharkawi I, Gozal D, Macklin EA, et al. Urinary biomarkers and obstructive sleep apnea in patients with Down syndrome. Sleep Med. 2017;34:84–89. doi: 10.1016/j.sleep.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population—a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311–1322. doi: 10.3978/j.issn.2072-1439.2015.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flora SJ. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid Med Cell Longev. 2009;2:191–206. doi: 10.4161/oxim.2.4.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalle-Donne I, Rossi R, Colombo R, et al. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 9.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 10.Donahoe SM, Stewart GC, McCabe CH, et al. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;298:765–775. doi: 10.1001/jama.298.7.765. [DOI] [PubMed] [Google Scholar]
- 11.Budanov AV, Shoshani T, Faerman A, et al. Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene. 2002;21:6017–6031. doi: 10.1038/sj.onc.1205877. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Budanov AV, Karin M. Sestrins orchestrate cellular metabolism to attenuate aging. Cell Metab. 2013;18:792–801. doi: 10.1016/j.cmet.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuang YC, Yang JL, Yang DI, et al. Roles of Sestrin2 and ribosomal protein S6 in transient global ischemia-induced hippocampal neuronal injury. Int J Mol Sci. 2015;16:26406–26416. doi: 10.3390/ijms161125963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rai N, Kumar R, Desai GR, et al. Relative alterations in blood-based levels of Sestrin in Alzheimer’s disease and mild cognitive impairment patients. J Alzheimers Dis. 2016;54:1147–1155. doi: 10.3233/JAD-160479. [DOI] [PubMed] [Google Scholar]
- 15.Zhou L, Chen P, Peng Y, et al. Role of oxidative stress in the neurocognitive dysfunction of obstructive sleep apnea syndrome. Oxid Med Cell Longev. 2016;2016:9626831. doi: 10.1155/2016/9626831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisele HJ, Markart P, Schulz R. Obstructive sleep apnea, oxidative stress, and cardiovascular disease: evidence from human studies. Oxid Med Cell Longev. 2015;2015:608438. doi: 10.1155/2015/608438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumitrascu R, Heitmann J, Seeger W, et al. Obstructive sleep apnea, oxidative stress and cardiovascular disease: lessons from animal studies. Oxid Med Cell Longev. 2013;2013:234631. doi: 10.1155/2013/234631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Report of an American Academy of Sleep Medicine Task Force Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–689. doi: 10.1093/sleep/22.5.667. [DOI] [PubMed] [Google Scholar]
- 19.Li K, Chen Z, Qin Y, et al. MiR-664a-3p expression in patients with obstructive sleep apnea: a potential marker of atherosclerosis. Medicine (Baltim) 2018;97:e9813. doi: 10.1097/MD.0000000000009813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye J, Wang M, Xu Y, et al. Sestrins increase in patients with coronary artery disease and associate with the severity of coronary stenosis. Clin Chim Acta. 2017;472:51–57. doi: 10.1016/j.cca.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Fu H, Song W, Wang Y, et al. Radiosensitizing effects of Sestrin2 in PC3 prostate cancer cells. Iran J Basic Med Sci. 2018;21:621–624. doi: 10.22038/IJBMS.2018.18283.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bae SH, Sung SH, Oh SY, et al. Sestrins activate Nrf2 by promoting p62-dependent autophagic degradation of Keap1 and prevent oxidative liver damage. Cell Metab. 2013;17:73–84. doi: 10.1016/j.cmet.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Zeng N, D’Souza RF, Sorrenson B, et al. The putative leucine sensor Sestrin2 is hyperphosphorylated by acute resistance exercise but not protein ingestion in human skeletal muscle. Eur J Appl Physiol. 2018;118:1241–1253. doi: 10.1007/s00421-018-3853-8. [DOI] [PubMed] [Google Scholar]
- 24.Lee M, Kim JH, Yoon I, et al. Coordination of the leucine-sensing Rag GTPase cycle by leucyl-tRNA synthetase in the mTORC1 signaling pathway. Proc Natl Acad Sci USA. 2018;115:E5279–E5288. doi: 10.1073/pnas.1801287115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfson RL, Sabatini DM. The dawn of the age of amino acid sensors for the mTORC1 pathway. Cell Metab. 2017;26:301–309. doi: 10.1016/j.cmet.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tóthová L, Hodosy J, Mucska I, et al. Salivary markers of oxidative stress in patients with obstructive sleep apnea treated with continuous positive airway pressure. Sleep Breath. 2014;18:563–570. doi: 10.1007/s11325-013-0919-z. [DOI] [PubMed] [Google Scholar]
- 27.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 28.Edwards KM, Tomfohr LM, Mills PJ, et al. Macrophage migratory inhibitory factor (MIF) may be a key factor in inflammation in obstructive sleep apnea. Sleep. 2011;34:161–163. doi: 10.1093/sleep/34.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang LL, Zhang ZJ. Sestrin2 aggravates oxidative stress of neurons by decreasing the expression of Nrf2. Eur Rev Med Pharmacol Sci. 2018;22:3493–3501. doi: 10.26355/eurrev_201806_15176. [DOI] [PubMed] [Google Scholar]
- 30.Essler S, Dehne N, Brune B. Role of Sestrin2 in peroxide signaling in macrophages. FEBS Lett. 2009;583:3531–3535. doi: 10.1016/j.febslet.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Tan H, Shi XN, et al. The effect of nasal continuous positive airway pressure on endothelial function in obstructive sleep apnea–hypopnea syndrome with coronary heart disease. Zhonghua Nei Ke Za Zhi. 2006;45:188–191. [PubMed] [Google Scholar]
- 32.Christou K, Kostikas K, Pastaka C, et al. Nasal continuous positive airway pressure treatment reduces systemic oxidative stress in patients with severe obstructive sleep apnea syndrome. Sleep Med. 2009;10:87–94. doi: 10.1016/j.sleep.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Schulz R, Mahmoudi S, Hattar K, et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2000;162:566–570. doi: 10.1164/ajrccm.162.2.9908091. [DOI] [PubMed] [Google Scholar]
- 34.Schäfer H, Pauleit D, Sudhop T, et al. Body fat distribution, serum leptin, and cardiovascular risk factors in men with obstructive sleep apnea. Chest. 2002;122:829–839. doi: 10.1378/chest.122.3.829. [DOI] [PubMed] [Google Scholar]
- 35.Gaines J, Vgontzas AN, Fernandez-Mendoza J, et al. Inflammation mediates the association between visceral adiposity and obstructive sleep apnea in adolescents. Am J Physiol Endocrinol Metab. 2016;311:E851–E858. doi: 10.1152/ajpendo.00249.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirotsu C, Albuquerque RG, Nogueira H, et al. The relationship between sleep apnea, metabolic dysfunction and inflammation: the gender influence. Brain Behav Immun. 2017;59:211–218. doi: 10.1016/j.bbi.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Nourbakhsh M, Sharifi R, Ghorbanhosseini SS. Evaluation of plasma TRB3 and Sestrin-2 levels in obese and normal-weight children. Child Obes. 2017;13:409–414. doi: 10.1089/chi.2017.0082. [DOI] [PubMed] [Google Scholar]
- 38.Kotani K, Tsuzaki K, Taniguchi N, et al. Correlation between reactive oxygen metabolites and atherosclerotic risk factors in patients with type diabetes mellitus. Indian J Med Res. 2013;137:742–748. [PMC free article] [PubMed] [Google Scholar]
- 39.Nigro E, Scudiero O, Monaco ML, et al. New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res Int. 2014;2014:658913. doi: 10.1155/2014/658913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christou GA, Kiortsis DN. Adiponectin and lipoprotein metabolism. Obes Rev. 2013;14:939–949. doi: 10.1111/obr.12064. [DOI] [PubMed] [Google Scholar]
- 41.Soontornniyomkij V, Soontornniyomkij B, Moore DJ, et al. Antioxidant sestrin-2 redistribution to neuronal soma in human immunodeficiency virus-associated neurocognitive disorders. J Neuroimmune Pharmacol. 2012;7:579–590. doi: 10.1007/s11481-012-9357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park HW, Park H, Ro SH, et al. Hepatoprotective role of Sestrin2 against chronic ER stress. Nat Commun. 2014;5:4233. doi: 10.1038/ncomms5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kotani K, Fujiwara S, Tsuzaki K, et al. The association between the uncoupling protein-1 gene A-3826G polymorphism and high-density lipoprotein cholesterol in a general japanese population: a consideration of the obesity status. J Clin Med Res. 2011;3:319–324. doi: 10.4021/jocmr738w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ro SH, Nam M, Jang I, et al. Sestrin2 inhibits uncoupling protein 1 expression through suppressing reactive oxygen species. Proc Natl Acad Sci USA. 2014;111:7849–7854. doi: 10.1073/pnas.1401787111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barter P. Effects of inflammation on high-density lipoproteins. Arterioscler Thromb Vasc Biol. 2002;22:1062–1063. doi: 10.1161/01.ATV.0000024683.73431.99. [DOI] [PubMed] [Google Scholar]
- 46.Yang JH, Kim KM, Kim MG, et al. Role of sestrin2 in the regulation of proinflammatory signaling in macrophages. Free Radic Biol Med. 2015;78:156–167. doi: 10.1016/j.freeradbiomed.2014.11.002. [DOI] [PubMed] [Google Scholar]