Abstract
Abstract
The medicinal plant, Terminalia bellirica (Gaertn.) Roxb. is widely used in the traditional Indian system of medicine like Ayurveda for centuries in the treatment of various ailments owing to it’s rejuvenating as well as health promoting effects. The present study evaluates protective role of aqueous acetone extract of T. bellirica fruits (AATB) against CCl4 induced liver toxicity in animal model. The liver damage was assessed by liver function markers including ALT, AST, ALP, GGT, LDH, total bilirubin, total protein, albumin, globulin and albumin-globulin ratio. The levels of MDA, ROS, and NO along with the tissue antioxidants were evaluated to assess hepatic oxidative stress and level of lipid peroxidation. Treatment with AATB prior to the exposure of CCl4 significantly reduced the damage when compared to the control rats. The outcome of the present study advocates the traditional use of the plant as ethnic food and health tonic.
Graphical Abstract
Keywords: Terminalia bellirica fruit, Carbon tetrachloride, Oxidative stress, Liver injury
Introduction
Liver diseases have emerged as a malady that affects the people around the globe irrespective of the geographical as well as life style changes. Liver as the metabolic hub in the body, regulates and coordinates various physiological processes and act as ‘metabolic clearing house’ that plays vital role in the detoxification of both endogenous and exogenous chemicals, drugs and xenobiotics. Owing to its prime role in the drug metabolism, it possess as equally higher risk of getting damaged by the toxic agents and chemicals. Overload of various harmful agents including biological as well as chemical substances and drugs collectively called hepatotoxicants disrupt normal functioning of the liver. Liver diseases are often caused by various toxins including xenobiotics, alcohols, free radicals, pollutants as well as food additives [1]. Free radicals generated from various toxins cause hepatic injury by the mechanism of covalent binding and lipid peroxidation. Oxidative stress induced by large number of highly reactive free radicals is the starting point for liver injury which later advances to serious chronic conditions.
Regular antioxidant intake has become an important prophylactic aspect in preventing as well as delaying the onset of oxidative liver diseases. Liver toxicity and associated complications can be counteracted to a great extent by natural antioxidant molecules [2]. Herbal antioxidants are of prime importance in preventing various liver diseases that arise due to the excessive free radical generation and oxidative damage and are widely prescribed as dietary supplements in the alternative systems of medicine, which originated from India including Ayurveda and Siddha [3]. Herbal remedies have been extensively prescribed as rejuvenators or health tonics in the alternative medicine systems as most of them possess prophylactic or therapeutic antioxidant potential [2]. Scientific studies have clearly advocates the use of antioxidant rich herbals and foods against oxidative stress induced liver damage [4–8]. Herbal antioxidants have attained much attention in the current scenario of surge in liver injuries and other non communicable diseases with a common oxidative stress induced onset and progression. Dietary as well as natural antioxidant intake may be an important strategy for inhibiting or delaying the oxidation of susceptible cellular substrates, and is thus relevant to disease prevention in many paradigms [9]. Natural antioxidants and hepatoprotective agents are widely accepted over conventional drugs with drastic side effects to be used in mitigating oxidative stress and further advancement into liver damage.
A wide array of medicinal plants with antioxidant potential has extensively been used in Indian traditional system of medicine for treating various disorders. Most of the medicinal plants contain various phytochemicals including triterpenes, triterpene glycosides, (also known as saponines), and other triterpenoids, representing one of numerous classes of natural compounds having very higher antioxidant activity [10]. Herbal antioxidants in the form of dietary supplements for promoting health and longevity has been in use for generations in the alternative medicine systems like Ayurveda and Siddha, which originated from India [2]. Triphala, ashwagandha, tulsi, ginger and neem are some of the herbal antioxidant preparations that are extensively used in Indian systems of alternative medicine, as they are considered to possess significant rejuvenating properties [2]. Triphala is widely used as a dietary supplement for promoting longevity in alternative medicine systems of Ayurveda and Siddha [11]. The constituents of triphala were reported to enhance the antioxidant status in aging animal models [12].
The plant, Terminalia bellirica (Gaertn.) Roxb. (Combretaceae) is native to Bangladesh, Bhutan, Cambodia, China, Indonesia, Laos, Malaysia, Nepal, Pakistan, Sri Lanka, Thailand, Vietnam and India. It is frequently found in monsoon forests, mixed deciduous forests or dry deciduous dipterocarp forests, associated with teak. It is known as Bhibhitaki in Sanskrit and locally called Behera in India is a large deciduous tree found throughout India except for the dry western region up to an elevation of 900 m [13]. The fruits, apart from being an integral part in Triphala, are widely used in various traditional therapeutic formulations in the indigenous system of medicine either alone or in combination with other plant-based drugs [14]. The fruit of T. bellirica has been consumed as food by several ethnic groups of Nepal [15]. The fruits have laxative, astringent, anthelmintic and antipyretic properties and are used in Ayurveda against various disorders like hepatitis, bronchitis, asthma, dyspepsia, piles, diarrhea, coughs, eye diseases [16]. The fruits elicit various pharmacological properties like antioxidant, antidiabetic, analgesic, antidiarrhoeal, anti-inflammatory [17] in its different extracts. The fruit possess strong antioxidant properties and has reported hepatoprotective activity in its ethanolic extract. Toxicity studies conducted in our laboratory proves the safe usage of T. bellirica fruits [18, 19]. In our screening study, aqueous acetone extract of T. bellirica fruits showed maximum antioxidant activity [20] and hence the present study was carried out to evaluate the protective effect of the extract against CCl4 induced liver damage in animal model.
Materials and Methods
Chemicals
All the chemicals used in this study were analytical grade reagents with highest purity. The chemicals such as thiobarbituric acid (TBA), 5,5′-dithiobis-2-nitrobenzoic acid (DTNB), pyrogallol, 1-chloro-2,4-dinitrobenzene (CDNB) and glutathione (GSH) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All the other reagents were procured from Sisco Research Laboratories, Mumbai, India. All the solvents used in the study were purchased from Merck, India.
Plant Material
Current season’s fruits of T. bellirica were purchased from Kerala Forest Research Institute (KFRI), Peechi, India. The fruits were de-seeded and dried in shade for few days before made into fine powder. The fruit powder stored in air tight containers was used for the preparation of the extract.
Preparation of Extract
Fruit powder of T.bellirica was extracted with 70% aqueous acetone in a mechanical shaker for 72 h after removing the fatty substances by treating with petroleum ether. After evaporating the solvent completely, the extract was filtered and lyophilized to get in the powder form. The dried extract was kept in the refrigerator at 4 °C till further use.
Experimental Animals
The male Wistar albino rats of 120–150 g, used in the study were purchased from the licensed breeder in Kerala, India (Nagarjuna Herbal Concentrates Private Limited, Thodupuzha). The animals were acclimatized to the laboratory conditions for 1 week prior to the experimentation. The animals were housed in polypropylene cages, maintained on a 12-h light/dark cycle at a temperature of 25 ± 2 °C and provided standard pellet diet and water ad libitum. The study protocol made in accordance with the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines (Reg No B23032014-02) was approved by the Institutional Animal Ethics Committee of School of Biosciences, Mahatma Gandhi University, Kottayam. All the procedures in the experiment were carried out humanely.
Experiment Design
The animals were grouped into six with each group contain 6 rats. Group I served as the normal control rats without any treatment. Group II animals that received 0.5% Tween-20 were kept as vehicle or placebo control. Group III received CCl4 alone was kept as negative (CCl4) control. Group IV and V were treatment groups were orally treated with AATB 200 and 400 mg/kg body weight respectively for 9 consecutive days. Group VI was treated with standard drug silymarin at a dose of 10 mg/kg p.o for 9 days. A single dose of CCl4 (1.5 ml/kg b.wt) was administered orally to all experimental animals except group I and II on the nineth day to induce hepatic damage. Blood and liver tissue required for various biochemical evaluations as well as histopathological studies were harvested after sacrificing the animals 24 h after the CCl4 intoxication. The liver tissue was thoroughly washed with normal saline before fixing in buffered formalin. The liver tissue required for biochemical analysis was stored at − 20 °C until use.
Biochemical Parameters of Liver Function
The blood collected in clot stimulating tubes were kept in room temperature for 30 min to separate the serum. The serum was transferred to fresh tubes and stored at − 20 °C until use. The serum samples were used to determine enzyme as well as other biochemical markers of liver function including aspartate transaminase (AST), alanine transaminase (ALT), gamma glutamate transaminase (GGT), alkaline phosphatase (ALP), lactate dehydrogenase(LDH), total bilirubin, total protein, levels of albumin and globulin and albumin/globulin ratio (A/G). All the assays were carried out in semi auto biochemical analyzer (Microlab-ARX-235) using standard diagnostic kits (Span Diagnostics Limited kits, Surat, India).
Hepatic Oxidative Stress Markers
A homogenate containing 10% liver tissue in 100 mM phosphate buffer containing 1 mM EDTA was used for all the assays. The homogenate was centrifuged at 12,000 g for 30 min at 4 °C and the supernatant obtained was used for the analysis of reduced glutathione (GSH) [21], superoxide dismutase (SOD) [22] and catalase (CAT) [23].
Determination of Lipid Peroxidation and Nitric Oxide Levels
The levels of lipid peroxidation (LPO) [24] and nitric oxide level (NO) [25] was determined using liver homogenate and serum respectively.
Level of Reactive Oxygen Species (ROS)
The quantification of reactive oxygen species (ROS) content in the tissue was determined according to the method of [26] to assess antioxidant status of the liver tissue.
Histological Evaluation of Liver
Hepatic tissue was dissected, washed in ice cold saline, fixed in 10% formalin, processed and embedded in paraffin. Tissue sections were prepared (5 µm thick), stained with hemotoxylin and eosin (H&E) and observed under microscope for histopathological studies (Olympus Q imaging Micropublisher). Histopathological scoring was perfomed according to the method of Jamshidzadeh et al. [27] where histological damage was given specific scores as 0: absent; +: mild; ++; moderate; +++: severe according to the severity. The liver activity index was calculated using the modified quantitative scoring system of Ishak et al. [28] and scores were assigned as follows: scores of 1–3 (minimal liver damage), scores of 4–8 (mild damage), scores of 9–12 (moderate damage), scores 13–18 (severe damage).
Statistical Analysis
All the data were expressed as mean ± standard deviation (n = 6) and the results were analysed by one way ANOVA followed by Tukey’s Post hoc analysis using IBM SPSS Statistics version 20 statistical program. A value of p < 0.05 was considered as statistically significant.
Results
Effect on Biochemical Parameters
The biochemical parameters used to assess liver functioning include both enzymatic and non enzymatic hepatic function markers. The serum level of enzyme markers (ALT, AST, ALP, LDH and GGT), when compared with normal rats were found significantly elevated in the CCl4 treated control which clearly reflected the liver damage caused by the toxin (Table 1). Comparing the values of other biochemical parameters of the study including total protein, albumin, globulin, and A/G ratio with untreated animals, exposure to the toxin drastically changed the characteristics (Table 2). The prior treatment with AATB significantly reduced the extent of liver damage which was evident from the levels of biochemical parameters of the treated groups. The extract could significantly reduce the levels of AST, ALT, ALP, LDH and GGT as compared to the CCl4 control. Similarly, treatment with AATB significantly improved the levels of other biochemical parameters towards the normal range. The elevated level of bilirubin was significantly reduced whereas the depleted levels of total protein, albumin, globulin and A/G ratio restored significantly upon treating with AATB. The changes in the test parameters were significant (p < 0.05) with corresponding control groups. The biochemical findings were indicative of the liver protective ability of the plant. The higher dose of AATB (400 mg/kg body weight) produced comparable protection with that of standard drug Silymarin.
Table 1.
Effect of AATB on liver function enzyme markers in CCl4 induced liver injury
| Groups | ALT (IU/L) | AST (IU/L) | ALP (IU/L) | LDH (IU/L) | GGT (IU/L) |
|---|---|---|---|---|---|
| Normal control | 81.78 ± 2.50 | 203.80 ± 4.16 | 629.30 ± 2.13 | 359.00 ± 2.41 | 9.11 ± 0.09 |
| Toxic control | 893.88 ± 4.95a | 496.55 ± 3.77a | 1023.47 ± 4.33a | 699.90 ± 3.32a | 22.83 ± 0.92 |
| Vehicle control | 81.73 ± 3.97b | 205.35 ± 3.71b | 630.40 ± 2.80b | 361.78 ± 3.49b | 9.09 ± 0.11b |
| AATB (200 mg/kg) | 381.20 ± 4.10ab | 363.70 ± 4.71ab | 823.58 ± 3.17ab | 489.88 ± 2.12ab | 17.18 ± 0.25ab |
| AATB (400 mg/kg) | 210.08 ± 3.24ab | 207.58 ± 3.73b | 725.45 ± 3.41ab | 382.75 ± 3.24ab | 11.23 ± 0.28ab |
| Silymarin (10 mg/kg) | 192.48 ± 3.41ab | 280.53 ± 3.78ab | 707.98 ± 3.61ab | 373.80 ± 3.46ab | 10.65 ± 0.31ab |
All values are expressed as Mean ± SD, n = 6
ap < 0.05, values compared to control group
bp < 0.05, values compared to CCl4 toxic group
Table 2.
Effect of AATB on biochemical parameters in CCl4 induced liver injury
| Groups | Bilirubin (mg/dL) | Total protein (mg/dL) | Albumin (mg/dL) | Globulin (mg/dL) | A/G ratio |
|---|---|---|---|---|---|
| Normal control | 0.82 ± 0.02 | 6.32 ± 0.16 | 3.72 ± 0.11 | 2.60 ± 0.06 | 1.43 ± 0.02 |
| Toxic control | 9.85 ± 0.25a | 2.77 ± 0.22a | 1.27 ± 0.14a | 1.51 ± 0.09a | 0.84 ± 0.05a |
| Vehicle control | 0.82 ± 0.02b | 6.56 ± 0.23b | 3.83 ± 0.12b | 2.73 ± 0.11b | 1.40 ± 0.01b |
| AATB (200 mg/kg) | 4.39 ± 0.38ab | 4.17 ± 0.02ab | 2.19 ± 0.01ab | 1.98 ± 0.01ab | 1.11 ± 0.01ab |
| AATB (400 mg/kg) | 2.23 ± 0.22ab | 6.09 ± 0.04b | 3.34 ± 0.01ab | 2.74 ± 0.03ab | 1.22 ± 0.01ab |
| Silymarin (10 mg/kg) | 2.01 ± 0.16ab | 6.18 ± 0.03b | 3.41 ± 0.02ab | 2.77 ± 0.01ab | 1.23 ± 0.01ab |
All values are expressed as Mean ± SD, n = 6
ap < 0.05, values compared to control group
bp < 0.05, values compared to CCl4 toxic group
Hepatic Oxidative Stress Markers
The level of tissue antioxidant system was determined to assess the status of hepatic oxidative stress. The levels of SOD, CAT and GSH in the CCl4 treated animals were found significantly depleted as compared with untreated animals (Fig. 1). Treatment with AATB prior to the administration of toxin prevented the depletion of tissue antioxidants up to significant level and this protective effect, especially in case of higher dose, was found equally good with that of standard drug used in the study.
Fig. 1.
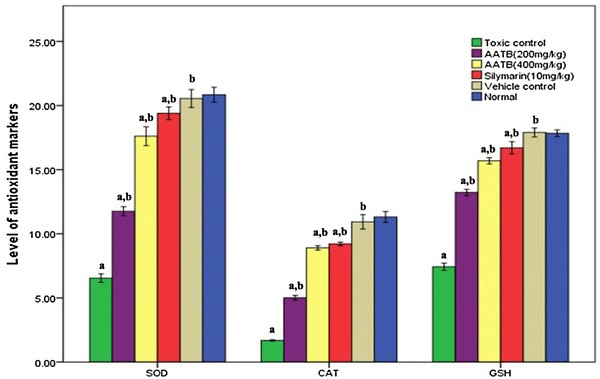
Level of tissue antioxidants (SOD, CAT, GSH), all values are expressed as Mean ± SD, n = 6. ap < 0.05, values compared to control group, bp < 0.05, values compared to CCl4 toxic group
Levels of LPO, ROS and NO
The level of lipid peroxidation as a measure of cellular damage by the free radicals indicated that CCl4 administration significantly elevated the levels of LPO with a concomitant increase in the hepatic NO level (Table 3). AATB treatment could minimize the surge of the above parameters which is indicative of the protective role of the plant extract in controlling tissue level oxidation with efficiency very close to that of silymarin, the standard drug. The results of the ROS quantification in the hepatic tissue clearly indicated the severe damage caused by the toxin since the values were significantly higher than the untreated rats. The higher level of ROS is indicative of the failure of radical scavenging mechanism in the liver. The plant extract could however keep the ROS significantly lower level when compared to CCl4 control rats.
Table 3.
Effect of AATB on LPO, ROS and NO levels CCl4 induced liver injury
| Groups | LPO (μmol MDA/mg protein) | ROS (μmol NBT/g tissue) | NO (μmol/g tissue) |
|---|---|---|---|
| Normal | 0.37 ± 0.02 | 488.78 ± 3.54 | 59.25 ± 0.75 |
| Toxic control | 6.94 ± 0.06a | 980.98 ± 4.22a | 96.38 ± 0.43a |
| Vehicle control | 0.35 ± 0.01b | 486.78 ± 5.68b | 59.48 ± 0.57b |
| AATB (200 mg/kg) | 1.59 ± 0.02ab | 762.15 ± 2.70ab | 81.25 ± 0.62ab |
| AATB (400 mg/kg) | 0.51 ± 0.01ab | 592.25 ± 3.76ab | 74.90 ± 0.42ab |
| Silymarin (10 mg/kg) | 0.49 ± 0.01b | 553.03 ± 4.83ab | 72.65 ± 1.74b |
All values are expressed as Mean ± SD, n = 6
ap < 0.05, values compared to control group
bp < 0.05, values compared to CCl4 toxic group
Histopathological Findings
The tissue level changes evident in the histopathological evaluation were shown Fig. 2 and the quantified effects through hepatic scoring were summarized in Table 4. Microscopic evaluation of liver sections of CCl4 treated animals showed signs of acute liver damage such as severe necrosis, sinusoidal dilation, fatty change, ballooning, binucleation and portal tract inflammation (Fig. 1c). Prior treatment with AATB 200 mg/kg showed moderate damage to hepatic architecture (Fig. 1d) while treatment with higher dose of the extract (AATB 400 mg/kg) and the standard drug Silymarin showed almost normalization of hepatocytes with very less extent of fatty changes, swelling and necrosis of the liver (Fig. 1e, f). Treatment with AATB reduced the toxic effects in a great extent that the hepatic architecture was found nearly normal with mild lesions and necrosis while central vein and sinusoids appeared almost normal. These observations are in tune with the early described biochemical parameters that were already suggestive of the protective role of the plant against oxidative stress and related hepatic toxicity.
Fig. 2.
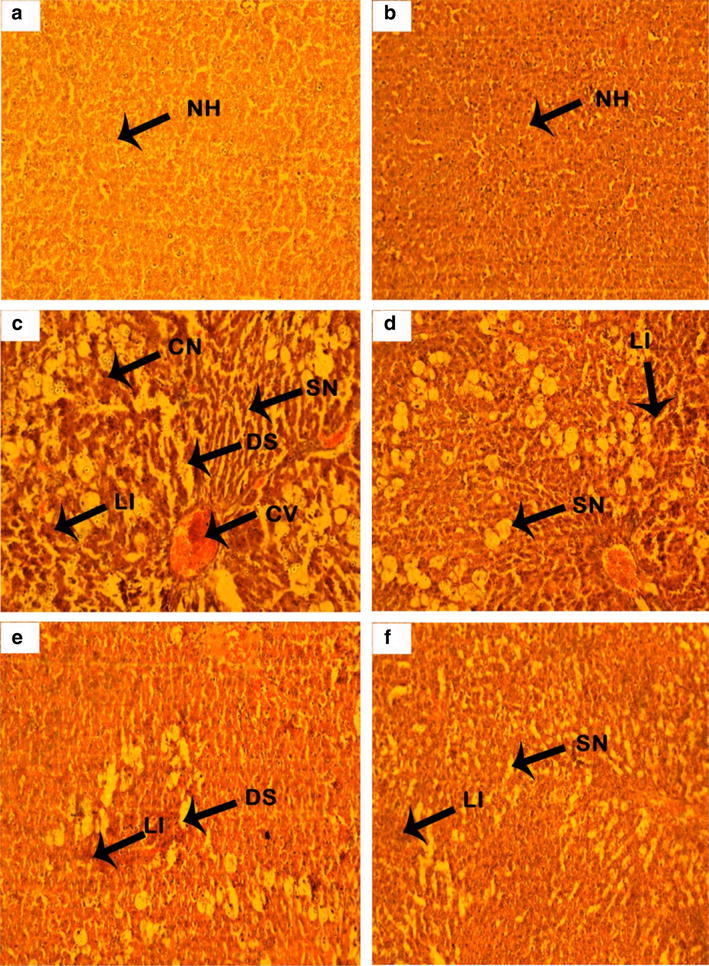
Effect of AATB on liver histology in CCl4 induced liver injury (40x). a Normal control, b vehicle control, c CCl4 control, d CCl4 + AATB(200 mg/kg), e CCl4 + AATB (400 mg/kg), f CCl4 + Silymarin (10 mg/kg)
Table 4.
Histological changes in CCl4 induced liver injury
| Group | Microscopic histological observations | |||||||
|---|---|---|---|---|---|---|---|---|
| Liver activity index | Necrosis | Sinusoid dilation | Lymphocyte infiltration | Fatty changes | Hemorrhage | Binucleate cells | Hepatocyte swelling | |
| Normal control | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Toxic control | 14 | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| Vehicle control | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AATB(200 mg/kg) | 11 | ++ | ++ | ++ | ++ | ++ | ++ | + |
| AATB(400 mg/kg) | 8 | + | + | + | + | + | 0 | + |
| Silymarin(10 mg/kg) | 7 | + | + | + | + | + | 0 | + |
Hepatocyte activity index: 1–3, minimal; 4–8, mild; 9–12, moderate; 13–18, hepatocyte score-severe
0, Absent; +, mild; ++, moderate; +++ severe
Discussion
Plants based antioxidants has become more popular and focussed ever than before as these plant based free radical scavenging formulations are of immense value in controlling oxidative stress related pathogenesis. It has been shown by various studies that the onset and progression of various chronic diseases can be controlled by minimising the cellular oxidative stress with the ultimate benefit of extending the life span by several years [29]. Plants are rich in bioactive compounds capable of eliminating free radicals and thereby reduce oxidative damage and its related complications [30]. The age old ayurvedic formulation ‘Triphala’ that contains T.bellirica fruit as one of the ingredients, has been widely used in the traditional medicine as dietary supplement for promoting longevity [11]. In our previous work, we have studied in vitro antioxidant properties of T.bellirica fruits and aqueous scetone extract was found to exhibit maximum antioxidant potential [20]. Therefore, the present study intends to evaluate the protective efficacy of T.bellirica fruit extract (AATB) against CCl4 induced liver injury in rodent model.
The Carbon tetrachloride (CCl4) induced liver damage in animal model is a scientific experimental method for liver toxicity studies. Carbon tetrachloride induces liver injury by generating the highly reactive trichloromethyl radical (CCl3) as a result of biotransformation by cytochrome P-450 system. The trichloromethyl radical (CCl3) is further converted to trichloromethyl peroxyl radical (CCl3O2). The highly reactive metabolites of CCl4 cause severe lipid peroxidation and membrane destruction that finally leads to liver damage [31].
The enzymatic markers ALT and AST are used to evaluate liver functioning and levels of these enzymes are found elevated in hepatic damage. The damaged hepatocytes release ALT and AST into the extracellular space and ultimately enter the blood stream thereby increase the serum levels of these biomarkers [32]. ALP, GGT and total bilirubin levels are additional biomarkers of liver function. Serum level of LDH is another enzyme biomarker of hepatocellular toxicity but with less specificity [33]. Elevated levels of these markers are indicative of hepatobilary dysfunction. Abnormal levels of biochemical markers were observed in CCl4 intoxicated group of the present study whereas treatment with AATB significantly improved the liver function status which was evident from the reversal of the biochemical parameters.
Liver is a major function of protein synthesis so abnormal levels of protein are a sign of liver injury [34]. Hypoproteinemia is a clear indication of liver injury [35]. Albumin and globulin levels also reveal the physiological status of the liver [36]. Altered Albumin to globulin (A/G) ratio is also indicate hepatic damage [37]. The levels of total protein, albumin, globulin and A/G ratio were found reduced during CCl4 intoxicated control groups of the present study whereas administration of the aqueous acetone extract regained the serum protein levels and A/G ratio and thus clearly indicated its protection against hepatotoxicity.
The levels of antioxidant molecules in the tissues like SOD, CAT and GSH indicate the antioxidant balance inside the cell as these molecules are primarily involved in the maintenance of steady state in the cell [38]. Tissue antioxidant levels were greatly depleted by the CCl4 administration as it was evident from the values observed in the control group animals. Reduced activity of the enzymes may results in deleterious effects due to the accumulation of superoxide radical and hydrogen peroxide. Treatment with AATB provided recovery of the SOD, catalase and GSH levels which clearly indicates antioxidant potential of the plant.
An increase in the level of malondialdehyde, the final product of lipid peroxidation articulates to the hepatic damage. MDA level was notably increased in the CCl4 treated rats compared to the control. Treatment with the aqueous acetone extract reverses these changes and thus prevents the peroxidation of membrane lipids as seen in the reduced MDA levels in the AATB treated groups. These results indicate that the aqueous acetone extract could attenuate oxidative stress by decreasing the level of free radicals and lipid peroxidation in CCl4 treated rats. ROS is known to play an important role in tissue damage in a variety of pathological processes [39]. Over-production of ROS leads to oxidative damage to macromolecules, resulting in lipid peroxidation and breakage of DNA [39, 40]. A significant reduction in the amount of ROS in the AATB treated animals’ points to the potential of the plant toward radical scavenging. Tissue NO plays a prime role in the promotion of various inflammatory pathways leading to further tissue damage by generate peroxynitrite and hydroxyl radicals after interaction with superoxide ions [41]. The inhibition of NO level by the plant is critical evidence that the plant can modify oxidative stress mediated inflammatory pathways.
Treatment with AATB prior to the intoxication significantly reduced the toxic effects in a great extent that the hepatic architecture was found nearly normal with lesions and necrosis while central vein and sinusoids appeared almost normal. The results were so prominent in the higher dose of AATB (400 mg/kg). These observations are in agreement with the early described biochemical parameters. The silymarin treated group also showed very minimal hepatic damage in terms of liver histology. The protective efficacy of the fruit extract against liver injury may be due to its high efficiency in scavenging the free radicals generated by the toxin.
The traditional medicinal plant T. bellirica is a component of many ayurvedic preparations and the fruit especially is often prescribed as a general tonic. In the present investigation, aqueous acetone extract of the fruit proved as an efficient natural agent in combating oxidative stress and hepatic injury. The fruit extract exhibited comparable results with the standard drug in both biochemical as well as histological evaluation. The extract could reverse toxic effects induced by the toxin in experimental animals. The biochemical markers of liver injury and oxidative stress markers in addition to the histological evaluation clearly revealed the ameliorative potential of the fruit extract against liver injury. The current data in turn validate the traditional knowledge that supports its wide use in the traditional medicine. Present study points to the need of elaborate studies in this regard so as to develop T. bellirica fruits as a herbal remedy or a plant based nutraceutics with protective role against oxidative damage and related onset of toxicity as well as chronic diseases of liver.
Acknowledgements
The authors are grateful to School of Biosciences and DBT-MSUB-IPLS (BUILDER) programme for providing the research facilities required for the study. J.K. gratefully acknowledges UGC for providing teacher fellowship under the UGC-FDP scheme. Authors acknowledge Dr. K.C. Pillai, Scientist, KFRI, Peechi, Kerala, India for providing the plant material.
Conflict of interest
The authors declare that they have no conflicts of interest.
Informed Consent
This article does not contain any studies with human performed by any of the authors.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
References
- 1.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Rashed K, Potočnjak I, Giacometti J, Škoda M, Domitrović R. Terminalia bellerica aerial parts ethyl acetate extract exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. J Funct Foods. 2014;31(8):319–330. doi: 10.1016/j.jff.2014.03.033. [DOI] [Google Scholar]
- 3.Jamesdaniel S, Samson A. Herbal antioxidants as rejuvenators in alternative medicine. In: Phytochemicals-bioactivities and impact on health, 2011. p. 297–312.
- 4.Wang BS, Lee CP, Chen ZT, Yu HM, Duh PD. Comparison of the hepatoprotective activity between cultured Cordyceps militaris and natural Cordyceps sinensis. J Funct Foods. 2012;4(2):489–495. doi: 10.1016/j.jff.2012.02.009. [DOI] [Google Scholar]
- 5.Vulić JJ, Ćebović TN, Čanadanović-Brunet JM, Ćetković GS, Čanadanović VM, Djilas SM, et al. In vivo and in vitro antioxidant effects of beetroot pomace extracts. J Funct Foods. 2014;31(6):168–175. [Google Scholar]
- 6.Hou F, Zhang R, Zhang M, Su D, Wei Z, Deng Y, et al. Hepatoprotective and antioxidant activity of anthocyanins in black rice bran on carbon tetrachloride-induced liver injury in mice. J Funct Foods. 2013;5(4):1705–1713. doi: 10.1016/j.jff.2013.07.015. [DOI] [Google Scholar]
- 7.Chen SY, Chyau CC, Chu CC, Chen YH, Chen TH, Duh PD. Hepatoprotection using sweet orange peel and its bioactive compound, hesperidin, for CCl4-induced liver injury in vivo. J Funct Foods. 2013;5(4):1591–1600. doi: 10.1016/j.jff.2013.07.001. [DOI] [Google Scholar]
- 8.Jeyadevi R, Sivasudha T, Rameshkumar A, Harnly JM, Lin LZ. Phenolic profiling by UPLC–MS/MS and hepatoprotective activity of Cardiospermum halicacabum against CCl4 induced liver injury in Wistar rats. J Funct Foods. 2013;5(1):289–298. doi: 10.1016/j.jff.2012.10.019. [DOI] [Google Scholar]
- 9.Maheshwari DT, Kumar MY, Verma SK, Singh VK, Singh SN. Antioxidant and hepatoprotective activities of phenolic rich fraction of Seabuckthorn (Hippophae rhamnoides L.) leaves. Food Chem Toxicol. 2011;49(9):2422–2428. doi: 10.1016/j.fct.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 10.Patočka J. Biologically active pentacyclic triterpenes and their current medicine signification. J Appl Biomed. 2003;1(1):7–12. doi: 10.32725/jab.2003.002. [DOI] [Google Scholar]
- 11.Jagetia GC, Baliga MS, Malagi KJ, Kamath MS. The evaluation of the radioprotective effect of Triphala (an ayurvedic rejuvenating drug) in the mice exposed to γ-radiation. Phytomedicine. 2002;9(2):99–108. doi: 10.1078/0944-7113-00095. [DOI] [PubMed] [Google Scholar]
- 12.Gupta R, Gupta A, Singh RL. Hepatoprotective activities of Triphala and its constituents. Int J Pharma Res Rev. 2015;4:34–55. [Google Scholar]
- 13.Hazra B, Sarkar R, Biswas S, Mandal N. Comparative study of the antioxidant and reactive oxygen species scavenging properties in the extracts of the fruits of Terminalia chebula, Terminalia belerica and Emblica officinalis. BMC Complement Altern Med. 2010;10(1):20. doi: 10.1186/1472-6882-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meena AK, Bansal P, Kumar S. Plants-herbal wealth as a potential source of ayurvedic drugs. Distribution. 2009;4(4):152–170. [Google Scholar]
- 15.Chalise JP, Acharya K, Gurung N, Bhusal RP, Gurung R, Skalko-Basnet N, et al. Antioxidant activity and polyphenol content in edible wild fruits from Nepal. Int J Food Sci Nutr. 2010;61(4):425–432. doi: 10.3109/09637481003591590. [DOI] [PubMed] [Google Scholar]
- 16.Deb A, Choudhury G, Barua S, Das B, Anindita C, Choudhury DG. Pharmacological activities of Baheda (Terminalia bellerica): a review. J Pharmacogn Phytochem. 2016;5(1):2278–4136. [Google Scholar]
- 17.Jayesh K, Helen LR, Vysakh A, Binil E, Latha MS. Ethyl acetate fraction of Terminalia bellirica (Gaertn.) Roxb. fruits inhibits proinflammatory mediators via down regulating nuclear factor-κB in LPS stimulated Raw 264.7 cells. Biomed Pharmacother. 2017;95:1654–1660. doi: 10.1016/j.biopha.2017.09.080. [DOI] [PubMed] [Google Scholar]
- 18.Jayesh K, Helen LR, Vysakh A, Binil E, Latha MS. In vivo toxicity evaluation of aqueous acetone extract of Terminalia bellirica (Gaertn.) Roxb. fruit. Regul Toxicol Pharmacol. 2017;86:349–355. doi: 10.1016/j.yrtph.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Jayesh K, Raisa Helen L, Vysakh A, Binil E, Latha Cytotoxicity evaluation of bioactive fraction from Terminalia bellirica (Gaertn.) Roxb. Fruits in L929 cells. Int J Adv Res. 2016;5(9):2320–5407. [Google Scholar]
- 20.Jayesh K, Helen LR, Binil E, Latha M, Latha MS. Comparative antioxidant properties of Terminalia bellirica. IjpprHuman. 2016;7(74):310–320. [Google Scholar]
- 21.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 22.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21(2):130–132. [PubMed] [Google Scholar]
- 23.Aebi H. [13] Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 24.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 25.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15 N] nitrate in biological fluids. Anal Biochem. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- 26.Vrablic A, Albright C, Craciunescu C, Salganik R, Warrell D. Chapter 26: Clinical toxicology of snakebite in Africa and the Middle East/Arabian peninsula. In: Handbook of clinical toxicology of animal venoms and poisons; 1995. p. 433–92.
- 27.Jamshidzadeh A, Baghban M, Azarpira N, Bardbori AM, Niknahad H. Effects of tomato extract on oxidative stress induced toxicity in different organs of rats. Food Chem Toxicol. 2008;46(12):3612–3615. doi: 10.1016/j.fct.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22(6):696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 29.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 30.Firuzi O, Miri R, Tavakkoli M, Saso L. Antioxidant therapy: current status and future prospects. Curr Med Chem. 2011;18(25):3871–3888. doi: 10.2174/092986711803414368. [DOI] [PubMed] [Google Scholar]
- 31.Szymonik-Lesiuk S, Czechowska G, Stryjecka-Zimmer M, SŁomka M, Mądro A, CeliŃski K, et al. Catalase, superoxide dismutase, and glutathione peroxidase activities in various rat tissues after carbon tetrachloride intoxication. J Hepato Biliary Pancreat Sci. 2003;10(4):309–315. doi: 10.1007/s00534-002-0824-5. [DOI] [PubMed] [Google Scholar]
- 32.Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245(3):194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 33.Ramaiah SK. A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem Toxicol. 2007;45(9):1551–1557. doi: 10.1016/j.fct.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Thapa BR, Walia A. Liver function tests and their interpretation. Indian J Pediatr. 2007;74(7):663–671. doi: 10.1007/s12098-007-0118-7. [DOI] [PubMed] [Google Scholar]
- 35.David AS. Intelligence and schizophrenia. Acta Psychiatr Scand. 1999;100(1):1–2. doi: 10.1111/j.1600-0447.1999.tb10907.x. [DOI] [PubMed] [Google Scholar]
- 36.Yakubu MT, Bilbis LS, Lawal M, Akanji MA. Evaluation of selected parameters of rat liver and kidney function following repeated administration of yohimbine. Biokemistri. 2003;15(2):50–56. [Google Scholar]
- 37.Hong KW, Jin HS, Song D, Kwak HK, Soo Kim S, Kim Y. Genome-wide association study of serum albumin: globulin ratio in Korean populations. J Human Genet. 2013;58(3):174. doi: 10.1038/jhg.2012.130. [DOI] [PubMed] [Google Scholar]
- 38.Khan RA, Khan MR, Sahreen S. CCl4-induced hepatotoxicity: protective effect of rutin on p53, CYP2E1 and the antioxidative status in rat. BMC Complement Altern Med. 2012;12(1):178. doi: 10.1186/1472-6882-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci IJBS. 2008;4(2):89. [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. 2012;24:2012. [Google Scholar]
- 41.Radi RB, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls: the cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266(7):4244–4250. [PubMed] [Google Scholar]


