Abstract
The ameliorative effects of dietary natural compounds have drawn increasing attention. Dietary antioxidant is considered a common practice adopted in traditional and alternative medicine. The current study was considered to assess the ameliorative effect of grape seed extract on dexamethasone-induced hepatotoxicity in rats. Rats were injected with dexamethasone, (0.1 mg/kg; i.m.), three times per week, for 30 days. The other groups; dexamethasone (0.1 mg/kg) and grape seed extract at a dose of 200 and 400 mg/kg were given orally to rats, respectively. Dexamethasone treatment resulted in a significant elevation in liver function markers activities, lipid profile, and hematological alterations; also, a remarkable increase in hepatic lipid peroxidation marker whereas decreased antioxidant activities in rats. However, administration of grape seed extract resulted in a reversal of dexamethasone-induced lipid peroxidation, antioxidant enzyme activities, liver function markers and lipid profile, and hematological alterations. Moreover, grape seed extract demonstrated preventive action against dexamethasone-induced histopathological changes in rat liver tissues. In conclusion, grape seed extract exhibited a protective effect in rats against oxidative stress, hyperlipidemia and hematological alterations induced by dexamethasone.
Keywords: Dexamethasone, Grape seed extract, Lipid peroxidation, Hematological, Hyperlipidemia, Oxidative stress
Introduction
The liver has an essential role in the metabolic pathways including carbohydrate metabolism, lipid metabolism and protein metabolism [1, 2]. Furthermore, the liver plays a crucial role in the detoxification and biotransformation of the metabolites and drugs; therefore, it is susceptible to the toxicity of these agents [2]. The adverse effects of drugs on liver, kidney, heart, lung, and brain tissue represents the crucial cause of the non-approval and withdrawal of drugs by the Food and Drug Administration [3].
Dexamethasone is a long-acting anti-inflammatory synthetic steroid. The main problem of dexamethasone is misuse through ingestion of overdose [4]. It was reported that dexamethasone overdose-induced hyperglycemia, hyperlipidemia, development of steatosis and fatty liver [5, 6]. Free radicals under oxidative stress are known to play an essential role in damage to the cell membrane through reactive oxygen radicals. Oxidative stress is defined as the unbalance between the intracellular formation of free radicals and cellular protection mechanisms. In experimental studies, dexamethasone toxicity has been shown to elevate malondialdehyde (MDA), an important index of lipid peroxidation [7]. Drugs can induce hematologic alterations, affecting levels of hemoglobin, leucocyte, erythrocyte, and platelets [8]. Hematological markers are perspective to be influenced by any disease disorder which disturbs the physiology of hematopoiesis [9].
The ameliorative effects of dietary natural compounds have drawn increasing attention; a variety of dietary antioxidant is often present in vegetables, seeds, and fruits. Moreover; therapy of this dietary antioxidant is considered a common practice adopted in traditional and alternative medicine [10]. Fruits provide a valid tool for the health benefits due to their powerful antioxidant content. Grape seed extract (GSE) contains a number of polyphenols, including procyanidins and proanthocyanidins, which are powerful free radical scavengers [5]. Various pharmacological studies demonstrated the anti-inflammatory, antioxidative, antitumor, antibacterial, and hepatoprotective properties of grape seed [11]. Aqueous extract of V. vinifera was found to be a potent inhibitor of lipid peroxide formation and scavenger of hydroxyl radicals’ in vitro [11].
Therefore, the aim of the current study was to elucidate the side effects of dexamethasone on the liver tissue and to assess the putative ameliorative effects of grape seed extract on hematological, biochemical and histopathological alterations induced by dexamethasone in albino rats.
Materials and Methods
Chemicals
Dexamethasone was purchased from EIPICO (10th of Ramadan City, Egypt). Dexamethasone was diluted with normal saline. The doses of the GSE used in this study are 200 mg, and 400 mg/kg BW and the dexamethasone (0.1 mg/kg b.wt.) according to the suggested dosage [12], All other chemicals were of analytical grade and obtained from standard commercial suppliers.
Preparation of Grape Seed Extract
Grapes (Vitis vinifera L) were obtained from local market Hail City, KSA. The seeds were dried and powdered. The grape seed powder was extracted according to the method of Hasona et al. [5].
Experimental Animals
Adult male Wistar rats weighing between 120 and 130 g, obtained from the animal house of the College of Pharmacy, King Saud University (Riyadh, KSA) were used in the current study. Rats were housed in well-aerated standard cages at a normal atmospheric temperature (25 ± 2 °C) and normal 12 h light/dark cycle and were kept under observation for 1 week for acclimatization. They were supplied daily with standard pellet diet of known composition and given access to water ad libitum. All animal procedures and policies were approved by the Animal Care and Institutional Ethics Committee, College of medicine, Hail University, KSA.
Animal Grouping and Treatments
Thirty-two rats were used to study the protective effects of grape seed extract against DEX-induced liver injury. According to the study protocol, animals were divided into four groups as follows:
Group 1: Animals were given a subcutaneous injection of normal saline three times per week for four consecutive weeks.
Group 2: Animals were given a subcutaneous injection of 0.1 mg/kg/day dexamethasone [12] three times per week for four consecutive weeks.
Group 3: animals were given dexamethasone and also received GSE (200 mg/kg BW) by oral gavage [5] three times per week for four consecutive weeks.
Group 4: animals were given dexamethasone and also received GSE (400 mg/kg BW) by oral gavage [5] three times per week for four consecutive weeks.
Rats were sacrificed by cervical decapitation under light ether anesthesia [2]. Approximately 0.2 ml of the blood was drawn into tubes containing anticoagulant (EDTA) shaken and taken for hematological analysis. Remaining blood samples were collected, left to coagulate and then centrifuged at 3000 rpm for 15 min. The clear non-hemolyzed serum was quickly removed and kept at − 20 °C for analysis. The liver was quickly removed, rinsed with ice-cold saline, washed, and kept frozen in liquid nitrogen. Frozen samples (10% w/v) were homogenized in cold phosphate-buffered saline (PBS) and the homogenates were centrifuged at 3000 rpm for 10 min [5]. The clear homogenates were collected and stored at − 80 °C for subsequent assays.
Hematological Analysis
Hematological profile (Erythrocytes, white blood cells, hemoglobin concentration, and platelets) were determined by using automated (SYSMX.KX-21n) hematology analyzer.
Biochemical Analysis
The activities of ALT, AST, and ALP were assayed according to the enzymatic colorimetric methods of [13] and [14] respectively. Serum levels of total cholesterol, triglycerides and HDL-cholesterol were assayed following the methods of [15], [16] and [17], respectively. LDL-C was calculated according to Friedewald et al. [18].
Oxidative Stress and Antioxidant Defenses Markers
Lipid peroxidation was assayed in liver homogenates by measurement of malondialdehyde (MDA) levels according to the method of Ohkawa et al. [19]. The activities of superoxide dismutase (SOD), and catalase (CAT) were measured according to the methods of [20] and [21] respectively.
Histopathological Study
Liver samples from each group were fixed in 10% buffered formalin after washing with cold normal saline for histopathological studies. The liver was embedded in paraffin; then sections were cut and stained with hematoxylin and eosin (H&E) according to the method of [22]. The stained slides were examined under light microscope.
Statistical Analysis
Data were done by one-way analysis of variance followed by Duncan’s Multiple Range Test as a post hoc test at the 5% probability level. The SPSS version 23 was used to analyze data. The significance level was regarded as P < 0.05. All data were presented as mean ± SE.
Results
Table 1 depicts the effect of DEX and GSE on liver function parameters in dexamethasone-induced rats. Dexamethasone-induced rats exhibited significantly (P < 0.001) elevated liver enzymes (ALT, AST, and ALP) activities. The treatment of the DEX-induced rats with either 200 or 400 mg/kg grape seed extract significantly (P < 0.001) decreased liver function enzymes (ALT, AST, and ALP) activities.
Table 1.
Effect of GSE on liver function parameters in DEX-induced rats (n = 8) (Mean ± Std. Error)
| Parameters | G1 | G2 | G3 | G4 | F‐ratio | P value |
|---|---|---|---|---|---|---|
| ALT (U/L) | 65.63a ± 2.06 | 161.50c ± 4.84 | 115.38b ± 2.15 | 70.88a ± 3.25 | 187.05 | P < 0.001 |
| AST (U/L) | 47.00a ± 2.29 | 192.13d ± 3.69 | 139.88c ± 2.29 | 84.75b ± 2.81 | 504.49 | P < 0.001 |
| ALP (U/L) | 78.25a ± 3.45 | 336.00d ± 4.00 | 160.50c ± 2.76 | 121.13b ± 2.38 | 1241.77 | P < 0.001 |
The different letters indicate statistically different means according to Duncan multiple range test
ALT alanine transaminase, AST aspartate transaminase, ALP alkaline phosphatase, G1 normal control group, G2 dexamethasone control group, G3 dexamethasone + GSE (200 mg/kg BW), G4 dexamethasone + GSE (400 mg/kg BW)
Table 2 summarizes the effect of dexamethasone and GSE on serum lipid profile parameters in DEX-induced rats. In the present study, dexamethasone-induced rats exhibited marked (P < 0.001) elevation in serum cholesterol, triglycerides, and LDL-cholesterol level as compared to that of normal rats. Both GSE doses administered after DEX significantly (P < 0.001) alleviated cholesterol, triglycerides, and LDL-cholesterol levels. On the other hand, dexamethasone induced rats showed a significant (P < 0.001) decline in serum HDL-cholesterol, an effect that was significantly (P < 0.001) reversed by GSE, as depicted in Table 2.
Table 2.
Effect of GSE on lipid profile in DEX-induced rats (n = 8) (Mean ± Std. Error)
| Parameters | G1 | G2 | G3 | G4 | F‐ratio | P value |
|---|---|---|---|---|---|---|
| Cholesterol (mmol/l) | 2.33 ± 0.17a | 4.69 ± 0.21d | 3.68 ± 0.14c | 3.13 ± 0.10b | 39.10 | P < 0.001 |
| Triglycerides (mmol/l) | 0.50 ± 0.04a | 1.05 ± 0.03c | 0.61 ± 0.03b | 0.56 ± 0.03ab | 61.52 | P < 0.001 |
| HDL-cholesterol (mmol/l) | 1.05 ± 0.04b | 0.85 ± 0.03a | 0.96 ± 0.03b | 1.02 ± 0.03b | 7.38 | P < 0.001 |
| LDL-cholesterol (mmol/l) | 1.05 ± 0.11a | 3.36 ± 0.22d | 2.45 ± 0.15c | 1.85 ± 0.11b | 39.59 | P < 0.001 |
The different letters indicate statistically different means according to Duncan multiple range test
HDL high-density lipoprotein, LDL low-density lipoprotein, G1 normal control group, G2 dexamethasone control group, G3 dexamethasone + GSE (200 mg/kg BW), G4 dexamethasone + GSE (400 mg/kg BW)
With respect to oxidative stress as depicted in Table 3, administration of dexamethasone significantly (P < 0.001) reduced antioxidant enzymes SOD and CAT and elevated lipid peroxidation marker MDA level in liver homogenate of DEX control animals. Treatment with low and high doses of GSE (200 and 400 mg/kg BW) restored SOD and CAT enzyme activities with a decline in MDA level when compared with that of DEX control group animals. Treatment with a high dose of GSE (400 mg/kg BW) seemed to be more effective in ameliorating antioxidant enzymes.
Table 3.
Effect of GSE on antioxidant parameters in DEX-induced rats (n = 8) (Mean ± Std. Error)
| Parameters | G1 | G2 | G3 | G4 | F‐ratio | P-value |
|---|---|---|---|---|---|---|
| CAT (μmol H2O2/min/mg Protein) | 146.56 ± 3.15d | 56.72 ± 2.86a | 89.83 ± 2.16b | 107.05 ± 3.10c | 172.55 | P < 0.001 |
| SOD (U/g tissue) | 22.09 ± 0.93d | 10.44 ± 0.42a | 12.97 ± 0.58b | 16.76 ± 0.82c | 49.96 | P < 0.001 |
| MDA (nmol/100 mg) | 18.33 ± 0.72a | 50.33 ± 1.12d | 33 ± 1.13c | 27.81 ± 1.06b | 172.60 | P < 0.001 |
The different letters indicate statistically different means according to Duncan multiple range test
CAT catalase, SOD superoxide dismutase, MDA Malondialdehyde, G1 normal control group, G2 dexamethasone control group, G3 dexamethasone + GSE (200 mg/kg BW), G4 dexamethasone + GSE (400 mg/kg BW)
Table 4 depicts the effect of dexamethasone and GSE on hematological parameters in DEX-induced rats. The dexamethasone-treated rats exhibited a significant elevation in hematological parameters compared to normal control rats. The data showed that GSE treatment with either 200 or 400 mg/kg showed a significant improvement in RBC, WBC, and PLT counts; also, Hb level reversed to near normal level. The GSE extract alleviated the adverse effects on WBC, Hb, RBC, and PLT counts caused by dexamethasone.
Table 4.
Effect of GSE on hematological parameters in DEX-induced rats (n = 8) (Mean ± Std. Error)
| Parameters | G1 | G2 | G3 | G4 | F‐ratio | P-value |
|---|---|---|---|---|---|---|
| RBC (× 106/μl) | 7.70 ± 0.22a | 8.91 ± 0.28b | 8.12 ± 0.11a | 7.99 ± 0.22a | 5.72 | P < 0.01 |
| Hb (g/dL) | 14.35 ± 0.16a | 15.98 ± 0.40c | 15.19 ± 0.19b | 14.30 ± 0.12a | 10.82 | P < 0.001 |
| WBC (103/μL) | 8.80 ± 0.60a | 10.93 ± 0.44b | 9.99 ± 0.10a | 9.50 ± 0.26a | 5.18 | P < 0.01 |
| PLT (103/μL) | 614.88 ± 15.81a | 842.00 ± 35.45c | 731.13 ± 22.25b | 654.63 ± 15.22a | 17.90 | P < 0.001 |
Data are expressed as mean ± SE; the different letters indicate statistically different means according to Duncan multiple range test
RBC red blood cell, Hb hemoglobin, WBC white blood cell, PLT platelets, G1 normal control group, G2 dexamethasone control group, G3 dexamethasone + GSE (200 mg/kg BW), G4 dexamethasone + GSE (400 mg/kg BW)
Microscopic examination of liver sections showed normal hepatocyte without any pathological changes in normal group (Fig. 1a). On the other hand, the liver sections of DEX-induced rats’ revealed remarkable histopathological alterations, and degenerative changes including cytoplasmic vacuolization of hepatocytes due to fatty change all over the hepatocytes with leucocytic infiltration in the portal area, as depicted in Fig. 1b and c. Treatment of the DEX-induced rats with 200 mg/kg (Fig. 1d) and 400 mg/kg (Fig. 1e) doses of GSE produced remarkable amelioration the liver tissue architecture. Only slight small fatty vacuoles in hepatocytes of 200 mg/kg GSE supplemented animals was observed (Fig. 1d).
Fig. 1.
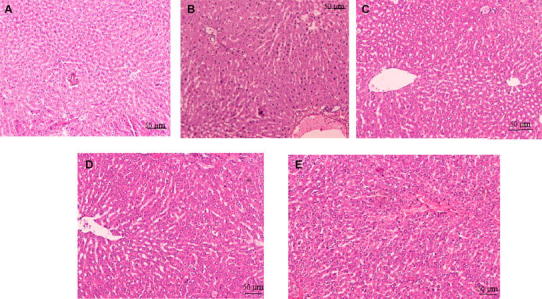
Effect of GSE on histological changes in liver of DEX-induced rats. Photomicrographs of H&E-stained liver sections of normal rats (a), DEX-induced rats revealing leucocyte infiltration, congestion, hepatic fatty vacuoles and cytoplasmic vacuolization of hepatocytes (b, c), DEX-induced rats treated with 200 mg/kg GSE, showing slight small fatty vacuoles (d), DEX-induced rats treated with 400 mg/kg GSE (e). (The original magnification was ×20)
Discussion
The current work deals with the effect of dexamethasone administration on rat liver, as well as the putative ameliorative afforded by grape seed extract. In the current investigation, DEX-induced hepatotoxicity is apparent by a remarkable elevation in serum activities of ALT, AST, and ALP. These findings are in agreement with our recent study representative elevated liver marker enzymes in serum of DEX-intoxicated rats [5]. The activities these enzymes are sensitive indications of liver damage and directly related to the degree of injury [2].
The hepatotoxicity induced by DEX was further confirmed by the remarkable histopathological alterations and degenerative changes including cytoplasmic vacuolization of hepatocytes due to fatty change all over the hepatocytes with leucocytic infiltration in the portal area. Our results in the line of those of Safaei et al. [23] who showed that DEX-induced inflammatory cells infiltration, severe hepatocyte degeneration, and necrosis.
Administration of the DEX-induced rats with either dose of GSE significantly ameliorated serum activities of liver enzymes in a dose-dependent manner, indicating the beneficial role of GSE to counteract the DEX‐induced liver injury. Moreover, GSE markedly alleviated the liver tissue architecture, where it was able to get back the normal liver histology. Only slight cytoplasmic vacuolization was observed. Consistent with our results, Zou et al. [24] found that GSE decreased serum transaminases in CCl4-induced hepatotoxicity in mice.
DEX-administration provoked a significant elevation in serum levels of total cholesterol, triglycerides and LDL-cholesterol and a decline in HDL-cholesterol, which indicated a dexamethasone impact on lipid profile. Our findings are in agreement with [25] who showed that dexamethasone administration induced a remarkable alteration in lipid profile.
DEX-administration is known to cause an elevation in the secretion of VLDL by the liver and stimulate VLDL formation by the intestine, so an imbalance in lipid metabolism occurred due to the low level of hepatic lipoprotein lipase inhibit removal of VLDL leading to elevation of triglyceride level [25].
In the current finding, administration of the DEX-induced rats with either dose of GSE has significantly normalized the serum level of cholesterol, triglyceride, and LDL-cholesterol and a remarkable increase in the level of HDL-cholesterol in a dose-dependent manner, indicating the ameliorative effect of GSE against hyperlipidemia induced by dexamethasone. Antihyperlipidemic effect of GSE due to an active ingredient, like polyphenol which inhibits absorption of triglyceride from the intestine, by inhibition of pancreatic lipase and stimulates lipoprotein lipase activity.
Regarding the oxidative stress, DEX-administration induced oxidative stress as indicated by the elevated hepatic lipid peroxidation marker MDA and a concomitant decline in activities of hepatic antioxidant enzymes SOD and CAT. Our findings were in agreement with the findings of [5] who stated that Oxidative stress is a major cause of dexamethasone‐induced liver injury due to the extreme production of free radicals.
Supplementation with either dose of GSE potentially reduces lipid peroxidation (MDA) and tends to bring the activities of the antioxidant enzymes (SOD, CAT) to the normal level. These findings were consistent with [26]. The antioxidant properties of GSE attributed to the catechin and epicatechin which are the major phenolic compounds in GSE [27], where catechin inhibits the oxidation of plasma lipids and, epicatechins are able to scavenge hydroxyl radicals, peroxyl radicals, and superoxide anion radicals.
Regarding, hematologic markers which can be used as valuable tools in evaluating physiological changes and good indicators of deleterious effects of drugs. DEX-administration induced leukocytosis and a remarkable increase in hemoglobin and red cell content. Our results are consistent with those of others [28] who state that glucocorticoid administration results in increased polymorphonuclear leukocytes in the blood due to an increased rate of the entrance from marrow and a decreased rate of removal from the vascular compartment.
Administration of the DEX-induced rats with either dose of GSE significantly improved the hematological parameters compared with rats treated with dexamethasone alone. Our results in the line of those of Abdou and Wahby [29] who demonstrated that GSE administration improves the hematological alteration induced by Triton.
Overall, DEX-induced hepatotoxic, hyperlipidemic and hematological alterations effects which were evidenced by elevation in the activities of liver enzymes and lipid profile in serum and decreases in antioxidant activities in liver homogenate in addition to liver histological perturbances. However, the GSE seemed to be more effective in ameliorating oxidative stress, hyperlipidemia and hematological alterations induced by dexamethasone. Further studies are needed to shed light on the molecular mechanisms that explain effects of GSE on gene expression in hepatotoxicity.
Conflict of interest
The author declares that he has no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The study protocol was approved by the Animal Ethics Committee in the College of medicine, Hail University.
References
- 1.Rui L. Energy metabolism in the liver. Compr Physiol. 2014;4(1):177–197. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastway M, Hasona N, Selemain H. Protective effects of extract from dates (Phoenix dactylifera L.) and ascorbic acid on thioacetamide-induced hepatotoxicity in rats. Iran J Pharm Res. 2008;7(3):193–201. [Google Scholar]
- 3.Iorga A, Dara L, Kaplowitz N. Drug-induced liver injury: cascade of events leading to cell death, apoptosis or necrosis. Int J Mol Sci. 2017;18:1018. doi: 10.3390/ijms18051018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luskin AT, Antonova EN, Broder MS, Chang EY, Omachi TA, Ledford DK. Health care resource use and costs associated with possible side effects of high oral corticosteroid use in asthma: a claims-based analysis. Clinicoecon Outcomes Res. 2016;8:641–648. doi: 10.2147/CEOR.S115025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasona NA, Alrashidi AA, Aldugieman TZ, Alshdokhi AM, Ahmed MQ. Vitis vinifera extract ameliorate hepatic and renal dysfunction induced by dexamethasone in albino rats. Toxics. 2017;5(2):11. doi: 10.3390/toxics5020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin G, Cao L, Du J, Jia R, Kitazawa T, Kubota A, et al. Dexamethasone-induced hepatomegaly and steatosis in larval zebrafish. J Toxicol Sci. 2017;42(4):455–459. doi: 10.2131/jts.42.455. [DOI] [PubMed] [Google Scholar]
- 7.Tayade PM, Jagtap SA, Borde S, Chandrasekar N, Joshi A. Effect of Psoralea corylifolia on dexamethasone-induced insulin resistance in mice. J King Saud Univ Sci. 2012;24:251–255. doi: 10.1016/j.jksus.2011.03.007. [DOI] [Google Scholar]
- 8.Kassa E, Enawgaw B, Gelaw A, Gelaw B. Effect of anti-tuberculosis drugs on hematological profiles of tuberculosis patients attending at University of Gondar Hospital, Northwest Ethiopia. BMC Hematol. 2016;16:1. doi: 10.1186/s12878-015-0037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasona N, Amer O, Raef A. Hematological alterations and parasitological studies among infected patients with Plasmodium vivax and Plasmodium falciparum in Hail, Kingdom of Saudi Arabia. Asian Pac J Trop Dis. 2016;6(9):695–698. doi: 10.1016/S2222-1808(16)61112-X. [DOI] [Google Scholar]
- 10.Hasona NA, Ahmed MQ, Alghassab TA, Alghassab MA, Alghabban AA. Ameliorative properties of Iranian Trigonella foenum-graecum L. seeds and Punica granatum L. peel extracts in streptozotocin-induced experimental diabetic guinea pigs. Asian Pac J Trop Biomed. 2017;7(3):234–239. doi: 10.1016/j.apjtb.2016.12.004. [DOI] [Google Scholar]
- 11.Patel AK, Davis A, Rodriguez ME, Agron S, Hackam AS. Protective effects of a grape-supplemented diet in a mouse model of retinal degeneration. Nutrition. 2016;32:384–390. doi: 10.1016/j.nut.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Z, Xue J, Shen T, Mu S, Fu Q. Curcumin alleviates glucocorticoid-induced osteoporosis through the regulation of the Wnt signaling pathway. Int J Mol Med. 2016;37:329–338. doi: 10.3892/ijmm.2015.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reitman S, Frankel S. The colorimetric method for determination of serum glutamic oxaloacetic transaminase and serum glutamic pyruvic transaminase. Am J Clin Pathol. 1957;28:56. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 14.Rec GS. A colorimetric method for the estimation of alkaline phosphatase. J Clin Chem Clin Biochem. 1972;10:18. [Google Scholar]
- 15.Allain CC, Poon LS, Chan CSG, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 16.Jacobs NJ, Van Denmark PJ. Enzymatic determination of serum triglycerides. J Arch Biochem. 1960;88:250–255. doi: 10.1016/0003-9861(60)90230-7. [DOI] [Google Scholar]
- 17.Gordon T, Gordon M. enzymatic method to determine the serum HDL-cholesterol. Am J Med. 1977;62:707–708. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RL, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative utracentrifugation. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 20.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 21.Cohen G, Dembiec D, Marcus J. Measurement of catalase activity in tissue. Anal Biochem. 1970;34:30–38. doi: 10.1016/0003-2697(70)90083-7. [DOI] [PubMed] [Google Scholar]
- 22.Drury R, Wallington E. Carleton’s histological techniques, vol 1. 5. London: Oxford University Press; 1980. pp. 653–661. [Google Scholar]
- 23.Safaei N, Shomali T, Taherianfard M. Niacin ameliorates lipid disturbances due to glucocorticoid administration in rats. Iran J Basic Med Sci. 2012;15(4):997–1002. [PMC free article] [PubMed] [Google Scholar]
- 24.Zou J, Qi F, Ye L, Yao S. Protective role of grape seed proanthocyanidins against Ccl4 induced acute liver injury in mice. Med Sci Monit. 2016;22:880–889. doi: 10.12659/MSM.895552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arab Dolatabadi A, Mahboubi M. A study of the influence of dexamethasone on lipid profile and enzyme lactate dehydrogenase. J Med Life. 2015;8(3):72–76. [PMC free article] [PubMed] [Google Scholar]
- 26.El Ayed M, Kadri S, Smine S, Elkahoui S, Limam F, Aouani E. Protective effects of grape seed and skin extract against high-fat-diet-induced lipotoxicity in rat lung. Lipids Health Dis. 2017;16:174. doi: 10.1186/s12944-017-0561-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yilmaz Y, Toledo RT. Major flavonoids in grape seeds and skins: antioxidant capacity of catechin, epicatechin, and gallic acid. J Agric Food Chem. 2004;52:255–260. doi: 10.1021/jf030117h. [DOI] [PubMed] [Google Scholar]
- 28.Danesh A, Janghorbani M, Khalatbari S. Effects of antenatal corticosteroids on maternal serum indicators of infection in women at risk for preterm delivery: a randomized trial comparing betamethasone and dexamethasone. J Res Med Sci. 2012;17:911–917. [PMC free article] [PubMed] [Google Scholar]
- 29.Abdou HM, Wahby MM. Neuroprotection of grape seed extract and pyridoxine against triton-induced neurotoxicity. Oxid Med Cell Longev. 2016;2016:8679506. doi: 10.1155/2016/8679506. [DOI] [PMC free article] [PubMed] [Google Scholar]


