Dear editor,
Staining of gels for esterase activity is a routinely performed procedure in any biological laboratory. The substrates popularly known for such purpose are α-naphthyl acetate or β-naphthyl acetate (BNA). After the electrophoresis run the gels are incubated with the ester substrates, and azo dye like Fast Red B. The separated protein having esterase function that cleaves the ester bond of the substrate concerned and forms either α-naphthol or β-naphthol which is subsequently stained by the azo dye. By the above procedure esterase activity can be easily visualised on gel [1].
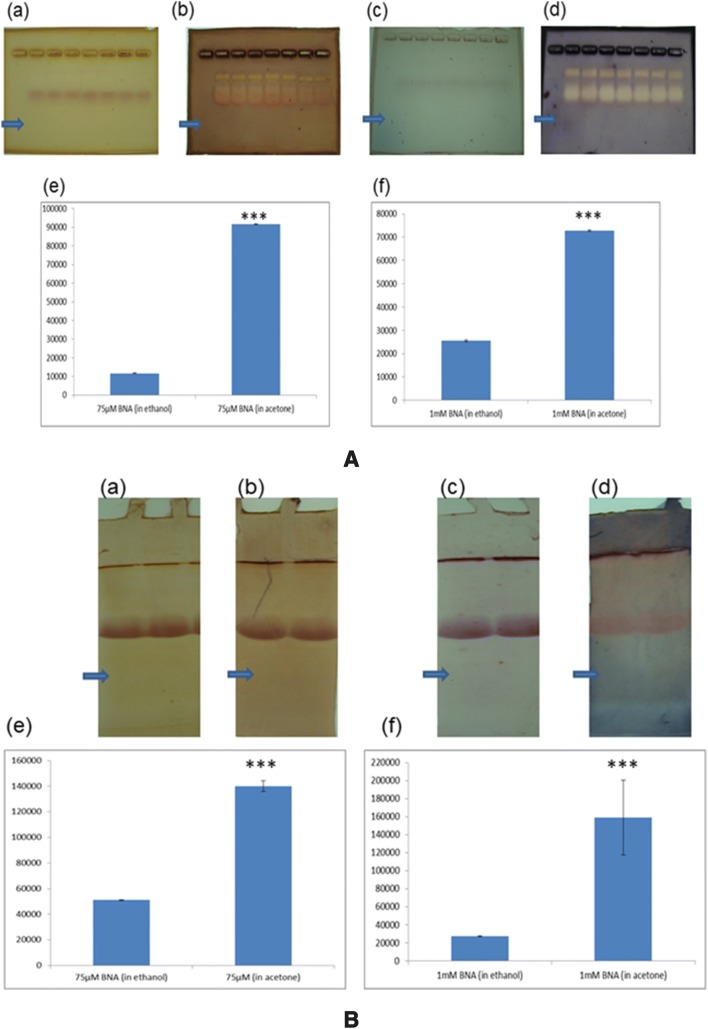
As on date, there is no consensus about the preparation of the ester substrate. Earlier workers have dissolved the substrates in acetone or ethanol and detected the enzyme activity [2–5]. While working in this line, we have observed that if BNA is dissolved in acetone, it produces a dark background compared to the situation when the substrate is prepared by dissolving in ethanol. Enzyme activity bands are visible in both the cases [Fig. 1A, B for agarose and native polyacrylamide gel electrophoresis (PAGE) respectively; for methods, please refer the supplementary]. It is needless to emphasise that low background staining is a prerequisite for electrophoresis experiment.
Fig. 1.
A Representative picture of agarose gel electrophoresis of serum proteins followed by staining with BNA for detection of esterase activity. The gel is incubated with (a) 75 µM BNA (in ethanol), (b) 75 µM BNA (in acetone), (c) 1 mM BNA (in ethanol), (d) 1 mM BNA (in acetone). The densitometry analysis of the background of the gel (→). Mean ± SD (n = 6) of the background density is shown in (e) for (a) & (b) and (f) for (c) & (d) respectively (Y-axis in square pixel where 96 pixel = 1 inch). ***p value less than 0.0001. B Representative picture of native PAGE of plasma proteins followed by staining with BNA for detection of esterase activity. The gel is incubated with (a) 75 µM BNA (in ethanol), (b) 75 µM BNA (in acetone), (c) 1 mM BNA (in ethanol), (d) 1 mM BNA (in acetone). The densitometry analysis of the background of the gel (→). Mean ± SD (n = 6) of the background density is shown in (e) for (a) & (b) and (f) for (c) & (d) respectively (Y-axis in square pixel where 96 pixel = 1 inch). ***p value less than 0.0001
It is interesting to note that destaining is sometimes necessary when the ester substrates are prepared in acetone [5]. This supports our observation that acetone as a dissolving medium of the ester substrate produces a dark background in the gel. With ethanol using BNA we have observed no (or very light) background staining. So if ethanol is used as a dissolving medium of BNA for esterase staining of gels, no destaining will be necessary.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
DK acknowledges Council of Scientific and Industrial Research, India for providing fellowship (File No: 09/141(0197)/2016-EMR-I). DB acknowledges Department of Science and Technology (DST), New Delhi for financial assistance.
Abbreviations
- BNA
β-Naphthyl acetate
- PAGE
Polyacrylamide gel electrophoresis
Compliance with Ethical Standards
Conflict of interest
Authors do not have any conflict of interest.
References
- 1.Nagpal N, Chowdhary S, Bhattacharyya R, Banerjee D. A color-reaction-based rapid screening for null activity of butyrylcholinesterase: a step toward point-of-care screening for succinylcholine apnea. Biotechnol Appl Biochem. 2015;62:154–163. doi: 10.1002/bab.1252. [DOI] [PubMed] [Google Scholar]
- 2.Lund BM. A comparison by the use of gel electrophoresis of soluble protein components and esterase enzymes of some group D Streptococci. J Gen Microbiol. 1965;40:413–419. doi: 10.1099/00221287-40-3-413. [DOI] [PubMed] [Google Scholar]
- 3.Thangthaeng N, Sumien N, Forster MJ, Shah RA, Yan LJ. Nongradient blue native gel analysis of serum proteins and in-gel detection of serum esterase activities. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:386–394. doi: 10.1016/j.jchromb.2010.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabriele M, Puccini P, Lucchi M, Vizziello A, Gervasi PG, Longo V. Presence and inter-individual variability of carboxylesterases (CES1 and CES2) in human lung. Biochem Pharmacol. 2018;150:64–71. doi: 10.1016/j.bcp.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 5.Chownk M, Kaur J, Singh K, Kaur J. mbtJ: an iron stress-induced acetyl hydrolase/esterase of Mycobacterium tuberculosis helps bacteria to survive during iron stress. Future Microbiol. 2018;13:547–564. doi: 10.2217/fmb-2017-0194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.