Abstract
Transplantation of hydrogel-encapsulated allogeneic islets has been explored to reduce or eliminate the need for chronic systemic immunosuppression by creating a physical barrier which prevents direct antigen presentation. Although successful in rodents, translation of alginate microencapsulation to large animals and humans has been hindered by large capsule sizes (≥500 μm diameter) that result in suboptimal nutrient diffusion in the intraperitoneal space. We developed a microfluidic encapsulation system which generates synthetic poly(ethylene glycol) (PEG)-based microgels with smaller diameters (310 ± 14 μm) that improve encapsulated islet insulin responsiveness over alginate capsules and allow transplantation within vascularized tissue spaces, thereby reducing islet mass requirements and graft volumes. By delivering PEG encapsulated islets to an isolated, retrievable, and highly vascularized site via a vasculogenic delivery vehicle, we demonstrate that a single pancreatic donor syngeneic islet mass exhibits improved long term function over conventional alginate capsules and close integration with transplant site vasculature. In vivo tracking of bioluminescent allogeneic encapsulated islets in an autoimmune type 1 diabetes murine model showed enhanced cell survival over unencapsulated islets in the absence of chronic systemic immunosuppression. This method demonstrates a translatable alternative to intraperitoneal encapsulated islet transplantation.
Introduction
Type 1 diabetes (T1D) requires life-long administration of daily insulin injections and vigilant blood glucose monitoring (1) which cannot completely eliminate secondary complications (2). Islet replacement therapy has long been proposed as an alternative to exogenous insulin, with the potential to eliminate secondary complications by restoring native insulin signaling for the lifetime of the patient (3). However, the necessity of chronic systemic immunosuppression limits the application of islet transplantation to patients with severe hypoglycemic episodes, where the benefits of the procedure outweigh the risks of long-term immunosuppression (4).
Islet immunoisolation within encapsulating materials may reduce or eliminate the need for chronic systemic immunosuppression, thereby enabling broader application of islet transplantation (5). Microencapsulation of islets within alginate capsules (500–1000 μm diameter) is the most investigated approach (6), including in pre-clinical non-human primate models (7, 8) and human trials (9–13). Considerable graft volumes imparted by both material and islet loading limit transplantation to the intraperitoneal (IP) cavity, where a minimum of 10,000 islet equivalents (IEQ) per kg body weight and multiple infusions (14) are required to achieve insulin independence. Whereas IP delivery has exhibited consistent success in allogeneic rodent models in the absence of immunosuppression (15), clinical translation has been limited by suboptimal graft performance in non-human primates and humans, safety concerns over the inability to retrieve capsules from the intraperitoneal space due to tissue adhesion and fibrotic responses, and high donor islet masses required for function (8–10, 12). Our previous work has demonstrated that delivery of islets to a non-vital extrahepatic transplant site such as the human omentum-equivalent murine epididymal fat pad (EFP) within a vasculogenic hydrogel delivery vehicle enhances islet vascularization, survival, and function while creating a retrievable islet graft (16, 17). As such, reducing capsule size, and therefore overall transplant volume, to enable transplantation within non-vital, retrievable tissues may enhance the safety and translatability of this therapy.
We previously reported on a microfluidic system to generate synthetic poly(ethylene glycol) (PEG) encapsulated islets (18) with tight control over microgel size as well as tunability of polymer composition. Synthetic materials such as PEG allow for greater consistency and reliability in production over biologically-derived alginate, which exhibits significant batch-to-batch variability (19). Herein, we demonstrate that microfluidic PEG-based encapsulation reduces microgel size over traditional alginate encapsulation techniques, reducing diffusion distances and transplant site limitations. We hypothesized that transplantation of PEG-encapsulated islets within a vasculogenic degradable hydrogel to a retrievable transplant site will reduce islet mass requirements by promoting vascularization in close proximity to encapsulated islets. We show that syngeneic single pancreatic donor PEG-encapsulated islet grafts exhibit improved performance over alginate-encapsulated islet grafts, and in vivo tracking of PEG-encapsulated allogeneic islets in a murine model of autoimmune diabetes demonstrates long-term protection imparted by encapsulation alone.
Research Design and Methods
Materials
Chemicals were obtained from Sigma-Aldrich, cell culture materials were obtained from Thermo Fisher, and peptides were obtained from Genscript, unless otherwise noted.
Islet isolation
Murine donors were anesthetized with ketamine (100 mg/kg)/xylazine (10 mg/kg) (Henry Schein) prior to pancreas perfusion with collagenase (153.8 μg/mL, Roche), thermolysin (5 μg/mL, Roche), and DNase (100 μg/mL) in HBSS. Pancreata were digested at 37 °C prior to Ficoll (Mediatech) density purification (1.11, 1.096, 1.069, 1.00 g/mL). Islets were counted by dithizone staining islet equivalent method (20). Islets were cultured in CMRL-1066 supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, 1% L-glutamine, 25 mM HEPES.
PEG encapsulation
A flow-focusing microfluidic device (18) was utilized to form crosslinked polymer droplets. Both shielding and crosslinker phases consisted of light mineral oil with 2% SPAN80. Crosslinker phase was an emulsion 1:15 of 30 mg/mL dithiothreitol (DTT) in PBS. PEG macromer (macromer refers to an oligomer that serves as a building block for further polymerization) was reacted for 20 min with 1.0 mM adhesive peptide in buffer solution containing 2 mg/mL F-127 surfactant and 1:1 Optiprep density gradient. A co-flowing shielding phase protected the cell-containing macromer solution, 5% PEG-4MAL (10 kDa or 20 kDa, > 95% functionalization, Laysan Bio), from the crosslinker phase until droplets were formed. Capsules were washed 5X and sized using FIJI (ImageJ) software. This method results in 1–2 islets per microgel.
Alginate encapsulation
Islets were dispersed in 1.6% alginate (UPMVG, Novomatrix) in PBS. Alginate-islet mixture was extruded through a 0.5 mm diameter needle into a 1.5% crosslinking buffer (50 mM BaCl2, 10 mM 3-(N-morpholino)propanesulfonic acid (MOPS), 140 mM D-mannitol, 0.025% vol/vol Tween-20, pH 7.4) using an electrostatic droplet generator (Nisco Engineering). Capsules were crosslinked 10 min, washed 3x with PBS, and resuspended in complete CMRL-1066 (1–2 islets per capsule).
Gel permeability studies and finite element modeling
Microgels/capsules were submerged in 0.5 mL of 0.15 mg/mL FITC-dextran solutions (10, 40, 70, or 150 kDa) in PBS on a glass slide at 37 ⁰C and imaged at 30 second intervals using a Nikon C2 confocal with a 10X objective. FITC intensity was measured using FIJI (ImageJ). The diffusion coefficient of FITC-dextran molecules was calculated assuming 3D transient diffusion using COMSOL Multiphysics 5.2a (COMSOL, Burlington, MA) as described in Supplementary Materials.
Islet viability and function
Islets (150 IEQ) were left unmodified or encapsulated in 100 μL PEG gels modified with 1.0 mM RGD (GRGDSPC), RDG (GRDGSPC), YIGSR (CGGEGYGEGYIGSR), AG73 (CGGRKRLQVQLSIRT), A2G10 (CGGSYWYRIEASRTG), C16 (CGGKAFDITYVRLKF), or IKVAV (CGQAASIKVAVSADR) and cultured for 7 days. For GSIR, islets were suspended in KREBs buffer (99 mM NaCl2, 5 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 2.6 mM CaCl2, 26 mM NaHCO3, and 20 mg/mL bovine serum albumin). Unencapsulated or encapsulated islets were exposed to sequential 1.0 mL D-glucose challenges (3.3 mM, 3.3 mM, 16 mM, 3.3 mM) in 60 minute intervals using transwell inserts in a 24 well plate. Islet viability was assessed by Live/Dead Imaging (Invitrogen; Live: Calcein, Dead: Toto-3-Iodide) at 48 hours by Live/Dead imaging. Metabolic activity (6 hours) was assessed via Alamar Blue (Invitrogen). For modified GSIR, islets were exposed to sequential D-glucose challenges in 30 minute intervals (3.3 mM, 6.6 mM, 9.0 mM, 13.2 mM, 16.0 mM, 13.2 mM, 9.0 mM, 6.6 mM, 3.3 mM) using the column method and supernatant evaluated by insulin ELISA (Mercodia).
Diabetes induction
Animal experiments were performed with the approval of the Georgia Tech Animal Care and Use Committee with veterinary supervision and within the guidelines of the Guide for the Care and Use of Laboratory Animals. C57BL/6J or Balb/c male mice (10–14 weeks) were used as recipients, and diabetes was induced by single dose streptozotocin i.p. injection (180–200 mg/kg) at pre-operative day 5. C57BL/6J or Balb/c female mice (10–14 weeks) were used as islet donors. B6;FVB-Ptprca Tg(CAG-luc,-GFP)L2G85Chco Thy1a/J female mice (8–12 weeks) were used as donors for imaging and tracking studies. Diabetic NOD females (16–22 weeks) were used as recipients in tracking studies. Diabetes was defined as > 2 consecutive blood glucose readings above 350 mg/dL, diabetes reversal as 2 consecutive readings < 250 mg/dL.
Epididymal fat pad (EFP) islet transplant
One day post-encapsulation, 600 IEQ (syngeneic) or 1200 IEQ (allogeneic) unencapsulated or encapsulated islets were dispersed within vasculogenic hydrogels to form two equal volume gels (25 μL) for delivery to two EFP per recipient. Vasculogenic hydrogel preparation: a sterile 5% (final, w/v) solution of 4-arm PEG-maleimide monomer (20 kDa, Laysan Bio) was functionalized with 1.0 mM RGD and 10 μg/mL VEGF (Invitrogen) at 37° C for 15 min in buffer (PBS, 25 mM HEPES). VPM (GCRDVPMSMRGGDRCG) peptide crosslinking solution was prepared separately. Solutions were adjusted to pH 7.0–7.5. Macromers with unencapsulated or encapsulated islets were rapidly mixed with crosslinker and swelled in complete CMRL for 30 min. Islet-containing vasculogenic gels were centered on the open EFP and wrapped in excess tissue before sealing with a degradable PEG gel (10% PEG 10 kDa, 1 mM RGD, VPM crosslinker). A typical EFP can accommodate an approximately 50 μL volume construct. An intraperitoneal glucose tolerance test was performed between day 90–100 post-transplant (2 gm/kg glucose). Recipients comprise individuals pooled from 5 separate isolations and transplants.
In vivo islet tracking
Islets were isolated from B6;FVB Luc+GFP+ transgenic and wild-type C57BL/6J mice and transplanted in the gonadal adipose tissue of diabetic, age-matched NOD recipients (1200 IEQ: 400 IEQ Luc+GFP+, 800 IEQ B6). Bioluminescence was detected by kinetic monitoring of signal (3-min intervals) after beetle luciferin (0.4 mg/recipient, Promega) injection under anesthesia, until peak signal, on an IVIS SpectrumCT (PerkinElmer) weekly. Total flux was measured over a circular region of interest around each graft. Results were pooled from two separate transplant and imaging experiments.
Lectin perfusion and imaging
DyLight 488-labeled lectin was injected intravenously (200 μL, Vector Labs), and the vasculature flushed with saline after 15 min prior to graft removal and fixation in 10% buffered formalin. For live graft imaging, grafts were incubated in saline for imaging prior to fixation. Grafts were stabilized between glass slides prior to whole-mount imaging on a confocal microscope. Z-stacks of each sample were acquired at 10–20 μm intervals via a 10X or 20X objective. Lectin was quantified within 10X image FOV using the Image J analyze particles module, n = 2–6 images per animal, N = 3 animals per group.
Histological evaluation
Formalin-fixed grafts were paraffin-embedded and sectioned. Antigen retrieval in citrate buffer was used before sequential blocking with Power Block (BioGenex) and goat serum (BioGenex). Primary antibodies against insulin (DAKO, 1:50) and isotype control antibodies were incubated overnight at 4°C, and secondary antibodies (Invitrogen, 1:200) were incubated for 2 hours at room temperature with DAPI before mounting. For hematoxylin and eosin staining, tissue sections were deparaffinized and sequentially stained using an automatic slide stainer.
Statistics
Microgel/capsule sizes were analyzed by two-way ANOVA with Tukey’s multiple comparison test. Adhesive peptide stimulation index and live/dead viability, modified perfusion assay, Alamar blue metabolic activity, IPGTT and average blood glucose > 50 days were analyzed by one-way ANOVA using Dunn’s multiple comparison test. Survival curve was analyzed by Log-rank (Mantel-Cox) test. Islet tracking values > day 7 were analyzed by one-way ANOVA with Tukey’s multiple comparison test. Vascularization data was analyzed by parametric t-test.
Results
Hydrogel diffusion kinetics and encapsulated islet insulin secretion
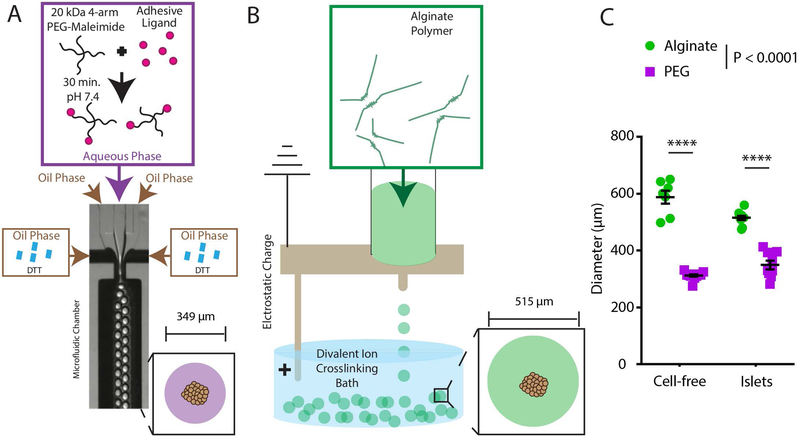
We determined the size distribution of PEG microgel-encapsulated islets generated by microfluidic encapsulation relative to electrostatic alginate encapsulation (Fig. 1). Alginate encapsulation (Fig. 1A) resulted in capsules of 588 ± 60 μm (average ± standard deviation) and 515 ± 24 μm average diameter for cell-free and islet-containing capsules, respectively. By comparison, microfluidic PEG encapsulation (Fig. 1B) resulted in microgels of 312 ± 14 μm and 349 ± 45 μm diameter for cell-free and islet-containing microgels, respectively. PEG microgels result in substantially reduced (32%) diameters for islet-containing microgels compared to alginate capsules (Fig. 1C).
Figure 1.
Schematic demonstrating (A) PEG encapsulation via microfluidic droplet generation and (B) alginate encapsulation via electrostatic droplet generation. (C) Quantification of size difference between alginate electrostatic and microfluidic PEG capsule generation with or without islets. Error bars = SEM. **** P < 0.0001 by two-way ANOVA with Tukey’s multiple comparison test.
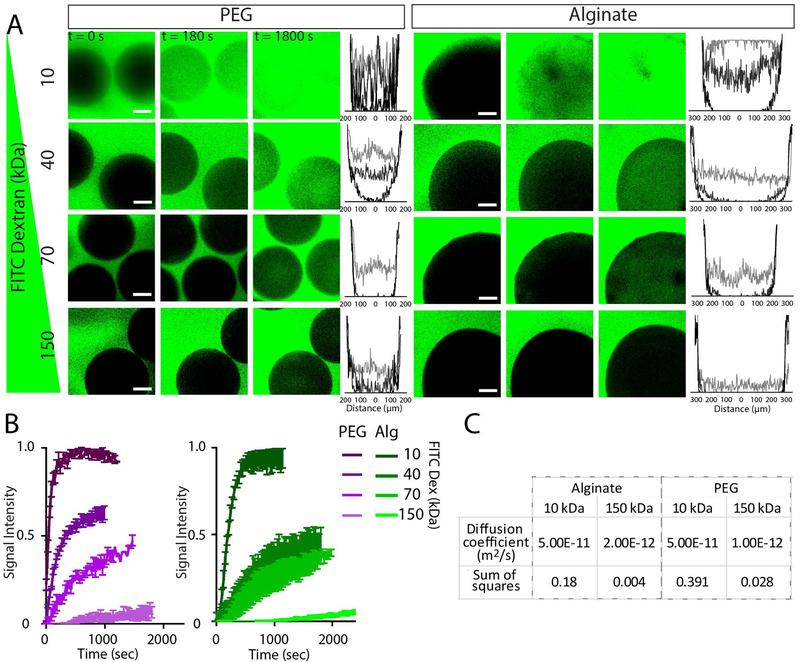
A crucial feature of an encapsulation material is selective permeability which should enable efficient transport of insulin (~7 kDa), and limit transport of pro-inflammatory cytokines (~5–50 kDa) and IgG (~150 kDa). We evaluated the diffusion kinetics for PEG microgels and alginate capsules using model FITC-dextran molecules (10, 40, 70, 150 kDa). Internal fluorescence was quantified as demonstrated by intensity histograms (Fig. 2A) and change of intensity over time (Fig. 2B). Finite element modeling of diffusivity via parametric sweeps and sum of the squares analysis against measured values (Fig. S1) provided diffusivity values comparable to those determined by others (21), and demonstrated comparable diffusivities between PEG and alginate hydrogels (Fig. 2C).
Figure 2.
Transport properties for PEG microgels and alginate capsules. FITC-dextran diffusion studies elucidate PEG microgel properties compared to traditional alginate capsules. Confocal images of FITC-dextran diffusion within materials at varied time points and accompanying histograms (B) illustrate spatio-temporal diffusion properties within materials. Quantification of central region of microgel/capsule fluorescence over time (C) allows approximation of diffusion coefficient (D) using finite element modeling. Scale bars = 100 μm. Error bars = SEM.
We determined whether the incorporation of adhesive peptides within PEG gels impacts the function or viability of encapsulated islets (Fig. 3 and Fig. S2). PEG gels (100 μL) were modified with fibronectin-derived adhesive peptide RGD, scrambled non-adhesive peptide RDG, or various peptides derived from laminin (YIGSR, AG73, A2G10, C16, IKVAV). A peptide-free control group was not used, as the omission of peptide from the hydrogel would alter the network crosslink density and therefore change the mechanical and structural properties of the hydrogel. After 7 days in culture, unencapsulated or encapsulated islets were subjected to GSIR, where all groups demonstrated a characteristic insulin response, with the exception of RDG and YIGSR peptides (Fig. 3A). Normalizing insulin values at high glucose to the first low glucose yields the islets’ stimulation index, a metric of the degree of glucose responsiveness (22). Stimulation indices of RDG- and YIGSR-functionalized PEG gels were significantly lower than that of PEG-RGD (P < 0.05 and P < 0.01, respectively; Fig. 3B). Live dead imaging of unencapsulated or encapsulated islets (Fig. S2A & B) showed different responses in islet viability to adhesive peptides, and quantification of relative live islet area (Fig. S2C) demonstrated significantly reduced viability in RDG, AG73, and IKVAV groups compared to unencapsulated islets (P < 0.005, P < 0.0005, and P < 0.005, respectively).
Figure 3.
Encapsulation matrix impact on islet viability and function in vitro. (A) Islet GSIR for unencapsulated islets or islets encapsulated in PEG modified with adhesive ligand RGD, scrambled non-adhesive RDG, and laminin derived peptides YIGSR, AG73, A2G10, C16, and IKVAV. (B) Stimulation index derived from GSIR in (A). (C) Live/Dead imaging demonstrates comparable viability between PEG-RGD- and alginate-encapsulated islets, and unencapsulated islets. (D) Evaluation of glucose stimulated insulin secretion profile via modified perfusion assay. (E) Metabolic activity was measured by Alamar Blue. (F) GSIR area under the curve normalized to metabolic activity illustrates overall insulin release relative to islet number. Error bars = SEM. Scale bars = 200 μm. * P < 0.05. All analyses by one-way ANOVA using Dunn’s multiple comparison test.
We evaluated in vitro viability and function for PEG microgel-encapsulated islets relative to alginate-encapsulated and unencapsulated islets. After 48 h of culture, live/dead staining indicated comparable viability across all groups (Fig. 3C), and insulin responsiveness by modified GSIR demonstrate a characteristic response in all groups (Fig. 3D). PEG-RGD encapsulated islets demonstrated comparable kinetics to unencapsulated islets, whereas alginate-encapsulated islets showed reduced overall insulin secretion (P = 0.0087 vs. unencapsulated islets). We posit that this reduction in insulin secretion could be partially explained by islet loss during the encapsulation process, as the electrostatic alginate encapsulation method results in greater loss of hydrogel and islets within the system during encapsulation compared to the microfluidic encapsulation method. To test this, we evaluated all groups via Alamar Blue metabolic activity assay (Fig. 3E), which demonstrated a significantly reduced activity for alginate-encapsulated islets (P < 0.05). As viability was comparable across all groups (Fig. 3C), the reduction in metabolic activity indicates an average loss during encapsulation of ~40% of islets. When we normalize insulin secretion area under the curve (AUC) to Alamar Blue activity, we find comparable values across all groups (Fig. 3F).
Diabetes reversal by syngeneic and allogeneic encapsulated islet transplantation in a vascularized, retrievable transplant site
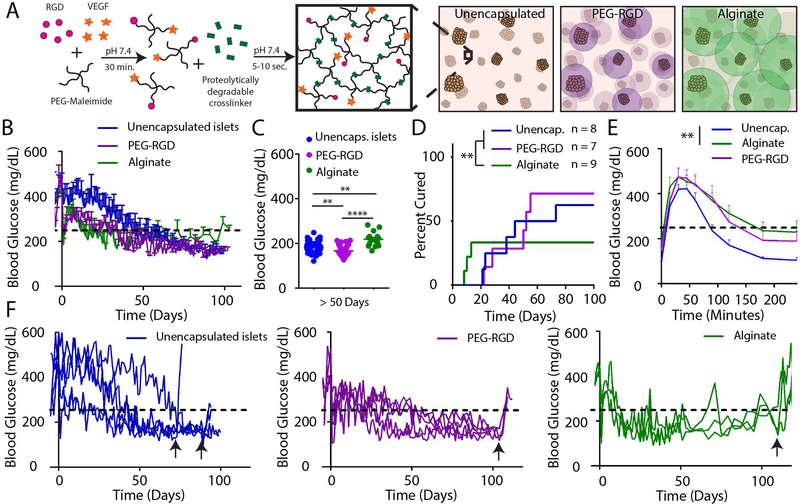
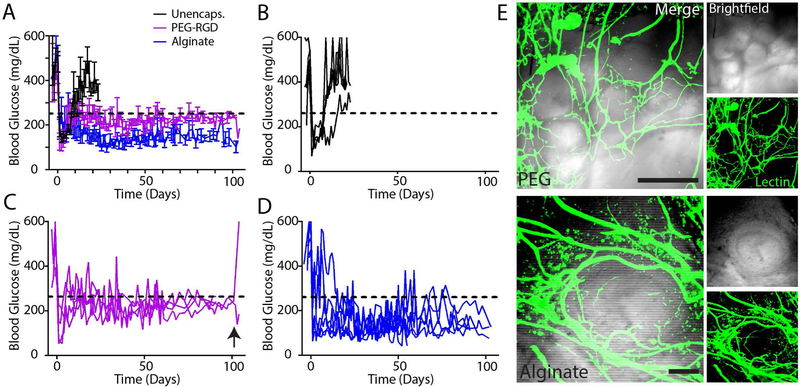
We first evaluated whether microfluidic PEG-RGD encapsulated islets could restore normoglycemia in chemically induced diabetic mice in a syngeneic islet transplantation model. We delivered a single pancreatic donor islet mass (600 IEQ) of encapsulated (PEG-RGD or alginate) or unencapsulated syngeneic islets to the EFP via a vasculogenic degradable hydrogel vehicle (Fig. 4A) and monitored normoglycemic recipients long term for average nonfasting blood glucose (Fig. 4B). Average blood glucose after day 50 (Fig. 4C) and survival analysis demonstrate that PEG-RGD encapsulated islets perform equivalently to unencapsulated islets, whereas alginate recipients exhibited a reduced rate of diabetes reversal (Fig. 4D, P < 0.01). PEG-RGD encapsulated islets performed equivalently to unencapsulated islets as assessed by an endpoint intraperitoneal glucose tolerance test (IPGTT) on functioning grafts, whereas the alginate IPGTT curve differed significantly from unencapsulated islets (P < 0.01, Fig. 4E). Individual transplant recipient blood glucose curves (Fig. 4F) of functional islet grafts demonstrate return to hyperglycemia upon graft removal (black arrows), confirming functioning islet grafts. Additionally, these curves further suggest the instability of alginate-encapsulated islet grafts after engraftment, where grafts exhibit dysfunction after 50 days post-transplantation. This trend is exhibited in significantly higher average alginate non-fasting blood glucose (P < 0.001) (Fig. 4C, Fig. S3).
Figure 4.
Syngeneic single pancreatic donor encapsulated islet transplantation in retrievable extrahepatic site. (A) Unencapsulated islets or islets encapsulated in PEG-RGD microgels or alginate capsules were transplanted in the EFP via a vasculogenic degradable hydrogel vehicle. (B) Nonfasting blood glucose of functioning grafts (n = 4 for unencapsulated, n = 5 PEG-RGD, n = 3 alginate) was monitored out to 100 days post-transplant, and (C) average blood glucose values post day 50 were averaged to evaluate overall graft glycemic control (one-way ANOVA using Dunn’s multiple comparison test). (D) Survival curves indicate overall diabetes reversal rates of single pancreatic donor (Log-rank Mantel-Cox test). IPGTT at endpoint (day 90–100, E) demonstrates average functioning graft response to bolus glucose (one-way ANOVA using Dunn’s multiple comparison test). (F) Individual recipient blood glucose traces of functioning grafts demonstrate function prior to graft removal (black arrows). ** P < 0.01, **** P < 0.001.
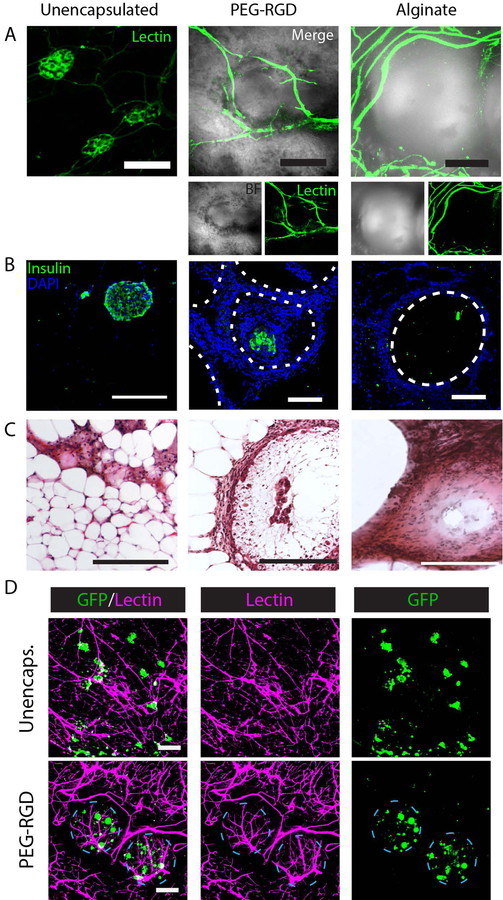
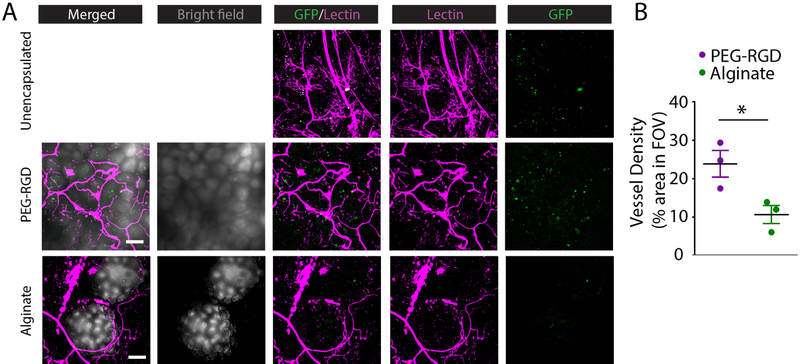
To examine the degree to which grafts were vascularized, recipients were lectin-perfused to visualize functional vasculature (Fig. 5A). For unencapsulated islets, the characteristic dense vasculature of islet organoids (16) is visualized. By comparison, encapsulated islet grafts demonstrated vasculature at the surface of the hydrogel. Vasculature typically surrounded the smaller diameter PEG microgels for higher surface coverage relative to larger alginate capsules which demonstrated poorer vascular penetration between capsules. Immunohistochemistry identified insulin-positive islets within all grafts (Fig. 5B), and hematoxylin and eosin (H&E) staining shows remodeling of tissue between and surrounding islets and capsules (Fig. 5C). GFP-expressing islets from a B6 background were unencapsulated or PEG-RGD encapsulated and transplanted into diabetic B6 recipients prior to lectin perfusion 4 weeks post-transplant to examine islet proximity to vasculature (Fig. 5D). Merged images of unencapsulated islets (green) and vasculature (magenta) demonstrate extensive islet integration with host vasculature. Imaging of PEG-RGD encapsulated islets demonstrates dense vascularization at the microgel surface and viable islets within well-defined microgels.
Figure 5.
Histological evaluation of EFP-transplanted unencapsulated and encapsulated islets. (A) Whole mount imaging of graft vasculature via lectin staining. (B) IHC staining of graft sections for insulin. (C) H&E staining of grafts. (D) Syngeneic B6/GFP (green) islets in B6 recipient at 4 weeks demonstrating islet or encapsulated islet integration with functional, lectin perfused vasculature (magenta). Scale bars = 200μm.
To examine islet viability and PEG microgel vascular integration in an allogeneic model, unencapsulated or encapsulated B6/GFP islets were transplanted into Balb/c recipient EFPs via vasculogenic degradable hydrogel vehicle for lectin perfusion and whole mount imaging. At 4 weeks post-transplantation (Fig. 6A), unencapsulated islets exhibit fragmented and reduced GFP signal, well integrated with host vasculature, whereas PEG-RGD encapsulated islets maintained integrity and robust signal. PEG-RGD encapsulated allogeneic recipients were also imaged after 120 days in vivo (Fig. 6B), demonstrating dense vascularization and robust GFP signal despite MHC mismatch. This encouraging response indicates that PEG-RGD encapsulation alone may serve to preserve islet viability in vivo over long time scales.
Figure 6.
Whole mount imaging of vascularized, PEG-RGD encapsulated allogeneic islet grafts. Allogeneic B6/GFP islets in Balb/c recipients at 4 (A) and 18 (B) weeks demonstrate vascularized encapsulated islet survival long-term. Scale bars = 200 μm.
Whereas B6 to Balb/c is an allogeneic MHC mismatch, this model is less robust than the Balb/c to B6 transplantation scheme, as B6 recipients exhibit more robust innate responses, potentially incurring a stronger adaptive response to transplanted islets (23). As such, we evaluated diabetes reversal of allogeneic Balb/c islets transplanted within B6 EFPs via vasculogenic hydrogel delivery vehicle (Fig. 7A). Individual non-fasting blood glucose values for unencapsulated (Fig. 7B), and PEG-RGD- (Fig. 7C) or alginate- (Fig. 7D) encapsulated islets show that unencapsulated islets exhibit rapid rejection (mean time to rejection 11.5 days), and islets encapsulated in either PEG-RGD or alginate exhibit long-term function out to 100 days. Graft procurement of select recipients after 100 days exhibited return to diabetic state. Lectin-perfused islet grafts within the EFP demonstrate a high degree of close apposition with vascular network (Fig. 7E), with PEG-RGD grafts exhibiting inter-capsule vascularization.
Figure 7.
Long-term allogeneic encapsulated islet function within the vascularized EFP. (A) Average nonfasting blood glucose of unencapsulated (n = 4), PEG-RGD (n = 4), or alginate (n = 6) encapsulated Balb/c islets transplanted within the EFP of B6 recipients using a vasculogenic hydrogel delivery vehicle. (B-D) Individual traces of unencapsulated (B), PEG-RGD- (C), or alginate-encapsulated (D) islets. (E) Imaging of PEG-RGD- (top) and alginate- (bottom) encapsulated islet grafts after day 100 demonstrate capsule integration with lectin-perfused (green) functional vasculature. Black arrow signifies selected graft removal. Error bars = SEM. Scale bars = 200 μm.
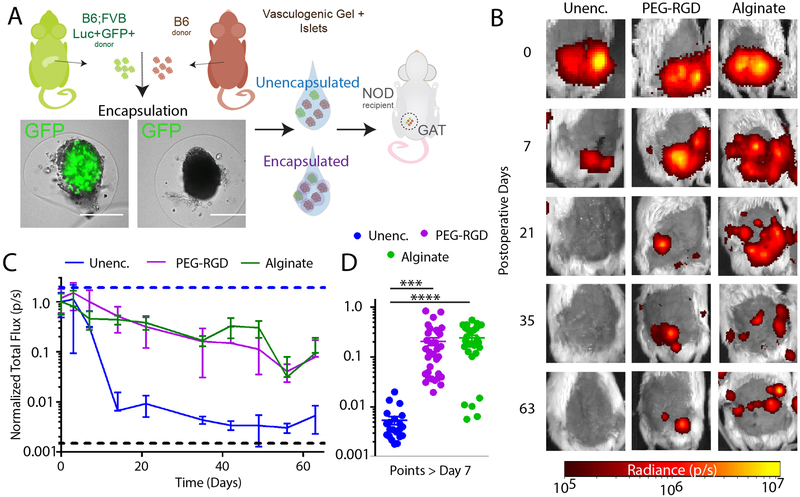
Tracking of allogeneic encapsulated islet survival in an autoimmune T1D murine model
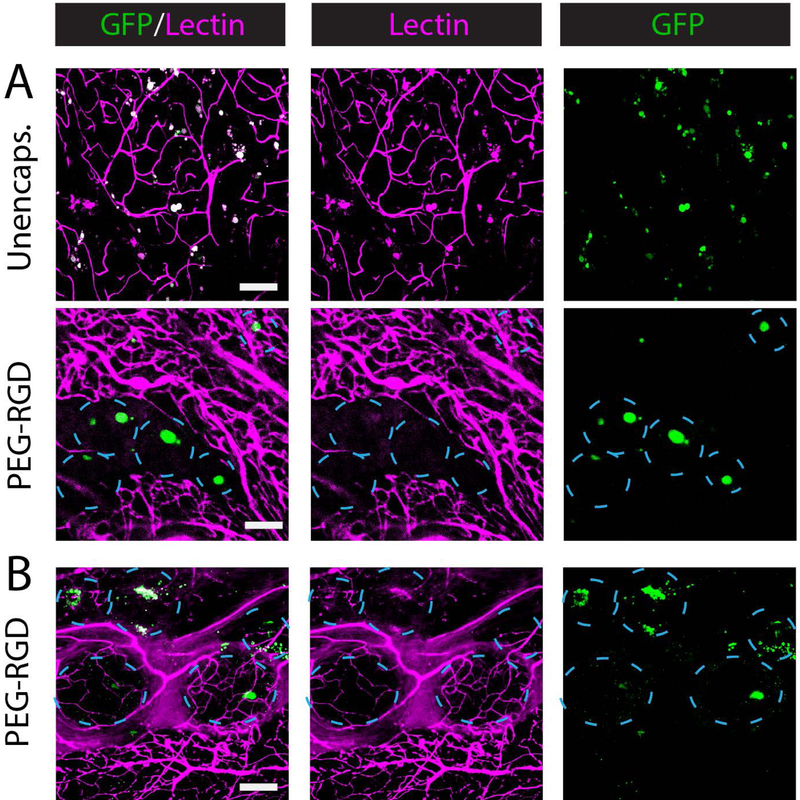
We next investigated the extent of PEG-RGD encapsulation protection of allogeneic islets in the autoimmune NOD mouse model. To evaluate encapsulated islet survival longitudinally in vivo, we utilized transgenic B6 donors constitutively expressing luciferase to track unencapsulated and encapsulated islet grafts in diabetic NOD recipients gonadal adipose tissue (GAT) (Fig. 8A). NOD females were used due to their higher rate of diabetes occurrence (~80%), and the equivalent structure to the EFP in the female mouse is the GAT. Recipients were imaged weekly for bioluminescence signal (Fig. 8B), and overall signal was quantified (Fig. 8C) as a measure of islet number. Unencapsulated islet grafts exhibited rapid rejection signified by signal reduction to less than 1% of original signal within 2 weeks of transplant. In contrast, both PEG-RGD- and alginate-encapsulated islets exhibited a slower decline in signal to approximately 10% of day 0 signal by 9 weeks post-transplant. Due to the greater size of alginate capsules, resulting in a greater overall graft volume than PEG-RGD grafts, all alginate recipients exhibited capsule escape from the transplantation site to the intraperitoneal cavity within two weeks post-transplant (confirmed at euthanasia and dissection), whereas PEG-RGD encapsulated groups exhibited consistent localization within the GAT. Overall, both PEG-RGD and alginate encapsulated groups exhibited significantly higher luciferase signal for the 9 week study duration compared to unencapsulated controls (Fig. 8D, P < 0.001). Lectin perfusion showed unencapsulated and encapsulated islet association with GAT vasculature via whole mount imaging (Fig. 9A). Limited GFP signal was detected in unencapsulated islet recipients, whereas a high density of GFP signal was seen in PEG-RGD encapsulated islets, with significant vascularization observed among PEG-RGD capsules. In the alginate group, GFP signal was detectable, although in lower density than PEG-RGD grafts due to the size of alginate capsules, which results in greater distance between encapsulated islets. Additionally, this greater size resulted in a reduced vascular penetration between capsules, as observed in syngeneic studies and quantified in Fig. 9B (P < 0.05).
Figure 8.
In vivo tracking of allogeneic islet survival in an immunocompetent autoimmune diabetic (NOD) murine model. (A) Schematic demonstrating transplant and imaging strategy. (B) In vivo imaging of bioluminescent unencapsulated (n = 4), PEG-RGD- (n = 6) or alginate- (n = 4) encapsulated islets over time. (C) Quantification of luciferase signal over 9 weeks, and summarized data points (D). Scale bars = 200 μm. *** P < 0.0005, **** P < 0.001 by one-way ANOVA with Tukey’s multiple comparison test.
Figure 9.
Whole mount imaging of vascularization in allogeneic islet grafts transplanted in NOD recipients. (A) Lectin (magenta) perfused grafts illustrate vascularization of unencapsulated or encapsulated islet (GFP, green) grafts. (B) Quantification of vascularization area within image field of view demonstrates significantly higher vascularization in PEG-RGD grafts compared to alginate. Scale bars = 200 μm. * P < 0.05 by Student’s t-test.
Discussion
Hydrogel encapsulation of allogeneic islets has long been proposed as a method of reducing or eliminating the need for chronic immunosuppression in islet cell replacement therapy (5), and as alginate is the most studied and leading candidate for islet encapsulation, it serves as the most appropriate reference with which to benchmark our new method. However, we note that there are significant structural and chemical differences between alginate and PEG hydrogels so these materials cannot be directly compared. Whereas intraperitoneal delivery of alginate encapsulated islets has demonstrated reproducible success in rodent models (6), translation to higher-order models such as non-human primates (7, 8) and humans (9–13) has been limited. Intraperitoneal capsule function is limited by capsule fibrosis and tissue adhesion over long time scales, exacerbated by capsule accumulation in bipedal recipients (8–10, 12), and islet necrosis due to nutrient diffusion limitations (24, 25). To address these limitations, we developed a microfluidic-based system to generate smaller diameter capsules than conventional techniques, capable of delivery to a highly vascularized transplantation site.
We compared synthetic PEG microgel performance to conventional alginate in vitro (Fig. 1 and 2). We generated synthetic microgels of reduced size, and approximated the permselectivity of alginate, allowing efficient diffusion of insulin-scale molecules while obstructing the diffusion of larger molecules. An advantage of this synthetic hydrogel system is facile incorporation of pro-survival adhesive peptides. Studies examining islets cultured on surfaces coated with pancreatic extracellular matrix components collagen I, collagen IV, fibronectin, and laminin showed that insulin responsiveness is highest in response to fibronectin and laminin (26). We demonstrate that incorporation of adhesive peptides into PEG hydrogel modulates islet viability and function, where RGD-functionalized PEG gels demonstrated the highest insulin responsiveness and islet viability (Fig. 3 and Fig S2), and as is supported by our previous studies exploring the impact of RGD on PEG hydrogel-encapsulated islets (16, 17, 27); thus this formulation was selected for subsequent studies. Modification of alginate with RGD is non-trivial, and most uses of alginate in the literature do not utilize RGD-modified alginate, and as a result this was not attempted in this study.
Because of the reduced microgel size imparted by the microfluidic method, overall graft volume is reduced up to 8-fold over alginate-encapsulated islet grafts. Thus, PEG-RGD encapsulated grafts are not limited to delivery within the intraperitoneal space. We therefore evaluated encapsulated islet function within a vascularized, retrievable transplant site. Transplantation within retrievable tissue may enhance the safety and translatability of encapsulated cell transplantation by enabling graft removal in case of complications. We demonstrated that islet delivery to the EFP using a degradable vasculogenic hydrogel vehicle results in rapid graft vascularization and return to normoglycemia for a single pancreatic donor syngeneic islet mass (16)(Fig. 4), indicating high clinical translatability by reducing the donor tissue required for a single recipient. We hypothesize that close proximity of vasculature to encapsulated islets (Fig. 5) provided by transplantation within highly vascularized tissue and smaller PEG-RGD microgels may improve long term viability and function by minimizing diffusion distances.
Whereas allogeneic islet function is a standard barrier in murine models of islet transplantation (Fig. 7), it does not fully recapitulate the allo- and autoimmune attack observed in a T1D recipient of an islet allograft. By contrast, the NOD mouse model involves both allogeneic and autoimmune responses to an islet transplant graft. Although groups have demonstrated allogeneic or even xenogeneic alginate-encapsulated islet function in NOD models (28, 29), the limited successful translation of this technology to large animal models, as well as the large grafts required to obtain long-term function, suggest that significant graft loss remains a potential barrier to translation. Additionally, while the primary goal of islet immunoisolation is the prevention of direct antigen recognition by circulating immune cells (30), capsule permselectivity designed for rapid insulin diffusion (~7 kDa) cannot prevent the passage of shed islet antigens (<10 kDa) which activate the immune system via the indirect antigen presentation pathway (31, 32), particularly in the presence of pre-existing autoimmunity against beta cells. We postulated that quantification of islet survival using transgenic luciferase islet imaging within the NOD transplant model would elucidate the degree of protection conferred by an encapsulating material alone (Fig. 8), and quantify graft destruction in the absence of direct antigen recognition. Quantification of this destruction demonstrates that both alginate- and PEG-RGD-encapsulated grafts exhibit a steady loss in graft viability to 10% of original signal over a period of 9 weeks, and may explain the difference in success observed between rodent and higher order animal models. Additionally, while diabetes reversal was observed in allogeneic recipients out to 100 days (Fig. 7), consistent with previous reports of allogeneic encapsulated islet function, in vivo imaging of NOD recipients (Fig. 8) suggests a more robust immune response to transplanted encapsulated islets due to the presence of pre-existing autoimmunity. Rodent studies typically use highly pure multiple-donor islet preparations for a single recipient, which may facilitate observations of “long-term” function, usually indicating the range of 6 months to 1 year, if residual function of 10% is still a sufficiently large islet loading to maintain function. Conversely, non-human primate and human transplantation is much more limited in donor tissue availability, resulting in limited function observed in short- (7, 8, 11, 12) and long-term studies (9, 10). This data demonstrates quantitatively that immunoisolation methods will ultimately require synergistic approaches to completely prevent the immune destruction of encapsulated islets.
The longitudinal tracking data demonstrate that alginate capsules escaped the transplant site over the duration of the study (Fig. 8B), confirmed by gross observations upon graft explantation. Whereas IP alginate capsules exhibit comparable survival to vascularized tissue-confined PEG-RGD capsules in the allogeneic/autoimmune model, non-retrievable capsules within the IP space present safety concerns due to potential fibrotic responses and undesirable capsule adhesion to vital organs in the abdominal cavity (8–10, 12). Therefore, reduced size PEG-RGD capsules enabling confinement to a defined transplant site may reduce long term complications of encapsulated islet therapy and enable graft retrievability.
Conclusion
We demonstrate a synthetic PEG-based microfluidic islet encapsulation method with reduced size and comparable permselectivity to alginate encapsulation. Reduced capsule size facilitates transplantation within isolated and retrievable transplant sites for enhanced safety and translatability of this therapy, and a vasculogenic hydrogel delivery vehicle enables capsule integration with host vasculature. Reduced diffusional distances resulting from smaller capsule size and enhanced vascular proximity resulted in improved encapsulated islet function in a single pancreatic donor syngeneic transplant model. PEG-RGD-encapsulated islets integrated within host tissue reduced immune-mediated islet destruction to a comparable degree as conventional alginate capsules, with detectable islet signal for 9 weeks. As such, this strategy may represent an attractive alternative to conventional alginate encapsulated islet transplantation due to enhanced safety and the potential for improved long-term islet viability.
Supplementary Material
Acknowledgments
The authors thank the Juvenile Diabetes Research Foundation (JDRF) for their support through the encapsulation consortium (2-SRA-2014–287-Q-R, 3-SRA-2015–38-Q-R) and Postdoctoral Fellowship Award, and the National Institutes of Health (NIH, U01 AI132817) for their financial support.
Abbreviations:
- PEG
poly(ethylene glycol)
- IPGTT
intraperitoneal glucose tolerance test
- EFP
epididymal fat pad
- NOD
non obese diabetic
- GAT
gonadal adipose tissue
- T1D
Type 1 Diabetes
- IEQ
islet equivalents
- IP
intraperitoneal
- DDT
dithiothreitol
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care 2011;34:S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tao B, Pietropaolo M, Atkinson M, Schatz D, Taylor D. Estimating the cost of type 1 diabetes in the US: a propensity score matching method. PLoS One 2010;5:e11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruni A, Gala-Lopez B, Pepper AR, Abualhassan NS, Shapiro AJ. Islet cell transplantation for the treatment of type 1 diabetes: recent advances and future challenges. Diabetes Metab Syndr Obes 2014;7:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan EA, Paty BW, Senior PA, Shapiro AJ. Risks and side effects of islet transplantation. Curr Diab Rep 2004;4:304–309 [DOI] [PubMed] [Google Scholar]
- 5.Orive G, Hernández RM, Gascón AR, Calafiore R, Chang TM, De Vos P, Hortelano G, Hunkeler D, Lacík I, Shapiro AJ. Cell encapsulation: promise and progress. Nat Med 2003;9:104–107 [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann H, Shirley SG, Zimmermann U. Alginate-based encapsulation of cells: past, present, and future. Curr Diab Rep 2007;7:314–320 [DOI] [PubMed] [Google Scholar]
- 7.Sun Y, Ma X, Zhou D, Vacek I, Sun AM. Normalization of diabetes in spontaneously diabetic cynomologus monkeys by xenografts of microencapsulated porcine islets without immunosuppression. J Clin invest 1996;98:1417–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott R, Escobar L, Tan P, Garkavenko O, Calafiore R, Basta P, Vasconcellos A, Emerich D, Thanos C, Bambra C. Intraperitoneal alginate-encapsulated neonatal porcine islets in a placebo-controlled study with 16 diabetic cynomolgus primates. Transplant Proc 2005;37:3505–3508. [DOI] [PubMed] [Google Scholar]
- 9.Elliott RB, Escobar L, Tan PL, Muzina M, Zwain S, Buchanan C. Live encapsulated porcine islets from a type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation 2007;14:157–161 [DOI] [PubMed] [Google Scholar]
- 10.Tuch BE, Keogh GW, Williams LJ, Wu W, Foster JL, Vaithilingam V, Philips R. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care 2009;32:1887–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calafiore R, Basta G, Luca G, Lemmi A, Montanucci MP, Calabrese G, Racanicchi L, Mancuso F, Brunetti P. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes: first two cases. Diabetes Care 2006;29:137–138 [DOI] [PubMed] [Google Scholar]
- 12.Jacobs-Tulleneers-Thevissen D, Chintinne M, Ling Z, Gillard P, Schoonjans L, Delvaux G, Strand BL, Gorus F, Keymeulen B, Pipeleers D. Sustained function of alginate-encapsulated human islet cell implants in the peritoneal cavity of mice leading to a pilot study in a type 1 diabetic patient. Diabetologia 2013;56:1605–1614 [DOI] [PubMed] [Google Scholar]
- 13.Soon-Shiong P, Heintz RE, Merideth N, Yao QX, Yao Z, Zheng T, Murphy M, Moloney MK, Schmehl M, Harris M. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet 1994;343:950–951 [DOI] [PubMed] [Google Scholar]
- 14.Markmann JF, Deng S, Huang X, Desai NM, Velidedeoglu EH, Lui C, Frank A, Markmann E, Palanjian M, Brayman K. Insulin independence following isolated islet transplantation and single islet infusions. Ann Surg 2003;237:741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scharp DW, Marchetti P. Encapsulated islets for diabetes therapy: history, current progress, and critical issues requiring solution. Adv Drug Deliv Rev 2014;67:35–73 [DOI] [PubMed] [Google Scholar]
- 16.Weaver JD, Headen DM, Aquart J, Johnson CT, Shea LD, Shirwan H, García AJ. Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites. Sci Adv 2017;3:e1700184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weaver JD, Headen DM, Hunckler MD, Coronel MM, Stabler CL, García AJ. Design of a vascularized synthetic poly (ethylene glycol) macroencapsulation device for islet transplantation. Biomaterials 2018;172:54–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Headen DM, Aubry G, Lu H, García AJ. Microfluidic-Based Generation of Size-Controlled, Biofunctionalized Synthetic Polymer Microgels for Cell Encapsulation. Adv Mater 2014;26:3003–3008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Vos P, Marchetti P. Encapsulation of pancreatic islets for transplantation in diabetes: the untouchable islets. Trends Mol Med 2002;8:363–366 [DOI] [PubMed] [Google Scholar]
- 20.Kissler H, Niland J, Olack B, Ricordi C, Hering B, Naji A, Kandeel F, Oberholzer J, Fernandez L, Contreras J. Validation of methodologies for quantifying isolated human islets: an islet cell resources study. Clin Transplant 2010;24:236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mobed-Miremadi M, Djomehri S, Keralapura M, McNeil M. Fickian-based empirical approach for diffusivity determination in hollow alginate-based microfibers using 2D fluorescence microscopy and comparison with theoretical predictions. Materials 2014;7:7670–7688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pipeleers D, Maes E, Van De Winkel M. Glucose-induced insulin release depends on functional cooperation between islet cells. Proc Natl Acad Sci USA 1982;79:7322–7325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe H, Numata K, Ito T, Takagi K, Matsukawa A. Innate immune response in Th1-and Th2-dominant mouse strains. Shock 2004;22:460–466 [DOI] [PubMed] [Google Scholar]
- 24.Buchwald P, Cechin SR, Weaver JD, Stabler CL. Experimental evaluation and computational modeling of the effects of encapsulation on the time-profile of glucose-stimulated insulin release of pancreatic islets. Biomed Eng Online 2015;14:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams SJ, Huang H-H, Kover K, Moore WV, Berkland C, Singh M, Smirnova IS, MacGregor R, Stehno-Bittel L. Reduction of diffusion barriers in isolated rat islets improves survival, but not insulin secretion or transplantation outcome. Organogenesis 2010;6:115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daoud J, Petropavlovskaia M, Rosenberg L, Tabrizian M. The effect of extracellular matrix components on the preservation of human islet function in vitro. Biomaterials 2010;31:1676–1682 [DOI] [PubMed] [Google Scholar]
- 27.Phelps EA, Templeman KL, Thulé PM, García AJ. Engineered VEGF-releasing PEG–MAL hydrogel for pancreatic islet vascularization. Drug Deliv Transl Res 2013;5:125–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duvivier-Kali VF, Omer A, Parent RJ, O’Neil JJ, Weir GC. Complete protection of islets against allorejection and autoimmunity by a simple barium-alginate membrane. Diabetes 2001;50:1698–1705 [DOI] [PubMed] [Google Scholar]
- 29.Lum Z-P, Tai IT, Krestow M, Norton J, Vacek I, Sun AM. Prolonged reversal of diabetic state in NOD mice by xenografts of microencapsulated rat islets. Diabetes 1991;40:1511–1516 [DOI] [PubMed] [Google Scholar]
- 30.Lanza R, Kuhtreiber W, Beyer A, Chick W. Humoral response to encapsulated islets. Transplant. Proc 1994;26:3346. [PubMed] [Google Scholar]
- 31.Gilla RG. Antigen presentation pathways for immunity to islet transplants: relevance to immunoisolation. Ann NY Acad Sci 1999;875:255–260 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.