Abstract
The transcriptional changes in Nicotiana attenuata Torr. ex Wats. elicited by attack from Manduca sexta larvae were previously characterized by mRNA differential display (D. Hermsmeier, U. Schittko, I.T. Baldwin [2001] Plant Physiol 125: 683–700). Because herbivore attack causes wounding, we disentangled wound-induced changes from those elicited by M. sexta oral secretions and regurgitant (R) with a northern analysis of a subset of the differentially expressed transcripts encoding threonine deaminase, pathogen-induced oxygenase, a photosystem II light-harvesting protein, a retrotransposon homolog, and three unknown genes. R extensively modified wound-induced responses by suppressing wound-induced transcripts (type I) or amplifying the wound-induced response (type II) further down-regulating wound-suppressed transcripts (type IIa) or up-regulating wound-induced transcripts (type IIb). It is interesting that although all seven genes displayed their R-specific patterns in the treated tissues largely independently of the leaf or plant developmental stage, only the type I genes displayed strong systemic induction. Ethylene was not responsible for any of the specific patterns of expression. R collected from different tobacco feeding insects, M. sexta, Manduca quinquemaculata, and Heliothis virescens, as well as from different instars of M. sexta were equally active. The active components of M. sexta R were heat stable and active in minute amounts, comparable with real transfer rates during larval feeding. Specific expression patterns may indicate that the plant is adjusting its wound response to efficiently fend off M. sexta, but may also be advantageous to the larvae, especially when R suppress wound-induced plant responses.
Plants are challenged by a variety of abiotic and biotic stresses. The differential activation of distinct sets of genes or gene products in response to these various challenges is referred to as specificity. The plant must be able to recognize the type of challenge and the recognition must be translated into distinct signals to elicit specific responses (Karban and Baldwin, 1997; Stout and Bostock, 1999). Plants clearly distinguish between pathogen- and wound-inducible responses and respond to different genotypes of pathogens in a gene-for-gene specific manner (Baron and Zambryski, 1995; Hammond-Kosack and Jones, 1996; Baker et al., 1997; De Wit, 1997). A gene-for-gene interaction between plants and insects has been reported for aphids (Rossi et al., 1998; Vos et al., 1998), which specialize on single cell types, indicating that some insect-inducible responses can be highly specific. Free-feeding herbivores cause extensive damage, and it is now abundantly clear that plants respond differentially to mechanical damage as compared with herbivore damage (for example Haukioja and Neuvonen, 1985; Hildebrand et al., 1989; Kendall and Bjostad, 1990; Shimoda et al., 1997; Reymond et al., 2000) even when the feeding is carefully mimicked (Baldwin, 1988, 1990). Moreover, feeding by different herbivore species results in different plant responses (for example, Hartley and Lawton, 1987; Felton et al., 1994; Stout et al., 1994; Takabayashi and Dicke, 1996; De Moraes et al., 1998). How plants recognize their herbivores is not completely clear, because chemical and mechanical stimuli may function as herbivore-specific cues, and every herbivore has its own mechanical feeding pattern and saliva composition.
To investigate saliva-specific defense activation, researchers have supplied larval oral secretions and regurgitant (R) to standardized mechanical wounds or intact leaves via the cut leaf petiole. R-induced specificity has been reported as changes in plant hormones (McCloud and Baldwin, 1997; Kahl et al., 2000), direct defenses (McCloud and Baldwin, 1997; Bernasconi et al., 1998), or indirect defenses, namely volatile emissions and their specific attractiveness to parasitoids or predators of the herbivore (Turlings et al., 1990, 1993; Mattiacci et al., 1994; Pare and Tumlinson, 1997), and herbivore performance (Lin et al., 1990). Korth and Dixon (1997) investigated R-induced specificity on a transcriptional level with standardized mechanical wounds and reported that transcripts for proteinase inhibitor II (PIN II) and 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGR) accumulated more rapidly in potato leaves treated with R of Manduca sexta than in mechanically damaged leaves.
Response specificity requires elicitors that are recognized by the plant. A few nonenzymatic (volicitin and related fatty acid-amino acid conjugates) and enzymatic (β-glucosidase, several cell wall degrading, and oxidative enzymes) insect-derived factors have been identified in R (Ma et al., 1990; Mattiacci et al., 1995; Alborn et al., 1997; Miles, 1999; Halitschke et al., 2001).
In this study we examined the interaction of the specialist lepidopteran herbivore M. sexta and its solanaceous host plant Nicotiana attenuata Torr. ex Wats., native to the North American Great Basin Desert. In Nicotiana species, herbivore-specific responses have been reported for hormone signals and secondary metabolites. Several volatile terpenoids are exclusively emitted after herbivory by M. sexta or R treatment (Halitschke et al., 2000). Both jasmonic acid (JA) and ethylene are known to convey specificity. Application of larval R to standardized mechanical wounds amplifies the local wound-inducible accumulation of JA (McCloud and Baldwin, 1997; Schittko et al., 2000), and an R-specific ethylene burst antagonistically interferes with systemic jasmonate-inducible nicotine biosynthesis in the roots (McCloud and Baldwin, 1997; Kahl et al., 2000).
The set of genes we investigated in this study was previously isolated by mRNA differential display of N. attenuata in response to M. sexta feeding (Hermsmeier et al., 2001). Here we analyzed their insect-specificity in response to R-derived cues and the effect of ethylene signaling on R-specific transcript accumulation. We present two comparisons: mechanical simulations of larval feeding with true herbivory; and water (W) applications to standardized mechanical wounds with applications of larval R. To determine the degree to which the herbivore contributes to the specificity, we applied R of different lepidopteran species or different larval instars of M. sexta. Given that defense expression is influenced by plant ontogeny (Takabayashi et al., 1994; Herbers et al., 1996; Stout et al., 1996; Ohnmeiss and Baldwin, 2000) and coordinately regulated with source-sink metabolism (Herbers and Sonnewald, 1998; Roitsch, 1999), we investigated local and systemic R-specific transcript accumulation in different leaf and plant developmental stages to characterize the response on a whole-plant scale.
RESULTS
The eight insect-responsive genes investigated in this study were originally isolated by mRNA differential display of N. attenuata plants that had been subjected to M. sexta larval feeding (Hermsmeier et al., 2001). Five of them share sequence similarity with known genes. One encodes Thr deaminase (TD; pDH14.2), which catalyzes the committed step in iso-Leu biosynthesis by converting Thr to 2-ketobutyrate (Samach et al., 1991; Azevedo et al., 1997). One encodes pathogen-induced oxygenase (PIOX; pDH41.6), which catalyzes α-oxidation of fatty acids to hydroperoxy fatty acids and may be involved in signal generation (Sanz et al., 1998; Hamberg et al., 1999). One shares sequence similarity with the tomato lhb C1 gene encoding a subunit of light-harvesting complex II (LHB C1; pDH61.1; Schwartz et al., 1991), one with the potato mRNA for the kinase cofactor GAL83 (pDH63.5; Lakatos et al., 1999), and one with a protein (T. Sasaki, T. Matsumoto, and K. Yamamoto, unpublished data) similar to the rice retrotransposon RIRE1 (pDH25.4; Noma et al., 1997; Hermsmeier et al., 2001).
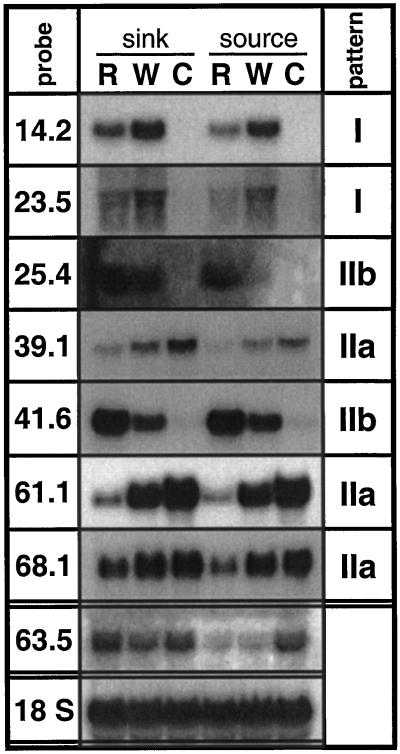
We monitored specific transcript accumulation in response to R by northern analysis. Two types of specific responses were observed. Transcript levels of TD (pDH14.2) and an unknown gene encoded by pDH23.5 were lower in leaves treated with R than in leaves treated with W (Fig. 1). Hence, for these genes the wound-induced transcript accumulation was reduced by R (type I genes). In contrast, the wound-induced response of the other five genes was amplified by R (type II genes). In this group of genes two subgroups could be distinguished: those transcripts that were down-regulated by wounding and were further suppressed by R (pDH39.1, the LHB C1 homolog [pDH61.1], and pDH68.1; type IIa), and those that were up-regulated by wounding and were further amplified by R (the retrotransposon homolog [pDH25.4] and PIOX [pDH41.6]; type IIb). Transcript accumulation of the LHB C1 homolog encoded by pDH61.1 was most strongly affected by applications of R (Fig. 1).
Figure 1.
Transcript accumulation in sink and source leaves. Leaves growing at node 1 and node 4 of separate plants with the youngest fully expanded leaf defining node 3 were analyzed. Leaves of five replicate N. attenuata plants were continuously wounded and supplied with W or R from M. sexta larvae for 105 min, creating one row of puncture wounds every 15 min and harvesting 15 min after the final treatment. Untreated leaves were harvested as controls (C). Probe pDH63.5, coding for a GAL83 homolog, was used as a positive indicator of source-sink differences. Hybridization with an 18S rRNA probe demonstrates equal loading. The type of expression pattern (I, IIa, and IIb) is indicated.
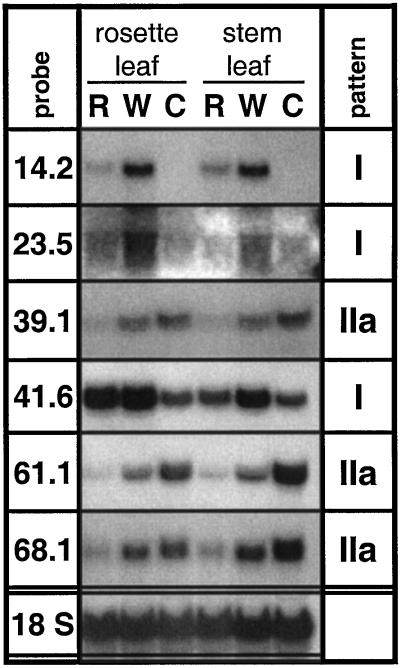
Induction of defense responses may depend on the metabolic state of a leaf, as shown for pDH63.5, coding for the GAL83 homolog, which responded strongly to wounding only in source leaves, but not in sink leaves. GAL83-like proteins form an accessory subunit of SNF1-like protein kinase complexes that may be important regulators of carbon metabolism in plants (Halford and Hardie, 1998). In contrast, for five of the seven genes (TD [pDH 14.2], pDH23.5, pDH39.1, the LHB C1 homolog [pDH61.1], and pDH68.1) R-specific transcript accumulation was independent of the developmental state of the leaf or the plant (Figs. 1 and 2). Apart from minor differences in quantity, similar transcript patterns were found in sink leaves as compared with source leaves of rosette-stage plants (Fig. 1), as well as in source leaves of flowering-stage plants harvested from a rosette or a stem position (Fig. 2). For the retrotransposon homolog (pDH25.4), no bands could be detected by northern analysis of flowering plants. Accumulation of PIOX transcripts (pDH41.6) differed between the experiments, showing a type II pattern in rosette stage plants (Fig. 1), but a type I pattern in flowering plants (Fig. 2). Moreover, constitutive expression of PIOX in control leaves was higher in flowering plants.
Figure 2.
Transcript accumulation in rosette or stem leaves of flowering plants. Leaves (being two positions older than the youngest rosette leaf or two positions younger than the first stem-born leaf) of five replicate N. attenuata plants were continuously wounded and supplied with W or R from M. sexta larvae for 80 min, creating one row of puncture wounds every 20 min and harvesting 20 min after the final treatment. Untreated leaves were harvested as controls (C). Hybridization with an 18S rRNA probe demonstrates equal loading. The type of expression pattern (I, IIa) is indicated.
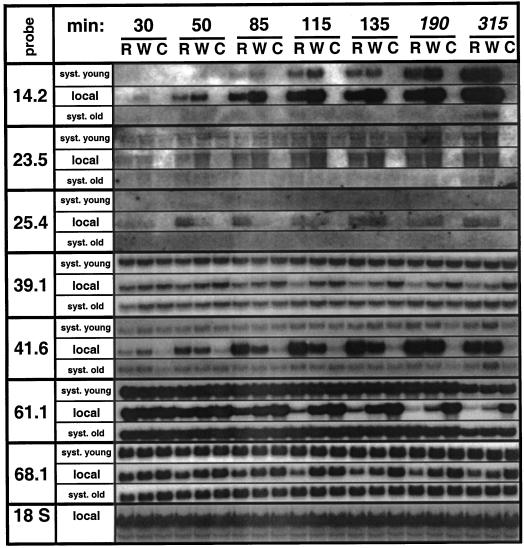
In a kinetic experiment (Fig. 3), the earliest changes in transcript levels were detectable for TD (pDH14.2), pDH23.5, and PIOX (pDH41.6). Within 50 min after the first treatment, R-specific transcript accumulation of all seven genes was detectable in the treated leaf. Accumulation of PIOX transcripts (pDH41.6) switched from a type I pattern 30 min after the first treatment to a type II pattern at every subsequent harvest (Fig. 3). To assess the persistence of the R-specific responses we harvested plants five times during the treatment period (Fig. 3, t = 30 to 135 min), as well as twice, 1 and 3 h, after the last treatment (Fig. 3, t = 190 min and t = 315 min, respectively). The R-specific amplification of the transcript accumulation of the retrotransposon homolog (pDH25.4) was extremely transient, being restricted to t = 50 min and t = 85 min. The transcriptional responses of pDH39.1, the LHB C1 homolog (pDH61.1), and pDH68.1 were long lasting, whereas for TD (pDH14.2), pDH23.5, and PIOX (pDH41.6) the impact of R ceased soon after the treatments had been stopped, leading to a reduced difference in transcript levels between leaves treated with R and leaves treated with W (Fig. 3).
Figure 3.
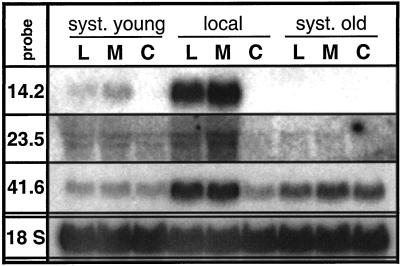
Local and systemic transcript accumulation in rosette stage plants. The node 4 leaf of four replicate N. attenuata plants was wounded and supplied with W or R from M. sexta larvae every 15 to 20 min, creating one row of puncture wounds at each harvest. The treated leaf (local), all leaves younger (syst. young), and all leaves older (syst. old) than the node 4 leaf were harvested at eight different time intervals (min) after the first treatment when they had received one, two, four, six, or eight (the last three harvests) rows of puncture wounds, respectively. Harvests at 30, 50, 85, 115, and 135 min were during the continuous treatment period, whereas the 190- and 315-min harvests were taken 1 and 3 h after the final treatment event, respectively. Untreated plants were harvested as controls (C). Hybridization with an 18S rRNA probe demonstrates equal loading.
It is interesting that although all genes showed strong R-specific patterns of expression in the treated leaf, only the type I genes, TD (pDH14.2), and pDH23.5 showed strong systemic expression. This systemic expression was more dramatic in leaves younger than the treated leaf, but expression in older leaves was also discernible (Fig. 3). Systemic expression of type II genes was weak for PIOX (pDH41.6) and absent for all others (Fig. 3).
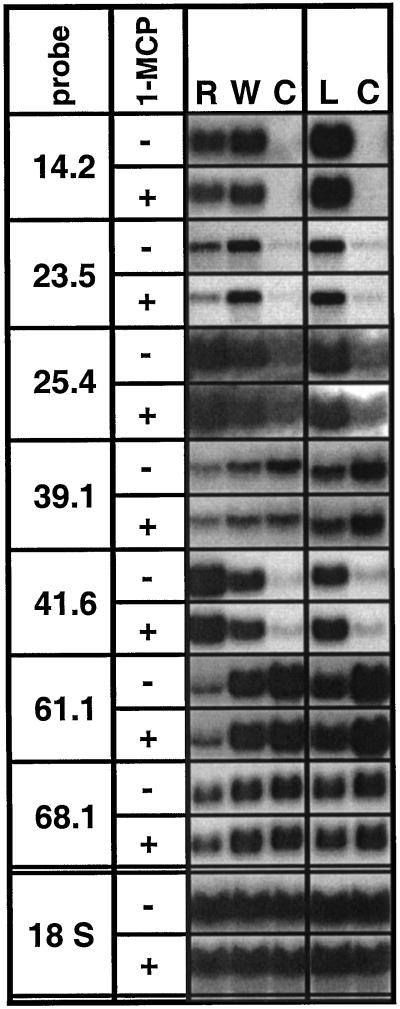
Differential transcript accumulation in response to R treatment is unlikely to be mediated by ethylene since it was not affected by treating plants with 1-methylcyclopropene (1-MCP), a competitive inhibitor at the plant's ethylene receptors (Fig. 4). In contrast, for TD (pDH14.2), ethylene appears to have a weak stimulatory effect on transcript up-regulation. It is interesting that only R-induced transcript levels were diminished by 1-MCP-treatment for pDH23.5, resulting in an increased difference between R- and W-treated leaves (Fig. 4).
Figure 4.
Transcript accumulation in the absence (−) or presence (+) of 1-MCP, the competitive inhibitor of ethylene receptors. The node 4 leaf of five replicate rosette-stage N. attenuata plants was continuously wounded and supplied with W or R from M. sexta larvae for 80 min, creating one row of puncture wounds every 20 min and harvesting 20 min after the final treatment. Alternatively, seven 2nd and 3rd instar M. sexta larvae (L) were allowed to systemically feed on four replicate plants for 4 h. Untreated node 4 leaves or plants were harvested as controls (C). Hybridization with an 18S rRNA probe demonstrates equal loading.
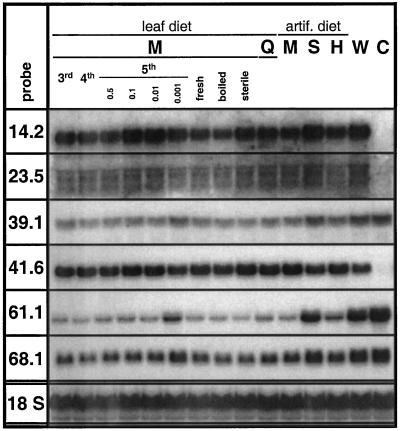
Specific transcript accumulation was also found for other lepidopteran species (Fig. 5). R from the tomato hornworm Manduca quinquemaculata and the tobacco budworm Heliothis virescens were as active as R from the tobacco hornworm M. sexta, whereas the response to R from Spodoptera littoralis was hardly different from a wound response (Fig. 5). However, activity of S. littoralis R was found to vary. In a replicate experiment using a different batch of S. littoralis R it was as active as R collected from M. sexta (data not shown). R of M. sexta from all instars tested induced a specific response, and activity did not depend on the food M. sexta larvae had been reared on. Storage at −80°C did not affect the activity of the R as indicated by the transcript accumulation in response to application of freshly collected R. Also, sterile filtration and boiling did not interfere with R activity, except for TD (pDH14.2), in which case boiling enhanced its specific transcript down-regulation slightly (Fig. 5).
Figure 5.
Transcript accumulation in response to different R solutions. The node 4 leaf of four replicate rosette-stage N. attenuata plants was continuously wounded and supplied with W or different R solutions for 80 min, creating one row of puncture wounds every 20 min and harvesting 20 min after the final treatment. R from Manduca quinquemaculata (Q), Spodoptera littoralis (S), Heliothis virescens (H) and 3rd to 5th instar M. sexta (M) larvae were tested, as well as boiled, sterile filtered, and freshly collected M. sexta R. Untreated node 4 leaves were harvested as controls (C). Hybridization with an 18S rRNA probe demonstrates equal loading.
Even if only small amounts of oral secretions are transferred during the feeding process, they are likely to be sufficient for specific induction. Even when R was diluted to 1/1,000 with W, specific transcript accumulation was still observed for all genes, even though clearly less pronounced than in response to more concentrated R solutions for type II genes [pDH39.1, PIOX (pDH41.6), the LHB C1 homolog (pDH61.1), and pDH68.1]. It is surprising that suppression of TD (pDH14.2) transcript accumulation by R diluted to 1/1,000 was more pronounced than by R diluted to 1/100 (Fig. 5). Transcript levels of the retrotransposon homolog (pDH25.4) were below the detection limit and are not shown.
With a M. sexta mandible fixed to a toothpick we simulated the spatial and temporal pattern and amount of damage caused by larval feeding (Fig. 6). As expected for type I genes, TD (pDH14.2) and pDH23.5, in which the wound-induced response is suppressed by R from the larvae, the response to larval feeding was slightly weaker than the response to mandibular damage. This pattern is probably not caused by unequal damage intensities since PIOX (pDH41.6) expression levels did not differ between the two treatments (Fig. 6). Specific transcript accumulation of PIOX may switch from a type I to a type II pattern (see Fig. 3) and is therefore difficult to monitor when harvesting at only one time point. Transcriptional changes in response to local larval feeding seemed to be slower or weaker than in response to our puncture wounding procedure. For pDH39.1, the LHB C1 homolog (pDH61.1), and pDH68.1, no response was observed within the 3.5-h time period of the feeding experiment and transcript levels of the retrotransposon homolog (pDH25.4) were below the detection limit of our northern analysis.
Figure 6.
Transcript accumulation in response to real and simulated herbivory. On seven replicate N. attenuata plants, one 3rd instar M. sexta larva (L) was allowed to feed on the node 4 source leaf for 150 to 180 min. Feeding was simultaneously mimicked with larval mandibles (M). The damaged leaf (local), as well as all leaves younger (syst. young) and all leaves older (syst. old) than the node 4 leaf were analyzed. Untreated node 4 leaves were harvested as controls (C). Hybridization with an 18S rRNA probe demonstrates equal loading.
DISCUSSION
Plants need to discriminate between different environmental challenges to optimize the allocation of their resources to growth, defense, and reproduction. Phytophagous insects display a great diversity of feeding modes and life histories, and chemical and physical attributes of herbivory could be used by the plant to distinguish attack from different insects. Disentangling these two attributes of herbivory is difficult. To exactly mimic the physical attributes may not be possible and the quantity of chemical signals transferred during feeding is unknown. In this study we used two experimental approaches to identify specificity to chemical cues contained in R. We analyzed transcript accumulation in response to standardized mechanical wounds treated with W or larval R. This approach, which controls exactly for the mechanical component, was complemented with a dilution series experiment, which revealed that a specific response is induced even if only very small amounts of oral secretions are transferred during feeding. The specificity in transcript accumulation corroborated earlier work on endogenous JA accumulation after applications of dilute R (Schittko et al., 2000). In a second approach herbivore-induced transcript accumulation was compared with transcript accumulation in response to a careful mechanical simulation of feeding using larval mandibles.
Both experimental approaches revealed that R- contained chemical cues extensively modify the plant's wound response. It is interesting that antagonistic and synergistic effects were found. The wound response of type I genes (pDH14.2 encoding TD and pDH23.5) was repressed, whereas the wound response of type II genes was amplified with wound-suppressed transcripts being further down-regulated (type IIa; pDH39.1, pDH61.1 encoding the LHB C1 homolog, and pDH68.1) and wound-induced transcripts being further up-regulated (type IIb; pDH25.4 encoding the RIRE1 homolog).
Similar experimental attempts were made to identify R specificity in potato plants. Transcript accumulation of proteinase inhibitor II and HMGR induced by M. sexta R was found to be faster than wound-induced transcript accumulation (Korth and Dixon, 1997).
Larval feeding is always accompanied by mechanical tissue damage, and accordingly we found R responses and wound responses to qualitatively overlap. The overlap of responses has been proposed to increase with an attendant decrease in specificity in the sequence of events from recognition downstream to the phenotypic changes that influence attackers and plant fitness (Paul et al., 2000). However, here we clearly demonstrate that R-specific transcript accumulation was quantitatively different from wound-induced transcript accumulation.
Because the fitness value of plant parts changes dramatically over ontogeny, evolutionary theory predicts that resource allocation to defense should also change (McKey, 1974; Feeny, 1976). In contrast to this prediction, a majority of genes displayed R-specific transcript accumulation at all plant developmental stages investigated, suggesting that they may encode central defense functions in N. attenuata. Transcripts of the retrotransposon homolog (pDH25.4) were not detectable in blots of flowering plants, and PIOX (pDH41.6) displayed type I and type IIb transcript accumulation patterns, depending on the time interval between treatment and harvest and on plant developmental stage.
The time course of local and systemic transcript accumulation suggested that each group of genes may share common regulatory elements since the respective genes were coordinately expressed. Type I genes including PIOX (pDH41.6), TD (pDH14.2), and pDH23.5 were systemically expressed and most stringently controlled by R; specific transcript accumulation was initiated shortly after the first treatment, but also vanished shortly after treatments had been stopped. Specific expression patterns of type IIa genes pDH39.1, the LHB C1 homolog (pDH61.1), and pDH68.1 were restricted to the treated leaf and waxed and waned more slowly. For the retrotransposon homolog (pDH25.4) specificity was observed only transiently at the beginning of the continuous treatment period.
The fine tuning of defense gene expression may result from antagonistic or synergistic crosstalk between salicylic acid, JA, and ethylene (Reymond and Farmer, 1998; Genoud and Metraux, 1999). In this study the impact of ethylene on R-specific transcript accumulation of N. attenuata plants was tested by inhibiting their ethylene perception with 1-MCP. 1-MCP had successfully been used to demonstrate that ethylene, which is specifically released by N. attenuata in response to M. sexta R treatment, suppresses JA-induced nicotine production in the roots (Kahl et al., 2000). Both type I genes, TD (pDH14.2) and pDH23.5, are inducible by methyl jasmonate (Hildmann et al., 1992; Peña-Cortés et al., 1993; Samach et al., 1995; Hermsmeier et al., 2001), but ethylene did not mediate the antagonistic effect of R on their wound-induced transcript accumulation. Ethylene was also not involved in type II gene expression. R-dependent amplification of type II genes parallels the R-dependent amplification of the local wound-inducible accumulation of JA in leaves of N. attenuata. This parallel suggests a specific signaling role for JA, although only two of the five type II genes, PIOX (pDH41.6) and the LHB C1 homolog (pDH61.1), have been found to be responsive to jasmonates (Sanz et al., 1998; Hermsmeier et al., 2001).
R from all three tobacco feeding larvae, the two closely related Manduca species and H. virescens were equally active. In a study examining a set of 150 genes, the feeding of two closely related Pieris species, P. rapae and P. brassicae, on Arabidopsis also elicited a similar transcript signature (Reymond et al., 2000). However, tobacco and cotton plants were found to produce distinct blends of volatiles in response to feeding by H. virescens and Helicoverpa zea (De Moraes et al., 1998), suggesting that a plant can distinguish even between closely related species. Although the broad host-plant use of S. littoralis does not naturally include N. attenuata, larval R induced specific expression patterns, however inconsistently. Variation in the active components of the saliva of different insects may account for the variation in the plant responses and the R of S. littoralis needs further characterization. Volicitin was identified from beet armyworm R (Alborn et al., 1997). Related fatty acid-amino acid conjugates have been reported from all species tested and constitute active components in R of M. sexta and M. quinquemaculata (Pohnert et al., 1999; Halitschke et al., 2001).
The R-specific plant response was independent of larval development and food. All instars of M. sexta induced the specific response as was found with JA induction (Schittko et al., 2000). In accordance with earlier studies on M. sexta R (Korth and Dixon, 1997; Schittko et al., 2000), activity did not depend on the food source, since R from larvae reared on artificial diet were as active as those from plant-fed larvae. Sterile filtration did not interfere with R activity, and boiling of R, which had been found to increase specific PIN II and HMGR transcript accumulation in potato (Korth and Dixon, 1997), slightly intensified specific down-regulation of TD (pDH14.2) only. The active components in M. sexta R must also be heat stable (Korth and Dixon, 1997).
Wound-induced transcript accumulation of type I genes, with PIOX (pDH41.6) and TD (pDH14.2) putatively being involved in defense signaling and accumulation (Samach et al., 1995; Hamberg et al., 1999; Van der Hoeven and Steffens, 2000; Hermsmeier et al., 2001 and refs. therein), was repressed by R treatment. In the process of plant-insect co-evolution, suppression of plant defenses might complement the detoxification capacity of herbivorous insects (Felton and Eichenseer, 1999; Reymond et al., 2000). The wound response of type II genes was amplified by R treatment. Wound-suppressed transcript accumulation of lhb C1 (pDH61.1) and two unknown genes (pDH39.1 and pDH68.1) was further down-regulated by R, whereas wound-induced transcript accumulation of PIOX (pDH41.6) and the retrotransposon homolog (pDH25.4) was further up-regulated. Down-regulation of photosynthesis is known to be coordinated with the up-regulation of defenses against pathogens or insects (Ehness et al., 1997; Roitsch, 1999; Hermsmeier et al., 2001). The amplified transcriptional response observed for type II genes may indicate that the plant “recognizes” the attacking insect and responds with intensified defense-gene activation. In short, the plant might adjust its wound response to effectively fend off the attacking insect, and the insect might in turn rely on being able to suppress it. It is interesting that compounds that might serve important digestive functions in the larvae, the detergent-like fatty acid-amino acid conjugates, were found to be sufficient to mediate the observed transcriptional responses (Halitschke et al., 2001). The significance of these R-specific transcriptional changes for the accumulation and activity of the encoded proteins eventually needs to be determined and the respective protein functions need to be elucidated to evaluate their impact on plant and larval performance in this plant-insect interaction.
MATERIALS AND METHODS
Plant Growth
Inbred line Nicotiana attenuata Torr. ex Wats. (originating from the DI ranch in southwest Utah, T40S R19W, section 10, 1988) plants were cultivated as described by Hermsmeier et al. (2001). Upon transfer into no-nitrogen hydroponic solution (Baldwin and Schmelz, 1994), each plant was supplied with 2 mL of 1 m KNO3, followed by another 1 mL 6 to 7 d later. Flowering plants additionally received 1 mL of 1 m KNO3 on d 13 and 24 after transfer. Experiments were initiated 1 to 3 d after the last nitrogen addition when plants were in the late rosette stage of growth or in the early flowering stage.
Insect Rearing and R Collection
Manduca sexta Linnaeus and Manduca quinquemaculata Haworth larvae were reared on foliage of N. attenuata. We tested R from different instars of M. sexta, as well as a dilution series of the last (5th) instar R in W. To eliminate enzyme activity, R from 5th instar M. sexta were incubated at 100°C for 20 min. To exclude elicitation by microbes, we tested sterile filtered R from 5th instar larvae. R from M. quinquemaculata were collected from 5th instar larvae. R from the last 3 instars of Spodoptera littoralis Boisduval and Heliothis virescens Fabricius were collected from larvae reared on artificial diet (modified from Bell and Joachim, 1976) after hatching. We also tested 5th instar M. sexta R from larvae fed on artificial diet following their last ecdysis.
R were collected with microcapillaries or teflon tubing connected to a vacuum and stored under argon at −80°C. To control for storage induced changes in R activity, we compared freshly collected R from 4th instar M. sexta larvae with frozen R. If not specified, R were always diluted 1:1 (v/v) with W. Except for the most dilute R solution, which was too dilute to be measured with pH paper, all R solutions had a pH value of approximately 8.5.
Experimental Design
R transfer from feeding larvae always involves mechanical tissue damage. We replaced the mechanical component of larval feeding with a standardized puncture-wound treatment and immediately supplied the wound sites with different test solutions. Transcript accumulation was compared between leaves that received R and leaves treated with autoclaved W to reveal how insect-specific chemical cues alter the transcriptional responses induced by mechanical damage. We used a fabric pattern wheel (Dritz, Spartanburg, SC) to create one row of puncture wounds, parallel to the mid rib, every 15 to 20 min and added 5 μL of the respective test solution to the wound sites. A total of five rows (flowering plants experiment [Fig. 2], ethylene experiment [Fig. 4], and different R experiment [Fig. 5]) or eight rows (source-sink experiment [Fig. 1] and kinetics [Fig. 3]) of puncture wounds were generated per leaf. The treated leaves were harvested 15 to 20 min after the last treatment event except in the kinetic experiment (Fig. 3), in which plants were continuously harvested 30, 50, 85, 115, 135, 190, or 315 min after the first treatment when they had received 1, 2, 4, 6, or 8 (the last three harvests) rows of puncture wounds, respectively.
Rosette-stage plants were treated on fully expanded node 4 source leaves of four to five replicate plants with the youngest fully expanded leaf defining node 3. To compare the responses of source and sink leaves, leaves growing at node 1 and node 4 from separate plants were compared (Fig. 1). We examined transcript accumulation in the treated leaves in all experiments of this study. In addition, for each harvest of the kinetic experiment (Fig. 3), we examined systemic responses by harvesting separately all (untreated) leaves younger and older than the treated leaf at node 4. Two different types of source leaves were treated on separate flowering-stage plants, one being two positions older than the youngest rosette leaf and one being two positions younger than the first stem-born leaf (Fig. 2).
We used 1-MCP, a competitive inhibitor of ethylene at the receptors of the hormone, to inhibit the plant's ethylene perception (Sisler et al., 1996). During 1-MCP exposure, plants were enclosed in 18.5-L plastic containers. Two vials with 1-MCP (0.5 g in 8 mL of 0.75% [w/v] NaOH, 0.75% [w/v] KOH) were placed in each container, one during the 9 h preceding the treatments and one during the course of the treatments. Seven 2nd and 3rd instar larvae were allowed to feed on all leaves for 4 h (Fig. 4).
To examine the effects of saliva-derived cues during larval feeding, one needs to precisely control for the mechanical attributes of the damage caused by feeding (Fig. 6). One 3rd instar larva that had starved for at least 1 h was allowed to feed on the node 4 leaf of seven replicate plants. Larvae initiated a feeding bout approximately every 20 min, six to nine times during the experiment and consumed about 2 cm2 of leaf tissue during each bout. In parallel we used a larval mandible glued to the tip of a toothpick in combination with a plastic support to carefully mimic the feeding bouts. We “cut” node 4 leaves of separate plants at the corresponding location on the leaf, for a corresponding time period, trying to remove a corresponding area of leaf tissue with a similar number of “bites.” The treated leaf and leaves both younger and older than the treated leaf at node 4 (to examine systemic responses) were separately harvested 150 to 180 min after larvae had started feeding and in every case 15 to 20 min after the last feeding bout (Fig. 6).
Molecular Techniques
Total cellular RNA was isolated according to Pawlowski et al. (1994). Gel electrophoresis of RNA, northern blotting, probe labeling, and hybridizations were performed as described in Hermsmeier et al. (2001). GenBank accession numbers of the template sequences are AW191811 (pDH14.2), AW191815 (pDH23.5), AW191816 (pDH25.4), AW191819 (pDH39.1), AW191821 (pDH41.6), AW191826 (pDH61.1), AW191827 (pDH63.5), AW191828 (pDH64.4, 18S), and AW191830 (pDH68.1).
ACKNOWLEDGMENTS
We thank Dieter Spiteller and Dr. Ursula Röse for providing S. littoralis and H. virescens larvae, and editor Carlos Ballaré, two anonymous reviewers, and Rayko Halitschke for improving the manuscript.
Footnotes
This work was supported by the Max Planck Gesellschaft.
LITERATURE CITED
- Alborn HT, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH. An elicitor of plant volatiles from beet armyworm oral secretion. Science. 1997;276:945–949. [Google Scholar]
- Azevedo RA, Arruda P, Turner WL, Lea PJ. The biosynthesis and metabolism of the aspartate derived amino acids in higher plants. Phytochemistry. 1997;46:395–419. doi: 10.1016/s0031-9422(97)00319-1. [DOI] [PubMed] [Google Scholar]
- Baker B, Zambryski P, Staskawicz B, Dinesh-Kumar SP. Signaling in plant-microbe interactions. Science. 1997;276:726–733. doi: 10.1126/science.276.5313.726. [DOI] [PubMed] [Google Scholar]
- Baldwin IT. The alkaloidal responses of wild tobacco to real and simulated herbivory. Oecologia. 1988;77:378–381. doi: 10.1007/BF00378046. [DOI] [PubMed] [Google Scholar]
- Baldwin IT. Herbivory simulations in ecological research. Trends Ecol Evol. 1990;5:91–93. doi: 10.1016/0169-5347(90)90237-8. [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Schmelz EA. Constraints on an induced defense: the role of leaf area. Oecologia. 1994;97:424–430. doi: 10.1007/BF00317335. [DOI] [PubMed] [Google Scholar]
- Baron C, Zambryski PC. The plant response in pathogenesis, symbiosis, and wounding: variations on a common theme? Annu Rev Genet. 1995;29:107–129. doi: 10.1146/annurev.ge.29.120195.000543. [DOI] [PubMed] [Google Scholar]
- Bell RA, Joachim FG. Techniques for rearing laboratory colonies of tobacco hornworms and pink bollworms. Ann Entomol Soc Am. 1976;69:365–373. [Google Scholar]
- Bernasconi ML, Turlings TCJ, Ambrosetti L, Bassetti P, Dorn S. Herbivore-induced emissions of maize volatiles repel the corn leaf aphid, Rhopalosiphum maidis. Entomol Exp Appl. 1998;87:133–142. [Google Scholar]
- De Moraes CM, Lewis WJ, Pare PW, Alborn HT, Tumlinson JH. Herbivore-infested plants selectively attract parasitoids. Nature. 1998;393:570–573. [Google Scholar]
- De Wit PJGM. Pathogen avirulence and plant resistance: a key role for recognition. Trends Plant Sci. 1997;2:452–458. [Google Scholar]
- Ehness R, Ecker M, Godt DE, Roitsch T. Glucose and stress independently regulate source and sink metabolism and defense mechanisms via signal transduction pathways involving protein phosphorylation. Plant Cell. 1997;9:1825–1841. doi: 10.1105/tpc.9.10.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeny P. Plant apparency and chemical defense. In: Wallace JW, Mansell RL, editors. Biochemical Interactions between Plants and Insects. New York: Plenum Press; 1976. pp. 1–40. [Google Scholar]
- Felton GW, Eichenseer H. Herbivore saliva and its effects on plant defense against herbivores and pathogens. In: Agrawal AA, Tuzun S, Bent E, editors. Induced Plant Defenses against Pathogens and Herbivores: Biochemistry, Ecology, and Agriculture. APS Press, St. Paul. 1999. pp. 19–36. [Google Scholar]
- Felton GW, Summers CB, Mueller AJ. Oxidative responses in soybean foliage to herbivory by bean leaf beetle and three-cornered alfalfa hopper. J Chem Ecol. 1994;20:639–650. doi: 10.1007/BF02059604. [DOI] [PubMed] [Google Scholar]
- Genoud T, Metraux J. Crosstalk in plant cell signaling: structure and function of the genetic network. Trends Plant Sci. 1999;4:503–507. doi: 10.1016/s1360-1385(99)01498-3. [DOI] [PubMed] [Google Scholar]
- Halford NG, Hardie DG. SNF1-related protein kinases: global regulators of carbon metabolism in plants. Plant Mol Biol. 1998;37:735–748. doi: 10.1023/a:1006024231305. [DOI] [PubMed] [Google Scholar]
- Halitschke R, Kessler A, Kahl J, Lorenz A, Baldwin IT. Eco-physiological comparison of direct and indirect defenses in Nicotiana attenuata. Oecologia. 2000;124:408–417. doi: 10.1007/s004420000389. [DOI] [PubMed] [Google Scholar]
- Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol. 2001;125:711–717. doi: 10.1104/pp.125.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M, Sanz A, Castresana C. α-Oxidation of fatty acids in higher plants: identification of a pathogen-inducible oxygenase (PIOX) as an alpha-dioxygenase and biosynthesis of 2-hydroperoxylinolenic acid. J Biol Chem. 1999;274:24503–24513. doi: 10.1074/jbc.274.35.24503. [DOI] [PubMed] [Google Scholar]
- Hammond-Kosack KE, Jones JDG. Resistance gene-dependent plant defense responses. Plant Cell. 1996;8:1773–1791. doi: 10.1105/tpc.8.10.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley SE, Lawton JH. Effects of different types of damage on the chemistry of birch foliage and the responses of birch feeding insects. Oecologia. 1987;74:432–437. doi: 10.1007/BF00378941. [DOI] [PubMed] [Google Scholar]
- Haukioja E, Neuvonen S. Induced long-term resistance of birch foliage against defoliators: defensive or incidental? Ecology. 1985;66:1303–1308. [Google Scholar]
- Herbers K, Meuwly P, Metraux J, Sonnewald U. Salicylic acid-independent induction of pathogenesis-related protein transcripts by sugars is dependent on leaf developmental stage. FEBS Lett. 1996;397:239–244. doi: 10.1016/s0014-5793(96)01183-0. [DOI] [PubMed] [Google Scholar]
- Herbers K, Sonnewald U. Altered gene expression brought about inter- and intracellulary formed hexoses and its possible implications for plant-pathogen interactions. J Plant Res. 1998;111:323–328. [Google Scholar]
- Hermsmeier D, Schittko U, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata: I. Large-scale changes in the accumulation of growth- and defense-related plant mRNAs. Plant Physiol. 2001;125:683–700. doi: 10.1104/pp.125.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand DF, Rodriguez JG, Legg CS, Brown GC, Bookjans G. The effects of wounding and mite infestation on soybean leaf lipoxygenase levels. Z Naturforsch (C) 1989;44:655–659. [Google Scholar]
- Hildmann T, Ebneth M, Peña-Cortés H, Sanchez-Serrano JJ, Willmitzer L, Prat S. General roles of abscisic and jasmonic acids in gene activation as a result of mechanical wounding. Plant Cell. 1992;4:1157–1170. doi: 10.1105/tpc.4.9.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl J, Siemens DH, Aerts RJ, Gabler R, Kuhnemann F, Preston CA, Baldwin IT. Herbivore-induced ethylene suppresses a direct defense but not a putative indirect defense against an adapted herbivore. Planta. 2000;210:336–342. doi: 10.1007/PL00008142. [DOI] [PubMed] [Google Scholar]
- Karban R, Baldwin IT. Induced Responses to Herbivory. Chicago: The University of Chicago Press; 1997. [Google Scholar]
- Kendall DM, Bjostad LB. Phytochemical ecology: herbivory by Thrips tabaci induces greater ethylene production in intact onions than mechanical damage alone. J Chem Ecol. 1990;16:981–991. doi: 10.1007/BF01016506. [DOI] [PubMed] [Google Scholar]
- Korth KL, Dixon RA. Evidence for chewing insect-specific molecular events distinct from a general wound response in leaves. Plant Physiol. 1997;115:1299–1305. doi: 10.1104/pp.115.4.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos L, Klein M, Hoefgen R, Banfalvi Z. Potato StubSNF1 interacts with StubGAL83: a plant protein kinase complex with yeast and mammalian counterparts. Plant J. 1999;17:569–574. doi: 10.1046/j.1365-313x.1999.00406.x. [DOI] [PubMed] [Google Scholar]
- Lin H, Kogan M, Fischer D. Induced resistance in soybean to the Mexican bean beetle (Coleoptera: Coccinellidae): comparisons of inducing factors. Environ Entomol. 1990;19:1852–1857. [Google Scholar]
- Ma R, Reese JC, Black IVWC, Bramel-Cox P. Detection of pectinesterase and polygalacturonase from salivary secretions of living greenbugs, Schizaphis graminum (Homoptera: Aphididae) J Insect Physiol. 1990;36:507–512. [Google Scholar]
- Mattiacci L, Dicke M, Posthumus MA. Induction of parasitoid attracting synomone in brussel sprouts plants by feeding of Pieris brassicae larvae: role of mechanical damage and herbivore elicitor. J Chem Ecol. 1994;20:2229–2247. doi: 10.1007/BF02033199. [DOI] [PubMed] [Google Scholar]
- Mattiacci L, Dicke M, Posthumus MA. β-Glucosidase: an elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc Natl Acad Sci USA. 1995;92:2036–2040. doi: 10.1073/pnas.92.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloud ES, Baldwin IT. Herbivory and caterpillar regurgitants amplify the wound-induced increases in jasmonic acid but not nicotine in Nicotiana sylvestris. Planta. 1997;203:430–435. [Google Scholar]
- McKey D. Adaptive patterns in alkaloid physiology. Am Nat. 1974;108:305–320. [Google Scholar]
- Miles PW. Aphid saliva. Biol Rev. 1999;74:41–85. [Google Scholar]
- Noma K, Nakajima R, Ohtsubo H, Ohtsubo E. RIRE1, a retrotransposon from wild rice Oryza australiensis. Genes Genet Syst. 1997;72:131–140. doi: 10.1266/ggs.72.131. [DOI] [PubMed] [Google Scholar]
- Ohnmeiss T, Baldwin IT. Optimal defense theory predicts the ontogeny of an induced nicotine defense. Ecology. 2000;81:1765–1783. [Google Scholar]
- Pare PW, Tumlinson JH. De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol. 1997;114:1161–1167. doi: 10.1104/pp.114.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul ND, Hatcher PE, Taylor JE. Coping with multiple enemies: an integration of molecular and ecological perspectives. Trends Plant Sci. 2000;5:220–225. doi: 10.1016/s1360-1385(00)01603-4. [DOI] [PubMed] [Google Scholar]
- Pawlowski K, Kunze R, De Vries S, Bisseling T. Isolation of total, poly(A) and polysomal RNA from plant tissue. In: Gelvin SB, Schilperoort RA, editors. Plant Molecular Biology Manual. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. , Section D5, pp 1–4. [Google Scholar]
- Peña-Cortés H, Albrecht T, Prat S, Weiler EW, Willmitzer L. Aspirin prevents wound-induced gene expression in tomato leaves by blocking jasmonic acid biosynthesis. Planta. 1993;191:123–128. [Google Scholar]
- Pohnert G, Jung V, Haukioja E, Lempa K, Boland W. New fatty acid amides form regurgitant of lepidopteran (Noctuidae, Geometridae) caterpillars. Tetrahedron. 1999;55:11275–11280. [Google Scholar]
- Reymond P, Farmer EE. Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol. 1998;1:404–411. doi: 10.1016/s1369-5266(98)80264-1. [DOI] [PubMed] [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE. Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell. 2000;12:707–719. doi: 10.1105/tpc.12.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitsch T. Source-sink regulation by sugar and stress. Curr Opin Plant Biol. 1999;2:198–206. doi: 10.1016/S1369-5266(99)80036-3. [DOI] [PubMed] [Google Scholar]
- Rossi M, Goggin FL, Milligan SB, Kaloshian I, Ullman DE, Williamson VM. The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proc Natl Acad Sci USA. 1998;95:9750–9754. doi: 10.1073/pnas.95.17.9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samach A, Broday L, Hareven D, Lifschitz E. Expression of an amino acid biosynthesis gene in tomato flowers: developmental up-regulation and MeJA response are parenchyma-specific and mutually compatible. Plant J. 1995;8:391–406. doi: 10.1046/j.1365-313x.1995.08030391.x. [DOI] [PubMed] [Google Scholar]
- Samach A, Hareven D, Gutfinger T, Ken-Dror S, Lifschitz E. Biosynthetic threonine deaminase gene of tomato: isolation, structure and up-regulation in floral organs. Proc Natl Acad Sci USA. 1991;88:2678–2682. doi: 10.1073/pnas.88.7.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz A, Moreno Juan I, Castresana C. PIOX, a new pathogen-induced oxygenase with homology to animal cyclooxygenase. Plant Cell. 1998;10:1523–1537. doi: 10.1105/tpc.10.9.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schittko U, Preston CA, Baldwin IT. Eating the evidence? Manduca sexta larvae can not disrupt specific jasmonate induction in Nicotiana attenuata by rapid consumption. Planta. 2000;210:343–346. doi: 10.1007/PL00008143. [DOI] [PubMed] [Google Scholar]
- Schwartz E, Stasys R, Aebersold R, McGrath JM, Green BR, Pichersky E. Sequence of a tomato gene encoding a third type of LHCII chlorophyll a/b-binding polypeptide. Plant Mol Biol. 1991;17:923–926. doi: 10.1007/BF00037074. [DOI] [PubMed] [Google Scholar]
- Shimoda T, Takabayashi J, Ashihara W, Takafuji A. Response of predatory insect Scolothrips takahashii toward herbivore-induced plant volatiles under laboratory and field conditions. J Chem Ecol. 1997;23:2033–2048. [Google Scholar]
- Sisler EC, Dupille E, Serek M. Effect of 1-methylcyclopropene and methylenecyclopropane on ethylene binding and ethylene action on cut carnations. Plant Growth Regul. 1996;18:79–86. [Google Scholar]
- Stout MJ, Bostock RM. Specificity of induced responses to arthropods and pathogens. In: Agrawal AA, Tuzun S, Bent E, editors. Induced Plant Defenses against Pathogens and Herbivores: Biochemistry, Ecology, and Agriculture. APS Press, St. Paul. 1999. pp. 183–210. [Google Scholar]
- Stout MJ, Workman J, Duffey SS. Differential induction of tomato foliar proteins by arthropod herbivores. J Chem Ecol. 1994;20:2575–2594. doi: 10.1007/BF02036193. [DOI] [PubMed] [Google Scholar]
- Stout MJ, Workman KV, Workman JS, Duffey SS. Temporal and ontogenetic aspects of protein induction in foliage of the tomato, Lycopersicon esculentum. Biochem Syst Ecol. 1996;24:611–625. [Google Scholar]
- Takabayashi J, Dicke M. Plant-carnivore mutualism through herbivore-induced carnivore attractants. Trends Plant Sci. 1996;1:109–113. [Google Scholar]
- Takabayashi J, Dicke M, Posthumus MA. Volatile herbivore-induced terpenoids in plant-mite interactions: variation caused by biotic and abiotic factors. J Chem Ecol. 1994;20:1329–1354. doi: 10.1007/BF02059811. [DOI] [PubMed] [Google Scholar]
- Turlings TCJ, McCall PJ, Alborn HT, Tumlinson JH. An elicitor in caterpillar oral secretions that induces corn seedlings to emit chemical signals attractive to parasitic wasps. J Chem Ecol. 1993;19:411–425. doi: 10.1007/BF00994314. [DOI] [PubMed] [Google Scholar]
- Turlings TCJ, Tumlinson JH, Lewis WJ. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 1990;250:1251–1253. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- Van der Hoeven RS, Steffens JC. Biosynthesis and elongation of short- and medium-chain-length fatty acids. Plant Physiol. 2000;122:275–282. doi: 10.1104/pp.122.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos P, Simons G, Jesse T, Wijbrandi J, Heinen L, Hogers R, Frijters A, Groenendijk J, Diergaarde P, Reijans M, Fierensonstenk J, Deboth M, Peleman J, Liharska T, Hontelez J, Zabeau M. The tomato Mi-1 gene confers resistance to both root-knot nematodes and potato aphids. Nat Biotechnol. 1998;16:1365–1369. doi: 10.1038/4350. [DOI] [PubMed] [Google Scholar]