Abstract
We present a case report of a patient with acute myeloid leukemia (AML) characterized by the simultaneous presence of nucleophosmin 1 (NPM1) mutation and the breakpoint cluster region-Abelson (BCR-ABL) fusion oncogene. Our findings emphasize the importance of routinely including BCR-ABL in the diagnostic workup of AML in order to offer to the patients the most appropriate risk category and treatment options.
1. Introduction
Mutations in the NPM1 gene are the most frequent genetic abnormalities occurring in AML and are highly specific for de novo AML [1]. The BCR-ABL fusion gene is the genetic hallmark of chronic myeloid leukemia (CML) but can also be found in approximately 30% of acute lymphoblastic leukemia (ALL) and rarely in AML (0.3–2% of newly diagnosed cases). In the updated WHO classification published in 2016, AML with BCR-ABL has been introduced as a provisional new entity [2, 3]. In BCR-ABL-positive AML, molecular and cytogenetic profile may help to distinguish de novo AML cases from CML-blastic phase (CML-BP).
To the best of our knowledge, the co-occurrence of BCR-ABL fusion gene and NPM1 mutations in de novo AML has been reported in only few cases [4]. Herein, we report a 74-year-old patient diagnosed with AML harboring a complex three-way translocation t(9;22;12)(q34;q13;q11) encoding for two isoforms of BCR-ABL transcript (b3a2;b2a2) and a concomitant type A mutation in the NPM1 gene.
2. Case Report
A 74-year-old male, with a history of type II diabetes and previous ischemic heart disease, was admitted on September 2014 to the emergency room of the hospital complaining severe asthenia and nasal bleeding. There was no previous history of hematological disorders. Blood cell count disclosed Hb 6.4 gr/dL (12.0–16.0 g/dL), Plts 35 × 109/L (150–450 × 109/L), a WBC of 62 × 109 (4.30–10.80 × 109/L), basophils <2% (0–1.5%), and with 50% of blasts. The coagulation profile showed INR 1.5 (0.8–1.2), fibrinogen 69 mg/dL (200–400 mg/dL), ATIII 77% (75–128%), and D-dimer 10757 ng/mL (0–500 ng/mL), suggesting a disseminated intravascular coagulopathy (DIC). Bone marrow aspirate showed infiltration by 89% of hypergranular leukemic blasts (Figures 1 and 2).
Figure 1.
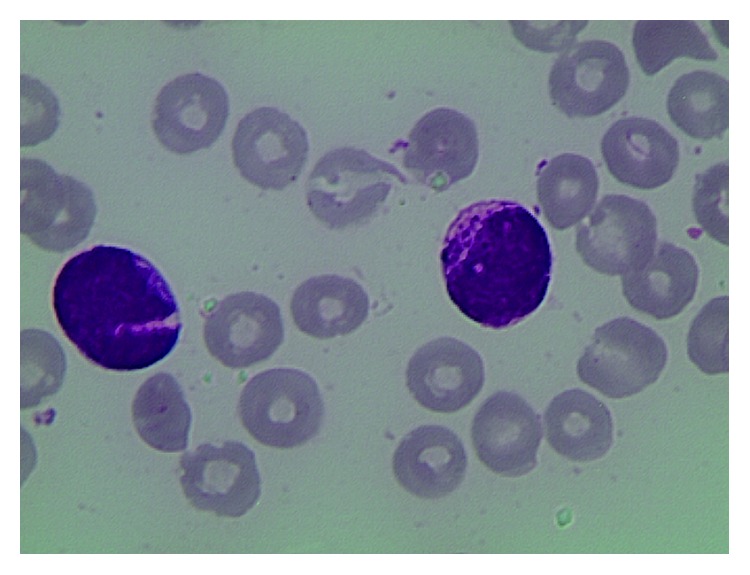
Figure 2.

Immunophenotyping of the leukemic population showed positivity for CD45, CD33, CD117, and MPO and negativity for CD34, HLA-DR, CD13, and CD56, compatible with a diagnosis of AML. Clinical examination showed mild splenomegaly (14 cm) and multiple thick and erythematous skin lesions localized on the back. A biopsy of one such lesion followed by histologic examination was consistent with extramedullary localization of AML.
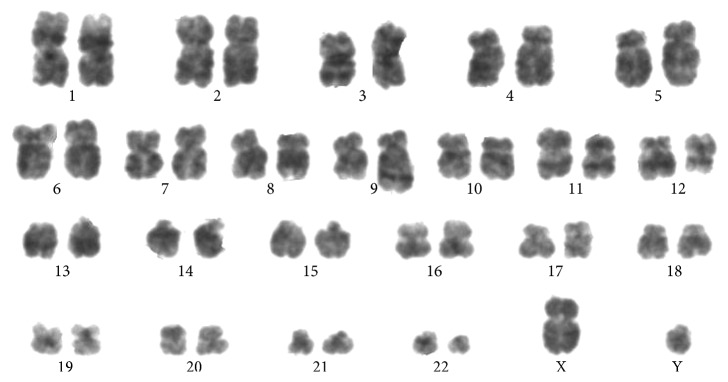
Conventional karyotyping (Figure 3) and FISH (Figure 4) showed the presence of a three-way translocation t(9;12;22)(q34;q13;q11) on 15/15 metaphases.
Figure 3.
G-banding karyotype from a bone marrow blood metaphase.
Figure 4.
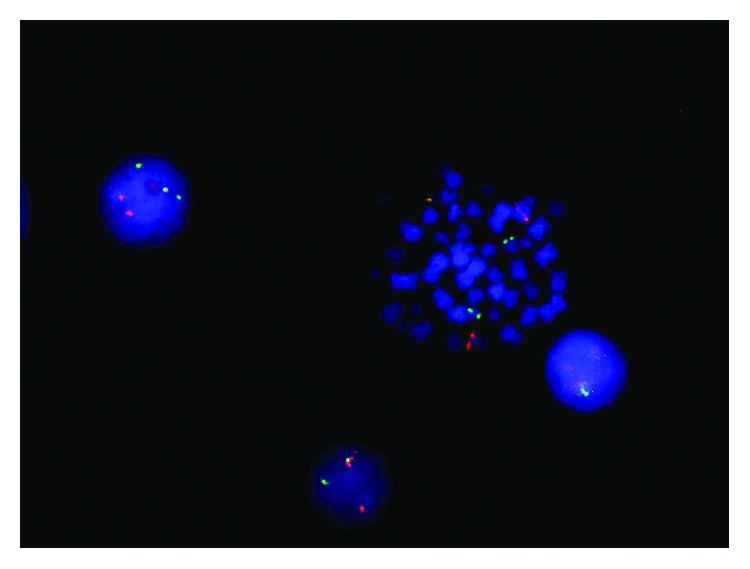
FISH BCR/ABL dual color dual fusion probe.
The p210 BCR-ABL fusion transcript was detected by standard RT-PCR, which allowed to identify both b3a2 and b2a2 transcript isoforms [5]. Nowadays, no data are available regarding prognostic value of these transcripts in AML; however, studies regarding CML have shown that in some cases transcript, b2a2 has slower molecular and inferior response rates to TKI and a poorer long‐term outcome [6]. In addition, molecular screening allowed to detect the presence of a type A mutation in the NPM1 gene [7] and the absence of BCR/ABL p190, RUNX1/RUNXT1, CBFbeta/MYH11, DEK/CAN, FLT3-ITD, and PML/RARalpha rearrangements. Quantitative RQ-PCR showed IS 36% of BCR-ABL, with a copy number of BCR-ABL p210 of 7,152 · 104/ABL [8] and NPM1 copies of 101,160 · 104/ABL [7].
The CT scan showed hepatic lesions suggestive for extramedullary involvement and disseminated venous thrombosis localized in the sovrahepatic veins and the right frontal sinus. Lumbar puncture showed no central nervous system involvement. The patient was started on initial cytoreductive treatment with hydroxyurea for ten days and was subsequently treated with second-generation tyrosine kinase inhibitor (TKI) dasatinib 140 mg PO daily. Dasatinib was preferred over imatinib, a first generation TKI, due to high risk of the central nervous system involvement, extramedullary localization [9, 10] and, as proven for CML, it gives deeper and longer molecular responses [11].
In December 2014, due to trilineage pancytopenia, research for point mutations in BCR/ABL fusion gene was detected and showed, unfortunately after patient died, T315I mutation.
In January 2015, three months after the start of dasatinib, he was hospitalized for neutropenic fever and was diagnosed with pneumonia. He died of sepsis few days later.
3. Discussion
To the best of our knowledge, only few cases of AML with concomitant BCR-ABL rearrangement and NPM1 mutation have been reported so far [4]. In clinical practice, the distinction between de novo BCR-ABL +ve AML and CML-BP is still challenging [12, 13]. In the present case, patient clinical features (no history of previous hematologic disorders, lack of basophilia, mild splenomegaly, and a lower bone marrow myeloid/erythroid ratio [14]) and molecular abnormalities (concomitant presence of NPM1 mutation) were more suggestive of a de novo AML rather than of CML-BP. In previous reports of simultaneous NPM1 mutation and BCR-ABL rearrangement, the p210 and p190 transcripts were detected in 2 and 4 cases, respectively. However, the concomitant presence of b3a2 and b2a2 BCR-ABL p210 isoforms combined to simultaneous NPM1 mutation, as observed in the present report, has been reported to date in only one case [12]. An additional peculiarity detected in leukemic cells of our patient is the presence of a complex three-way translocation involving chromosomes 9, 22, and 12 a karyotypic aberration previously unreported in either AML or CML.
BCR-ABL seems to cooperate with several AML-specific aberrations, including CBFB-MYH11 and NPM1, although the precise molecular interaction among the altered proteins remains poorly understood [15].
In our case, RQ-PCR analysis of NPM1 and BCR-ABL detected in both instances a high transcript copy number. Together with the elevated number of metaphases harboring the Philadelphia chromosome (15/15), these findings suggest that the two molecular aberrations were present within the same leukemic clone rather than occurring in two separate clones. However, in recently reported patients with Ph-positive AML, targeted treatment with TKIs was able to abrogate the BCR-ABL +ve clone but did not lead to complete hematologic response, indicating that the BCR-ABL lesion was present at the subclonal level [16]. This highlights the more complex clonal architecture of Ph-positive AMLs as compared to CML. In addition, BCR-ABL has been described only occasionally as a minor subclone arising during AML progression. Unfortunately, due to early death of our patients, we were not able to assess the cytogenetic/molecular response to TKI therapy.
At the moment, there is no standardized treatment for patients with Ph-positive AML although few case reports suggested a favorable response to TKIs [17]. In light of the new ELN classification [2], our findings further highlight the need of including BCR-ABL screening in routine diagnostic workup of AML. BCR-ABL and NPM1 mutation are considered distinct entities in the WHO classification of AML with recurrent genetic abnormalities. According to the updated ELN criteria, the presence of BCR-ABL fusion gene classifies AML in the adverse risk category while NPM1 mutation is generally regarded as a favorable prognostic in absence of the FLT3-ITD aberration. This case report highlights the possibility to concomitantly detect these two alterations in rare cases of AML and emphasizes the importance of including BCR-ABL screening in routine AML diagnostic panel in order to better assign the patient to the correct risk category and targeted treatment.
Acknowledgments
The authors thank Associazione Italiana Ricerca sul Cancro (AIRC Grant no. 21267).
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Papaemmanuil E., Gerstung M., Bullinger L., et al. Genomic classification and prognosis in acute myeloid leukemia. New England Journal of Medicine. 2016;374(23):2209–2221. doi: 10.1056/NEJMoa1516192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Döhner H., Estey E., Grimwade D., et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arber D. A., Orazi A., Hasserjian R., et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 4.Neuendorff N. R., Burmeister T., Dörken B., Westermann J. BCR-ABL-positive acute myeloid leukemia: a new entity? Analysis of clinical and molecular features. Annals of Hematology. 2016;95(8):1211–1221. doi: 10.1007/s00277-016-2721-z. [DOI] [PubMed] [Google Scholar]
- 5.van Dongen J. J. M., Macintyre E. A., Gabert J. A., et al. Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease. Leukemia. 1999;13(12):1901–1928. doi: 10.1038/sj.leu.2401592. [DOI] [PubMed] [Google Scholar]
- 6.Castagnetti F., Gugliotta G., Breccia M., et al. The BCR-ABL1 transcript type influences response and outcome in Philadelphia chromosome-positive chronic myeloid leukemia patients treated frontline with imatinib. American Journal of Hematology. 2017;92(8):797–805. doi: 10.1002/ajh.24774. [DOI] [PubMed] [Google Scholar]
- 7.Gorello P., Cazzaniga G., Alberti F., et al. Quantitative assessment of minimal residual disease in acute myeloid leukemia carrying nucleophosmin (NPM1) gene mutations. Leukemia. 2006;20(6):1103–1108. doi: 10.1038/sj.leu.2404149. [DOI] [PubMed] [Google Scholar]
- 8.Gabert J., Beillard E., van der Velden V. H. J., et al. Standardization and quality control studies of “real-time” quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia—a Europe against cancer program. Leukemia. 2003;17(12):2318–2357. doi: 10.1038/sj.leu.2403135. [DOI] [PubMed] [Google Scholar]
- 9.Isobe Y., Sugimoto K., Masuda A., Hamano Y., Oshimi K. Central nervous system is a sanctuary site for chronic myelogenous leukaemia treated with imatinib mesylate. Internal Medicine Journal. 2009;39(6):408–411. doi: 10.1111/j.1445-5994.2009.01947.x. [DOI] [PubMed] [Google Scholar]
- 10.Alimena G., Breccia M., Latagliata R., et al. Dasatinib in the management of lymphoid blast crisis of Philadelphia-positive chronic myeloid leukemia with multiple extra-medullary and intracranial localizations. Leukemia Research. 2009;33(8):e134–e136. doi: 10.1016/j.leukres.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Cortes J. E. A second-generation TKI should always be used as initial therapy for CML. Blood Advances. 2018;2(24):3653–3655. doi: 10.1182/bloodadvances.2018018655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konoplev S., Yin C. C., Kornblau S. M., et al. Molecular characterization ofde novoPhiladelphia chromosome-positive acute myeloid leukemia. Leukemia & Lymphoma. 2013;54(1):138–144. doi: 10.3109/10428194.2012.701739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soupir C. P., Vergilio J. A., Dal Cin P., et al. Philadelphia chromosome-positive acute myeloid leukemia: a rare aggressive leukemia with clinicopathologic features distinct from chronic myeloid leukemia in myeloid blast crisis. American Journal of Clinical Pathology. 2007;127(4):642–650. doi: 10.1309/B4NVER1AJJ84CTUU. [DOI] [PubMed] [Google Scholar]
- 14.Nacheva E. P., Grace C. D., Brazma D., et al. DoesBCR/ABL1positive acute myeloid leukaemia exist? British Journal of Haematology. 2013;161(4):541–550. doi: 10.1111/bjh.12301. [DOI] [PubMed] [Google Scholar]
- 15.Han E., Lee H., Kim M., et al. Characteristics of hematologic malignancies with coexisting t(9;22) and inv(16) chromosomal abnormalities. Blood Research. 2014;49(1):p. 22. doi: 10.5045/br.2014.49.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aoki J., Kakihana K., Kobayashi T., et al. Tyrosine kinase inhibitor therapy for acute myeloid leukemia with late-appearing Philadelphia chromosome. Leukemia Research. 2012;36(1):e41–e42. doi: 10.1016/j.leukres.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Ueda K., Horiike S., Zen K., Misawa S., Taniwaki M. Complete cytogenetic and molecular response to treatment with imatinib mesylate for philadelphia chromosome positive acute myeloid leukemia with multilineage dysplasia. Leukemia & Lymphoma. 2006;47(9):1967–1969. doi: 10.1080/16066350600687749. [DOI] [PubMed] [Google Scholar]