Abstract
Cadmium contamination in soils and waters can lead to food chain accumulation and ultimately human health degradation; means for reducing bioavailable Cd are desperately required; biochars may play a role. Long-term lab incubation experiments aimed at explaining wheat straw-derived biochar (0, 5, and 15% by wt.) effects on Cd sorption, decreasing soil and solution Cd bioavailability, and identifying Cd forms present using both the European Community Bureau of Reference (BCR) wet chemical sequential extraction procedure and synchrotron-based X-ray absorption spectroscopy (XAS). Cadmium removal was 88 and 90% in the Cd-contaminated soil and Cd-containing solution, respectively, when using the 15% biochar application rate as compared to the control; significantly less Cd removal was observed with 5% biochar application rate. Based on the wet chemical sequential extraction procedure in conjunction with XAS, biochar application promoted the formation of (oxy)hydroxide, carbonate, and organically bound Cd phases. As a material, biochar may be promoted as a tool for reducing and sequestering bioavailable Cd from soils and solutions, potentially improving environmental and human health.
Keywords: BCR method, Biochar (BC), Cadmium, Contaminated paddy soil, Synchrotron, X-ray absorption spectroscopy
1. Introduction
Soil heavy metal pollution is a serious problem globally, leading to food chain accumulation and ultimately human health risk. In China specifically, soil heavy metal pollution can be linked to recent rapid industrialization, urbanization, and subsequently widespread solid, liquid, and gaseous metal emissions (Wang, 1998; Zhao et al., 2015). China’s Ministry of Environmental Protection (MEP, 2014) has estimated that almost 16% of all agricultural soils are contaminated with heavy metals. Of those heavy metals present, soil cadmium (Cd) concentrations exceed China’s environmental standards in 7% of all arable lands (98,000 km2) based on limits that protect agricultural production, heavy metal accumulation in the food chain, and consequently human health. When ingested, Cd may lead to kidney dysfunction, skeletal damage, and cancers (Satarug & Moore, 2004). According to the World Health Organization (2011), oral ingestion of as little as 0.125 mg Cd kg−1 body weight can cause health issues. Given the amount of arable land contaminated with Cd, the potential for human health issues is exacerbated in China.
A “cancer villages” map published online identified 247 locations in China with major health issues likely associated with man-induced pollution (China.org.cn, 2013). In China and other locations where soil heavy metal pollution is an issue, it is generally considered that human exposure to Cd is most often through the consumption of contaminated food (Zhao et al., 2013). In order to reduce the risk of heavy metal accumulation from soils, to plants, and ultimately humans, soil heavy metal bioavailability needs to be reduced. One such novel approach for reducing soil bioavailable metals is the use of biochar.
Biochar (BC), a carbon enriched material, is produced via pyrolysis of biomass at moderate temperature (~500°C) under limited oxygen concentrations (Lehmann et al., 2006). Because of its unique physical-chemical characteristics, BC is recognized as a multifunctional material for treating heavy metal polluted soils and water (Lehmann et al., 2011). Many studies (e.g., Devi & Saroha, 2014; Ippolito et al., 2017; Uchimiya et al., 2011) have reported that BC significantly sorbed heavy metals, leading to reduced heavy metal bioavailability and leaching. Multiple mechanisms of heavy metal sorption onto biochar have been proposed.
Ippolito et al. (2012) used synchrotron radiation to show that under slightly acidic or basic pH conditions, KOH steam-activated pecan shell biochar sequestered Cu as either bound to organic functional groups or precipitated as (hydr)oxides/carbonate phases, respectively. Lee et al. (2017) optimized pyrolysis conditions for Pb sorption onto palm oil sludge biochar. As with the Ippolito et al. (2012) findings, the authors showed that under acidic conditions the biochar sorbed Pb via cation exchange through various functional groups present. Peanut hull biochar was shown to be efficient in removing Cd (6.74 mg g−1) and Pb (63.09 mg g−1) from solution in the form of Cd carbonate (> 50%) and organo-Pb complexation (~40%) (Cui et al., 2014). Cui et al. (2016) utilized wheat straw biochar to reduce bioavailable soil Cd and Pb to more stable forms (e.g., residual forms), subsequently reducing Cd and Pb uptake by rice from contaminated paddy soil. Similarly, Bian et al. (2014) utilized a paddy soil contaminated with Cd and Pb from nearby smelter emissions, adding wheat straw biochar up to 40 Mg ha−1. Rice uptake of Cd and Pb were reduced by up to 69%, likely due to heavy metal interactions with surface functional groups, Fe oxyhydroxide phases, and Ca and P present in biochar. Ippolito et al. (2017) found that lodgepole pine or tamarisk biochar could reduce bioavailable Cd, Zn, Cu, and Pb concentrations by raising soil pH and subsequently forming carbonate and oxyhydroxide metal precipitates.
The above studies illustrate that biochars can reduce heavy metal bioavailability via a number of processes. However, the above studies also utilized either relatively short batch shaking periods (<24 hours) or relatively long field studies (3 to 5 years). We hypothesized that maximum biochar heavy metal sorption likely requires more than 24 hours to attain equilibrium, potentially on the order of days or weeks. Furthermore, we hypothesized that, given time, biochar-heavy metal interactions may lead from sorption via surface functional groups to precipitation of heavy metal mineral phases. Our objectives were to 1) utilize biochar to reduce bioavailable soil Cd and solution Cd, 2) investigate biochar-metal sorption and 3) identify form(s) present as affected by biochar application rate and time using both a sequential extraction procedure and X-ray absorption spectroscopy.
2. MATERIALS AND METHODS
2.1. Biochar and soil
Biochar was created from wheat straw pyrolyzed at ~450°C at the Sanli New Energy Company, Henan Province, China. After collection, BC was ground to pass through a 2-mm sieve.
The experimental soil was collected from a rice paddy field (31°24.434’N and 119°41.605’E) where atmospheric fallout and effluent discharges from a local smelter had contaminated the soil with Cd over several years. The paddy soil was air-dried and then passed through 2-mm sieve. Basic BC and soil properties are listed in Table 1 and were determined using methods described by Lu (2000). Briefly, pH was determined using a soil:water ratio of 1:2.5, cation exchange capacity (CEC) was detected via the ammonium acetate exchange method, total C using the potassium dichromate method, total N via Kjeldahl digestion and analyzed colorimetrically, total P via HClO4—H2SO4 digestion and detected using the molybdate blue method, total K via HClO4–HNO3–HF digestion and detected by atomic absorption spectrometry (FAAS; TAS-986, Persee, China), and total Ca, Mg and Cd using a HClO4–HNO3–HF digestion and analyzed using inductively coupled plasma–optical emission spectroscopy (ICP-OES, Perkin Elmer Optima 7300 DV, Shelton, USA).
Table 1.
Wheat straw biochar (BC) and paddy soil (0 to 15 cm depth) basic properties and total elemental analyses.
| pH | CEC† | C | N | P | K | Ca | Mg | Cd | |
|---|---|---|---|---|---|---|---|---|---|
| cmolc kg−1 | g kg−1 | mg kg−1 | |||||||
| BC | 10.4 | 21.7 | 467 | 5.90 | 14.4 | 11.5 | 10.4 | 6.49 | 0.03 |
| Soil | 6.1 | 18.0 | 20.7 | 3.19 | 0.82 | 11.4 | 1.46 | 5.38 | 22.6 |
CEC = Cation Exchange Capacity.
2.2. Experimental design
2.2.1. Biochar Sorption of Cd from Solution
In triplicate, 0.00, 0.10, or 0.30g BC were weighed into 50 mL centrifuge tubes (equivalent to 0, 5, and 15% BC by wt.). Next, 40 mL of a Cd solution containing either 0, 5, or 15 mg Cd L−1, in 0.01 M KCl, was added to each tube; the solution pH was not buffered. The tubes were shaken at 120 rpm for 1h, 2h, 4h, 7d, 14d, 30d, 60d, 120d, and 240d. After each shaking period, the tubes were centrifuged (40 g) and solution pH was measured. The supernatant was filtered through a 0.45 μm membrane filter, two to three drops of concentrated HNO3 were added, and the solutions analyzed for Cd concentrations using ICP–OES. The biochar solid fraction was air-dried, crushed using a mortar and pestle, and then saved for X-ray absorption spectroscopy (XAS) analysis.
2.2.2. Biochar Sorption of Cd from Contaminated Soil
In quadruplicate, 2.00 g of contaminated soil and either 0.00, 0.10, or 0.30g BC (equivalent to 0, 5, and 15% BC by weight) were placed into 50 mL centrifuge tubes. Next, 40 mL of 0.01 M KCl (a proxy for metal bioavailability, as compared to Ippolito et al., 2017) was added to each tube; the solution pH was not buffered. The tubes were shaken at 120 rpm for 1h, 2h, 4h, 7d, 14d, 30d, 60d, 120d, and 240d. Following each shaking period, the tubes were centrifuged, the solution pH determined, and then the supernatant was filtered through a 0.45 μm membrane filter. Two to three drops of concentrated HNO3 were added, and the solutions analyzed for bioavailable Cd concentrations using ICP-OES. The biochar-soil solid fraction from 1h, 1d, 7d, 14d, and 120d were air-dried, crushed using a mortar and pestle, and saved for wet chemical-heavy metal sequential extraction and XAS analysis.
2.2.3. Biochar Effects on Soil Cd Fractionation (BCR Sequential Extraction)
The air-dried, crushed soil-BC composite samples from 1h, 1d, 14d, and 120d, were extracted using the four-step European Community Bureau of Reference (BCR) sequential extraction procedure according to Ippolito et al., (2017). Briefly, Step 1 (B1; soluble, carbonates, exchangeable fraction): 40 mL of 0.11 M acetic acid was added to 1 g of the soil-BC samples in a 50 mL centrifuge tube and shaken for 16 h at room temperature. The extract was then separated from the solid residue by centrifugation, decantation, and filtration through a 0.45 μm membrane filter. Step 2 (B2; iron and manganese oxyhydroxides fraction): 40 mL of a freshly prepared 0.1 M hydroxylamine hydrochloride was added to the residue from step 1 in the centrifuge tube and re-suspended by shaking for 16 h at room temperature. The extract was separated from the solid phase as described above. Step 3 (B3; organically bound and sulfides fraction): 10 mL of 30% H2O2 was added to the residue from step 2 in the centrifuge tube at room temperature for 1 h with occasional manual shaking, and then digested at 85 °C until the volume was reduced to about 3 mL. An additional 10 mL of 30% H2O2 was added and the digestion was continued at 85°C until the volume was reduced to 1 mL; the samples were allowed to cool. Finally, 40 mL of 1 M ammonium acetate was added and the tubes shaken for 16 h at room temperature. The extract was separated from the solid phase as described above. Step 4 (B4; residual fraction): the residue from step 3 was allowed to air dry and then it was digested in a 50 mL digestion tube using 1 mL deionized water to make a slurry, followed by aqua regia addition (7 mL concentrated HCl: 2 mL of concentrated HNO3). The mixtures were allowed to predigest overnight at room temperature and then digested at 105°C for 2 h the next day. Then, the mixtures were brought to a 50 mL final volume, filtered through a 0.45 μm membrane filter, and analyzed for Cd concentrations using ICP–OES.
2.2.4. Biochar-Cd Solution and Contaminated Soil X-Ray Absorption Spectroscopy (XAS) Analysis
Air-dried, crushed biochar from the 1h, 1d, 14d, and 120d Cd solution study, and air-dried crushed biochar amended soils from 1h, 1d, 7d, and 120d, were analyzed using XAS spectroscopy to elucidate solid-phase Cd speciation. The XAS experiments were conducted at the Materials Research Collaborative Access Team’s beamline 10-BM, Sector 10 located at the Advanced Photon Source, Argonne National Laboratory, Argonne, Illinois (Kropf et al., 2010). The electron storage ring operated at 7 GeV in top-up mode. A liquid N2-cooled, double crystal Si (111) monochromator was used to select incident photon energies, and a platinum-coated mirror was used for harmonic rejection. The samples were prepared as thin pellets with a hand-operated IR pellet press, and the samples were secured by Kapton tape in sample holders. Five XAS spectra were collected in fluorescence (Si drift detector) and transmission mode at room temperature from −200 to 800 eV relative to the absorption edge position of Cd. Data processing and linear combination fitting (LCF) of X-ray absorption near-edge structure (XANES) derivative spectra were done with the program Athena (Ravel & Newville, 2005).
2.2.5. Statistics
All data were expressed as means plus or minus one standard deviation. Differences between the treatments were examined using a Student’s t-test in a two-way analysis of variance (ANOVA), and considered significant when p < 0.05. All statistical analyses were carried out using SPSS (version 20.0, USA).
3. RESULTS AND DISCUSSION
3.1. Biochar Sorption of Cd from solution
As compared to the control, solution pH was significantly elevated over time (by ~1 to 4 pH units) with both 5 and 15% biochar applications in either 0 or 5 mg Cd L−1 (all p < 0.001) (Fig. 1A). When placed into the Cd solution containing 15 mg L−1, the 15% biochar application rate maintained about a 2 to 5 unit pH increase over the control over time (all p < 0.001); lesser increases were observed with the 5% biochar application rate. The biochar pH was 10.4 (Table 1), likely elevated due to the presence of oxides, hydroxides, and carbonate phases in the biochar (as shown by Ippolito et al., 2017). If biochar was removing Cd from solution, it may have occurred due to precipitation of Cd oxides, hydroxides, and carbonate mineral phases. Greater biochar application rates would have introduced greater oxides, hydroxides, and carbonate phases into solution, thus helping maintain an elevated pH over the control or lower biochar application rates. Concomitantly, increasing Cd solution concentrations would allow potentially allow for solid phase Cd precipitation reactions to occur, lowering solution pH as sorption sites and precipitation reactions occurred.
Fig. 1.
Increasing wheat straw biochar (0, 5, and 15% by wt.) in solutions containing 0, 5, or 15 mg Cd L−1, on (A) solution pH and (B) residual solution Cd concentration after sorption onto biochar over shaking time.
Neither biochar application rate caused appreciable Cd loss from biochar itself when placed in solution containing 0 mg Cd L−1 (Fig. 1B), with Cd concentrations near or below detection. When placed in solution containing either 5 or 15 mg Cd L−1, there was always significantly less Cd in solution with the 15% versus 5% biochar application rate (p < 0.001). Over time, the 15% biochar application sorbed and removed practically all Cd from the 5 mg L−1 solution (>90% removal after 4 h), and removed almost 75% of the Cd from the 15 mg L−1 solution. As with the previous pH findings, as well as those of others (e.g., Ippolito et al., 2017; Xu et al., 2013; Kolodyńska et al., 2012), greater biochar application rates likely provided more sites for Cd sorption and precipitation reactions to occur.
Biochar dosage, contact time, and solution Cd concentration, all played a key role in Cd removal; others have found similar results. Bogusz et al. (2017) reported that the biogas residue biochar (600 °C) effectively removed Cd from solution, with sorption potentially affected by interfering anions (e.g., Cl−, NO3−). However, the authors suggested that Cd-(hydr)oxide precipitates blocked active sites on biochar surfaces, reducing interfering ion effects. Other inorganic ions (e.g., Ca2+, Mg2+, PO43-) from biochar were positively correlated to Cd removal from solution, promoting sorption or precipitation reactions (Clemente et al., 2017). Usman et al. (2016) also found that the palm waste biochar (700 °C) could remove almost 100% of Cd from solution, attributed to biochar functional groups (such as -OH, -CO32-) that promoted CdCO3 precipitation. Similarly, Štefelová et al., (2017) found that spruce sawdust wood biochar could sorb 13.4 mg Cd g−1, attributed to biochar surface functional groups (-OH) as pyrolysis temperature increased (400–700°C), promoting Cd precipitation reactions.
3.2. Biochar Sorption of Cd from Contaminated Soil
Increasing biochar application rate caused significant upward shifts in pH (p < 0.001) (Fig. 2A), likely due to the wheat straw biochar pH being 10.4 (Table 1). Additionally, when biochar is used as amendment, elements such as Ca, Mg, and K (Table 1) found on biochar sorption sites can be replaced with heavy metals such as Cd, causing a rise in soil solution pH (Huggins et al., 2016; Zambon et al., 2016). As suggested in the biochar-solution pH results above, biochar application likely supplied oxides, hydroxides, and carbonate phases to the soil and caused the pH to increase (Ippolito et al., 2017).
Fig. 2.
Increasing wheat straw biochar (0, 5, and 15% by wt.) on Cd contaminated soil (A) pH and (B) bioavailable Cd concentration after sorption onto biochar over shaking time.
Biochar addition of 5 and 15% (by wt.) to the Cd contaminated soil reduced bioavailable Cd concentration by 53.4%−87.9% over the 240 d experiment as compared to the control soil (p < 0.001)(Fig. 2B). Supporting this result, Beesley et al. (2010) showed that an 8% biochar application rate (w/w) reduced the water-soluble Cd concentration in a multi-heavy metal contaminated soil in 56 days incubation pot experiment. A Chinese herb medicine biochar immobilized 81% Cd in contaminated soil following a 28 days incubation (Qiao et al., 2017). Lahori et al. (2017) reported that a 1% tobacco biochar significantly reduced plant-available Cd by 32% (Cd) over a control following ~ 10 weeks of incubation. Ippolito et al. (2017) mixed lodgepole pine biochar into four heavy metal contaminated soils, showing between 74 to 81% bioavailable Cd removal after only 2 h; the authors showed that Cd removal was due to the formation of Cd oxides, hydroxides, and carbonate phases as shown via the use of a BCR sequential extraction procedure.
3.3. Biochar Effects on Soil Cd Fractionation
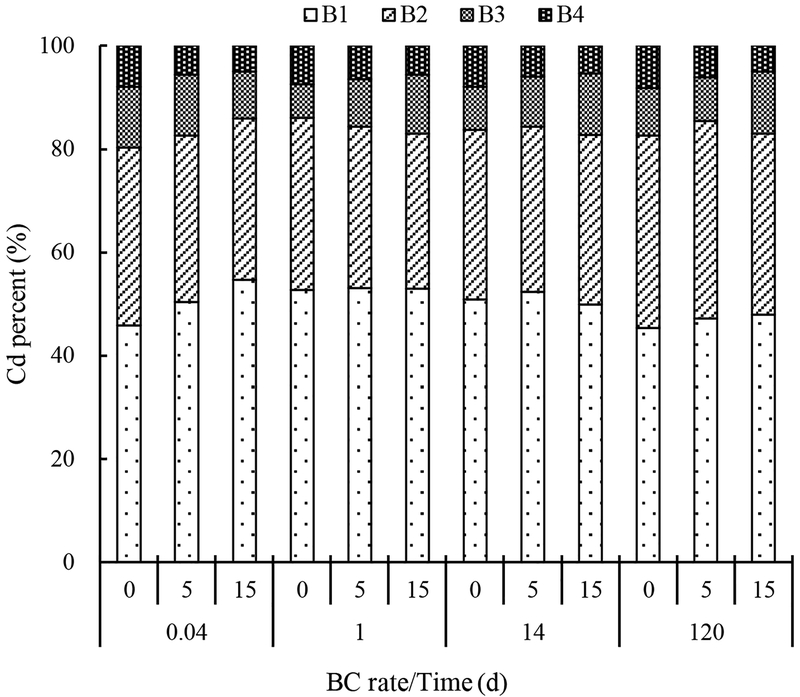
Soil Cd fractions were operationally defined using the BCR sequential extraction procedure, with: step 1 (B1) associated with the soluble/carbonates/exchangeable fraction; step 2 (B2) associated with the iron and manganese oxyhydroxides fraction; step 3 (B3) associated with the organically bound and sulfides fraction; and step 4 (B4) associated with the residual fraction (Fig. 3). This approach is justifiable when the goal is to compare differences in a soil as affected by different treatments (Ippolito et al., 2009), or in the current study, by increasing biochar application rate.
Fig. 3.
The effect of increasing biochar rate (0, 5, or 15% by wt.) over time (0.04 days [1h], 1, 14, and 120 d) on the ratio of (B1) soluble/exchangeable/carbonate, (B2) Fe/Mn oxyhydroxide, (B3) organically bound and sulfide fraction, and (B4) residual fractions of Cd in a Cd-contaminated soil.
In the current study, Cd increased in the soluble/carbonate/exchangeable fraction when 5 or 15% biochar was added as compared to the control (p < 0.001), while the 1 h, 1 d, and 14 d contained greater Cd in this phase as compared to 120 d (p < 0.001). There were no statistical treatment differences between Cd in steps 2 (iron/manganese oxyhydroxides fraction; p = 0.069) or 3 (organically bound/sulfide fraction; p = 0.365), while over time the iron/manganese oxyhydroxides fraction increased (p < 0.001). Cadmium content decreased in the residual fraction when 5 or 15% biochar was added as compared to the control (p < 0.001), while no differences over time were observed p = 0.965); this decrease may have contributed to the Cd increase in soluble/carbonate/exchangeable and the conversion of Cd bound in the iron/manganese oxyhydroxides fraction over time.
The increase in the soluble/carbonate/exchangeable fraction with biochar application might be attributed to Cd weakly sorbed onto the biochar surface or in the unstable biochar forms, as shown by Cui et al. (2014). Yet weakly sorbed or unstable forms would not likely not lead to a reduction in Cd bioavailability. Others (e.g., Egene et al., 2018; Cui et al., 2016) have shown water-soluble and exchangeable Cd reductions due to biochar application, but did not specify where Cd eventually resided.
However, others have identified Cd transformations from bioavailable to other soil forms. The reduction in Cd bioavailability has been associated with increasing soil pH and the formation of carbonate and oxyhydroxide precipitates when biochars were introduced into Cd-contaminated soil, as shown by Ippolito et al. (2017) using the BCR technique. Houben and Sonnet (2015) used an extraction procedure to show that miscanthus straw biochar shifted the Cd exchangeable pool to the carbonate pool primarily due to increasing pH. Moreno-Barriga et al. (2017) showed that biochar could reduce metal mobility due to increasing soil pH, and shift metals toward Fe/Mn oxyhydroxide phases. Shen et al. (2017) showed that biochar application could cause heavy metal shifts towards carbonate and carbonate-hydroxide phases. Thus, it appears that increasing and maintaining soil pH may help prevent dissolution and release of Cd back into the soil solution, which would re-promote Cd bioavailability.
3.4. Biochar-Cd X-Ray Absorption Spectroscopy (XAS) Analysis
Cadmium speciation in both the biochar-Cd solution sorption study and Cd in biochar amended soil investigation was determined through linear combination fitting (LCF) of the derivative XANES spectra. Four primary Cd species were identified, which include Cd associated with typical organic matter functional groups (OM/Biochar), cadmium sulfate (Cd Sulfate), cadmium bound to thiol (Cd Thiol), and Cd sorbed to Fe/Mn/Al (oxy)hydroxides or clay minerals (Mineral Bound).
Speciation results for the biochar-Cd solution sorption study were similar regardless of the biochar or Cd rate or as a function of treatment time. On average, OM/Biochar accounted for 66% (±2.16%), Cd Sulfate averaged 19% (±1.76%), and Cd Thiol measured 15% (±2.20%) (Table 2).
Table 2.
Speciation results of biochar sorption of Cd from solution.
| Cd speciation distribution (%) | ||||||
|---|---|---|---|---|---|---|
| Biochar rate (%) | Treatment time (d) | OM/Biochar | Cd sulfate | Cd thiol | Mineral Bound | R-factor* |
| 5 | 0.04 | 69 | 16 | 15 | 0.0021 | |
| 15 | 66 | 18 | 16 | 0.0021 | ||
| 5 | 1 | 65 | 19 | 16 | 0.0032 | |
| 15 | 62 | 20 | 18 | 0.0029 | ||
| 5 | 14 | 65 | 21 | 14 | 0.0019 | |
| 15 | 66 | 20 | 14 | 0.0029 | ||
| 5 | 120 | 67 | 21 | 12 | 0.0078 | |
| 15 | 68 | 20 | 12 | 0.0014 | ||
A measure of mean square sum of the misfit at each data point.
Speciation results for biochar sorption of Cd from the contaminated soil varied relative to biochar rate and treatment time (Table 3, Figure S1). The control soil (0% biochar rate) showed consistent results as a function of treatment time, demonstrating that Cd was associated with 37±2.02% OM/Biochar, 17±3.15% Cd Sulfate, 20±2.18% Cd Thiol, and 26±1.68% Mineral Bound. In contrast, upon biochar amendment into soil there was a modest decrease in Mineral Bound Cd and Cd Thiol at each treatment time interval. Cd Sulfate showed a sharp increase at the 0.04-d treatment time relative to the 0% biochar value; however, Cd Sulfate in both the control and biochar treatments reduced in percentage over the 120-d reaction period to about 15%. Finally, the amount of Cd associated with OM/Biochar steadily increased as a function of treatment time, with slightly greater OM/Biochar values for the 15% biochar rate over the 5% rate.
Table 3.
Speciation results of biochar sorption of Cd from contaminated soil.
| Cd speciation distribution (%) | ||||||
|---|---|---|---|---|---|---|
| Biochar rate (%) | Treatment time (d) | OM/Biochar | Cd sulfate | Cd thiol | Mineral Bound | R-factor* |
| 0 | 0.04 | 37 | 21 | 18 | 24 | 0.0031 |
| 5 | 39 | 30 | 14 | 18 | 0.0022 | |
| 15 | 38 | 29 | 19 | 15 | 0.0019 | |
| 0 | 1 | 40 | 15 | 19 | 26 | 0.0011 |
| 5 | 46 | 20 | 13 | 22 | 0.0013 | |
| 15 | 47 | 22 | 12 | 20 | 0.0017 | |
| 0 | 14 | 37 | 16 | 20 | 27 | 0.0019 |
| 5 | 45 | 19 | 14 | 23 | 0.0023 | |
| 15 | 50 | 17 | 11 | 23 | 0.0025 | |
| 0 | 120 | 35 | 15 | 23 | 28 | 0.0015 |
| 5 | 48 | 15 | 13 | 25 | 0.0023 | |
| 15 | 55 | 14 | 11 | 20 | 0.0037 | |
A measure of mean square sum of the misfit at each data point.
4. Conclusions
Some biochars are recognized for treating and removing heavy metals in contaminated waters and soils, reducing metal bioavailability and lessening metal effects on humans and the environment. We tested the ability of a wheat straw biochar, pyrolyzed at ~450 °C, to sequester Cd from solution and a contaminated soil. Based on wet chemical and XAS analysis, it appeared that functional groups present on wheat straw biochar helped to sorb, retain, and remove excess bioavailable Cd from solution and Cd contaminated soil. This particular form of Cd may be re-released back to the environment if biochar was to oxidize. Luckily, biochars pyrolyzed at modest temperatures (e.g., close to 500 °C) tend to be stable over the long-term (e.g., 100s of years), and thus this particular biochar may be beneficial for reducing solution and soil Cd bioavailability, and ultimately reducing bioavailable Cd exposure to humans and the environment.
Supplementary Material
Acknowledgements
This study was partially supported by National Natural Science Foundation of China under a grant number of 41501339, 21677119, 21277115, 41301551, 21407123, 41501353, Jiangsu Province Science Foundation for Youths under a grant number of BK20140468, sponsored by Qing Lan Project. MRCAT operations are supported by the Department of Energy and the MRCAT member institutions. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02–06CH11357. Although EPA contributed to this article, the research presented was not performed by or funded by EPA and was not subject to EPA’s quality system requirements. Consequently, the views, interpretations, and conclusions expressed in this article are solely those of the authors and do not necessarily reflect or represent EPA’s views or policies.
References
- Abdelhadi SO, Dosoretz CG, Rytwo G, Gerchman Y, Azaizeh H, 2017. Production of biochar from olive mill solid waste for heavy metal removal. Bioresour. Technol 244, 759–767. [DOI] [PubMed] [Google Scholar]
- Aslam Z, Khalid M, Naveed M, Shahid M, Aon M, 2017. Evaluation of Green Waste and Popular Twigs Biochar Produced at Low and High Pyrolytic Temperature for Efficient Removal of Metals from Water. Water Air Soil Pollut. 228(11), 432. [Google Scholar]
- Beesley L, Moreno-Jiménez E, Gomez-Eyles JL 2010. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ. Pollut 158(6), 2282–2287. [DOI] [PubMed] [Google Scholar]
- Bian R, Joseph S, Cui L, Pan G, Li L, Liu X, Zhang A, Rutlidge H, Wong S, Chia C, 2014. A three-year experiment confirms continuous immobilization of cadmium and lead in contaminated paddy field with biochar amendment. J. Hazard. Mater 272, 121–128. [DOI] [PubMed] [Google Scholar]
- Bogusz A, Nowak K, Stefaniuk M, Dobrowolski R, Oleszczuk P, 2017. Synthesis of biochar from residues after biogas production with respect to cadmium and nickel removal from wastewater. J. Environ. Manage 201, 268–276. [DOI] [PubMed] [Google Scholar]
- China.org.cn. 2013. China’s mainland home to 247 ‘cancer villages’. Available at: http://www.china.org.cn/environment/2013-02/25/content_28047976.htm.
- Clemente JS, Beauchemin S, MacKinnon T, Martin J, Johnston CT, Joern B, 2017. Initial biochar properties related to the removal of As, Se, Pb, Cd, Cu, Ni, and Zn from an acidic suspension. Chemosphere 170, 216–224. [DOI] [PubMed] [Google Scholar]
- Cui L, Pan G, Li L, Bian R, Liu X, Yan J, Quan G, Ding C, Chen T, Liu Y, 2016. Continuous immobilization of cadmium and lead in biochar amended contaminated paddy soil: A five-year field experiment. Ecol. Eng 93, 1–8. [Google Scholar]
- Cui L, Yan J, Li L, Quan G, Ding C, Chen T, Yin C, Gao J, Hussain Q, 2014. Does biochar alter the speciation of Cd and Pb in aqueous solution? Bioresources 10(1), 88–104. [Google Scholar]
- Devi P, Saroha AK 2014. Risk analysis of pyrolyzed biochar made from paper mill effluent treatment plant sludge for bioavailability and eco-toxicity of heavy metals. Bioresour. Technol 162, 308–315. [DOI] [PubMed] [Google Scholar]
- Egene CE, Van Poucke R, Ok YS, Meers E, Tack FMG, 2018. Impact of organic amendments (biochar, compost and peat) on Cd and Zn mobility and solubility in contaminated soil of the Campine region after three years. Sci. Total Environ. 626, 195–202. [DOI] [PubMed] [Google Scholar]
- Houben D, Sonnet P, 2015. Impact of biochar and root-induced changes on metal dynamics in the rhizosphere of Agrostis capillaris and Lupinus albus. Chemosphere 139, 644–651. [DOI] [PubMed] [Google Scholar]
- Huggins TM, Haeger A, Biffinger JC, Ren ZJ, 2016. Granular biochar compared with activated carbon for wastewater treatment and resource recovery. Water Res. 94, 225–232. [DOI] [PubMed] [Google Scholar]
- Ippolito J, Barbarick K, Brobst R, 2009. Fate of biosolids Cu and Zn in a semi-arid grassland. Agric. Ecosyst. Environ 131(3), 325–332. [Google Scholar]
- Ippolito J, Berry C, Strawn D, Novak J, Levine J, Harley A, 2017. Biochars reduce mine land soil bioavailable metals. J. Environ. Qual 46(2), 411–419. [DOI] [PubMed] [Google Scholar]
- Ippolito J, Strawn D, Scheckel K, Novak J, Ahmedna M, Niandou M, 2012. Macroscopic and molecular investigations of copper sorption by a steam-activated biochar. J. Environ. Qual 41(4), 1150–1156. [DOI] [PubMed] [Google Scholar]
- Karunanayake AG, Todd OA, Crowley M, Ricchetti L, Pittman CU Jr, Anderson R, Mohan D, Mlsna T, 2018. Lead and cadmium remediation using magnetized and nonmagnetized biochar from Douglas fir. Chem. Eng. J 331, 480–491. [Google Scholar]
- Kolodyńska D, Wnetrzak R, Leahy JJ, Hayes MHB, Kwapiński W, and Hubicki Z. 2012. Kinetic and adsorptive characterization of biochar in metal ions removal. Chem. Engineer. J 197:295–305. [Google Scholar]
- Kropf AJ, Katsoudas J, Chattopadhyay S, Shibata T, Lang EA, Zyryanov VN, Ravel B, McIvor K, Kemner KM, Scheckel KG, Bare SR, Terry J, Kelly SD, Bunker BA, and Segre CU. 2010. The new MRCAT (Sector 10) Bending Magnet beamline at the Advanced Photon Source. AIP Conference Proceedings 1234, 299–302 (2010). [Google Scholar]
- Lahori AH, Zhang Z, Guo Z, Li R, Mahar A, Awasthi MK, Wang P, Shen F, Kumbhar F, Sial TA, 2017. Beneficial effects of tobacco biochar combined with mineral additives on (im) mobilization and (bio) availability of Pb, Cd, Cu and Zn from Pb/Zn smelter contaminated soils. Ecotoxicol. Environ. Saf 145, 528–538. [DOI] [PubMed] [Google Scholar]
- Lee XJ, Lee LY, Hiew BYZ, Gan S, Thangalazhy-Gopakumar S, Ng HK, 2017. Multistage optimizations of slow pyrolysis synthesis of biochar from palm oil sludge for adsorption of lead. Bioresour. Technol 245, 944–953. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Gaunt J, Rondon M 2006. Bio-char sequestration in terrestrial ecosystems–a review. Mitig. Adapt. Strat. Gl 11(2), 395–419. [Google Scholar]
- Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D, 2011. Biochar effects on soil biota–a review. Soil Biol. Biochem 43(9), 1812–1836. [Google Scholar]
- Liang J, Yang Z, Tang L, Zeng G, Yu M, Li X, Wu H, Qian Y, Li X, Luo Y, 2017. Changes in heavy metal mobility and availability from contaminated wetland soil remediated with combined biochar-compost. Chemosphere 181, 281–288. [DOI] [PubMed] [Google Scholar]
- Lu K, Yang X, Gielen G, Bolan N, Ok YS, Niazi NK, Xu S, Yuan G, Chen X, Zhang X 2017. Effect of bamboo and rice straw biochars on the mobility and redistribution of heavy metals (Cd, Cu, Pb and Zn) in contaminated soil. J. Environ. Manage 186, 285–292. [DOI] [PubMed] [Google Scholar]
- Lu R, 2000. Methods of inorganic pollutants analysis. Soil and agro-chemical analysis methods, 205–266. [Google Scholar]
- MEP MLR. 2014. The national soil contamination survey. 1–5. [Google Scholar]
- Mohamed I, Ali M, Ahmed N, Abbas MHH, Abdelsalam M, Azab A, Raleve D, Fang C 2018. Cow manure-loaded biochar changes Cd fractionation and phytotoxicity potential for wheat in a natural acidic contaminated soil. Ecotoxicol. Environ. Saf 162, 348–353. [DOI] [PubMed] [Google Scholar]
- Moreno-Barriga F, Faz Á, Acosta JA, Soriano-Disla M, Martínez-Martínez S, Zornoza R 2017. Use of Piptatherum miliaceum for the phytomanagement of biochar amended Technosols derived from pyritic tailings to enhance soil aggregation and reduce metal (loid) mobility. Geoderma 307, 159–171. [Google Scholar]
- Qiao Y, Wu J, Xu Y, Fang Z, Zheng L, Cheng W, Tsang EP, Fang J, Zhao D, 2017. Remediation of cadmium in soil by biochar-supported iron phosphate nanoparticles. Ecol. Eng 106, 515–522. [Google Scholar]
- Ravel B, Newville M, 2005. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12(4), 537–541. [DOI] [PubMed] [Google Scholar]
- Satarug S, Moore MR, 2004. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ. Health Perspect. 112(10), 1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z, Zhang Y, Jin F, McMillan O, Al-Tabbaa A, 2017. Qualitative and quantitative characterisation of adsorption mechanisms of lead on four biochars. Sci. Total Environ. 609, 1401–1410. [DOI] [PubMed] [Google Scholar]
- Štefelová J, Zelenka T, Slovák V, 2017. Biosorption (removing) of Cd (II), Cu (II) and methylene blue using biochar produced by different pyrolysis conditions of beech and spruce sawdust. Wood Sci. Technol 51(6), 1321–1338. [Google Scholar]
- Uchimiya M, Chang S, Klasson KT, 2011. Screening biochars for heavy metal retention in soil: role of oxygen functional groups. J. Hazard. Mater 190(1), 432–441. [DOI] [PubMed] [Google Scholar]
- Usman A, Sallam A, Zhang M, Vithanage M, Ahmad M, Al-Farraj A, Ok YS, Abduljabbar A, Al-Wabel M, 2016. Sorption process of date palm biochar for aqueous Cd (II) removal: Efficiency and mechanisms. Water Air Soil Pollut. 227(12), 449. [Google Scholar]
- Wang Q, 1998. Integrated amendment and ecological restoration of polluted soils by heavy metals. Proc. Strategy Soil Environ. Protect. New Century of China, 26–29. [Google Scholar]
- Webb S, 2005. SIXpack: a graphical user interface for XAS analysis using IFEFFIT. Phys. Scr 2005(T115), 1011. [Google Scholar]
- World Health Organization. 2011. Cadmium in drinking-water. Available at: http://www.who.int/water_sanitation_health/dwq/chemicals/cadmium.pdf.
- Xu X, Cao X, Zhao L, Wang H, Yu H, and Gao B. 2013. Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ. Sci. Pollut. Res 20:358–368. [DOI] [PubMed] [Google Scholar]
- Zambon I, Colosimo F, Monarca D, Cecchini M, Gallucci F, Proto AR, Lord R, Colantoni A, 2016. An innovative agro-forestry supply chain for residual biomass: Physicochemical characterisation of biochar from olive and hazelnut pellets. Energies 9(7), 526. [Google Scholar]
- Zhao FJ, Ma Y, Zhu YG, Tang Z, McGrath SP, 2015. Soil Contamination in China: Current Status and Mitigation Strategies. Environ. Sci. Technol, 49(2), 750–759. [DOI] [PubMed] [Google Scholar]
- Zhao FJ, Zhu YG, Meharg AA, 2013. Methylated arsenic species in rice: geographical variation, origin, and uptake mechanisms. Environ. Sci. Technol 47(9), 3957–3966. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Liao B, Lin L, Qiu W, Song Z 2018. Adsorption of Cu(II) and Cd(II) from aqueous solutions by ferromanganese binary oxide–biochar composites. Sci. Total Environ. 615, 115–122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.