Abstract
Background:
Patients with primary refractory Hodgkin lymphoma or early relapse have a poor prognosis. Although many salvage regimens have been developed, there is no standard of care.
Methods.
Children’s Oncology Group protocol AHOD1221 (NCT01780662) tested Brentuximab vedotin with gemcitabine in children and young adults with primary refractory Hodgkin Lymphoma or early relapse. Eligibility criteria included age ≤30 years; no prior Brentuximab vedotin exposure; and relapse <1 year from completion of initial therapy. Each 21-day cycle consisted of intravenous gemcitabine 1000mg/m2 on days 1 and 8 and Brentuximab vedotin on day 1 at 1·4 mg/kg or 1·8 mg/kg. The primary objectives were to determine the recommended phase 2 dose of Brentuximab vedotin in this combination, and the complete response rate among those treated at this dose level, within four cycles of therapy, on an intention to treat basis.
Findings:
46 patients enrolled between 5 February 2013 and 19 August 2016, including one who was found to be ineligible. The recommended phase 2 dose of Brentuximab vedotin was 1·8 mg/kg. Twenty-four of 42 patients (57%; 95% CI 41–72%) treated at this dose level experienced a complete response within the first four cycles. Four of 13 (31%) patients with partial response or stable disease had all target lesions with Deauville scores ≤ 3 after cycle 4. By modern response criteria, these are also complete responses, increasing the complete response rate to 28 of 42 (67%, 95% CI, 51–80%). There were no treatment-related deaths. The most common grade 3–4 adverse events among all subjects treated at the recommended phase 2 dose included neutropenia (15 of 42, 36%), rash (15 of 42, 36%), transaminitis (9 of 42, 21%), and pruritus (4 of 42, 10%).
Interpretation:
Brentuximab vedotin with gemcitabine is a highly active combination for patients with primary refractory Hodgkin lymphoma or high-risk relapse. Peripheral blood stem cells can be collected successfully following Brentuximab vedotin with gemcitabine, making this an effective regimen when autologous stem cell transplantation is indicated. Compared to alternate second-line regimens, Brentuximab vedotin with gemcitabine offers the advantage of avoiding agents associated with late treatment sequelae, such as anthracyclines, alkylators, or epipodophyllotoxins.
Funding:
National Institutes of Health and the St. Baldrick’s Foundation
Keywords: Brentuximab vedotin, gemcitabine, Hodgkin lymphoma, relapsed, refractory disease, chemotherapy, thymus and activation regulated cytokine, salvage therapy, peripheral blood stem cells
Introduction
Patients with early relapse of Hodgkin lymphoma or primary refractory disease have historically experienced poor disease-free survival.1 Although there exist second-line chemotherapy regimens with high overall response rates, these typically include alkylators (e.g. ifosfamide or bendamustine), anthracyclines and/or epipodophyllotoxins, which may be associated with significant treatment-related toxicity or result in secondary malignancy.1,2
Children’s Oncology Group protocol AHOD1221 evaluated a novel therapeutic combination: Brentuximab vedotin with gemcitabine. Brentuximab vedotin is an antibody-drug conjugate containing an anti-CD30 murine/human chimeric monoclonal antibody (cAC10; brentuximab) covalently linked by an enzyme-cleavable peptide linked to monomethylauristatin E (vedotin), a microtubule disrupting agent. Brentuximab vedotin produced a 34% complete response rate among adults with relapsed Hodgkin lymphoma,3 and has been tested in combination with conventional chemotherapy.4,5 In addition, Brentuximab vedotin has demonstrated safety in pediatric patients.6 Children’s Oncology Group protocol AHOD0321 demonstrated that gemcitabine can be safely combined with a microtubule inhibitor, vinorelbine, among patients with Hodgkin lymphoma refractory to two or more lines of therapy.7,8 The clinical study of the combination of Brentuximab vedotin and gemcitabine is further supported by preclinical data demonstrating that CD30 targeting sensitizes lymphoma cells to gemcitabine.9,10
Patients and Methods
Study design and participants
AHOD1221 was a single-arm, non-randomized trial, registered at ClinicalTrials.gov ( NCT01780662). Pediatric and young adult patients less than 30 years of age were eligible if they had primary refractory Hodgkin lymphoma (i.e. never achieved a complete response with frontline therapy), or high-risk relapse (relapse ≤ 6 months from the completion of initial therapy, or advanced disease (stages III or IV) at initial diagnosis with relapse < 1 year from the completion of initial therapy). Exclusion criteria included prior Brentuximab vedotin exposure, prior stem cell transplantation, or low-stage initial disease (stages IA or IIA) treated with less than four cycles of chemotherapy or radiation alone. Additional eligibility requirements included measurable disease, life expectancy of at least 8 weeks, adequate bone marrow, pulmonary, neurologic, renal and hepatic function, and performance status (≥50%, evaluated by the Karnofsky scale for subjects over 16 years of age, and Lansky score for patients under 16 years of age). All subjects were required to have fully recovered from the acute toxic effects of all prior therapy. At least 14 days must have elapsed after the last dose of myelosuppressive chemotherapy, and 28 days after the last dose of nitrosourea or bleomycin. To exclude progression at inclusion, imaging studies to determine eligibility must have been completed within 14 days of the start of study therapy. Patients with clinical or radiographic evidence of progression (increase in any target lesion by more than 25%) prior to the start of therapy would be excluded. Informed consent was obtained from the patient or guardian in accordance with institutional policies and as approved by the US Department of Health and Human Services and in accordance with the Declaration of Helsinki.
Procedures
A limited-institution phase 1 trial evaluated two Brentuximab vedotin dose levels. The starting dose was 1.4 mg/kg, with a second, maximum dose level of 1.8 gm/kg. The recommended phase 2 dosse was defined as the maximum dose at which fewer than one third of patients experience dose limiting toxicity. Escalation from dose level 1 to 2 proceeded according to the rules of a 3+3 design. If none of the initial three patients at dose level 1 experienced a toxicity, the dose is escalated to dose level 2. If one of the first three patients at dose level 2 experienced a dose limiting toxicity, three more would be accrued at the same level. On 23 December 2013, the protocol was amended to expand dose level 2, with accrual of at least six additional subjects, because two of the first six subjects at this level experienced reversible non-hematologic dose limiting toxicities (described in detail below).
Brentuximab vedotin was given on day 1 of every 21-day cycle, and gemcitabine was administered on days 1 and 8 of each cycle, at 1000 mg/m2/dose over 100 minutes. Filgrastim (GCSF) was recommended only for patients who experienced prolonged grade 4 neutropenia (5 micrograms/kg/dose intravenously or subcutaneously once daily from day 9 until the post-nadir neutrophil count increases to ≥1,500/uL), or in preparation for collection of peripheral blood stem cells (per institutional guidelines).
In order to monitor for adverse events, a history and physical examination were required weekly during cycle 1, and days 1 and 8 of each subsequent cycle. Complete blood counts, electrolytes, calcium, magnesium and phosphate were measured weekly during cycle 1 and on day 1 of every subsequent cycle. Additional evaluations were conducted as clinically indicated. Expedited reporting of adverse events was required for any pulmonary toxicity, any grade 3 or 4 toxicity that precipitates hospitalization, and death within 30 days of study therapy.
Definitions of dose limiting toxicity are provided in the Appendix, page 1. The dose of brentuximab vedotin was reduced to 1.2 mg/kg for grade 4 thrombocytopenia that does not resolve to platelets >20,000/uL within 7 days of the next scheduled dose, for non-hematologic toxicity meeting the definition for dose limiting toxicity, or for grade 2–3 peripheral neuropathy.
Response was evaluated after every even cycle of therapy, with both functional imaging (flurodeoxyglucose-positron emission tomography; FDG-PET) and computed tomography (CT). complete response was defined by FDG-PET negativity (Deauville score 1–2) regardless of residual lesion size. Partial response was defined by residual FDG avidity (Deauville score 3–5) in at least one involved site, and at least a 50% decrease in the sum of the product of the perpendicular diameters of up to six of the largest target lesion, or reduction in involved lymph nodes to normal size. Progressive disease was defined by an increase ≥ 50% in the product of the perpendicular diameters for any target lesion or development of a new measurable site of disease. Stable disease was defined by not meeting criteria for complete response, partial response, or progressive disease. All images were reviewed centrally at the Imaging and Radiation Oncology Core / Quality Assurance Review Center (IROC/QARC; Providence, RI), for confirmation of response assessment. A second, retrospective analysis was conducted after the publication of updated response criteria,14 which refined the definition of complete response to include subjects where all lesions were Deauville score ≤ 3.
In the absence of progressive disease patients were required to receive a minimum of four cycles of therapy, with the exception that patients with complete response after two cycles of therapy were allowed the option to come off study therapy to pursue autologous stem cell transplantation. A maximum of 16 cycles of therapy was allowed.
Patients were removed from protocol therapy if they had evidence of radiographic disease, experienced adverse events requiring cessation of therapy, completed 16 cycles of therapy, elected to undergo stem cell transplantation with a complete response after 2 cycles, or with stable disease or any response after four cycles.
Peripheral blood stem cell collection was allowed after any cycle of therapy, following the standard operating procedures of each institution. Daily filgrastim administration, 10 micrograms/kg/dose, beginning on day 9 of a cycle, was recommended to mobilize peripheral blood stem cells. A successful collection was defined by a minimum of 2 × 106 CD34 positive cells/kg.
Serum TARC was measured using singleplex ELISA (ThermoFisher/Life Technologies) calibrated against known standards. Genomic DNA was isolated from peripheral blood mononuclear cells collected at baseline. The presence of the FcγRIIIa valine allotype (rs396991) was detected by PCR-based allelic discrimination (Applied Biosystems, Waltham MA).
Outcomes
The primary objectives for AHOD1221 were to define the recommended phase 2 dose of Brentuximab vedotin when given in combination with gemcitabine, to describe the toxicity of this combination, and to determine the complete response rate within four cycles of treatment with Brentuximab vedotin and gemcitabine. The recommended phase 2 dose was defined by the dose at which fewer than one third of patients experience a dose limiting toxicity in cycle 1 of therapy. The maximum dose tested for brentuximab vedotin was set, a priori, at the dose approved for use in adults, 1.8 mg/kg/dose.
Secondary objectives included a description of the proportion of patients able to mobilize an adequate yield of CD34 positive stem cells, as well as analysis of biomarkers that may relate to treatment response or toxicity: thymus and activation-regulated chemokine (TARC; CCL17), and the prevalence of the FcγRIIIa receptor 158 valine allotype (rs396991).
Statistical Analysis
The sample size for the phase 1, dose finding component of the study was determined by a 3+3 dose escalation design. All patients treated with at least one dose of Brentuximab vedotin and gemcitabine were eligible for analysis of toxicity. Patients were evaluable for response if they were treated with at least four cycles of therapy at the recommended phase 2 dose. Patients with progressive disease within the first four cycles, or had a centrally-confirmed complete response after 2 cycles and were taken off study therapy for autologous stem cell transplantation were also evaluable for response.
The phase 2 component of the study was designed to test the hypothesis that the complete response rate after Brentuximab vedotin plus gemcitabine is greater than the 46% complete response rate observed among patients treated with gemcitabine plus vinorelbine on AHOD0321.8 All subjects treated at the recommended phase 2 dose of brentuximab vedotin were evaluable for this primary objective, on an intention to treat basis, including those enrolled in the phase 1, dose finding component of the study The phase 2 component was designed to accrue up to 41 patients evaluable for the promary response endpoint. Considering a 15% rate for ineligible/inevaluable patients, this component could have enrolled a maximum of 48 subjects. A Simon 2-stage MinMax rule was used to test whether the complete response rate within four cycles of Brentuximab vedotin plus gemcitabine was at least 60%, resulting in an interim analysis after the first 28 eligible patients underwent central response review. With this rule, combination therapy would have been deemed insufficiently effective if ≤ 11 CRs were observed in the first 28 patients, or ≤ 20 CRs were observed in a maximum of 41 evaluable patients. The type I and type II error were both 0·1.
A Wilcoxon rank sum test was used to compare TARC levels after each cycle, relative to baseline, and to compare the change in levels from baseline between patients with a complete response versus those without a complete response. Longitudinal mixed model analyses of absolute reduction in serum TARC and of proportional reduction in serum TARC relative to baseline were conducted, considering complete response status and cycle number as fixed effects, and patient identification number as a random effect.
Overall survival was defined as time from study entry to death due to any cause. Patients alive at last contact were censored. Kaplan-Meier product-limit method was used to estimate overall survival probability, along with the Greenwood standard error estimates and 95% confidence intervals based on the log-log transformation. All analyses were conducted using SAS 9·4 (SAS Inc. Cary, North Carolina).
Role of the funding sources.
The funding sources had no role in study design, collection, analysis or interpretation of the data, or writing of this report. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
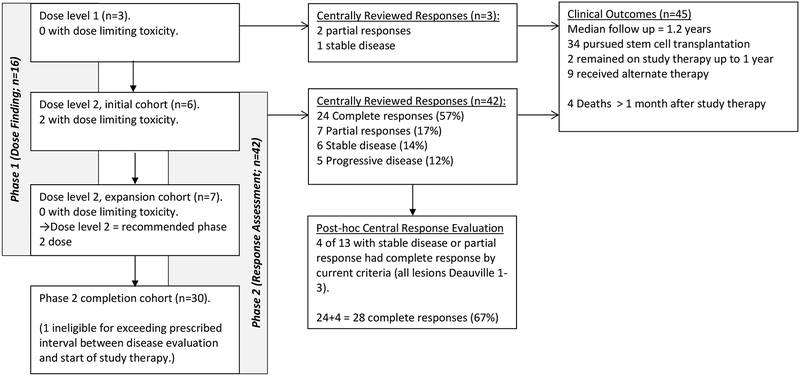
Figure 1 illustrates the trial diagram and outcomes for 46 patients enrolled on AHOD1221 between 5 February 2013 and 19 August 2016, including one who was found to be ineligible, due to exceeding the prescribed interval between disease evaluation and study entry. Baseline characteristics are shown in Table 1. Median follow up for all eligible patients is 15 months (IQR, 9–24 months). Sites from which patients were recruited are listed in the Appendix, page 2
Figure 1. Trial Diagram.
Outcome for all patients enrolled on study. Subjects in both the Phase 1 (dose finding) and Phase 2 (complete response rate) portions are included. Subjects were treated at either dose level 1 (1.4 mg/kg/dose of Brentuximab vedotin) or dose level 2 (1.8 mg/kg/dose of Brentuximab vedotin). Results of primary outcomes and clinical outcomes are indicated.
Table 1.
Baseline Characteristics of the Eligible Subjects (N=45)
| Characteristic | |
|---|---|
| Age, years | |
| Median | 17·6 |
| Range | 5·4 – 28·7 |
| Interquartile Range | 15·3 – 18·6 |
| Sex, N (%) | |
| Male | 21 (47%) |
| Female | 24 (53%) |
| Race, N (%) | |
| Black | 8 (18%) |
| White | 31 (69%) |
| Other or Unknown | 6 (13%) |
| Ethnicity, N (%) | |
| Hispanic | 10 (22%) |
| Non-Hispanic | 35 (78%) |
| Country of Residence, N (%) | |
| Canada | 2 (4%) |
| USA | 43 (96%) |
| Disease Status at enrollment, N (%) | |
| Primary refractory (i.e. no prior complete response) | 29 (64%) |
| Early relapse < 6 months after initial therapy | 9 (20%) |
| Advance stage (III or IV) with relapse > 6 months after initial therapy | 7 (16%) |
| Performance Status at Enrollment, N (%) | |
| Normal (100%) | 21 (47%) |
| Minor symptoms or restrictions in activity (90%) | 19 (42%) |
| Tires more quickly; some signs or symptoms of disease (80%) | 5 (11%) |
| Stage at start of therapy with Brentuximab vedotin + gemcitabine | |
| IA, IIA, IIIA | 19 (42%) |
| IIB, IIIB | 9 (20%) |
| IVA, IVB | 17 (38%) |
None of the three (0%) patients treated at dose level 1 experienced dose-limiting toxicity. Two of the first six patients (33%) treated at dose level 2 experienced a dose limiting toxicity: one had asymptomatic elevation of hepatic transaminases that required longer than three weeks to return to baseline, and a second had hypotension after each gemcitabine dose, and was subsequently demonstrated to have adrenal insufficiency due to prior corticosteroid therapy. Both of these patients remained on study therapy, at a reduced dose of Brentuximab vedotin (1.2 mg/kg), and received a total of 4 and 5 cycles of therapy respectively. None of seven patients (0%) treated in a dose level 2 expansion cohort experienced a dose-limiting toxicity. Dose level 2, 1·8 mg/kg/dose of Brentuximab vedotin, was selected as the recommended phase 2 dose, because fewer than one third of subjets treated at this dose (2 of 13, 15%) experienced dose limiting toxicity.
Common adverse events reported among patients treated at the recommended phase 2 dose are shown in Table 2. In addition, any grade 1–2 toxicity occurring in ≥10% of patients in any cycle of therapy and all grade 3 and 4 adverse events are shown in Table 3, stratified by grade. Grade 4 neutropenia was the most frequent hematologic toxicity, reported in 15 (36%) of 42 subjects during cycle 1. Dermatologic reactions and elevations of hepatic transaminases were the most frequent non-hematologic adverse events. Maculopapular rash and/or pruritus was reported in 16 of 42 patients treated at the recommended phase 2 dose (38%) and were more common among female (13 of 23; 57%) than male subjects (3 of 19; 16%; P=0·007). Thirteen of 45 treated patients (29%) experienced serious adverse events that were considered possibly or probably attributable to study therapy, and met criteria for expedited reporting. Among these, hypotension was most frequent, occurring in 3 of 45 (7%). One patient developed myositis during the fifth cycle of therapy and was taken off study therapy.
Table 2: Common Adverse Events during therapy with Brentuximab vedotin and gemcitabine.
A. All Grade 4 hematologic toxicity. B. Non-hematologic toxicity ≥ Grade 3, occurring in 10% of subjects or more.
| Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | |
| Leucopenia | 5 (12%) | 3 (7%) | 3 (12%) | 3 (9%) |
| Neutropenia | 15 (36%) | 11 (26%) | 5 (19%) | 9 (35%) |
| Febrile neutropenia | 2 (5%) | |||
| Anemia | 1 (2%) | 2 (5%) | 0 (0%) | 1 (4%) |
| Thrombocytopenia | 3 (7%) | 4 (10%) | 4 (15%) | 6 (22%) |
| Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | |
| Rash, maculopapular | 15 (36%) | 4 (10%) | 1 (4%) | 1 (4%) |
| Pruritus | 4 (10%) | 2 (5%) | 0 (0%) | 0 (0%) |
| Alanine aminotransferase, increased | 9 (21%) | 6 (14%) | 2 (8%) | 0 (0%) |
| Aspartate aminotransferase, increased | 7 (17%) | 4 (10%) | 2 (8%) | 0 (0%) |
Table 3: Adverse events occurring in any cycle of therapy.
Included in this table are any Grade 1 or 2 adverse event occurring in more than 10% of subjects, as well as all grade 3 and grade 4 adverse events. There were no grade 5 adverse events. For each adverse event, the number and percentage of 45 treated subjects is shown.
| CTCAE4.0 Grade | CTCAE4.0 Adverse Events | N (%) |
|---|---|---|
| 1 & 2 | Alanine aminotransferase increased | 11 (24%) |
| 1 & 2 | Aspartate aminotransferase increased | 10 (22%) |
| 1 & 2 | Nausea | 10 (22%) |
| 1 & 2 | Lymphocyte count decreased | 7 (16%) |
| 1 & 2 | Platelet count decreased | 7 (16%) |
| 1 & 2 | Rash maculo-papular | 7 (16%) |
| 1 & 2 | White blood cell decreased | 7 (16%) |
| 1 & 2 | Anemia | 6 (13%) |
| 1 & 2 | Alanine aminotransferase increased | 5 (11%) |
| 1 & 2 | Aspartate aminotransferase increased | 5 (11%) |
| 3 | Alanine aminotransferase increased | 14 (31%) |
| 3 | Neutrophil count decreased | 14 (31%) |
| 3 | Aspartate aminotransferase increased | 11 (24%) |
| 3 | White blood cell decreased | 10 (22%) |
| 3 | Lymphocyte count decreased | 8 (18%) |
| 3 | Platelet count decreased | 7 (16%) |
| 3 | Anemia | 5 (11%) |
| 3 | Diarrhea | 3 (7%) |
| 3 | Hypotension | 3 (7%) |
| 3 | Rash maculo-papular | 3 (7%) |
| 3 | Febrile neutropenia | 2 (4%) |
| 3 | Hypophosphatemia | 2 (4%) |
| 3 | Nausea | 2 (4%) |
| 3 | Abdominal pain | 1 (2%) |
| 3 | Adrenal insufficiency | 1 (2%) |
| 3 | Anorexia | 1 (2%) |
| 3 | BACTEREMIA | 1 (2%) |
| 3 | Dehydration | 1 (2%) |
| 3 | Dental caries | 1 (2%) |
| 3 | GGT increased | 1 (2%) |
| 3 | Hemolytic uremic syndrome | 1 (2%) |
| 3 | Lung infection | 1 (2%) |
| 3 | Myositis | 1 (2%) |
| 3 | NEUTROPHIL COUNT DECREASE | 1 (2%) |
| 3 | Non-cardiac chest pain | 1 (2%) |
| 3 | Pain in extremity | 1 (2%) |
| 3 | Pneumonitis | 1 (2%) |
| 3 | Pruritus | 1 (2%) |
| 3 | Skin infection | 1 (2%) |
| 3 | Urinary tract infection | 1 (2%) |
| 4 | Neutrophil count decreased | 25 (56%) |
| 4 | White blood cell decreased | 12 (27%) |
| 4 | Platelet count decreased | 9 (20%) |
| 4 | Lymphocyte count decreased | 5 (11%) |
| 4 | Hypotension | 1 (2%) |
| 4 | Lung infection | 1 (2%) |
| 4 | Pericardial effusion | 1 (2%) |
Pulmonary events were reported in two of the 45 treated subjects (4%). Both were taken off study therapy. An 18-year-old with a preexisting paralyzed hemidiaphragm (secondary to biopsy of Hodgkin lymphoma nodules within the pulmonary parenchyma) developed low grade fever, cough, and hypoxia on day 16 of the third cycle of therapy, with radiographic evidence of atelectasis and pericardial and pleural effusions. Her condition improved within 24 hours of initiating corticosteroid therapy and supportive care. A second 18-year-old was found to have an asymptomatic FDG-avid pulmonary consolidation during a scheduled response evaluation after the second cycle of therapy. Severe respiratory compromise developed over the following weeks. An open biopsy documented active Mycobacterium avium-intracellulare infection. Review of both cases by the study team in collaboration with the NCI/Cancer Therapy Evaluation Program concluded that neither case was consistent with pneumonitis caused by study therapy.
The three patients treated at dose level 1 received two, four and eight cycles of therapy, respectively. Two had a partial response and one had stable disease.
Patients treated at dose level 2 received a median of four cycles of therapy (range 2–16). Of the 18 subjects treated at dose level 2 with only two cycles, five were taken off study therapy due to progression. One with stable disease was taken off study due to pulmonary infection, described above. The remaining 12 had complete response and were taken off study therapy to undergo autologous stem cell transplantation.
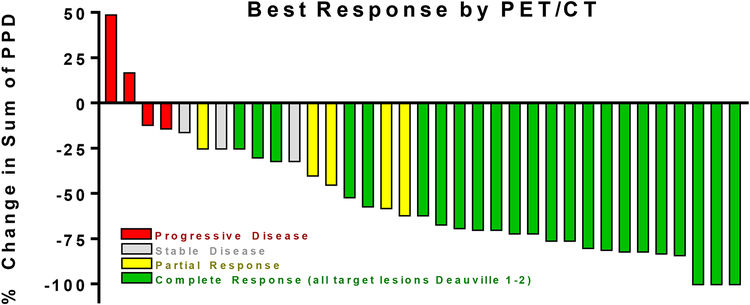
Twenty-four of 42 evaluable patients (57%; 95% CI, 41–72%) had a complete response meeting protocol criteria (all target lesions were Deauville score 1–2 on FDG-PET scan), confirmed on central review. Twenty-one of these 24 (88%) had their complete response after the first two cycles of therapy. The overall response rate (24 complete responses plus 7 partial responses) was 31 of 42 evaluable patients (74%; 95% CI, 58–86%). Five patients (12·2%) experienced progressive disease at or before the first response evaluation. The remaining six patients had stable disease. A waterfall plot, illustrating reduction in target lesion size is shown in Figure 2, for 35 subjects with interpretable CT measurements of all target lesions at baseline and follow-up imaging. No subject first experienced a complete response later than the evaluation after the fourth cycle.
Figure 2. Maximum tumor volume reduction after treatment with Brentuximab vedotin and gemcitabine.
Thirty-five of 42 patients treated at the recommended phase 2 dose had centrally reviewed CT measurements of all target lesions at baseline and following the last cycle of therapy. The maximum change in tumor volume, estimated by the change from baseline in the sum of the product of the perpendicular diameters, is shown in this waterfall plot. For seven patients, tumor volume reduction could not be accurately calculated for one or more of the following reasons: Pretreatment target lesion(s) included FDG-avid non-measurable extranodal sites; pre-or post-treatment target lesions were too small to accurately measure in 2 dimensions on CT; or post treatment progression was diagnosed by clinical symptoms, physical examination, and FDG-PET scan without CT measurement.
For four of the 13 patients with stable disease or partial response, all target lesions were Deauville score ≤ 3 on central review, and would thus be considered CRs by response criteria published after AHOD1221 opened.14 By these criteria, the complete response rate observed on AHOD1221 was 28 of 42 patients (67%; 95% CI, 51–80%).
There were no treatment-related deaths. There have been a total of four deaths among study participants, reported at 3, 9, 18, and 36 months after completion of study therapy. Two were related to complications of progressive disease, and two were related to complications of subsequent therapy (chimeric antigen T-cell therapy, and stem cell transplantation, respectively). Overall survival was conducted as a post-hoc analysis, as it was neither a primary nor secondary objective of the study. One year overall survival is 95% (95% CI, 80–99%) among all eligible patients (Appendix, page 3). Thirty-four patients who came off study therapy have undergone stem cell transplantation.
Peripheral blood stem cell collection was attempted for 24 of 45 subjects (53%), most often after two cycles of therapy (n=16), with a range of 1 to 5 cycles. The median yield was 8·7 × 106 CD34 positive cells/kg (range 3·5–36·8 × 106 cells/kg). Successful stem cell collection, defined by protocol as a collection of more than 2×106 CD34 positive cells, was achieved in all 24 subjects (100%). For 21 subjects, peripheral blood stem cell collection was not attempted during protocol therapy, either because stem cells had been collected prior to study enrollment, or no autologous stem cell transplant was planned.
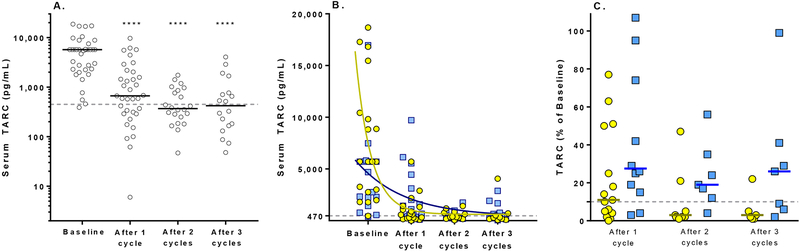
Median serum TARC at baseline was 5700 pg/mL (range 389–18,667 pg/mL). Thirty-eight patients had baseline TARC measurements, and 36 (95%) had baseline values higher than the upper limit of normal in healthy controls (470 pg/mL).15 The median serum TARC decreased to 668 pg/mL (range 6–9718; n=35) after one cycle of therapy (Figure 3A). Thirty three patients had serum TARC measurements at both baseline and after one cycle. Paired analysis demonstrated a significant decrease of serum TARC from baseline (P < 0·0001; Wilcoxon signed rank test). After two cycles, the median serum TARC decreased further to 368 pg/mL (range 47–1749; n=22), and remained significantly lower than baseline (P <0·0001; Wilcoxon signed rank test). A longitudinal mixed model analysis of changes in serum TARC with treatment indicated a greater absolute reduction from baseline among patients who experienced a complete response than among patients without a complete response (progressive disease, stable disease or partial response; p=0·0002; Figure 3B). There was no statistically significant difference in the median proportional reduction over time between these two groups. However, patients who experienced a complete response tended to be more likely to have ≥90% reduction in serum TARC after two cycles than those without a complete response (8 of 12 with a complete response vs. 1 of 7 without a complete response; P = 0·04; one-sided Fisher’s exact test; Figure 3C).
Figure 3. Change in serum TARC after treatment with Brentuximab vedotin and gemcitabine.
A. Serum TARC decreases significantly from baseline, within one cycle of therapy. Each circle represents an individual patient. Solid bars indicate the median value. The dotted green line indicates the upper limit of serum TARC among healthy controls, 470 pg/mL. ****, P<0·0001, compared to baseline. B. Serum TARC after treatment with Brentuximab vedotin and gemcitabine among individual patients who experienced a complete response (yellow circles) vs. remaining patients (progressive disease, partial response, stable disease; blue squares). Solid lines indicate the nonlinear fit of serum TARC data for each group. The dotted gray line indicates the upper limit of serum TARC among healthy controls, 470 pg/mL. C. Change in serum TARC after treatment with Brentuximab vedotin and gemcitabine, shown as % of baseline (100 × serum TARC at the indicated timepoint divided by baseline TARC) among patients with a complete response (open white circles) vs. remaining patients (progressive disease, partial response, stable disease; shaded squares). Lower values indicate a greater reduction from baseline. Values above 100 indicate an increase in serum TARC from baseline. Bold horizontal bars indicate median values. The dotted horizontal line indicates 10% of baseline, i.e. a 90% reduction with therapy.
Forty-one subjects consented for genetic studies and had germline DNA suitable for analysis of the FcγRIIIa polymorphism. Twenty-two (54%) were homozygous for the wild-type phenylalanine allele; five (12%) were homozygous for the variant valine allele; and the remaining 14 (34%) were heterozygous. Of these 41 subjects, 37 were treated at the recommended phase 2 dose and were evaluable for response. Subjects who were homozygous for the wild-type allele were more likely to have a radiographic complete response within four cycles of Brentuximab vedotin and gemcitabine therapy (defined by all lesions Deauville score ≤3) than those who carried at least one variant allele (80% vs. 47%; P=0·04; one-sided Fisher’s exact test). Because there was no pulmonary toxicity attributable to protocol therapy, we could not associate the FcγRIIIa polymorphism with this adverse event.
Discussion
Brentuximab vedotin with gemcitabine is a highly active combination for pediatric and young adult patients with primary refractory or early relapsed Hodgkin lymphoma, a population at higher risk for being refractory to second-line chemotherapy.2 The clinical objective for most patients with primary refractory or early relapse of Hodgkin lymphoma is to achieve a complete response with salvage therapy. This study was designed to define the complete response rate within four cycles of brentuximab vedotin and gemcitabine, with the rationale that the absence of a rapid complete response indicates therapy-refractory disease and the need for an alternate salvage regimen. Here we showed that more than 80% of the complete responses observed in this study occurred after just two cycles, allowing subjects to rapidly proceed to consolidation with high-dose chemotherapy with autologous stem cell rescue. Although cross-trial comparisons must be done with caution, the observed complete response rate is comparable to that seen after other contemporary second-line regimens,4,17–20 Brentuximab vedotin with gemcitabine offers the additional advantages of outpatient administration, and of not including alkylating agents, anthracyclines, or epipodophyllotoxins. This combination therefore offers a high complete response rate, with the potential for decreased acute and long-term toxicity in a population likely to become long-term survivors. Grade 3–4 neutropenia, asymptomatic elevation of hepatic transaminases, and rash were the most frequently observed adverse events, and were not typically associated with delays in therapy. Hypersensitivity reactions were uncommon, compared to the frequency described after Brentuximab vedotin with bendamustine.18,21–23 Significant peripheral neuropathy was similarly uncommon, presumably because risk of this toxicity is associated with increasing cumulative exposure to Brentuximab vedotin, while subjects on this study received a median of only four cycles. Our observation that maculopapular rash was more common among female subjects echoes an earlier report of cutaneous reactions after a different gemcitabine-containing regimen (ifosfamide, gemcitabine, and vinorelbine; IGEV).24 The etiology of this sex difference remains unknown.
Pulmonary events were monitored closely, because grade 3–5 pneumonitis has been described in five of 23 adults treated with the unconjugated monoclonal antibody to CD30 (SGN30) in combination with gemcitabine, vinorelbine, and pegylated liposomal doxorubicin.13 Excess pulmonary toxicity was also observed when Brentuximab vedotin was combined with bleomycin, doxorubicin, vinblastine and dacarbazine.5 However, no pulmonary toxicity attributable to study therapy was observed following Brentuximab vedotin with gemcitabine in this study. This result suggests (1) that the antibody-drug conjugate, Brentuximab vedotin, may trigger less off-target toxicity than the unconjugated monoclonal anti-CD30 antibody, and (2) that, unlike bleomycin, gemcitabine can be safely combined with Brentuximab vedotin.
Responses on this study were associated with two biomarkers, TARC and a FcγRIIIa polymorphism. Decreases in serum TARC have been correlated with disease response in adults with advanced stage11,25 or relapsed Hodgkin lymphoma.26 To our knowledge, this is the first report demonstrating the utility of this biomarker specifically in pediatric patients with relapsed or refractory Hodgkin lymphoma. Further evaluation is necessary to define the optimal timing for evaluating change in serum TARC, as well as the threshold for TARC reduction that maximizes sensitivity and specificity for predicting radiographic complete response.
Subjects in this study were screened for the FcγRIIIa 158 valine allotype (present in 28% of the general population), because all five adults who developed pulmonary toxicity after treatment with SGN30, gemcitabine, vinorelbine and pegylated liposomal doxorubicin carried this variant. In addition to a potential impact on toxicity, presence of this polymorphism could impact the efficacy of Brentuximab vedotin. The valine allotype displays a higher affinity for immunoglobulin G1 than the wild-type phenylalanine, resulting in increased antibody-dependent cytotoxicity,12 thereby increasing response rates for monoclonal antibodies such as rituximab in some studies.27,28 Interestingly, we observed the opposite relationship: a lower complete response rate among subjects with one or more variant valine alleles. As an antibody-drug conjugate, Brentuximab vedotin is thought to exert cytotoxicity primarily through selective delivery of a microtubule disrupting agent to CD30 positive cells,29 a mechanism independent of antibody-dependent cellular cytotoxicity. It is possible that NK cells expressing the variant FcγRIIIa bind Brentuximab vedotin more tightly, preventing internalization and release of the cytotoxic monomethylauristatin E, thus reducing cytotoxicity. Confirmation of this hypothesis will require additional study.
The primary limitation to this study is that patients with prior exposure to Brentuximab vedotin were excluded from enrolling. Brentuximab vedotin is increasingly being incorporated into initial therapy for patients with Hodgkin lymphoma. Although some patients with relapsed Hodgkin lymphoma will respond to Brentuximab vedotin more than once30, it is possible that the Brentuximab complete response rate will be lower among Hodgkin lymphoma patients who relapse after prior Brentuximab vedotin therapy than what was observed in this study of Brentuximab vedotin-naive patients. An additional limitation is that this Phase 1/2 study was not designed as a randomized trial, prohibiting any direct comparison of results after this combination with other recently-published salvage regimens. The numbers of patients required for statistical power to detect significant differences in outcomes among regimens would have required many additional years for completion, and a randomized design would not have been consistent with the primary purpose of this study, to define the safety and complete response rate of Brentuximab vedotin with gemcitabine among pediatric and young adult patients with refractory hodgkin lymphoma.
Conclusions
Brentuximab vedotin with gemcitabine is a highly active, outpatient salvage regimen for children and young adults with primary refractory Hodgkin lymphoma or early relapse, with tolerable toxicity.
Supplementary Material
Research In Context.
Evidence before this study
Patients with primary refractory Hodgkin lymphoma or early relapse have a poor prognosis. Although many salvage regimens have been developed, there is no agreed standard of care. We searched PubMed on 01/02/2018 for clinical studies, with no restrictions on language or publication date, using the search terms “brentuximab vedotin” and “gemcitabine” and did not find any studies reporting on the use of this combination. Each of these two agents has been previously shown to be active among patients with relapsed or refractory Hodgkin lymphoma when used as monotherapy, and each has been successfully used in combination with other agents. Preclinical data suggest that brentuximab vedotin can sensitize lymphoma cells to gemcitabine, supporting the use of the combination.
Added value of this study
Given the activity of each agent in patients with relapsed or refractory Hodgkin lymphoma, with non-overlapping clinical toxicity profiles, we tested the combination. The safety profile of this outpatient regimen and high complete response rate are a major advance in the field of treatment for patients with Hodgkin lymphoma.
Implications of all the available evidence
Highly active salvage regimens for patients with relapsed or refractory Hodgkin lymphoma typically include anthracyclines, platinums, alkylating agents, and/or epipodophyllotoxins, which can increase risk for post-transplant morbidity as well as long term complications. In contrast, the combination of brentuximab vedotin with gemcitabine, an outpatient regimen with a favorable toxicity profile, reduces the toxicity burden and may be expected to reduce long-term complications of curative therapy. The immediate implication is that this regimen can be considered a viable first-line salvage regimen for children and young adults with relapsed or refractory Hodgkin lymphoma.
Acknowledgements of research support:
Supported in part by a grant from the National Institutes of Health to the Children’s Oncology Group (U10CA098543), NCTN Operations Center Grant U10CA180886, NCTN Statistics & Data Center Grant U10CA180899, Phase 1/Pilot Consortium Grant UM1CA097452, and the St. Baldrick’s Foundation. The authors declared no conflicts of interest.
Footnotes
Publisher's Disclaimer: Disclaimers: None
References
- 1.Kelly KM. Hodgkin lymphoma in children and adolescents: improving the therapeutic index. Blood. 2015;126(22):2452–8. [DOI] [PubMed] [Google Scholar]
- 2.Harker-Murray PD, Drachtman RA, Hodgson DC, Chauvenet AR, Kelly KM, Cole PD. Stratification of treatment intensity in relapsed pediatric Hodgkin lymphoma. Pediatric Blood & Cancer. 2014;61(4):579–86. [DOI] [PubMed] [Google Scholar]
- 3.Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a Pivotal Phase II Study of Brentuximab Vedotin for Patients With Relapsed or Refractory Hodgkin’s Lymphoma. Journal of Clinical Oncology. 2012;30(18):2183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michallet A-S, Guillermin Y, Deau B, Lebras L, Harel S, Amorin S, et al. Sequential combination of gemcitabine, vinorelbine, pegylated liposomal doxorubicin and brentuximab as a bridge regimen to transplant in relapsed or refractory Hodgkin lymphoma. Haematologica. 2015;100(7):e269–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younes A, Connors JM, Park SI, Fanale M, O’Meara MM, Hunder NN, et al. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin’s lymphoma: a phase 1, open-label, dose-escalation study. The Lancet Oncology. 2013;14(13):1348–56. [DOI] [PubMed] [Google Scholar]
- 6.Flerlage JE, Metzger ML, Wu J, Panetta JC. Pharmacokinetics, immunogenicity, and safety of weekly dosing of brentuximab vedotin in pediatric patients with Hodgkin lymphoma. Cancer Chemotherapy and Pharmacology. 2016;78(6):1217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole PD, Schwartz CL, Drachtman RA, de Alarcon PA, Chen L, Trippett TM. Phase II study of weekly gemcitabine and vinorelbine for children with recurrent or refractory Hodgkin’s disease: a children’s oncology group report. J Clin Oncol. 2009;27(9):1456–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole PD, McCarten KM, Drachtman RA, Alarcon Pd, Chen L, Trippett TM, et al. Early [18F]fluorodeoxyglucose positron emission tomography-based response evaluation after treatment with gemcitabine and vinorelbine for refractory Hodgkin disease: a children’s oncology group report. Pediatric Hematology & Oncology. 2010;27(8):650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerveny CG, Law CL, McCormick RS, Lenox JS, Hamblett KJ, Westendorf LE, et al. Signaling via the anti-CD30 mAb SGN-30 sensitizes Hodgkin’s disease cells to conventional chemotherapeutics. Leukemia. 2005;19(9):1648–55. [DOI] [PubMed] [Google Scholar]
- 10.Heuck F, Ellermann J, Borchmann P, Rothe A, Hansen H, Engert A, et al. Combination of the human anti-CD30 antibody 5F11 with cytostatic drugs enhances its antitumor activity against Hodgkin and anaplastic large cell lymphoma cell lines. Journal of Immunotherapy. 2004;27(5):347–53. [DOI] [PubMed] [Google Scholar]
- 11.Plattel WJ, Alsada ZN, Imhoff GW, Diepstra A, Berg A, Visser L. Biomarkers for evaluation of treatment response in classical Hodgkin lymphoma: comparison of sGalectin‐1, sCD163 and sCD30 with TARC. British Journal of Haematology. 2016;175(5):868–875. [DOI] [PubMed] [Google Scholar]
- 12.Koene HR, Kleijer M, Algra J, Roos D, von dem Borne AE, de Haas M. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood. 1997;90(3):1109–14. [PubMed] [Google Scholar]
- 13.Blum KA, Jung S-H, Johnson JL, Lin TS, Hsi ED, Lucas DM, et al. Serious pulmonary toxicity in patients with Hodgkin’s lymphoma with SGN-30, gemcitabine, vinorelbine, and liposomal doxorubicin is associated with an FcγRIIIa-158 V/F polymorphism. Annals of Oncology. 2010;21(11):2246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for Initial Evaluation, Staging, and Response Assessment of Hodgkin and Non-Hodgkin Lymphoma: The Lugano Classification. Journal of Clinical Oncology. 2014; 32(27):3059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plattel WJ, van den Berg A, Visser L, van der Graaf AM, Pruim J, Vos H, et al. Plasma thymus and activation-regulated chemokine as an early response marker in classical Hodgkin lymphoma. Haematologica. 2011; 97:410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santoro A, Bredenfeld H, Devizzi L, Tesch H, Bonfante V, Viviani S, et al. Gemcitabine in the treatment of refractory Hodgkin’s disease: results of a multicenter phase II study. Journal of Clinical Oncology. 2000;18(13):2615–9. [DOI] [PubMed] [Google Scholar]
- 17.Santoro A, Mazza R, Pulsoni A, Re A, Bonfichi M, Zilioli VR, et al. Bendamustine in Combination With Gemcitabine and Vinorelbine Is an Effective Regimen As Induction Chemotherapy Before Autologous Stem-Cell Transplantation for Relapsed or Refractory Hodgkin Lymphoma: Final Results of a Multicenter Phase II Study. Journal of Clinical Oncology. 2016;34(27):3293–9. [DOI] [PubMed] [Google Scholar]
- 18.Kalac M, Lue JK, Lichtenstein E, Turenne I, Rojas C, Amengual JE, et al. Brentuximab vedotin and bendamustine produce high complete response rates in patients with chemotherapy refractory Hodgkin lymphoma. British journal of haematology. 2016; 180(5):757–60. [DOI] [PubMed] [Google Scholar]
- 19.Trippett TM, Schwartz CL, Guillerman RP, Gamis AS, Gardner S, Hogan S, et al. Ifosfamide and vinorelbine is an effective reinduction regimen in children with refractory/relapsed Hodgkin lymphoma, AHOD00P1: A children’s oncology group report. Pediatric Blood & Cancer. 2014;62(1):60–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moskowitz CH, Bertino JR, Glassman JR, Hedrick EE, Hunte S, Coady-Lyons N, et al. Ifosfamide, carboplatin, and etoposide: a highly effective cytoreduction and peripheral-blood progenitor-cell mobilization regimen for transplant-eligible patients with non-Hodgkin’s lymphoma. Journal of Clinical Oncology. 1999;17(12):3776–85. [DOI] [PubMed] [Google Scholar]
- 21.Cerchione C, Di Perna M, Della Pepa R, Pugliese N, Pane F, Picardi M. High-dose bendamustine plus brentuximab combination is effective and has a favourable toxicity profile in the treatment of refractory and relapsed Hodgkin lymphoma. Hematological Oncology. 2017;35:317–8.26450521 [Google Scholar]
- 22.Gallamini A, Bijou F, Viotti J, Rossi A, Perrot A, Patti C, et al. Brentuximab-vedotin And Bendamustine Is A Feasible And Effective Drug Combination As First-Line Treatment Of Hodgkin Lymphoma In The Elderly (HALO TRIAL). Hematological Oncology. 2017;35:170. [Google Scholar]
- 23.LaCasce AS, Bociek G, Sawas A, Caimi PF, Agura E, Matous J, et al. Brentuximab vedotin plus bendamustine: a highly active salvage treatment regimen for patients with relapsed or refractory Hodgkin lymphoma. Am Soc Hematology; 2015;126:3982. [Google Scholar]
- 24.Paszkiewicz-Kozik E, Romejko-Jarosinska J, Brzeska B, Osiadacz W, Swierkowska-Czeneszew M, Osowiecki M, et al. Unexpected cutaneous toxicity of IGEV regimen in patients with Hodgkin lymphoma. Journal of Clinical Oncology. 2011;29(15_suppl):e18543. [Google Scholar]
- 25.Plattel W, Van Den Berg A, Van der Graaf AM, Vos H, Visser L, Diepstra A, et al. , editors. Mid-Treatment Plasma Levels of Thymus Activated and Regulated Chemokine (TARC) Predict Treatment Outcome In Classical Hodgkin Lymphoma Patients [Abstract 748] American Society of Hematology Annual Meeting; 2010; Orlando, FL. [Google Scholar]
- 26.Moskowitz AJ, Cho S, Fleisher M, Woo KM, Zhang Z, Fox S, et al. TARC Predicts PET-Normalization and Event Free Surival in Relapsed/Refractory Hodgkin Lymphoma Patients Treated with Brentuximab Vedotin. Blood. 2015;126(23):18. [Google Scholar]
- 27.Burkhardt B, Yavuz D, Zimmermann M, Schieferstein J, Kabickova E, Attarbaschi A, et al. Impact of Fc gamma-receptor polymorphisms on the response to rituximab treatment in children and adolescents with mature B cell lymphoma/leukemia. Annals of Hematology. 2016;95(9):1503–12. [DOI] [PubMed] [Google Scholar]
- 28.Mellor JD, Brown MP, Irving HR, Zalcberg JR, Dobrovic A. A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. Journal of hematology & oncology. 2013;6(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katz J, Janik JE, Younes A. Brentuximab Vedotin (SGN-35). Clinical Cancer Research. 2011;17(20):6428–36. [DOI] [PubMed] [Google Scholar]
- 30.Bartlett NL, Chen R, Fanale MA, Brice P, Gopal A, Smith SE, et al. Retreatment with brentuximab vedotin in patients with CD30-positive hematologic malignancies. Journal of Hematology & Oncology. 2014;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.