Abstract
Objective
This study evaluates the roles of medical and social complexity in health care use outcomes in cystic fibrosis (CF) after transfer from pediatric to adult care.
Methods
Retrospective cohort design included patients with CF who were transitioned into adult care at Indiana University from 2005 to 2015. Predictor variables included demographic and comorbidity data, age at transition, treatment complexity score (TCS), and an objective scoring measure of their social complexity (Bob’s Level of Social Support, BLSS). Outcome variables included outpatient visit rates and hospitalization rates. Pearson’s correlations and linear regression were used to analyze the data.
Results
The median age of the patients (N=133) at the time of transition was 20 (IQR 19–23) years. The mean FEV1 % predicted at transition was 69 ± 24%. TCS correlated with outpatient visit rates (r = 0.3, p = 0.003), as well as hospitalization rates (r = 0.4, p < 0.001); while the BLSS only correlated with hospitalization rates (r = 0.7, p < 0.001). After adjusting for covariates, the strongest predictors of post-transfer hospitalizations are BLSS (p <0.0001) and pre-transfer hospitalization rate (p <0.0001).
Conclusion
Greater treatment complexity is associated with greater healthcare utilization overall, while greater social complexity is associated with increased hospitalizations (but not planned outpatient visits). Screening young adults for social complexity will identify high-risk subpopulations and allow for patient centered interventions to support them and prevent avoidable health care use.
Keywords: Social Dimensions of Pulmonary Medicine, Cystic Fibrosis (CF), health care transition, social complexity, health care outcomes
INTRODUCTION
Cystic fibrosis (CF) is the most common multi-organ genetic disease in the Caucasian population affecting more than 70,000 people worldwide1. Due to advances in medical treatment, outcomes have improved over the last several decades2. In 2014, 50.7% of patients in the US CF Patient Registry (CFPR) were 18 years old and over, and this number has continued to increase1,3. The rising adult demographic underscores the need for adult-oriented care and age-appropriate management. The US Cystic Fibrosis Foundation (CFF) has long supported the development of adult CF programs. However, best practices surrounding transfer of care from pediatric to adult programs rely heavily on expert opinion, and the lack of outcomes data has left providers to make transition-related decisions without evidence-based guidelines4.
Adolescence is a vulnerable developmental period where puberty and brain maturation lead to new behaviors that set the future trajectory toward health and well-being5. The literature regarding social determinants of health (SDH) in adolescence is expanding5,6. Income, education, and family support influence adolescent health throughout the transition into adulthood5. SDH add another layer to medical complexity for patients with CF, likely influencing a patient’s readiness to transition, increasing the support required to successfully transfer care, and affecting general health outcomes.
While data demonstrate that transfer to adult care does not necessarily exacerbate lung function decline in patients with CF, there is a paucity of data exploring health care utilization after transfer7,8. Additionally, the effects of SDH on health services outcomes are poorly understood. Most of the current research with SDH in CF explores associations between clinical outcomes and various patient or family psychosocial factors. Health care utilization data, such as adherence, hospitalizations, emergency department visits, and outpatient visits may better reflect outcomes related to social and psychological characteristics, rather than clinical outcomes alone9-12.
This study evaluates the roles of medical and social complexity on health care utilization outcomes in CF after transfer into adult care. We hypothesize that individuals with high disease complexity or high social complexity experience increased health care utilization after transfer.
METHODS
A retrospective cohort study was conducted using data from patients in the CFF-accredited CF program at Indiana University (IU). All patients with a diagnosis of CF who transferred to the adult CF program at IU from January 2005 to December 2015 were included. Patients were diagnosed with CF by clinical features confirmed with either elevated sweat chloride (≥ 60 mmol/L), or genotype with two identifiable CFTR mutations. Patients were included if they had received care at Riley Hospital for Children and then transferred to the IU Adult CF Program at University Hospital; they were excluded if they had transferred to the adult program from a center outside of IU. Overall 133 patients were eligible to be included in the study.
Data Collection
Data from the electronic medical record, CFPR, paper charting, and storage on body plethysmographers (Morgan Scientific, Inc., Haverhill, MA) was extracted to obtain the longitudinal data used in this study. Data points were collected at the time of transfer (time 0); the preceding 24 months, 12 months, and 6 months prior to transfer; and the subsequent 6 months, 12 months, and 24 months after transfer. Time 0 was determined to be the date at which a patient had the first visit with an adult provider. This occurred either as a transition visit (when the adult provider saw the patient at the children’s hospital), the first adult hospital admission without a previous adult outpatient visit, or the first appointment at the adult CF outpatient center.
General demographic data that is included in the CFPR such as age, gender, race, marital status, employment status, living situation, education, income and insurance were collected at time 0. CF specific comorbidities, such as Pseudomonas aeruginosa microbiology, sinus disease, CF-related diabetes, pancreatic insufficiency, liver disease, as well as anxiety and depression were collected as categorical variables (present or not) within 2 years of time 0. Anxiety and depression were considered present if the patient had a corresponding diagnosis code or there was documentation in the clinical notes mentioning one of these diagnoses. Absolute FEV1 at time 0 was converted to FEV1 % predicted using Hankinson’s formula for standardization13.
Outcome variables included average yearly number of outpatient visits pre- and post- transfer and average yearly number of hospitalizations pre- and post- transfer. Study data were collected and managed using REDCap electronic data capture tools14. One hundred and thirty-three patients were eligible for data collection; all were included in analysis for demographic data. Eleven patients did not have enough information to score the social complexity scale and were excluded from health services outcomes analyses.
Defining Treatment Complexity and Social Complexity
The treatment complexity score (TCS) has been previously published and is a composite number derived from 37 chronic CF therapies15. Higher scores signify a more complex medical regimen. The more labor intensive medications, such as insulin or multiple daily dosing of inhaled antibiotics, are given a score of 3; those that are less onerous, such as vitamins and thrice weekly azithromycin, are given a score of 1; and those intermediate, such as dornase alfa and pancreatic enzymes, are given a score of 215. In our study TCS was calculated based on the medication list at time 0 and used in correlations and as a covariate in logistic regression.
The Bob’s Level of Social Support scale (BLSS) was designed for use in a developmental pediatrics clinic to screen families’ needs for increased care management support (Nickel R. Bob’s Level of Social Support.; 2011)(Supplementary Data 1). We utilized BLSS as a measure of social complexity, defined as SDH having a direct or indirect impact on health outcomes. The BLSS is divided into five dimensions: overall health, family, behavioral and mental health, education, and special issues. Each is rated from 1–3, with total BLSS scores ranging from 5–15. Special issues include ability to follow through with recommendations, cultural issues as barriers to care, and ability to proactively manage care. Higher scores indicate the presence of more complex psychosocial factors. The BLSS is not a validated tool; it has not been studied to measure health outcomes. However, it is a complexity tool that is both available for public use and includes family and social factors. The BLSS score is based on documentation from social work, care management, and physician notes at time 0. BLSS scores were compiled retrospectively by EC, and CB performed a reliability audit of these scores.
Statistical Analysis
Averages and frequencies of demographic data were determined using descriptive statistics. Inter-rater reliability for the audited BLSS and TCS scores were calculated using intra-class correlation. Pearson product moment correlation was used to determine relationships between TCS, BLSS, and hospitalization rates and outpatient visit rates. Multivariable linear regression was used to predict post- transfer hospitalizations from several variables that were significant with bivariate linear regression: age at transfer, FEV1 % predicted at transfer, BLSS, TCS, depression, pre-transfer hospitalizations, post-transfer outpatient visits, and gaps in care.
22Covariates were included in the model if the p-value ≤ 0.05. All analyses were conducted with SPSS versions 23 and 24 (IBM Corp. New York, 2015) statistical software. The Institutional Review Board at IU approved this study (Protocol ID 1509912571).
RESULTS
Median age at transfer was 20 (IQR 19–23) years, and female patients comprised 48% of the group (Table 1). The demographic characteristics were comparable to the general adult CF population as reported in the CFPR1; however, we had more patients with Pseudomonas aeruginosa infection (64% vs 47.5%), and depression (32% vs 24.1%), and fewer patients with CF-related diabetes (25% vs 34.9%).
Table 1:
Characteristics of the study population in comparison to national population
| Study Population, N = 133 |
CFPR Data1 N= 28,983 |
|
|---|---|---|
| Age at transfer in years (IQR) | 20 (19–23) | N/A |
| Female, % | 48 | 48.4 |
| Caucasians, % | 97 | 93.8 |
| Employed, % | 47 | 48.8 |
| Living independently, % | 33 | N/A |
| High school or less education, % | 34 | 31 |
| Public insurance, % | 55 | 44.8 |
| FEV1, % predicted (SD) | 69 (24) | 67.1 |
| TCS (IQR) | 14 (10–18) | N/A |
| BLSS (SD) | 7 (6–9) | N/A |
Data presented as median (IQR, interquartile range) and mean (SD, standard deviation) unless otherwise specified
Public insurance includes Medicare/Medicaid and Children’s special health care
N/A, not collected for CFPR
CFPR, Cystic Fibrosis Patient Registry
TCS, treatment complexity score
BLSS, Bob’s level of social support
FEV1, Forced Expiratory Volume in 1 second
Inter-rater Reliability for Scales
Audited inter-rater reliability was completed for both the TCS and BLSS for a random sample of 50% (67/133) of the subjects. A high degree of reliability was found between the total scores on the TCS (κ = 0.93, CI 0.89, 0.96) and BLSS (κ = 0.93, CI 0.89, 0.96). Reliability was also tested between the individual scores of the different domains of the BLSS: health (κ = 0.71, CI 0.52, 0.83), family (κ = 0.87, CI 0.78, 0.92), behavioral and mental health (κ = 0.85, CI 0.75, 0.91), education (κ = 0.77, CI 0.62, 0.87), special issues (κ = 0.52, CI 0.19, 0.72).
Health Services Outcomes Analysis
Among the overall population, there was a decrease in the number of outpatient visits per year after transfer and an increase in the number of hospitalizations per year. However, this was not statistically significant (Table 2). A higher TCS correlated with outpatient visit rates before and after transfer (r = 0.31, p = 0.003, and r = 0.33, p < 0.001, respectively as well as hospitalization rate before and after transfer (r = 0.45, p < 0.001, and r = 0.41, p < 0.001, respectively). A higher BLSS did not correlate with outpatient visit rates before or after transfer (r = −0.03, p = 0.8, and r = 0.08, p = 0.36, respectively). However, there was a strong positive correlation between BLSS and hospitalization rates before and after transfer (r = 0.7, p < 0.001, and r = 0.72, p < 0.001, respectively).
Table 2:
Social complexity and treatment complexity associations with health care use outcomes
| Study Population, N = 122 |
Correlation with TCS (R) |
Correlation with BLSS (R) |
||
|---|---|---|---|---|
| Average annual | ||||
| outpatient visits (SD) | ||||
| Pre-transfer | 4.0 (2.8) | 0.31† | −0.028 | |
| Post-transfer | 3.6 (2.0) | 0.33* | 0.08 | |
| Average annual | ||||
| hospitalizations (SD) | ||||
| Pre-transfer | 1.1 (1.7) | 0.45* | 0.70* | |
| Post-transfer | 1.4 (2.3) | 0.41* | 0.72* | |
Data presented as mean (SD, standard deviation) unless otherwise specified
p <0.001
p <0.05
Multivariable linear regression was performed to ascertain the effects of gap in care (categorical variable defined as the number of days between the last pediatric and first adult visit or hospitalization: ≤ 100 days, 101–364 days, or ≥ 365 days), age at transfer, outpatient visits post-transfer, pre-transfer hospitalization rate, FEV1 % predicted at transfer, depression, BLSS, and TCS on the number of post-transfer hospitalizations. The model demonstrated BLSS and pre-transfer hospitalization rate to be the only significant predictors of post- transfer hospitalization (Table 3). After adjusting for covariates, for every one point increase in the BLSS, the number of hospitalizations will increase by 0.5 and for every hospitalization prior to transfer, the number of post-transfer hospitalizations will increase by 0.6. This relationship is illustrated graphically (Figure 1).
Table 3:
Social complexity has a strong influence on post-transfer hospitalization rate1
| Risk Factor | Coefficient (β) | Confidence Interval | P-value |
|---|---|---|---|
| BLSS | 0.46 | (0.28, 0.64) | < 0.0001 |
| Gap in Care | −0.26 | (−0.68, 0.16) | 0.22 |
| Outpatient Visit Rate (post-transfer, per year) | 0.08 | −0.08, 0.23 | 0.33 |
| FEV1 % predicted at transfer | −0.004 | (−0.02, 0.008) | 0.54 |
| Depression | 0.09 | (−0.27, 0.45) | 0.61 |
| TCS | 0.005 | (−0.05, 0.06) | 0.86 |
| Age at Transfer | −0.003 | (−0.05, 0.04) | 0.88 |
| Hospitalization Rate (pre-transfer, per year) |
0.60 | (0.36, 0.83) | < 0.0001 |
Variables included had a significant relationship with post-transfer hospitalization rate in bivariate analysis. adjusted R2 = 0.72, N = 122
Post-transfer Hospitalizations = (0.46 * BLSS) + (0.6 * pre-transfer hospitalization rate) – 2.7
BLSS, Bob’s level of social support
TCS, Treatment Complexity Scale
Figure 1:
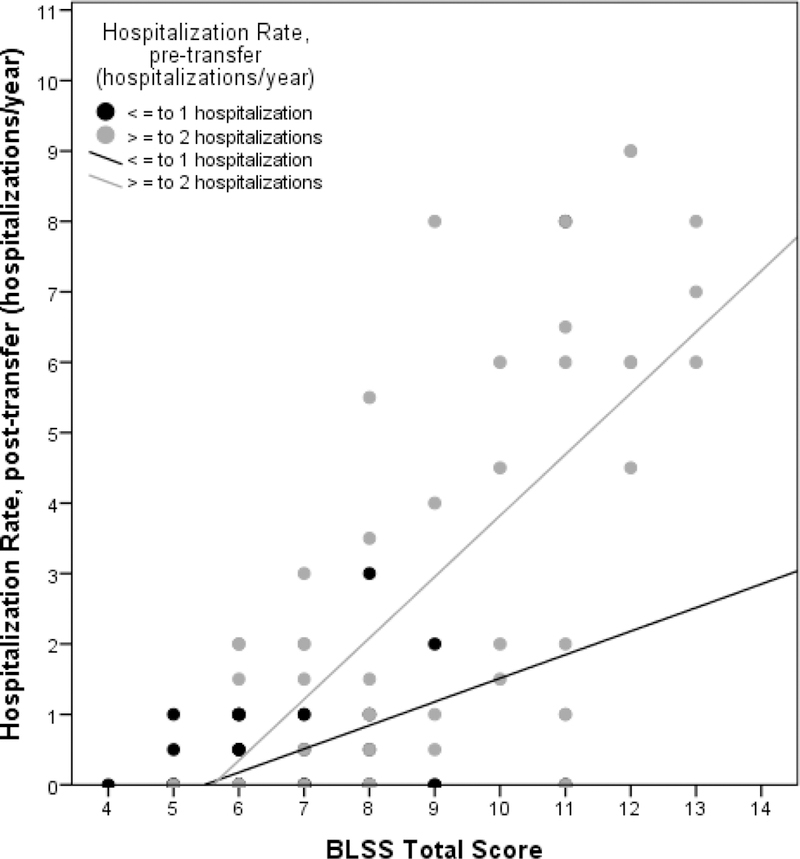
Subjects with increased social complexity and increased pre-transfer hospitalizations had increased hospitalizations after transfer to adult care to a greater degree than those with increased social complexity and fewer pre-transfer hospitalizations. This graph shows the distribution of patients when divided into 2 groups: ≤ 1 hospitalization in the 2 years prior to transfer (black) or ≥ 2 hospitalizations in the 2 years prior to transfer (grey). As the social complexity increases (BLSS score), so does the number of overall hospitalizations both before and after transfer. It also shows that in the patients with high BLSS, pre-transfer hospitalization seems to have an influence on the number of post-transfer hospitalizations.
Several points are overlapping.
BLSS, Bob’s level of social support
DISCUSSION
In adults with CF, treatment complexity correlated with health care utilization for both outpatient visits and hospitalizations, and social complexity significantly correlated with hospitalizations but not outpatient visits. Increased social complexity is a strong predictor of hospitalization after transfer to adult care even after accounting for treatment complexity and hospitalization rate prior to transfer.
Most commonly used disease complexity scores use medical data alone without consideration of environmental influences16. Previous literature in CF regarding SDH explores maternal education attainment, family discord, and socioeconomic status (SES) as factors affecting outcomes in CF. Low SES is associated with significantly poorer clinical outcomes in CF, including: lower FEV1, lower BMI, and more frequent pulmonary exacerbations17-20. We used a novel scale to measure social complexity, which incorporates several SDH, rather than SES alone.
Both treatment complexity and social complexity influenced health care utilization outcomes. It is known that SDH can negatively affect health care use despite equal access to care21. A study of the effect of SES, Medicaid status, and maternal education attainment in pediatric patients with CF showed that individuals with Medicaid were found to have the most outpatient sick visits. It further demonstrated that lower maternal education attainment was associated with having fewer exacerbations treated with home IV antibiotics, despite the number of stable visits being consistent across groups19. In a study of pediatric patients performed in Western Australia, marital discord and family psychological distress were significant predictors of increased hospitalizations, emergency, and outpatient visits in early life CF after adjusting for disease severity22. In a population of pediatric patients with noncomplex chronic conditions, it was found that having social complexity risk factors were significantly associated with the need for care coordination23.
As previously defined, the TCS was studied to evaluate change in medical complexity over time and site-specific health outcomes, but has not been used as a predictor of health-related outcomes15. This study demonstrated that the TCS significantly correlated with both outpatient visit rates and hospitalization rates before and after transfer. These results are intuitive, as patients’ disease progresses, their medical regimen is likely to become more complicated. Additionally, they are likely to be seen more frequently and have more frequent hospitalizations for disease-related issues.
A recent study validated social complexity risk factors in relation to care-giver reported need for care coordination23. This study used state administrative data to collect social complexity risk factors; several of these are the SDH captured by the BLSS. Like the TCS, the BLSS has not been studied or used as a predictor of health care outcomes. Strikingly, the BLSS correlated more strongly with hospitalization rates before and after transfer, while it did not correlate with outpatient visit rates. Arguably, hospitalizations are unplanned sequela of poor disease control, while outpatient visits are planned attempts to prevent these sequelae. These results support the hypotheses that patients with higher BLSS scores are more likely to have a lack of ability to navigate the health care system, unstable disease due to psychosocial factors out of proportion to disease phenotype, and a lack of resources or support outside of the healthcare environment that leads to hospitalization rather than outpatient visits.
When transferring a patient with CF into adult care, it is imperative to recognize and acknowledge not only the developmental needs but also the relevant SDH in order to communicate the need and provide appropriate levels of support to foster independence and successful self-care. While prospective validation of the BLSS needs to be performed, this study suggests that the BLSS may have value as a screening tool for health care use outcomes among this population. Prior observational analyses have shown that hospitalizations in patients with cystic fibrosis are independent predictors of lung function decline and that a pulmonary exacerbation treated with intravenous antibiotics is a strong risk factor for future courses of intravenous antibiotics24,25. Furthermore, SES, environmental factors, and insurance status may influence whether a patient receives intravenous antibiotics at home or is hospitalized for the duration of therapy. Taking the risk of a prior hospitalization leading to a future hospitalization into account, we included this in our multivariable analysis to see how it interacted with social complexity. We found that along with pre-transfer hospitalization rate, the BLSS is a strong contributor to the post-transfer hospitalization rate. This result is consistent with the correlations between BLSS scores and hospitalizations, both before and after transfer of care, as those patients with high BLSS sustained increased hospitalizations.
Our study has several limitations. First, it is a retrospective review from a single center. One of the challenges of research surrounding transition is the variability across center practices. While the transition practices at Indiana University might not be generalizable, our patient population was consistent with that in the 2015 CFPR. Second, the time period of our study was over 4 years and this might not be enough time to fully appreciate a clinical decline in otherwise stable patients, and two years after transfer might not be long enough to capture those patients who become fully independent. We collected the CF-specific comorbidities and demographic variables of marital status, employment status, schooling, etc at the time of transfer. We chose this approach in an attempt to better understand the population transferring into our adult program. However, it is likely that these specific demographics would change in the four years of this study, particularly over the age range of transfer (19–23 years old) in this study. Transition and transfer not only reflect changes in medical care but also in life, specifically as an adolescent moves towards independence. These changes would likely have an influence on the outcomes measured in the two years after transfer, in either a positive or negative way. For instance, if a subject was employed, you would hypothesize that this could be a positive factor - in that they now have steady insurance through an employer. This could also be a negative effect, in that now they have less time for treatments. Likewise, you might hypothesize that the subject’s educational attainment might heavily influence their health literacy. Thirdly, the TCS score was based on a medication list, rather than patient interview or refill records and does not account for patient adherence. Lastly, neither the TCS nor BLSS have been validated to predict health care outcomes. While the BLSS appears to be a valuable tool, and we worked to operationalize definitions for each domain relevant to the cystic fibrosis population, another limitation of this scale is the “Special Issues” domain. This domain is a broad category that is probably influenced most by subjective assessment. It was scored in the moderate range, as the least closely aligned item and may benefit from more clarity of anchor definitions.
CONCLUSION
Our study demonstrates a strong correlation between complicated social situations and increased, sustained hospitalization rates before and after transfer to adult care. Additionally, social complexity as measured by the BLSS, along with pre-transfer hospitalizations are significant predictors of future hospitalizations. From this study, we can conclude that those subjects with increased social complexity sustain an increased rate of hospitalization before and after transfer, whereas outpatient visits decrease. This study is an early step towards understanding the important psychosocial and family factors that influence health care utilization outcomes in the CF population. Future studies are required to externally validate the BLSS, as a quick and complete screening tool to identify patients who are at risk for increased health care utilization. This could lead to successful interventions delivered through a high-risk period of time to mitigate worsening illness and its associated health care costs by avoiding preventable crises.
Supplementary Material
ACKNOWLEDGMENTS
• Erin Crowley is the guarantor of the content of the manuscript, including the data and analysis.
• EC had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. CB, MC, BK, and GB contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
• The authors have indicated they have no financial relationships relevant to this article to disclose.
• The authors would like to thank Dr. Robert Nickel for providing his permission and support to use the BLSS.
Funding Source: Dr. Brown is supported by the Cystic Fibrosis Foundation.
Dr. Khan is supported through awards from the National Institute on Aging (K23AG043476), and the National, Heart, Lung, and Blood Institute (R01HL131730)
Footnotes
Financial Disclosure: The authors have indicated they have no financial relationships relevant to this article to disclose.
Conflict of Interest: The authors have indicated they have no potential conflicts of interest to disclose.
Prior Abstract Publication/Presentation:
Crowley, E; Bosslet, G; Khan, B; Ciccarelli, M; Brown, C. Social Complexity as a Determinant of Outcomes in Cystic Fibrosis after Transition to Adult Care [abstract]. Pediatric Pulmonology. 2016;51(suppl):450S.
North American Cystic Fibrosis Conference, Orlando, Florida. October 2016
REFERENCES
- 1.Foundation CF. Patient Registry Annual Data Report. Bethesda, Maryland 2015. [Google Scholar]
- 2.Spoonhower KA, Davis PB. Epidemiology of Cystic Fibrosis. Clin Chest Med 2016;37:1–8. [DOI] [PubMed] [Google Scholar]
- 3.Foundation CF. Patient Registry Annual Data Report. Bethesda, Maryland 2014. [Google Scholar]
- 4.American Academy of P, American Academy of Family P, American College of P, Transitions Clinical Report Authoring G, Cooley WC, Sagerman PJ. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics 2011;128:182–200. [DOI] [PubMed] [Google Scholar]
- 5.Viner RM, Ozer EM, Denny S, et al. Adolescence and the social determinants of health. Lancet 2012;379:1641–52. [DOI] [PubMed] [Google Scholar]
- 6.Braveman P, Egerter S, Williams DR. The social determinants of health: coming of age. Annu Rev Public Health 2011;32:381–98. [DOI] [PubMed] [Google Scholar]
- 7.Dugueperoux I, Tamalet A, Sermet-Gaudelus I, et al. Clinical changes of patients with cystic fibrosis during transition from pediatric to adult care. J Adolesc Health 2008;43:459–65. [DOI] [PubMed] [Google Scholar]
- 8.Tuchman L, Schwartz M. Health outcomes associated with transition from pediatric to adult cystic fibrosis care. Pediatrics 2013;132:847–53. [DOI] [PubMed] [Google Scholar]
- 9.Fair C, Cuttance J, Sharma N, et al. International and Interdisciplinary Identification of Health Care Transition Outcomes. JAMA Pediatr 2016;170:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fredericks EM, Magee JC, Opipari-Arrigan L, Shieck V, Well A, Lopez MJ. Adherence and health-related quality of life in adolescent liver transplant recipients. Pediatr Transplant 2008;12:289–99. [DOI] [PubMed] [Google Scholar]
- 11.Kipps S, Bahu T, Ong K, et al. Current methods of transfer of young people with Type 1 diabetes to adult services. Diabet Med 2002;19:649–54. [DOI] [PubMed] [Google Scholar]
- 12.Yeung E, Kay J, Roosevelt GE, Brandon M, Yetman AT. Lapse of care as a predictor for morbidity in adults with congenital heart disease. Int J Cardiol 2008;125:62–5. [DOI] [PubMed] [Google Scholar]
- 13.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159:179–87. [DOI] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawicki GS, Ren CL, Konstan MW, et al. Treatment complexity in cystic fibrosis: trends over time and associations with site-specific outcomes. J Cyst Fibros 2013;12:461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon TD, Cawthon ML, Stanford S, et al. Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics 2014;133:e1647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quittner AL, Schechter MS, Rasouliyan L, Haselkorn T, Pasta DJ, Wagener JS. Impact of socioeconomic status, race, and ethnicity on quality of life in patients with cystic fibrosis in the United States. Chest 2010;137:642–50. [DOI] [PubMed] [Google Scholar]
- 18.Schechter MS, Margolis PA. Relationship between socioeconomic status and disease severity in cystic fibrosis. J Pediatr 1998;132:260–4. [DOI] [PubMed] [Google Scholar]
- 19.Schechter MS, McColley SA, Silva S, Haselkorn T, Konstan MW, Wagener JS. Association of socioeconomic status with the use of chronic therapies and healthcare utilization in children with cystic fibrosis. J Pediatr 2009;155:634-9.e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schechter MS, Shelton BJ, Margolis PA, Fitzsimmons SC. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. Am J Respir Crit Care Med 2001;163:1331–7. [DOI] [PubMed] [Google Scholar]
- 21.Pincus T, Esther R, DeWalt DA, Callahan LF. Social conditions and self-management are more powerful determinants of health than access to care. Ann Intern Med 1998;129:406–11. [DOI] [PubMed] [Google Scholar]
- 22.Douglas T, Green J, Park J, Turkovic L, Massie J, Shields L. Psychosocial characteristics and predictors of health-care use in families of young children with cystic fibrosis in Western Australia. J Paediatr Child Health 2016;52:34–9. [DOI] [PubMed] [Google Scholar]
- 23.Schrager SM, Arthur KC, Nelson J, et al. Development and Validation of a Method to Identify Children With Social Complexity Risk Factors. Pediatrics 2016;138. [DOI] [PubMed] [Google Scholar]
- 24.Sanders DB, Li Z, Laxova A, et al. Risk factors for the progression of cystic fibrosis lung disease throughout childhood. Ann Am Thorac Soc 2014;11:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubin JL, Thayer S, Watkins A, Wagener JS, Hodgkins PS, Schechter MS. Frequency and costs of pulmonary exacerbations in patients with cystic fibrosis in the United States. Curr Med Res Opin 2017;33:667–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


