Abstract
Feeding by the tobacco specialist Manduca sexta (Lepidoptera, Sphingidae) and application of larval oral secretions and regurgitant (R) to mechanical wounds are known to elicit: (a) a systemic release of mono- and sesquiterpenes, (b) a jasmonate burst, and (c) R-specific changes in transcript accumulation of putatively growth- and defense-related mRNAs in Nicotiana attenuata Torr. ex Wats. We identified several fatty acid-amino acid conjugates (FACs) in the R of M. sexta and the closely related species Manduca quinquemaculata which, when synthesized and applied to mechanical wounds at concentrations comparable with those found in R, elicited all three R-specific responses. Ion-exchange treatment of R, which removed all detectable FACs and free fatty acids (FAs), also removed all detectable activity. The biological activity of ion-exchanged R could be completely restored by the addition of synthetic FACs at R-equivalent concentrations, whereas the addition of FAs did not restore the biological activity of R. We conclude that the biological activity of R is not related to the supply of FAs to the octadecanoid cascade for endogenous jasmonate biosynthesis, but that FACs elicit the herbivore-specific responses by another mechanism and that the insect-produced modification of plant-derived FAs is necessary for the plant's recognition of this specialized herbivore.
Feeding by Manduca sexta (Lepidoptera, Sphingidae) larvae on Nicotiana attenuata Torr. ex Wats. elicits responses clearly different from those induced by careful mechanical simulation of larval feeding. The wound-induced increase in jasmonic acid (JA) levels is amplified by herbivore feeding and by application of larval oral secretions and regurgitant (R) to mechanical wounds (McCloud and Baldwin, 1997; Schittko et al., 2000), whereas the wound-induced increase in nicotine-accumulation, which strongly correlates with wound-induced JA-levels (Baldwin et al., 1994a, 1997) is suppressed (Baldwin, 1988; McCloud and Baldwin, 1997) by an ethylene-burst released by the plant after herbivore attack (Kahl et al., 2000). Furthermore, herbivore feeding and R application to plant wounds are also known to induce the release of several mono- and sesquiterpenes in N. attenuata (Halitschke et al., 2000; Kahl et al., 2000), which, in turn, are thought to function as an indirect defense, guiding parasitoids to feeding larvae. Moreover, whereas both the volatile release and nicotine-accumulation can be elicited by the application of jasmonates to plants (Baldwin, 1999; Halitschke et al., 2000; Kahl et al., 2000), only the wound-induced nicotine-response is suppressed by application of inhibitors of endogenous JA-biosynthesis (Baldwin et al., 1997; Halitschke et al., 2000). In short, R results in direct and indirect defense responses in this specialist herbivore-plant system, and these responses appear to involve the octadecanoid cascade.
In addition to these well-described phenotypic responses to herbivory, extensive transcriptional re-organization was recently revealed by mRNA differential display of N. attenuata in response to M. sexta feeding. In 1/20th of the insect-responsive transcriptome, 27 genes displayed altered expression patterns (Hermsmeier et al., 2001). A subset of seven genes was found to differentially respond to R as compared with mechanical damage. Larval R of M. sexta and Manduca quinquemaculata antagonistically (type I genes) or synergistically (type II genes) modified wound-induced transcriptional responses of these seven genes (Schittko et al., 2001). Given that chemical attributes of larval feeding mediate extensive changes in transcript accumulation and phenotypic responses, characterization of active components of R is of great interest.
Two types of elicitors have been identified in lepidopteran R that result in the release of plant volatiles responsible for attracting parasitic wasps. First, an enzymatic elicitor, β-glucosidase, isolated from Pieris brassicae R, was shown to elicit the release of parasitoid-attracting volatile emissions from cabbage leaves. This elicitor is thought to release signal compounds by cleaving stored glycosidic precursors (Hopke et al., 1994; Mattiacci et al., 1995). Second, volicitin, N-(17-hydroxylinolenoyl)-l-Gln, a fatty acid-amino acid conjugate (FAC) identified in the R of Spodoptera exigua induces the release of volatiles in corn plants (Zea mays) comparable with that induced by larval feeding (Alborn et al., 1997; Turlings et al., 2000). Volicitin and several structurally related FACs have been identified in R of different lepidopteran species (Paré et al., 1998; Pohnert et al., 1999a; Alborn et al., 2000).
Because linolenic acid is a precursor of JA in the octadecanoid cascade, the inducing activity of the FACs may be due to the supply of fatty acid substrates introduced to the plant after hydrolytic cleavage of the FAC amide-bond (Koch et al., 1999). This mechanism is supported by investigations with the lima bean (Phaseolus lunatus) in which (a) Treatment of leaves with free linolenic acid results in the release of volatiles comparable with that elicited by treatments with N-linolenoyl-l-Gln (18:3-Gln), and (b) treatment of the leaves with inhibitors of the octadecanoid pathway suppresses the volatile response elicited by the application of free fatty acids (FAs; Koch et al., 1999). Unfortunately, in these studies the FAs and FACs were supplied in concentrations far exceeding those found in larval R. Moreover, other mechanisms that do not invoke substrate supply for the octadecanoid pathway can account for the activity of the conjugates. For example, the conjugates may be recognized by specific receptors that subsequently trigger the octadecanoid pathway.
Here we identify and quantify the FAs and FACs in the R of M. sexta and M. quinquemaculata larvae, synthesize these FACs, and investigate their role in eliciting the volatile release, endogenous JA-accumulation and changes in transcript accumulation of six mRNAs of N. attenuata that are known to be specifically altered by R from M. sexta and M. quinquemaculata. We critically evaluate the biological roles of the identified compounds by removing all FAs and FACs in R by anion-exchange chromatography and add back synthetic FAs and FACs to the ion-exchanged R (exR) at naturally occurring concentrations.
RESULTS AND DISCUSSION
Chemical Analysis of R
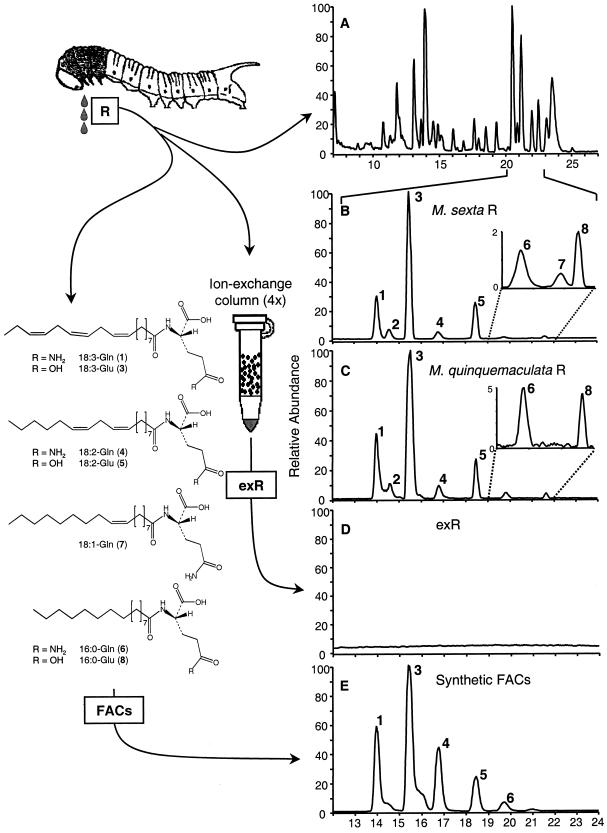
Oral secretions and regurgitant of M. sexta larvae fed on N. attenuata foliage were separated by HPLC (gradient C-18: CH3CN/H2O; 0.5% [v/v] HAc; 0.7 mL/min: 0% [v/v] CH3CN, 20–25 min 100% [v/v] CH3CN) and analyzed by atmospheric pressure chemical ionization-mass spectrometry. Analysis of fragmentation patterns (Pohnert et al., 1999a) revealed the presence of a series of FACs as minor constituents in the medium polar region of the chromatogram (Fig. 1A). Adjustment of the separation conditions (HPLC gradient C18: CH3CN/H2O; 0.5% [v/v] HAc; 0.7 mL/min: 40% [v/v] CH3CN, 7 min 68% [v/v] CH3CN, 18 min 80% [v/v] CH3CN, 28 min 100% [v/v] CH3CN) allowed us to separate the FACs (Fig. 1, B and C) and identify seven structurally related FACs by comparison of their retention times and APCI mass spectra with synthetic references (see “Materials and Methods”). The FACs in the R of M. sexta are dominated by Glu conjugates of C16- and C18-fatty acids (Fig. 1B, compounds 3, 5, and 8), which contrasts with the composition of seven other lepidopteran larvae R, which in turn, are dominated by the Gln conjugates of these fatty acids (Paré et al., 1998; Pohnert et al., 1999a; Alborn et al., 2000). The Gln conjugates (Fig. 1B, compounds 1, 4, and 6) are relatively minor components of M. sexta R compared with the corresponding Glu conjugates. This unusual composition was also found in nearly identical relative ratios in the R of another specialist herbivore of N. attenuata, M. quinquemaculata (Fig. 1C). Remarkably, no functionalized FACs (e.g. volicitin), often present in the R of lepidopteran larvae (Alborn et al., 1997, 2000; Pohnert et al., 1999a; Turlings et al., 2000), could be detected in the R of M. sexta or M. quinquemaculata.
Figure 1.
Scheme of experimental setup of the ion-exchange approach and structures of identified FACs (left) and HPLC-MS-Base peak profiles of 10-μL injections of test solutions (right): A, oral secretions and regurgitant (R) from M. sexta larvae. HPLC gradient (C18): CH3CN/H2O; 0.5% (v/v) HAc; 0.7 mL/min: 0% (v/v) CH3CN, 20 to 25 min 100% (v/v) CH3CN. Separation of the FACs in M. sexta (B) and M. quinquemaculata (C) R: HPLC gradient (C18): CH3CN/H2O; 0.5% (v/v) HAc; 0.7 mL/min: 40% (v/v) CH3CN, 7 min 68% (v/v) CH3CN, 18 min 80% (v/v) CH3CN, 28 min 100% (v/v) CH3CN. 1, N-linolenoyl-l-Gln; 2, unidentified; 3, N-linolenoyl-l-Glu; 4, N-linoleoyl-l-Gln; 5, N-linoleoyl-l-Glu; 6, N-palmitoyl-l-Gln; 7, N-oleoyl-l-Gln; and 8, N-palmitoyl-l-Glu. Base peak profiles of ion-exchanged M. sexta R (D) and mixture of synthetic FACs at concentrations found in M. sexta R (E) analyzed with the HPLC gradient as in B and C.
The total concentration of FACs in the R of M. sexta reared on fresh N. attenuata foliage in the laboratory varied from 0.6 to 1.2 mm. Analysis of free fatty acids showed two major FAs, linolenic acid (18:3) at a concentration of 1.3 mm, and linoleic acid (18:2) at a concentration of 0.4 mm. Ion-exchange chromatography on R and the synthetic mixture of FACs removed all detectable amounts of FACs (Fig. 1D, detection limit = 30 nm) and FAs (detection limit = 200 nm).
Induction of cis-α-Bergamotene Release
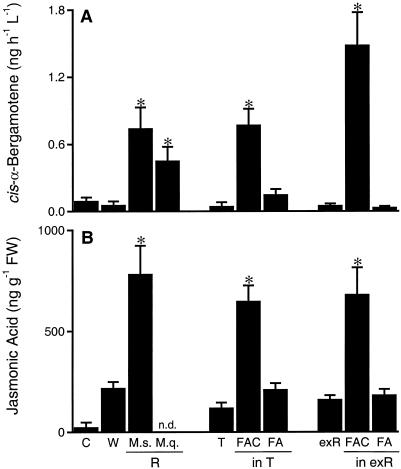
Treatment of standard puncture wounds on a single leaf with M. sexta R elicited significant increases in whole-plant (WP) emissions of cis-α-bergamotene compared with untreated plants or plants that were comparably damaged but had water applied to their puncture wounds (Fig. 2A, ANOVA F9, 70 = 14.565, P < 0.0001). Application of M. quinquemaculata R induced elevated WP cis-α-bergamotene emissions comparable with those elicited by R of the closely related species M. sexta. The volatile-inducing activity of R was completely eliminated by ion-exchange chromatography. Application of the exR, which did not contain any detectable amounts of the analyzed FAs or FACs (Fig. 1D), did not induce cis-α-bergamotene emissions. However, the addition of synthetic FACs (Fig. 1E) to the ion-exchanged R at their original concentrations completely restored the volatile-inducing activity. Moreover, an aqueous solution of synthetic FACs at concentrations found in R was as active as the larval R. The free fatty acids, when applied as aqueous solution or dissolved in the exR at concentrations found in M. sexta R, did not induce volatile emissions, even though the molar concentrations of the applied test solutions were higher than those of the FAC treatment. From these results, we conclude that the FACs found in M. sexta R, but not the FAs, are necessary and sufficient for the elicitation of the volatile release in N. attenuata plants. However, in excised leaves of lima beans, linolenic acid and its amino acid conjugate 18:3-Gln both induce homoterpene emissions (Koch et al., 1999), which were not detected among the volatiles released by N. attenuata (Halitschke et al., 2000). Even though the compounds were applied at higher concentrations than in this study, different mechanisms of volatile-induction may exist in different plant-herbivore systems.
Figure 2.
Mean (±se) WP cis-α-bergamotene trapped per hour, per liter air sampled from individual (eight per treatment) N. attenuata (A) plants and mean (±se) JA concentrations (n.d., not determined) of node two leaves of four replicate plants per treatment 35 min (time of maximum JA induction; B) after the node two leaf was wounded and treated with 20 μL of the following test solutions: water (W), oral secretions and regurgitant from M. sexta larvae (M.s.) or M. quinquemaculata (M.q.), exR, Triton X-100 in water (T), FAC in concentrations found in R and FA in concentrations found in R in the triton solution or in exR. Control plants (C) remained undamaged. Stars represent significantly (P < 0.05) increased emissions as compared with wounded plants treated with water (W) as determined by Fisher's protected least significant difference from ANOVAs.
Induction of Endogenous JA Burst
As previously described (Kahl et al., 2000; Schittko et al., 2000), application of M. sexta R to puncture wounds on N. attenuata leaves transiently elicits higher JA concentrations than does the addition of water to identical puncture wounds (Fig. 2B, ANOVA F8, 26 = 14.551, P < 0.0001). The ion-exchange treatment removed the JA inducing activity of R so that application of exR did not amplify the wound-induced JA accumulation. We tested the mixture of synthetic FACs (Fig. 1E) in a triton-containing aqueous solution and in exR at concentrations comparable to those found in R. Both solutions elicited a dramatic amplification of the wound-induced increase in JA concentrations as observed after application of larval R. No amplification of JA induction was observed after treatments with triton control solution and FA mixtures in exR or triton-containing aqueous solution compared with the wound treatment. These results demonstrate that other induction-mechanisms than a simple supply of FA as substrate for endogenous JA biosynthesis must account for the response activation processes in the N. attenuata-M. sexta system. The JA response is known to be very sensitive to M. sexta R, which retain their activity even when diluted to 1/1,000 with water (Schittko et al., 2000). This sensitivity also argues against a substrate supply mechanism, because the quantity of FAs delivered to a leaf as FACs in this highly diluted, but still active R is not sufficient to supply the quantity of fatty acid substrate required for the observed endogenous JA burst.
Changes in Transcript Accumulation
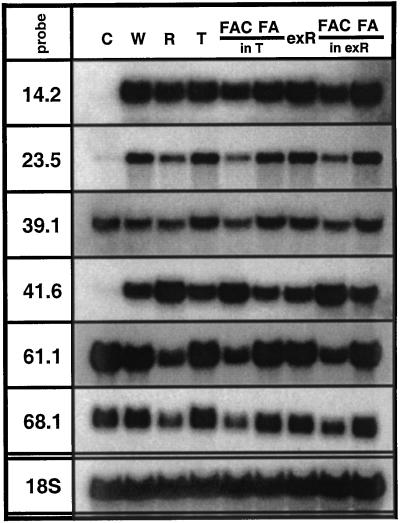
We also investigated the effect of the identified R-components on transcript accumulation. We applied the same test solutions as described for volatile and JA analysis and examined changes in transcript accumulation of a set of genes that specifically respond to R (Schittko et al., 2001). We found that FACs, supplied either in triton-containing aqueous solution or exR, caused specific changes in transcript accumulation exactly as larval R did, whereas transcript accumulation in response to FA solutions (in triton-containing aqueous solution or exR) did not differ from wound-induced transcript levels (Fig. 3). As described by Schittko et al. (2001), two types of expression patterns were distinguished. Wound-induced transcript accumulation was specifically repressed (type I) by R or FAC treatments for Thr deaminase (pDH14.2; Hermsmeier et al., 2001) and an unknown gene encoded by pDH23.5, whereas the wound response of the other four investigated genes was amplified (type II; Fig. 3). Wound-induced transcript accumulation of pathogen-induced oxygenase (pDH41.6; Hermsmeier et al., 2001) was further up-regulated, and wound-suppressed transcript accumulation of genes encoded by pDH61.1 (similar to the tomato gene for a light harvesting complex II subunit, lhb C1; Schwartz et al., 1991), pDH39.1, and pDH68.1 was further down-regulated (Fig. 3). No changes in transcript accumulation compared with the wound-treatment were observed after application of exR or triton control solution (Fig. 3).
Figure 3.
Northern analysis of transcript accumulation in response to different test solutions. The node two leaf of five replicate rosette-stage plants was continuously wounded and supplied with water (W), M. sexta larval oral secretions and regurgitant (R), exR, Triton X-100 in water (T), FACs or FAs in concentrations found in R, dissolved in either T or exR for 80 min, creating one row of puncture wounds every 20 min, and harvesting 20 min after the final treatment. Untreated node two leaves were harvested as controls (C). Hybridization with an 18S rRNA probe indicates equal loading.
CONCLUSION
In this study we identified FACs in R of the closely related herbivores M. sexta and M. quinquemaculata that, when applied to leaves of N. attenuata, are sufficient to activate the three investigated herbivore-specific plant responses in the signal transduction hierarchy (JA accumulation, changes in transcript accumulation, and volatile release). The chromatographic inactivation of a complex mixture of elicitors by ion exchange, and the restoration of activity by re-addition of synthetic FACs, powerfully demonstrates the biological activity of FACs. Future investigations are necessary to examine the contribution of each individual compound to the activity of R and to elucidate the structure-function relationship of the identified FACs.
Because FAs in Manduca R were not active elicitors of herbivore-induced responses in N. attenuata, the process of their conjugation with amino acids to form FACs in the insect (Paré et al., 1998) suggests that the insect controls the production of its own elicitors. Although the function of FACs in insects is not absolutely clarified, FACs are likely to function as emulsifiers and detergents (Collatz and Mommsen, 1974). Hence, the plant distinguishes the feeding activity of this herbivore from other agents that cause leaf damage by recognizing compounds essential for the insect's digestive processes.
The FACs could serve as useful tools for the study of plant-herbivore interactions because they allow researchers to uncouple herbivore-specific plant responses from herbivory and the damage it causes.
MATERIALS AND METHODS
Plant Growth and Insect Rearing
Nicotiana attenuata Torr. ex Wats. seeds (collected at the DI ranch, UT, T40S R19W, section 10, 1988) were germinated in smoke-treated soil (Baldwin et al., 1994b). For JA experiments, seedlings were transferred to soil and grown for 3 to 4 weeks. Plants for volatile experiments and northern analysis were grown as described in Hermsmeier et al. (2001) in no-nitrogen hydroponic solution (Baldwin and Schmelz, 1994). To provide nitrogen, 2 mL of 1 mm KNO3 solution were added to each 1-L chamber, followed by another 1 mL, 10 to 12 d later (the day before the experiment started). All plants were grown under a 32°C, 16-h/27°C, 8-h day/night regime and were in the rosette-stage of growth at the time of the experiment.
Manduca sexta (Lepidoptera, Sphingidae) larvae were hatched from eggs (Carolina Biological Supply, Burlington, NC) and reared on fresh N. attenuata foliage under a 28°C, 16-h/8-h day/night regime. Eggs of Manduca quinquemaculata Haworth were collected at Pahcoon Springs Burn in Utah in 1999, and the larvae were reared in the laboratory as described for M. sexta.
Analysis of Oral Secretions and Regurgitant
Atmospheric pressure chemical ionization-HPLC-MS analyses of M. sexta and M. quinquemaculata R and synthetic FAC mixtures were performed as previously described (Pohnert et al., 1999a) using a reversed-phase HPLC separation (LiChrospher 100 RP-18, 5 μm, 250 × 4 mm, Merck, Darmstadt, Germany) with acetonitrile, water, and acetic acid as eluent. Detection and identification of the FACs was performed with a Finnigan (San Jose, CA) LCQ ion trap MS (Atmospheric pressure chemical ionization, vaporizer 560°C) by comparison with synthetic standards. Details on the LC-MS procedure, the synthesis and spectroscopic data of FACs 1 and 3 to 7 are published elsewhere (Pohnert et al., 1999a). Synthesis of the newly identified FAC 8, found in Manduca R, proceeded from free palmitic acid and unprotected Glu following a published protocol (Pohnert et al., 1999b).
The following selected spectroscopic data were obtained by MS and NMR analyses of n-palmitoyl-l-glutamate (16:0-Glu, 8): [1H]NMR (CD3OD, 500 MHz) δ: 0.9 (t, J = 7.1, 3H); 1.25 to 1.35 (m, 26H); 1.62 (t, J = 7.2, 2H); 1.89 to 1.97 (m, 1H); 2.14 to 2.22 (m, 1H); 2.25 (t, J = 7.5, 1H; 2.4 (t, J = 7.8, 1H); 4.43 (dd, J = 5, 9.17, 1H); [13C]NMR (CD3OD, 125 MHz) δ: 14.54; 23.83; 27.01; 27.94; 30.36; 30.56; 30.57; 30.74; 30.83; 30.83; 30.85; 30.86; 30.87; 30.88; 30.89; 30.9; 31.35; 53.01; 175.08; 176.39; 176.58, MS (70 eV); 385(M+•, 8); 3.67(10); 341(8); 256(7); 239(10); 189(89); 171(30); 130(47); 102(100); 84(59); 57(65); and HR-MS: m/z calculated for C21H39NO5: 385.2828, observed: 385.2828.
Free fatty acids were extracted from 80 μL of R after addition of 4 μg cis-10-nonadecenoic acid as an internal standard with a ternary solvent composition (water-methanol-chloroform), as described by Bligh and Dyer (1959). The extract was derivatized with 600 μL of freshly prepared solution of diazomethane in ether. The solvent was evaporated and the residue dissolved in 20 μL of N-methyl-N-trimethylsilyltrifluoroacetamide (Macherey-Nagel, Düren, Germany). One-microliter aliquots were injected and analyzed on a Saturn 2000 GC-MS (Varian, Walnut Creek, CA). Methylated FAs were separated on a 30-m × 0.25-mm DB-Wax column (0.25-μm film thickness; J&W Scientific, Folsom, CA). The injector temperature was held at 225°C, and the column oven temperature was programmed as follows: initial column temperature 120°C held for 3 min, ramped from 120°C to 170°C at 10°C/min, held at 170°C for 6 min, ramped from 170°C to 230°C at 3°C/min, ramped from 230°C to 240°C at 20°C/min, and finally held at 240°C for 10 min. The carrier gas flow throughout the program was maintained at 1 mL/min.
Test Solutions
Oral secretions and regurgitant were collected with Teflon tubing connected to a vacuum from 4th to 5th instar M. sexta and M. quinquemaculata larvae reared on N. attenuata leaves and stored under argon at −80°C. They were diluted 1:1 (v:v) with water prior to the treatment. To remove FAs and FACs, 400 μL of R were eluted consecutively through four ion-exchange columns containing 400 mg of the basic anion-exchange resin Amberlite IRA-400 (Sigma, Steinheim, Germany). The final eluate was called “ion-exchanged oral secretions and regurgitant” (exR). For application of FAs and FACs at concentrations similar to those found in R, aqueous solutions containing 0.005% (w/w) Triton X-100 (Fluka, Buchs, Switzerland) were prepared and diluted 1:1 (v:v) with water or exR prior to the treatment. The FA solution contained 120 ng μL−1 (0.4 mm) of linoleic acid and 350 ng μL−1 (1.3 mm) of linolenic acid. A mixture of the four main FACs was prepared at concentrations of 50 ng μL−1 (0.12 mm) N-linolenoyl-l-Gln (18:3-Gln, 1), 138 ng μL−1 (0.34 mm) N-linolenoyl-l-Glu (18:3-Glu, 3), 41 ng μL−1 (0.10 mm) N-linoleoyl-l-Gln (18:2-Gln, 4), and 26 ng μL−1 (0.06 mm) N-linoleoyl-l-Glu (18:2-Glu, 5). The FAC mixture differed from the FAC composition of R in that it contained approximately 4 times the amount of FAC 4 and did not contain FAC 8. An aqueous solution containing 0.0025% (w/w) Triton X-100 was used to control for the potential inducing activity of this detergent.
Volatile and JA Analysis
To determine the JA- and volatile-inducing activity of different test solutions, 20-μL samples were added to the leaf lamina immediately after three rows of puncture wounds were created on each leaf half with a fabric pattern wheel (Dritz, Spartanburg, SC). All treatments were applied to a single leaf node (node two) of each plant with the youngest fully expanded leaf, the leaf that had just completed the source-sink transition (as defined in Wait et al., 1998) defining node one.
Leaves scheduled for JA analysis were harvested 35 min after the induction of four replicate plants per treatment. Jasmonate concentrations were determined with 13C1,2-JA as an internal standard and analyzed by GC-MS as described by Schittko et al. (2000).
Volatile collection commenced 24 h after the treatment and lasted for 8 h. Eight replicate plants per treatment were covered with 1-L open-top WP volatile collection chambers, and volatiles were collected by adsorption on 30 mg of SuperQ at a mean flow rate of 300 mL min−1 through the WP-chamber and analyzed by GC-MS as previously described (Halitschke et al., 2000). Because cis-α-bergamotene is the most consistently systemically released volatile from different genotypes of N. attenuata (Halitschke et al., 2000), we used the WP emission of this sesquiterpene to quantify the induced volatile response. The released amounts were calculated from peak areas using calibration curves with tetraline as an internal standard and normalized to trapping efficiencies by peak areas of a trapped sesquiterpene that was abundant in the surrounding growth room air (Halitschke et al., 2000). Given that induced volatile emissions are known to be influenced by many environmental factors (Loughrin et al., 1994; Takabayashi and Dicke, 1996; Paré and Tumlinson, 1998; Halitschke et al., 2000), our open-flow trapping system has the distinct experimental advantage of allowing the simultaneous analysis of 80 plants. Statistical comparisons of volatile and JA data were performed with protected contrasts (Fisher's protected least significant difference) from ANOVAs.
Northern Analysis
A fabric pattern wheel (Dritz, Spartanburg, SC) was used to create one row of puncture wounds in parallel to the leaf midrib every 20 min, and 5-μL aliquotes of the respective test solution were applied to the fresh wounds. A total number of five rows were applied to a leaf at node two and the treated leaf of five replicate plants per treatment was harvested 20 min after the last wounding. Total cellular RNA was isolated according to Pawlowski et al. (1994). Agarose gel electrophoresis, northern blotting, probe labeling, and hybridizations were performed as described in Hermsmeier et al. (2001). GenBank accession numbers of the template sequences are AW191811 (pDH14.2), AW191815 (pDH23.5), AW191819 (pDH39.1), AW191821 (pDH41.6), AW191826 (pDH61.1), AW191828 (pDH64.4), and AW191830 (pDH68.1). Hybridization with an 18S rRNA probe (pDH64.4) was used to monitor loading. The wound-induced response of the other six mRNAs of N. attenuata is known to be specifically altered by M. sexta and M. quinquemaculata R (Schittko et al., 2001).
ACKNOWLEDGMENTS
We thank André Kessler and Dieter Spiteller for assistance with the volatile collection experiments and analysis of R. Support by the Max-Planck Gesellschaft is gratefully acknowledged. We thank editor Carlos Ballaré and the two anonymous reviewers whose insights substantially improved the manuscript.
Footnotes
This work was supported by the Max Planck Gesellschaft.
LITERATURE CITED
- Alborn HT, Jones TH, Stenhagen GS, Tumlinson JH. Identification and synthesis of volicitin and related components from beet armyworm oral secretions. J Chem Ecol. 2000;26:203–220. [Google Scholar]
- Alborn HT, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH. An elicitor of plant volatiles from beet armyworm oral secretion. Science. 1997;276:945–949. [Google Scholar]
- Baldwin IT. The alkaloidal responses of wild tobacco to real and simulated herbivory. Oecologia. 1988;77:378–381. doi: 10.1007/BF00378046. [DOI] [PubMed] [Google Scholar]
- Baldwin IT. Inducible nicotine production in native Nicotiana as an example of adaptive phenotypic plasticity. J Chem Ecol. 1999;25:3–30. [Google Scholar]
- Baldwin IT, Schmelz EA. Constraints on an induced defense: the role of leaf area. Oecologia. 1994;97:424–430. doi: 10.1007/BF00317335. [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Schmelz EA, Ohnmeiss TE. Wound-induced changes in root and shoot jasmonic acid pools correlate with induced nicotine synthesis in Nicotiana sylvestris. J Chem Ecol. 1994a;20:2139–2157. doi: 10.1007/BF02066250. [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Staszak-Kozinski L, Davidson R. Up in smoke: I. Smoke-derived germination cues for postfire annual, Nicotiana attenuata Torr. Ex. Watson. J Chem Ecol. 1994b;20:2345–2371. doi: 10.1007/BF02033207. [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Zhang Z-P, Diab N, Ohnmeiss TE, McCloud ES, Lynds GY, Schmelz EA. Quantification, correlations and manipulations of wound-induced changes in jasmonic acid and nicotine in Nicotiana sylvestris. Planta. 1997;201:397–404. [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Collatz K-G, Mommsen T. Die struktur der emulgierenden substanzen verschiedener invertebraten. J Comp Physiol. 1974;94:339–352. [Google Scholar]
- Halitschke R, Keβler A, Kahl J, Lorenz A, Baldwin IT. Eco-physiological comparison of direct and indirect defenses in Nicotiana attenuata. Oecologia. 2000;124:408–417. doi: 10.1007/s004420000389. [DOI] [PubMed] [Google Scholar]
- Hermsmeier D, Schittko U, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata: I. Large-scale changes in the accumulation of growth- and defense-related plant mRNAs. Plant Physiol. 2001;125:683–700. doi: 10.1104/pp.125.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopke J, Donath J, Blechert S, Boland W. Herbivore-induced volatiles: the emission of acyclic homoterpenes from leaves of Phaseolus lunatus and Zea mays can be triggered by a beta-glucosidase and jasmonic acid. FEBS Lett. 1994;352:146–150. doi: 10.1016/0014-5793(94)00948-1. [DOI] [PubMed] [Google Scholar]
- Kahl J, Siemens DH, Aerts RJ, Gäbler R, Kühnemund F, Preston CA, Baldwin IT. Herbivore-induced ethylene suppresses a direct defense but not a putative indirect defense against an adapted herbivore. Planta. 2000;210:336–342. doi: 10.1007/PL00008142. [DOI] [PubMed] [Google Scholar]
- Koch T, Krumm T, Jung T, Engelberth J, Boland W. Differential induction of plant volatile biosynthesis in the lima bean by early intermediates of the octadecanoid-signaling pathway. Plant Physiol. 1999;121:153–162. doi: 10.1104/pp.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughrin JH, Manukian A, Heath R, Turlings TCJ. Diurnal cycle of emission of induced volatile terpenoids herbivore-injured cotton plants. Proc Natl Acad Sci USA. 1994;91:11836–11840. doi: 10.1073/pnas.91.25.11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiacci L, Dicke M, Posthumus MA. Beta-glucosidase: an elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc Natl Acad Sci USA. 1995;92:2036–2040. doi: 10.1073/pnas.92.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloud ES, Baldwin IT. Herbivory and caterpillar regurgitants amplify the wound-induced increases in jasmonic acid but not nicotine in Nicotiana sylvestris. Planta. 1997;203:430–435. [Google Scholar]
- Paré PW, Alborn HT, Tumlinson JH. Concerted biosynthesis of an insect elicitor of plant volatiles. Proc Natl Acad Sci USA. 1998;95:13971–13975. doi: 10.1073/pnas.95.23.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré PW, Tumlinson JH. Plant volatiles as a defense against insect herbivores. Plant Physiol. 1998;121:325–331. [PMC free article] [PubMed] [Google Scholar]
- Pawlowski K, Kunze R, deVries S, Bisseling T. Isolation of total, poly(A) and polysomal RNA from plant tissue. In: Gelvin SB, Schilperoort RA, editors. Plant Molecular Biology Manual. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 1–4. [Google Scholar]
- Pohnert G, Jung V, Haukioja E, Lempa K, Boland W. New fatty acid amides from regurgitant of lepidopteran (Noctuidae, Geometridae) caterpillars. Tetrahedron. 1999a;55:11275–11280. [Google Scholar]
- Pohnert G, Koch T, Boland W. Synthesis of volicitin: a novel three-component Wittig approach to chiral 17-hydroxylinolenic acid. Chem Commun. 1999b;12:1087–1088. [Google Scholar]
- Schittko U, Hermsmeier D, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata: II. Accumulation of plant mRNAs responding to insect-derived cues. Plant Physiol. 2001;125:701–710. doi: 10.1104/pp.125.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schittko U, Preston CA, Baldwin IT. Eating the evidence? Manduca sexta larvae can not disrupt specific jasmonate induction in Nicotiana attenuata by rapid consumption. Planta. 2000;210:343–346. doi: 10.1007/PL00008143. [DOI] [PubMed] [Google Scholar]
- Schwartz E, Stasys R, Aebersold R, McGrath JM, Green BR, Pichersky E. Sequence of a tomato gene encoding a third type of LHCII chlorophyll a/b-binding polypeptide. Plant Mol Biol. 1991;17:923–926. doi: 10.1007/BF00037074. [DOI] [PubMed] [Google Scholar]
- Takabayashi J, Dicke M. Plant-carnivore mutualism through herbivore-induced carnivore attractants. Trends Plant Sci. 1996;1:109–113. [Google Scholar]
- Turlings TCJ, Alborn HT, Loughrin JH, Tumlinson JH. Volicitin, an elicitor of maize volatiles in oral secretions of Spodoptera exigua: isolation and bioactivity. J Chem Ecol. 2000;26:189–202. [Google Scholar]
- Wait DA, Jones CG, Coleman JS. Effects of nitrogen fertilization on leaf chemistry and beetle feeding are mediated by leaf development. Oikos. 1998;82:502–514. [Google Scholar]