Abstract
Objective:
Foundational knowledge on neural circuitry underlying pediatric obsessive- compulsive disorder (OCD) and how it changes during standard treatment is needed to provide the basis for conceptualization and development of novel, targeted treatments. This study explored the effects of sertraline, a selective serotonin reuptake inhibitor, on resting-state functional connectivity (RSFC) in cortico-striatal-thalamic-cortical circuits in pediatric OCD. Method: Medication-free youths with OCD (n=14) and healthy controls (n=14) were examined at baseline and 12 weeks with resting-state fMRI. Between scan sessions, participants with OCD received 12 weeks of sertraline. For each scan, we conducted seed-based whole-brain RSFC analyses with 6 striatal seeds. Analysis of variance (ANOVA) examined the interaction between group and time on striatal connectivity, including cluster-based thresholding to correct for multiple tests. Connectivity changes within circuits identified in group analyses were correlated with clinical change.
Results:
Two significant group x time effects in the OCD group showed increased striatal connectivity from baseline to 12 weeks compared with controls. Circuits demonstrating this pattern included right putamen with left frontal cortex/insula and left putamen with left frontal cortex and pre- and post-central cortices. Increase in connectivity in left putamen circuit was significantly correlated with clinical improvement on Children’s Yale-Brown Obsessive- Compulsive Scale (r = −0.58, p = .03).
Conclusion:
Sertraline appears to affect specific striatal-based circuits in pediatric OCD, and in part, these changes may account for clinical improvement. Future work is needed to confirm these preliminary findings, which would facilitate identification of circuit-based targets for novel treatment development.
Clinical trial registration information:
Clinical trial registration information: Effects of Sertraline on Brain Connectivity in Adolescents with OCD. https://clinicaltrials.gov/; NCT02797808
Keywords: obsessive-compulsive disorder, neuroimaging, functional MRI, SSRIs
Introduction
Obsessive-compulsive disorder (OCD) is a debilitating condition with intrusive, unwanted thoughts (i.e., obsessions) and repetitive behaviors (i.e., compulsions). The lifetime prevalence rate of OCD is approximately 2–3%1 Neuroimaging studies in OCD implicate abnormalities in cortico-striatal-thalamic-cortical (CSTC) circuitry as key in OCD pathogenesis.2 CSTC networks connect regions in the frontal cortex, striatum (caudate, putamen, nucleus accumbens), and thalamus.3–5 Other brain circuits identified as important in OCD include the salience network6–8 and the default mode network.9,10 OCD likely has a neurodevelopmental etiology, and the networks of interest in OCD undergo substantial change throughout childhood and adolescence.2 Unfortunately, up to 62% of youths with OCD do not show clinical remission with current treatments,11 highlighting the need for new treatments. Research examining the abnormal neural circuits underlying OCD in youth and how these circuits change with treatment will provide the foundational knowledge necessary to develop new treatments and guide experimental therapeutic approaches.
One approach for examining neural circuitry in youth that is suitable for longitudinal assessments is resting-state functional magnetic resonance imaging (R-fMRI), which measures spontaneous slow wave fluctuations of blood-oxygen-level-dependent (BOLD) signals in the brain during rest.12 Resting-state functional connectivity (RSFC) is calculated by measuring inter-regional correlations of these temporal patterns.13 Meta-analyses of cross-sectional RSFC studies in adults with OCD have implicated CSTC14,15 as well as fronto-parietal, salience and default mode networks.14 In the only published study of selective serotonin reuptake inhibitor (SSRI) effects on RSFC in OCD, Shin et al. reported that after 16 weeks of escitalopram, adults with OCD exhibited clinical improvement and normalization of functional connectivity differences (derived from graph theory) in frontoparietal networks.16
Six R-fMRI studies in pediatric OCD4,6,9,17–19 show diverse findings in youth with OCD versus healthy controls including lower RSFC in participants with OCD in the dorsal anterior cingulate cortex (dACC) with right anterior operculum, and ventral medial frontal cortex with posterior cingulate.4,9 Subsequently, Fitzgerald et al.4 demonstrated lower RSFC in rostral ACC-dorsal striatum and dACC-medial dorsal thalamus connections that were specific to children with OCD compared with controls. We found lower RSFC in participants with OCD compared to controls between the left putamen and a single cluster of right-sided areas including parts of orbitofrontal cortex (OFC), inferior frontal gyrus, insula, and operculum.6 Using independent components analysis (ICA), lower connectivity was reported in cingulate network in youths with OCD compared with controls;18 and greater ICA expression scores were found in participants with OCD compared with controls in the middle frontal dorsal anterior cingulate and anterior/posterior cingulate networks and lower scores in the visual network.17 Abnormalities in graph theory-derived global and local network architecture were demonstrated in participants with OCD in comparison with controls.19 These studies support RSFC abnormalities in pediatric OCD. However, most included both medicated and unmedicated youths with OCD, which is an important limitation since other work has shown that psychiatric medications impact RSFC.20–23 Furthermore, none tracked brain connectivity longitudinally or included a treatment phase, precluding examination of how existing treatments for OCD alter connectivity.
Our primary aim was to investigate how sertraline, an SSRI and first-line treatment for pediatric OCD,11 impacts neural circuitry in youths with OCD. Although numerous neural networks have been implicated in OCD, we focused on CSTC circuitry as a core network that has consistently been considered primary to OCD pathology6,24.We collected R-fMRI data in medication-free participants with OCD before and after 12 weeks of sertraline. To confirm that neural changes were due to drug effects versus the effects of naturally-occurring changes over time, we also scanned a group of matched healthy youths at baseline and 12 weeks later. We examined CSTC circuitry by conducting seed-based, whole brain analyses using 6 a priori selected striatal areas (bilateral caudate, putamen, nucleus accumbens). We hypothesized that following 12 weeks of sertraline, RSFC within the CSTC circuitry would change in the youths with OCD compared with healthy controls. We explored whether change in RSFC in children and adolescents with OCD correlated with change in symptom severity on Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS).25
Method
Participants:
Inclusion criteria for participants with OCD were: OCD as primary DSM-IV diagnosis based on Anxiety Disorders Interview Schedule (ADIS) for DSM-IV, Child Version26 and Total CY- BOCS25 severity score >15, which represents a moderate level of symptom severity and is the same criterion used in prior pediatric OCD trials.11 Since children are more likely to be medication-free than adolescents, we included a wide age range of participants (8–17 years). Exclusion criteria were lifetime diagnosis of bipolar disorder, schizophrenia, or substance abuse on ADIS; IQ <80 on Wechsler Abbreviated Scales of Intelligence (WASI);27 positive urine drug screen or pregnancy test; MRI-incompatible features (e.g., dental braces, claustrophobia); current or recent trial of psychotropic medication (within past 4 weeks or 6 weeks for fluoxetine); current psychotherapy (e.g., cognitive-behavioral therapy); non-response in ≥2 SSRI trials of adequate dose/duration; and history of seizure disorder or head injury. Additional exclusion criteria for controls were any lifetime psychiatric diagnosis on ADIS and family history of OCD in first-degree relative based on Family Interview for Genetic Studies.28
Procedure:
The study was approved by the University of Minnesota Institutional Review Board. After written informed consent and written assent were obtained by the Project Coordinator, diagnostic assessment including interviews and rating scales was administered by trained interviewers. Participants subsequently underwent neuroimaging at the Center for Magnetic Resonance Research. Assessment instruments and neuroimaging were obtained at baseline and 12 weeks later in all participants.
Assessment Instruments:
ADIS is a semi-structured clinician interview with good inter-rater reliability and validity29 that was used to document OCD and comorbid diagnoses.30,31 CY-BOCS is a clinician-rated semi-structured instrument assessing OCD severity; Total Score ranges from 0–40.25 Dimension scores (e.g., forbidden thoughts, contamination/cleaning, ordering/repeating, hoarding) can be calculated based on endorsement of CY-BOCS Checklist items.32 Child Obsessive-Compulsive Impact Scale - Revised (COIS-R) - Parent and Child Versions are self- and parent-rated questionnaires measuring functional impairment in daily activities due to OCD33 with total scores ranging from 0–99. WASI provided a measure of cognitive functioning; total IQ scores were used in the current study.
Training of Raters:
Raters blind to study hypotheses and content of medication management sessions administered the CY-BOCS to parent and child together at baseline, week 6, and week 12. Raters included 1 PhD psychologist, 5 clinical psychology PhD students, and 2 BA-level project coordinators. Raters were trained and supervised by a PhD psychologist. Two interviewers simultaneously rated 17.5% of CY-BOCS interviews and inter-rater reliability was 0.98 for Obsession and 0.99 Compulsion subscales.
Medication Management:
After baseline assessment and scans, participants with OCD received 12 weeks of sertraline which was titrated using an individualized flexible dosing schedule that maximized efficacy and minimized side effects. The target dose was 100–200 mg/day by week 6 of the 12-week trial.11 Medication management sessions were conducted by the first author for 30 minutes for the first 4 weeks and every other week thereafter. General support and encouragement were provided but no elements of CBT or exposure therapy were permitted. Sessions included vital signs, monitoring side effects with a checklist, and safety evaluation. Compliance was monitored with pill counts at medication appointments and with sertraline blood level at week 12. No concurrent psychotherapies (e.g., CBT, family therapy) were permitted.
MRI Acquisition:
Imaging was conducted using a 32 channel head coil on a Siemens Prisma 3T scanner. Resting- state BOLD data were collected using a multi-band gradient echo planer imaging pulse sequence. One 12-minute resting scan was obtained (TR = 710ms, TE = 30ms, flip angle 55 degrees, 2mm isotropic voxel, 72 slices, MB = 8, 1014 volumes). Participants were instructed to close their eyes and try to stay awake. A field map with identical voxel parameters was acquired to correct for distortions in the R-fMRI caused by magnetic field inhomogeneity. A T1 weighted volume was acquired for anatomic registration and to identify brain structures (TR = 2530, TE = 3.65, TI = 1100ms, flip angle = 7 degrees, 1mm isotropic voxel).
Image Processing
T1:
FreeSurfer 5.3.0 processing stream was applied to each participant’s high resolution T1 weighted scan, resulting in standardized subcortical volume segmentation and cortical parcellation.
R-fMRI:
FSL FEAT was used to preprocess the R-fMRI data, including distortion and motion correction, brain extraction, registration to Montreal Neurological Institute space, and smoothing with a 3mm Gaussian kernel. The methods of Power et al.34 were used to determine if an R-fMRI dataset had adequate quality to include in the analysis. Framewise displacement (FD) and derivative variance (DVARS) were computed for each R-fMRI volume, those that exceeded FD (0.5 mm) and/or DVARS (1% Δ BOLD) thresholds and the preceding volume and following two volumes were flagged; participants with >30% flagged volumes were excluded. R-fMRI scans that passed the quality threshold were not subjected to volume censoring. Rather, to remove spurious correlations caused by participant head motion, respiration, and heartbeat, a denoising procedure was performed on the R-fMRI data using FSL FIX.35,36
Data Analysis
CSTC Functional Connectivity Analysis:
Whole brain connectivity of six striatal regions of interest (ROIs) in the CSTC circuit implicated in OCD (bilateral caudate, putamen, and nucleus accumbens) was examined using a seed-based approach similar to Bernstein et al.6 The six individual anatomically-based ROIs determined by FreeSurfer on the participant T1 volume were aligned to each individual’s fMRI data using bbregister.37 Time series for each ROI was computed by taking the mean value across time from the preprocessed R-fMRI data. Time series of each ROI was then used as the primary regressor carried out in FEAT version 6.0, part of FSL (FMRIB’s software library, www.fmrib.ox.ac.uk/fsl), using a general linear model; the lateral ventricle CSF and white matter time courses were used as regressors of no interest.
Group by Time Analyses:
To identify (at the whole-brain level) group differences (OCD versus control) in the pattern of change (baseline versus 12 weeks) in RSFC, two-way mixed effects Analyses of Variance ([ANOVA] (one for each ROI) were carried out using FEAT. Because we had no a priori hypothesis of the direction of the group by time change in RSFC, 2 tests (OCD > control and OCD < control) were performed for each ROI. The FEAT “cluster” option was used on voxelwise whole brain Z (Gaussianized T/F) statistic images, with contiguous clusters determined by using a voxelwise z-threshold of 2.3. Each cluster’s estimates significance level was determined from Gaussian Random Fields theory and was compared to a probability threshold Bonferroni corrected for the number of tests performed. Findings were therefore considered significant if the cluster p value was < .0042 based on p < .05/(6 ROIs x 2 tests per ROI).
Given our small sample size, the study was designed to mitigate the effects of age by matching the groups by age. However, to explore the effect of age on our findings, we performed a secondary two-step analysis that included age as a covariate. For each ROI, we first ran a fixed effects analysis in FEAT for each participant to compute the change in connectivity between the two time points. A group level ANOVA was then performed in FEAT to measure the group difference in connectivity change over time with age added as a covariate.
Post-Hoc Analyses of Group by Time Interaction:
To further investigate the group by time interaction from the two-way mixed effects ANOVA analyses, we compared mean z-scores within each of the significant clusters from both the baseline and 12-week first-level connectivity maps for each participant. Binary masks from the two significant clusters were applied to participant’s baseline and 12-week MNI space z-statistics maps and the FSL tool fslstats was used to determine the mean z-value within each cluster of each participant run. The resulting numbers represented striatal connectivity with that cluster for each individual at each time point. Independent sample t-tests were used to compare these RSFC metrics between groups at baseline and at 12 weeks. Within-group paired-sample t-tests examined RSFC change over time in these metrics for each group. Bonferroni correction was applied to the alpha level (two-tailed, p < .05/6 = .0083) for multiple testing.
Associations Between Symptom Score Changes and Connectivity Changes:
We explored the relations between clinical improvement (change from baseline to 12 weeks on CY-BOCS Total Score, CY-BOCS Obsession subscale, CY-BOCS Compulsion subscale, and CY-BOCS dimension scores) and change in connectivity within the circuits identified in the two-way mixed effects ANOVA. Change scores for connectivity metrics were calculated by subtracting the baseline mean z-score within group analysis-defined clusters for each participant from the 12-week value. Pearson’s correlation coefficients were calculated between the two change scores. Alpha level was not corrected (two-tailed, p < .05) in these comparisons due to the exploratory nature of these analyses.
Results
Participants:
Twenty-three children and adolescents with OCD (ages 8 to 17 years) and 18 age- and gender- matched controls were enrolled (Figure 1). Of the 41 participants, 6 with OCD and 2 controls did not qualify to continue in the study (e.g., diagnostic exclusion criteria, did not complete baseline scan due to anxiety) and 1 with OCD and 1 control dropped out after their baseline scan. Of 31 completers, 1 with OCD and 1 control were excluded because their MRI data were acquired using incompatible scan parameters. Finally, 1 participant with OCD was excluded due to motion effects on the 12-week scan. Analyses included 14 each in the OCD and control groups.
Figure 1:
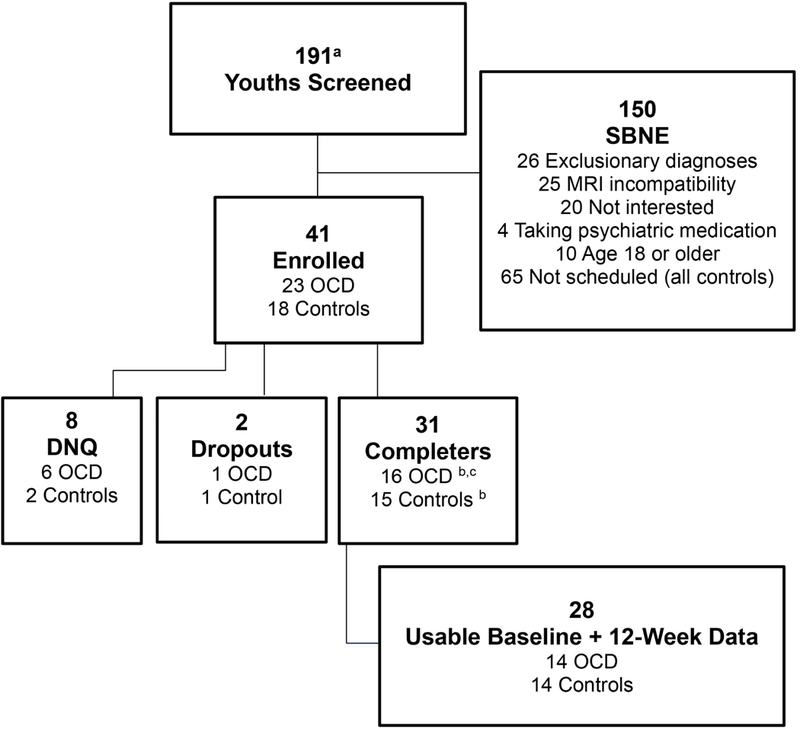
Consort Diagram
Note: DNQ = did not qualify; MRI = magnetic resonance imaging; OCD = obsessive-compulsive disorder; SBNE = screened but not enrolled.
a Includes 65 controls who were screened but not scheduled
b One participant’s data excluded because data were acquired on different scanner
c One participant’s week 12 data excluded due to motion artifact
There were no significant differences between OCD and control groups on age at assessment, gender, socioeconomic status,38 handedness, ethnicity, or IQ (Table 1). All 14 participants with OCD were medication-free with 11 being medication-naïve. All urine pregnancy and toxicology tests were negative. Mean sertraline dose was 115.2 ± 43.9 mg at 6 weeks and 121.4 ± 58.5 mg at 12 weeks. Mean sertraline trough blood level (approximately 24 hours after last dose) at 12 weeks was 44.2 ng/mL.
TABLE 1.
Demographic and Clinical Characteristics of Participants
| Characteristic | OCD (n = 14) | Control (n = 14) | t or χ2 | p | ||
|---|---|---|---|---|---|---|
| Age at onset in yrs, mean (SD) | 9.8 | (3.5) | ||||
| Age at assessment in yrs, mean (SD) | 13.4 | (2.6) | 13.4 | (2.9) | t = 0.068 | .95 |
| Male, n (%) | 8 | (57.1) | 5 | (35.7) | χ2 = 1.292 | .45 |
| SES, mean (SD) | 1.7 | (0.8) | 1.3 | (0.6) | t = −1.479 | .15 |
| WASI IQ, mean (SD) | 111.2 | (118) | 114.1 | (8.6) | t = 0.735 | .47 |
| Ethnicity, n (%) | χ2 = 3.391 | .34 | ||||
| Caucasian | 10 | (71.4) | 13 | (92.9) | ||
| Asian | 2 | (14.3) | 0 | (0) | ||
| African-American | 1 | (7.1) | 1 | (7.1) | ||
| Hispanic/Latino | 1 | (7.1) | 0 | (0) | ||
| Right handed, n (%) | 11 | (78.6) | 13 | (92.9) | χ2 = 1167 | .60 |
| 1st-degree family history of OCD, n (%) | χ2 = 1037 | 1.00 | ||||
| Yes | 1 | (7.1) | 0 | (0) | ||
| No | 13 | (92.9) | 14 | (100) | ||
| Medication status at baseline, n (%) | ||||||
| Medication Free | 3 | (21.4) | ||||
| Medication Naïve | 11 | (78.6) | ||||
| CY-BOCS, Total, mean (SD) | 24.7 | (66) | 0 | (0) | t = −14.921 | <−.001 |
| COIS-R, parent, mean (SD)a | 32.3 | (24.2) | 0 | (0) | t = −5.354 | <−.001 |
| COIS-R, child, mean (SD)a | 27.1 | (16.4) | 0 | (0) | t = −6.621 | <.001 |
| Current comorbidity, n (%) | ||||||
| ADHD | 4 | (26.7) | 0 | (0) | ||
| MDD | 4 | (26.7) | 0 | (0) | ||
| GAD, SAD, and/or Social Anxiety | 12 | (80) | 0(0) | |||
Note: ADHD = attention-deficit/hyperactivity disorder; COIS-R = Child Obsessive-Compulsive Impact Scale - Revised; CY-BOCS = Children’s Yale-Brown Obsessive Compulsive Scale; GAD = generalized anxiety disorder; MDD = major depressive disorder; OCD = obsessive-compulsive disorder; SAD = separation anxiety disorder; SES = socioeconomic status; WASI = Wechsler Abbreviated Scale of Intelligence
Missing data on 1 participant with OCD
Clinical Change:
We defined treatment response as 33% reduction in CY-BOCS Total. Eight of 14 (57%) participants with OCD were responders after 12 weeks of sertraline. Mean CY-BOCS Total at baseline was 24.8 ± 6.9 and at post-treatment was 14.1 ± 8.5 [t (df = 13) = 5.21, p < 0.001] (43% improvement). Mean COIS-R Parent score at baseline was 30.2 ± 23.8 and post-treatment was 14.5 ± 13.0 [t (df = 12) = 3.33, p = 0.006] (52% improvement). Mean COIS-R Child score at baseline was 26.9 + 17.1 and post-treatment was 16.1 + 11.0 [t (df = 12) = 3.03, p = .011] (40% improvement).
Longitudinal Two Group RSFC Analyses:
The two-way mixed effects ANOVA results showed two significant group by time interactions. There were increases in RSFC between baseline and 12 weeks in youths with OCD compared with controls between right putamen ROI and a left-sided cluster including parts of the insula; frontal pole; and temporal, parietal, and opercular cortices (p = .00001) (Figure 2). A similar pattern was found for the left putamen analysis, although the resulting left-sided cluster involved less of the frontal cortex and more of the pre- and post-central cortices adjacent to the insula (p < .00001) (Figure 2).
Figure 2:
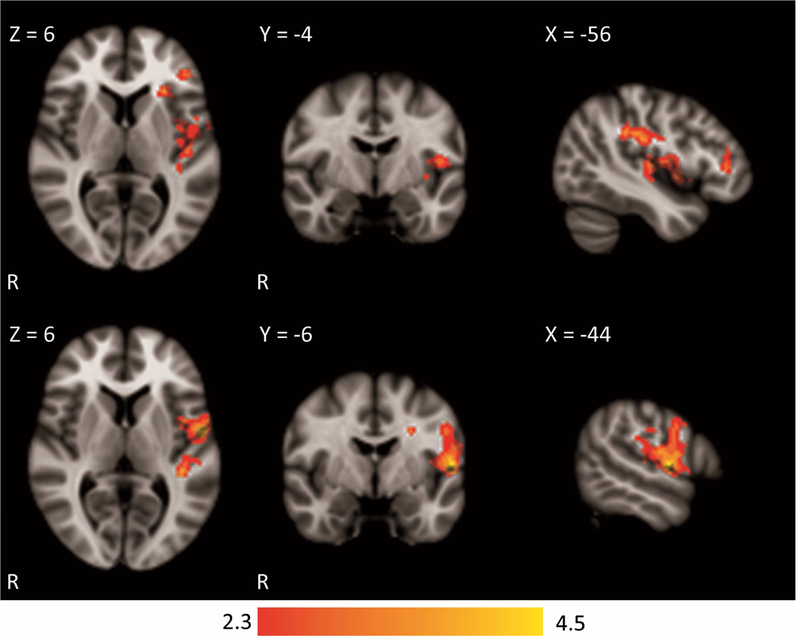
Significant Clusters from the Two-Way Mixed Effects Analysis of Variance (ANOVA) Note: Coordinates represent the location of the slices displayed. Top row: results from right putamen cluster with maximum z-score of 3.85 located at x = −40, y = −30, z = 30. Bottom row: results from left putamen cluster with maximum z-score of 4.6 located at x = −40, y = −34, z = 28.
The two-step ANOVAs with age included as a covariate also showed significant group by time interactions in the right putamen and left putamen analyses. In both cases, the significant clusters were somewhat smaller clusters which were subsets of the findings from the two-way mixed effects ANOVA without age covariance. The right putamen cluster significance dropped to p = .0001 while the left putamen cluster remained at p < .00001.
Post-Hoc Analyses:
Independent sample t-tests comparing groups at baseline and then at 12 weeks on mean z-scores in the clusters identified in group-by-time interactions revealed that at baseline, the OCD group showed significantly lower connectivity scores between right putamen and the identified cluster compared with control group (p = .003). The same comparisons in left putamen ROI revealed no significant group differences at baseline (p = .04). At 12 weeks, the OCD group showed significantly greater mean RSFC z-scores between right putamen ROI and the identified cluster compared with the control group (p = .001) and significantly greater mean z-score in connectivity between left putamen and identified cluster compared with the control group (p < .001).
Paired t-tests examining within-group change over time revealed that following sertraline treatment, mean z-score significantly increased in the OCD group between the right putamen ROI and the identified cluster (p < .001) (Figure 3). There was no significant change from baseline to 12 weeks in right putamen RSFC with the identified cluster in controls (p = .053). The OCD group showed a significant increase in RSFC following treatment between the left putamen and identified cluster (p = .001). In contrast, the control group showed a significant decrease in connectivity in the left putamen and identified cluster from baseline to 12 weeks (p = .002).
Figure 3:
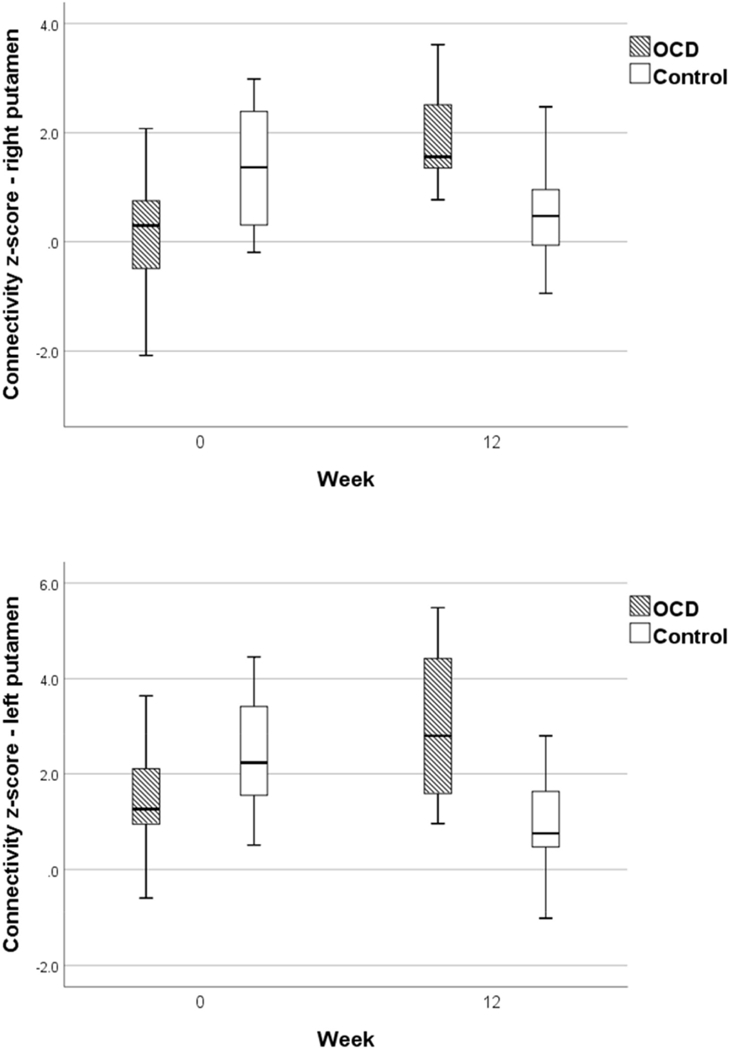
Boxplots of Connectivity Z-Scores for Right Putamen and Left Putamen at Baseline and 12 Weeks for Obsessive-Compulsive Disorder (OCD) and Control Groups
Association of Change in Connectivity with Change in OCD Severity:
There was a significant correlation between change in connectivity z-scores in the left putamen- left frontal cortex/insula circuit and change in CY-BOCS Total Score from pre- to post-treatment in the OCD group (r = −0.58, p = .03) (Table 2). As RSFC change increased, severity of OCD decreased (Figure 4). There were no significant correlations between change in connectivity z- scores in right putamen circuit and change in CY-BOCS Total Score (Table 2). Due to the significant correlation in the left putamen circuit, further correlations were computed to explore whether connectivity change was related to specific types of OCD symptoms. Change in left putamen connectivity and change in CY-BOCS Compulsion subscale was significant (r = −0.61, p = .02) but correlation between change in connectivity and change in CY-BOCS Obsession subscale was not significant (r = −0.50, p = .07) (Table 3). Correlations between change in connectivity in the left putamen circuit and change in the CY-BOCS dimension scores were not significant (Table 3).
TABLE 2.
Correlations Between Change in Connectivity Z-Scores and Change in CY-BOCSa Total Score Over 12 Weeks
| ROI | Correlation | p |
|---|---|---|
| Right putamen | 0.21 | .48 |
| Left putamen | −0.58 | .03 |
Note: CY-BOCS =Children‘s Yale-Brown Obsessive Compulsive Scale; ROI = region of interest
n = 14 participants with OCD
Figure 4:
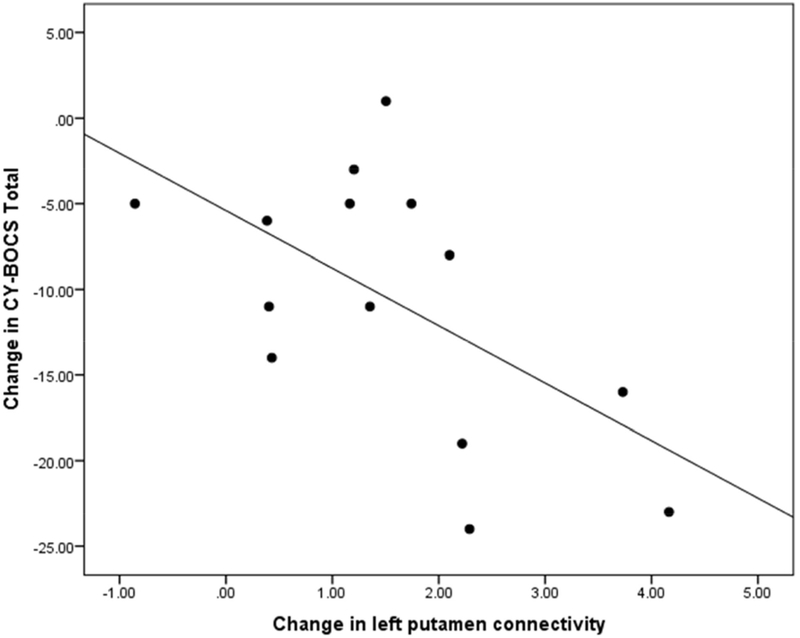
Change in Left Putamen Connectivity Z-Scores (Baseline to Week 12) Correlated with Change in Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS) Total Scores (Baseline to Week 12)
TABLE 3.
Correlations Between Change in Left Putamen Connectivity Z-Scores and Change in CY-BOCSa Over 12 Weeks
| CY-BOCS Scales | Correlation | p |
|---|---|---|
| Total | −0.55 | .03 |
| Obsessions | −0.50 | .07 |
| Compulsions | −0.61 | .02 |
| Forbidden thoughts | −0.53 | .053 |
| Contamination/cleaning | −0.29 | .30 |
| Ordering/repeating | −0.16 | .58 |
| Hoarding | 0.21 | .48 |
Note: CYBOCS = Children’s Yale-Brown Obsessive Compulsive Scale
n = 14 participants with OCD
Discussion
To our knowledge, this is the first study to investigate longitudinal SSRI effects on RSFC in youths with OCD. Following sertraline treatment, RSFC significantly increased between both the right and left putamen and clusters in the left hemisphere including parts of the frontal cortex, insula, and operculum in youths with OCD compared to controls. These findings add to prior work implicating the CSTC and salience networks in OCD,6,8,39 and provide new evidence regarding how these circuits may be malleable in response to treatment. Further, increased connectivity in the left putamen circuit was significantly correlated with greater symptom improvement on CY-BOCS Total Score and CY-BOCS Compulsion subscale in the OCD group, highlighting the clinical significance of these RSFC changes and suggesting that increases in RSFC may represent an important mechanism of action underlying sertraline’s clinical effects in youths with OCD.
The phenotypic expression of OCD is highly heterogeneous, leading researchers to question whether particular symptom subtypes differ in terms of underlying neuropathology and treatment response. While we did not find significant associations between increase in left putamen connectivity and improvement on CY-BOCS dimension scores, prior research has demonstrated that distinct neural substrates mediate different OCD symptom dimensions using both structural40 and functional imaging. Further research is needed to determine if change in OCD dimension scores is associated with connectivity changes in specific circuits.
The current study highlights the importance of careful control of participant medication status in studies of brain connectivity in pediatric OCD. Without such control, it is difficult to discriminate RSFC patterns linked to disorder etiology versus medication effects. In our study, patients with OCD showed significantly lower right putamen RSFC than controls at baseline and significant increases in RSFC after 12 weeks of sertraline treatment, including greater RSFC than controls in several brain regions post-treatment. In our previous cross-sectional study, which included adolescents with OCD on SSRIs and/or clomipramine without remission of OCD symptoms (mean CY-BOCS = 19.7), individuals with OCD showed a significantly lower RSFC between left putamen and a right-sided cluster including parts of OFC, inferior frontal gyrus, insula, and operculum.6 It is possible that different patterns of results reflect medication confounds in the cross-sectional study. Furthermore, the observed connectivity patterns may have reflected nonresponse to serotonergic antidepressants or the longer-term effects of medication. Additional longitudinal studies of medication-free youths with OCD will help us understand if increase in RSFC in specific striatal-frontal or striatal-insular circuits may serve as a biomarker of positive response to SSRIs.
It was unexpected that the control group showed significant reduction of RSFC with time in the left putamen circuit. This may be partially due to natural fluctuations of RSFC over time,41,42 to acclimation to being scanned and/or to changes related to normative development.2 Our understanding of how RSFC networks change across time is still nascent. One prior cross-sectional study of healthy children, adolescents, and adults showed that RSFC between dorsal striatum and posterior cingulate increases with age (to the mid-twenties), but that RSFC between the ventral striatum and the anterior insula and dorsal anterior cingulate cortex decreases with age, well into adulthood.43 This is potentially in line with the direction of change in RSFC in the left putamen circuit noted in our healthy controls. Analysis of large R-fMRI datasets from longitudinal studies (with larger time windows) of children and adolescents such as the ABCD study44 are required to better understand how RSFC circuits evolve over the natural course of time and across development. The presence of a significant RSFC change detected in our control group underscores the relevance of including a control arm in study design. One consideration is that RSFC change over time in youths with OCD compared with healthy youths may differ irrespective of treatment. Future longitudinal work incorporating a placebo arm would be useful to disentangle existing questions.
The current study indicates that abnormal neural networks in pediatric OCD extend beyond CSTC circuitry, and involve parts of the salience network. The salience network signals the presence of events that are important to the individual, assists with conflict monitoring, and is involved with behavioral responses to stimuli.45 Salience network is comprised of insula, operculum, OFC, dACC, amygdala, ventral pallidostriatum, thalamus, and temporal pole. Insular regions are involved in emotional and motivational processes.46 Previous research has identified circuitry involving the dACC and insula as important in emotional, motivational, and cognitive processing in OCD.10,47 Our earlier cross-sectional study also identified parts of the salience network as involved in OCD circuitry.6 The current study suggests that striatal-salience circuits are malleable to treatment and that these changes relate to clinical improvement.
Strengths of this study include that all participants were assessed with validated semi-structured psychiatric interviews and multi-informant symptom ratings. This is the only longitudinal study to assess changes in RSFC from pre- to post-SSRI treatment in youths with OCD. Only a limited number of medication treatment studies have scanned healthy controls twice (simultaneously with the patient group) as comparators. At baseline, all participants with OCD were medication- free and most (79%) were medication-naive. Participants received a carefully monitored 12- week sertraline trial with target dose achieved by week 6. Pill counts and sertraline blood levels documented compliance.
The primary shortcoming of the study is the small sample size. Recruitment of medication-free youths with OCD who met criteria for the study and were willing to postpone other treatments for 12 weeks was challenging. Future work to confirm these findings with larger samples will require a significant investment of resources. Larger samples will have the power to characterize specific neural differences in participants who respond to SSRIs versus those who do not and to examine potential moderators of medication-induced RSFC changes, including age, gender, and interactions between age and gender, such as differences within groups of males and females based on pubertal status, which has been shown to be a moderator of function of neural fear systems.48 Second, although we collected longitudinal data from a healthy control group to provide a background of normal variation in RSFC metrics over time, a more optimal approach would have been to include a placebo arm, since longitudinal change in an OCD group in the absence of sertraline may be different from longitudinal change in untreated healthy controls. Inclusion of a placebo arm would confirm that changes over time in the OCD brain are due to medication and not effects of development, regression to the mean, or treatment expectation. This approach presents an ethical challenge since the beneficial effects of sertraline are already known; however future efforts might consider offering another standard treatment such as cognitive-behavioral therapy in both placebo and drug arms to mitigate this concern. Finally, our whole-brain analyses included voxel-wise z-threshold of 2.3 (which corresponds to p < .0107) and cluster threshold of p < .0042 to correct for 6 ROIs and 2 tests per ROI. Although these are commonly used thresholds in fMRI analyses, we acknowledge that in the context of recent debates in the literature,49 these may be considered liberal. Given the small sample size, we were under-powered to apply stricter thresholding and therefore, the results should be considered preliminary.
Functional connectivity changes may occur early in the course of treatment. Changes in brain connectivity have been reported after 1–7 days of antidepressants in healthy adults.21,23 Future directions may include obtaining multiple longitudinal scans during the course of SSRI treatment to investigate the trajectory of neural circuit change, which may eventually help to identify biomarkers of response and non-response earlier in the course of treatment (e.g., as opposed to waiting the several weeks typically needed to observe change in clinical symptoms).
In conclusion, we used a standard treatment (sertraline) as a probe to investigate the pathophysiology of pediatric OCD. This pilot study suggests that sertraline treatment in pediatric OCD is associated with increases in RSFC in CSTC circuits (i.e., between putamen and frontal cortex) and between the putamen and the insula/operculum (parts of salience network). We also demonstrated that greater improvement in OCD severity was associated with increase in RSFC in left putamen-frontal cortex/insular circuit. This pilot study elucidates the circuitry involved in SSRI treatment (i.e., CSTC and salience networks) and the direction of change (increase in RSFC after sertraline treatment). Additional research is needed to confirm our preliminary findings, which would pave the way for identification of circuit-based treatment targets for future novel treatment development.
Acknowledgments
Funding:
This study was supported by grants from the National Institutes of Health: R21MH101395 (G A B.), K23MH103617 (C.A.C.), 1P30NS076408, and P41EB015894. The work was carried out in part using computing resources at the University of Minnesota Supercomputing Institute.
The authors extend special thanks to the children and adolescents and their parents who participated. The authors gratefully acknowledge Mark Fiecas, PhD, School of Public Health, University of Minnesota, for statistical consultation.
Footnotes
Disclosures:
Drs. Bernstein, Cullen, Conelea, Zagoloff, Lee, and Mueller; Ms. Harris; and Ms. Carstedt reported no biomedical financial interests or potential conflicts of interest.
This data was presented in part at the American Academy of Child and Adolescent Psychiatry Annual Meetings in New York, NY, October 24–29, 2016, and Washington, DC, October 23–28, 2017.
Dr. Lee served as the statistical expert for this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry January 2010; 15(1):53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huyser C, Veltman DJ, de Haan E, Boer F. Paediatric obsessive-compulsive disorder, a neurodevelopmental disorder? Evidence from neuroimaging. Neurosci Biobehav Rev June 2009;33(6):818–830. [DOI] [PubMed] [Google Scholar]
- 3.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986;9:357–381. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald KD, Welsh RC, Stern ER, et al. Developmental alterations of frontal-striatal- thalamic connectivity in obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry September 2011;50(9):938–948 e933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maia TV, Cooney RE, Peterson BS. The neural bases of obsessive-compulsive disorder in children and adults. Dev Psychopathol Fall 2008;20(4):1251–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein GA, Mueller BA, Schreiner MW, et al. Abnormal striatal resting-state functional connectivity in adolescents with obsessive-compulsive disorder. Psychiatry Res January 30 2016;247:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shapira NA, Liu Y, He AG, et al. Brain activation by disgust-inducing pictures in obsessive-compulsive disorder. Biol Psychiatry October 1 2003;54(7):751–756. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Y, Fan Q, Zhang H, et al. Altered intrinsic insular activity predicts symptom severity in unmedicated obsessive-compulsive disorder patients: a resting state functional magnetic resonance imaging study. BMC psychiatry April 16 2016; 16:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzgerald KD, Stern ER, Angstadt M, et al. Altered function and connectivity of the medial frontal cortex in pediatric obsessive-compulsive disorder. Biol Psychiatry December 1 2010;68(11): 1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stern ER, Welsh RC, Fitzgerald KD, et al. Hyperactive error responses and altered connectivity in ventromedial and frontoinsular cortices in obsessive-compulsive disorder. Biol Psychiatry March 15 2011;69(6):583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: The pediatric OCD treatment study (POTS) randomized controlled trial. JAMA 2004;292:1969–1976. [DOI] [PubMed] [Google Scholar]
- 12.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med October 1995;34(4):537–541. [DOI] [PubMed] [Google Scholar]
- 13.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience September 2007; 8(9):700–711. [DOI] [PubMed] [Google Scholar]
- 14.Gursel DA, Avram M, Sorg C, et al. Frontoparietal areas link impairments of large-scale intrinsic brain networks with aberrant fronto-striatal interactions in OCD: a meta-analysis of resting-state functional connectivity. Neurosci Biobehav Rev February 3 2018;87:151–160. [DOI] [PubMed] [Google Scholar]
- 15.Peterson A, Thome J, Frewen P, Lanius RA. Resting-state neuroimaging studies: a new way of identifying differences and similarities among the anxiety disorders? Can J Psychiatry June 2014;59(6):294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin DJ, Jung WH, He Y, et al. The effects of pharmacological treatment on functional brain connectome in obsessive-compulsive disorder. Biol Psychiatry April 15 2014;75(8):606–614. [DOI] [PubMed] [Google Scholar]
- 17.Gruner P, Vo A, Argyelan M, et al. Independent component analysis of resting state activity in pediatric obsessive-compulsive disorder. Hum Brain Mapp October 2014;35(10):5306–5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber AM, Soreni N, Noseworthy MD. A preliminary study of functional connectivity of medication naive children with obsessive-compulsive disorder. Prog Neuropsychopharmacol Bol Psychiatry August 4 2014;53:129–136. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong CC, Moody TD, Feusner JD, et al. Graph-theoretical analysis of resting-state fMRI in pediatric obsessive-compulsive disorder. J Affect Disord March 15 2016;193:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heller AS, Johnstone T, Light SN, et al. Relationships between changes in sustained fronto-striatal connectivity and positive affect in major depression resulting from antidepressant treatment. Am J Psychiatry February 2013;170(2):197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCabe C, Mishor Z, Filippini N, et al. SSRI administration reduces resting state functional connectivity in dorso-medial prefrontal cortex. Mol Psychiatry June 2011; 16(6):592–594. [DOI] [PubMed] [Google Scholar]
- 22.Posner J, Hellerstein DJ, Gat I, et al. Antidepressants normalize the default mode network in patients with dysthymia. JAMA psychiatry April 2013;70(4):373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaefer A, Burmann I, Regenthal R, et al. Serotonergic modulation of intrinsic functional connectivity. Curr Biol October 6 2014;24(19):2314–2318. [DOI] [PubMed] [Google Scholar]
- 24.Stein DJ, Goodman WK, Rauch SL. The cognitive-affective neuroscience of obsessive- compulsive disorder. Curr Psychiatry Rep August 2000;2(4):341–346. [DOI] [PubMed] [Google Scholar]
- 25.Scahill L, Riddle MA, McSwiggin-Hardin M, et al. Children’s yale-brown obsessive compulsive scale: reliability and validity. J Am Acad Child Adolesc Psychiatry June 1997;36(6):844–852. [DOI] [PubMed] [Google Scholar]
- 26.Silverman WK, Albano AM. Anxiety Disorders Interview Schedule for DSM-IV, Child and Parent Versions. San Antonio, TX: Psychological Corporation, 1996. [Google Scholar]
- 27.Wechsler D Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio, TX: Harcourt Assessment, 1999. [Google Scholar]
- 28.Maxwell ME. Family Interview for Genetic Studies (FIGS): Manual for FIGS. Bethesda, MD: Clinical Neurogenetics Branch, Intramural Research Program, National Institute of Mental Health, 1992. [Google Scholar]
- 29.Storch EA, Murphy TK, Adkins JW, et al. The children’s yale-brown obsessive- compulsive scale: psychometric properties of child- and parent-report formats. J Anxiety Disord 2006;20(8):1055–1070. [DOI] [PubMed] [Google Scholar]
- 30.Silverman WK, Saavedra LM, Pina AA. Test-retest reliability of anxiety symptoms and diagnoses with the Anxiety Disorders Interview Schedule for DSM-IV: Child and parent versions. J Am Acad Child Adolesc Psychiatry August 2001;40(8):937–944. [DOI] [PubMed] [Google Scholar]
- 31.Wood JJ, Piacentini JC, Bergman RL, et al. Concurrent validity of the anxiety disorders section of the Anxiety Disorders Interview Schedule for DSM-IV: Child and parent versions. Journal of Clinical Child & Adolescent Psychology September 2002;31(3):335–342. [DOI] [PubMed] [Google Scholar]
- 32.Bernstein GA, Victor AM, Nelson PM, Lee SS. Pediatric obsessive-compulsive disorder: symptom patterns and confirmatory factor analysis. J Obsessive Compuls Relat Disord 2013;2:299–305. [Google Scholar]
- 33.Piacentini J, Peris TS, Bergman RL, et al. Functional impairment in childhood OCD: development and psychometrics properties of the child obsessive-compulsive impact scale-revised (COIS-R). Journal of Clinical Child and Adolescent Psychology Oct-Dec 2007;36(4):645–653. [DOI] [PubMed] [Google Scholar]
- 34.Power JD, Barnes KA, Snyder AZ, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage February 01 2012;59(3):2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salimi-Khorshidi GDG, Beckmann CF, Glasser MF, Griffanti L, Smith SM Automatic denoising of functional MRI data: Combining independent component analysis and hierarchical fusion of classifiers. NeuroImage 2014;90:449–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffanti L, Salimi-Khorshidi G, Beckmann CF, et al. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. Neuroimage July 15 2014;95:232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage October 15 2009;48(1):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale University Department of Sociology, 1975. [Google Scholar]
- 39.Qiu L, Fu X, Wang S, et al. Abnormal regional spontaneous neuronal activity associated with symptom severity in treatment-naive patients with obsessive-compulsive disorder revealed by resting-state functional MRI. Neurosci Lett February 15 2017;640:99–104. [DOI] [PubMed] [Google Scholar]
- 40.Lazaro L, Calvo A, Ortiz AG, et al. Microstructural brain abnormalities and symptom dimensions in child and adolescent patients with obsessive-compulsive disorder: a diffusion tensor imaging study. Depress Anxiety December 2014;31(12):1007–1017. [DOI] [PubMed] [Google Scholar]
- 41.Bernstein GA, Mueller BA, Glass TW, et al. The dynamic nature of functional brain connectivity in adolescents with OCD. Poster presented at the Annual Meeting of the American Academy of Child and Adolescent Psychiatry San Diego, CA 2014. [Google Scholar]
- 42.Handwerker DA, Roopchansingh V, Gonzalez-Castillo J, Bandettini PA. Periodic changes in fMRI connectivity. Neuroimage November 15 2012;63(3):1712–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porter JN, Roy AK, Benson B, et al. Age-related changes in the intrinsic functional connectivity of the human ventral vs. dorsal striatum from childhood to middle age. Developmental cognitive neuroscience February 2015;11:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volkow ND, Koob GF, Croyle RT, et al. The conception of the ABCD study: From substance use to a broad NIH collaboration. Developmental cognitive neuroscience October 10 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain structure & function June 2010;214(5–6):655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Craig AD. Emotional moments across time: a possible neural basis for time perception in the anterior insula. Philos Trans R Soc Lond B Biol Sci July 12 2009;364(1525):1933–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stern ER, Fitzgerald KD, Welsh RC, et al. Resting-state functional connectivity between fronto-parietal and default mode networks in obsessive-compulsive disorder. PLoS One 2012;7(5):e36356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramikie TS, Ressler KJ. Mechanisms of Sex Differences in Fear and Posttraumatic Stress Disorder. Biol Psychiatry May 15 2018;83(10):876–885. [DOI] [PubMed] [Google Scholar]
- 49.Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A July 12 2016;113(28):7900–7905 [DOI] [PMC free article] [PubMed] [Google Scholar]


