Abstract
Objective:
The aim of this project was to determine the effects of lower extremity aerobic exercise coupled with upper extremity repetitive task practice (RTP) on health related quality of life (HRQOL) and depressive symptomology in individuals with chronic stroke.
Design:
Secondary analysis of data from two randomized controlled trials.
Setting:
Research laboratory.
Participants:
Individuals (N=40) with chronic stroke.
Interventions:
Participants received one of the following interventions: forced exercise + RTP (FE+RTP, n=16), voluntary exercise + RTP (VE+RTP, n=16), or stroke education + RTP (EDU+RTP, n=8). All groups completed 24 sessions, each session lasting 90 minutes.
Main Outcome Measure:
The Center for Epidemiological Studies-Depression Scale (CES-D) and Stroke Impact Scale (SIS) were used to assess depressive symptomology and HRQOL.
Results:
There were no significant group-by-time interactions for any of the SIS domains or composite scores. Examining the individual groups following the intervention, those in the FE+RTP and VE+RTP groups demonstrated significant improvements in the following SIS domains: strength, mobility, hand function, activities of daily living, and the physical composite. Additionally, the FE+RTP group demonstrated significant improvements in memory, cognitive composite, and percent recovery from stroke. The HRQOL did not change in the EDU+RTP group. While CES-D scores improved predominately for those in the FE+RTP group, these improvements were not statistically significant. Overall, results were maintained at the four week follow-up.
Conclusion:
Aerobic exercise, regardless of mode, preceding motor task practice improved HRQOL in patients with stroke. The potential of aerobic exercise to improve cardiorespiratory endurance, motor outcomes, and HRQOL following stroke justifies its use to augment traditional task practice.
Keywords: stroke, depression, health related quality of life, aerobic exercise, forced exercise, repetitive task practice
Every year, approximately 795,000 people in the United States incur a stroke1 with approximately 25–50% of survivors experiencing long-term disability.2,3 Rehabilitation interventions following stroke aim to improve physical limitations, restore health-related quality of life (HRQOL), and reduce depressive symptomology. Investigating outcomes that extend beyond motor function is imperative, as an increasing body of literature has shown that survivors of stroke experience feelings of social isolation, difficulty re-integrating into the community,4 and post-stroke depression (PSD).5 Post-stroke depression is associated with increased disability, lower HRQOL, and higher mortality,6–8 and approximately 1 in 3 survivors of stroke exhibit symptoms of PSD.6,9,10 A challenge to reducing depression and improving HRQOL after stroke is the persistent upper extremity (UE) paresis experienced by >60% of survivors of stroke,11 which leads to greater dependency in mobility and activities of daily living (ADLs). Dependence in mobility and ADLs is associated with an increased risk of developing PSD.6,12,13 Interestingly, the degree of PSD or HRQOL experienced by survivors of stroke has not been shown to be affected by whether the stroke led to impairment in the dominant versus non-dominant UE.14,15
It is plausible that HRQOL and depressive symptomology may improve if a survivor of stroke can improve UE function. It has been theorized that aerobic exercise may be administered immediately preceding motor task practice to enhance motor recovery.17 To examine this theory, a preliminary trial was conducted examining the impact of pairing a high intensity aerobic exercise intervention, termed forced exercise (FE), with UE repetitive task practice (RTP).18,19 Results indicated that FE followed by UE RTP resulted in significant improvements in UE motor function compared to those who performed voluntary exercise (VE) followed by RTP and those who performed RTP alone.19 Improvements in motor recovery following a FE intervention provided initial support for the concept that high intensity aerobic exercise may facilitate motor recovery via an endogenous increase in central neurotrophic factor levels.18,19
It is unclear if a high intensity aerobic exercise combined with RTP leads to improvements in HRQOL and depressive symptomology following stroke. The aim of this project was to determine whether two different modes of aerobic exercise coupled with UE RTP affected HRQOL and depressive symptomology following stroke. Previous data indicated that FE was a more effective mode of exercise in terms of enhancing motor recovery;19 therefore, it was hypothesized that individuals who completed FE coupled with UE RTP would exhibit greater improvements in depressive symptomology and HRQOL compared to those who completed VE or those completing RTP in the absence of an aerobic intervention.
METHODS
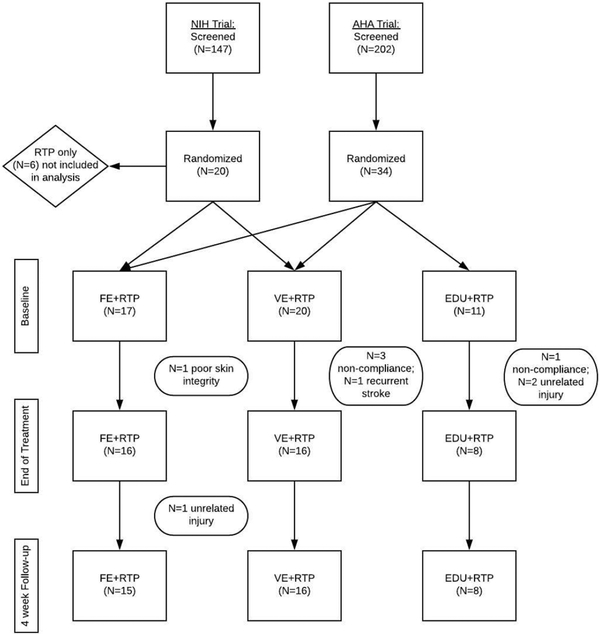
This report is a secondary analysis of data from two randomized controlled trials (clinicaltrials.gov registration numbers NCT02076776; National Institute of Health and NCT02494518; American Heart Association). Both studies recruited from the same demographic area and included individuals with chronic stroke and residual UE impairment (see inclusion/exclusion below). Figure 1 displays the participant flow for both studies. The most common reasons for exclusion were: level of impairment (too high or low functioning UE), lack of reliable transportation, and the individual declining to participate.
Figure 1:
Flow chart of the two randomized controlled clinical trials used for data analysis.
The FE and VE aerobic exercise protocols were identical in both studies. The studies differed in the intervention of the non-aerobic exercise control group. Six individuals in the NIH trial were randomized to a non-dose matched group (i.e. 90 minutes of RTP), thus were not included in this analysis. A dose-matched RTP group was included in the American Heart Association study; thus, those patients served as a control group in this analysis (i.e. stroke education + RTP; N=8). Both preliminary trials were approved by the (blinded) Institutional Review Board and participants completed the informed consent process prior to participation. All testing and interventions occurred at the main campus of the (blinded) located in (blinded).
Subjects
Inclusion criteria were as follows: 1) single, unilateral stroke ≥ 6 months prior, 2) 19–55 on the Upper Extremity Motor section of the Fugl-Meyer Assessment, 3) between 18–85 years of age, 4) able to provide informed consent, and 5) approval from the primary care physician to undergo cardiopulmonary exercise test. Exclusion criteria were: 1) hospitalization for myocardial infarction, congestive heart failure, or heart surgery within 3 months of study enrollment, 2) serious cardiac arrhythmia, 3) hypertrophic cardiomyopathy, 4) severe aortic stenosis, 5) cardiac pacemaker, 6) pulmonary embolus, 7) other medical or musculoskeletal contraindication to exercise, 8) significant cognitive impairment (inability to follow 1–2 step commands) or major psychiatric disorder resulting in difficulty participating in the study, 9) anti-spasticity injection in the upper extremity within 3 months of study enrollment, 10) pregnancy, 11) resting systolic blood pressure >200mmHg or diastolic blood pressure >110 mmHg, and 12) symptomatic fall in systolic blood pressure >20 mmHg with exercise.20,21 To ensure safe cardiac response to high-intensity exercise, participants meeting enrollment criteria underwent a cardiopulmonary exercise test.a
Outcome Measures
The Stroke Impact Scale (SIS) version 3.0 and the Center for Epidemiological Studies-Depression Scale (CES-D) were administered by a blinded rater at three time points: baseline, end of treatment (EOT) and four weeks following EOT (EOT+4).
Stroke Impact Scale 3.0 (SIS)
The SIS version 3.0 is a validated, self-reported questionnaire that uses a 5-point Likert scale to measure HRQOL. The 59 item questionnaire assesses eight domains: strength (4 items), hand function (5 items), mobility (9 items), ADL (10 items), communication (7 items), emotions (9 items), memory (7 items), and meaningful activities (8 items) for individuals after stroke.22,23 The domains can be combined to form two composite categories: physical (strength, hand function, mobility, ADL) and cognitive (communication, memory).24 Scores are normalized to a 100 point scale with a higher score indicating better self-rating in that category. Individuals also rank their global assessment of percentage of recovery on a 0–100 scale, with 100 being full recovery from the stroke.
Center for Epidemiological Studies-Depression (CES-D)
The CES-D is a 20-item measure that assesses self-reported symptoms of depression. It is a recommended screening tool for PSD,25 with established validity and reliability in a stroke population.26 Participants rate how frequently they experience various symptoms, with 0 indicating “rarely” and 3 indicating “most or almost all the time.” The highest possible score is 60, and scores ≥16 are indicative of depressive symptomology.26
Intervention
Following baseline testing, participants were randomized via an envelope pull to one of three groups: 1) Forced exercise + UE RTP (FE+RTP), 2) Voluntary exercise + UE RTP (VE+RTP), or 3) Stroke education + RTP (EDU+RTP). All participants attended a total of 24 sessions, each lasting 90 minutes.
Forced Exercise + Repetitive Task Practice (FE+RTP)
Individuals randomized to the FE+RTP group exercised for 45 minutes on a recumbent stationary cycle ergometer equipped with an electric motor and control algorithm to mechanically augment pedaling rate by 30% greater than the participant’s voluntary pedaling rate achieved during the exercise stress test.27–29 Each 45-minute FE session was monitored by an exercise physiologist or physical therapist and included a five-minute warm-up, 35-minute main exercise set, and a five-minute cool-down. While assistance was provided in the FE mode via the motor to augment pedaling rate, it is important to note that the participant was actively contributing to the pedaling action. Participants were instructed to maintain their heart rate between 60–80% of their heart rate reserve calculated using the Karvonen formula,21 and heart rate was continuously monitored.b Blood pressure and rate of perceived exertion were obtained every 10 minutes, while cadence and power (Watts) was recorded every five minutes. Following a 10-minute rest period, participants completed a 45-minute session of UE RTP.
Repetitive task practice emphasizes highly-repetitious blocked practice tasks that are goal-oriented and relevant to the individual.30 The RTP activities were administered by a neurologic physical therapist who tailored each task to ensure appropriate difficulty and relevance. During the 45 minute RTP session, the therapist typically selected 3–5 tasks and targeted 70–100 repetitions of each task.
Voluntary Exercise + Repetitive Task Practice
The VE+RTP sessions were conducted in an identical manner to the FE+RTP sessions, except participants in the VE+RTP group exercised for 45 minutes on a stationary recumbent cycle ergometer at a self-selected cadence without assistance from a motor. Target heart rate range was identical to the FE+RTP group, at 60–80% of heart rate reserve. Exercise monitoring and RTP sessions were also conducted in an identical manner to the FE+RTP group.
Stroke Education +Repetitive Task Practice Group
The control group underwent a time-matched intervention consisting of a 45-minute session of stroke-related education followed by a 45-minute session of UE RTP. Each education session covered a different stroke-related topic such as stroke pathology, nutrition, pharmacology, fatigue, etc. The RTP sessions were administered in an identical manner to the FE+RTP and VE+RTP groups.
Statistical Analysis
The analysis included participants who completed all study-related interventions and clinical testing. A total of 48 individuals were randomized into the two studies and eight participants withdrew from the study (Figure 1). At EOT, there were a total of 16, 16, and 8 individuals included in the FE+RTP, VE+RTP, and EDU+RTP groups, respectively. At EOT+4, one participant in the FE+RTP group experienced an unrelated injury and was excluded from analysis at that time point.
Demographic variables were summarized per group using summary statistics. The intervention effects were assessed using a linear mixed effects model including a random intercept, a main effect for time, and the group-by time interaction. Normality assumptions of the residuals were met, as normality was checked visually using a QQ-plot and fitted values were plotted against residuals. The model adjusted for baseline differences between groups by incorporating the baseline metric in the outcome vector and by excluding the group main effect term from the model.31 The model estimated the changes from baseline to EOT and from baseline to EOT+4.
The group-by-time interaction was assessed from this model at the 0.05 significance level. Regardless of interaction statistical significance, post hoc contrasts were performed estimating the change from baseline to EOT and EOT+4 within each randomized group. The CES-D outcome was not normally distributed, and paired Wilcoxon tests were performed within each group from baseline to EOT and baseline to EOT+4. Within each outcome, comparisons were Bonferroni corrected to maintain a 5% type I error rate per outcome. Analyses were conducted using R version 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria) and the “nlme” package.32
RESULTS
A total of 40 participants successfully completed the intervention and were included in the analyses. Participant demographics and baseline characteristics are summarized in Table 1. There were no significant differences among demographic variables across groups (p>0.05).
Table 1.
Subject demographics and baseline characteristics by group
| Factor | FE+RTP (N = 16) |
VE+RTP (N = 16) |
EDU+RTP (N = 8) |
|---|---|---|---|
| Age (years) | 51 ± 12 | 60 ± 14 | 58 ± 12 |
| Male | 12 (75%) | 10 (62%) | 7 (88%) |
| Race | |||
| African American | 8 (50%) | 3 (19%) | 4 (50%) |
| Asian | 1 (6%) | 1 (6%) | 0 (0%) |
| Other | 2 (12%) | 0 (0%) | 1 (12%) |
| White | 5 (31%) | 12 (75%) | 3 (38%) |
| Hispanic Ethnicity | 1 (6%) | 0 (0%) | 1 (12%) |
| Months since stroke | 12 [7, 16] | 16 [11, 32] | 17 [12, 35] |
| Baseline Fugl Meyer | 37 ± 8 | 33 ± 11 | 33 ± 9 |
| Assessment score |
EDU, education; FE, forced exercise; RTP, repetitive task practice; VE, voluntary exercise Summary statistics presented as mean ± standard deviation (normally distributed characteristics), median [first quartile, third quartile] (characteristics with skewed data), or N (%) (categorical data). T-tests were used for normally distributed variables, while Wilcoxon rank sum tests were used for skewed data.
There was no significant difference between groups (p>0.05)
Effect of exercise on the Stroke Impact Scale (SIS)
The mean ± standard deviation for the SIS outcome variables are summarized in Table 2. In contrast to our hypothesis, there were no significant group-by-time interactions for any of the SIS domains or the SIS composite scores (p>0.05). Post hoc contrasts to examine within group differences over time revealed those in the FE+RTP group demonstrated a significant improvement in the following domains of the SIS from baseline to EOT: strength (p=0.002), mobility (p<0.001), hand function (p<0.001), ADL (p=0.005), and memory (p=0.04). With the exception of memory, improvements in these domains persisted at the 4-week follow-up, and the communication domain became significant (p=0.03). Participants in the VE+RTP group exhibited significant improvements in the physical domains of strength (p=0.03), mobility (p=0.001), hand function (p<0.001), and ADL (p<0.001) following the intervention. All of these improvements were maintained at the 4-week follow-up assessment. The EDU+RTP group only exhibited an improvement in hand function from baseline to EOT+4 (p<0.001).
Table 2.
Stroke Impact Scale (SIS) and Center for Epidemiological Studies-Depression Scale (CES-D) results by group
| FE + RTP (N = 16) | VE +RTP (N = 16) | EDU+RTP (N = 8) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Baseline | EOT | EOT+4* | Baseline | EOT | EOT+4 | Baseline | EOT | EOT+4 |
| SIS domains | |||||||||
| Strength | 55 ± 15 | 69 ± 13† | 66 ± 18† | 53 ± 18 | 64 ± 17† | 66 ± 18† | 51 ± 17 | 56 ± 12 | 62 ± 15 |
| Mobility | 75 ± 13 | 87 ± 8† | 85 ± 11† | 78 ± 19 | 88 ± 10† | 88 ± 10† | 77 ± 15 | 81 ± 10 | 86 ± 11 |
| Hand Function | 56 ± 23 | 72 ± 14† | 73 ± 17† | 43 ± 26 | 58 ± 30† | 61 ± 31† | 52 ± 23 | 61 ± 16 | 73 ± 18† |
| Activities of Daily Living | 75 ± 10 | 82 ± 10† | 84 ± 10† | 72 ± 17 | 81 ± 15† | 80 ± 14† | 73 ± 10 | 78 ± 11 | 81 ± 7 |
| Memory | 77 ± 19 | 85 ± 16† | 83 ± 18 | 82 ± 21 | 81 ± 22 | 85 ± 21 | 91 ± 6 | 88 ± 13 | 95 ± 8 |
| Emotions | 81 ± 14 | 89 ± 13 | 86 ± 13 | 87 ± 9 | 85 ± 14 | 89 ± 13 | 83 ± 15 | 85 ± 12 | 91 ± 8 |
| Communication | 84 ± 19 | 91 ± 12 | 92 ± 13† | 83 ± 26 | 84 ± 24 | 87 ± 19 | 96 ± 5 | 93 ± 6 | 95 ± 5 |
| Meaningful Activities | 63 ± 20 | 71 ± 16 | 68 ± 19 | 64 ± 19 | 70 ± 22 | 71 ± 21 | 58 ± 20 | 58 ± 17 | 71 ± 19 |
| SIS Physical Composite | 58 ± 10 | 69 ± 9† | 67 ± 10† | 57 ± 14 | 66 ± 15† | 67 ± 14† | 56 ± 10 | 60 ± 8 | 68 ± 7† |
| SIS Cognitive Composite | 80 ± 18 | 88 ± 13† | 87 ± 15† | 82 ± 22 | 82 ± 21 | 86 ± 19 | 94 ± 5 | 91 ± 7 | 95 ± 6 |
| Stroke Recovery (%) | 67 ± 10 | 77 ± 10† | 73 ± 14† | 58 ± 19 | 63 ± 15 | 68 ± 14† | 62 ± 11 | 64 ± 18 | 68 ± 12 |
| CES-D | 15 ± 10 | 9 ± 8 | 10 ± 8 | 10 ± 8 | 10 ± 10 | 8 ± 7 | 10 ± 9 | 8 ± 7 | 7 ± 6 |
CES-D, Center for Epidemiological Studies-Depression Scale; EDU, education; FE, forced exercise; RTP, repetitive task practice; SIS, stroke impact scale; VE, voluntary exercise
Data are presented as mean ± SD. There was no interaction effect among any of the outcome variables (p>0.05)
indicates a significant difference from baseline (p<0.05)
One FE patient did not complete an EOT + 4 follow-up and was thus excluded from analyses.
The CES-D outcome was not normally distributed so paired Wilcoxon tests were performed within each group.
The FE+RTP group displayed significant improvements in the cognitive composite from baseline to EOT (p=0.007) and baseline to EOT+4 (p=0.01). When examining the physical composite score, the FE+RTP and VE+RTP groups improved from baseline to EOT (p<0.001 for both) and from baseline to EOT+4 (p<0.001 for both). The EDU+RTP group exhibited a significant improvement in the physical composite from baseline to EOT+4 only (p<0.001). The FE+RTP group displayed a significant improvement in percent recovery from stroke for baseline to EOT (p<0.001) and EOT+4 (p=0.02). The VE+RTP group displayed a significant improvement in percent recovery from baseline to EOT+4 (p=0.02), while the EDU+RTP group did not significantly change.
Effect of exercise on the Center for Epidemiological Studies-Depression (CES-D)
There was no statistically significant change in any of the groups from baseline to EOT and EOT+4 (p>0.05). Notably, the FE+RTP group demonstrated a mean improvement of 6 points in the CES-D from baseline to EOT, while the VE+RTP and EDU+RTP groups improved by a mean of 0 and 2 points, respectively.
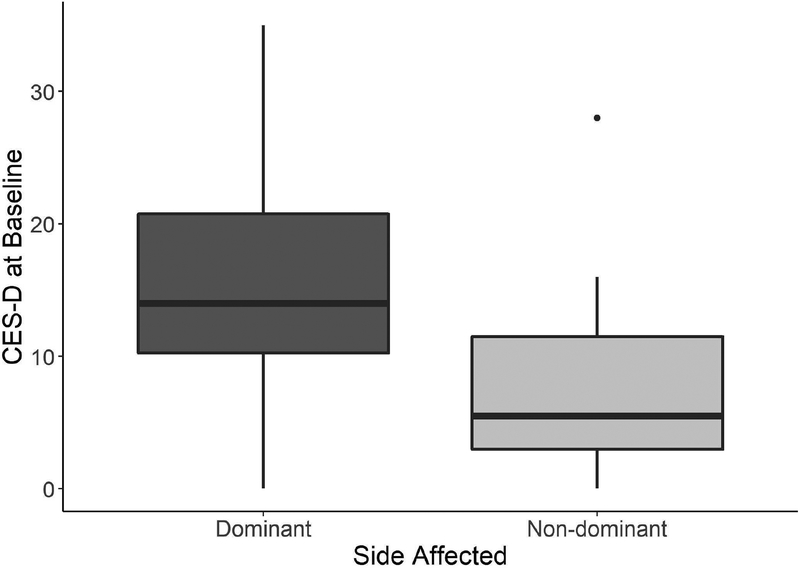
An exploratory analysis revealed a difference in baseline CES-D scores between individuals with hemiparesis in the dominant (n = 22; 15.7 ± 9.3 points) versus non-dominant side (n = 18; 7.8 ± 6.8 points) (Figure 2). A multivariable linear regression model with baseline CES-D as a function of affected side, age, gender, race, baseline Fugl-Meyer Assessment score, and time since stroke was used to determine association. Dominant affected side was significantly associated with increased CES-D at baseline, with an estimated increase in mean CES-D (95% CI) of 7.7 (2.8, 12.8) compared to non-dominant affected side after controlling for baseline demographic variables (p=0.004).
Figure 2:
Box plot of baseline CES-D scores separated by dominant (n=22) and non-dominant (n=18) side. Those whose dominate UE was affected by stroke experienced significantly higher CES-D scores, indicating increased depressive symptomology.
DISCUSSION
Stroke rehabilitation traditionally targets motor impairments, and the impact of physical activity on mood and HRQOL are often secondary or not measured. Our primary analysis did not support the hypothesis that FE+RTP would result in the greatest improvements in HRQOL and depressive symptomology. However, when each group was examined separately, our post hoc analysis indicated that aerobic exercise, both VE and FE, when combined with RTP resulted in improvements in multiple domains of the SIS after the intervention, while motor task practice coupled with education did not impact SIS. Overall, this pattern of results was maintained at the 4-week follow-up.
It is intuitive that a motor intervention leads to improvements in physical domains of the SIS; yet a significant improvement was found in the memory domain and cognitive composite in the FE+RTP group from baseline to EOT and in the communication domain from baseline to EOT+4. The SIS is a self-reported questionnaire; thus, we cannot conclude that an individual’s perception of improved cognition, memory, or communication resulted in an actual improvement. Notably, the role of intensive aerobic exercise in improving cognitive function in individuals with mild cognitive impairment is well documented.33,34 While our results are encouraging in demonstrating the potential benefits of intensive aerobic exercise in impacting memory and communication after stroke, our study was underpowered to detect change in these domains and further study is warranted using standardized neuropsychological measures of multiple components of executive function such as working memory, information processing, processing speed, attention, and learning.
Stroke often causes a dramatic shift in lifestyle, with almost 1/3 of survivors reporting social isolation, rarely leaving their home, or not engaging in social activities.35 Active participation in interventions such as in this study where participants traveled to a rehabilitation laboratory and interacted with a therapist (and potentially other study participants) for a minimum of 4½ hours per week for an 8-week period, may reduce feelings of social isolation.36,37 Therefore, ensuring comparable total contact time and dosage of UE rehabilitation across groups was a critical component of our experimental approach. All three groups experienced identical contact time during their respective interventions; thus, it is unlikely that therapist interaction alone was a defining factor in HRQOL improvements. Given previous reports of aerobic exercise contributing to improvements in mood,38 cognition,33,34 and motor improvements,18,19 it is plausible that the aerobic exercise interventions played a role in the observed improvements.
Although there was no statistically significant effect of group assignment on change in depressive symptomology, several observations can be made from the data that warrant further investigation. Those in the FE+RTP group improved by a mean of 6 points on the CES-D while modest or no changes were evident in the remaining groups. Aerobic exercise has been recommended as an efficacious, non-pharmacological adjunct to the treatment of depression.38 The exact mechanism as to how aerobic exercise impacts depression is not known; however, it is theorized that exercise-induced neurotrophic growth factors promote neurogenesis in the hippocampus, an area of the brain which plays a prominent role in depression.39 The precise type, intensity, and dosage needed for optimal effect in humans are not known, although in stroke it appears that high intensity exercise has a greater effect on depressive symptomology than low intensity.40 The intensive aerobic exercise intervention prescribed for the FE+RTP group may have contributed to the positive, albeit not statistically significant, outcomes observed in our study.
An unexpected and potentially meaningful finding was observed when participants’ levels of depression was evaluated as a function of dominant versus non-dominant side affected by the stroke. Individuals with hemiparesis in their dominant UE were more likely to exhibit depressive symptomology at baseline, presenting with a mean score of approximately 8 points greater on the CES-D than their counterparts. This finding may appear intuitive – those whose dominant side was affected likely experienced greater difficulty performing unimanual ADL’s such as brushing teeth and hair, and ADL dependence is related to PSD.12 However, our findings are in contrast with Nam and colleagues who found that dominance and side of hemiparesis had no bearing on HRQOL.15 Further investigation is warranted to examine the relationship between hand dominance, PSD, and HRQOL, and how those factors may potentially be used to trigger additional neuropsychological or behavioral therapy.
Study Limitations
While including HRQOL and depressive symptomology outcomes is justified when examining the impact of aerobic exercise on survivors of stroke, these studies were underpowered to detect differences between groups in these outcomes. It is likely that the EDU+RTP group experienced a ceiling effect in several of the SIS cognitive domains (Memory, Communication, and Cognitive Composite), and thus limited the ability to detect improvement in that group. Future studies should consider including individuals with more severe depressive disorder, as that patient subgroup was excluded from this study and thus limits the generalizability. A larger randomized controlled trial powered to investigate the impact of aerobic exercise on HRQOL and depressive symptomology in individuals with chronic stroke would address these limitations and provide significant value to the stroke community.
CONCLUSION
The results from this study indicated that a combined intervention of aerobic exercise and RTP may improve HRQOL in individuals with chronic stroke. While we cannot claim superiority due to the lack of interaction effect, following the intervention both aerobic exercise groups demonstrated enhanced HRQOL in the motor domains of the SIS and FE combined with UE RTP improved in the non-motor domains of the SIS. Changes in depressive symptomology did not change statistically following the intervention; however, there was a numerical improvement in the FE+RTP group, indicating that the impact of high intensity aerobic exercise on depression in stroke may be improve with a FE intervention. These results are promising and warrant further investigation with a larger clinical trial powered to detect differences in these outcomes. Our future work includes examining these outcomes in a larger randomized clinical trial and investigating the economic impact of these interventions form the perspectives of the individual, healthcare sector, and society. The additional benefits of aerobic exercise training in improving aerobic functional capacity and reducing cardiovascular risk factors cannot be overstated,41–43 and thus it should be considered as an adjunct to traditional motor rehabilitation.
Acknowledgements:
The authors would like to thank Amanda L. Penko for her assistance implementing the cycling intervention.
Funding Source: This study was supported by the National Institute of Neurological Disorders and Stroke (R03HD073566) and the American Heart Association (15MCPRP25700312). The funders had no role in data collection and analysis or preparation of the manuscript.
Clinical Trail Registration: This trial is registered at clinicaltrials.gov, registration numbers NCT02076776; NIH and NCT02494518; AHA
Abbreviations:
- ADLs
activity of daily living
- CES-D
Center for Epidemiological Studies-Depression Scale
- EDU
education
- EOT
end of treatment
- FE
forced exercise
- FMA
Fugl-Meyer assessment
- PSD
post-stroke depression
- HRQOL
health-related quality of life
- RTP
repetitive task practice
- SIS
Stroke Impact Scale
- UE
upper extremity
- VE
voluntary exercise
Footnotes
Previous Scientific Presentation: A subset of the results have been presented in poster format at the American Physical Therapy Association Combined Sections Meeting 2018.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: JLA has authored intellectual property associated with the algorithm used in the control of the FE bicycle. The remaining authors declare no conflicts of interest.
Suppliers:
Lode upright bicycle ergometer, Groningen, Netherlands;
Garmin™Edge® 800, Olathe, KS
REFERENCES
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D’Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis. 2003;12(3):119–126. [DOI] [PubMed] [Google Scholar]
- 3.do Carmo JF RLM, Pinto HP, de Oliveira ERA. Disability after stroke: a systematic review. Fisioter Mov. 2015;28(2):407–418. [Google Scholar]
- 4.Walsh ME, Galvin R, Loughnane C, Macey C, Horgan NF. Factors associated with community reintegration in the first year after stroke: a qualitative meta-synthesis. Disabil Rehabil. 2015;37(18):1599–1608. [DOI] [PubMed] [Google Scholar]
- 5.Esparrago Llorca G, Castilla-Guerra L, Fernandez Moreno MC, Ruiz Doblado S, Jimenez Hernandez MD. Post-stroke depression: an update. Neurologia. 2015;30(1):23–31. [DOI] [PubMed] [Google Scholar]
- 6.Ayerbe L, Ayis S, Wolfe CD, Rudd AG. Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br J Psychiatry. 2013;202(1):14–21. [DOI] [PubMed] [Google Scholar]
- 7.Pohjasvaara T, Vataja R, Leppavuori A, Kaste M, Erkinjuntti T. Depression is an independent predictor of poor long-term functional outcome post-stroke. Eur J Neurol. 2001;8(4):315–319. [DOI] [PubMed] [Google Scholar]
- 8.Razmara A, Valle N, Markovic D, et al. Depression Is Associated with a Higher Risk of Death among Stroke Survivors. J Stroke Cerebrovasc Dis. 2017;26(12):2870–2879. [DOI] [PubMed] [Google Scholar]
- 9.Hackett ML, Yapa C, Parag V, Anderson CS. Frequency of depression after stroke - A systematic review of observational studies. Stroke. 2005;36(6):1330–1340. [DOI] [PubMed] [Google Scholar]
- 10.Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. International Journal of Stroke. 2014;9(8):1017–1025. [DOI] [PubMed] [Google Scholar]
- 11.Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34(9):2181–2186. [DOI] [PubMed] [Google Scholar]
- 12.Allan LM, Rowan EN, Thomas AJ, Polvikoski TM, O’Brien JT, Kalaria RN. Long-term incidence of depression and predictors of depressive symptoms in older stroke survivors. Brit J Psychiat. 2013;203(6):453–460. [DOI] [PubMed] [Google Scholar]
- 13.De Ryck A, Brouns R, Fransen E, et al. A prospective study on the prevalence and risk factors of poststroke depression. Cerebrovasc Dis Extra. 2013;3(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris JE, Eng JJ. Individuals with the dominant hand affected following stroke demonstrate less impairment than those with the nondominant hand affected. Neurorehabil Neural Repair. 2006;20(3):380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nam HU, Huh JS, Yoo JN, et al. Effect of dominant hand paralysis on quality of life in patients with subacute stroke. Ann Rehabil Med. 2014;38(4):450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ploughman M, Attwood Z, White N, Dore JJ, Corbett D. Endurance exercise facilitates relearning of forelimb motor skill after focal ischemia. The European journal of neuroscience. 2007;25(11):3453–3460. [DOI] [PubMed] [Google Scholar]
- 17.Mang CS, Campbell KL, Ross CJ, Boyd LA. Promoting neuroplasticity for motor rehabilitation after stroke: considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor. Phys Ther. 2013;93(12):1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linder SM, Rosenfeldt AB, Rasanow M, Alberts JL. Forced Aerobic Exercise Enhances Motor Recovery After Stroke: A Case Report. Am J Occup Ther. 2015;69(4):6904210010p6904210011–6904210018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linder SM, Rosenfeldt AB, Dey T, Alberts JL. Forced Aerobic Exercise Preceding Task Practice Improves Motor Recovery Poststroke. Am J Occup Ther. 2017;71(2):7102290020p7102290021–7102290020p7102290029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yates JS, Studenski S, Gollub S, et al. Bicycle ergometry in subacute-stroke survivors: feasibility, safety, and exercise performance. Journal of aging and physical activity. 2004;12(1):64–74. [DOI] [PubMed] [Google Scholar]
- 21.American College of Sports Medicine. Resource Manual for Guidelines for Exercise Testing and Prescription. 10th ed: Wolters Kluwer; 2018. [Google Scholar]
- 22.Duncan PW, Bode RK, Min Lai S, Perera S, Glycine Antagonist in Neuroprotection Americans I. Rasch analysis of a new stroke-specific outcome scale: the Stroke Impact Scale. Arch Phys Med Rehabil. 2003;84(7):950–963. [DOI] [PubMed] [Google Scholar]
- 23.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30(10):2131–2140. [DOI] [PubMed] [Google Scholar]
- 24.Vellone E, Savini S, Fida R, et al. Psychometric evaluation of the Stroke Impact Scale 3.0. J Cardiovasc Nurs. 2015;30(3):229–241. [DOI] [PubMed] [Google Scholar]
- 25.Meader N, Moe-Byrne T, Llewellyn A, Mitchell AJ. Screening for poststroke major depression: a meta-analysis of diagnostic validity studies. J Neurol Neurosurg Psychiatry. 2014;85(2):198–206. [DOI] [PubMed] [Google Scholar]
- 26.Shinar D, Gross CR, Price TR, Banko M, Bolduc PL, Robinson RG. Screening for depression in stroke patients: the reliability and validity of the Center for Epidemiologic Studies Depression Scale. Stroke. 1986;17(2):241–245. [DOI] [PubMed] [Google Scholar]
- 27.Ridgel AL, Vitek JL, Alberts JL. Forced, not voluntary, exercise improves motor function in Parkinson’s disease patients. Neurorehabil Neural Repair. 2009;23(6):600–608. [DOI] [PubMed] [Google Scholar]
- 28.Shah C, Beall EB, Frankemolle AM, et al. Exercise Therapy for Parkinson’s Disease: Pedaling Rate Is Related to Changes in Motor Connectivity. Brain Connect. 2016;6(1):25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alberts JL, Linder SM, Penko AL, Lowe MJ, Phillips M. It is not about the bike, it is about the pedaling: forced exercise and Parkinson’s disease. Exerc Sport Sci Rev. 2011;39(4):177–186. [DOI] [PubMed] [Google Scholar]
- 30.Hubbard IJ, Parsons MW, Neilson C, Carey LM. Task-specific training: evidence for and translation to clinical practice. Occup Ther Int. 2009;16(3–4):175–189. [DOI] [PubMed] [Google Scholar]
- 31.Fitzmaurice G, Laird N, JH W. Applied Longitudinal Analysis. 2nd ed ed. Hoboken NJ: Wiley; 2011. [Google Scholar]
- 32.Pinheiro J, Bates D, DebRoy S, Sarkar D. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–131. 2017; https://CRAN.R-project.org/package=nlme.
- 33.Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cammisuli DM, Innocenti A, Franzoni F, Pruneti C. Aerobic exercise effects upon cognition in Mild Cognitive Impairment: A systematic review of randomized controlled trials. Arch Ital Biol. 2017;155(1–2):54–62. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Yan T, You L, Li K, Gao Y. Social Isolation and Physical Barriers in the Houses of Stroke Survivors in Rural China. Arch Phys Med Rehabil. 2016;97(12):2054–2060. [DOI] [PubMed] [Google Scholar]
- 36.Dickens AP, Richards SH, Greaves CJ, Campbell JL. Interventions targeting social isolation in older people: a systematic review. BMC Public Health. 2011;11:647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Health Quality O Social isolation in community-dwelling seniors: an evidence-based analysis. Ont Health Technol Assess Ser. 2008;8(5):1–49. [PMC free article] [PubMed] [Google Scholar]
- 38.Schuch FB, Vancampfort D, Richards J, Rosenbaum S, Ward PB, Stubbs B. Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. J Psychiatr Res. 2016;77:42–51. [DOI] [PubMed] [Google Scholar]
- 39.Alenina N, Klempin F. The role of serotonin in adult hippocampal neurogenesis. Behav Brain Res. 2015;277:49–57. [DOI] [PubMed] [Google Scholar]
- 40.Eng JJ, Reime B. Exercise for depressive symptoms in stroke patients: a systematic review and meta-analysis. Clin Rehabil. 2014;28(8):731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kendall BJ, Gothe NP. Effect of Aerobic Exercise Interventions on Mobility among Stroke Patients: A Systematic Review. Am J Phys Med Rehabil. 2016;95(3):214–224. [DOI] [PubMed] [Google Scholar]
- 42.Hackam DG, Spence JD. Combining multiple approaches for the secondary prevention of vascular events after stroke: a quantitative modeling study. Stroke. 2007;38(6):1881–1885. [DOI] [PubMed] [Google Scholar]
- 43.Billinger SA, Arena R, Bernhardt J, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(8):2532–2553. [DOI] [PubMed] [Google Scholar]