Introduction
The prevalence of erectile dysfunction (ED) among men in the US ranges from 10–18% but rates are as high as 50% in men with HIV. [1–5] The most commonly prescribed medications used to treat ED are phosphodiesterase-5 enzyme inhibitors (PDE-I): sildenafil, tadalafil, and vardenafil. Sildenafil (viagra) has been available on the market since 1998 and a generic version is now available at reduced cost. More than 12 million prescriptions for sildenafil and tadalafil were filled in the US in 2016. [6] Limited data suggests that erectile dysfunction medication (EDM) use is correlated with risky sexual behavior, particularly among men-who-have-sex-with-men (MSM), although few studies focus on men living with HIV. [7–13] In general, US providers fail to ask patients routinely about sexual behaviors or screen for sexually transmitted infection (STI) in clinic; this extends to men prescribed EDM. [14–16] HIV/STI coinfection is particularly important among men with HIV since the probability of HIV transmission to a sexual partner increases by 2–4 fold in the presence of STI coinfection. [17] Within the US, the southern states account for 44% of all people living with an HIV diagnosis. Among new HIV diagnoses, 54% occur among black Americans, 59% occur among black MSM. [18] The prevalence of gonorrhea and chlamydia are also highest in the southern region of the US; within Alabama, incidence rose to 246 per 100,000 for gonorrhea and 616 per 100,000 for chlamydia. [19, 20]
Since bacterial STI rates are currently at peak levels in the US (particularly among MSM) and EDM is a commonly prescribed medication, it is critical to understand any association between EDM and sexual risk behaviors and infection outcomes. [21–23] We conducted a retrospective cohort study of HIV-infected men in care to assess whether or not prescription of EDM was followed by a change in bacterial STI testing rates, infection rates and/or sexual behavior patterns.
Materials and Methods
Study Population
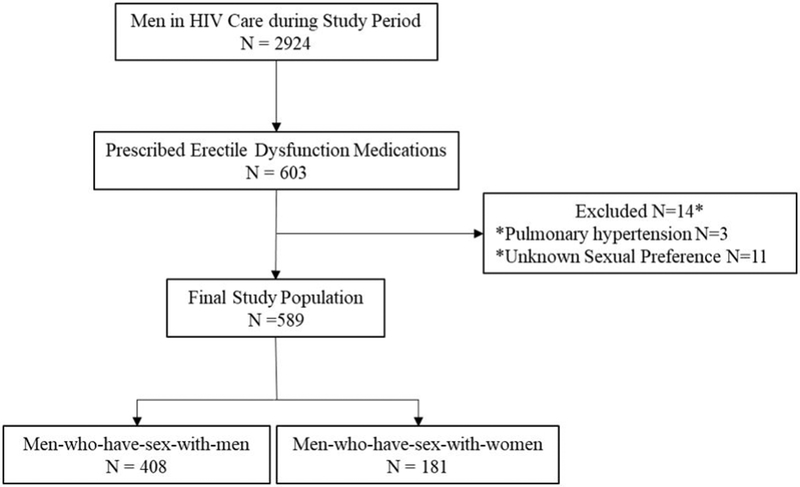
Study participants were adult men (age ≥18) who were engaged in care at the 1917 HIV clinic at the University of Alabama at Birmingham (UAB) during 2008–2016(Fig 1). The 1917 clinic is the largest HIV clinic in Alabama with approximately 3800 active patients. Care engagement was defined by the Health Resources and Services Administration and HIV/AIDS Bureau (HRSA-HAB) criterion: at least two provider visits separated by 90 days during the 12-month period before and after EDM prescription. Participants were eligible for this retrospective cohort study if they had documentation of a new prescription for medication to treat ED (sildenafil, tadalafil, and/or vardenafil) from a clinic provider between August 2008 and June 2016 and documentation of sexual preference. Patients were excluded if they were receiving PDE-I for indications other than erectile dysfunction.
Figure 1.
Flow diagram of Study Participants
Study Database
Data extracted from electronic medical records included demographic characteristics (age, race, and insurance status), medical comorbidities, laboratory data and patient-reported outcomes (PRO). PRO data is self-reported information about sexual preferences, practices and substance use/alcohol use in the past three to six months. This data is collected using touch-screen computer surveys performed in clinic every six months. The PRO questionnaire offers standardized, validated instruments; the alcohol use disorders identification test (AUDIT), the AUDIT alcohol consumption questions (AUDIT-C), the HIV risk assessment for positives (HRAP), and the alcohol, smoking and substance involvement screening test (ASSIST). [24–27] CD4 and HIV viral load count were included within 6 months of the date of EDM prescription.
Study Outcomes
The main study outcomes were bacterial STI (CT, GC, and incident syphilis) testing and infection rates and sexual behavior was assessed before and after EDM prescription in clinic. STI data was collected during 12-month periods before and after EDM prescription. STI testing was defined as any test performed in clinic for CT, GC or syphilis and results are presented separately for each infection. Symptom information was not captured. CT and GC were diagnosed by PCR with nucleic acid amplification testing (NAAT) at the UAB STI Diagnostic Laboratory (Aptima Hologic, San Diego, CA). CT/GC antigen tests were available but rarely performed. Samples were collected from urogenital (urine) and extragenital (rectal and oropharyngeal) sites. Routine syphilis testing with the traditional testing algorithm (RPR screen and TPPA confirmation) was used between 2008 and February 2015. The reverse algorithm (treponemal IgG enzyme immunoassay (EIA) screen and RPR follow up testing) was used between March 2015 and December 2016. Incident syphilis was classified based on medical record review and the standard CDC case-definition (new positive RPR with treponemal confirmatory testing or a 4-fold increase in RPR titer with positive treponemal testing with clinically compatible syndromes, when present). [28] For the measurement of STI screening and STI detection, only the first test (performed or positive) during the 12-month period for each individual STI was used. Repeated positive or persistently positive tests for the same pathogen during the 12-month period were not included.
PRO questionnaires have been offered since 2008: thus, the study period for sexual behavior data was restricted to 2009–2015 in order to allow for data collection 12 months before and after EDM prescription. Risky sexual behaviors were defined as: unprotected sex without a condom, more than one partner in the past six months and sex after illicit drugs/alcohol. [29]
Statistical Analysis
For descriptive data, continuous variables were reported as mean (standard deviation, SD) and median (with first and third quartiles) and categorical variables were reported as frequencies and percentages. Paired data analysis was used to compare the outcomes during the 12-month period before and after EDM prescription. Conditional odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using conditional logistic regression. In addition to the overall comparison, stratification by sexual preference was performed (MSM and men-who-have-sex-with-women [MSW]).
Missing data was encountered, particularly for PROs. In order to account for this and to explore the robustness of the results, a sensitivity analysis was conducted using a generalized mixed model approach with poisson distribution for the number of partners and logit distribution (dichotomized outcome) for sexual activity, unprotected sex and sex after drugs/alcohol.
Statistical significance was set at 0.05 (two-tailed) and the analysis was conducted using SAS statistical software, version 9.4 (Cary, NC).
Ethics Approval
The study was approved by the University of Alabama at Birmingham Institutional Review Board with a waiver of informed consent.
Results
Patient characteristics
Of 2924 HIV-infected men in care, 603 (20.1 %) had newly prescribed EDM during the study period (Figure 1). Fourteen men were excluded: 11 were missing self-reported sexual preference and three were prescribed PDE-I medication for pulmonary hypertension. The final study population was comprised of 589 participants (69.3% MSM, and 30.7% MSW).
Demographic, clinical and behavioral characteristics of participants are shown in Table 1. Mean age was 47.6 years and MSM were younger than MSW, on average. In terms of race, black and white men were equally represented, although a higher proportion of MSW (81.2%) were black. HIV viral load was undetectable (<200 copies/mL) in 79.8% of participants but approximately 10% had AIDS (with CD4 count <200 cells/mmΛ3) during the study period. The mean CD4 count was 520 cells/mm3. Problem alcohol use was more common in MSM than MSW (17% vs 13.8%) and substance use was approximately 7.5% in both groups. Historical STI was more common among MSM compared to MSW (32.3% vs 12.1%) and prior syphilis was the most common STI reported by both groups (26.7% in MSM vs 7.7% in MSW).
Table 1.
Baseline Characteristics of Men in HIV Care Prescribed Erectile Dysfunction Medication (EDM) (n=589)
| Characteristic | Total N=589 N (%) |
MSM N=408 N (%) |
MSW N=181 N (%) |
|---|---|---|---|
| Demographics | |||
| Age (years), mean (SD) | 47.6 (9.5) | 46.3 (9.5) | 50.6 (9.0) |
| Age (years) | |||
| 18–29 | 20 (3.4) | 19 (4.7) | 1 (0.6) |
| 30–39 | 93 (15.8) | 75 (18.4) | 18 (9.9) |
| 40–49 | 218 (37.0) | 152 (37.2) | 66 (36.5) |
| ≥50 | 258 (43.8) | 162 (39.7) | 96 (53.0) |
| Race | |||
| Black | 303 (51.4) | 156 (38.2) | 147 (81.2) |
| White | 278 (47.2) | 245 (60.1) | 33 (18.2) |
| Other | 8 (1.4) | 7 (1.7) | 1 (0.6) |
| Insurance Status | |||
| Public | 171 (29.0) | 103 (25.2) | 68 (37.6) |
| Private | 218 (37.0) | 168 (41.2) | 50 (27.6) |
| Uninsured | 159 (27.0) | 109 (26.7) | 50 (27.6) |
| Unknown | 41 (7.0) | 28 (6.9) | 13 (7.2) |
| Clinical Information | |||
| CD4 Count (cells/mm3) | |||
| <200 | 57 (9.7) | 33 (8.1) | 24 (13.3) |
| ≥200 | 470 (79.8) | 331 (81.1) | 139 (76.8) |
| Unknown | 62 (10.5) | 44 (10.8) | 18 (9.9) |
| HIV Viral Load (copies/mL) | |||
| <200 | 476 (80.8) | 331 (81.1) | 145 (80.1) |
| ≥200 | 81 (13.8) | 57 (14.0) | 24 (13.3) |
| Unknown | 32 (5.4) | 20 (4.9) | 12 (6.6) |
| History of any STIa | 154 (26.1) | 132 (32.3) | 22 (12.1) |
| History of Chlamydia | 17 (2.9) | 14 (3.4) | 3 (1.7) |
| History of Gonorrhea | 55 (9.3) | 49 (12.0) | 6 (3.3) |
| History of Syphilis | 123 (20.9) | 109 (26.7) | 14 (7.7) |
| Alcohol abuseb | |||
| At risk | 56 (16.2) | 44 (17.0) | 12 (13.8) |
| Low risk | 27 (7.8) | 16 (6.2) | 11 (12.6) |
| Unknown | 263 (76.0) | 199 (76.8) | 64 (73.6) |
| Substance usec | |||
| Current | 44 (7.5) | 30 (7.4) | 14 (7.7) |
| Prior | 140 (23.8) | 105 (25.7) | 35 (19.3) |
| Never | 149 (25.3) | 111 (27.2) | 38 (21.0) |
| Unknown | 256 (43.4) | 162 (39.7) | 94 (52.0) |
EDM=erectile dysfunction medication; HIV=human immunodeficiency virus; MSM=men having sex with men; MSW=men having sex with women; Q1=first quartile; Q3=third quartile; SD=standard deviation; STI: sexually transmitted infection; UAB=University of Alabama at Birmingham (Birmingham, AL).
Any STI refers to prior chlamydia, gonorrhea and/or syphilis infection
Using Alcohol use disorder identification test (AUDIT-C).
Using Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST).
STI Testing
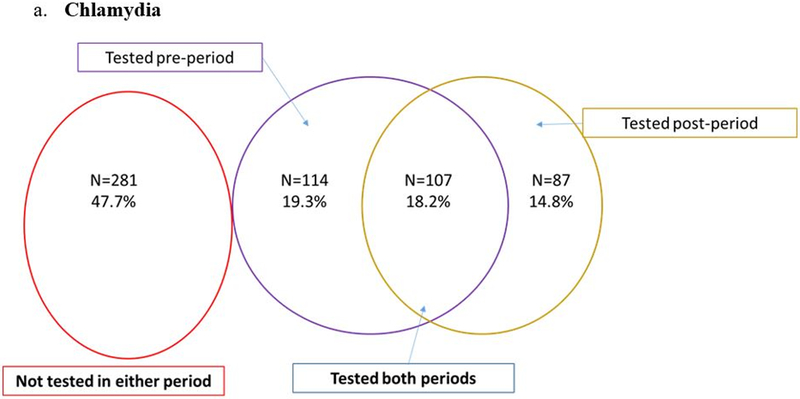
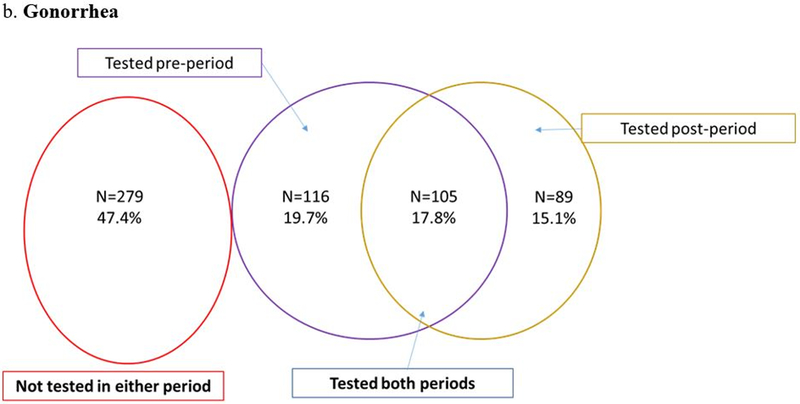
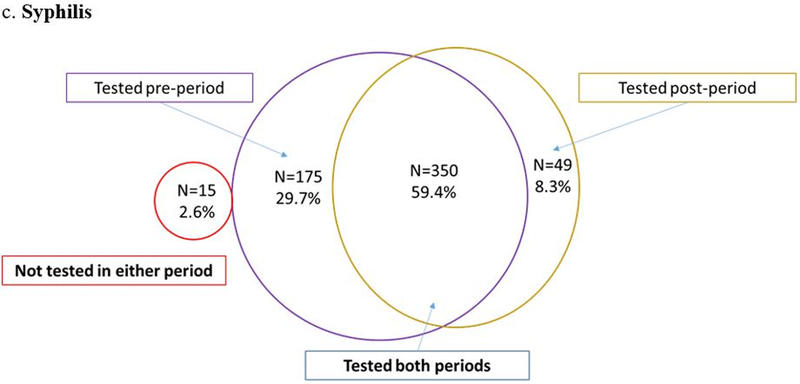
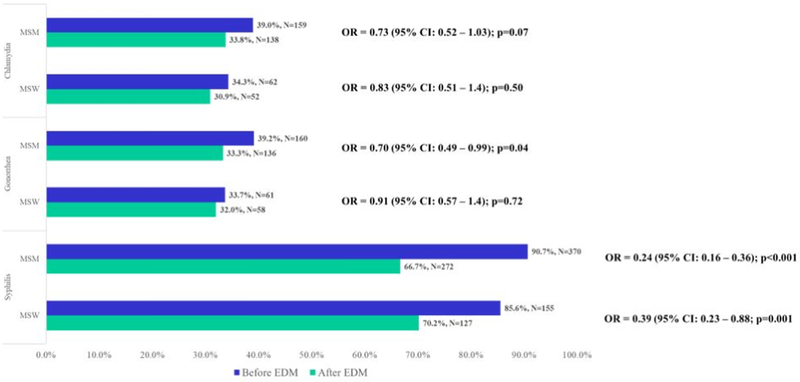
Despite engagement in HIV care, CT/GC screening rates were low. Nearly half of participants (47.7% for CT and 47.4% for GC) were not tested in the year before nor after EDM and only one in five men (18.2% for CT and 17.8% for GC) were tested in both time periods (Figures 2a and 2b). The odds of being screened for chlamydia after EDM prescription were lower when compared to the prior period (0R=0.76; 95% CI: 0.58 – 1.01; p=0.06) (Figure 2a). The odds for testing were similar for gonorrhea (0R=0.77; 95% CI: 0.58 – 1.01; p=0.06) (Figure 2b). Testing for syphilis was much more frequent: 59.4% were tested in both periods and only 2.6% were not tested in either period (Figure 2c). The odds of being tested for syphilis after EDM prescription were significantly lower than the period before EDM prescription (OR = 0.28; 95% CI: 0.20 – 0.38; p <0.001) (Figure 2c). When STI testing rates were stratified by sexual preference, MSM had lower odds of testing compared to MSW (Figure 3).
Figure 2.
STI testing in Men with HIV before and after EDM prescription (N=589)
Conditional logistic regression: Odds ratio=0.76 (95% CI: 0.58 – 1.01); p=0.06.
Conditional logistic regression: Odds ratio=0.77 (95% CI: 0.58 – 1.01); p=0.06.
Conditional logistic regression: Odds ratio=0.28 (95% CI: 0.20 – 0.38); p<0.0001.
CI=confidence interval; EDM=erectile dysfunction medication; OR=odds ratio; STI=sexually transmitted infections.
Figure 3.
STI testing in MSM and MSW with HIV Before and After EDM Prescription, N=589
CI=confidence interval; EDM=erectile dysfunction medication; MSM=men having sex with men; MSW: men having sex with women; OR=odds ratio; UAB= University of Alabama at Birmingham (Birmingham, AL).
Note: Paired data analysis using conditional logistic regression used to calculate ORs with corresponding 95% CIs and p-values.
STI Positivity
Forty-three STIs (25 incident syphilis infections, 10 chlamydia infections and 8 gonorrhea infections) were detected during the 2-year period before and after EDM prescription; 42/43 occurred in MSM (Table 2) and one infection (GC) occurred in MSW. There were no statistically significant differences in STI positivity before and after EDM. However, the odds of GC infection (0R=6.00; 95% CI: 0.72 – 49.84; p=0.10) and incident syphilis infection (0R=1.27; 95% CI: 0.58 – 2.80; p=0.55) were higher after EDM while the odds of CT infection were lower after EDM (0R=0.25; 95% CI: 0.05 – 1.18; p=0.08) (Table 2).
Table 2.
STI before and after EDM prescription among MSM in HIV Care
| Before EDM Prescription | ||||
|---|---|---|---|---|
| Positive N (%) |
Negative N (%) |
Total N (%) |
ORa (95% CI), p-value |
|
| After EDM | ||||
| Prescription | ||||
| Chlamydia | ||||
| Positive | 0 (0) | 2 (2.5) | 2 (2.5) | 0.25 (0.05–1.18) |
| Negative | 8 (9.9) | 71 (87.7) | 79 (97.5) | p= 0.08 |
| Total | 8 (9.9) | 73 (90.1) | 81 (100) | |
| Gonorrhea | ||||
| Positive | 1 (1.3) | 6 (7.5) | 7 (8.8) | 6.00 (0.72–49.84) |
| Negative | 1 (1.3) | 72 (90.0) | 73 (91.2) | p= 0.10 |
| Total | 2 (2.5) | 78 (97.5) | 80 (100) | |
| Syphilis | ||||
| Positive | 2 (0.8) | 14 (5.8) | 16 (6.6) | 1.27 (0.58–2.80) |
| Negative | 11 (4.6) | 214 (88.8) | 225 (93.4) | p= 0.55 |
| Total | 13 (5.4) | 228 (94.6) | 241 (100) | |
CI=confidence interval, EDM=erectile dysfunction/medication, OR=odds ratio; STI=sexually transmitted infection Bolded data highlights STIs detected before and after EDM.
Paired data analysis using conditional logistic regression used to calculate ORs with corresponding 95% CIs and p-values.
Sexual Behaviors
Only 234/589 (39.7%) participants had PRO data from pre and post EDM time periods for inclusion in the analysis of sexual behavior variable. Patients with no sexual behavior data (n=119), data before EDM only (N=54) or after EDM only (N=85) were excluded and a sensitivity analysis was performed comparing both groups.
Among 234 participants, 180 (77%) were sexually active before and after EDM. The proportion of men who were sexually active before versus after EDM was similar (87.6% vs 82.9%; p=0.08) (Table 3). The median number of sexual partners in the past 6 months decreased from 2 to 1 among MSM after EDM when compared to prior (p=0.19) and was stable at 1 among MSW (Table 3). Data on condom use was available for 122/180 (68%) sexually active patients. Consistent condom use was rare: only 6.6% of men reported consistent condom use before and after EDM (Table 3). Sex after drugs/alcohol was common before and after EDM prescription in MSM (41.1% vs 40.2%; p=0.84) and MSW (41.7% vs 30.6%; p=0.18).
Table 3.
Patient reported outcomes in the past six months before and after EDM among men in HIV care (n=234)a
| Behavior change | Total (N=234) |
MSM (N =176) |
MSW (N =58) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Before EDM | After EDM | ORb (95% CI) |
Before EDM | After EDM | ORb (95% CI) |
Before EDM | After EDM | ORb (95% CI) |
|
| Sexually active | |||||||||
| N (%) | 205 (87.6) | 194 (82.9) | 0.56 (0.29–1.08) | 154 (87.5) | 145 (82.4) | 0.50 (0.23–1.11) | 51 (87.9) | 49 (84.5) | 0.71 (0.23–2.25) |
| Number of sex partners, median (Q1, Q3) | 1 (1,2) | 1 (1,2) | p=0.12c | 2 (1,2) | 1 (1,2) | p=0.19c | 1 (1,2) | 1 (1,2) | p=0.47c |
| Unprotected sex | |||||||||
| N (%) | 114 (93.4) | 114 (93.4) | 1.00 (0.06–15.99) | 83 (97.6) | 83 (97.6) | No changed | 31 (83.8) | 31 (83.8) | 1.00 (0.06–15.99) |
| Sex after drugs/alcohol | |||||||||
| N (%) | 59 (41.3) | 54 (37.8) | 0.74 (0.37–1.47) | 44 (41.1) | 43 (40.2) | 0.92 (0.42–2.02) | 15 (41.7) | 11 (30.6) | 0.33 (0.06–1.65) |
NOTE: For the categorical variables, the number presented are the marginal frequencies and percentages (%) of the 2×2 paired data table.
CI=confidence interval, EDM=erectile dysfunction medication; MSM=men having sex with men; MSW=men having sex with women; OR=odds ratio; Q1=first quartile, Q3=third quartile; UAB=University of Alabama at Birmingham.
Missing data: Unprotected sex = 112 (MSM=91, MSW=21), Sex after alcohol/drug = 91 (MSM=69, MSW=22).
Among men who had data available on sexual activity in both before and after EDM periods.
Paired data analysis using conditional logistic regression used to calculate ORs with corresponding 95% CIs and p-values.
Wilcoxon signed rank test for paired data.
As there was no change (discordant cells=0) between before and after periods, the OR could not be calculated.
The sensitivity analysis for missing data was based on 373 patients with behavioral data before or after EDM (MSM=270, MSW=103). Findings were similar to the main cohort in terms of partner number, unprotected sex and sex after drugs/alcohol and persisted with stratification by sexual preference (Suppl. Tables 1B and 1C). This relationship remained the same when patients with missing data were included in the mixed model analysis.
Discussion
Among the 2,924 men engaged in HIV care at the 1917 clinic, 1 in 5 received a new prescription for ED medication between 2008–2016. Despite CDC recommendations to screen all sexually active adults with HIV for STI, there was relatively low testing for chlamydia or gonorrhea after EDM prescription. [30] In contrast, syphilis testing rates were consistently high. ED medication did not have a significant impact on STI rates nor was it associated with an increase in risky sexual behaviors. This ran counter to our study hypothesis and similar studies in high-risk populations. [7, 11, 13, 31, 32]
In terms of STI screening rates, scant literature exists on the impact of EDM prescription on STI screening practices. One VA-based study documented minimal STI testing in the period after EDM prescription. [16] Chlamydia and gonorrhea screening rates were around 40% in a large cohort of HIV-infected adults in the US in 2013 [33] Our study is aligned with these findings which reflect inadequate STI screening rates in high risk groups despite access to care. Syphilis screening rates in the current study were higher than CT/GC (60% before and after EDM). This discrepancy is likely due to: 1) syphilis screening as a clinic performance measure; 2) syphilis screening rates are tracked by the federal government as part of the Ryan White Program; 3) the Deep South (and the US overall) has experienced a resurgence of syphilis, particularly among MSM. This awareness and frequent diagnosis of active syphilis by clinic providers leads to frequent screening for asymptomatic infection. Since the historic low of syphilis cases within the US (2.1 cases per 100,000), syphilis incident cases have increased dramatically through 2017 (9.5 per 100,000); the southern region has the second highest rates of syphilis (9.7 per 100,000). Among men in 2017, syphilis incidence rose to 16.9 cases per 100,000; MSM accounted for 57.9% of the 30,644 incident cases. [34] National data shows similar syphilis screening rates in other HIV clinics with annual testing rates documented at 69% among MSM and 61% among MSW. [33]
Nearly all STIs detected in this cohort occurred among MSM yet MSM had lower odds of STI testing compared to heterosexual men. In the most recent CDC STD surveillance report, MSM accounted for 18% of chlamydia cases (vs 15% for MSW) and 42% of gonorrhea cases (vs 26% for MSW) across the STD Surveillance Network (SSuN), and 80% of primary and secondary syphilis cases among men. [35] It was surprising that the likelihood of GC was higher than the likelihood of CT post EDM. Screening was performed for both infections simultaneously and the route of transmission is the same. Since gonorrhea infection is more often symptomatic in men, it is possible that men with GC were more likely to present for screening compared to men with CT. The high rate of incident syphilis and prior syphilis infection among MSM in our population is relevant since uninfected male partners who acquire syphilis have a significant short-term risk of HIV acquisition (3.6% within first year). [36, 37]
This study does not confirm findings from 2005 and 2016 showing that HIV-infected MSM are more likely to engage in unprotected sex after EDM prescription. [11, 13] This deviation from the literature may be due to a benefit of EDM prescription within a community care clinic that focuses on HIV treatment and prevention. This study did not measure the use of prescribed EDM in a “party” context. Research has shown that ED drugs are commonly used in the party scene in conjunction with other recreational substances, particularly among younger MSM. This mixture of EDM and recreation substances has been associated with increases in risky sexual behavior. [32, 38–40] Our study population is different since men were middle-aged (median age 48), engaged in HIV care, and EDM medications were prescribed by a provider. The PRO questionnaire is a well-validated method of collecting private information on a frequent and longitudinal basis compared to other studies using national sampling, anonymous questionnaires or telephone surveys. [9, 11, 32, 41, 42] Our study population was also unique since it was performed in the Southeastern portion of the US, which may have led to some of the differences noted. [9, 41–43] Data collection from the 6 months before and after EDM prescription may have led to a difference in results compared to other studies using a larger window or a longitudinal design. [7, 11] Other EDM survey studies include dissimilar populations of men with HIV who are not engaged in care or men without HIV who may access care more sporadically and survey studies are more limited in the ascertainment of STI outcomes. [9, 32, 41, 42, 44]
In terms of limitations, our results may not be generalized to younger men, men who obtain EDM without a prescription or men without HIV infection. However, findings from this single site study in the southeastern US are expected to apply to other southern US sites where HIV and STI prevalence is high. This study did not include an assessment of ED medication adherence or patterns of use and self-reported sexual preferences and behaviors may have led to social desirability bias. Also, this study could not verify the pattern of condom use or frequency of sex. The study sample size was limited by the paired study design, which only uses discordant pairs in order to calculate the odds ratio. This limitation led to wide confidence intervals and lack of statistical significance. Furthermore, the data for STI positivity was restricted to only those who were tested in both the periods. Therefore, in spite of a strong association between EDM prescription and CT and GC positivity (albeit in opposite directions), it seems premature to make definitive statements about STI trends. Although some PRO data was missing, the results were robust in the sensitivity analysis. An important study strength is the sizable population of men engaged in HIV care with pharmacy and laboratory records and risk behavior information collected at multiple points in time.
In terms of study implications, innovative research is needed to reach universal STI screening among men in HIV clinic in order to prevent STI transmission. This could include automated screening for sexually active adults at HIV clinic visits or self-collected swabs for STI testing that are performed by the patient at exposure sites before the provider enters the room. Also, the context in which EDM (when, how often, why, and what setting) is used may be invaluable in assessing the relationship between EDM and sexual health outcomes.
Conclusions
EDM prescription did not lead to any detectable change in risk behavior in this setting. Despite inadequate testing rates, bacterial STI was common among MSM, particularly incident syphilis. The management of ED in HIV clinic provides an excellent opportunity to discuss risk reduction, safer sex practices and the importance of routine STI screening to prevent HIV/STI transmission.
Supplementary Material
Supplementary Table 1. Sensitivity analyses comparing of measures of association with and without including patients with missing data.
Funding and Conflicts of Interest:
Funding was provided by The University of Alabama at Birmingham, Center for AIDS Research Grant: P30 AI027767, CNICS R24 AI067039, NIH/NIHCD 1K23HD090993 (JD).
Dr. Burkholder has received research support from Amgen, Inc. and Bristol-Myers Squibb and has consulted for Definicare, LLC and Medscape.
A portion of this work was presented as a poster at the 2018 CDC STD Prevention Conference in Washington DC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cook RL, McGinnis KA, Samet JH, Fiellin DA, Rodriguez-Barradas MC, Kraemer KL, Gibert CL, Braithwaite RS, Goulet JL, Mattocks K, Crystal S, Gordon AJ, Oursler KK, Justice AC, Erectile dysfunction drug receipt, risky sexual behavior and sexually transmitted diseases in HIV-infected and HIV-uninfected men. J Gen Intern Med, 2010. 25(2)115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santi D, Brigante G, Zona S, Guaraldi G, Rochira V, Male sexual dysfunction and HIV--a clinical perspective. Nat Rev Urol, 2014. 11(2)99–109. [DOI] [PubMed] [Google Scholar]
- 3.Perez I, Moreno T, Navarro F, Santos J Palacios R, Prevalence and factors associated with erectile dysfunction in a cohort of HIV-infected patients. Int J STD AIDS, 2013. 24(9)712–715. [DOI] [PubMed] [Google Scholar]
- 4.Zona S, Guaraldi G, Luzi K, Beggi M, Santi D, Stentarelli C, Madeo B Rochira V, Erectile dysfunction is more common in young to middle-aged HIV-infected men than in HIV-uninfected men. J Sex Med, 2012. 9(7)1923–1930. [DOI] [PubMed] [Google Scholar]
- 5.Selvin E, Burnett AL Platz EA, Prevalence and risk factors for erectile dysfunction in the US. Am J Med, 2007. 120(2)151–157. [DOI] [PubMed] [Google Scholar]
- 6.Johnson LA Viagra goes generic: Pfizer to launch own little white pill. The Washington Post, 2017. [Google Scholar]
- 7.Jena AB, Goldman DP, Kamdar A, Lakdawalla DN Lu Y, Sexually transmitted diseases among users of erectile dysfunction drugs: analysis of claims data. Ann Intern Med, 2010. 153(1)1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finlayson T, Le B, Smith A, Bowles K, Cribbin M, Miles I, Oster A, Martin T, Edwards A DiNenno E, Sexually Transmitted Disease Surveillance 2011, in Morbidity and Mortality Weekly Report. 2012, Centers for Disease Control and Prevention: Atlanta, Georgia: p. 11. [PubMed] [Google Scholar]
- 9.Paul JP, Pollack L, Osmond D Catania JA, Viagra (sildenafil) use in a population-based sample of U.S. men who have sex with men. Sex Transm Dis, 2005. 32(9)531–533. [DOI] [PubMed] [Google Scholar]
- 10.Marks G, Richardson JL, Milam J, Bolan R, Stoyanoff S McCutchan A, Use of erectile dysfunction medication and unsafe sex among HIV+ men who have sex with men in care. Int J STD AIDS, 2005. 16(3)271–272. [DOI] [PubMed] [Google Scholar]
- 11.Lin X, Mattson CL, Freedman M Skarbinski J, Erectile Dysfunction Medication Prescription and Condomless Intercourse in HIV-Infected Men Who have Sex with Men in the United States. AIDS Behav, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spindler HH, Scheer S, Chen SY, Klausner JD, Katz MH, Valleroy LA Schwarcz SK, Viagra, methamphetamine, and HIV risk: results from a probability sample of MSM, San Francisco. Sex Transm Dis, 2007. 34(8)586–591. [DOI] [PubMed] [Google Scholar]
- 13.Swearingen SG Klausner JD, Sildenafil use, sexual risk behavior, and risk for sexually transmitted diseases, including HIV infection. Am J Med, 2005. 118(6)571–577. [DOI] [PubMed] [Google Scholar]
- 14.Wimberly YH, Hogben M, Moore-Ruffin J, Moore SE Fry-Johnson Y, Sexual history-taking among primary care physicians. J Natl Med Assoc, 2006. 98(12)1924–1929. [PMC free article] [PubMed] [Google Scholar]
- 15.Wong EY, Jordan WC, Malebranche DJ, DeLaitsch LL, Abravanel R, Bermudez A Baugh BP, HIV testing practices among black primary care physicians in the United States. BMC Public Health, 2013. 1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holman KM, Carr JA, Baddley JW Hook EW 3rd, Sexual history taking and sexually transmitted infection screening in patients initiating erectile dysfunction medication therapy. Sex Transm Dis, 2013. 40(11)836–838. [DOI] [PubMed] [Google Scholar]
- 17.Galvin SR Cohen MS The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol, 2004. 2(1)33–42. [DOI] [PubMed] [Google Scholar]
- 18.HIV in the Southern States of the US, in CDC issue brief. 2016, Centers of Disease Prevent and Control. [Google Scholar]
- 19.Gonorrhea. Sexually Transmitted Disease Surveillance 2017 2018; Available from: https://www.cdc.gov/std/stats17/gonorrhea.htm.
- 20.Chlamydia. Sexually Transmitted Disease Surveillance 2017 2018. [cited 2019; Available from: https://www.cdc.gov/std/stats17/chlamydia.htm.
- 21.Kalichman SC, Pellowski J Turner C, Prevalence of sexually transmitted co-infections in people living with HIV/AIDS: systematic review with implications for using HIV treatments for prevention. Sex Transm Infect, 2011. 87(3)183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rieg G, Lewis RJ, Miller LG, Witt MD, Guerrero M Daar ES, Asymptomatic sexually transmitted infections in HIV-infected men who have sex with men: prevalence, incidence, predictors, and screening strategies. AIDS Patient Care STDS, 2008. 22(12)947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raifman JR, Gebo KA, Mathews WC, Korthuis PT, Ghanem KG, Aberg JA, Moore RD, Nijhawan AE, Monroe AK Berry SA, Gonorrhea and Chlamydia Case Detection Increased When Testing Increased in a Multisite US HIV Cohort, 2004–2014. J Acquir Immune Defic Syndr, 2017. 76(4)409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Babor Thomas F., JCH-B, Saunders John B. & Monteiro Maristela G., The Alcohol Use Disorders Identification Test, in Guidelines for Use in Primary Care. 2001, World Health Organization. [Google Scholar]
- 25.Bush K, Kivlahan DR, McDonell MB, Fihn SD Bradley KA, The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med, 1998. 158(16)1789–1795. [DOI] [PubMed] [Google Scholar]
- 26.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R Dunbar GC, The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry, 1998. 59 Suppl 2022–33;quiz 34–57. [PubMed] [Google Scholar]
- 27.Humeniuk R, Ali R, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, de Lacerda RB, Ling W, Marsden J, Monteiro M, Nhiwatiwa S, Pal H, Poznyak V Simon S, Validation of the Alcohol, Smoking And Substance Involvement Screening Test (ASSIST). Addiction, 2008. 103(6)1039–1047. [DOI] [PubMed] [Google Scholar]
- 28.Syphilis. 2015. Sexually Transmitted Diseases Treatment 2015 July 27, 216; Syphilis testing guidelines]. Available from: https://www.cdc.gov/std/tg2015/syphilis.htm.
- 29.Slaymaker E, Walker N, Zaba B Collumbien M, Unsafe sex. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors, 2004. 21177–1254. [Google Scholar]
- 30.Workowski KA Bolan GA Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep, 2015. 64(Rr-03)1–137. [PMC free article] [PubMed] [Google Scholar]
- 31.Cachay E, Mar-Tang M Mathews WC, Screening for potentially transmitting sexual risk behaviors, urethral sexually transmitted infection, and sildenafil use among males entering care for HIV infection. AIDS Patient Care STDS, 2004. 18(6)349–354. [DOI] [PubMed] [Google Scholar]
- 32.De Ryck I, Van Laeken D, Noestlinger C, Platteau T Colebunders R, The use of erection enhancing medication and party drugs among men living with HIV in Europe. AIDS Care, 2013. 25(8)1062–1066. [DOI] [PubMed] [Google Scholar]
- 33.Mattson CL, Bradley H, Beer L, Johnson C, Pearson WS Shouse RL, Increased Sexually Transmitted Disease Testing Among Sexually Active Persons Receiving Medical Care for Human Immunodeficiency Virus Infection in the United States, 2009–2013. Clin Infect Dis, 2017. 64(5)629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Syphilis. Sexually Transmitted Disease Surveillance 2017 2018. [cited 2019; Available from: https://www.cdc.gov/std/stats17/Syphilis.htm.
- 35.Sexually Transmitted Disease Surveillance 2017. 2018, Centers for Disease Control and Prevention: Atlanta, Georgia: p. 3–23. [Google Scholar]
- 36.STDs and HIV - CDC Fact Sheet, in Sexually Transmitted Diseases (STDs) 2017, Centers for Disease Control and Prevention. [Google Scholar]
- 37.Peterman TA, Newman DR, Maddox L, Schmitt K Shiver S, High Risk for HIV following Syphilis Diagnosis among Men in Florida, 2000–2011. Public Health Reports, 2014. 129(2)164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colfax GN, Mansergh G, Guzman R, Vittinghoff E, Marks G, Rader M Buchbinder S, Drug use and sexual risk behavior among gay and bisexual men who attend circuit parties: a venue-based comparison. J Acquir Immune Defic Syndr, 2001. 28(4)373–379. [DOI] [PubMed] [Google Scholar]
- 39.Halkitis PN Green KA, Sildenafil (Viagra) and club drug use in gay and bisexual men: the role of drug combinations and context. Am J Mens Health, 2007. 1(2)139–147. [DOI] [PubMed] [Google Scholar]
- 40.Semple SJ, Strathdee SA, Zians J Patterson TL, Sexual risk behavior associated with co-administration of methamphetamine and other drugs in a sample of HIV-positive men who have sex with men. Am J Addict, 2009. 18(1)65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nettles CD, Benotsch EG Uban KA, Sexual risk behaviors among men who have sex with men using erectile dysfunction medications. AIDS Patient Care STDS, 2009. 23(12)1017–1023. [DOI] [PubMed] [Google Scholar]
- 42.Goltz HH, Coon DW, Catania JA Latini DM, A pilot study of HIV/STI risk among men having sex with men using erectile dysfunction medications: challenges and opportunities for sexual medicine physicians. J Sex Med, 2012. 9(12)3189–3197. [DOI] [PubMed] [Google Scholar]
- 43.Kim AA, Kent CK Klausner JD, Increased risk of HIV and sexually transmitted disease transmission among gay or bisexual men who use Viagra, San Francisco 2000–2001. Aids, 2002. 16(10)1425–1428. [DOI] [PubMed] [Google Scholar]
- 44.Kramer SC, Schmidt AJ, Berg RC, Furegato M, Hospers H, Folch C Marcus U, Factors associated with unprotected anal sex with multiple non-steady partners in the past 12months: results from the European Men-Who-Have-Sex-With-Men Internet Survey (EMIS 2010). BMC Public Health, 2016. 1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Sensitivity analyses comparing of measures of association with and without including patients with missing data.