Abstract
Aim:
Helminth infections inflict negatively on the production and well-being of animals including poultry. This study was carried out to determine the prevalence, species diversity, intensity, and risk factors associated with the gastrointestinal helminths of intensively raised poultry in Kwara Central senatorial district of Kwara State.
Materials and Methods:
Fecal samples were collected from 502 poultry species from 15 farms. The samples were subjected to floatation and the formalin-ethyl acetate concentration techniques of examination. The intensity of infections was determined using McMaster counting technique.
Results:
Seven helminth species were detected with Heterakis gallinarum (10.2%) and Ascaridia galli (6.0%) been the most prevalent, while Capillaria species was the least prevalent (0.8%). Physiological status, bird type, production purpose, farm age (years), presence of other animals in the farm, flock size (birds), farm size (acres), housing type, farm type, frequency of anthelmintic use, distance to waste area (meters), level of biosecurity, and frequency of cleaning the pen were the risk factors significantly (p<0.05) associated with the presence of helminth infections.
Conclusion:
This study shows that helminth infections are endemic in the study area, as 66.7% of the sampled farms were infected with one or more helminth species. Findings from this study provide information that will assist in improving the poultry sector in Kwara State, Nigeria in general, for better production and profitability.
Keywords: epidemiology, gastrointestinal helminths, Kwara State, Nigeria, poultry
Introduction
Poultry are domesticated birds kept by man for the purpose of obtaining meat, eggs, sometimes feathers, and as a means of livelihood. They include birds such as chicken, duck, goose, and turkey [1]. It is one of the most important sources of protein and farm manure, for man and so their importance cannot be overemphasized [1,2]. Poultry production has increased constantly throughout the world over the past decades, and according to the Food and Agriculture Organization [3], around 75% of a total of 15 billion chickens is found in the developing countries [4]. In Nigeria, poultry is an important component of the livestock subsector with a total population of over 200 million [5,6]. This sector has developed to the level of commercial enterprise which provides employment, income, and animal protein for urban and rural dwellers as well as manure for crop production [6]. It is an important instrument for alleviating problems associated with poverty in Nigeria and other developing countries (in terms of food security and malnutrition) [6].
The diseases condition caused by helminth infections is known as helminthosis. This condition has been considered as an important problem of poultry in Nigeria and other parts of the world [7-9]. Helminth parasites have been incriminated as a major cause of ill-health and loss of productivity through decreased feed conversion ratio, reduced weight gain and weight loss in broilers, poor egg lay in layers, and mortalities. Helminthoses are also associated with catarrh, diarrhea, intestinal obstruction, loss of appetite, anemia, weakness, paralysis, and poor feathering in birds [1,2,7,9,10]. Helminth parasites of poultry are commonly divided into three main groups; nematodes, cestodes, and trematodes. Nematodes constitute the most important group of helminth parasites of poultry, both in number of species and the extent of damage they cause. Few numbers of cestodes and trematodes are known to parasitize poultry [7,11,12]. The prevalence and intensity of helminth infections may be influenced by several factors, such as climatic conditions (temperature and humidity) which may alter the population dynamics of the parasites, resulting in dramatic changes in the prevalence and intensity of helminthic infections [12]. Many insects that may act as vectors for helminths are also favored by high temperatures and to some extent humidity [1,12].
To the best of our knowledge, there is no report on gastrointestinal helminths of poultry in this part of the country. This study is, therefore, carried out to determine the prevalence, species diversity, intensity, and risk factors associated with the gastrointestinal helminths of intensively raised poultry with the aim of providing information on this subject matter that will help in better profitability in the poultry sector in the state and country.
Materials and Methods
Ethical approval
All applicable international, national, and/or institutional guidelines for the collection of fecal samples from avian species were correctly followed.
Informed consent
Informed consent was obtained from all participants.
Description of study location
This study was conducted in Kwara Central senatorial district of Kwara State. Kwara Central lies almost in the middle of Nigeria, and it is one of the major linkages between the northern and southern part of the country. Kwara Central comprises four local government areas (Asa, Ilorin East, Ilorin South and Ilorin West). Kwara State is located between latitude 8°05N and 10°15N and longitude 2° 73E and 6°13E. It is located in the middle belt (North Central) within the forest-savanna region of Nigeria (Figure-1). The state is bordered in the west by Benin Republic, in the east by Kogi State, and the south by Oyo, Osun, and Ekiti States. Kwara State population is about 3 million people, and it covers a total area of 34,500 km2 comprising rainforest in the south and wooded savannah in the larger part of the state. It has 16 local government areas. The state has two seasons, the dry and wet season, with heavier rainfall in September and October. The state has a mean annual rainfall of between 112.8 cm and 146.9 cm and an average annual temperature ranging from 22.1°C to 33.3°C. It records a mean relative humidity of 49.6% [5,13].
Figure-1.
Map of Kwara Central (the study location). The insert map shows Kwara State within Nigeria (Designed using QGIS Version 2.6.1).
Study design and sampling
A total of 502 fecal samples were collected from 15 poultry farms located within Kwara Central (Figure-1), comprising layers, broilers, and mixed species farms. The farms were visited between December 2017 and May 2018 following permission by the farm owners. Birds were monitored and individually freshly voided fecal samples were immediately collected from the ground and placed into well-labeled sterile sample bottles and put in a cool box. The samples were immediately transported to the Parasitology Laboratory of the Faculty of Veterinary Medicine, University of Ilorin, Nigeria, for further processing.
Processing of fecal sample
Fecal samples were processed using the simple floatation and the formalin-ethyl acetate concentration techniques. The floatation technique was carried out as described by Soulsby [14]. Briefly, 2 g of each fecal sample was mixed with quantity of saturated sodium chloride solution and filtered (using a sieve) into a glass test tube. Afterward, the mixture was filled to the brim (forming a meniscus) with the saturated sodium chloride solution, and a clean coverslip was gently placed on top of the test tube, thereby avoiding spillage. The coverslip was left for about 20 min; afterward, the coverslip (having the harvested eggs) was placed on a clean glass slide and examined with the light microscope using the 10× and 40× objective lenses.
The formalin-ethyl acetate concentration technique was carried out as described by Cheesbrough [15]. Briefly, about 2 g of each feces was dissolved in 10% formalin and sieved into a plastic test tube to the 7 ml mark and allowed to stand for a few minutes. 3 ml of ethyl acetate was added. The tube was closed, vigorously shaken by hand for 1 min, and centrifuged at 3000 rpm for 5 min. The debris plug was loosened, and the top three layers were discarded. Iodine stain preparation was made with the sediment, and the entire sediment was examined on a clean glass slide and covered with a clean coverslip. The covered slides were examined using 10× and 40× objective lenses.
Fecal egg counts
Samples that were positive for helminth eggs were subjected to the McMaster counting technique as described by Soulsby [14], with modifications to make use of a smaller volume of saturated sodium chloride solution. Briefly, 2 g of feces was properly dissolved in 12 ml of saturated sodium chloride solutions (as against 60 ml). The solution was then filtered through a tea sieve into a beaker. The filtrate was pipetted into the two chambers of the McMaster slide. Afterward, it was allowed to stand for 30 s. Finally, it was examined using the 10× objective lens. All the eggs seen within the ruled areas were counted. The eggs per gram (EPG) was estimated using the formulae below:

Identification of helminth eggs
The eggs from the processing methods described above were identified using the helminthological keys as described by Soulsby [14] and Taylor et al. [16].
Determination of positivity
Samples that were positive in one or both of the tests carried out were considered positive for the helminth(s) detected.
Determination of prevalence (%) and mean intensity (EPG)
The total prevalence (%) of each helminth species was calculated as the total number of poultry infected with each helminth detected divided by the total number of poultry sampled (502), while the farm-based prevalence (%) was calculated as the total number of poultry infected with a particular helminth detected in each farm divided by the total number of poultry sampled in that farm. The mean intensity (EPG) was calculated by summing the total EPG from all infected birds having a particular helminth species in a farm divided by the number of birds infected with the particular helminth parasite in that farm.
Questionnaire design and administration
A well-structured, interviewer-administered questionnaire containing open-ended and closed-ended (dichotomous or multiple choices) questions was designed to obtain information on individual bird that sample was collected from, the poultry farm demography, environmental and management factors, and biosecurity. A respondent was someone who was actively involved in the daily activities of the farm and was not necessarily the farm owner.
Statistical analysis
The data were statistically analyzed using the “Microsoft Excel 2010 and SPSS-Version 22.0” (SPSS Inc., Chicago). Descriptive statistics were conducted to estimate the prevalence using percentages in tables. The univariate analysis (Chi-square) test and odds ratios (ORs) with 95% confidence interval (CI) were used to determine the association between each risk factor and the presence and absence of helminth parasites. The ORs were calculated with respect to a reference category as indicated in the respective tables. p<0.05 was considered statistically significant for all analyses.
Results
Overall prevalence (%) of helminth parasites
Seven different species of helminth (six nematodes and one cestode) were detected from our study. Of the 502 birds sampled, 10.2% (51/502; 95% CI=7.7-13.0) were infected with Heterakis gallinarum, while 0.4% (2/502; 95% CI=0.1-1.3) were infected with Syngamus trachea. The prevalence of the other helminths ranged between 0.8% and 6.0% (Figure-2).
Figure-2.
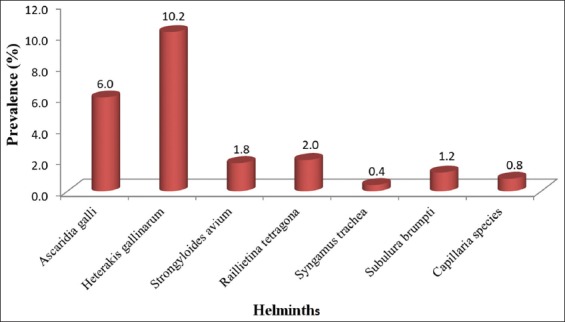
Prevalence (%) of gastrointestinal helminths of intensively managed poultry in Kwara Central, Kwara State.
Prevalence (%) of helminth parasites coinfection among poultry
Of the sampled birds, 84 (16.7%) were infected with one helminth or the other. 66 of them were infected with one helminth species representing 13.2% (95% CI=10.4-16.3). Of this category, H. gallinarum was the most prevalent (33/502; 6.6%; 95% CI=4.6-9.0), while Subulura brumpti was the least prevalent (2/502; 0.4%; 95% CI=0.1-1.3). Ascaridia galli + H. gallinarum combination was the most prevalent in the two helminth parasites coinfection representing 1.6% of the sampled population. Two birds had three helminth parasites coinfection representing 0.4% (2/502; 95% CI=0.1-1.3) of the sampled population. Four of the sampled birds were infected with four helminth parasites at the same time, with the infection been A. galli + H. gallinarum + S. brumpti + Capillaria species (2/502; 0.4%) and H. gallinarum + S. trachea + S. brumpti + Capillaria species (2/502; 0.4%) (Table-1).
Table-1.
Prevalence (%) of gastrointestinal helminths coinfection among intensively managed poultry in Kwara Central, Kwara State.
| Gastrointestinal helminth (s) | Number positive (%) | 95% CI |
|---|---|---|
| One helminth infection | 66 (13.2) | 10.4; 16.3 |
| Ascaridia galli | 18 (3.6) | 2.2; 5.5 |
| Heterakis gallinarum | 33 (6.6) | 4.6; 9.0 |
| Strongyloides avium | 5 (1.0) | 0.4; 2.2 |
| Raillietina tetragona | 8 (1.6) | 0.7; 3.0 |
| Subulura brumpti | 2 (0.4) | 0.1; 1.3 |
| Two helminths infection | 12 (2.4) | 1.3; 4.0 |
| Ascaridia galli+Heterakis gallinarum | 8 (1.6) | 0.7; 3.0 |
| Heterakis gallinarum+Raillietina tetragona | 2 (0.4) | 0.1; 1.3 |
| Heterakis gallinarum+Strongyloides avium | 2 (0.4) | 0.1; 1.3 |
| Three helminths infection | 2 (0.4) | 0.1; 1.3 |
| Ascaridia galli+Heterakis gallinarum+Strongyloides avium | 2 (0.4) | 0.1; 1.3 |
| Four helminths infection | 4 (0.8) | 0.3; 1.9 |
| Ascaridia galli+Heterakis gallinarum+Subulura brumpti+Capillaria species | 2 (0.4) | 0.1; 1.3 |
| Heterakis gallinarum+Syngamus trachea+Subulura brumpti+Capillaria species | 2 (0.4) | 0.1; 1.3 |
CI=Confidence interval
Prevalence (%) of helminth parasites in the different poultry farms
The farm prevalence of helminth infections is presented in Table-2. Of the 15 farms visited, five were free from helminth infections representing 33.3%. One farm had birds that were infected with six of the seven helminth species detected, while other farms were infected with between one to three helminth species. H. gallinarum and A. galli had the widest spread, been detected in eight and seven farms, respectively, while the other helminth species were detected in one or two farms. In general, individual helminth prevalence within farms ranged between 6.3% (A. galli in farm 3) and 56.7% (H. gallinarum in farm 8).
Table-2.
The prevalence (%) of gastrointestinal helminth parasites from the different poultry farms in Kwara Central, Kwara State.
| Farms | n | Gastrointestinal helminths (number infected [prevalence %]) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ascaridia galli | Heterakis gallinarum | Strongyloides avium | Raillietina tetragona | Syngamus trachea | Subulura brumpti | Capillaria species | Number of helminths | ||
| 1 | 80 | nf | nf | nf | nf | nf | nf | nf | nf |
| 2 | 30 | 4 (13.3) | nf | nf | nf | nf | nf | nf | 1 |
| 3 | 32 | 2 (6.3) | 4 (12.5) | 6 (18.8) | nf | nf | nf | nf | 3 |
| 4 | 30 | nf | 4 (13.3) | nf | nf | nf | nf | nf | 1 |
| 5 | 30 | 2 (6.7) | 9 (30.0) | 3 (10.0) | nf | 2 (6.7) | 6 (20.0) | 4 (13.3) | 6 |
| 6 | 30 | 3 (10.0) | 5 (16.7) | nf | nf | nf | nf | nf | 2 |
| 7 | 30 | 2 (6.7) | 3 (10.0) | nf | 5 (16.7) | nf | nf | nf | 3 |
| 8 | 30 | 13 (43.3) | 17 (56.7) | nf | nf | nf | nf | nf | 2 |
| 9 | 33 | nf | nf | nf | nf | nf | nf | nf | nf |
| 10 | 30 | nf | nf | nf | nf | nf | nf | nf | nf |
| 11 | 30 | 4 (13.3) | nf | nf | nf | nf | nf | nf | 1 |
| 12 | 27 | nf | nf | nf | nf | nf | nf | nf | nf |
| 13 | 30 | nf | nf | nf | nf | nf | nf | nf | nf |
| 14 | 30 | nf | 5 (16.7) | nf | 5 (16.7) | nf | nf | nf | 2 |
| 15 | 30 | nf | 4 (13.3) | nf | nf | nf | nf | nf | 1 |
nf=Not found
Mean intensity of infections (EPG of feces) of helminth parasites in the different poultry farms
The highest mean intensity of infections was recorded in H. gallinarum (981.0) and closely followed by A. galli (751.4). Strongyloides avium, Raillietina tetragona, S. trachea, S. brumpti, and Capillaria species had a mean intensity of 360.0, 90.0, 60.0, 60.0, and 30.0, respectively. The individual mean intensity of infections within farms was highest in farm 4 with H. gallinarum recording 290.0 (±127.0) and lowest in farm 5 with Capillaria species recording 30.0 (±11.5) (Table-3).
Table-3.
The mean (±SD) intensity of gastrointestinal helminths (EPG of feces) of intensively managed poultry in individual farms in Kwara Central, Kwara State.
| Farms | Gastrointestinal helminths (mean [±SD] intensity of infections [EPG]) | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| Ascaridia galli | Heterakis gallinarum | Strongyloides avium | Raillietina tetragona | Syngamus trachea | Subulura brumpti | Capillaria species | ||
| 1 | nf | nf | nf | nf | nf | nf | nf | nf |
| 2 | 160.0 (8.2) | nf | nf | nf | nf | nf | nf | 160.0 |
| 3 | 100.0 (14.14) | 160.0 (23.1) | 180.0 (99.6) | nf | nf | nf | nf | 440.0 |
| 4 | nf | 290.0 (127.0) | nf | nf | nf | nf | nf | 290.0 |
| 5 | 160.0 (23.1) | 96.0 (63.1) | 180.0 (28.3) | nf | 60.0 (28.3) | 60.0 (35.7) | 30.0 (11.5) | 586.0 |
| 6 | 70.0 (11.5) | 80.0 (46.2) | nf | nf | nf | nf | nf | 150.0 |
| 7 | 60.0 (14.1) | 70.0 (11.5) | nf | 50.0 (11.5) | nf | nf | nf | 180.0 |
| 8 | 151.4 (55.3) | 145.0 (64.3) | nf | nf | nf | nf | nf | 296.4 |
| 9 | nf | nf | nf | nf | nf | nf | nf | nf |
| 10 | nf | nf | nf | nf | nf | nf | nf | nf |
| 11 | 50.0 (11.5) | nf | nf | nf | nf | nf | nf | 50.0 |
| 12 | nf | nf | nf | nf | nf | nf | nf | nf |
| 13 | nf | nf | nf | nf | nf | nf | nf | nf |
| 14 | nf | 60.0 (16.3) | nf | 40.0 (17.9) | nf | nf | nf | 100.0 |
| 15 | nf | 80.0 (69.3) | nf | nf | nf | nf | nf | 80.0 |
| Total | 751.4 | 981.0 | 360.0 | 90.0 | 60.0 | 60.0 | 30.0 | 2332.4 |
nf=Not found, SD=Standard deviation, EPG=Eggs per gram
Risk factors associated with helminth parasitic infection
The association between bird type and the occurrence of helminth infections was statistically significant (p<0.05). Layers (18.5%) were 6.6 times more likely to be infected with helminth parasites compared to turkeys (3.3%). Spent layers (80.0%) were significantly (p<0.05) more prone to helminth infections compared to productive (16.1%) and unproductive (14.3%) birds. Poultry birds raised for the purpose of meat were twice less prone to the infection than those raised for egg purpose. Farms that are <15 years of the establishment are of higher risk of infection compared to farms older than 15 years. The presence of other animal types in the farm increases the chances of helminth infections (OR=2.0). The risk of helminth infections decreases with the increase in flock size, farm size, and distance to waste area. Birds raised in the deep litter had a lower risk (0.4) of infection compared to those raised in a battery cage. Birds raised in farms in the presence of other bird types were of higher risk (3.3) of infection compared to birds raised in farms with single bird species. Frequency of anthelmintic use, level of biosecurity, and frequency of cleaning the pen were other risk factors significantly associated (p<0.05) with the occurrence of helminth infections (Table-4).
Table-4.
Risk factors associated with helminth infections among intensively managed poultry in Kwara Central, Kwara State.
| Variables | Helminth+ve (%) | Helminth –ve (%) | OR (95% CI) | p-value |
|---|---|---|---|---|
| Age (weeks) | ||||
| Chick (0-8) | 1 (3.85) | 25 (96.15) | 0.19 (0.01, 1.07) | 0.06 |
| Grower (>8-16) | 13 (19.70) | 53 (80.30) | 1.19 (0.60, 2.27) | 0.59 |
| Adult (>16) a | 70 (17.07) | 340 (82.93) | 1.00 | |
| Sex | ||||
| Male | 4 (7.84) | 47 (92.16) | 0.40 (0.12, 1.05) | 0.06 |
| Femalea | 80 (17.74) | 371 (82.26) | 1.00 | |
| Bird type | ||||
| Layers | 74 (18.50) | 326 (81.50) | 6.6 (1.2, 13.6) | 0.02b |
| Broilers | 9 (12.50) | 63 (87.50) | 4.1 (0.63, 9.53) | 0.17 |
| Turkeya | 1 (3.33) | 29 (96.67) | 1.00 | |
| Physiological status | ||||
| Unproductive | 26 (14.29) | 156 (85.71) | 0.04 (0.01, 0.20) | <0.01b |
| Productive | 50 (16.13) | 260 (83.87) | 0.05 (0.01, 0.22) | <0.01b |
| Spent layersa | 8 (80.00) | 2 (20.00) | 1.00 | |
| Production purpose | ||||
| Meat | 10 (9.80) | 92 (90.20) | 0.48 (0.23, 0.94) | 0.03b |
| Egga | 74 (18.50) | 326 (81.50) | 1.00 | |
| Farm age (years) | ||||
| <5 | 32 (21.05) | 120 (78.95) | 28.85 (5.36, 60.34) | <0.01b |
| >5-10 | 28 (18.67) | 122 (81.33) | 24.83 (4.58, 52.4) | <0.01b |
| >10-15 | 23 (25.56) | 67 (74.44) | 36.92 (6.64, 78.53) | <0.01b |
| >15-20a | 1 (0.91) | 109 (99.09) | 1.00 | |
| Presence of other animals in the farm | ||||
| Yes | 68 (19.32) | 284 (80.68) | 2.00 (1.14, 3.68) | 0.02b |
| Noa | 16 (10.67) | 134 (89.33) | 1.00 | |
| Flock size (birds) | ||||
| <1000 | 68 (22.67) | 232 (77.33) | 24.23 (4.64, 49.87) | <0.01b |
| 1000-2000 | 15 (13.56) | 103 (86.44) | 11.99 (2.08, 25.98) | <0.01b |
| >2000a | 1 (1.19) | 83 (98.81) | 1.00 | |
| Farm size (acres) | ||||
| <5 | 79 (23.80) | 253 (76.20) | 24.58 (4.72, 50.50) | <0.01b |
| 5-10 | 4 (4.44) | 86 (95.56) | 3.65 (0.45, 9.99) | 0.26 |
| >10a | 1 (1.25) | 79 (98.75) | 1.00 | |
| Housing type | ||||
| Deep litter | 8 (8.33) | 88 (91.67) | 0.40 (0.17, 0.82) | 0.01b |
| Battery cagea | 76 (18.72) | 330 (81.28) | 1.00 | |
| Farm type | ||||
| Multiple bird species | 68 (22.52) | 234 (77.48) | 3.34 (1.90, 6.11) | <0.01b |
| Single bird speciesa | 16 (8.00) | 184 (92.00) | 1.00 | |
| Frequency of anthelmintic use | ||||
| Every 2 months | 22 (32.35) | 46 (67.65) | 4.41 (1.70, 12.82) | <0.01b |
| Every 3 months | 52 (19.12) | 220 (80.88) | 2.20 (0.94, 5.91) | 0.07 |
| Occasionally | 4 (4.00) | 96 (96.00) | 0.39 (0.09, 1.49) | 0.17 |
| No at alla | 6 (9.68) | 56 (90.32) | 1.00 | |
| Distance to waste area (meters) | ||||
| <250 | 11 (17.74) | 51 (82.26) | 5.74 (0.91, 13.09) | 0.07 |
| 250-500 | 72 (17.48) | 340 (82.52) | 5.71 (1.05, 11.99) | 0.04b |
| >500a | 1 (3.57) | 27 (96.43) | 1.00 | |
| Level of biosecurity (%) | ||||
| 0-25 | 64 (19.39) | 266 (80.61) | 1.83 (1.08, 3.20) | 0.03b |
| 25-50a | 20 (11.63) | 152 (88.37) | 1.00 | |
| Frequency of cleaning the pen | ||||
| Twice a week | 35 (13.16) | 231 (86.84) | 8.30 (1.54, 17.41) | 0.01b |
| Weekly | 48 (26.67) | 132 (73.33) | 19.86 (3.70, 41.47) | <0.01b |
| Occasionallya | 1 (1.79) | 55 (98.21) | 1.00 | |
Reference category.
Significant. OR=Odds ratio, CI=Confidence interval
Discussion
Little or nothing is known about gastrointestinal helminth parasites of intensively managed poultry in Kwara Central as it pertains to its diversity, prevalence, intensity, and risk factors. The overall prevalence of 16.7% reported in this study is much lower than previous studies conducted in Nigeria: 42.5% [1], 81.0% [17], and 100.0% [7] and outside Nigeria: 84.6% in Bangladesh [4] and 91.9% in Iran [18]. The reason for the low prevalence recorded may be associated with the fact that the birds recruited for this study were intensively managed where they get better treatments in terms of biosecurity, hygiene, feeding, and appropriate preventive medical programs and general management. Higher helminth infections have been reported in extensively and semi-intensively raised birds compared to intensively raise domestic chickens [10].
The result of this study showed that seven species of helminths affect intensively managed poultry in the study area. Similar to our finding, Adang et al. [17] reported seven gastrointestinal helminth species in domestic chickens in Gombe State, Nigeria. Contrary to our finding, Yoriyo et al. [19] reported eight gastrointestinal helminth species among chickens in Bauchi State, six helminth species in Abuja [11], five species in Akure, Ondo State [10], and four species in Sokoto State [20]. Outside Nigeria, 16 helminth species have been reported among chickens in South Africa [8], eight helminth species in India [21], seven species in Trinidad [9], six species in Bangladesh [18], four species in Iran [22], and three helminth species in Poland [23]. The differences in the number of helminth species detected in this study vis-à-vis those of other studies could be attributed to environmental and climatic differences. Our finding shows that there are diverse species of gastrointestinal helminths affecting poultry in Kwara Central of Kwara State.
This study reported that H. gallinarum and A. galli were the predominant helminth species, with S. avium, R. tetragona, S. trachea, S. brumpti, and Capillaria species been of lesser prevalence. This is comparable with that recorded by other authors [7,10,24], who recorded H. gallinarum and A. galli as the most prevalent helminth species of poultry in their studies conducted in Nigeria and Iran [25], respectively. Low prevalence of S. avium, R. tetragona, S. trachea, S. brumpti, and Capillaria species has also been reported in Nigeria [1,11,24,26]. The high prevalence of H. gallinarum and A. galli may be associated with the peculiarity in their life cycle as the eggs of both helminths can remain viable in the soil for several months [16], whereby prolonging the contamination time in the environment as birds constantly pick up viable eggs from the droppings that contaminate the environment as they feed, and this also predisposes them to high prevalence and heavy parasite burden.
The helminths coinfection observed in this study is a common phenomenon in most poultry species [9,17,27]. This may be associated with the fact that some helminth infections require intermediate/paratenic host (e.g., earthworm) which can harbor and transmit more than one helminth species at a time [16].
The presence of helminth parasites in 66.7% of the visited farms shows that helminth infections are endemic among farms in the study area. Helminth has been known to cause reduced weight gain and weight loss in broilers, poor egg lay in layers, and mortalities [2,10], thereby resulting in production loss. The widespread of H. gallinarum and A. galli among farms confirms that these helminth species are the most endemic in the area and most parts of Nigeria as documented by previous researchers [1,11].
The mean intensity of infections showed not to be alarming as no helminth count was above 300 EPG. This low count may be attributed to the management system (intensive) in which the birds were raised. Nevertheless, this does not translate to optimum production in poultry as low intensity of helminth infections may also cause problems in a flock.
Higher prevalence of helminth infections was recorded in layers and broilers compared to turkey. This may not be readily explained, although prevalence of 68.3% has been reported in turkeys [26] compared to 88.4% in layers [10] with both works done in Nigeria. Spent layers had a higher prevalence of helminth infections compared to unproductive and productive birds. A study conducted in Germany reported that free-range chickens at the end of the laying period (spent layers) had greater intensity and prevalence of infection with A. galli and H. gallinarum compared to hens kept in closed poultry houses (in preparation for production or during production) [28]. This finding may be attributed to the fact that spent layers are not given much care in terms of medications and biosecurity as these birds are merely kept to be sold for meat. Moreover, one cannot rule out the effect of immunosuppression associated with aging which may also be a reason. Birds raised for the purpose of meat production had a lower prevalence of helminth infections compared to birds raised for egg production. Similarly, Afolabi et al. [10] reported a significantly lower prevalence of helminth infections in broilers compared to layers in their study carried out in Nigeria.
Birds raised in farms where other animals are present showed to be more prone to helminth infections compared to those raised in farms without other animal species. This is expected as other animals may serve as a vehicle in the transfer of viable helminth eggs into the farm [10,16]. Furthermore, the biosecurity in poultry farms where other animals are present (e.g., dogs) will be seriously compromised. Contrary to the expected outcome, birds raised in the deep litter had a lower prevalence of helminth infections compared to those raised in a battery cage. Bachaya et al. [29] and Teni et al. [30] reported that birds raised on deep litter were more infected with helminths than those raised in a battery cage. The frequent use of anthelminthic drugs by farms that raise their birds on deep litter may be the reason behind the contrary report between our study and other previous studies. The higher risk of infection seen in birds raised in the presence of other avian species may be associated with cross infections between different bird species.
Interestingly, birds that are occasionally treated with anthelmintics had the lowest prevalence of helminth infections while those that are treated every 2 months were most infected. This report may be attributed to anthelmintics resistance that is associated with its frequent use. Frequent use of anthelmintics increases the resistant population of nematodes [31,32].
Strunz et al. [33], Ikpeama et al. [34], and Taiwo et al. [35] have associated higher prevalence of helminth infections with proximity to waste area and level of sanitation and hygiene. These set of researchers reported that proximity, presence of waste, and poor level of hygiene and sanitation trigger the availability of helminth infections in man and livestock. In line with the report of these aforementioned researchers, we discovered an indirect relationship between the distance of to waste areas and level of biosecurity with the prevalence of helminth infections. Our findings may be attributed to the fact that helminths are known to require warmth, good humidity, and optimum temperature for eggs to hatch and develop to infective stage(s). Studies have shown that poor biosecurity provides favorable conditions for helminth infections in poultry [36,37].
Conclusion
The findings of this study show that helminth infections are endemic in the study area, with H. gallinarum and A. galli been the most prevalent among the seven species detected. Two-third of the sampled farms was infected with one helminth parasite or the other. There was a low mean intensity of infections, and this will not rule out the economic effect, helminthosis cause on production. A number of factors were significantly associated with the positivity of helminth infections. This study will be essential for policy-making in other to improve poultry production in Kwara State and Nigeria as poultry occupy a pivotal aspect of the national livestock sector.
Authors’ Contributions
SDO designed the study and was involved in sample collections and laboratory work. He also did the data analysis and drafted the manuscript. PIU was involved in the sampling and in laboratory work. IMS, IAG, KH, MR, and MOA were involved in the laboratory work. NE designed the map of the study location (figure-1) and was involved in sampling. All authors read and approved the final manuscript.
Acknowledgments
The authors would like to express their profound gratitude to the poultry farm owners for giving us attention and access to their birds. We are also grateful to the Department of Veterinary Parasitology and Entomology, Faculty of Veterinary Medicine, University of Ilorin, for making use of the laboratory equipment. The study was funded by the authors.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published map and institutional affiliation.
References
- 1.Jegede O.C, Asadu I.A, Opara M, Obeta S.S, Olayemi D.O. Gastrointestinal parasitism in local and exotic breeds of chickens reared in Gwagwalada Guinea Savannah zone of Nigeria. Sokoto J. Vet. Sci. 2015;13(3):25–30. [Google Scholar]
- 2.Ngongeh L.A, Chiejina S.N, Lawal A.I. Prevalence of gastrointestinal helminth infections in slaughtered chickens reared in the Nsukka area of Enugu state, Nigeria. IOSR J. Agric. Vet. Sci. 2014;7(11):51–54. [Google Scholar]
- 3.FAO. FAOSTAT. 2004. [Last accessed on 18-08-2018]. Available from: http://www.fao.org .
- 4.Ferdushy T, Hasan M.T, Kadir A.K.M. Cross-sectional epidemiological investigation on the prevalence of gastrointestinal helminths in free-range chickens in Narsingdi district, Bangladesh. J. Parasit. Dis. 2016;40(3):818–822. doi: 10.1007/s12639-014-0585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Bureau of Statistics. Annual Abstract of Statistics. Nigeria: Federal Republic of Nigeria; 2016. [Google Scholar]
- 6.Ola-Fadunsin S.D. Investigations on the occurrence and associated risk factors of avian coccidiosis in Osun state, Southwestern Nigeria. J. Parasitol. Res. 2017;2017(8):6. doi: 10.1155/2017/9264191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uhuo A.C, Okafor F.C, Odikamnoro O.O, Onwe C.S, Abarike M.C, Elom J.N. Common gastrointestinal parasites of local chicken (Gallus domesticus) slaughtered in some selected eatery centers in Abakaliki, Ebonyi state:Implication for meat quality. Int. J. Dev. Sustain. 2013;2(2):1416–1422. [Google Scholar]
- 8.Mukaratirwa S, Khumaloa M.P. Prevalence of helminth parasites in free-range chickens from selected rural communities in KwaZulu-Natal province of South Africa. J. S. Afr. Vet. Assoc. 2010;81(2):97–101. doi: 10.4102/jsava.v81i2.113. [DOI] [PubMed] [Google Scholar]
- 9.Baboolal V, Suratsingh V, Gyan L, Brown G, Offiah N.V, Adesiyun A.A, Basu AK. The prevalence of intestinal helminths in broiler chickens in Trinidad. Vet. Arhiv. 2012;82(6):591–597. [Google Scholar]
- 10.Afolabi O.J, Simon-Oke I.A, Olasunkanmi A.O. Intestinal parasites of domestic chicken (Gallus gallus domesticus) in Akure, Nigeria. J. Biomed. 2016;1(4):1–4. [Google Scholar]
- 11.Matur B.M, Dawam N.N, Malann Y.D. Gastrointestinal helminth parasites of local and exotic chickens slaughtered in Gwagwalada, Abuja (FCT), Nigeria. N. Y. Sci. J. 2010;3(5):96–99. [Google Scholar]
- 12.Naphade S.T. Studies on the prevalence of helminthic Infection in broiler poultry birds from Marathwada region, (MS) India. Sci. Res. Report. 2013;3(2):233–238. [Google Scholar]
- 13.Kwara State Government News. Kwara State Diary. Nigeria: Kwara State of Nigeria; 2012. [Google Scholar]
- 14.Soulsby E.J.L. Helminths, Arthropods and Protozoa of Domestic Animals. 7th ed. London: Bailliere Tindall Publishers; 1982. pp. 787–792. [Google Scholar]
- 15.Cheesbrough M. District Laboratory Practice in Tropical Countries. United Kingdom: Cambridge University Press; 2009. pp. 196–198. [Google Scholar]
- 16.Taylor M.A, Coop R.L, Wall R.L. Veterinary Parasitology. 3rd ed. Oxford: Blackwell Publishing Ltd; 2007. p. 809. [Google Scholar]
- 17.Adang K.L, Asher R, Abba R. Gastrointestinal helminths of domestic chickens Gallus gallus domestica and ducks Anas platyrhynchos slaughtered at Gombe main market, Gombe state, Nigeria. Asian J. Poult. Sci. 2014;8(2):32–40. [Google Scholar]
- 18.Alam M.N, Mostofa M, Khan M.A.H, Alim M.A, Rahman A.K.M, Trisha A.A. Prevalence of gastrointestinal helminth infections in indigenous chickens of selected areas of Barisal district, Bangladesh. Bangladesh J. Vet. Med. 2014;12(2):135–139. [Google Scholar]
- 19.Yoriyo K.P, Adang K.L, Fabiyi J.P, Adamu S.U. Helminths parasites of local chickens in Bauchi state, Nigeria. Sci. World J. 2008;3(2):35–37. [Google Scholar]
- 20.Attah D.D, Danladi Y.K, Abdullahi K, Ibrahim S. A survey of gastrointestinal helminths of chickens and guinea fowls slaughtered at Sokoto, Nigeria. Equity J. Sci. Tech. 2013;1(1):1–5. [Google Scholar]
- 21.Kumar S, Garg R, Ram H, Maurya P.S, Banerjee P.S. Gastrointestinal parasitic infections in chickens of upper Gangetic plains of India with special reference to poultry coccidiosis. J. Parasit. Dis. 2015;39(1):22–26. doi: 10.1007/s12639-013-0273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badparva E, Ezatpour B, Azami M, Badparva M. First report of birds infection by intestinal parasites in Khorramabad, West Iran. J. Parasit. Dis. 2015;39(4):720–724. doi: 10.1007/s12639-014-0427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomza-Marciniak A, Pilarczyk B, Tobiańska B, Tarasewicz N. Gastrointestinal parasites of free-range chickens. Ann Parasitol. 2014;60(4):305–308. [PubMed] [Google Scholar]
- 24.Nnadi P.A, George S.O. A cross-sectional survey on parasites of chickens in selected villages in the subhumid zones of South-Eastern Nigeria. J. Parasitol. Res. 2010;2010(5):6. doi: 10.1155/2010/141824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebrahimi M, Asadpour M, Khodaverdi M, Borji H. Prevalence and distribution of gastrointestinal helminths in free-range chickens in Mashhad, Northeast of Iran. Sci. Parasitol. 2014;15(1-4):38–42. [Google Scholar]
- 26.Dauda J, Lawal J.R, Bello A.M, Mustapha M, Ndahi J.J, Biu A.A. Survey on the prevalence of gastrointestinal nematodes and associated risk factors in domestic Turkeys (Meleagris gallopavo) slaughtered in poultry markets in Bukuru Jos, Plateau state, Nigeria. Int. J. Innov. Agric. Biol Res. 2016;4(4):27–36. [Google Scholar]
- 27.Junaidu H.I, Luka S.A, Mijinyawa A. Prevalence of gastrointestinal helminth parasites of the domestic fowl (Gallus gallus domesticus) slaughtered in Giwa market, Giwa local government, area, Kaduna state, Nigeria. J. Nat. Sci. Res. 2014;19(4):120–125. [Google Scholar]
- 28.Gawęcka K. New views about maintenance of hen on playgrounds. Pol. Poult. J. 2005;11(3):14–15. [Google Scholar]
- 29.Bachaya H.A, Raza M.A, Anjum M.A, Khan I.A, Aziz A, Manzoor Z, Munawar S.H. Prevalence of Ascaridia galli in white leghorn layers and Fayoumi-Rhode Island red crossbred flock at government poultry farm Dina, Punjab, Pakistan. Trop. Biomed. 2015;32(1):11–16. [PubMed] [Google Scholar]
- 30.Teni F, Adugna S, Keffale M. Prevalence of Ascaridia galli in the intensive poultry production system in Eastern Hararghe zone, Eastern Ethiopia. Adv. Biol. Res. 2017;11(3):139–143. [Google Scholar]
- 31.Waller P.J. Anthelmintic resistance and future for roundworm control. Vet. Parasitol. 1987;15(2):177–191. doi: 10.1016/0304-4017(87)90103-8. [DOI] [PubMed] [Google Scholar]
- 32.Salam S.T. Ascariasis in backyard chicken prevalence, pathology and control. Int. J Recent Sci. Res. 2015;6(4):3361–3365. [Google Scholar]
- 33.Strunz E.C, Addiss D.G, Stocks M.E, Ogden S, Utzinger J, Freeman M.C. Water, sanitation, hygiene, and soil-transmitted helminth infection:A systematic review and meta-analysis. PLoS Med. 2014;11(3):e1001620. doi: 10.1371/journal.pmed.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikpeama C.A, Obiajuru I.O.C, Ogomaka A.I. The impact of refuse disposal dump sites on the spread of intestinal helminthiasis in Owerri metropolis, Imo state, South Eastern Nigeria. Int. J. Clin. Chem. Lab. Med. 2016;2(2):13–18. [Google Scholar]
- 35.Taiwo O.T, Sam-Wobo S.O, Taiwo A.M. Spatial distribution of helminth infections in Nigeria (2005-2015) and the need for attitudinal and behavioral changes in the water, sanitation and hygiene interventions. Ife J. Sci. 2016;18(4):913–930. [Google Scholar]
- 36.Permin A, Bisgaard M, Frandsen F, Pearman M, Nansen P, Kold J. The prevalence of gastrointestinal helminths in different poultry production systems. Br. Poult. Sci. 1999;40(4):439–443. doi: 10.1080/00071669987179. [DOI] [PubMed] [Google Scholar]
- 37.Kaufmann F, Gauly M. Prevalence and burden of helminths in local free range laying hens. Book of Abstracts of the 60thAnnual Meeting of the European Association for Animal Production. Wageningen: Wageningen Academic Publishers; 2009. p. 553. [Google Scholar]


