Abstract
Background: Urothelial carcinoma associated 1 (UCA1), a novel long noncoding RNA (lncRNA) which is first discovered in 2006 in human bladder cancer and has become a hot spot in recent years. UCA1 has been demonstrated correlated with clinical outcomes in various cancers. However, the results from each study are insufficient and not completely consistent. Therefore, we perform a systematic meta-analysis to evaluate the value for a feasible biomarker for metastasis and prognosis of cancer. Methods: Relevant English literatures were searched in PubMed, Cochrane Library, Web of science, Embase databases and Chinese literatures were searched in Chinese National Knowledge Infrastructure Wanfang from inception up to 17 April 2018. The pooled odds ratio (OR) and hazard ratio (HR) with 95% confidence interval (CI) using random/fixed-effect were used to identify the relationship between UCA1 and lymph node metastasis (LNM) or overall survival (OS) of cancer patients. Subgroup analysis and sensitivity analysis were performed. The current meta-analysis was performed using Review Manager 5.3 and Stata 12.0 software. Results: A total of 3411 patients from 38 studies were finally included. Patients who with high UCA1 expression suffered from an increased risk of LNM (OR = 2.50; 95% CI: 1.93–3.25). UCA1 was also significantly associated with OS (HR = 2.05; 95% CI: 1.77–2.38). Subgroup analyses across several different variables also showed the similar results in LNM and OS of cancer patients. Conclusion: High expression of UCA1 was linked with poor clinical outcome. UCA1 can serve as a potential molecular marker for metastasis and prognosis in different types of cancers.
Keywords: cancer, lncRNA, lymph node metastasis, prognosis meta-analysis, UCA1
Introduction
Cancer is a major global public health problem that seriously threatens human health. In recent years, the incidence and mortality of cancer are also increasing year by year. According to GLOBCAN 2012, there were 14.1 million new cancer cases, 8.2 million cancer deaths and 32.6 million people living with cancer (within 5 years of diagnosis) in 2012 worldwide [1]. In the United States, cancer is the second leading cause of death with an estimated 1,685,210 new cases and 595,690 deaths cancer in 2016 [2]. In China, cancer has been the leading cause of death with an estimated 4,292,000 new cases and 2,814,000 death cases in 2015 [3]. The current strategies to cancer therapy have significantly improved in some types of cancer, such as surgery, radiotherapy or chemotherapy. However, the outcome still remains undesirable. Therefore, looking for effective molecular biomarkers which can be used to evaluate potential risk of cancer is becoming imminent.
With the development of second-generation sequencing technology, more and more long noncoding RNAs (lncRNAs) was been found. LncRNAs were defined as non-protein coding RNAs with the length of more than 200 nucleotides. Recent studies have shown that lncRNAs are closely associated with diverse biological processes, especially in various types of cancer and played an indispensable role in the metastasis and prognosis of cancer [4]. Noteworthily, lncRNAs either could be acted as oncogenes or tumor suppressors in multiple cancers, such as HOPPIP [5] and MEG3 [6]. Urothelial carcinoma associated 1 (UCA1), also known as cancer-resistant drug resistance gene, a 2314-bp lncRNA encoded on human chromosome 19p13.12. UCA1 was a novel lncRNA which was first discovered in 2006 in human bladder cancer and has become a hot spot in recent years [7,8]. Accumulating evidence revealed that UCA1 was dysregulated in cancer tissues and participated in the malignant progression of cancers, including bladder cancer, breast cancer, gastric cancer (GC), colorectal cancer (CRC) and lung cancer [9]. Studies have shown that the dysregulation of UCA1 is closely associated with the clinicopathological characteristics of cancer, such as lymph node metastasis (LNM) and overall survival (OS). However, since the results of the studies were not consistent and small sample size in individual study, we collected relevant publications and performed a meta-analysis to investigate the relationship between UCA1 expression and lymph node metastasis or prognosis, aiming to further evaluate whether the UCA1 could be served as a potential molecular biomarker for cancers.
Materials and methods
Literature collection
We searched the electronic databases PubMed, Cochrane Library, Web of science, Embase databases, Chinese National Knowledge Infrastructure (CNKI) and Wanfang, by using ‘UCA1 or urothelial carcinoma associated 1’ as the keywords, in order to obtain potential articles referenced in the publications. Retrieval time for the last update is up to 17 April 2018.
Inclusion and exclusion criteria
Inclusion criteria for the articles were as the following: (1) Evaluation of the relationship between UCA1 expression and metastasis, or prognosis of patients in human cancer. (2) Patients were divided into high and low expression group according to the expression levels of UCA1. (3) Related clinicopathologic parameters and outcomes were described, such as LNM and OS. (4) Sufficient data for calculating odds ratio (OR), hazard ratio (HR) and its corresponding 95% confidence intervals (CI).
Exclusion criteria for the articles were as follows: (1) Nonhuman research, reviews, editorials, expert opinions, letters and case reports. (2) Duplicate publications. (3) Studies without valuable data.
Date extraction
Two investigators (H.Y.T. and L.C.M.) extracted and reviewed the essential data from the included studies independently, according to the inclusion and exclusion criteria. Disagreements were solved by two investigators (J.J. and S.J.) by discussions. For each eligible study, we extracted the following information: first author, publication year, tumor type, country, total number of patients, detection method of UCA1, UCA1 expression levels, number of high UCA1 expression group and low UCA1 expression group, number of patients with LNM, follow-up duration, reference control, HRs as well as their 95% CIs.
Quality assessment
The quality of all included studies was assessed by two investigators (W.L.Q. and G.Z.Y.) according to the Newcastle–Ottawa Scale (NOS) independently. For any divergence, a consensus was reached by a third investigator (GTT). NOS scores ranged from 0 to 9 points, with higher scores indicated a better quality and all included eligible studies were assessed to be of high quality by using the NOS in this meta-analysis.
Statistical analysis
The association between UCA1 and cancer lymph node metastasis or prognosis was assessed by OR and HR with its corresponding 95% CI. The current meta-analysis was performed through Review Manager 5.3 and Stata 12.0 software. We use the χ2-based Q test and I2 statistics evaluate the heterogeneity of the eligible studies. The random-effects model was used to analyze the results when heterogeneity was present (I2 > 50%, P<0.05); while the fixed-effects model was applied for this meta-analysis when the heterogeneity was absent in eligible studies (I2 < 50%, P>0.05). The potential publication bias was assessed with the Begg’s funnel-plot. The P-value less than 0.05 was considered to be statistically significant.
Results
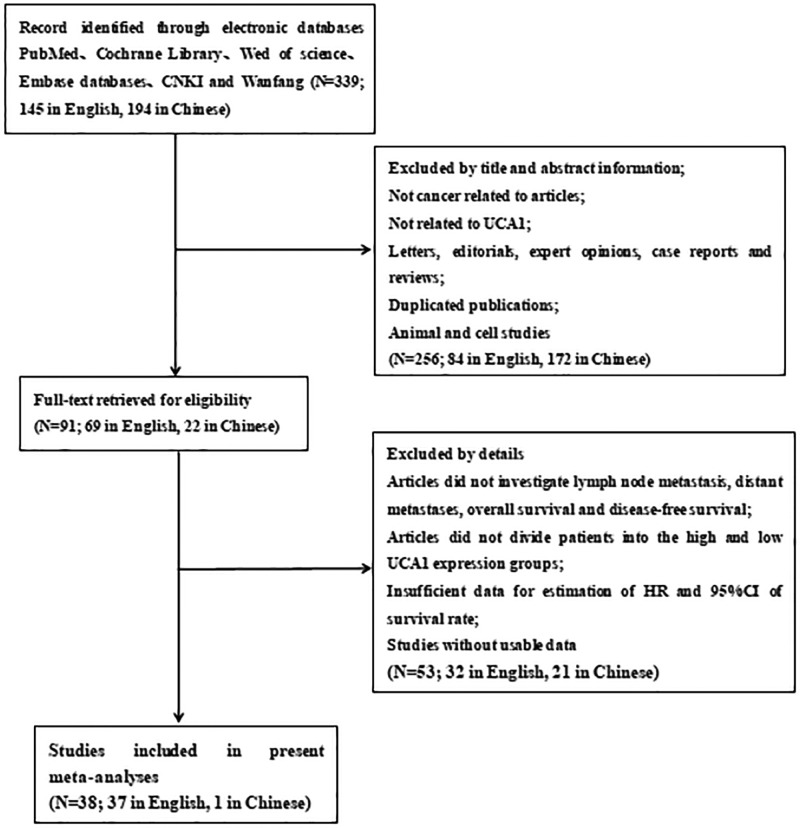
As shown in Figure 1, a total of 339 published articles were identified from the first attempt to search by using the keywords, of which 145 in English and 194 in Chinese. After removing duplicates, then screening the title and abstract carefully, 248 articles were excluded. After further inspection of the full articles, 53 articles were excluded. Eventually, according to the criteria for selection, a total of 38 studies, of which 1 is in Chinese and the others are in English, were included in the current meta-analysis.
Figure 1. The flow diagram of the meta-analysis.
Literature search and study characteristics
Tables 1 and 2 showed the main characteristics of the included researches. A total of 38 studies [10–47] involving 3411 cancer patients were included. The average patient sample size is 89.76, the maximum sample size is 384, and the minimum sample size is 20. Among the 38 studies, UCA1 was tested in 19 types of cancers, six studies focused on GC, four studies focused on CRC, three studies concentrated on prostate cancer and hepatocellular carcinoma (HCC), respectively; two study on renal cell carcinoma (RCC), ovarian cancer, (OC) pancreatic ductal adenocarcinoma (PDAC) and glioma, respectively, one study on non-small-cell lung cancer (NSCLC), lung cancer, esophageal cancer, esophageal squamous cell carcinoma, gallbladder cancer (GBC), oral squamous cell carcinoma, hypopharyngeal squamous cell carcinoma, urothelial carcinoma (UC), pancreatic cancer, endometrial cancer, breast cancer, osteosarcoma, colon cancer, cholangiocarcinoma (CCA) and multiple myeloma (MM), respectively. All the diagnoses of LNM were based on pathology. In all of the included studies, the patients were divided into two groups: high and low expression of UCA1. All studies used quantitative real-time PCR (qRT-PCR) to detect the expression of UCA1. The main characteristics of the eligible studies were summarized in Tables 1 and 2.
Table 1. Characteristics of studies about prognosis in this meta-analysis.
| Author | Year | Tumor type | Country | Sample size | UCA1 assay | Reference controls | UCA1 expression | Cut-off value | Research type of the studies | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High expression | High with LNM | Low expression | Low with LNM | |||||||||
| Bian | 2016 | CRC | China | 90 | qRT-PCR | β-Actin | 45 | 30 | 45 | 23 | Median | Case–control study |
| Cai Q. | 2017 | GBC | China | 45 | qRT-PCR | GAPDH | 23 | 12 | 22 | 18 | Median | Case–control study |
| Chen P. | 2016 | Pancreatic | China | 128 | qRT-PCR | GAPDH | 64 | 42 | 64 | 32 | Median | Case–control study |
| Fu | 2016 | PDAC | China | 80 | qRT-PCR | GAPDH | 40 | 17 | 40 | 17 | Median | Case–control study |
| Han | 2014 | CRC | China | 80 | qRT-PCR | GAPDH | 37 | 17 | 43 | 18 | Mean | Case–control study |
| He | 2017 | Glioma | China | 80 | qRT-PCR | β-Actin | 51 | 28 | 29 | 8 | NA | Case–control study |
| Khakiani | 2017 | GC | Iran | 40 | qRT-PCR | GUSB | 20 | 9 | 20 | 5 | Median | Case–control study |
| Li | 2014 | ESCC | China | 90 | qRT-PCR | GAPDH | 41 | 22 | 49 | 12 | Mean | Case–control study |
| Li L. | 2017 | GC | China | 102 | qRT-PCR | GAPDH | 73 | 44 | 29 | 10 | NA | Case–control study |
| Lu | 2016 | EC | China | 45 | qRT-PCR | GAPDH | 12 | 7 | 33 | 5 | Median | Case–control study |
| Ni | 2015 | CRC | China | 54 | qRT-PCR | GAPDH | 27 | 12 | 27 | 5 | Median | Case–control study |
| Nie | 2016 | NSCLC | China | 112 | qRT-PCR | β-Actin | 39 | 14 | 73 | 21 | Youden index | Case–control study |
| Qian | 2017 | HCC | China | 53 | qRT-PCR | β-Actin | 26 | 17 | 27 | 9 | Median | Case–control study |
| Tao | 2015 | CRC | China | 80 | qRT-PCR | β-Actin | 20 | 13 | 60 | 21 | Fourth quartile of the expression of UCA1 | Case–control study |
| Wang F. | 2015 | HCC | China | 98 | qRT-PCR | RNU6B | 49 | 30 | 49 | 11 | Median | Case–control study |
| Wang H. | 2015 | LC | China | 60 | qRT-PCR | GAPDH | 36 | 26 | 24 | 8 | Median | Case–control study |
| Wang Z. | 2017 | GC | China | 39 | qRT-PCR | GAPDH | 22 | 18 | 17 | 7 | Relative expression ratios <0.5 | Case–control study |
| Wen | 2017 | Osteosarcoma | China | 151 | qRT-PCR | GAPDH | 75 | 44 | 76 | 28 | NA | Case–control study |
| Xu | 2017 | CCA | China | 68 | qRT-PCR | GAPDH | 38 | 26 | 30 | 12 | NA | Case–control study |
| Yang Y.J. | 2016 | OC | China | 53 | qRT-PCR | GAPDH | 27 | 13 | 26 | 5 | Median | Case–control study |
| Yang Y.T. | 2016 | OSCC | China | 124 | qRT-PCR | GAPDH | 62 | 35 | 62 | 20 | NA | Case–control study |
| Zhang L. | 2016 | OC | China | 110 | qRT-PCR | GAPDH | 57 | 26 | 53 | 12 | Median | Case–control study |
| Zheng | 2015 | GC | China | 112 | qRT-PCR | RNU6B | 56 | 35 | 56 | 37 | Median | Case–control study |
| Zhou | 2017 | PC | China | 72 | qRT-PCR | GAPDH | 25 | 9 | 47 | 5 | Median | Case–control study |
| Zuo | 2017 | GC | China | 37 | qRT-PCR | RNU6B | 18 | 13 | 19 | 6 | Median | Case–control study |
Abbreviation: CCA, cholangiocarcinoma; CRC, colorectal cancer; EC, endometrial cancer; PC, pancreatic carcinoma.
Table 2. Subgroup analysis of the role of UCA1 in LNM in different types of cancer.
| Author | Year | Tumor type | Country | Sample size | Detection method | Reference Control | Cut-off value | Survival analysis | Multivariate analysis | HR statistic | Hazard ratios (95%) | Follow-up (months) | Research type of the studies |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bian | 2016 | CRC | China | 90 | qRT-PCR | β-Actin | Median | OS | Yes | Data in paper | 2.40 (1.04–5.50) | 75 | Case–control study |
| Bian | 2016 | CRC | China | 105 | qRT-PCR | β-Actin | Median | OS | NO | Survival curve | 1.62 (0.90–2.91) | 125 | Case–control study |
| Cai Q. | 2017 | GBC | China | 45 | qRT-PCR | GAPDH | Median | OS | NO | Survival curve | 2.08 (1.01–4.29) | 40 | Case–control study |
| Chen D. | 2015 | PDAC | U.S.A. | 63 | qRT-PCR | NA | Median | OS | NO | Survival curve | 2.76 (1.15–6.61) | 21 | Case–control study |
| Chen P. | 2016 | Pancreatic | China | 128 | qRT-PCR | GAPDH | Median | OS | Yes | Data in paper | 1.50 (1.01–2.24) | 60 | Case–control study |
| Fu | 2015 | PDAC | China | 80 | qRT-PCR | GAPDH | Median | OS | Yes | Data in paper | 2.02 (1.02–4.01) | 40 | Case–control study |
| Gao | 2015 | GC | China | 20 | qRT-PCR | GAPDH | NA | OS | Yes | Data in paper | 2.02 (1.02–3.37) | 40 | Case–control study |
| Han | 2014 | CRC | China | 80 | qRT-PCR | GAPDH | Mean | OS | NO | Survival curve | 7.44 (1.84–30.15) | 42.6 | Case–control study |
| He | 2017 | Glioma | China | 80 | qRT-PCR | β-Actin | NA | OS | NO | Survival curve | 1.52 (0.61–3.78) | 35 | Case–control study |
| Jiao | 2016 | Esophageal | China | 66 | qRT-PCR | NA | Median | OS | NO | Survival curve | 3.36 (1.48–7.61) | 30 | Case–control study |
| Johanna | 2017 | UC | Germany | 106 | qRT-PCR | SDHA/TBP | Median | OS | Yes | Data in paper | 0.57 (0.37–0.90) | 200 | Case–control study |
| Khakiani | 2017 | GC | Iran | 40 | qRT-PCR | GUSB | Median | OS | NO | Survival curve | 4.08 (1.63–10.22) | 100 | Case–control study |
| Li | 2014 | ESCC | China | 90 | qRT-PCR | GAPDH | Mean | OS | Yes | Data in paper | 2.63 (1.42–5.87) | 60 | Case–control study |
| Liu | 2016 | BC | China | 54 | qRT-PCR | GAPDH | Median | OS | NO | Survival curve | 2.08 (1.04–4.15) | 60 | Case–control study |
| Lu | 2016 | EC | China | 45 | qRT-PCR | GAPDH | Median | OS | NO | Survival curve | 3.95 (1.20–12.96) | 60 | Case–control study |
| Lu Y. | 2017 | RCC | China | 50 | qRT-PCR | GAPDH | Median | OS | NO | Survival curve | 3.20 (1.41–7.26) | 60 | Case–control study |
| Na | 2015 | PC | China | 40 | qRT-PCR | GAPDH | Median | OS | Yes | Survival curve | 1.52 (1.23–1.88) | 60 | Case–control study |
| Ni | 2015 | CRC | China | 54 | qRT-PCR | GAPDH | Median | OS | NO | Survival curve | 3.14 (1.17–8.41) | 50 | Case–control study |
| Nie | 2016 | NSCLC | China | 112 | qRT-PCR | β-Actin | Youden index | OS | Yes | Data in paper | 1.41 (1.08–1.84) | 60 | Case–control study |
| Qian | 2017 | HSCC | China | 53 | qRT-PCR | β-Actin | Median | OS | NO | Survival curve | 1.83 (0.89–3.78) | 60 | Case–control study |
| Sedlarikova | 2017 | MM | Czech | 64 | qRT-PCR | RPLP0 | NA | OS | Yes | Data in paper | 1.94 (1.17–3.22) | 60 | Case–control study |
| Tao | 2015 | CC | China | 80 | qRT-PCR | β-Actin | Fourth quartile of the expression level of UCA1. | OS | Yes | Data in paper | 2.00 (1.01–3.98) | 60 | Case–control study |
| Wang F. | 2015 | HCC | China | 98 | qRT-PCR | RNU6B | Median | OS | Yes | Data in paper | 1.86 (1.08–3.21) | 60 | Case–control study |
| Wang H. | 2015 | LC | China | 60 | qRT-PCR | GAPDH | Median | OS | Yes | Data in paper | 1.94 (1.06–3.26) | 60 | Case–control study |
| Wang Y. | 2017 | RCC | China | 384 | qRT-PCR | NA | NA | OS | Yes | Data in paper | 1.92 (1.36–2.70) | 150 | Case–control study |
| Wen | 2017 | Osteosarcoma | China | 151 | qRT-PCR | GAPDH | NA | OS | Yes | Data in paper | 2.52 (1.35–4.83) | 60 | Case–control study |
| Xu | 2017 | CCA | China | 68 | qRT-PCR | GAPDH | NA | OS | Yes | Data in paper | 2.27 (1.31–3.94) | 60 | Case–control study |
| Yang Y.J. | 2016 | OC | China | 53 | qRT-PCR | GAPDH | Median | OS | Yes | Data in paper | 6.32 (1.12–35.68) | 50 | Case–control study |
| Yang Z. | 2015 | HCC | Korea | 240 | qRT-PCR | NA | Median | OS | Yes | Data in paper | 1.99 (0.84–4.69) | 120 | Case–control study |
| Zhang L. | 2016 | OC | China | 110 | qRT-PCR | GAPDH | Median | OS | Yes | Data in paper | 1.69 (1.01–2.83) | 60 | Case–control study |
| Zhang S. | 2017 | PC | China | 47 | qRT-PCR | GAPDH | NA | OS | NO | Survival curve | 2.09 (0.80–5.46) | 60 | Case–control study |
| Zhao | 2017 | Glioma | China | 64 | qRT-PCR | GAPDH | >22.20 | OS | NO | Data in paper | 7.37 (3.03–17.90) | 48 | Case–control study |
| Zheng | 2015 | GC | China | 112 | qRT-PCR | RNU6B | Median | OS | Yes | Data in paper | 2.35 (1.22–4.52) | 60 | Case–control study |
| Zheng Z. | 2018 | HCC | China | 105 | qRT-PCR | GAPDH | Median | OS | Yes | Data in paper | 3.65 (1.17–4.65) | 60 | Case–control study |
| Zhou | 2017 | PC | China | 72 | qRT-PCR | GAPDH | Median | OS | NO | Survival curve | 1.87 (0.54–6.53) | 60 | Case–control study |
| Zuo | 2017 | GC | China | 37 | qRT-PCR | RNU6B | Median | OS | Yes | Data in paper | 2.92 (1.07–7.96) | 40 | Case–control study |
Abbreviation: CCA, cholangiocarcinoma; CRC, colorectal cancer; EC, endometrial cancer; PC, pancreatic carcinoma.
Meta-analysis results
Association between UCA1 and LNM
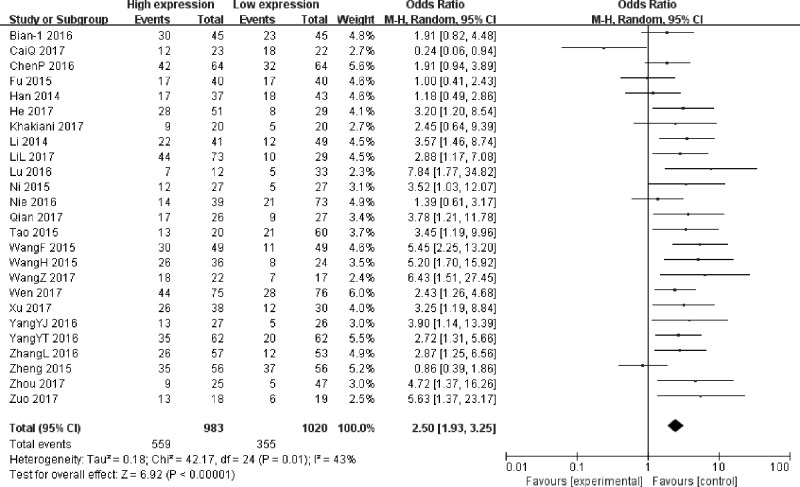
The 25 studies (Table 1) reported a total of 2003 patients with LNM based on different UCA1 expression levels. The random-effects model was adopted as the moderate heterogeneity (I2= 43%, P=0.01). Analysis showed that the OR of high UCA1 expression group versus low UCA1 expression group was 2.50 (95% CI: 1.93–3.25; P<0.00001) (Figure 2), which revealed that a higher UCA1 expression predicted more LNM. The result indicated that patients with high UCA1 expression in cancer tissues were more susceptible to LNM.
Figure 2. Forest plot for the association between UCA1 expression levels with LNM.
Association between UCA1 and OS
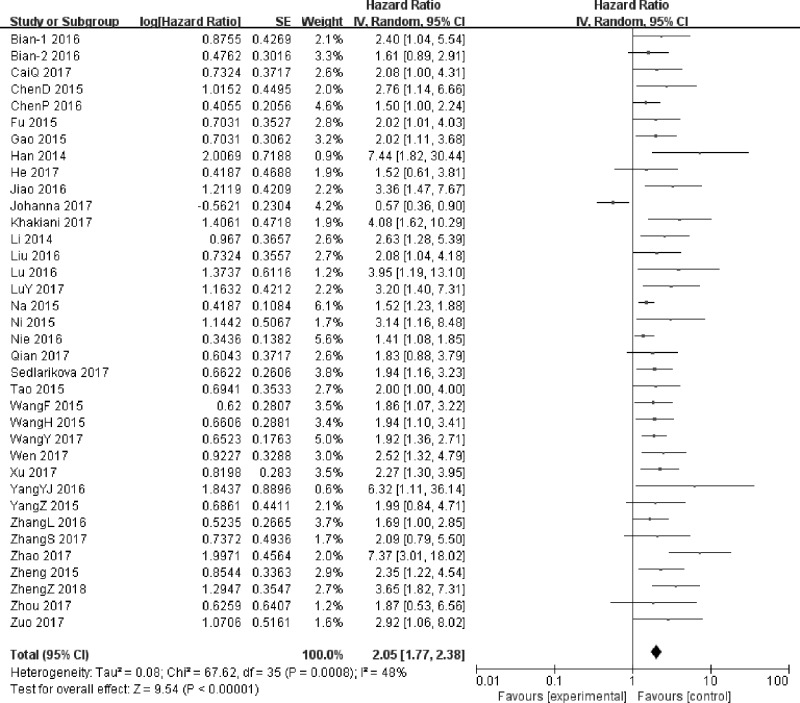
A total of 36 studies including 3146 patients were assessed for the correlation between UCA1 and OS (Table 2), High UCA1 expression was significantly correlated with poor prognosis, compared with low UCA1 expression in a pooled analysis of all studies (HR = 2.05; 95% CI: 1.77–2.38; P<0.00001) (Figure 3). The random-effects model was used because of the moderate heterogeneity (I2 = 48%, P=0.0008). In other words, high UCA1 expression group shortened the OS compared with low UCA1 expression group.
Figure 3. Forest plot for the association between UCA1 expression levels with OS.
Subgroup analysis
Subgroup analyses across several different variables were further performed to investigate the heterogeneity of the studies for meta-analysis of UCA1 and LNM or OS. The LNM-related data were stratified into subgroups based on sample size, tumor type, cut-off value and reference control. The assessment results in each subgroup are also shown in Table 3. Subgroup analysis by sample size explored that high UCA1 expression status was related to high LNM numbers both in big (n≥100, OR = 1.99, 95% CI: 1.50–2.65, P<0.0001) and small sample size group (n<100, OR = 2.71, 95% CI: 2.12–3.47, P<0.00001). And we also found a significantly positive correlation between UCA1 expression and LNM when grouped by different cut-off value [By median (OR = 2.48, 95% CI: 1.63–3.78, P<0.0001) and By others (OR = 2.53, 95% CI: 1.92–3.34, P<0.00001)]. However, when conducting subgroup analyses on tumor type, we found no significant correlation between high UCA1 expression and LNM among the studies in respiratory system (OR = 2.54, 95% CI: 0.70–9.23, P=0.16). According to the results presented in Table 3, when divided by reference control, the subgroup analysis showed that up-regulated UCA1 was associated with more LNM in GAPDH group (OR = 2.41, 95% CI: 1.91–3.04, P<0.00001) and β-actin group (OR = 2.35, 95% CI: 1.54–3.57, P<0.0001), while no significant association in RNU6B/GUSB group (OR = 2.69, 95% CI: 0.95–7.56, P=0.06).
Table 3. Subgroup analysis of the role of UCA1 in LNM in different types of cancer.
| Subgroup analysis | No. of studies | No. of patients | Test of relationship | Test of heterogeneity | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | I2 (%) | Q-value | |||
| Overall | 25 | 2003 | 2.50 (1.93–3.25) | <0.00001 | 43 | 0.01 |
| Sample size | ||||||
| <100 | 18 | 1164 | 2.71 (2.12–3.47) | <0.00001 | 46 | 0.02 |
| ≥100 | 7 | 839 | 1.99 (1.50–2.65) | <0.00001 | 23 | 0.25 |
| Tumor type | ||||||
| Respiratory system | 2 | 172 | 2.54 (0.70–9.23) | 0.16 | 71 | 0.06 |
| Digestive system | 17 | 1320 | 2.27 (1.61–3.20) | <0.00001 | 52 | 0.006 |
| Reproductive system | 3 | 208 | 3.65 (1.96–6.81) | <0.0001 | 0 | 0.51 |
| Others | 3 | 303 | 2.90 (1.77–4.77) | <0.0001 | 0 | 0.63 |
| Cut off | ||||||
| Median | 15 | 1077 | 2.48 (1.63–3.78) | <0.0001 | 59 | 0.002 |
| Others | 10 | 926 | 2.53 (1.92–3.34) | <0.00001 | 0 | 0.54 |
| Reference control | ||||||
| GAPDH | 16 | 1301 | 2.41 (1.91–3.04) | <0.00001 | 45 | 0.03 |
| β-Actin | 5 | 415 | 2.35 (1.54–3.57) | <0.0001 | 0 | 0.5 |
| RNU6B/GUSB | 4 | 287 | 2.69 (0.95–7.56) | 0.06 | 74 | 0.009 |
The OS-related data were stratified into subgroups based on sample size, tumor type, cut-off value, follow-up time, analysis method, race and reference control. The detailed assessment results in each subgroup are also shown in Table 4. Subgroup analysis by sample size, cut-off value, follow-up time, analysis method and reference control all revealed that high UCA1 expression was significantly associated with poor OS in each groups. However, when conducting subgroup analyses on tumor type, we found high UCA1 expression was remarkably related to poor OS among respiratory system, digestive system, reproductive system and other systems but no significant correlation between high UCA1 expression and OS among the studies in urinary system (HR = 1.54, 95% CI: 0.98–2.40, P=0.06). As for different race for UCA1, the relationship between UCA1 expression and OS was significant in Asian group (HR = 1.90, 95% CI: 1.72–2.10, P<0.00001), but not in others group (HR = 1.39, 95% CI: 0.52–3.71, P = 0.51).
Table 4. Subgroup analysis of the role of UCA1 in OS in different types of cancer.
| Subgroup analysis | No. of studies | No. of patients | Test of relationship | Test of heterogeneity | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | I2 (%) | Q-value | |||
| Overall | 36 | 3146 | 2.05 (1.77–2.38) | <0.00001 | 48 | 0.0008 |
| Sample size | ||||||
| <100 | 26 | 1593 | 2.03 (1.80–2.30) | <0.00001 | 14 | 0.26 |
| ≥100 | 10 | 1553 | 1.67 (1.25–2.22) | 0.0005 | 71 | 0.0003 |
| Tumor type | ||||||
| Respiratory system | 2 | 172 | 1.50 (1.17–1.91) | 0.001 | 0 | 0.32 |
| Digestive system | 20 | 1654 | 2.18 (1.87–2.55) | <0.00001 | 0 | 0.76 |
| Urinary system | 6 | 699 | 1.54 (0.98–2.40) | 0.06 | 78 | 0.0003 |
| Reproductive system | 3 | 208 | 2.10 (1.32–3.33) | 0.002 | 39 | 0.19 |
| others system | 5 | 413 | 2.37 (1.75–3.22) | <0.00001 | 49 | 0.10 |
| Region | ||||||
| Asian | 33 | 2913 | 1.90 (1.72–2.10) | <0.00001 | 21 | 0.14 |
| Non Asian | 3 | 233 | 1.39 (0.52–3.71) | 0.51 | 88 | 0.0002 |
| Cut off | ||||||
| Median | 24 | 1906 | 2.01 (1.65–2.45) | <0.00001 | 51 | 0.0002 |
| Others | 12 | 1240 | 1.92 (1.65–2.24) | <0.00001 | 43 | 0.06 |
| Analysis method | ||||||
| Non-multivariable analysis | 15 | 918 | 2.55 (2.04–3.18) | <0.00001 | 9 | 0.35 |
| Multivariable analysis | 21 | 2228 | 1.83 (1.55–2.16) | <0.00001 | 51 | 0.004 |
| Reference control | ||||||
| GAPDH | 19 | 1416 | 1.96 (1.72–2.22) | <0.00001 | 35 | 0.06 |
| β-actin | 6 | 520 | 1.57 (1.27–1.93) | <0.0001 | 0 | 0.81 |
| Other controls | 10 | 1210 | 2.00 (1.37–2.93) | 0.0004 | 72 | 0.0002 |
| Follow-up (months) | ||||||
| <60 | 11 | 642 | 2.71 (2.09–3.51) | <0.00001 | 14 | 0.31 |
| ≥60 | 25 | 2504 | 1.71 (1.54–1.89) | <0.00001 | 47 | 0.005 |
Sensitivity analysis
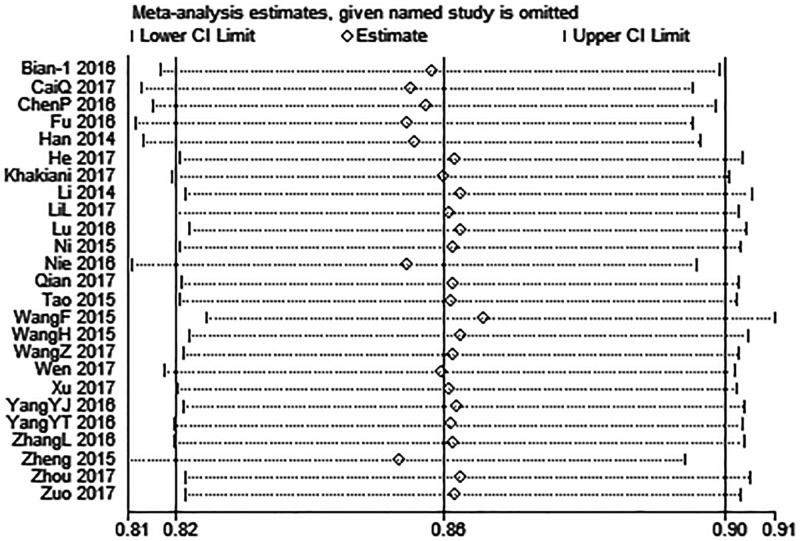
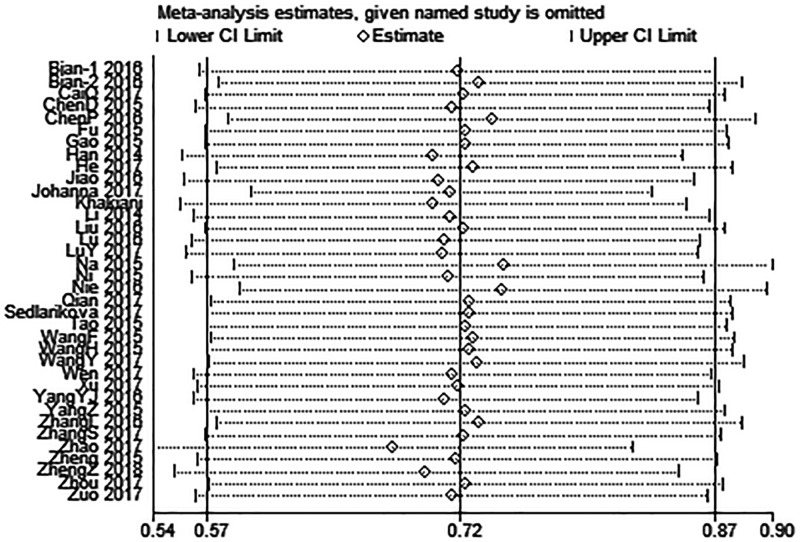
Multiple sensitivity analyses were carried out to evaluate whether individual study influenced pooled ORs or HRs by excluding one study by turns. It was found that none of the exclusions of a specific study would change the magnitude or direction of the summary effect for the correlation between UCA1 expression and LNM or OS, which further confirmed the validity of the results (Figures 4 and 5).
Figure 4. Sensitivity analysis for the association between UCA1 expression levels with LNM.
Figure 5. Sensitivity analysis for the association between UCA1 expression levels with OS.
Publication bias
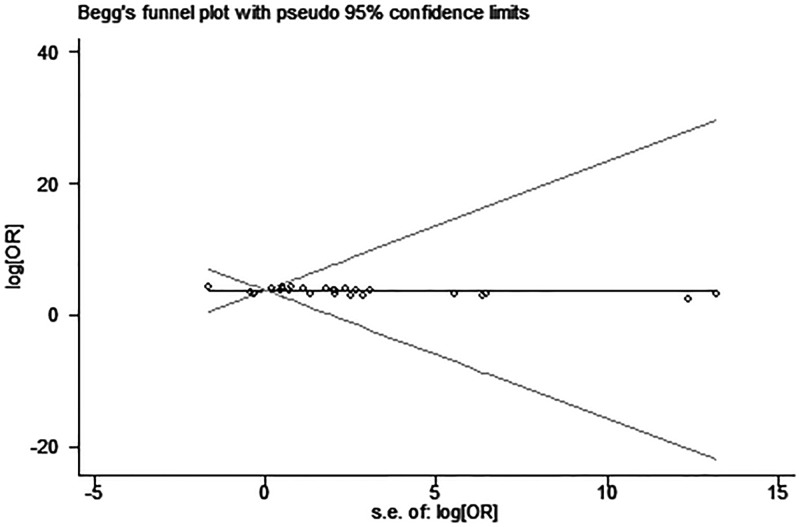
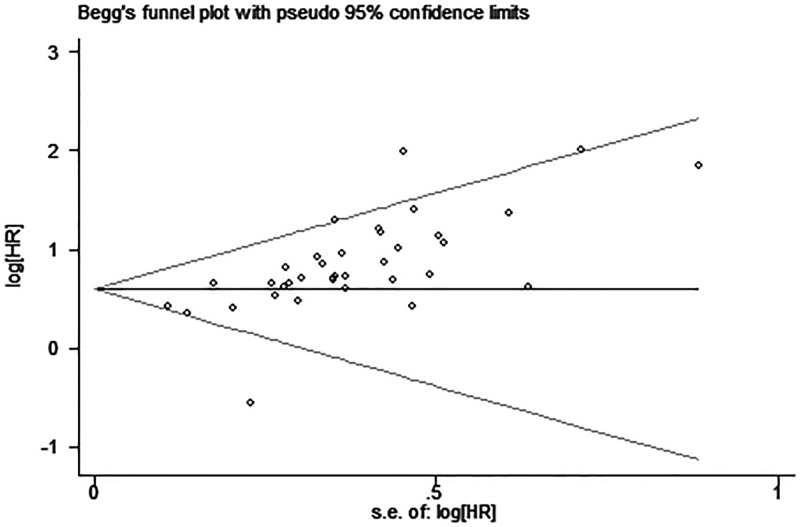
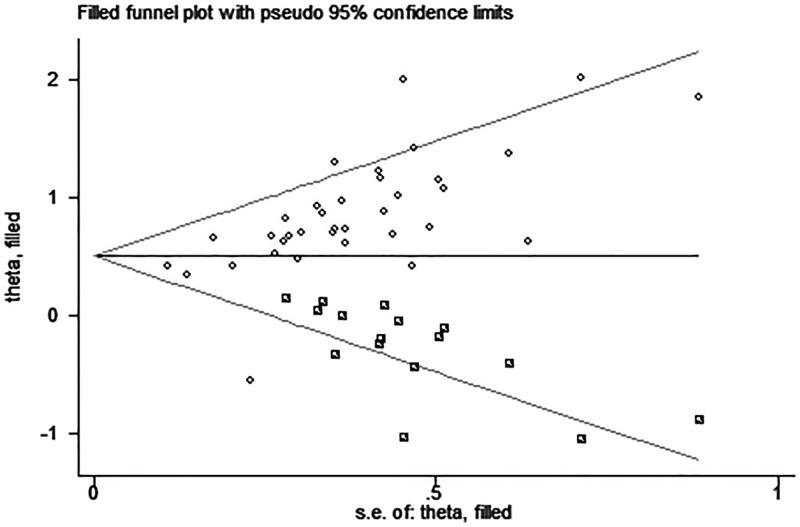
Egger’s test and funnel plot were introduced to evaluate potential publication bias in our present meta-analysis. No evidence supporting publication bias was found in analysis between UCA1 and LNM (Egger’s test, t = 1.31, P=0.202) (Figure 6). However, the shapes of funnel plot were asymmetric and Egger’s test displayed slightly publication bias for the HR evaluation of OS (Egger’s test, t = 4.76, P<0.05) (Figure 7). Because of this, trim and fill was used to perform a sensitivity analysis. This method conservatively conjectures hypothetical negative unpublished studies to reflect positive studies that lead to funnel plot asymmetry, and then a symmetrical funnel plot appears (Figure 8). While the statistically significant relationship between UCA1 expression and OS was also shown in pooled analysis incorporating the hypothetical studies, indicating that the result was stable and publication did not have an impact on it though publication bias exists.
Figure 6. Funnel plot analysis of potential publication bias for LNM.
Figure 7. Funnel plot analysis of potential publication bias for OS.
Figure 8. Funnel plot analysis of potential publication bias for OS with trim and fill.
Discussion
Evidence from multiple publications demonstrated that lncRNAs, similar to protein-coding genes, can act as oncogenes or tumor suppressor genes, which involving in a variety of tumorigenesis processes, including proliferation, invasion, migration and apoptosis. With the rapid development of high-throughput genome-wide analysis technology, more and more functional lncRNAs have been found to have potential value on predicting cancer progression. UCA1, a novel functional lncRNA which was first discovered in 2006 in human bladder cancer. In recent years, a growing number of studies have shown that UCA1 up-regulated in several cancers, including HCC, GC and lung cancer [7]. UCA1 involved in tumor proliferation, invasion, migration and apoptosis, and played an important role in tumor progression, metastasis and prognosis. However, a persuasive support of the UCA1 in clinical practice is still controversial, partially due to the uncertainty of the relationship between UCA1 and metastasis or prognosis implication. Several literatures established a statistically significant relationship between high UCA1 expression and lymph node metastasis or prognosis. Nevertheless, some studies showed no statistical impact of UCA1 dysregulation on cancer metastasis and prognosis. In order to combine previous research results about UCA1 and cancers to arrive at a summary conclusion, a comprehensive study is performed.
In the present meta-analysis, we systematically explore the relationship between UCA1 and cancer metastasis or prognosis. The results of the current study demonstrated that high UCA1 expression level was positively related to increasing the risk of LNM in cancer patients. Moreover, we also identified that there was a significantly positive correlation between high UCA1 expression and short OS in cancer patients. In multiple sensitivity analyses, we did not detect any substantial difference in pooled estimates, and there was no excessive influence on the overall results in any individual study.
The exact mechanisms underlying the association between elevated UCA1 expression and more LNM or poor prognosis is poorly understood, and the related reports are not the same, but many similarities were still existed. Several literatures have suggested potential mechanisms that could be involved in the metastasis and prognostic impact of UCA1 on carcinogenesis. First, UCA1 could act as a key competing endogenous RNA (ceRNA) or sponge for miR-204-5p, miR-193a-3p, miR-145, miR-143, miR-216b, miR-203, miR-196a-5p and miR-135a in several different cancers. For example, Zhang et al. found that UCA1 could directly interacted with miR-204 and functioned as a ceRNA, thus regulating the expression of ATF2 and promoting cell proliferation and metastasis in prostate cancer [16]. Nie et al. found UCA1 up-regulated the expression level of miR-193a-3p target gene ERBB4 by competitively ‘spongeing’ miR-193a-3p in NSCLC [26]. In bladder cancer, Xue et al. demonstrated that UCA1 induced epithelial–mesenchymal transition (EMT) of bladder cancer cells through up-regulating the expression of zinc finger E-box binding homeobox 1 and 2 (ZEB1 and ZEB2), and also regulated cell migration and invasion of bladder cancer by suppressing miR-145 and its target gene the actin-binding protein fascin homologue 1 (FSCN1) [48]. In HCC, Wang et al. found UCA1 acted as an endogenous sponge through binding to miR-216b directly and down-regulated the expression of miR-216b. UCA1 reversed the inhibitory effect on the growth and metastasis of miR-216b of HCC, which might be involved in the suppression of fibroblast growth factor receptor 1 (FGFR1) expression, a target gene of miR-216b, and the activation of ERK signaling pathway [42]. In addition, Xiao et al. showed that UCA1 up-regulation promoted cell EMT in HCC via sponging to miR-203 effectively and thus activating the expression of transcription factor Snail2 and promote HCC progression [49]. In bladder cancer, UCA1 could promote glycolysis by up-regulating hexokinase 2 through both activation of STAT3 and repression of miR-143 [50]. UCA1-activated transcription factor CREB which resulting in miR-196a-5p expression by binding with its promoter and thereby modulating the influence on cisplatin/gemcitabine resistance [51]. Second, UCA1 promoted the progression of different cancer by activating of the Wnt/β-catenin signaling pathway. UCA1 down-regulation increased the tamoxifen sensitivity through inhibiting Wnt/β-catenin pathway in breast cancer cells while UCA1 up-regulation promoted EMT of breast cancer cells by activating Wnt/β-Catenin signaling pathway [31,52]. Silence UCA1 suppressed cell proliferation and metastasis and induced cell apoptosis of oral squamous cell carcinoma, which might be significantly correlated with the activation level of the Wnt/β-Catenin signaling pathway [11]. Fan et al. indicated that UCA1 could increase the cisplatin resistance of bladder cancer cells by regulating the Wnt signaling [53]. Third, UCA1 overexpression could promote cancer metastasis by activation of metastasis-related genes including GRK2/ERK-MMP9, EZH2/AKT, p21/E-cadherin, iASPP, KLF4-KRT6/13, FGFR1/ERK and ZEB1/2-FSCN1. UCA1 overexpression could increase the metastatic ability of GC cells through regulating GRK2 protein stability by promoting Cbl-c-mediated GRK2 ubiquitination and degradation, thus activate the ERK-MMP9 signaling pathway [54]. Mechanically, UCA1 promoted the cell proliferation and metastasis of GBC by recruiting enhancer of zeste homolog 2 (EZH2) to the promoter of p21 and E-cadherin, and epigenetically suppressing their transcript [43]. He et al. demonstrated that UCA1 overexpression promoted cell proliferation and migration of glioma, to regulate the tumor growth and metastasis via miR-182 dependent iASPP regulation [35]. Wang et al. suggested that UCA1 overexpression contributed to the growth and metastasis of HCC via inhibiting miR-216b and activating FGFR1/ERK signaling pathway [42]. Simultaneously, UCA1 also remarkably associated with prognosis of patients with different cancer and may be a potential diagnosis biomarker in hepatocellular cancer, CRC and GC.
Otherwise, some limitations to this meta-analysis should be taken into account. First, the cut-off values of UCA1 high and low expression were lack of uniform standard due to different methods and criteria in different types of cancer, which may result in some heterogeneity and affect the results of the study. Second, most studies tended to report positive results rather than negative results; our meta-analysis may overestimate the significance of UCA1 to some extent. Third, some of the HRs were estimated from survival curves rather than directly obtained from the primary studies. Lastly, most of the included studies were performed in the population from Asian countries rather than worldwide population; our results should be substantiated by additional studies in other races. Although there are some limitations, but this current meta-analysis still has its noteworthy advantages. First, 38 literatures which including a total of 3411 cases and 19 types of cancer were included in this meta-analysis. The sample size included was the largest, which significantly improved the statistical efficiency and accuracy of the test. Second, the number of search databases were greater and cancer types were more comprehensive in this meta-analysis compared with previous reports. Finally, the inclusion and exclusion criteria were more stringent and the quality of the literatures incorporated was higher.
Conclusion
In conclusion, even some limitations mentioned above, our meta-analysis reveals that the expression level of UCA1 was significantly associated with metastasis and prognosis in different types of cancer. The higher expression of UCA1, the higher probability of occurrence of LNM cancer patients suffer with. Meanwhile, shorter OS may be observed in the patients with high UCA1 expression. Thus, UCA1 might be a novel predictive marker for estimating the metastasis and prognosis in different types of cancer. However, the significance of UCA1 in LNM in respiratory system cancers and RNU6B/GUSB reference control group should incorporate more studies to validate this result, and so does in urinary system prognosis and non-Asian people prognosis.
Abbreviations
- BC
bladder cancer
- CCA
cholangiocarcinoma
- ceRNA
competing endogenous RNA
- CI
confidence interval
- CRC
colorectal cancer
- CREB
cAMP response element-binding protein
- EC
endometrial cancer
- EMT
epithelial–mesenchymal transition
- EZH2
enhancer of zeste homolog 2
- FGFR1
fibroblast growth factor receptor 1
- FSCN1
fascin homologue 1
- GBC
gallbladder cancer
- GC
gastric cancer
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- iASPP
inhibitor of apoptosis-stimulating protein of p53
- lncRNA
long noncoding RNA
- LNM
lymph node metastasis
- MM
multiple myeloma
- NOS
Newcastle–Ottawa Scale
- NSCLC
non-small-cell lung cancer
- OC
ovarian cancer
- OR
odd ratio
- OS
overall survival
- PC
pancreatic carcinoma
- PDAC
pancreatic ductal adenocarcinoma
- qRT-PCR
quantitative real-time PCR
- RCC
renal cell carcinoma
- STAT3
phospho-signal transducer and transcription activator 3
- UC
urothelial carcinoma
- UCA1
urothelial carcinoma associated 1
Author contribution
Y.H. and C.L. conceived of the idea, designed the study and wrote the paper. J.J. and J.S. performed the experiments and solved the discussion. L.W., Z.G. and T.G. contributed to the quality assessment, confirmed statistical analyses and draw the figures. All authors approved the final version of the manuscript.
Funding
The authors declare that there are no sources of funding to be acknowledged.
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.International Agency for Research on Cancer. GLOBOCAN 2012. 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx [Google Scholar]
- 2.Siegel R.L., Miller K.D. and Jemal A. (2016) Cancer statistics, 2016. CA Cancer J. Clin. 66, 7–30 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 3.Chen W., Zheng R., Baade P.D.. et al. (2016) Cancer statistics in China, 2015. CA Cancer J. Clin. 66, 115–132 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 4.Yang B., Luo T., Zhang M., Lu Z., Xue X. and Fang G. (2017) The novel long noncoding RNA RP11-357H14.17 acts as an oncogene by promoting cell proliferation and invasion in diffuse-type gastric cancer. Onco. Targets Ther. 10, 2635–2643 10.2147/OTT.S134121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Y., Zhou Y., Bai Y.. et al. (2017) A long non-coding RNA HOTTIP expression is associated with disease progression and predicts outcome in small cell lung cancer patients. Mol. Cancer 16, 017–0729 10.1186/s12943-017-0729-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei G.H. and Wang X. (2017) lncRNA MEG3 inhibit proliferation and metastasis of gastric cancer via p53 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 21, 3850–2856 [PubMed] [Google Scholar]
- 7.Xue M., Chen W. and Li X. (2016) Urothelial cancer associated 1: a long noncoding RNA with a crucial role in cancer. J. Cancer Res. Clin. Oncol. 142, 1407–1419 10.1007/s00432-015-2042-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X.S., Zhang Z., Wang H.C.. et al. (2006) Rapid identification of UCA1 as a very sensitive and specific unique marker for human bladder carcinoma. Clin. Cancer Res. 12, 4851–4858 10.1158/1078-0432.CCR-06-0134 [DOI] [PubMed] [Google Scholar]
- 9.Li F. and Hu C.P. (2015) Long non-coding RNA urothelial carcinoma associated 1 (UCA1): insight into its role in human diseases. Crit. Rev. Eukaryot. Gene Expr. 25, 191–197 10.1615/CritRevEukaryotGeneExpr.2015013770 [DOI] [PubMed] [Google Scholar]
- 10.Wang Z.Q., Cai Q., Hu L.. et al. (2017) Long noncoding RNA UCA1 induced by SP1 promotes cell proliferation via recruiting EZH2 and activating AKT pathway in gastric cancer. Cell Death Dis. 8, 143 10.1038/cddis.2017.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y.T., Wang Y.F., Lai J.Y.. et al. (2016) Long non-coding RNA UCA1 contributes to the progression of oral squamous cell carcinoma by regulating the WNT/beta-catenin signaling pathway. Cancer Sci. 107, 1581–1589 10.1111/cas.13058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuo Z.K., Gong Y., Chen X.H.. et al. (2017) TGFbeta1-induced LncRNA UCA1 upregulation promotes gastric cancer invasion and migration. DNA Cell Biol. 36, 159–167 10.1089/dna.2016.3553 [DOI] [PubMed] [Google Scholar]
- 13.Zhou Y., Wang X., Zhang J.. et al. (2017) Artesunate suppresses the viability and mobility of prostate cancer cells through UCA1, the sponge of miR-184. Oncotarget 8, 18260–18270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Q., Wu F., Dai W.Y.. et al. (2015) Aberrant expression of UCA1 in gastric cancer and its clinical significance. Clin. Transl. Oncol. 17, 640–646 10.1007/s12094-015-1290-2 [DOI] [PubMed] [Google Scholar]
- 15.Zhao W., Sun C. and Cui Z. (2017) A long noncoding RNA UCA1 promotes proliferation and predicts poor prognosis in glioma. Clin. Transl. Oncol. 19, 735–741 10.1007/s12094-016-1597-7 [DOI] [PubMed] [Google Scholar]
- 16.Zhang S., Dong X., Ji T., Chen G. and Shan L. (2017) Long non-coding RNA UCA1 promotes cell progression by acting as a competing endogenous RNA of ATF2 in prostate cancer. Am. J. Transl. Res. 9, 366–375 [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L., Cao X., Zhang L., Zhang X., Sheng H. and Tao K. (2016) UCA1 overexpression predicts clinical outcome of patients with ovarian cancer receiving adjuvant chemotherapy. Cancer Chemother. Pharmacol. 77, 629–634 10.1007/s00280-016-2963-4 [DOI] [PubMed] [Google Scholar]
- 18.Yang Z., Lu Y., Xu Q., Tang B., Park C.K. and Chen X. (2015) HULC and H19 played different roles in overall and disease-free survival from hepatocellular carcinoma after curative hepatectomy: a preliminary analysis from gene expression omnibus. Dis. Markers 2015, 191029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y., Jiang Y., Wan Y.. et al. (2016) UCA1 functions as a competing endogenous RNA to suppress epithelial ovarian cancer metastasis. Tumour Biol. 37, 10633–10641 10.1007/s13277-016-4917-1 [DOI] [PubMed] [Google Scholar]
- 20.Wen J.J., Ma Y.D., Yang G.S. and Wang G.M. (2017) Analysis of circulating long non-coding RNA UCA1 as potential biomarkers for diagnosis and prognosis of osteosarcoma. Eur. Rev. Med. Pharmacol. Sci. 21, 498–503 [PubMed] [Google Scholar]
- 21.Wang Y., Gao W., Xu J., Zhu Y. and Liu L. (2017) The long noncoding RNA urothelial carcinoma-associated 1 overexpression as a poor prognostic biomarker in clear cell renal cell carcinoma. Tumour Biol. 39, 1010428317698377 [DOI] [PubMed] [Google Scholar]
- 22.Wang H.M., Lu J.H., Chen W.Y. and Gu A.Q. (2015) Upregulated lncRNA-UCA1 contributes to progression of lung cancer and is closely related to clinical diagnosis as a predictive biomarker in plasma. Int. J. Clin. Exp. Med. 8, 11824–11830 [PMC free article] [PubMed] [Google Scholar]
- 23.Tao K., Yang J., Hu Y.. et al. (2015) Clinical significance of urothelial carcinoma associated 1 in colon cancer. Int. J. Clin. Exp. Med. 8, 21854–21860 [PMC free article] [PubMed] [Google Scholar]
- 24.Sedlarikova L., Gromesova B., Kubaczkova V.. et al. (2017) Deregulated expression of long non-coding RNA UCA1 in multiple myeloma. Eur. J. Haematol. 99, 223–233 10.1111/ejh.12908 [DOI] [PubMed] [Google Scholar]
- 25.Qian Y., Liu D., Cao S.. et al. (2017) Upregulation of the long noncoding RNA UCA1 affects the proliferation, invasion, and survival of hypopharyngeal carcinoma. Mol. Cancer 16, 017–0635 10.1186/s12943-017-0635-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nie W., Ge H.J., Yang X.Q.. et al. (2016) LncRNA-UCA1 exerts oncogenic functions in non-small cell lung cancer by targeting miR-193a-3p. Cancer Lett. 371, 99–106 10.1016/j.canlet.2015.11.024 [DOI] [PubMed] [Google Scholar]
- 27.Ni B., Yu X., Guo X.. et al. (2015) Increased urothelial cancer associated 1 is associated with tumor proliferation and metastasis and predicts poor prognosis in colorectal cancer. Int. J. Oncol. 47, 1329–1338 10.3892/ijo.2015.3109 [DOI] [PubMed] [Google Scholar]
- 28.Na X.Y., Liu Z.Y., Ren P.P., Yu R. and Shang X.S. (2015) Long non-coding RNA UCA1 contributes to the progression of prostate cancer and regulates proliferation through KLF4-KRT6/13 signaling pathway. Int. J. Clin. Exp. Med. 8, 12609–12616 [PMC free article] [PubMed] [Google Scholar]
- 29.Lu Y., Liu W.G., Lu J.H.. et al. (2017) LncRNA UCA1 promotes renal cell carcinoma proliferation through epigenetically repressing p21 expression and negatively regulating miR-495. Tumour Biol. 39, 1010428317701632 10.1177/1010428317701632 [DOI] [PubMed] [Google Scholar]
- 30.Lu L., Shen Y., Tseng K.F., Liu W., Duan H. and Meng W. (2016) Silencing of UCA1, a poor prognostic factor, inhibited the migration of endometrial cancer cell. Cancer Biomark. 17, 171–177 10.3233/CBM-160628 [DOI] [PubMed] [Google Scholar]
- 31.Liu H., Wang G., Yang L., Qu J., Yang Z. and Zhou X. (2016) Knockdown of long non-coding RNA UCA1 increases the tamoxifen sensitivity of breast cancer cells through inhibition of Wnt/beta-Catenin pathway. PLoS ONE 11, 10.1371/journal.pone.0168406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J.Y., Ma X. and Zhang C.B. (2014) Overexpression of long non-coding RNA UCA1 predicts a poor prognosis in patients with esophageal squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 7, 7938–7944 [PMC free article] [PubMed] [Google Scholar]
- 33.Droop J., Szarvas T., Schulz W.A.. et al. (2017) Diagnostic and prognostic value of long noncoding RNAs as biomarkers in urothelial carcinoma. PLoS ONE 12, e0176287 10.1371/journal.pone.0176287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiao C., Song Z., Chen J.. et al. (2016) lncRNA-UCA1 enhances cell proliferation through functioning as a ceRNA of Sox4 in esophageal cancer. Oncol. Rep. 36, 2960–2966 10.3892/or.2016.5121 [DOI] [PubMed] [Google Scholar]
- 35.He Z., Wang Y., Huang G., Wang Q., Zhao D. and Chen L. (2017) The lncRNA UCA1 interacts with miR-182 to modulate glioma proliferation and migration by targeting iASPP. Arch. Biochem. Biophys. 624, 1–8 10.1016/j.abb.2017.01.013 [DOI] [PubMed] [Google Scholar]
- 36.Han Y., Yang Y.N., Yuan H.H.. et al. (2014) UCA1, a long non-coding RNA up-regulated in colorectal cancer influences cell proliferation, apoptosis and cell cycle distribution. Pathology 46, 396–401 10.1097/PAT.0000000000000125 [DOI] [PubMed] [Google Scholar]
- 37.Gao J., Cao R. and Mu H. (2015) Long non-coding RNA UCA1 may be a novel diagnostic and predictive biomarker in plasma for early gastric cancer. Int. J. Clin. Exp. Pathol. 8, 12936–12942 [PMC free article] [PubMed] [Google Scholar]
- 38.Fu X.L., Liu D.J., Yan T.T.. et al. (2016) Analysis of long non-coding RNA expression profiles in pancreatic ductal adenocarcinoma. Sci. Rep. 6, 33535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen P., Wan D., Zheng D., Zheng Q., Wu F. and Zhi Q. (2016) Long non-coding RNA UCA1 promotes the tumorigenesis in pancreatic cancer. Biomed. Pharmacother. 83, 1220–1226 10.1016/j.biopha.2016.08.041 [DOI] [PubMed] [Google Scholar]
- 40.Chen D.T., Davis-Yadley A.H., Huang P.Y.. et al. (2015) Prognostic fifteen-gene signature for early stage pancreatic ductal adenocarcinoma. PLoS ONE 10, e0133562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bian Z., Jin L., Zhang J.. et al. (2016) LncRNA-UCA1 enhances cell proliferation and 5-fluorouracil resistance in colorectal cancer by inhibiting miR-204-5p. Sci. Rep. 6, 10.1038/srep23892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang F., Ying H.Q., He B.S.. et al. (2015) Upregulated lncRNA-UCA1 contributes to progression of hepatocellular carcinoma through inhibition of miR-216b and activation of FGFR1/ERK signaling pathway. Oncotarget 6, 7899–7917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai Q., Jin L., Wang S.. et al. (2017) Long non-coding RNA UCA1 promotes gallbladder cancer progression by epigenetically repressing p21 and E-cadherin expression. Oncotarget 8, 47957–47968 10.18632/oncotarget.18204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu Y., Yao Y., Leng K.. et al. (2017) Long non-coding RNA UCA1 indicates an unfavorable prognosis and promotes tumorigenesis via regulating AKT/GSK-3beta signaling pathway in cholangiocarcinoma. Oncotarget 8, 96203–96214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nasrollahzadeh-Khakiani M., Emadi-Baygi M. and Nikpour P. (2017) Augmented expression levels of lncRNAs ecCEBPA and UCA1 in gastric cancer tissues and their clinical significance. Iran J. Basic Med. Sci. 20, 1149–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li C., Liang G., Yang S.. et al. (2017) Dysregulated lncRNA-UCA1 contributes to the progression of gastric cancer through regulation of the PI3K-Akt-mTOR signaling pathway. Oncotarget 8, 93476–93491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng Z.K., Pang C., Yang Y., Duan Q., Zhang J. and Liu W.C. (2018) Serum long noncoding RNA urothelial carcinoma-associated 1: A novel biomarker for diagnosis and prognosis of hepatocellular carcinoma. J. Int. Med. Res. 46, 348–356 10.1177/0300060517726441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xue M., Pang H., Li X., Li H., Pan J. and Chen W. (2016) Long non-coding RNA urothelial cancer-associated 1 promotes bladder cancer cell migration and invasion by way of the hsa-miR-145-ZEB1/2-FSCN1 pathway. Cancer Sci. 107, 18–27 10.1111/cas.12844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao J.N., Yan T.H., Yu R.M.. et al. (2017) Long non-coding RNA UCA1 regulates the expression of Snail2 by miR-203 to promote hepatocellular carcinoma progression. J. Cancer Res. Clin. Oncol. 143, 981–990 10.1007/s00432-017-2370-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Z., Li X., Wu S., Xue M. and Chen W. (2014) Long non-coding RNA UCA1 promotes glycolysis by upregulating hexokinase 2 through the mTOR-STAT3/microRNA143 pathway. Cancer Sci. 105, 951–955 10.1111/cas.12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pan J., Li X., Wu W.. et al. (2016) Long non-coding RNA UCA1 promotes cisplatin/gemcitabine resistance through CREB modulating miR-196a-5p in bladder cancer cells. Cancer Lett. 382, 64–76 10.1016/j.canlet.2016.08.015 [DOI] [PubMed] [Google Scholar]
- 52.Xiao C., Wu C.H. and Hu H.Z. (2016) LncRNA UCA1 promotes epithelial-mesenchymal transition (EMT) of breast cancer cells via enhancing Wnt/beta-catenin signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 20, 2819–2824 [PubMed] [Google Scholar]
- 53.Fan Y., Shen B., Tan M.. et al. (2014) Long non-coding RNA UCA1 increases chemoresistance of bladder cancer cells by regulating Wnt signaling. Febs. J. 281, 1750–1758 10.1111/febs.12737 [DOI] [PubMed] [Google Scholar]
- 54.Wang Z.Q., He C.Y., Hu L.. et al. (2017) Long noncoding RNA UCA1 promotes tumour metastasis by inducing GRK2 degradation in gastric cancer. Cancer Lett. 408, 10–21 10.1016/j.canlet.2017.08.013 [DOI] [PubMed] [Google Scholar]