Abstract
It has been shown that CD40 is required for optimal B cell activation. Casitas-B-lineage lymphoma-b (Cbl-b), a RING finger E3 ubiquitin ligase, inhibits B cell activation. In this report, we demonstrate that CD40 stimulation markedly enhances IgM-induced B cell proliferation in wild-type (WT) mice, whereas this cell proliferation was reduced in CD40-deficient (Cd40−/−) mice. Interestingly, CD40 ligation strongly augments IgM-induced Cbl-b ubiquitination and degradation in primary mouse B cells, which closely correlates with their proliferation capacity. Cbl-b deficiency uncouples BCR-induced B cell proliferation from CD40 costimulation. Our results indicate that Cbl-b negatively regulates costimulation of BCR and CD40, possibly by setting the threshold for B cell activation via controlling Cbl-b expression.
Highlights
-
•
CD40 is required for optimal B cell activation.
-
•
B cell activation threshold is controlled by Cbl-b.
-
•
CD40 costimulation promotes Cbl-b ubiquitination and degradation.
1. Introduction
It has been documented that autoreactive B cells can be regulated via the induction of a state of hyporesponsiveness or anergy [[1], [2], [3]]. In this mechanism of tolerance, autoreactive B cells are inactivated so that they fail to participate in an immune response. The breakdown of peripheral B cell tolerance leads to the development of autoimmune disease [1], and it has been reported that autoreactive B cells become activated if they receive help from T cells. Similar to T cells, optimal B cell activation requires two signals [4]. The autoreactive B cells encounter their antigens (Ags) in the periphery (signal 1) and, if provided with T cell help (signal 2), are activated to produce antibody (Ab) [[2], [3], [4]]. However, if the autoreactive B cells do not receive T cell help (i.e., they receive signal 1 without signal 2), an overall anergic state ensues [2,4]. CD40 plays an essential role in this process as blocking CD40−CD40 ligand (CD40L) interaction suppresses the follicular entry of autoreactive B cells [4]. The interaction of CD40 on B cells with the CD40L on activated CD4+ T cells is the key process in the initiation of Ab responses to T-dependent Ags [5]. BCR or CD40 stimulation is weakly mitogenic alone, while signals generated through CD40, together with BCR, synergistically unleash the program of activation and proliferation of B cells, isotype switching, germinal center (GC) formation, and B cell memory development [3,6,7]. It has also been shown that high-affinity rheumatoid factor production by autoreactive B cells in rheumatoid arthritis is dependent on CD40−CD40L interaction [8]. The critical role of CD40 in autoimmunity is supported by the fact that Cd40−/− mice are resistant to several models of autoimmune diseases [9,10].
Cbl-b, a RING finger E3 ubiquitin ligase, consists of a phosphotyrosine-binding domain (PTB), a RING finger domain, a C-terminal proline-rich region with potential tyrosine phosphorylation sites, and a ubiquitin-associated region (UBA) [[11], [12], [13]]. We and others have demonstrated that CD28 controls the threshold for T cell activation by targeting Cbl-b for ubiquitination [[14], [15], [16]], which is mediated by protein tyrosine phosphatase SHP-1 or PKC-θ [16,17]. In B cells, Cbl-b has been shown to inhibit CD40-induced B cell responses by hindering the recruitment of TRAF-2 to CD40, thus attenuating CD40-mediated NF-κB and JNK activation [6]. Whether Cbl-b also controls the B cell activation threshold is currently unknown.
In this study, we show that BCR/CD40 costimulation induces stronger ubiquitination and degradation of Cbl-b than that of BCR stimulation alone. Consistent with this finding, BCR-induced Cbl-b degradation is significantly reduced in Cd40−/− B cells compared to wild-type (WT) B cells. The levels of Cbl-b expression in WT and Cd40−/− B cells correlate with their rates of B cell proliferation, and Cbl-b deficiency completely restores defective Cd40−/− B cell proliferation induced by IgM stimulation. Taken together, our data indicate that Cbl-b is a checkpoint regulator of BCR and CD40 signaling pathways.
2. Methods
2.1. Mice
Female WT and Cd40−/− BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Cblb–/– mice were described previously (29) and have been backcrossed to BALB/c mice 12 generations. Cblb–/– mice were bred with Cd40−/− mice to obtain Cblb–/–Cd40−/− double mutant mice. Mice used for experiments were aged from 6 to 12 weeks and all procedures and care of the animals were in accordance with guidelines provided by the Institutional Animal Care and Use Committees of the Hunan Normal University, Central South University, and University of Iowa.
2.2. Reagents
Anti-mouse IgM F(ab’)2 fragment antibodies were purchased from Zymed (San Francisco,CA). Anti-mouse CD40 (clone 3/23) was purchased from BD PharMingen (San Diego, CA). Abs against Cbl-b, Lyn, Fyn, PI3-K (p85), and ubiquitin (Ub), were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Murine B cell isolation kit was obtained from Miltenyi Biotec. (Auburn, CA). HRP-conjugated goat anti-rabbit IgG or rabbit anti-mouse IgG were purchased from Kirkegaard & Perry Laboratories (Gaithersburg, MD).
2.3. B cell isolation and activation
Splenic B cells from WT and Cd40−/− mice were isolated (purity > 95% as determined by FACS analysis of B220 cell surface expression) using a B cell isolation kit (AutoMACS; Miltenyi Biotec.). Contaminating T cells were less than 1% as determined by CD3 staining. For in vitro activation, B cells (5 × 106/ml) were stimulated for various time periods indicated by F(ab’)2 anti-IgM (2 μg/ml) and/or anti-CD40 (2 μg/ml) monoclonal Abs (mAbs). The cells were lysed in 1% NP-40 lysis buffer [10 mM Tris, pH 7.5, 150 mM NaCl, 2 mM EGTA, 50 mM β-glycerophosphate, 2 mM Na3VO4, 10 mM NaF, 1 mM dithiothreitol (DTT), 1 mM PMSF, 10 μg/ml leupeptin, and 10 μg/ml aprotinin] or in radioimmunoprecipitation assay (RIPA) buffer (25 mM Tris, pH 7.4, 150 mM NaCl, 5 mM EDTA, 1% NP-40, 1% sodium deoxycholate, 1% SDS, 100 μM Na3VO4, 1 mM NaF, 1 mM PMSF, 10 μg/ml aprotonin, 10 μg/ml leupeptin) where indicated.
2.4. B cell proliferation assay
Splenic B cells (1 × 106/ml) from WT and Cd40−/− or WT, Cd40−/−, Cblb–/–, and Cblb–/–Cd40−/− mice were cultured for 56 h at 37oC in round-bottomed 96-well plates with F(ab’)2 anti-IgM with or without anti-CD40 mAb as indicated. The cells were pulsed with 1 μCi [3H]-thymidine, and harvested 16 h later. The radioactivity was quantitated using a Wallac 1205 Betaplate™ β-liquid scintillation counter (Perkin Elmer-Wallac, Gaithersburg, MD).
2.5. Immunoprecipitation and western blotting
Protein concentrations in the cell lysates were determined using a bicinchoninic acid assay kit (Pierce, Rockford, IL). Cell lysates were precleared, postnuclear cell lysates were normalized for protein concentration levels, and immunoprecipitated (3 h, 4oC) with the specific polyclonal Abs or control isotype-matched preimmune immunoglobulin coupled to protein A CL-4B Sepharose (Pharmacia Biotech Inc., Sweden). The immunoprecipitates were resolved on SDS-PAGE and transferred to nitrocellulose membranes (Hybond C Super, Amersham, Piscataway, NJ). In some cases, the cell lysates were blotted with specific Abs. Blots were blocked for 1 h at room temperature in PBS containing 2% BSA and 0.05% Tween-20. Membranes were incubated overnight with specific Abs, then washed 3x in PBS containing 0.05% Tween-20, and detected using HRP-conjugated goat anti-rabbit IgG or rabbit anti-mouse IgG. After 3 washes in PBS containing 0.05% Tween-20, signals were revealed by enhanced chemiluminescence detection system (Amersham) and visualized by autoradiography.
2.6. Statistical analysis
Data are expressed as the mean ± SD. Student's t-test is used to determine differences between groups. The level of significance is set as *p < 0.05, **p < 0.01.
3. Results and discussion
3.1. Loss of Cbl-b relieves B cells from the requirement for CD40 costimulation
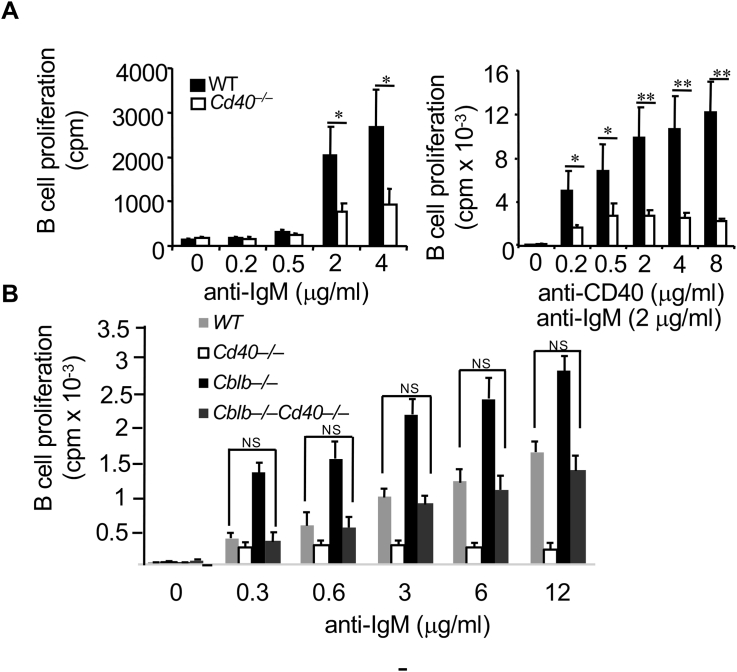
It has been shown optimal B cell activation requires two signals: one from the BCR and another one from costimulatory receptors [3,5]. BCR and CD40 synergize in B cell activation [7,18] where CD40 costimulation may provide the second signal for optimal B cell activation [5]. To confirm this, WT and Cd40−/− splenic B cells were stimulated for 72 h with different doses of F(ab’)2 anti-IgM or anti-CD40, or both, and B cell proliferation was determined by [3H]thymidine incorporation. As shown in Fig. 1, CD40 costimulation strongly promoted IgM-induced B cell proliferation in WT B cells, whereas B cell proliferation was significantly reduced in Cd40−/− mice.
Fig. 1.
Loss of Cbl-b completely restores IgM-induced B cell proliferation in Cd40−/− mice. A. Splenic B cells (2 × 105/ml) from WT and Cd40−/− BALB/c mice were stimulated for 72 h with different concentrations of F(ab’)2anti-IgM mAb (left panel) or constant anti-IgM (2 μg/ml) with different concentrations of anti-CD40 mAb (0.2–8 μg/ml) (right panel). B cell proliferation was determined by [3H]-thymidine incorporation (meansSD). B. Splenic B cells (2 × 106) from WT, Cd40−/−, Cblb–/– and Cblb–/–Cd40−/− mice were stimulated for 72 h with F(ab’)2anti-IgM (2 μg/ml). B cell proliferation was determined. Data represent mean ± SD cpm for B cells from three individual mice. *p < 0.05; **p < 0.01; Student t-test.
Previously we have shown that Cblb–/– B cells hyper-proliferate in responses to BCR and CD40 stimulation [6], suggesting that Cbl-b negatively regulates B cell activation. Since loss of Cbl-b results in hyper-proliferation of B cells in response to IgM and CD40 stimulation [6], we tested the hypothesis that loss of Cbl-b restores the proliferation of Cd40−/− B cells. To this end, we introduced Cbl-b deficiency into Cd40−/− mice. B cells were isolated from WT, Cd40−/−, and Cblb–/–Cd40−/− mice, and stimulated with F(ab’)2 anti-IgM and B cell proliferation was determined as in Fig. 1A. Indeed, loss of Cbl-b fully restored IgM-induced B cell proliferation to WT levels in Cd40−/− mice (Fig. 1B). These results suggest that loss of Cbl-b uncouples the requirement of CD40 costimulation for B cell proliferation.
3.2. CD40 stimulation promotes BCR-induced Cbl-b ubiquitination
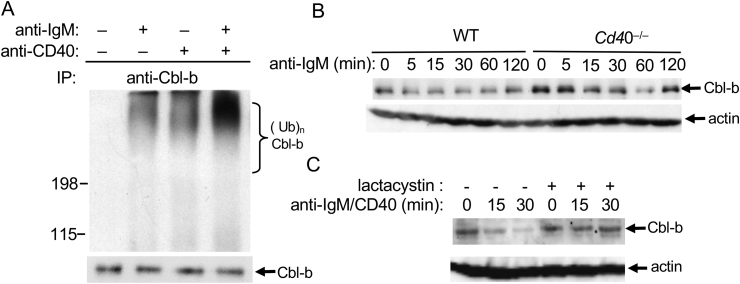
We have previously shown that CD28 costimulation of T cells can promote Cbl-b ubiquitination and degradation [15]. To investigate whether this effect on Cbl-b also occurs in CD40-mediated B cell activation, WT splenic B cells were stimulated with F(ab’)2 anti-IgM, with or without anti-CD40. Cell lysates were immunoprecipitated with anti-Cbl-b and blotted with anti-Ub mAb. Ligation of either IgM or CD40 induced weak Cbl-b ubiquitination (Fig. 2A), whereas co-ligation of IgM and CD40 synergistically promoted Cbl-b ubiquitination (Fig. 2A). Although Cbl-b was equally expressed in resting B cells from WT and Cd40−/− mice, Cbl-b degradation kinetics was significantly slower in B cells from Cd40−/− mice compared to WT B cells in response to IgM stimulation (Fig. 2B). Cbl-b degradation occurred in a proteasome-dependent pathway since a proteasome inhibitor, lactacystin, completely abrogated Cbl-b degradation (Fig. 2C).
Fig. 2.
CD40 stimulation enhances Cbl-b ubiquitination induced by IgM stimulation in B cells. A. Splenic B cells (5 × 106) from BALB/C mice were stimulated for 5 min with different concentrations of F(ab’)2anti-IgM (2 μg/ml) and anti-CD40 (2 μg/ml), or both, and lysed in RIPA buffer. The cell lysates were immunoprecipitated with anti-Cbl-b, and blotted with anti-Ub mAb. B. Splenic B cells (5 × 106) from WT and Cd40−/− mice were stimulated for 5, 15, 30, 60, and 120 min with F(ab’)2anti-IgM (2 μg/ml) and anti-CD40 (2 μg/ml), and lysed. Cbl-b expression was determined by immunoblotting. C. WT B cells were pre-treated for 1 h with lactacystin (20 μM), stimulated for 0, 15 and 30 min with F(ab’)2anti-IgM and anti-CD40, and lysed. Cell lysates were blotted with anti-Cbl-b mAb. These data are representative of three independent experiments.
3.3. Cbl-b degradation is not a general phenomenon associated with CD40 costimulation
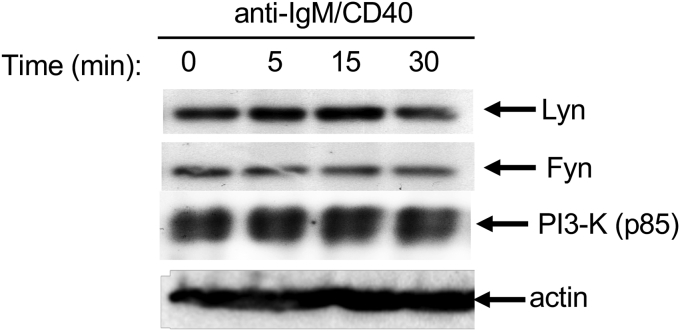
It is possible that Cbl-b ubiquitination and degradation is only a general phenomenon associated with CD40 costimulation, and many molecules may also undergo ubiquitination and degradation. To address whether this is the case, WT B cells were stimulated for different times with F(ab’)2 anti-IgM and anti-CD40. At each time-point, cells were collected, and lysed. The cell lysates were blotted with anti-Lyn, anti-Fyn, and anti-PI3-K (p85), revealing that none of these proteins was degraded (Fig. 3). Our data therefore indicate that ligation of CD40 may favor elimination of the negative regulator Cbl-b, thus allowing optimal activation of B cells.
Fig. 3.
Cbl-b degradation may not be a general phenomenon associated with CD40 costimulation. Splenic BALB/c B cells (5 × 106) were stimulated with anti-IgM and anti-CD40 for 5, 15 and 30 min, and lysed. The cell lysates were blotted with anti-Lyn, Fyn and PI3-K (p85), respectively. The data shown is representative of three independent experiments.
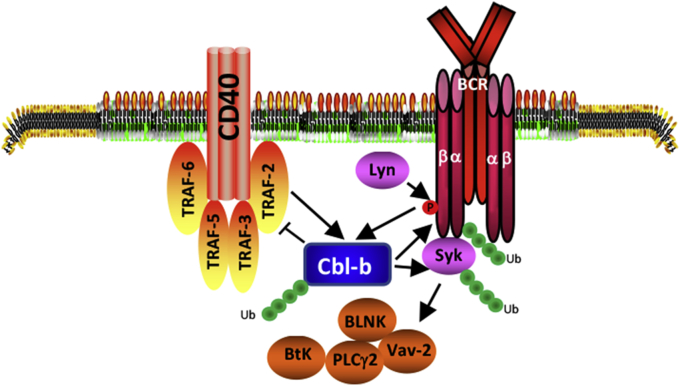
In summary, our data provide the first evidence for Cbl-b in BCR and CD40-mediated signaling pathways. Cbl-b deficiency uncouples the requirement for CD40 costimulation for B cells proliferation. Moreover, Cbl-b itself undergoes ubiquitination upon BCR stimulation, and CD40 costimulation significantly enhances BCR-induced Cbl-b ubiquitination which correlates with its costimulatory capacity for B cell activation (Fig. 4).
Fig. 4.
Schematic model for Cbl-b in BCR- and CD40-mediated signaling pathways Activation of CD40-dependent signaling pathway is mediated primarily by several TNFR-associated factor (TRAF) protein family members including TRAF2, 3, 5, and 6. TRAFs serve as adaptor proteins that connect the cytoplasmic domain of CD40 to downstream effector molecules, such as NF-κB, JNK and p38 MAPK. CD40 stimulation may also activate several protein tyrosine kinases (PTKs) including Syk and Btk in B cells. Cbl-b inhibits the recruitment of TRAF-2 to CD40, and targets Igα, Igβ, and Syk for ubiquitination, thus inhibiting both BCR- and CD40-mediated signaling pathways. CD40 costimulation facilitates BCR-induced Cbl-b ubiquitination and degradation, thus eliminating the negative regulator Cbl-b from the BCR- and CD40-signaling pathways, and allowing optimal B cell activation to occur.
Acknowledgements
We thank Dr. Wallace Y. Langdon for critical reading and editing of the manuscript. This work was supported in part by grants from the National Institutes of Health (K02 AR49047, R01 AR49775, R01 AI090901, and R01 AI123253 to JZ).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2019.100641.
Contributor Information
Rushi Liu, Email: 709382795@qq.com.
Jian Zhang, Email: jian-zhang@uiowa.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Goodnow C.C. Balancing immunity and tolerance: deleting and tuning lymphocyte repertoires. Proc. Natl. Acad. Sci. U. S. A. 1996;93:2264–2271. doi: 10.1073/pnas.93.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buhlmann J.E., Foy T.M., Aruffo A., Crassi K.M., Ledbetter J.A., Green W.R., Xu J.C., Shultz L.D., Roopesian D., Flavell R.A. In the absence of a CD40 signal, B cells are tolerogenic. Immunity. 1995;2:645–653. doi: 10.1016/1074-7613(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 3.Elgueta R., Benson M.J., de Vries V.C., Wasiuk A., Guo Y., Noelle R.J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seo S.J., Fields M.L., Buckler J.L., Reed A.J., Mandik-Nayak L., Nish S.A., Noelle R.J., Turka L.A., Finkelman F.D., Caton A.J., Erikson J. The impact of T helper and T regulatory cells on the regulation of anti-double-stranded DNA B cells. Immunity. 2002;16:535–546. doi: 10.1016/s1074-7613(02)00298-4. [DOI] [PubMed] [Google Scholar]
- 5.Lee B.O., Moyron-Quiroz J., Rangel-Moreno J., Kusser K.L., Hartson L., Sprague F., Lund F.E., Randall T.D. CD40, but not CD154, expression on B cells is necessary for optimal primary B cell responses. J. Immunol. 2003;171:5707–5717. doi: 10.4049/jimmunol.171.11.5707. [DOI] [PubMed] [Google Scholar]
- 6.Qiao G., Lei M., Li Z., Sun Y., Minto A., Fu Y.X., Ying H., Quigg R.J., Zhang J. Negative regulation of CD40-mediated B cell responses by E3 ubiquitin ligase Casitas-B-lineage lymphoma protein-B. J. Immunol. 2007;179:4473–4479. doi: 10.4049/jimmunol.179.7.4473. [DOI] [PubMed] [Google Scholar]
- 7.Ying H., Li Z., Yang L., Zhang J. Syk mediates BCR- and CD40-signaling integration during B cell activation. Immunobiology. 2011;216:566–570. doi: 10.1016/j.imbio.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyburz D., Corr M., Brinson D.C., Von Damm A., Tighe H., Carson D.A. Human rheumatoid factor production is dependent on CD40 signaling and autoantigen. J. Immunol. 1999;163:3116–3122. [PubMed] [Google Scholar]
- 9.Grewal I.S., Foellmer H.G., Grewal K.D., Xu J., Hardardottir F., Baron J.L., Janeway C.A., Jr., Flavell R.A. Requirement for CD40 ligand in costimulation induction, T cell activation, and experimental allergic encephalomyelitis. Science. 1996;273:1864–1867. doi: 10.1126/science.273.5283.1864. [DOI] [PubMed] [Google Scholar]
- 10.Gerritse K., Laman J.D., Noelle R.J., Aruffo A., Ledbetter J.A., Boersma W.J., Claassen E. CD40-CD40 ligand interactions in experimental allergic encephalomyelitis and multiple sclerosis. Proc. Natl. Acad. Sci. U. S. A. 1996;93:2499–2504. doi: 10.1073/pnas.93.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Q., Zhou H., Langdon W.Y., Zhang J. E3 ubiquitin ligase Cbl-b in innate and adaptive immunity. Cell Cycle. 2014;13:1875–1884. doi: 10.4161/cc.29213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang R., Langdon W.Y., Zhang J. Cell Immunol; 2018. Regulation of Immune Responses by E3 Ubiquitin Ligase Cbl-B.https://doi:10.1016/j.cellimm.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J. Ubiquitin ligases in T cell activation and autoimmunity. Clin. Immunol. 2004;111:234–240. doi: 10.1016/j.clim.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J., Bardos T., Li D., Gal I., Vermes C., Xu J., Mikecz K., Finnegan A., Lipkowitz S., Glant T.T. Cutting edge: regulation of T cell activation threshold by CD28 costimulation through targeting Cbl-b for ubiquitination. J. Immunol. 2002;169:2236–2240. doi: 10.4049/jimmunol.169.5.2236. [DOI] [PubMed] [Google Scholar]
- 15.Li D., Gal I., Vermes C., Alegre M.L., Chong A.S., Chen L., Shao Q., Adarichev V., Xu X., Koreny T., Mikecz K., Finnegan A., Glant T.T., Zhang J. Cutting edge: Cbl-b: one of the key molecules tuning CD28- and CTLA-4-mediated T cell costimulation. J. Immunol. 2004;173:7135–7139. doi: 10.4049/jimmunol.173.12.7135. [DOI] [PubMed] [Google Scholar]
- 16.Gruber T., Hermann-Kleiter N., Hinterleitner R., Fresser F., Schneider R., Gastl G., Penninger J.M., Baier G. PKC-theta modulates the strength of T cell responses by targeting Cbl-b for ubiquitination and degradation. Sci. Signal. 2009;2 doi: 10.1126/scisignal.2000046. ra30. [DOI] [PubMed] [Google Scholar]
- 17.Xiao Y., Qiao G., Tang J., Tang R., Guo H., Warwar S., Langdon W.Y., Tao L., Zhang J. Protein tyrosine phosphatase SHP-1 modulates T cell responses by controlling Cbl-b degradation. J. Immunol. 2015;195:4218–4227. doi: 10.4049/jimmunol.1501200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haxhinasto S.A., Bishop G.A. Synergistic B cell activation by CD40 and the B cell antigen receptor: role of B lymphocyte antigen receptor-mediated kinase activation and tumor necrosis factor receptor-associated factor regulation. J. Biol. Chem. 2004;279:2575–2582. doi: 10.1074/jbc.M310628200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.