Abstract
Postharvest senescence in broccoli (Brassica oleracea L. var Italica) florets results in phenotypic changes similar to those seen in developmental leaf senescence. To compare these two processes in more detail, we investigated molecular and biochemical changes in broccoli florets stored at two different temperatures after harvest. We found that storage at cooler temperatures delayed the symptoms of senescence at both the biochemical and gene expression levels. Changes in key biochemical components (lipids, protein, and chlorophyll) and in gene expression patterns occurred in the harvested tissue well before any visible signs of senescence were detected. Using previously identified senescence-enhanced genes and also newly isolated, differentially expressed genes, we found that the majority of these showed a similar enhancement of expression in postharvest broccoli as in developmental leaf senescence. At the biochemical level, a rapid loss of membrane fatty acids was detected after harvest, when stored at room temperature. However, there was no corresponding increase in levels of lipid peroxidation products. This, together with an increased expression of protective antioxidant genes, indicated that, in the initial stages of postharvest senescence, an orderly dismantling of the cellular constituents occurs, using the available lipid as an energy source. Postharvest changes in broccoli florets, therefore, show many similarities to the processes of developmental leaf senescence.
Senescence in plants is a complex, highly regulated process that involves a decline in photosynthesis, dismantling of chloroplasts, degradation of macromolecules such as proteins, nucleic acids and lipids, loss of chlorophyll, and mobilization of nutrients to developing parts of the plant (Smart, 1994; Buchanan-Wollaston, 1997). Over the last few years, the study of leaf senescence has resulted in the identification of a large number of senescence-enhanced genes (for review, see Buchanan-Wollaston, 1997; Gan and Amasino, 1997) and characterization of these has helped to elucidate some of the processes that are occurring.
Vegetables that are harvested when immature, before growth has ceased, are subjected to considerable stress due to the sudden disruption in energy, nutrient, and hormone supplies (Huber, 1987; King et al., 1990). Produce such as asparagus and broccoli consequently senesces rapidly on storage and has a very short shelf life. The physiological, biochemical, and molecular events that occur in this rapid deterioration merit more attention since, in general, more focus has been placed on the analysis of postharvest changes in mature fruit tissues that senesce more slowly.
Broccoli (Brassica oleracea L. var Italica) is an important vegetable with floral heads composed of hundreds of immature florets arranged in whorls on a fleshy stem. Each floret consists of an immature flower enclosed within chlorophyll-containing sepals, and it is the chlorophyll degradation within these sepals that results in the rapid yellowing of the heads during storage (Tian et al., 1994; Pogson et al., 1995). There has been extensive research into methods for improving the shelf life of broccoli by delaying postharvest senescence. Various techniques, including modified atmosphere (Makhlouf et al., 1989; Zhuang et al., 1994), different types of packaging (Zhuang et al., 1994; Toivonen, 1997), treatment with chemicals and cytokinins (Wang, 1977; Rushing, 1990; Downs et al., 1997), and refrigeration (Gillies and Toivonen, 1995) have been investigated with varying degrees of success. However, few studies on the genetic elements that could control postharvest senescence in this crop have been carried out. Differences in storage quality between genotypes have been observed, indicating that genetic factors are likely to be involved (Toivonen and Sweeney, 1998). Also changes in expression patterns of certain genes, such as those encoding 1-amino-cyclopropane-1-carboxylic acid oxidase, which are involved in ethylene biosynthesis, have been shown in senescing broccoli florets (Pogson et al., 1995). Ethylene appears to have an important role in regulating the yellowing of sepals after harvest, since chlorophyll loss is associated with an increase in floret ethylene synthesis (Tian et al., 1994). It was proposed that the increase in 1-amino-cyclopropane-1-carboxylic acid oxidase levels may contribute to ethylene production in the florets and thus regulate aspects of postharvest senescence (Pogson et al., 1995).
Many of the changes seen during storage of green vegetables, such as loss of chlorophyll, deterioration of cellular structure, and, finally, cell death, show similarities to changes seen during developmental leaf senescence. In this paper, we have analyzed postharvest senescence in broccoli florets and compare the process with developmental leaf senescence. Our aims were: (a) to investigate gene expression in postharvest broccoli and correlate this with some of the key biochemical changes that occur; (b) to compare these molecular events with those in leaf senescence; and (c) to identify novel genes expressed in postharvest broccoli.
RESULTS
Phenotypic Changes in Harvested Plant Material
Broccoli heads were harvested from field-grown plant trials grown at Horticulture Research International (Wellesbourne, Warwickshire, UK) during the summer of 1998 and stored in plastic bags at either room temperature or at 4°C. At regular intervals after harvest, samples were taken for RNA isolation and evaluation of various biochemical parameters (changes in lipids, chlorophyll, and protein). The material stored at room temperature remained visibly green until d 3 and then deteriorated, showing rapid yellowing between d 4 and 5. The material sampled at d 5 was uniformly yellow. In contrast, the material stored at 4°C showed no signs of yellowing until d 11 and even at d 18 was not uniformly yellow. Thus, low temperature markedly delays visible senescence in broccoli.
Changes in Lipids, Chlorophyll, and Proteins during Postharvest Storage
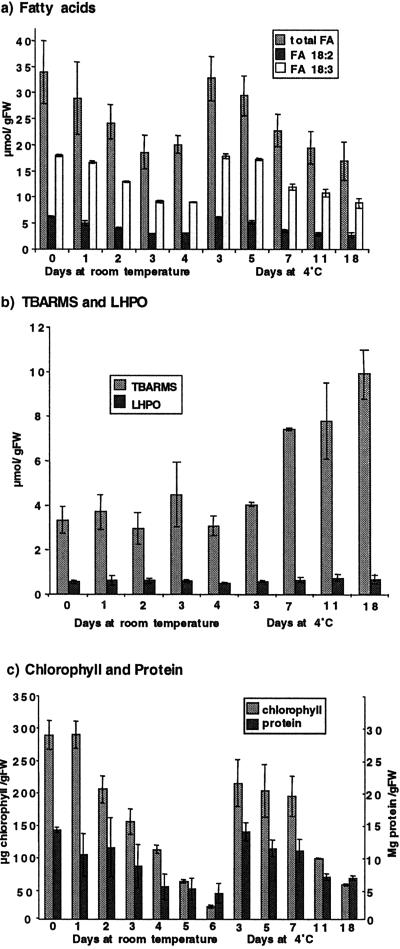
It has been suggested that lipid degradation and peroxidation are early events in the postharvest deterioration of broccoli (Zhuang et al., 1997). To investigate this claim, we used three independent methods to measure the extent of lipid peroxidation during storage, namely (a) measurement of decreases in the polyunsaturated fatty acid content, (b) assessment of the level of primary oxidation products, fatty acid (lipid-linked) hydroperoxides, and (c) measurement of thiobarbituric acid reactive materials (TBARMs, a relatively stable end product of lipid and other macromolecular-derived peroxidation reactions). The major fatty acid components of freshly harvested broccoli florets were α-linolenic acid (C18:3, Δ9, 12, 15; 53% [v/v]), linoleic acid (C18:2, Δ9, 12; 18%[v/v]), and palmitic acid (C16:0, 16%[v/v]) with small amounts of other C16 unsaturated fatty acids (C16:1, C16:2, and C16:3), stearate (C18:0), and oleate (C18:1) (data not shown). Following storage at room temperature for 3 d, the total fatty acids level and the levels of the C18:3 and C18:2 declined by approximately 50% (Fig. 1a). In comparison, when the broccoli heads were stored at 4°C, the loss of fatty acids was considerably delayed with 18:2 levels declining to 50% of original value (day of harvest) by d 14 and 18:3 declining to this level by d 18 (Fig. 1a). The rapid decline in fatty acids in the first 3 d, from tissues stored at room temperature, preceded visible signs of yellowing.
Figure 1.
Biochemical analysis of changes in postharvest broccoli. a, Fatty acid levels were measured by gas liquid chromatography in broccoli florets removed from heads that had been stored for different times at room temperature (up to 4 d) or at 4°C (up to 18 d). Total fatty acids, linolenic (18:2), and linoleic (18:3) per gram fresh weight were measured, and the data shown are the result of two different experiments (where n = 3 for each experiment) and include se bars. b, Lipid peroxidation products LHPO and TBARMs were measured in broccoli florets removed from heads that had been stored at room temperature or at 4°C. Results shown with se bars and from two different experiments (where n = 3 for each experiment). c, Chlorophyll and protein levels were measured in broccoli florets removed from heads that had been stored at room temperature or at 4°C (n = 3 ± se). Chlorophyll levels are measured as μg/g fresh weight shown on the axis on the left, and protein levels were measured as mg/g fresh weight shown on the axis on the right.
The metabolic fate of the fatty acids was investigated by measuring levels of the fatty acid hydroperoxides and TBARMs. The level of hydroperoxides remained almost constant and at similar levels in material stored under both temperature regimes (Fig. 1b). Because the total polyunsaturated fatty acids declined and hydroperoxide levels remained almost constant, the data expressed as a percent of total fatty acids oxidized in the tissue show that the basal level of oxidized fatty acids in freshly harvested broccoli was 1.7% and this increased to a maximum of 4.5% by d 3. At room temperature the TBARM levels measured in the stored material remained fairly constant; at 4°C, the levels increased approximately 3-fold over 18 d.
Chlorophyll and protein levels showed a rapid decline in the broccoli florets stored at room temperature; after 6 d of storage, chlorophyll levels had fallen by more than 90% and protein levels by over 70% (Fig. 1c). The reduction in levels of chlorophyll and protein that occurred during storage were similar to those previously reported (Zhuang et al., 1997). Both chlorophyll and protein were degraded at a slower rate in the material stored at 4°C when compared with the material stored at room temperature. The chlorophyll content declined markedly (by approximately 50%) after 3 d of storage at room temperature (although this was not evident on visual inspection of the tissue), whereas in samples maintained at 4°C, significant chlorophyll loss was only evident at d 11 postharvest. A similar retardation of protein loss was also observed (Fig. 1c).
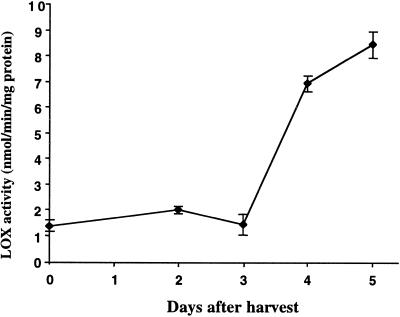
To determine whether lipoxygenases (LOX) played a role in the turnover of lipids in the plant material stored at room temperature, extracts of tissues were prepared and assayed for LOX activity using linoleic acid as a substrate (Fig. 2). LOX activity remained largely unchanged over the first 3 d of storage (during the time when fatty acids were rapidly declining in the tissues) and then increased markedly between d 3 and 5. Thus, LOX activity does not appear to be related to the early turnover of the C18:2 and C18:3 fatty acids during postharvest storage.
Figure 2.
Change in LOX activity during storage of broccoli at room temperature. LOX activity was measured in broccoli florets removed from heads that had been stored for different times at room temperature using linoleic acid as a substrate. Results are combined from two experiments (where n = 3 for each experiment) and ses are shown.
Gene Expression Postharvest
A number of cDNA clones representing genes that show enhanced expression during leaf senescence in Brassica napus have been identified by differential screening and subtractive hybridization (Buchanan-Wollaston, 1994; Hanfrey et al., 1996; Buchanan-Wollaston and Ainsworth, 1997). To examine whether genes expressed during leaf senescence in B. napus are also expressed during postharvest senescence in broccoli, we used a selection of the clones for RNA-blot analysis. Expression of these genes was also examined in mature green leaves and senescing leaves of broccoli.
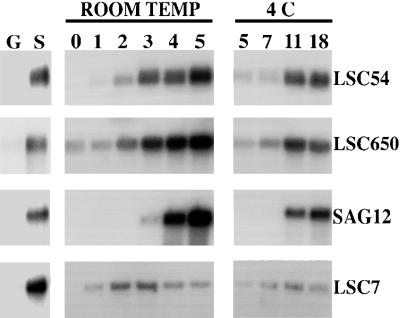
All four of the B. napus cDNA clones tested showed significantly increased levels of expression in senescing broccoli leaves compared with the mature green leaves (Fig. 3). An increase in transcript levels in the senescing harvested florets was also observed in all cases with the different genes showing a range of expression patterns. The expression pattern detected for each gene was similar whether the florets were stored at room temperature or at 4°C, but the increase in mRNA levels was delayed under the cooler conditions.
Figure 3.
RNA-blot analysis in broccoli florets postharvest. RNA-blot analysis showing transcript levels of four different leaf senescence enhanced genes in RNA isolated from broccoli florets at different times of storage at room temperature (0, 1, 2, 3, 4, and 5 d) and 4°C (5, 7, 11, and 18 d). G, Green leaves; S, senescent leaves. Ten micrograms of RNA was separated on formaldehyde agarose gels, blotted to nylon membrane, and hybridized with 32P-labeled cDNA fragments. LSC54, metallothionein; LSC650, catalase; SAG12, Cys protease; LSC7, Cys protease.
LSC54 encodes a metallothionein protein (Buchanan-Wollaston, 1994). In postharvest broccoli this gene showed induced expression first detectable at d 2 at room temperature. LSC650 encodes a catalase (Buchanan-Wollaston and Ainsworth, 1997). This gene was expressed at a low level in florets at d 0, and transcript levels started to increase 2 d after harvest with the highest level of expression being observed at d 4 and 5. Both genes are expressed before the florets showed any visible signs of deterioration. SAG12 is a gene originally identified in Arabidopsis, encoding a Cys protease, which is highly senescence specific, only being expressed in yellowing tissue (Lohman et al., 1994; Noh and Amasino, 1999). Expression of this gene was induced later in postharvest senescence than LSC650 and LSC54; under room temperature conditions expression is just detectable 3 d after harvest. Strongly induced expression of SAG12 was not seen until the broccoli florets were showing signs of yellowing (d 4). LSC7, also a Cys protease (Buchanan-Wollaston, 1997), showed a different pattern of expression to the other genes analyzed. Increased levels of transcript were detected only 1 d after harvest, with a further increase at d 2 and 3. However, the levels then declined in the yellowing tissue at d 4 and 5. LSC7 expression in the tissue stored at 4°C showed a small increase between 5 and 11 d, which then declined slightly by d 18.
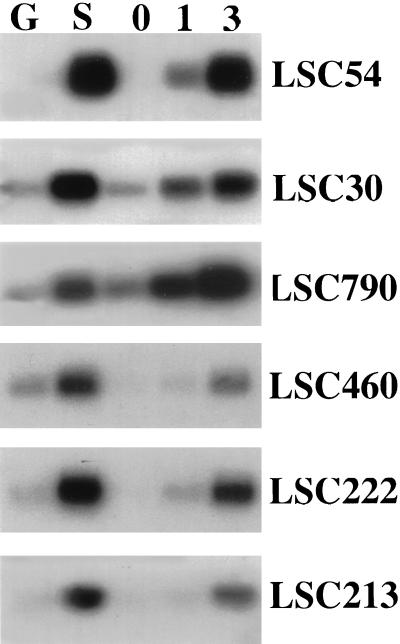
The expression analysis was extended to examine a number of other genes shown to be senescence enhanced in B. napus (Buchanan-Wollaston, 1997). We carried out these experiments by cDNA-blot hybridization, using membranes carrying cDNA made from five of the RNA samples described above. This method increases the sensitivity of the analysis. cDNA was made from RNA isolated from mature green leaves, senescing leaves, and broccoli florets sampled on the day of harvest and those stored at room temperature for 1 and 3 d after harvest. The cDNA was then hybridized with a selection of B. napus senescence-enhanced genes (Fig. 4). In all cases, the genes tested showed enhanced levels of expression in both senescing leaves and in postharvest broccoli florets. LSC54 was included in this experiment to allow comparison with the data shown in Figure 3. The hybridization pattern seen with LSC54 was comparable with that shown in the RNA-blot experiments except that, due to the increased sensitivity of this method, enhanced expression of LSC54 was clearly detectable 1 d after harvest. Two of the other genes, LSC30 (ferritin) and LSC790 (a third type of Cys protease) also showed a significant increase in expression 1 d after harvest. Other genes such as LSC460 (Gln synthetase), LSC222 (chitinase), and LSC213 (pyruvate ortho phosphate dikinase) showed a very small increase after d 1; however, a larger enhancement of expression was detected between d 1 and 3. In general, expression levels of all genes were higher in the d 3 tissue.
Figure 4.
Expression of leaf senescence-enhanced genes in broccoli postharvest. cDNA-blot analysis of gene expression. cDNA was synthesized from a selection of the RNA samples isolated from green (G) and senescing (S) leaves and florets from broccoli heads stored for 0, 1, and 3 d at room temperature. PCR-amplified cDNA (1 μg) was separated on an agarose gel, blotted to nylon membrane, and hybridized with 32P-labeled cDNA fragments representing different senescence enhanced genes. LSC54, metallothionein; LSC30, ferritin; LSC790, Cys protease; LSC460, Gln synthetase; LSC222, chitinase; LSC213, pyruvate ortho phosphate dikinase.
Identification of Novel Genes Expressed during Postharvest
All genes tested that had been identified previously as leaf senescence-enhanced in B. napus, also showed enhanced expression in postharvest broccoli (Figs. 3 and 4). This was somewhat unexpected because, although postharvest senescence shows many similarities to developmental leaf senescence, we were anticipating some differences in gene expression patterns. Therefore, we decided to use cDNA amplified fragment length polymorphism (AFLP) techniques to identify novel transcripts showing senescence-enhanced expression in postharvest broccoli. Using this technique, it is possible to compare the expression of a large number of gene products simultaneously using RNA isolated from several different tissues (Bachem et al., 1996). We used cDNA made from RNA isolated from the five tissues described above (green and senescing leaves and florets 0, 1, and 3 d after harvest) as the template for the AFLP analysis. Genes showing differential expression patterns were excised from the gel, cloned, and sequenced.
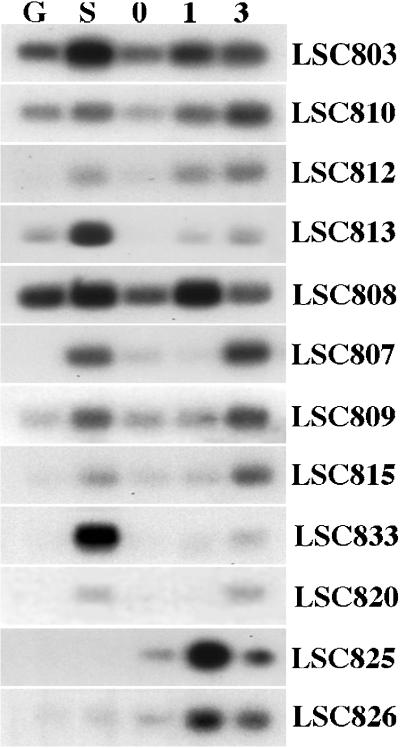
The sequences of most of the genes isolated showed close similarity with regions of the Arabidopsis genomic sequence (Table I). Only one of the genes (LSC 807, encoding an aspartic protease) had been previously isolated as a senescence-enhanced gene (Buchanan-Wollaston and Ainsworth, 1997). The other genes encode a range of different proteins, some of which could be assigned potential functions in senescence. For example, LSC803 has similarity to a phospholipid hydroperoxide dependent glutathione peroxidase that is likely to have a role in protection against oxidative stress. LSC833 encodes a vacuolar processing Cys protease (γVPE) similar to SAG23, a senescence-enhanced gene identified in Arabidopsis (Quirino et al., 1999) and See2, the gene that was identified by Smart et al. (1995) as being senescence enhanced in maize. This enzyme may have a role in the activation of hydrolytic enzymes stored in the vacuole. Other genes such as LSC808, similar to an RNA-binding protein, LSC809, encoding a cytochrome P450, LSC810, a putative Pro-rich APG, LSC815, similar to a signal recognition particle receptor protein, LSC820, a putative ABC transporter, and LSC826, some similarity to a histone deacetylase, may encode known proteins, but the role of these in senescence cannot easily be postulated. The other genes (LSC812, LSC813, and LSC825) have no putative homologs.
Table I.
Genes showing increased expression in broccoli florets postharvest
| Gene Name | Accession No. | Possible Function (by Similarity to Known Genes) | Equivalent Arabidopsis Gene and/or Reference |
|---|---|---|---|
| LSC803 | AJ293420 | Phospholipid hydroperoxide dependent glutathione peroxidase | AB001568. Sugimoto and Sakamoto (1997) |
| LSC807 | X80067 | Aspartic protease | Buchanan-Wollaston and Ainsworth (1997) |
| LSC808 | AJ293421 | Putative RNA binding protein | At4g20030 |
| LSC809 | AJ293422 | Cytochrome P450 | At2g46960 or At2g46950 |
| LSC810 | AJ293423 | Proline rich protein. APG precursor | T4012.11 |
| LSC812 | AJ293424 | Unknown | None |
| LSC813 | AJ293425 | Unknown | P1 clone, MEB5 |
| LSC815 | AJ293426 | Signal recognition particle receptor-like protein | At4g30600 |
| LSC820 | AJ293427 | Putative ABC transporter | F23H11.19 |
| LSC825 | AJ293428 | Unknown protein | MJK13.11 |
| LSC826 | AJ293429 | Histone deacetylase | T1N24.9 |
| LSC833 | AJ293430 | Gamma vacuolar processing enzyme | D61395. Kinoshita et al. (1995) |
The sequences of cDNA fragments that detected enhanced expression in postharvest broccoli florets were compared with sequences in the DNA databases. Potential Arabidopsis homologues were detected, some of which showed similarity to known genes.
The expression patterns of the cDNA clones identified were then analyzed on blots carrying cDNA from broccoli leaves and florets (as described above) (Fig. 5). The majority of genes showing enhanced expression in postharvest broccoli were also enhanced in senescing leaves. However, differences in expression patterns were observed with some of the genes.
Figure 5.
Expression of newly isolated genes in broccoli postharvest. cDNA-blot analysis of gene expression. Membranes prepared with the same samples as described for Figure 4 were hybridized with 32P-labeled fragments from the newly identified AFLP clones that are described in Table I.
The genes LSC803, LSC810, LSC812, and LSC813 all showed senescence-enhanced expression both in leaves and in postharvest broccoli florets. Enhanced expression of these four genes was detectable 1 d after harvest with little further increase at d 3. LSC810 was expressed in green leaves with only a small increase seen in the senescing leaves. However, expression of this gene showed a more significant enhancement in the postharvest broccoli florets. LSC808 also showed more increase in expression in the postharvest broccoli than it did in the senescing leaves. This gene was only enhanced at d 1; transcript levels were lower by d 3. Other genes identified, LSC807, LSC809, LSC815, LSC820, and LSC833, also showed clear senescence-enhanced expression in leaves and florets, but expression in florets was not enhanced until d 3. The third type of expression pattern was seen with the clones representing the genes LSC825 and LSC826. Expression of these genes was barely detectable in green or senescing leaves (LSC826 showed a very low level of expression in senescing leaves) but both genes showed enhanced expression in postharvest broccoli florets, particularly 1 d after harvest.
DISCUSSION
The degradative processes that occur in harvested leaves and organs, such as broccoli florets, show many similarities to the events that occur during natural leaf senescence. Chlorophyll degradation is the most obvious visual change, and this is accompanied by losses in membrane lipid and proteins eventually resulting in cell death. The main role of the process of leaf senescence during development is to mobilize nutrients from the leaf for use in other parts of the plant. However, in harvested broccoli, senescence is induced artificially, probably as a stress response resulting from the removal of nutrient supplies. Therefore, the gene expression patterns induced may be significantly different from those observed in natural developmental senescence. The stress experienced by a harvested immature broccoli head could be considered to be comparable with that occurring in a leaf that has been detached from the plant. Previous studies examining gene expression in detached leaves have shown that, although many senescence-enhanced genes were induced, there were also significant differences in expression patterns (Becker and Apel, 1993; Weaver et al., 1998). The Cys protease gene SAG12, for example, was only very weakly induced in detached Arabidopsis leaves (Weaver et al., 1998). In our studies we have shown that all the senescence-enhanced genes tested (including SAG12) also showed increased expression in postharvest broccoli indicating a close similarity between developmental leaf senescence and postharvest senescence in this system.
Lipid degradation is a common feature of many tissues undergoing senescence. Earlier studies with broccoli florets, stored at 5°C, showed that fatty acid levels decreased during postharvest senescence, and levels of peroxidation products increased at both 5°C and room temperature (Zhuang et al., 1995, 1997). These authors concluded that lipid peroxidation was correlated with the rapid deterioration of broccoli in storage. The nature of this relationship has been examined more closely in the present study. When broccoli florets were stored at room temperature, the lipid loss (primarily resulting from a loss of the main polyunsaturated fatty acids, linolenic acid, and linoleic acid) was not accompanied by a corresponding increase in the levels of primary oxidation products, namely fatty acid hydroperoxides or by an increase in the levels of a secondary end products of peroxidation measured as TBARMs. Thus, lipid peroxidation per se appears to be low in these tissues. The metabolic fate of the C18:2 and C18:3 fatty acids was not followed further but it is likely that they serve as substrates for β-oxidation for the generation of acetyl coenzyme A to be used in respiration or in gluconeogenesis. The intermediates of this β-oxidation spiral would not have been detected in either the FOX2 or the TBARM assays. That fatty acids released from degrading membranes can be converted back to Glc for transport or respiration via the glyoxylate pathway and gluconeogenesis is supported by the enhanced expression of LSC213. LSC213 encodes pyruvate orthophosphate dikinase, an enzyme in the gluconeogenesis pathway, which catalyzes the formation of phosphoenolpyruvate from pyruvate. Senescence-enhanced expression of this gene has previously been observed in B. napus (Buchanan-Wollaston, 1997) and maize (Smart et al., 1995). Also, in rice expression of a cytosolic pyruvate orthophosphate dikinase has been shown to increase in seedlings subjected to water stress (Moons et al., 1998).
In contrast to the observations made on the material stored at room temperature, we observed a significant increase in the TBARM content in tissues stored at 4°C similar to that reported by Zhuang et al. (1995). The reasons for this difference are not clear. Because TBARMs can arise from non-lipid sources it is possible that other macromolecules have undergone peroxidation. Also, differences in the respiration rate as a simple function of temperature would be expected. This may lead to a diversion of metabolites into different pathways (β-oxidation versus lipid peroxidation) with resultant differences in the pattern of TBARM accumulation.
The activity of LOX, the enzyme that generates fatty acid hydroperoxides, was low in the broccoli samples during the first 3 d of storage at room temperature, which indicates that the enzymatic generation of hydroperoxides would be low in this tissue. Furthermore, expression of the phospholipid hydroperoxide glutathione peroxidase (LSC 803) gene, which encodes an enzyme involved in the detoxification of fatty acid hydroperoxides (Beeor-Tzahar et al., 1995), is up-regulated and this would also reduce the endogenous hydroperoxide content. There are at least three genes encoding this enzyme in Arabidopsis; LSC803 is most closely similar (92% DNA sequence identity) to the cytosolic gene that was identified by Sugimoto and Sakamoto (1997) and shown to be induced in response to salt and other oxidative stress treatments. This gene has not been shown previously to be expressed during senescence.
Lipid peroxidation is also stimulated by iron. A build up of unbound ferrous/ferric ions is detrimental as they promote hydroxyl radical formation and rapid oxidative damage (Alscher et al., 1997). The observed up regulation of the LSC30 gene, encoding ferritin, which sequesters this metal, may also limit lipid peroxidation. In addition, we have shown that other genes that might play an antioxidant role in senescing leaves, also demonstrate senescence-enhanced expression in postharvest broccoli florets. These include LSC650, which encodes a catalase, and LSC54, which encodes a metallothionein (Buchanan-Wollaston, 1994).
Protein degradation is rapid in harvested broccoli and the expression patterns of several protease genes are senescence enhanced. These include the Cys protease encoding genes SAG12, LSC790, and LSC7 and the aspartic protease gene LSC807. LSC833 encodes a protein similar to a vacuolar-processing enzyme, γVPE, from Arabidopsis, which is thought to be involved in converting vacuolar proteins to their more mature forms. Kinoshita et al. (1999) also found that the expression of γVPE was up-regulated during senescence and under stress conditions and suggested that vegetative vacuolar processing enzymes (VPEs) may regulate the activation of senescence-associated hydrolytic enzymes in the lytic vacuoles of cells undergoing senescence.
Cytoplasmic Gln synthetase (GS1) is thought to have a role in the conversion of free amino acids released from degraded proteins during senescence into Gln for transport (Kamachi et al., 1991). Studies have shown that ammonium levels increase in senescing broccoli and also Gln and Asn accumulate to high levels during senescence (King and Morris, 1994). Our results show an increased expression of the LSC460 gene, which encodes GS1 (Buchanan-Wollaston and Ainsworth, 1997), indicating that as the florets deteriorate and protein degradation progresses, there is a requirement for greater Gln synthetase activity to convert ammonia to Gln.
The results presented here indicate that the processes occurring in harvested broccoli, especially during the first 3 d at room temperature, are highly comparable with the degradative and mobilization processes that occur during leaf senescence. The mobilization of membrane lipids is accompanied by the enhanced expression of genes encoding enzymes, which would act to limit lipid peroxidation and offer antioxidant protection. The coordinated catabolism of proteins, similarly, is evident from the senescence-enhanced expression of protease genes. It is noteworthy that the molecular and biochemical changes commence in the harvested broccoli tissue well before any visible signs of senescence. Within 24 h of harvesting, the expression of many senescence-enhanced genes has been induced. Storage at cooler temperatures delays the symptoms of senescence at the biochemical level and in relation to gene expression patterns. Thus, postharvest senescence, like natural leaf senescence, is under genetic control and future identification of the key genes that control this process may allow the manipulation of postharvest quality attributes.
MATERIALS AND METHODS
Plant Material
Field-grown Brassica oleracea cv Marathon was used as a source of material for RNA isolation and biochemical analyses. Mature green leaves and senescing leaves were harvested in the field and immediately frozen in liquid nitrogen. Mature broccoli (B. oleracea L. var Italica) heads were harvested and stored in the dark at either room temperature (20°C) or at 4°C. These heads were firm and compact in texture with no signs of yellowing or flower opening. At intervals after harvest, the outer flower buds were trimmed from the heads, and this material was analyzed. No stem material was included in the samples. For the RNA isolation and protein and chlorophyll assays, this material was immediately frozen in liquid nitrogen, and frozen material was then stored at −70°C until use. For lipid determinations and LOX activity, freshly harvested material was used throughout.
Chemicals
Ammonium ferrous sulfate, butylated hydroxytoluene (BHT), xylenol orange [o-cresol-sulfonphthalein-3,3-bis (methyliminodiacetic acid sodium salt)], catalase, triphenylphosphine (TPP), and linoleic acid were purchased from Sigma-Aldrich (Poole, Dorset, UK). All reagents were of the highest purity available.
RNA Isolation
RNA was isolated from mature green leaves and senescing leaves. RNA was also isolated from broccoli florets collected on the day of harvest, florets stored at room temperature for 1, 2, 3, 4, and 5 d after harvest, and florets stored at 4°C for 5, 7, 11, and 18 d after harvest. The RNA was isolated as described in Buchanan-Wollaston (1994).
RNA Blotting and Hybridization
This was carried out as described by Buchanan-Wollaston and Ainsworth (1997).
cDNA Synthesis and AFLP
First and second strand cDNA was synthesized from total RNA isolated from green and senescing leaves and florets stored at room temperature for 0, 1, and 3 d, using the SMART PCR cDNA Synthesis Kit (CLONTECH Laboratories, Palo Alto, CA). One microgram of RNA was used as a template. The cDNA was digested with MseI and MseI adapters (Life Technologies/Gibco-BRL, Cleveland) were ligated to the digested DNA. The cDNA was then used in AFLP reactions using a method based on the AFLP analysis system 1 (Life Technologies/Gibco-BRL). Selective primers based on the MseI adapter + 3 sequence were used with the SMART PCR primer (end labeled with 33P) to amplify a subset of cDNA fragments. Samples were denatured and separated on 6% (w/v) acrylamide gels and visualized by autoradiography. Bands were eluted in 100 μL of elution buffer (0.5 m ammonium acetate, 10 mm magnesium acetate, 1 mm EDTA, pH 8, 0.1% [w/v] SDS) at 37°C overnight. The eluted DNA was purified by spin chromatography, ethanol precipitated, and dissolved in 10 μL of Tris-EDTA. The DNA was ligated into the pGEM T-vector (Promega, Madison, WI) and transformed into JM109 (Promega).
cDNA Blots
PCR amplification of the cDNA for 20 cycles, using MSE1 core primer and the SMART PCR primer, provided the cDNA for the cDNA blots (CLONTECH Laboratories, Smart PCR cDNA kit, User Manual). PCR-amplified cDNA was run on 1.5% (w/v) agarose gels and blotted on to nylon membranes (Hybond N+, Amersham) using 0.4 m NaOH. Filters were hybridized with 32P-labeled, PCR-amplified cDNA fragments as described above.
DNA Sequence Analysis
Sequencing was carried out using a DNA sequencing kit (Big Dye Terminator Cycle sequencing Ready Reaction Kit, Perkin-Elmer Applied Biosystems, Foster City, CA) with an ABI 377 DNA sequencer (Perkin-Elmer Applied Biosystems). Homology searches were done using BLASTN and BLASTX programs (Altschul et al., 1997) in the National Center for Biotechnology Information database.
Preparation of FOX2 Reagent
FOX2 reagent was used to determine the lipid hydroperoxide (LHPO) content of the tissues and was prepared according to Nourooz-Zadeh et al. (1995) and Griffiths et al. (2000) by dissolving xylenol orange (Sigma-Aldrich) and ammonium ferrous sulfate in 250 mm H2SO4 to final concentrations of 1 and 2.5 mm, respectively. One volume of this concentrated reagent was added to 9 volumes of HPLC-grade methanol containing 4.4 mm BHT to make the working reagent that comprised 250 μm ammonium ferrous sulfate, 100 μm xylenol orange, 25 mm H2SO4, and 4 mm BHT in 90% (v/v) methanol. The reagents were calibrated with enzymatically synthesized 13S-hydroperoxy-9Z, 11E-octadecadienoic acid using soybean LOX (Sigma, lipoxidase type 1-S) essentially according to the method of Gardner (1997).
Lipid Extraction and LHPO Quantification
Total lipids were rapidly extracted from tissues by a modification of the method of Bligh and Dyer (1959) according to Griffiths et al. (1997, 2000). All procedures were performed in dim light at 4°C using chilled solvents (containing BHT, 0.01% [w/v]) and glassware. Florets (approximately 0.2 g fresh weight) were homogenized with a pestle and mortar containing 0.15 m acetic acid (1 mL) and chloroform/methanol (1:2 v/v; 7.5 mL) for approximately 2 min and transferred to culture tubes (Pyrex, Merck, Poole, UK). The pestle and mortar were rinsed with chloroform (2.25 mL) and combined with the extract to which was added distilled water (2.25 mL). Phase separation was facilitated by low-speed centrifugation, the lower chloroform (CHCl3) phase containing the lipids was removed, and aliquots dispensed into amber vials (Hewlett-Packard, Palo Alto, CA) and evaporated under N2. Vials were capped and stored on ice until all samples had been evaporated to dryness. Samples were resuspended in HPLC-grade methanol either in 100 μL for samples without TPP or in 90 μL of methanol to which was added 10 μL of TPP (25 mm in methanol). Samples were allowed to incubate at room temperature for 30 min in the dark and then for a further 30 min following the addition of the working FOX 2 reagent. Absorbances were determined spectrophotometrically at 560 nm, and the concentration of LHPOs determined using a molar absorption coefficient derived for standard linoleate hydroperoxide (ε = 6.0 × 104 m−1 cm−1; Gay et al., 1999).
Lipids were quantified as their fatty acid methyl ester derivatives obtained by transmethylation performed in 2.5% (v/v) sulfuric acid in anhydrous methanol (2 mL) and separated on a gas liquid chromatograph equipped with a flame ionization detector. Heptadecanoic acid (17:0) was used as the internal standard and separation was achieved on a 10% DEGS CW AW column (Jones Chromatography, Kenfig, UK) at 170°C with nitrogen (30 mL/min) as the carrier gas.
TBARM Determination
TBARM (of which malondialdehydes are considered to be a significant component) was measured using an assay modified from Hagage et al. (1990). Plant material (0.5 g) was homogenized with 1 mL of trichloroacetic acid (10% w/v). The homogenate was washed with 10 mL of acetone, vortexed, and then centrifuged at 1,300g for 15 min. The pellet was washed in 5 mL of acetone, vortexed, and then centrifuged at 1,300g for 10 min (4 times). The pellet was dried under nitrogen and incubated at 100°C for 30 min with 3 mL of H3PO4 (1% [w/v]) and 1 mL of thiobarbituric acid (0.6% [w/v]). The reaction was terminated by rapidly cooling the tubes on ice. Butan-1-ol (3 mL) was added, the mixture was vortexed, and then centrifuged to achieved separation of the phases. Absorbance of the aqueous phase was measured at 532 and 590 nm using a Uvikon 930 Spectrophotometer (Kontron Instruments, Watford, UK).
LOX Assays
Broccoli florets (2.5 g) were ground in a pestle and mortar in ice-cold ultrapure water (5 mL) and centrifuged at 12,000g for 10 min. The supernatant was removed and applied to a PD10 gel filtration column (Pharmacia, Uppsala) equilibrated with potassium phosphate buffer (50 mm, pH 5.7), the optimum pH for broccoli LOX activity (Zhuang et al., 1994). Aliquots of the eluate (equivalent to 0.2–0.3 mg of protein) were assayed in the same buffer (1 mL) using linoleic acid sodium salt (80 nmol) as substrate by following the production of conjugated dienes at 234 nm.
Chlorophyll Determination
Chlorophyll determinations were made on broccoli florets based on the method of Porra et al. (1989). Broccoli florets (0.3 g) were extracted in 10 mL of 80% (v/v) acetone. After centrifugation, absorbance (A) was read at 646.6, 663.6 and 750 nm on a Uvikon spectrophotometer (Kontron Instruments). Chlorophyll a (Chl a), chlorophyll b (Chl b) and total chlorophyll (TChl) were calculated using the following formulas: Chl a (μg mL−1) = 12.25 A663.6 − 2.55 A646.6; Chl b (μg mL−1) = 20.31 A646.6 − 4.91 A663.6; and TChl (μg mL−1) = 17.76 A646.6 + 7.34 A663.6. The readings for 663.6 and 646.6 nm were corrected for by A750 (Porra et al., 1989).
Protein Determination
Protein was extracted from broccoli florets (0.5 g) in 1.5 mL of extraction buffer (50 mm Tris [tris(hydroxymethyl)aminomethane]-HCL, pH 7.5, 2 mm EDTA, pH 8, 0.04% [v/v] mercaptoethanol). Samples were centrifuged at 12,000g for 20 min at 4°C, and proteins determined using a Coomassie protein assay reagent (Pierce Chemical, Rockford, IL) based on a modified Bradford method (Bradford, 1976) with bovine serum albumin as a standard.
ACKNOWLEDGMENTS
We would like to thank the United Kingdom Biotechnological and Biological Research Council (BBSRC) and the United Kingdom Ministry of Agriculture, Fisheries and Food (MAFF) for financial support. We thank Dr. Richard Napier for critical reading of the manuscript and Dr. David Wurr, Jayne Akehurst, and Angela Hambridge for providing the broccoli material. Technical support from M. Leverentz and N. Gill is gratefully acknowledged.
LITERATURE CITED
- Alscher RG, Donahue JL, Cramer CL. Reactive oxygen species and antioxidants: relationships in green cells. Physiol Plant. 1997;100:224–233. [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachem C, Hoeven R, de Bruijn S, Vreugdenhil D, Zabeau M, Visser R. Visualisation of differential gene expression using a novel method of RNA fingerprinting based on AFLP: analysis of gene expression during potato tuber development. Plant J. 1996;9:745–753. doi: 10.1046/j.1365-313x.1996.9050745.x. [DOI] [PubMed] [Google Scholar]
- Becker W, Apel K. Differences in gene-expression between natural and artificially induced leaf senescence. Planta. 1993;189:74–79. [Google Scholar]
- Beeor-Tzahar T, Ben-Hayyim G, Holland D, Faltin Z, Eshdat Y. A stress associated citrus protein is a distinct plant phospholipid hydroperoxide glutathione peroxidase. FEBS Lett. 1995;366:151–155. doi: 10.1016/0014-5793(95)00521-a. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid ans sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V. Isolation of cDNA clones for genes that are expressed during leaf senescence in Brassica napus. Plant Physiol. 1994;105:839–846. doi: 10.1104/pp.105.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan-Wollaston V. The molecular biology of leaf senescence. J Exp Bot. 1997;48:181–199. [Google Scholar]
- Buchanan-Wollaston V, Ainsworth C. Leaf senescence in Brassica napus: cloning of senescence related genes by subtractive hybridization. Plant Mol Biol. 1997;33:821–834. doi: 10.1023/a:1005774212410. [DOI] [PubMed] [Google Scholar]
- Downs CG, Somerfield SD, Davey MC. Cytokinin treatment delays senescence but not sucrose loss in harvested broccoli. Postharvest Biol Technol. 1997;11:93–100. [Google Scholar]
- Gan S, Amasino R. Making sense of senescence: molecular genetic regulation and manipulation of leaf senescence. Plant Physiol. 1997;113:313–319. doi: 10.1104/pp.113.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner HW. Analysis of plant lipoxygenase metabolites. In: Christie WW, editor. Advances in Lipid Methodology-Four. Dundee, UK: The Oily Press; 1997. pp. 1–43. [Google Scholar]
- Gay C, Collins J, Gebicki JM. Hydroperoxide assay with the ferric-xylenol orange complex. Anal Biochem. 1999;273:149–155. doi: 10.1006/abio.1999.4208. [DOI] [PubMed] [Google Scholar]
- Gillies SL, Toivonen PMA. Cooling method influences the postharvest quality of broccoli. HortScience. 1995;30:313–315. [Google Scholar]
- Griffiths G, Jones HE, Eaton CL, Stobart AK. Effect of n-6 polyunsaturated fatty acids on growth and lipid composition of neoplastic and non-neoplastic canine prostate epithelial cell cultures. Prostate. 1997;31:29–36. doi: 10.1002/(sici)1097-0045(19970401)31:1<29::aid-pros5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Griffiths G, Leverentz M, Silkowski H, Gill N, Sanchez-Serrano JJ. Lipid hydroperoxide levels in plant tissues. J Exp Bot. 2000;51:1363–1370. [PubMed] [Google Scholar]
- Hagage D, Nouvelot A, Boucard J, Gaspar T. Malondialdehyde titration with thiobarbiturate in plant extracts: avoidance of pigment interference. Phytochem Anal. 1990;1:86–89. [Google Scholar]
- Hanfrey C, Fife M, Buchanan-Wollaston V. Leaf senescence in Brassica napus: expression of genes encoding pathogenesis-related proteins. Plant Mol Biol. 1996;30:597–609. doi: 10.1007/BF00049334. [DOI] [PubMed] [Google Scholar]
- Huber DJ. Postharvest senescence: an introduction to the symposium. HortScience. 1987;22:853–859. [Google Scholar]
- Kamachi K, Yamaya T, Mae T, Ojima K. A role for glutamine synthetase in the remobilisation of leaf nitrogen during natural senescence in rice leaves. Plant Physiol. 1991;96:411–417. doi: 10.1104/pp.96.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GA, Morris SC. Early compositional changes during postharvest senescence of broccoli. J Am Soc Hort Sci. 1994;119:1000–1005. [Google Scholar]
- King GA, Woollard DC, Irving DE, Borst WM. Physiological changes in asparagus tips after harvest. Physiol Plant. 1990;80:393–400. [Google Scholar]
- Kinoshita T, Yamada K, Hiraiwa N, Kondo M, Nishimura M, Hara-Nishimura I. Vacuolar processing enzyme is up-regulated in the lytic vacuoles of vegetative tissues during senescence and under various stressed conditions. Plant J. 1999;19:43–53. doi: 10.1046/j.1365-313x.1999.00497.x. [DOI] [PubMed] [Google Scholar]
- Lohman KN, Gan S, John MC, Amasino RM. Molecular analysis of natural leaf senescence in Arabidopsis thaliana. Physiol Plant. 1994;92:322–328. [Google Scholar]
- Makhlouf J, Castaigne F, Arul J, Willemot C, Gosselin A. Long-term storage of broccoli under controlled atmosphere. HortScience. 1989;24:637–639. [Google Scholar]
- Moons A, Valcke R, VanMontagu M. Low-oxygen stress and water deficit induce cytosolic pyruvate orthophosphate dikinase (PPDK) expression in roots of rice, a C-3 plant. Plant J. 1998;15:89–98. doi: 10.1046/j.1365-313x.1998.00185.x. [DOI] [PubMed] [Google Scholar]
- Noh Y-S, Amasino R. Identification of a promoter region responsible for the senescence specific expression of SAG12. Plant Mol Biol. 1999;41:181–194. doi: 10.1023/a:1006342412688. [DOI] [PubMed] [Google Scholar]
- Nourooz-Zadeh J, Tajaddini-Sarmadi J, Birlouez-Aragon I, Wolff SP. Measurement of hydroperoxides in edible oils using the ferrous oxidation in xylenol orange assay. J Agric Food Chem. 1995;43:17–21. [Google Scholar]
- Pogson BJ, Downs CG, Davies KM. Differential expression of two 1-aminocyclopropane-1-carboxylic acid oxidase genes in broccoli after harvest. Plant Physiol. 1995;108:651–657. doi: 10.1104/pp.108.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedmann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochem Biophys Acta. 1989;975:384–394. [Google Scholar]
- Quirino B, Normanly J, Amasino R. Diverse range of gene activity during Arabidopsis thaliana leaf senescence includes pathogen independent induction of defense related genes. Plant Mol Biol. 1999;40:267–278. doi: 10.1023/a:1006199932265. [DOI] [PubMed] [Google Scholar]
- Rushing JW. Cytokinins affect respiration, ethylene production, and chlorophyll retention of packaged broccoli florets. HortScience. 1990;25:88–90. [Google Scholar]
- Smart CM. Gene expression during leaf senescence. New Phytol. 1994;126:419–448. doi: 10.1111/j.1469-8137.1994.tb04243.x. [DOI] [PubMed] [Google Scholar]
- Smart CM, Hosken SE, Thomas H, Greaves JA, Blair BG, Schuch W. The timing of maize leaf senescence and characterization of senescence-related cDNAs. Physiol Plant. 1995;93:673–682. [Google Scholar]
- Sugimoto M, Sakamoto W. Putative phospholipid hydroperoxide glutathione peroxidase gene from Arabidopsis thaliana induced by oxidative stress. Genes Genet Syst. 1997;72:311–316. doi: 10.1266/ggs.72.311. [DOI] [PubMed] [Google Scholar]
- Tian MS, Downs CG, Lill RE, King GA. A role for ethylene in the yellowing of broccoli after harvest. J Am Soc Hort Sci. 1994;119:276–281. [Google Scholar]
- Toivonen PMA. The effects of storage temperature, storage duration, hydro-cooling, and micro-perforated wrap on shelf life of broccoli (Brassica oleracea L., Italica Group) Postharvest Biol Technol. 1997;10:59–65. [Google Scholar]
- Toivonen PMA, Sweeney M. Differences in chlorophyll loss at 13°C for two broccoli cultivars associated with antioxidant enzyme activities. J Agric Food Chem. 1998;46:20–24. doi: 10.1021/jf970490n. [DOI] [PubMed] [Google Scholar]
- Wang CY. Effect of aminoethoxy analog of rhizobitoxine and sodium benzoate on senescence of broccoli. HortScience. 1977;12:54–56. [Google Scholar]
- Weaver LM, Gan S, Quirino B, Amasino RM. A comparison of the expression patterns of several senescence associated genes in response to stress and hormone treatment. Plant Mol Biol. 1998;37:455–469. doi: 10.1023/a:1005934428906. [DOI] [PubMed] [Google Scholar]
- Zhuang H, Barth MM, Hildebrand DF. Packaging influenced total chlorophyll, soluble protein, fatty acid composition and lipoxygenase activity in broccoli florets. J Food Sci. 1994;59:1171–1174. [Google Scholar]
- Zhuang H, Hildebrand DF, Barth MM. Senescence in broccoli buds is related to changes in lipid peroxidation. J Agric Food Chem. 1995;42:2585–2591. [Google Scholar]
- Zhuang H, Hildebrand DF, Barth MM. Temperature influenced lipid peroxidation and deterioration in broccoli buds during postharvest storage. Postharvest Biol Technol. 1997;10:49–58. [Google Scholar]