Abstract
Individuals with Turner syndrome (TS) are at risk for a constellation of neurocognitive and psychosocial differences, although there is significant individual variability in these features. TS is associated with an increased risk for difficulties with visual-spatial reasoning, visual-spatial memory, attention, executive functioning, motor, and math skills. Additionally, increased rates of social difficulties, anxiety and depression are observed. There can be significant interplay between all of these factors contributing to the behavioral phenotype. Neuropsychological features and previous research are reviewed. Clinical considerations and recommendations for evaluation and treatment of psychological and behavioral difficulties are provided, including consideration of medical features in TS, as well as therapies, educational supports, and medication treatment. Future research is needed to evaluate effectiveness of different treatments for neuropsychological and psychosocial features of TS, including modification and validation of existing evidence-based treatments and new approaches to care.
Keywords: Turner syndrome, ADHD, Anxiety, Executive Functioning, Social Development, Autism, Depression
Background:
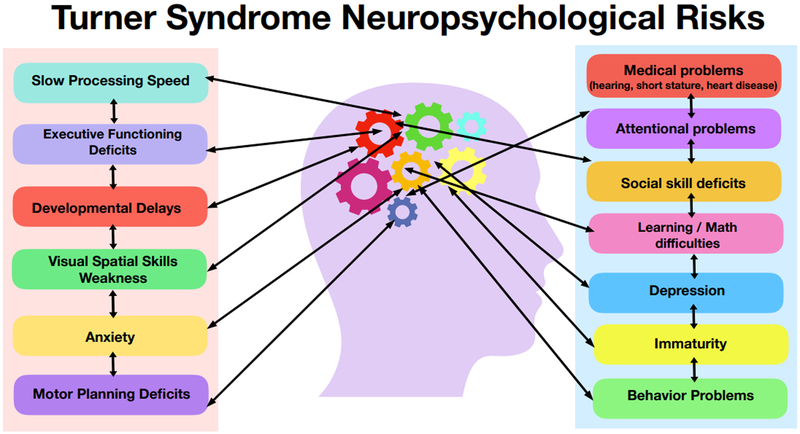
Turner syndrome (TS) is a common genetic condition which occurs in 1:2500 live born females due to the complete or partial absence of the second X-chromosome, with or without mosaicism. The physical and medical phenotype of TS has been well described to include short stature, webbed neck, characteristic facial features, cardiac defects, renal abnormalities, endocrine problems, hearing problems, and infertility. Although there is significant heterogeneity among girls with TS, there is also a commonly reported neuropsychological profile that can contribute to challenges in various aspects of daily life including academics, social, and emotional functioning. Overall cognitive functioning is typically within the average range, with a profile of relative strengths in verbal abilities and relative weakness in visuospatial skills, mathematics, and processing speed (Mazzocco, 2006). In addition, girls with TS have an increased likelihood of having executive functioning deficits, attention deficit hyperactivity disorder (ADHD), and increased symptoms of anxiety (Green et al., 2015; Kilic, Ergur, & Ocal, 2005). They may also have more vulnerability socially, with increased rates of difficulties in peer relationships (D. S. Hong, Dunkin, & Reiss, 2011; Wolstencroft & Skuse, 2018). This psychological profile contributes to a variable clinical presentation that ranges from girls who are markedly affected to those with minimal or no challenges in these different domains. While each construct (i.e. IQ, math skills, attention, anxiety) can be measured separately and diagnoses such as Specific Learning Disability in Mathematics or ADHD can be applied, when present in any combination they can interact with each other in ways that can be complex such that they may compound each other and it can be difficult to disentangle how each contributes to an individual’s unique clinical challenges. For example, behavioral symptoms of inattention may include a primary attentional deficit, however additional factors such as anxiety, slow processing speed, or learning deficits can also exacerbate attentional challenges in different settings and complicate the approach to treatments and supports. Figure 1 displays the multiple domains that can be affected in the TS neuropsychological profile to stimulate consideration of: (1) how these constructs interplay when evaluating psychological symptoms, and (2) how they contribute to the variability in the overall neuropsychological phenotype in girls with TS.
Figure 1:
Females with TS are at risk for different neuropsychological deficits and social-emotional difficulties that can interact with each other and that contribute to the variability in the overall neuropsychological phenotype in girls with TS.
In 2016, the management guidelines for Turner syndrome were revised to direct current practice in the care for girls and women with TS (Gravholt et al., 2017). These guidelines include a description of the psychological and cognitive profiles for girls with TS, acknowledging that possible educational interventions directed toward identified learning or attentional problems may improve educational outcomes, and highlight that even in the context of variable degrees and areas of learning difficulties, many girls with TS are able to successfully complete post-secondary education. Furthermore, these guidelines reference the emotional challenges faced by many girls with TS, and the increased risk for social isolation, immaturity and anxiety. Given these psychosocial risks, the current guidelines recommend initial and possibly continuous psycho-educational evaluations for cognitive, social and behavioral skills in order to ensure appropriate classroom accommodations are being considered and promote appropriate interventions to reinforce self-esteem, as well as maximize social, educational and vocational potential. This review seeks to expand upon the neuropsychological and social-emotional features described in girls with TS and further elucidate clinical recommendations for targeted evaluation and possible interventions, as well as identify additional research directions that may be pivotal in effectively addressing the psychosocial needs of girls with TS.
EARLY NEURODEVELOPMENT IN TS:
While there are limited data, what is known about the early natural history of neurodevelopment in TS comes from different cohorts of infants and young children clinically ascertained either during the prenatal period (from prenatal genetic testing performed either as part of routine genetic screening or based on structural abnormalities noted on ultrasound) or the neonatal period (often tested due to lymphedema, physical features, or findings of congenital heart disease). There is also a subgroup of children with TS who were studied as part of a larger research project performed at multiple centers in the US, Canada, and the UK where infants were identified by newborn screening (Robinson, Bender, & Linden, 1990). In all of these TS subgroups, a higher rate of developmental delays have been reported across all domains of development including fine motor, gross motor skills, and language skills. Medical problems and surgical intervention(s) in the first years of life can impact early development, and it is difficult to ascertain aspects of the developmental profile that may be related to medical features of TS. For example, infants with medical complications such as congenital heart disease requiring surgical intervention, anesthesia, and hospitalizations are at increased risk for neurodevelopmental issues (Marino et al., 2012). Further, infants and toddlers with an early environment involving frequent medical care and an essential focus on physical health maintenance have a very different experience than that of an infant/toddler without these health problems. In addition, medical features of middle ear disease and subsequent conductive hearing loss as well as sensorineural hearing loss are more common among children with TS (Kubba, Smyth, Wong, & Mason, 2017). It is important to consider the increased risk for hearing loss when a child presents with delays in language and/or social development. In the limited reports of early neurodevelopment in TS, these historical medical factors have not been frequently analyzed as potential contributors to developmental outcomes.
There is a need to better understand the natural history of neurodevelopment in infants and toddlers with TS, including the role of TS-specific medical features, early hormonal differences, genetic factors, and other environmental and family factors as predictors of differences in developmental outcomes. Clinical genetic testing has increased steadily in the past decades as genetic technology has advanced, and more recently prenatal genetic diagnoses of TS are increasing with the availability of prenatal testing of fetal DNA through maternal blood (see Bianchi 2019 in this journal). These practices have led to an increased rate of early diagnosis and a need for more detailed understanding of predictors of developmental outcomes to inform genetic counseling and to establish guidelines for early developmental assessments and clinical care for infants and toddlers with TS.
Currently, clinical practices for early developmental evaluations in TS are informed by protocols established for other populations at risk for developmental delays including premature infants or other genetic disorders. For all infants with TS, standardized screenings of developmental and social milestones/ASD at well-child care visits are recommended as per American Academy of Pediatrics (AAP) guidelines (AAP, 2006)(Zwaigenbaum et al., 2015), If screening measures show any areas of risk or “red flags” then referral for full neurodevelopmental evaluation is indicated, which could be performed by a developmental pediatrician, child psychologist, or pediatric neuropsychologist with expertise in early development, or through for community-based developmental monitoring and early intervention programs. Further, for all infants with TS, due to the overall increased risk for delays, neurodevelopmental evaluation including domains of cognitive, language, motor, social, and adaptive functioning should be performed at around 12, 24, and 36 months of age so that early intervention therapies can be initiated if delays are identified. During these evaluations, an understanding of the neurodevelopmental profile of TS is important in interpretation of results. For example, if there is early evidence of weaknesses in fine motor or pre-academic numeracy skills, then the threshold for initiation of services should be lower since these are known areas of weakness in TS that can be targeted at an early age. In the US, most early intervention therapies such as developmental therapy, speech, occupational or physical therapy from 0–3 years of age are delivered through government IDEA programs in the home setting. Private, clinic-based services for motor or speech development may also be indicated if there is lack of progress or in complex cases.
COGNITIVE ABILITIES:
A specific cognitive profile has been identified in individuals with TS, where neuropsychological studies of cognitive abilities have consistently shown a distinct profile of stronger verbal skills and weaker nonverbal/visual-spatial abilities (B. Pennington et al., 1985). Consensus is found across studies that most girls with TS have only mild decreases in full scale IQ when compared with the general population. The mean IQ in one of the largest studies of girls with TS was 94.6, contrasted with a mean IQ of 103.9 in the control group (J. F. Rovet, 1990). Multiple other studies show mean full-scale scores ranging from 92 to 102, with scores following a normal distribution. These overall full-scale scores, however, obscure the pattern found across most studies, in which girls with TS reliably perform better on verbal than nonverbal tasks, often with one or more standard deviations of difference between their verbal and nonverbal scores (J. F. Rovet, 1990). There is also a subgroup of individuals with TS with more significant cognitive involvement, and it is estimated that about 5–10 percent have overall cognitive skills in the intellectual disability range (Bender, Linden, & Robinson, 1994; Bispo et al., 2013; Swillen et al., 1993).
Studies further exploring specific nonverbal neurocognitive skills have identified relative weaknesses in spatial reasoning, mental rotations, visual discrimination, visual sequencing, and visual-spatial memory in TS compared to age-matched peers. This pattern of relatively weaker performance on visual-spatial reasoning tasks can be found as young as 4 years of age, and is generally consistent across development. While individuals continue to make gains in their visual-spatial reasoning abilities, the rate of their development in these areas often lags behind their age-matched peers. Neuroimaging studies have supported the neuropsychological findings, with reduced gray matter volume in areas associated with visual-spatial reasoning (i.e., superior parietal and the postcentral gyri) in TS compared to age-matched peers (Brown et al., 2004). See the article by Rebecca Knickmeyer in this journal collection for a review of neuroimaging studies in TS (Knickmeyer 2019).
EXECUTIVE FUNCTIONING & PROCESSING SPEED:
Specific difficulties in executive functioning skills have also been identified in individuals with TS. Executive functions are defined as a set of skills that assist with goal-directed behavior including planning, organization, task initiation, inhibition, attention, self-regulation, working memory, and sustained motivation to complete tasks. In addition to weaknesses in attention and short-term or working memory, increased rates of difficulties with inhibition, flexibility, organization, and planning can also be observed in girls and women with TS (Green et al., 2015; J. Ross, Zinn, & McCauley, 2000; J. F. Rovet, 1990; Temple, 2002). Executive function challenges can continue to affect how well an individual can complete daily tasks throughout life, and formal supports and/or interventions for difficulties with executive functioning (e.g. organization and planning) can be beneficial for individuals with TS in school, vocational, and home settings. (Martin, Quintin, Hall, & Reiss, 2016)
Individuals with TS are also at a greater risk for reduced processing speed (Kesler et al., 2004; J. L. Ross et al., 2002; Temple, 2002). Accommodations for the reduced processing speed (e.g. extended time for classwork and examinations) are likely beneficial for most individuals with TS.
ATTENTION:
Individuals with TS are also at increased risk for difficulties with sustained attention (Green et al., 2015). A significant increase in rates of Attention-Deficit/Hyperactivity Disorder (ADHD) have been reported in individuals with TS, with approximately 25 percent of individuals with TS meeting criteria for ADHD compared to approximately 1.3 percent of the general female population (Russell et al., 2006). Interestingly, there appears to be a higher risk for the Hyperactive/Impulsive subtype of ADHD in girls with TS, which is less common in females and in the general population overall (Green et al., 2015; Russell et al., 2006).
Standardized questionnaires that screen for symptoms of ADHD are recommended as part of the behavioral and psychosocial evaluation during routine TS behavioral health care, and most primary care practices are familiar with use of these measures such as the NICHQ Vanderbilt Assessment Scales, the Conners-3 Rating Scales, or other DSM-5 based symptom rating scales. It is important to consider that other factors commonly seen in TS such as hearing loss, learning disabilities, and anxiety can present with behaviors that appear to be inattentive, and thus it is important that these other domains are also considered for their contribution to ADHD symptoms since these problems are not likely to improve with ADHD treatments. When there are sufficient impairments in attention span, distractibility, hyperactivity, and/or impulsivity such that these symptoms are causing impairment in academic functioning and daily life, then applying a diagnosis of ADHD is important to guide supports and treatment recommendations for these challenges.
Currently, recommendations for the treatment of ADHD in the general population includes the combination of behavioral supports in the academic and home settings with medication treatment for ADHD symptoms (Wolraich et al., 2011). There have not been specific studies of ADHD treatments in TS, and the standard recommendations for ADHD treatments and supports should be applied in TS with some additional considerations. Clinical experience across multiple TS centers supports that ADHD medication is often a critical component in a successful ADHD treatment plan, however medical history and current cardiac and growth status need to be considered when deciding on the type of ADHD medication used due to possible side effects as elaborated below.
ADHD medications fall in categories of stimulant and nonstimulant medications. Stimulant medications can be effective, however consultation with cardiology should occur prior to beginning stimulant medications since there can be cardiac side effects in patients with a history of structural heart malformations or arrythmias, and there is also an increased risk of elevated blood pressure with stimulant medications (Hamilton et al., 2012). Stimulants can also decrease appetite and thus weight and growth should be monitored closely to make sure that caloric intake is sufficient to support ongoing growth (especially if also treated with growth hormone). Nonstimulant medications such as guanfacine or clonidine are other options for ADHD medication treatment that can also be effective in TS, and may be selected in various clinical situations such as young age (<6 years), inability to take stimulants due to side effects or health history, comorbid anxiety, or parental/patient preference. Buproprion or venlafaxine may also be considered, and can also be helpful in patients with comorbid anxiety. Due to genetic differences and lack of research specific to TS, medication treatment should begin at conservative doses and be increased slowly but persistently to obtain symptom control while monitoring side effects.
ACADEMIC SKILLS:
Research has suggested that up to 75 percent of women with TS will experience some level of math difficulties (Mazzocco, 2009), including greater risk for difficulties with math calculation and applied math skills. Difficulties with understanding early math and numeracy concepts can be seen during early school years for some children, but become most apparent as developmental demands increase. More specifically, difficulties with number recognition or understanding of 1:1 number correspondence issues can be seen in preschool and kindergarten, with reduced understanding of time and money concepts. In early grade school, response time for retrieving math facts can be longer compared to controls (Dennis, Berch, & Mazzocco, 2009). As math concepts increase in complexity, girls and young women with TS tend to require additional time and repetition to acquire specific math skills. Additionally, specific areas of math (e.g., geometry) can be especially difficult due to additional visual perceptual demands.
A better understanding of primary numeracy skills and concepts underlying math and visual-spatial learning has been explored in TS to better understand the math and visual spatial difficulties. For example, subitization is the ability to visually “see” a small number of objects without counting them, and has been identified as a core deficit in girls with TS compared to age-matched peers (Simon et al., 2008). This weakness in subitization is a component of an overall reduced neuropsychological “spatiotemporal resolution” that has been described in TS (Beaton et al., 2010). This term describes a reduced ability to perceive spatial details at the same resolution as peers when looking at an image or scene in the visual field (how much spatial detail can be resolved per unit of distance), and also a weakness in the how much detail can be perceived in a certain amount of time. Understanding these primary deficits in TS is important so that specific interventions can be developed including specific neuropsychological exercises directed at improving these core deficits, as well as teaching alternative strategies to their areas of weakness.
When considering academic reading and writing domains in TS, most individuals with TS will demonstrate at or above age expected performance in reading decoding and spelling skills. However, reading comprehension and reading tasks requiring a student to form hypotheses, make predictions, or understand nonliteral language may be challenging (Temple & Carney, 1996). While neurodevelopmental differences resulting from TS genetic differences are the primary factors contributing to difficulties in academic skills in this population, it is also important to consider other familial genetic factors when considering an individual’s risk for learning disabilities. Thus, a family history of learning disabilities will often compound with the TS learning profile to cause more severe academic difficulties. Alternatively, strengths in familial academic and cognitive abilities may also serve as protective factors contributing to overall academic and cognitive outcomes in TS.
Given the complexity of neuropsychological and academic profiles, current recommendations for TS include routine neuropsychological assessments at several time points throughout childhood and adolescence, including in preschool, upon school entry, and then at transition timepoints from elementary to middle school, middle to high school, and high school to post-secondary education (Gravholt et al., 2017; Heilbronner et al., 2010). Many children and young women with TS benefit from modifications and accommodations to the general educational curriculum, (e.g., additional academic instruction in math, extra time on tests due to slower processing speeds or attentional deficits) or academically-related therapeutic interventions (e.g., occupational therapy for motor skills). These are most commonly provided through special educational services with development of an Individualized Education Plan (IEP) specific to the needs of the student. Many individuals with TS also benefit from formal academic accommodations for difficulties with attention and executive functioning, which can be provided through an IEP or a Section 504 Plan. Such accommodations can include preferential seating, distraction-free test environment, extended time for assignments, or assistance with planning and organizational skills. Women with TS in the college and university settings may also continue to benefit from programming and supports offered through the academic support or learning resource centers that exist at most institutions of higher education to support students with additional learning needs. These programs can help to facilitate services and supports such as additional counseling, tutoring, and accommodations such as extended time for examinations. While IEPs do not transfer to post-secondary settings, most colleges and universities outline formal academic accommodations through Section 504 Plans.
MOTOR SKILLS:
Individuals with TS are also at increased risk for difficulties with motor functioning, of which fine motor problems can become apparent during early school years as they are beginning to learn how to write. Various aspects of challenges with motor coordination, motor speed, timing, and processing speed have also been described (M. W. Nijhuis-van der Sanden, Eling, & Otten, 2003; R. W. Nijhuis-van der Sanden, Smits-Engelsman, & Eling, 2000; Salbenblatt, Meyers, Bender, Linden, & Robinson, 1989), which can sometimes affect participation in sports or activities requiring high levels of motor proficiency. Occupational and/or physical therapy evaluations and treatments can be helpful for all types of motor skills difficulties, either for development of home-based strategies and exercises, or for direct therapy when needed.
LANGUAGE AND SOCIAL DEVELOPMENT:
Although verbal skills in general are repeatedly found to be similar to same-aged peers and an area of relative strength in people with TS (J. Rovet, 1993; J. F. Rovet, 1990), certain components of language development appear to be impacted by the social and executive function deficits in TS. For example, there can be difficulties with understanding the big picture or gestalt during reading or other verbal tasks, with a subsequent focus on details rather than the larger story.
Language, reading and verbal tasks that require sustained attention or complex search strategies can tax weaker executive function skills, resulting in below age-expected performance. Further, verbal tasks that require extensive use of spatial constructs including sequence and temporal relationships can also appear impaired (D. Hong, Scaletta Kent, & Kesler, 2009). Because social deficits can also be present in individuals with TS, it has been hypothesized that pragmatic aspects of language (i.e. higher level social language skills) may also be impaired and underlie some of the social difficulties. There is very limited research on social aspects of language in individuals with TS, however one study investigating brief conversational ability did not show differences between the TS and control groups (Mazzocco et al., 2006). In contrast to many other common genetic syndromes (Fragile X, Down syndrome, Trisomy X) and autism spectrum disorder, pragmatic language abilities have not yet been well-studied in TS.
Social skills, however, have received more attention by TS researchers. There is research demonstrating that many aspects of social functioning may be different in some people with TS (D. S. Hong et al., 2011; Wolstencroft & Skuse, 2018), including difficulties forming and maintaining social relationships (McCauley, Sybert, & Ehrhardt, 1986), having fewer close friends (Lagrou et al., 2006), and being seen as less socially competent than their peers (McCauley, Feuillan, Kushner, & Ross, 2001). Specific components of social processing that have been studied showed people with TS to have higher risk for impairments in areas of: facial recognition (Romans, Stefanatos, Roeltgen, Kushner, & Ross, 1998; J. L. Ross, Kushner, & Zinn, 1997), facial matching and fear recognition (D. S. Hong, Bray, Haas, Hoeft, & Reiss, 2014; Lawrence, Kuntsi, Coleman, Campbell, & Skuse, 2003; Mazzola et al., 2006), gaze processing (Elgar, Campbell, & Skuse, 2002; Lawrence, Campbell, et al., 2003) and interpretation of subtle social cues (McCauley, Kay, Ito, & Treder, 1987). Findings of the above research studies provide support for development of a social skills intervention specifically for TS targeted at improving skills in these areas of deficit, however this has not yet been accomplished. Many schools, medical centers, and psychology practices offer more general social skills programs or therapies that may be appropriate for helping individuals with TS, and it is recommended that families share background information about TS and these previous research findings to professionals leading the treatment so that these domains can receive additional attention according to individual need.
Currently, when social deficits are present, the question of whether these deficits are consistent with a diagnosis of autism spectrum disorder often arises. At least one study has found the incidence of autism not to be increased in TS (Lepage, Lortie, Deal, & Théoret, 2014), while an older study suggested a sharply increased incidence in a smaller sample (Creswell & Skuse, 1999). However, even in the general population autism spectrum disorder is increasingly common, occurring in approximately 1 in 151 girls in the US (Baio et al., 2018). And while there is no reason to assume people with TS would be at decreased risk, given the complexity of the social deficits that have been identified there is likely an increased risk compared to the general population. It is therefore important that clinicians be familiar with the typical social deficits found in TS, and not fail to diagnose ASD when it is present. Therefore, children with TS who have social skills deficits should receive the same screenings for ASD as children without TS (Zwaigenbaum et al., 2015), and further professional evaluation should be completed if concerns exist using clinical history and sensitive instruments such as the ADOS-2 (Lord & Rutter, 2012). Overall, the social deficits in TS tend to be more subtle compared to idiopathic ASD, with immaturity and social anxiety as prominent features (Swillen et al., 1993). A diagnosis of ASD requires both social communication deficits and restricted/repetitive patterns of behavior (RRB) (Association, 2013), and behaviors in the RRB domain are typically less common in TS. However, if ASD is present then treatment programs for ASD should be utilized, with professionals, educators and therapists considering the TS phenotype as well as the child’s individual strengths and weaknesses when developing individualized treatment plans.
Many of the evidence-based social skills curriculums developed for children and adolescents with high-functioning ASD may also be helpful to address some of the social difficulties in TS since they also often target areas such as emotion recognition and reading social cues, however research studies to validate these programs in TS are needed to determine if they are effective or require modifications for the TS population. Techniques such as The Hidden Curriculum by Brenda Smith Myles (Myles, Trautma, & Schelvan, 2013), Michelle Garcia Winner’s extensive “Social Detective” work (Crooke & Winner, 2016; Winner, 2018), and strategies in the book “The Science of Making Friends”, by Elizabeth Laugeson may be a good fit for often highly verbal girls with TS (Laugeson, 2013).
ANXIETY AND DEPRESSION IN TURNER SYNDROME:
The lifetime incidence of anxiety or depression in Turner syndrome is as high as 52%, a sharp increase when compared to the population incidence of these conditions in women (Cardoso et al., 2004; Lesniak-Karpiak, Mazzocco, & Ross, 2003; Schmidt et al., 2006). Overall, many subtypes of anxiety have been reported, including generalized anxiety, social anxiety, specific phobias, and obsessive-compulsive behaviors (Cardoso et al., 2004; El Abd, Patton, Turk, Hoey, & Howlin, 1999; Kilic et al., 2005; Moonga, Pinkhasov, & Singh, 2017). Despite many descriptive studies of anxiety and self-concept in TS (Kilic et al., 2005; Schmidt et al., 2006), a more precise breakdown of anxiety often results in conflicting information and may be affected by methodology including a reliance on self-report (Kilic et al., 2005; Lagrou et al., 1998). Because of the frequency of social anxiety in individuals with TS, it is important to consider that social desirability may be a confounding factor in the response pattern of self-report measures.
The interplay of social deficits and other psychological and medical domains affected in TS also need to be considered in evaluation of anxiety and depression. A lack of success with peers can increase social anxiety, and unfortunately this may decrease social practice and become a vicious cycle leading to more social isolation and symptoms of depression. If attention and difficulty with math make certain aspects of academics more difficult, test anxiety and performance/social anxiety (especially fear of being called on in class) can add to the anxiety burden of the school experience, and subsequently also decrease attentional abilities and/or academic performance. Because of their medical needs, individuals with TS may also experience medical or health anxiety. Frequent medical procedures and medical visits, blood draws for hormone levels or growth hormone injections can sometimes result in fears in medical settings, specific needle phobias, or symptoms of depression.
Standardized questionnaires that screen for symptoms of anxiety and depression are recommended as part of the behavioral and psychosocial evaluation in routine TS health care. If symptoms are present, further evaluation by a psychologist or mental health professional is recommended. Successful treatment of anxiety and depression often involves a combination of psychotherapy and medication. Psychological techniques based in Cognitive Behavioral Therapy (CBT) are effective in children, adolescents, and adults with anxiety and depressive disorders (Hofmann & Smits, 2017; Kreuze, Pijnenborg, de Jonge, & Nauta, 2018). In TS, a pilot study using CBT techniques targeting anxiety was helpful in improving self-esteem in 18 to 60 year old women with TS (Chadwick, Smyth, & Liao, 2014), although additional studies are needed targeting all aspects of anxiety and depression across the lifespan. Overall, because the strategies used in CBT rely on verbal strategies such as identifying thought distortions and learning to challenge them, the frequent verbal strengths of people with TS can be a good fit with this technique.
Medications are also often an important part of the treatment plan for symptoms of anxiety and depression. As with the ADHD medications described above, it is important to consider medical history and current cardiac status when deciding on the type of anxiety medication used, as some can have side effects such as prolongation of cardiac QT interval that may be contraindicated for some patients with TS. SSRI medications are the most commonly used class of medications for treatment and can be very effective for generalized anxiety, social anxiety, obsessive-compulsive behaviors, and depression in childhood and adulthood. Other considerations such as buproprion, norepinephrine-serotonin reuptake inhibitors, alpha-agonist medications and propranolol could also be considered. Additional study of the effects of medication treatments for anxiety and depression are needed, and research on other psychological strategies and therapies such as biofeedback should be explored.
PHENOTYPIC VARIABILITY
There can be significant variability in the severity of cognitive, social, and emotional concerns among individuals with TS, and the medical, genetic, and environmental factors underlying this variability are areas of active study. There are some genetic TS variations that are known to be associated with differences in the cognitive profile. For example, young women with the mosaic form of TS appear to be at a lower risk for difficulties with visual-spatial and math learning skills compared to individuals with the 45, X karyotype (Temple & Carney, 1993). In contrast, those with the 45,X/46X,+mar karyotype or those with ring X [r(X)] mosaicism are more likely to have more significant cognitive involvement (Cole et al., 1994; Leppig et al., 2004; Swillen et al., 1993).
DIAGNOSTIC CONSIDERATIONS:
The constellation of deficits in visual-spatial processing, math skills, and motor functioning, as well as issues with social cognition within the context of relatively preserved language skills was previously identified as a nonverbal learning disability (NVLD). Over the past several decades, there have been multiple attempts to define the primary deficits in NVLD and distinguish it from other clinical groups (e.g., autism spectrum disorders). However, research on the impairments in the multiple cognitive domains associated with the NVLD diagnosis found that they were difficult to consistently define, evaluate, and replicate (Fine, Semrud-Clikeman, Bledsoe, & Musielak, 2013). In comparison, the domains associated with language-based learning disabilities / reading disabilities including early language development, phonemic awareness, reading fluency, and overall reading skills have more clearly and consistently shown to be associated in psychological research (B. F. Pennington & Bishop, 2009; Peterson et al., 2016). NVLD was not included in the most recent 2013 publication of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (APA, 2013) or the ICD-10 (WHO, 1992), but has been widely discussed in popular literature. For example, there are a number of books that have been published since 2000 discussing educational and parenting strategies for children with NVLD. While the concept may help parents better understand the set of vulnerabilities, the lack of recognition by educational, psychological, and medical fields limits the utility of the diagnosis at this time. Further, to advance research on educational strategies or treatment programs specific to the neuropsychological profile in TS, it is important that the TS community shift from using the term NVLD and utilize terminology currently used in the fields of psychology and education.
CONCLUSION
Overall, the different domains of neuropsychological and social-emotional functioning that can been affected in TS have mostly been well-defined, with risks for many clinical diagnoses including specific learning disability in mathematics, ADHD, and anxiety or depressive disorders. The idea that neuropsychological domains overlap and affect each other in different ways to produce behavioral symptoms and overall functioning is well established in general psychological literature. However, there are few examples of studies aimed at understanding how the different neuropsychological constructs relate to one another in TS. However, in one study, LePage et al. (2013) explored factors related to social functioning in young girls with TS, and found that overall executive function skills contributed to social deficits more significantly than verbal IQ or visuospatial skills. Further, even after considering the impact of executive and cognitive skills on social functioning, there was still a core deficit in social skills compared to the control group without TS (Lepage, Dunkin, Hong, & Reiss, 2013). These results would suggest that targeting executive functioning skills and their contribution to social skills difficulties may be more effective than more traditional social skills interventions, and such programs have been shown to be effective in improving both EF and social skills in other populations such as ASD (Kenworthy et al., 2014). This is an example of how research exploring the relationships of psychological features in TS may guide treatment approaches, and additional research of this type is needed in TS for developing novel approaches to treatment. Overall, there is also a great need for research to study existing evidence-based interventions developed for treatment of math learning difficulties, ADHD, executive functioning difficulties, social deficits, and anxiety in TS, as well as to better understand what differences and modifications may be needed to validate these interventions in the TS population.
With a diagnosis of TS at any age, once health and medical features are addressed and stabilized, the challenges with academic and social-emotional functioning associated with TS often become the factors that have more significant ongoing impact on adaptive functioning, self-esteem, and overall quality of life. We strongly support further research evaluating current therapies and medications targeting these symptoms in TS, as well as developing interventions to support the specific needs of children and women with TS.
ACKNOWLEDGEMENTS:
Authors wish to acknowledge support for contributions and funding of clinical care in the eXtraOrdinary Kids Clinic at Children’s Hospital Colorado by TSSUS and TSGA, most significantly the Colorado groups led by Marybel Good. This publication has also received support from the NICHD (R01 HD091251; P.I. Tartaglia) and supported by NIH/NCATS Colorado CTSA Grant Number UL1 TR002535 (CCTSI). The symposium was supported by NICHD (NICHD R13 HD096857–01) and Patient-Centered Outcomes Research Institute (PCORI) Eugene Washington PCORI Engagement Award (#10460). Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
REFERENCES
- American Academy of Pediatrics, Council on Children With Disabilities, Section on Developmental Behavioral Pediatrics, Bright Futures Steering Committee, Medical Home Initiatives for Children With Special Needs Project Advisory Committee (2006). Identifying infants and young children with developmental disorders in the medical home: an algorithm for developmental surveillance and screening. Pediatrics, 118(1), 405–420. doi: 10.1542/peds.2006-1231 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, A. P. (2013). Diagnostic and statistical manual of mental disorders (5th ed.) (DSM-5). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, … Dowling NF (2018). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill Summ, 67(6), 1–23. Retrieved from 10.15585/mmwr.ss6706a1. doi: 10.15585/mmwr.ss6706a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaton EA, Stoddard J, Lai S, Lackey J, Shi J, Ross JL, & Simon TJ (2010). Atypical functional brain activation during a multiple object tracking task in girls with Turner syndrome: neurocorrelates of reduced spatiotemporal resolution. Am J Intellect Dev Disabil, 115(2), 140–156. doi: 10.1352/1944-7558-115.2.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender B, Linden M, & Robinson A (1994). Neurocognitive and Psychosocial Phenotypes Associated with Turner Syndrome In Broman SH & Grafman J (Eds.), Atypical Cognitive Deficits in Developmental Disorders: Implications for Brain Function. Hillside, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Bianchi D (2019). Turner Syndrome: New Insights From Prenatal Genomics and Transcriptomics. Am J Med Genet C Semin Med Genet (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bispo AV, Dos Santos LO, Buregio-Frota P, Galdino MB, Duarte AR, Leal GF, … Santos N (2013). Effect of chromosome constitution variations on the expression of Turner phenotype. Genet Mol Res, 12(4), 4243–4250. Retrieved from 10.4238/2013.March.13.13. doi: 10.4238/2013.March.13.13 [DOI] [PubMed] [Google Scholar]
- Cardoso G, Daly R, Haq NA, Hanton L, Rubinow DR, Bondy CA, & Schmidt P (2004). Current and lifetime psychiatric illness in women with Turner syndrome. Gynecol Endocrinol, 19(6), 313–319. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15726728. [PubMed] [Google Scholar]
- Chadwick PM, Smyth A, & Liao LM (2014). Improving self-esteem in women diagnosed with Turner syndrome: results of a pilot intervention. J Pediatr Adolesc Gynecol, 27(3), 129–132. Retrieved from 10.1016/j.jpag.2013.09.004. doi: 10.1016/j.jpag.2013.09.004 [DOI] [PubMed] [Google Scholar]
- Cole H, Huang B, Salbert BA, Brown J, Howard-Peebles PN, Black SH, … Jackson-Cook C (1994). Mental retardation and Ullrich-Turner syndrome in cases with 45,X/46X,+mar: additional support for the loss of the X-inactivation center hypothesis. Am J Med Genet, 52(2), 136–145. Retrieved from 10.1002/ajmg.1320520204. doi: 10.1002/ajmg.1320520204 [DOI] [PubMed] [Google Scholar]
- Creswell C, & Skuse D (1999). Autism in association with Turner syndrome: Genetic implications for male vulnerability to pervasive developmental disorders. Neurocase, 5, 511–518. [Google Scholar]
- Crooke PJ, & Winner MG (2016). Social Thinking(R) Methodology: Evidence-Based or Empirically Supported? A Response to Leaf et al. (2016). Behav Anal Pract, 9(4), 403–408. Retrieved from 10.1007/s40617-016-0151-y. doi: 10.1007/s40617-016-0151-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Berch DB, & Mazzocco MM (2009). Mathematical Learning Disabilities in Special Populations: Phenotypic Variation and Cross-Disorder Comparisons. Dev Disabil Res Rev, 15(1), 80–89. Retrieved from 10.1002/ddrr.54. doi: 10.1002/ddrr.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Abd S, Patton MA, Turk J, Hoey H, & Howlin P (1999). Social, communicational, and behavioral deficits associated with ring X turner syndrome. Am J Med Genet, 88(5), 510–516. Retrieved from http://dx.doi.org/. [DOI] [PubMed] [Google Scholar]
- Elgar K, Campbell R, & Skuse D (2002). Are you looking at me? Accuracy in processing line-of-sight in Turner syndrome. Proc Biol Sci, 269(1508), 2415–2422. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12495483. doi: 10.1098/rspb.2002.2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine JG, Semrud-Clikeman M, Bledsoe JC, & Musielak KA (2013). A critical review of the literature on NLD as a developmental disorder. Child Neuropsychol, 19(2), 190–223. Retrieved from 10.1080/09297049.2011.648923. doi: 10.1080/09297049.2011.648923 [DOI] [PubMed] [Google Scholar]
- Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, … Backeljauw PF (2017). Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol, 177(3), G1–g70. Retrieved from 10.1530/eje-17-0430. doi: 10.1530/eje-17-0430 [DOI] [PubMed] [Google Scholar]
- Green T, Bade Shrestha S, Chromik LC, Rutledge K, Pennington BF, Hong DS, & Reiss AL (2015). Elucidating X chromosome influences on Attention Deficit Hyperactivity Disorder and executive function. J Psychiatr Res, 68, 217–225. Retrieved from 10.1016/j.jpsychires.2015.06.021. doi: 10.1016/j.jpsychires.2015.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RM, Rosenthal E, Hulpke-Wette M, Graham JG, Sergeant J, & European Network of Hyperkinetic, D. (2012). Cardiovascular considerations of attention deficit hyperactivity disorder medications: a report of the European Network on Hyperactivity Disorders work group, European Attention Deficit Hyperactivity Disorder Guidelines Group on attention deficit hyperactivity disorder drug safety meeting In Cardiol Young (Vol. 22, pp. 63–70). England. [DOI] [PubMed] [Google Scholar]
- Heilbronner RL, Sweet JJ, Attix DK, Krull KR, Henry GK, & Hart RP (2010). Official position of the American Academy of Clinical Neuropsychology on serial neuropsychological assessments: the utility and challenges of repeat test administrations in clinical and forensic contexts. Clin Neuropsychol, 24(8), 1267–1278. Retrieved from 10.1080/13854046.2010.526785. doi: 10.1080/13854046.2010.526785 [DOI] [PubMed] [Google Scholar]
- Hofmann SG, & Smits JAJ (2017). The Evolution of Cognitive Behavioral Therapy for Anxiety and Depression. Psychiatr Clin North Am, 40(4), xi–xii. Retrieved from 10.1016/j.psc.2017.08.011. doi: 10.1016/j.psc.2017.08.011 [DOI] [PubMed] [Google Scholar]
- Hong D, Scaletta Kent J, & Kesler S (2009). Cognitive profile of Turner syndrome. Dev Disabil Res Rev, 15(4), 270–278. Retrieved from 10.1002/ddrr.79. doi: 10.1002/ddrr.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong DS, Bray S, Haas BW, Hoeft F, & Reiss AL (2014). Aberrant neurocognitive processing of fear in young girls with Turner syndrome. Soc Cogn Affect Neurosci, 9(3), 255–264. Retrieved from 10.1093/scan/nss133. doi: 10.1093/scan/nss133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong DS, Dunkin B, & Reiss AL (2011). Psychosocial functioning and social cognitive processing in girls with Turner syndrome. J Dev Behav Pediatr, 32(7), 512–520. Retrieved from 10.1097/DBP.0b013e3182255301. doi: 10.1097/DBP.0b013e3182255301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy L, Anthony LG, Naiman DQ, Cannon L, Wills MC, Luong-Tran C, … Wallace GL (2014). Randomized controlled effectiveness trial of executive function intervention for children on the autism spectrum. J Child Psychol Psychiatry, 55(4), 374–383. Retrieved from 10.1111/jcpp.12161. doi: 10.1111/jcpp.12161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Haberecht MF, Menon V, Warsofsky IS, Dyer-Friedman J, Neely EK, & Reiss AL (2004). Functional neuroanatomy of spatial orientation processing in Turner syndrome. Cereb Cortex, 14(2), 174–180. Retrieved from http://dx.doi.org/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic BG, Ergur AT, & Ocal G (2005). Depression, levels of anxiety and self-concept in girls with Turner’s syndrome. J Pediatr Endocrinol Metab, 18(11), 1111–1117. Retrieved from http://dx.doi.org/. [DOI] [PubMed] [Google Scholar]
- Knickmeyer R (2019). The deep biology of cognition: Moving toward a comprehensive neurodevelopmental model of Turner syndrome. Am J Med Genet C Semin Med Genet (this issue). [DOI] [PubMed] [Google Scholar]
- Kreuze LJ, Pijnenborg GHM, de Jonge YB, & Nauta MH (2018). Cognitive-behavior therapy for children and adolescents with anxiety disorders: A meta-analysis of econdary outcomes. J Anxiety Disord, 60, 43–57. Retrieved from 10.1016/j.janxdis.2018.10.005. doi: 10.1016/j.janxdis.2018.10.005 [DOI] [PubMed] [Google Scholar]
- Kubba H, Smyth A, Wong SC, & Mason A (2017). Ear health and hearing surveillance in girls and women with Turner’s syndrome: recommendations from the Turner’s Syndrome Support Society. Clin Otolaryngol, 42(3), 503–507. Retrieved from 10.1111/coa.12750. doi: 10.1111/coa.12750 [DOI] [PubMed] [Google Scholar]
- Lagrou K, Froidecoeur C, Verlinde F, Craen M, De Schepper J, François I, … Endocrinology B. S. G. o. P. (2006). Psychosocial functioning, self-perception and body image and their auxologic correlates in growth hormone and oestrogen-treated young adult women with Turner syndrome. Horm Res, 66(6), 277–284. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16946621. doi: 10.1159/000095547 [DOI] [PubMed] [Google Scholar]
- Lagrou K, Xhrouet-Heinrichs D, Heinrichs C, Craen M, Chanoine JP, Malvaux P, & Bourguignon JP (1998). Age-related perception of stature, acceptance of therapy, and psychosocial functioning in human growth hormone-treated girls with Turner’s syndrome. J Clin Endocrinol Metab, 83(5), 1494–1501. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9589645. doi: 10.1210/jcem.83.5.4807 [DOI] [PubMed] [Google Scholar]
- Laugeson E (2013). The Science of Making Friends: Helping Socially Challenged Teens and Young Adults. San Francisco: JosseyBass, John Wiley & Sons. [Google Scholar]
- Lawrence K, Campbell R, Swettenham J, Terstegge J, Akers R, Coleman M, & Skuse D (2003). Interpreting gaze in Turner syndrome: impaired sensitivity to intention and emotion, but preservation of social cueing. Neuropsychologia, 41(8), 894–905. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12667526. [DOI] [PubMed] [Google Scholar]
- Lawrence K, Kuntsi J, Coleman M, Campbell R, & Skuse D (2003). Face and emotion recognition deficits in Turner syndrome: a possible role for X-linked genes in amygdala development. Neuropsychology, 17(1), 39–49. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12597072. [PubMed] [Google Scholar]
- Lepage JF, Dunkin B, Hong DS, & Reiss AL (2013). Impact of cognitive profile on social functioning in prepubescent females with Turner syndrome. Child Neuropsychol, 19(2), 161–172. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22372383. doi: 10.1080/09297049.2011.647900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage JF, Lortie M, Deal CL, & Théoret H (2014). Empathy, autistic traits, and motor resonance in adults with Turner syndrome. Soc Neurosci, 9(6), 601–609. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25079009. doi: 10.1080/17470919.2014.944317 [DOI] [PubMed] [Google Scholar]
- Leppig KA, Sybert VP, Ross JL, Cunniff C, Trejo T, Raskind WH, & Disteche CM (2004). Phenotype and X inactivation in 45,X/46,X,r(X) cases. Am J Med Genet A, 128a(3), 276–284. Retrieved from 10.1002/ajmg.a.30002. doi: 10.1002/ajmg.a.30002 [DOI] [PubMed] [Google Scholar]
- Lesniak-Karpiak K, Mazzocco MM, & Ross JL (2003). Behavioral assessment of social anxiety in females with Turner or fragile X syndrome. J Autism Dev Disord, 33(1), 55–67. Retrieved from http://dx.doi.org/. [DOI] [PubMed] [Google Scholar]
- Lord C, & Rutter M (2012). Autism Diagnostic Observation Scales - 2nd Edition (ADOS-2). Torrance, CA: Western Psychological Services. [Google Scholar]
- Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, … Mahle WT (2012). Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation, 126(9), 1143–1172. Retrieved from 10.1161/CIR.0b013e318265ee8a. doi: 10.1161/CIR.0b013e318265ee8a [DOI] [PubMed] [Google Scholar]
- Martin A, Quintin EM, Hall SS, & Reiss AL (2016). The Role of Executive Function in Independent Living Skills in Female Adolescents and Young Adults With Fragile X Syndrome. Am J Intellect Dev Disabil, 121(5), 448–460. Retrieved from 10.1352/1944-7558-121.5.448. doi: 10.1352/1944-7558-121.5.448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzocco MM (2006). The cognitive phenotype of Turner syndrome: Specific learning disabilities. Int Congr Ser, 1298, 83–92. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19750135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzocco MM (2009). Mathematical learning disability in girls with Turner syndrome: a challenge to defining MLD and its subtypes. Dev Disabil Res Rev, 15(1), 35–44. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19213015. [DOI] [PubMed] [Google Scholar]
- Mazzocco MM, Thompson L, Sudhalter V, Belser RC, Lesniak-Karpiak K, & Ross JL (2006). Language use in females with fragile X or Turner syndrome during brief initial social interactions. J Dev Behav Pediatr, 27(4), 319–328. [DOI] [PubMed] [Google Scholar]
- Mazzola F, Seigal A, MacAskill A, Corden B, Lawrence K, & Skuse DH (2006). Eye tracking and fear recognition deficits in Turner syndrome. Soc Neurosci, 1(3–4), 259–269. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18633792. doi: 10.1080/17470910600989912 [DOI] [PubMed] [Google Scholar]
- McCauley E, Feuillan P, Kushner H, & Ross JL (2001). Psychosocial development in adolescents with Turner syndrome. J Dev Behav Pediatr, 22(6), 360–365. [DOI] [PubMed] [Google Scholar]
- McCauley E, Kay T, Ito J, & Treder R (1987). The Turner syndrome: cognitive deficits, affective discrimination, and behavior problems. Child Dev, 58(2), 464–473. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/3829787. [PubMed] [Google Scholar]
- McCauley E, Sybert VP, & Ehrhardt AA (1986). Psychosocial adjustment of adult women with Turner syndrome. Clin Genet, 29(4), 284–290. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/3720006. [DOI] [PubMed] [Google Scholar]
- Moonga SS, Pinkhasov A, & Singh D (2017). Obsessive-Compulsive Disorder in a 19-Year-Old Female Adolescent With Turner Syndrome. J Clin Med Res, 9(12), 1026–1028. Retrieved from 10.14740/jocmr3195w. doi: 10.14740/jocmr3195w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles BS, Trautma ML, & Schelvan RL (2013). The Hidden Curriculum for Understanding Unstated Rules in Social Situations for Adolescents and Young Adults. Shawnee, Kansas: Autism Asperger Publishing Company. [Google Scholar]
- Nijhuis-van der Sanden MW, Eling PA, & Otten BJ (2003). A review of neuropsychological and motor studies in Turner Syndrome. Neurosci Biobehav Rev, 27(4), 329–338. [DOI] [PubMed] [Google Scholar]
- Nijhuis-van der Sanden RW, Smits-Engelsman BC, & Eling PA (2000). Motor performance in girls with Turner syndrome. Dev Med Child Neurol, 42(10), 685–690. Retrieved from http://dx.doi.org/. [DOI] [PubMed] [Google Scholar]
- Pennington B, Heaton R, Karzmark P, Pendleton MG, Lehman R, & Shucard DW (1985). The neuropsychological phenotype in Turner syndrome. Cortex, 21, 391–404. [DOI] [PubMed] [Google Scholar]
- Pennington BF, & Bishop DV (2009). Relations among speech, language, and reading disorders. Annu Rev Psychol, 60, 283–306. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18652545. [DOI] [PubMed] [Google Scholar]
- Peterson RL, Boada R, McGrath LM, Willcutt EG, Olson RK, & Pennington BF (2016). Cognitive Prediction of Reading, Math, and Attention: Shared and Unique Influences. J Learn Disabil. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/26825667. doi: 10.1177/0022219415618500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A, Bender BG, & Linden MG (1990). Summary of clinical findings in children and young adults with sex chromosome anomalies. Birth Defects Orig Artic Ser, 26(4), 225–228. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2090320. [PubMed] [Google Scholar]
- Romans SM, Stefanatos G, Roeltgen DP, Kushner H, & Ross JL (1998). Transition to young adulthood in Ullrich-Turner syndrome: neurodevelopmental changes. Am J Med Genet, 79(2), 140–147. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9741472. [PubMed] [Google Scholar]
- Ross J, Zinn A, & McCauley E (2000). Neurodevelopmental and psychosocial aspects of Turner syndrome. Ment Retard Dev Disabil Res Rev, 6(2), 135–141. Retrieved from . doi: [DOI] [PubMed] [Google Scholar]
- Ross JL, Kushner H, & Zinn AR (1997). Discriminant analysis of the Ullrich-Turner syndrome neurocognitive profile. Am J Med Genet, 72(3), 275–280. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9332653. [DOI] [PubMed] [Google Scholar]
- Ross JL, Stefanatos GA, Kushner H, Zinn A, Bondy C, & Roeltgen D (2002). Persistent cognitive deficits in adult women with Turner syndrome. Neurology, 58(2), 218–225. Retrieved from http://dx.doi.org/. [DOI] [PubMed] [Google Scholar]
- Rovet J (1993). The psychoeducational characteristics of children with Turner syndrome. Journal of Learning Disabilities, 26, 333–341. [DOI] [PubMed] [Google Scholar]
- Rovet JF (1990). The Cognitive and Neuropsychological Characteristics of Females with Turner Syndrome In Berch DB & Bender BB (Eds.), Sex Chromosome Abnormalities and Human Behavior: Psychological Studies (pp. 38–77). Boulder, Colorado: Westview Press, Inc. [Google Scholar]
- Russell HF, Wallis D, Mazzocco M, Moshang T, Zackai E, Zinn A, … Muenke M (2006). Increased prevalence of ADHD in Turner syndrome with no evidence of imprinting effects. Journal of Pediatric Psychology, 31(9), 945–955. [DOI] [PubMed] [Google Scholar]
- Salbenblatt JA, Meyers DC, Bender BG, Linden MG, & Robinson A (1989). Gross and fine motor development in 45,X and 47,XXX girls. Pediatrics, 84(4), 678–682. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2780130. [PubMed] [Google Scholar]
- Schmidt PJ, Cardoso GM, Ross JL, Haq N, Rubinow DR, & Bondy CA (2006). Shyness, social anxiety, and impaired self-esteem in Turner syndrome and premature ovarian failure In Jama (Vol. 295, pp. 1374–1376). United States. [DOI] [PubMed] [Google Scholar]
- Simon TJ, Takarae Y, DeBoer T, McDonald-McGinn DM, Zackai EH, & Ross JL (2008). Overlapping numerical cognition impairments in children with chromosome 22q11.2 deletion or Turner syndromes. Neuropsychologia, 46(1), 82–94. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/17920087. doi: 10.1016/j.neuropsychologia.2007.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillen A, Fryns JP, Kleczkowska A, Massa G, Vanderschueren-Lodeweyckx M, & Van den Berghe H (1993). Intelligence, behaviour and psychosocial development in Turner syndrome. A cross-sectional study of 50 pre-adolescent and adolescent girls (4–20 years). Genet Couns, 4(1), 7–18. Retrieved from http://dx.doi.org/. [PubMed] [Google Scholar]
- Temple CM (2002). Oral fluency and narrative production in children with Turner’s syndrome. Neuropsychologia, 40(8), 1419–1427. Retrieved from http://dx.doi.org/. [DOI] [PubMed] [Google Scholar]
- Temple CM, & Carney R (1996). Reading skills in children with Turner’s syndrome: an analysis of hyperplexia. Cortex, 32(2), 335–345. [DOI] [PubMed] [Google Scholar]
- Temple CM, & Carney RA (1993). Intellectual functioning of children with Turner syndrome: a comparison of behavioural phenotypes. Dev Med Child Neurol, 35(8), 691–698. [DOI] [PubMed] [Google Scholar]
- Winner MG (2018). Social Thinking Series, www.socialthinking.com.
- Wolraich M, Brown L, Brown RT, DuPaul G, Earls M, Feldman HM, … Visser S (2011). ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics, 128(5), 1007–1022. Retrieved from 10.1542/peds.2011-2654. doi: 10.1542/peds.2011-2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstencroft J, & Skuse D (2018). Social skills and relationships in Turner syndrome. Curr Opin Psychiatry. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30407217. doi: 10.1097/YCO.0000000000000472 [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bauman ML, Fein D, Pierce K, Buie T, Davis PA, … Wagner S (2015). Early Screening of Autism Spectrum Disorder: Recommendations for Practice and Research. Pediatrics, 136 Suppl 1, S41–59. Retrieved from 10.1542/peds.2014-3667D. doi: 10.1542/peds.2014-3667D [DOI] [PMC free article] [PubMed] [Google Scholar]