Abstract
BACKGROUND
Nivolumab has been associated with longer overall survival than docetaxel among patients with previously treated non-small-cell lung cancer (NSCLC). In an open-label phase 3 trial, we compared first-line nivolumab with chemotherapy in patients with programmed death ligand 1 (PD-Ll)-positive NSCLC.
METHODS
We randomly assigned, in a 1:1 ratio, patients with untreated stage IV or recurrent NSCLC and a PD-L1 tumor-expression level of 1% or more to receive nivolumab (administered intravenously at a dose of 3 mg per kilogram of body weight once every 2 weeks) or platinum-based chemotherapy (administered once every 3 weeks for up to six cycles). Patients receiving chemotherapy could cross over to receive nivolumab at the time of disease progression. The primary end point was progression-free survival, as assessed by means of blinded independent central review, among patients with a PD-L1 expression level of 5% or more.
RESULTS
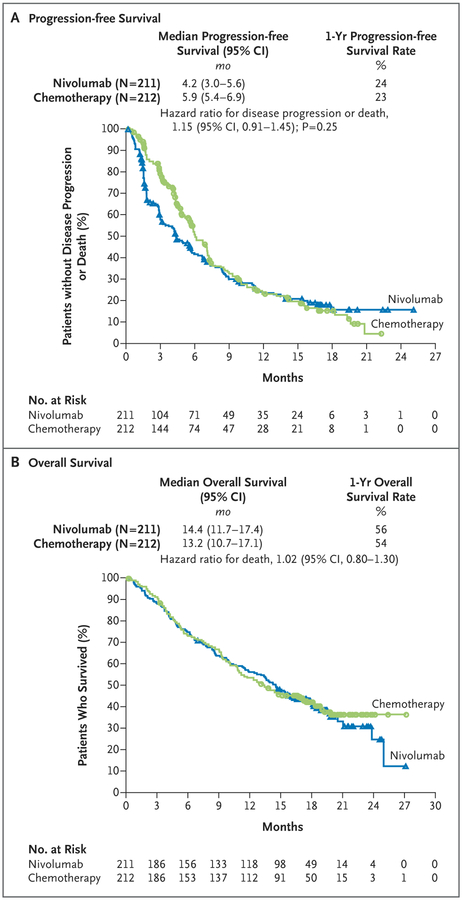
Among the 423 patients with a PD-L1 expression level of 5% or more, the median progression-free survival was 4.2 months with nivolumab versus 5.9 months with chemo-therapy (hazard ratio for disease progression or death, 1.15; 95% confidence interval [CI], 0.91 to 1.45; P=0.25), and the median overall survival was 14.4 months versus 13.2 months (hazard ratio for death, 1.02; 95% CI, 0.80 to 1.30). A total of 128 of 212 patients (60%) in the chemotherapy group received nivolumab as subsequent therapy. Treatment-related adverse events of any grade occurred in 71% of the patients who received nivolumab and in 92% of those who received chemotherapy. Treatment-related adverse events of grade 3 or 4 occurred in 18% of the patients who received nivolumab and in 51% of those who received chemotherapy.
CONCLUSIONS
Nivolumab was not associated with significantly longer progression-free survival than chemotherapy among patients with previously untreated stage IV or recurrent NSCLC with a PD-L1 expression level of 5% or more. Overall survival was similar between groups. Nivolumab had a favorable safety profile, as compared with chemotherapy, with no new or unexpected safety signals. (Funded by Bristol-Myers Squibb and others; CheckMate 026 ClinicalTrials.gov number, NCT02041533.)
FOR THE PAST TWO DECADES, PLATINUM-based combination chemotherapy has been the standard-of-care, first-line treatment for patients with advanced non-small-cell lung cancer (NSCLC) without mutations that were sensitive to targeted therapy.1,2 However, chemo-therapy has provided only a moderate benefit, with a limited safety profile. In phase 3 clinical trials, the median progression-free survival with platinum-based chemotherapy was 4 to 6 months, and the median overall survival was 10 to 13 months.3–8
In two phase 3 trials, nivolumab, a programmed death 1 (PD-1) immune-checkpoint-inhibitor antibody, resulted in significantly longer overall survival than docetaxel among patients with metastatic NSCLC who had disease progression during or after platinum-based chemo-therapy.9–11 Benefit was seen regardless of the PD-1 ligand 1 (PD-L1) expression level but was enhanced in patients with nonsquamous NSCLC with increasing PD-L1 expression.9,10
In a multicohort phase 1 study involving previously untreated patients with NSCLC (Check-Mate 012),12 preliminary data from a cohort of 20 patients who received nivolumab monotherapy showed durable responses and a favorable safety profile. Among the 10 patients with a PD-L1 expression level of 5% or more, the objective response rate was 50%, the rate of progression-free survival at 24 weeks was 70%, and the median progression-free survival was 10.6 months.13 Although an increasing PD-L1 expression level was associated with greater benefit in the expanded cohort, clinical activity was also seen in patients with a low PD-L1 expression level or with no PD-L1 expression.12 On the basis of this preliminary data set and the finding that approximately 12 to 15% of the patients had a PD-L1 result showing expression between 1% and 4% across studies of nivolumab involving patients with NSCLC (Bristol-Myers Squibb, data on file), progression-free survival among patients with a PD-L1 expression level of 5% or more was chosen as the primary end point because this population was thought to be more likely to show a progression-free survival benefit with nivolumab than patients with a lower (<5%) PD-L1 expression level.
Owing to the complexity of the immune system, biomarkers for response to immuno-onco-logic agents beyond PD-L1 expression levels are being explored. Early data support the hypothesis that a high tumor-mutation burden may in crease the likelihood of benefit from immune-therapy, because a high tumor-mutation burden may enhance tumor immunogenicity by increasing the number of neoantigens, which are recognized by T cells as nonself, leading to an anti-tumor immune response.14
We report the results of an international, randomized, open-label, phase 3 trial (CheckMate 026) that compared the efficacy and safety of nivolumab with those of platinum-based chemo-therapy as first-line therapy in patients with stage IV or recurrent NSCLC with a PD-L1 expression level of 5% or more (primary efficacy analysis population) and those with a PD-L1 expression level of 1% or more (secondary efficacy analysis population). Furthermore, we report an exploratory analysis to assess the effects of the tumor-mutation burden on treatment outcomes.
METHODS
PATIENTS
Eligible adult patients had histologically confirmed squamous-cell or nonsquamous stage IV or recurrent NSCLC, an Eastern Cooperative Oncology Group (ECOG) performance-status score of 0 or 1 (on a 5-point scale, with higher numbers indicating greater disability), and measurable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1,15 and had received no previous systemic anti-cancer therapy as primary therapy for advanced or metastatic disease. Patients with central nervous system metastases were eligible if they had been adequately treated and had been asymptomatic for at least 2 weeks before randomization. Eligible patients had to not be taking glucocorticoids or had to be taking a stable or decreasing dose of 10 mg or less of prednisone daily (or its equivalent). Previous palliative radiotherapy, if completed at least 2 weeks before randomization, and previous adjuvant or neoadjuvant chemo-therapy that was completed at least 6 months before enrollment were permitted. Patients with an autoimmune disease or known EGFR mutations or ALK translocations that were sensitive to available targeted therapy were excluded.
Fresh or archival tumor-biopsy specimens obtained within 6 months before enrollment were tested for PD-L1 by a centralized laboratory with the use of the anti-PD-L1 antibody (28–8 antibody).9,10 Only patients with a PD-L1 expression level of 1% or more underwent randomization. Written informed consent was provided by all the patients before enrollment.
TRIAL DESIGN AND TREATMENT
Patients were enrolled from March 2014 through April 2015. Eligible patients were randomly assigned in a 1:1 ratio to receive nivolumab (at a dose of 3 mg per kilogram of body weight every 2 weeks) or the investigator’s choice of platinum doublet chemotherapy (every 3 weeks for four to six cycles) (Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). Chemotherapy was continued until disease progression, the occurrence of an unacceptable level of toxic effects, or the completion of permitted cycles. Maintenance therapy with pemetrexed was allowed in patients with nonsquamous NSCLC who had stable disease or a response after cycle 4. Treatment with nivolumab beyond progression was permitted if protocol-defined criteria were met, including investigator-assessed clinical benefit, no rapid disease progression, no unacceptable level of adverse events related to nivolumab, and a stable performance status, and if there was no interference with imminent intervention to prevent serious complications of disease progression. Concomitant systemic glucocorticoid treatment (courses lasting <3 weeks) was allowed for nonautoimmune conditions, including but not limited to treatment-related adverse events with a potential immune-logic cause.
Randomization was stratified according to PD-L1 expression level (<5% vs. >5%) and tumor histologic findings (squamous vs. nonsquamous). Patients in the chemotherapy group who had disease progression according to RECIST, as assessed by the investigator and confirmed by an independent radiologist, could cross over to receive nivolumab, provided that eligibility criteria were met. For patients in the chemotherapy group, dose delays and two or fewer dose reductions because of toxic effects were allowed. For patients in the nivolumab group, dose delays because of toxic effects were allowed, but dose reductions were not allowed.
END POINTS AND ASSESSMENTS
The primary end point was progression-free survival, as assessed by blinded independent central review, among patients with a PD-L1 expression level of 5% or more. Secondary end points included progression-free survival, as assessed by means of blinded independent central review, among all the patients who had undergone randomization (of whom all had a PD-L1 expression level of >1%), overall survival among patients with a PD-L1 expression level of 5% or more and among all the patients who had undergone randomization, and the independently assessed response rate among patients with a PD-L1 expression level of 5% or more.
Tumor response was assessed every 6 weeks until week 48 and every 12 weeks thereafter. Safety assessments included the recording of adverse events, which were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. The investigators determined whether an adverse event was related to a trial drug.
EXPLORATORY BIOMARKER ANALYSIS OF TUMOR-MUTATION BURDEN
The tumor-mutation burden, which was defined as the total number of somatic missense mutations present in a baseline tumor sample, was determined in patients with tumor and blood samples sufficient for whole-exome sequencing. For efficacy analyses, patients were grouped in thirds according to tumor-mutation burden. The boundaries for these three groups were a tumor-mutation burden of 0 to less than 100 mutations (low burden), 100 to 242 mutations (medium burden), and 243 or more mutations (high burden). All the testing and analyses of tumor-mutation burden were exploratory and not prespecified, including the evaluation according to distribution into the three groups. The testing was conducted in a research laboratory, and the methodologic approach that we used has not been approved by the Clinical Laboratory Improvement Amendments program as a clinical diagnostic test. Details are provided in the Supplementary Appendix.
TRIAL OVERSIGHT
The trial was designed and data were analyzed jointly by the sponsor (Bristol-Myers Squibb) and a steering committee, with the participation of individual authors. All the investigators collected data. The trial protocol, available at NEJM.org, was approved by the institutional review board or independent ethics committee at each center. The trial was conducted in accordance with the International Conference on Harmonisation Guidelines on Good Clinical Practice and the Declara tion of Helsinki. An independent data and safety monitoring committee provided oversight of safety and efficacy. This report is based on the final data analysis (database locked on August 2, 2016).
All the authors attest that the trial was conducted in accordance with the protocol and vouch for the accuracy and completeness of the data and analyses. All the authors signed a confidentiality agreement with the sponsor. Medical writing support, including writing of the first draft of the manuscript, was provided by Evidence Scientific Solutions, with funding from the sponsor.
STATISTICAL ANALYSIS
The sample-size estimation for the primary efficacy analysis population (patients with a PD-L1 expression level of >5%) was based on an expected median progression-free survival of 7 months in the chemotherapy group and an overall hazard ratio for disease progression or death of 0.71 favoring nivolumab. We estimated that a sample of approximately 415 patients would provide the trial with 80% power to detect a difference in treatment effect on the primary end point with the use of a log-rank test with a two-sided significance level of 5% after a minimum follow-up of approximately 18 months in patients with no disease progression or death.
The between-group comparisons of progression-free survival and overall survival were performed by means of two-sided log-rank tests stratified according to PD-L1 expression level (<5% vs. >5%, for end points in all the patients who had undergone randomization) and tumor histologic findings. We used a stratified Cox proportional-hazards model that included the randomized treatment group as a single covariate to estimate hazard ratios and their associated 95% confidence intervals. The Kaplan-Meier method was used to estimate survival curves. Response rates were compared between treatment groups with the use of a two-sided, stratified Cochran-Mantel-Haenszel test. The Clopper-Pearson method was used to estimate response rates and their exact 95% confidence intervals.
RESULTS
PATIENTS AND TREATMENT
Of 1325 patients enrolled in the trial, 541 (41%) underwent randomization, with 271 assigned to receive nivolumab and 270 assigned to receive chemotherapy. A total of 784 patients (59%) did not undergo randomization because their PD-L1 samples could not be evaluated (6% of patients), because the PD-L1 expression level was less than 1% (23%), or because they did not meet other trial criteria (30%). During screening, 746 of 1047 patients (71%) who had PD-L1 results that could be evaluated had a PD-L1 expression of 1% or more. Overall, 530 patients (98% of all the patients who had undergone randomization) received treatment (Fig. S1A and Table S1 in the Supplementary Appendix).
The primary efficacy analysis population (423 patients with a PD-L1 expression level of >5%) constituted 78% of all the patients who had undergone randomization. The median time from diagnosis to randomization of all the patients was 1.9 months (range, 0.3 to 214.9) in the nivolumab group and 2.0 months (range, 0.5 to 107.3) in the chemotherapy group, with 76% and 72% of patients, respectively, being assigned to the corresponding treatment groups within 3 months after diagnosis. Overall, 39% of the patients had received radiotherapy previously.
The baseline characteristics of all the patients who underwent randomization were similar to those of the patients who were included in the primary efficacy analysis (Table 1, and Tables S2 and S3 in the Supplementary Appendix). Among all the patients, the baseline characteristics were generally balanced between the treatment groups. However, in the nivolumab group, the percentage of women was lower than that in the chemo-therapy group (32% vs. 45%), as was the percentage of patients with a PD-L1 expression level of 50% or more (32% vs. 47%); the percentage of patients with liver metastases was slightly higher in the nivolumab group (20% vs. 13%). In addition, patients in the nivolumab group had a greater tumor burden (on the basis of the median sum of target-lesion diameters) than those in the chemotherapy group (Table 1).
Table 1.
Characteristics at Baseline of All the Patients Who Underwent Randomization.*
| Characteristic | Nivolumab (N = 271) |
Chemotherapy (N=270) |
Total (N = 541) |
|---|---|---|---|
| Age — yr | |||
| Median | 63 | 65 | 64 |
| Range | 32–89 | 29–87 | 29–89 |
| Age ≥75 yr — no. (%) | 30 (11) | 32 (12) | 62 (11) |
| Female sex — no. (%) | 87 (32) | 122 (45) | 209 (39) |
| Disease stage — no. (%) | |||
| Stage IV | 255 (94) | 244 (90) | 499 (92) |
| Recurrent | 16 (6) | 25 (9) | 41 (8) |
| Not reported | 0 | 1 (<1) | 1 (<1) |
| ECOG performance-status score — no. (%)† | |||
| 0 | 85 (31) | 93 (34) | 178 (33) |
| 1 | 183 (68) | 174 (64) | 357 (66) |
| ≥2 | 2 (1) | 3 (1) | 5 (1) |
| Not reported | 1 (<1) | 0 | 1 (<1) |
| Smoking status — no. (%) | |||
| Never smoked | 30 (11) | 29 (11) | 59 (11) |
| Former smoker | 186 (69) | 182 (67) | 368 (68) |
| Current smoker | 52 (19) | 55 (20) | 107 (20) |
| Unknown | 3 (1) | 4 (1) | 7 (1) |
| Previous systemic therapy — no. (%) | |||
| Adjuvant | 22 (8) | 25 (9) | 47 (9) |
| Neoadjuvant | 5 (2) | 4 (1) | 9 (2) |
| Previous radiotherapy — no. (%) | 102 (38) | 107 (40) | 209 (39) |
| Tumor histologic findings — no. (%) | |||
| Squamous-cell carcinoma | 66 (24) | 64 (24) | 130 (24) |
| Nonsquamous-cell carcinoma | 205 (76) | 206 (76) | 411 (76) |
| Selected site of metastatic lesions — no. (%) | |||
| Brain | 33 (12) | 36 (13) | 69 (13) |
| Liver | 54 (20) | 36 (13) | 90 (17) |
| Sum of target-lesion diameters — mm | |||
| Median | 82 | 68 | 76 |
| Range | (14–218) | (15–272) | (14–272) |
| PD-L1 expression level — no. (%) | |||
| ≥5% | 208 (77) | 210 (78) | 418 (77) |
| ≥50% | 88 (32) | 126 (47) | 214 (40) |
PD-L1 denotes programmed death ligand 1.
The Eastern Cooperative Oncology Group (ECOG) performance-status score is assessed on a 5-point scale, with higher numbers indicating greater disability. Patients were required to have an ECOG performance-status score of 0 or 1 during screening. However, at baseline the score had worsened to 2 in five patients and was not reported in one patient.
The minimum follow-up for overall survival was 13.7 months, and the median follow-up was 13.5 months (the minimum follow-up was computed as the time from randomization of the last patient to the database lock, and the median follow-up was computed for all the patients from randomization to the last known vital-status date). The median duration of therapy was 3.7 months (range, 0.0 to 26.9+ [the plus sign indicates an ongoing status at the time of the database lock]) in the nivolumab group and 3.4 months (range, 0.0 to 20.9+) in the chemo-therapy group. Details regarding the chemotherapy regimens are provided in Table S4 in the Supplementary Appendix. A total of 38% of treated patients received maintenance pemetrexed. A total of 77 of 267 patients (29%) who were treated with nivolumab received nivolumab beyond investigator-assessed progression according to RECIST. A total of 26 patients received more than six doses of nivolumab after progression.
Among the 211 patients with a PD-L1 expression level of 5% or more in the nivolumab group, 92 (44%) received subsequent systemic cancer therapy, and 39 (18%) continued receiving nivolumab at the time of the database lock. Among the corresponding 212 patients in the chemotherapy group, 136 (64%) received subsequent systemic therapy, including 128 (60%) who received nivolumab — 58% as crossover treatment within the trial and 3% in clinical practice after the trial; 1 patient received the drug both within the trial and after the trial (Table S5 in the Supplementary Appendix).
EFFICACY
Primary Efficacy Analysis Population and All Patients
In the primary efficacy analysis population (patients with a PD-L1 expression level of >5%), there was no significant difference in progression-free survival between treatment groups (Fig. 1A). The median progression-free survival was 4.2 months (95% confidence interval [CI], 3.0 to 5.6) in the nivolumab group and 5.9 months (95% CI, 5.4 to 6.9) in the chemotherapy group (hazard ratio for disease progression or death, 1.15; 95% CI, 0.91 to 1.45; P = 0.25). The median overall survival in the primary efficacy analysis population was 14.4 months (95% CI, 11.7 to 17.4) in the nivolumab group and 13.2 months (95% CI, 10.7 to 17.1) in the chemotherapy group (hazard ratio for death, 1.02; 95% CI, 0.80 to 1.30) (Fig. 1B). Similar results regarding progression-free survival and overall survival were found in the analyses that included all the patients who had undergone randomization (Figs. S2 and S3 in the Supplementary Appendix).
Figure 1. Progression-free Survival and Overall Survival among Patients with a Programmed Death Ligand 1 Expression Level of 5% or More.
CI denotes confidence interval.
The response rate among patients with a PD-L1 expression level of 5% or more was 26% in the nivolumab group and 33% in the chemotherapy group (Table 2). The nivolumab group had a higher percentage of patients than the chemo-therapy group with a best response of progressive disease (27% vs. 10%). The median time to response was similar in the nivolumab group and the chemotherapy group (2.8 months and 2.6 months, respectively), whereas the median duration of response was more than twice as long with nivolumab as with chemotherapy (12.1 vs. 5.7 months) (Table 2).
Table 2.
Tumor Response in Patients with a PD-L1 Expression Level of 5% or More.*
| Variable | Nivolumab (N = 211) |
Chemotherapy (N = 212) |
|---|---|---|
| Objective response† | ||
| No. of patients with response | 55 | 71 |
| % of patients (95% CI) | 26 (20–33) | 33 (27–40) |
| Estimated odds ratio (95% CI) | 0.70 (0.46–1.06) | |
| Best overall response — no. (%) | ||
| Complete response | 4 (2) | 1 (<1) |
| Partial response | 51 (24) | 70 (33) |
| Stable disease | 81 (38) | 100 (47) |
| Progressive disease | 58 (27) | 21 (10) |
| Could not be determined | 17 (8) | 20 (9) |
| Time to response — mo‡§ | ||
| Median | 2.8 | 2.6 |
| Range | 1.2–13.2 | 1.2–9.8 |
| Duration of response — mo‡¶ | ||
| Median | 12.1 | 5.7 |
| Range | 1.7–19.4+ | 1.4–21.0+ |
Data are based on an August 2, 2016, database lock.
Objective response was assessed according to the Response Evaluation Criteria in Solid Tumors, version 1.1, by independent central review. The 95% confidence interval is based on the Clopper-Pearson method. The analysis was stratified according to tumor histologic findings. The strata-adjusted odds ratio was calculated with the use of the Cochran-Mantel-Haenszel method.
The analysis was performed with data from all the patients who had a response (55 patients in the nivolumab group and 71 in the chemotherapy group).
The time to response was defined as the time from randomization to the date of the first documented complete or partial response.
Results were calculated with the use of the Kaplan-Meier method. The duration of response was defined as the time between the date of the first response and the date of the first documented event of progression, death, or last tumor assessment that was evaluated before subsequent therapy (data-censoring date). The plus sign indicates that the response was ongoing at the time of data analysis; ongoing responses are censored at the date of the most recent scan obtained before the data analysis.
Selected Subgroups
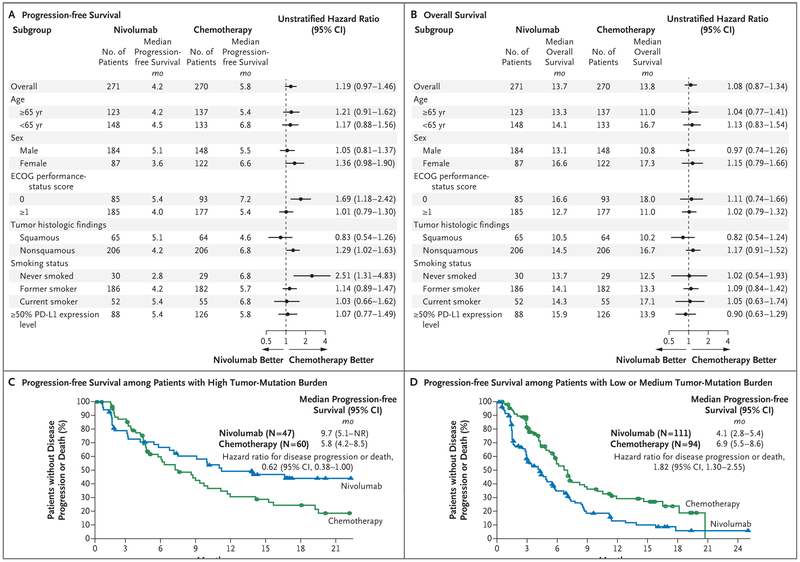
Across most planned subgroups (which included all the patients who had undergone randomization), the results of the analyses of progression-free survival and overall survival were consistent with the overall trial results (Fig. 2A and 2B). The only prespecified subgroup was patients defined according to histologic findings (a stratification factor); patients with histologic results showing squamous-cell NSCLC had slightly longer progression-free survival and overall survival with nivolumab than with chemotherapy, although the results were not significant (Fig. 2A and 2B).
Figure 2 (facing page). Exploratory Subgroup Analyses of Progression-free Survival and Overall Survival.
Panel A shows the subgroup analysis of progression-free survival involving all the patients who underwent random ization, and Panel B the subgroup analysis of overall survival. PD-L1 denotes programmed death ligand 1. The Eastern Cooperative Oncology Group (ECOG) per formance-status score is assessed on a 5-point scale, with higher numbers indicating greater disability. Panel C shows the analysis of progression-free survival among patients who could be evaluated for tumor-mutation burden and who had a high burden. NR denotes not reached. Panel D shows the analysis of progression-free survival among patients who could be evaluated for tumor-mutation burden and who had a low or medium burden. The data for patients with a low or medium tumor-mutation burden were pooled.
In the exploratory subgroup analysis involving patients with a PD-L1 expression level of 50% or more, the hazard ratio for disease progression or death was 1.07 (95% CI, 0.77 to 1.49), and the hazard ratio for death was 0.90 (95% CI, 0.63 to 1.29). In this subgroup, the response rate was 34% (95% CI, 24 to 45) in the nivolumab group and 39% (95% CI, 30 to 48) in the chemotherapy group. Because patients were not stratified according to whether they had a PD-L1 expression level of 50% or more, the nivolumab group had fewer patients than the chemotherapy group (88 vs. 126), and the imbalance in sex that was noted in the overall population (32% of the patients in the nivolumab group vs. 45% in the chemotherapy group were women) was even more pronounced in this subgroup (25% of the patients in the nivolumab subgroup vs. 44% in the chemotherapy subgroup were women). The corresponding findings for the subgroups of the primary efficacy analysis population are provided in Figure S4 in the Supplementary Appendix.
An exploratory analysis was conducted in 312 patients (58% of the patients who had undergone randomization) to assess the effect of the tumor-mutation burden on outcomes (Fig. 2C and 2D). The percentage of patients with a high tumor-mutation burden was imbalanced between the treatment groups (30% in the nivolumab group vs. 39% in the chemotherapy group). The characteristics at baseline and the results regarding progression-free survival and overall sur vival were generally consistent with those in the total population. Details are provided in Tables S6, S7, and S8 and in Figures S5 through S14 in the Supplementary Appendix.
Among the patients with a high tumor-mutation burden, the response rate was higher in the nivolumab group than in the chemotherapy group (47% vs. 28%), and progression-free survival was longer (median, 9.7 vs. 5.8 months; hazard ratio for disease progression or death, 0.62; 95% CI, 0.38 to 1.00) (Fig. 2C). Overall survival was similar between groups regardless of the tumor-mutation burden. However, 68% of the patients with a high tumor-mutation burden in the chemotherapy group received subsequent nivolumab because of treatment crossover, access to nivolumab after the trial, or both. There was no significant association between tumor-mutation burden and PD-L1 expression level (Pearson’s correlation coefficient = 0.059). However, in the nivolumab group, patients with both a high tumor-mutation burden and a PD-L1 expression level of 50% or more had a higher response rate (75%) than those with only one of these factors (32% among patients with a high tumor-mutation burden only and 34% among those with a PD-L1 expression level of >50% only) or neither factor (16%). However, this comparison was not powered for statistical analysis. Details are provided in Figures S8, S9, S12, S13, and S14 in the Supplementary Appendix.
SAFETY
Treatment-related adverse events of any grade occurred in 71% of the patients treated with nivolumab and in 92% of those treated with chemotherapy. The percentage of patients with treatment-related adverse events of grade 3 or 4 was lower with nivolumab than with chemotherapy (18% vs. 51%) (Table 3, and Table S9 in the Supplementary Appendix). The rates of treatment-related serious adverse events were similar in the two groups. Treatment-related adverse events leading to discontinuation of the study drug were 10% with nivolumab and 13% with chemo-therapy (Table 3, and Tables S10, S11, and S12 in the Supplementary Appendix). The most common selected adverse events (those with a potential immunologic cause) that were adjudicated as being related to treatment were skin-related events in the nivolumab group and gastrointestinal events in the chemotherapy group (Table S13 in the Supplementary Appendix).
Table 3.
Treatment-Related Adverse Events.*
| Event | Nivolumab (N = 267) | Chemotherapy (N = 263) | ||
|---|---|---|---|---|
| Any Grade | Grade 3 or 4 | Any Grade | Grade 3 or 4 | |
| number of patients with event (percent) | ||||
| Any event | 190 (71) | 47 (18) | 243 (92) | 133 (51) |
| Any serious event | 46 (17) | 35 (13) | 48 (18) | 41 (16) |
| Any event leading to discontinuation of therapy | 26 (10) | 21 (8) | 35 (13) | 17 (6) |
| Fatigue | 56 (21) | 3 (1) | 93 (35) | 14 (5) |
| Diarrhea | 37 (14) | 3 (1) | 34 (13) | 5 (2) |
| Decreased appetite | 32 (12) | 1 (<1) | 73 (28) | 4 (2) |
| Nausea | 31 (12) | 1 (<1) | 127 (48) | 5 (2) |
| Rash | 26 (10) | 2 (1) | 15 (6) | 1 (<1) |
| Vomiting | 15 (6) | 0 | 60 (23) | 5 (2) |
| Constipation | 9 (3) | 0 | 29 (11) | 0 |
| Anemia | 9 (3) | 1 (<1) | 113 (43) | 46 (17) |
| Asthenia | 8 (3) | 0 | 28 (11) | 4 (2) |
| Thrombocytopenia | 2 (1) | 1 (<1) | 38 (14) | 22 (8) |
| Platelet count decreased | 2 (1) | 0 | 33 (13) | 9 (3) |
| Neutrophil count decreased | 1 (<1) | 1 (<1) | 36 (14) | 20 (8) |
| Neutropenia | 0 | 0 | 48 (18) | 29 (11) |
Data are based on an August 2, 2016, database lock. Safety analyses included all the patients who had received at least one dose of nivolumab or chemotherapy. Included are events that were reported in at least 10% of the patients in either trial group from the time of the first dose of nivolumab or chemotherapy to 30 days after the receipt of the last dose or to the time of the first dose of nivolumab crossover, whichever came first. The relatedness of adverse events to treatment was adjudicated by the investigators.
Five deaths were attributed to study treatment. There were two deaths in the nivolumab group (one each from multiorgan failure and pneumonitis) and three in the chemotherapy group (one from sepsis and two from febrile neutropenia).
DISCUSSION
In the primary efficacy population in this trial involving patients with stage IV or recurrent NSCLC and a PD-L1 expression level of 5% or more, patients who received first-line mono-therapy with nivolumab did not have longer progression-free survival than those who received chemotherapy. Overall survival was similar in the two treatment groups, comparing favorably with historical controls of first-line platinum-based chemotherapy.3–8 Given that nivolumab therapy prolongs survival among previously treated patients with advanced NSCLC,9,10 the high frequency of subsequent nivolumab treatment may have contributed to the favorable overall survival in the chemotherapy group. In addition, imbalances in the characteristics of the patients at baseline may have favored the chemotherapy group, including disease characteristics that are associated with a better prognosis (i.e., slightly fewer liver metastases, smaller tumor burden, and a higher proportion of women). Two factors that appear in retrospect to have had an influence on the response to nivolumab (i.e., a PD-L1 expression level of >50% and a high tumor-mutation burden) also disfavored the nivolumab group, which had lower proportions of such patients than did the chemotherapy group.3,4,16
Two additional observations worth noting are the high percentage of patients in this trial who had received radiotherapy previously (39%) and the median time from diagnosis to randomization of approximately 2 months. Both these results may be attributed in part to the patients with recurrent disease who enrolled in this trial or to protocol criteria that allowed previous palliative radiotherapy up to 2 weeks before randomization, with further language encouraging patients with symptomatic tumor lesions to receive this therapy before randomization. This approach may have selected for a population of patients who had a poorer prognosis because of a high tumor burden and advanced disease; however, the results in the chemotherapy group with regard to response rate and progression-free survival do not support this interpretation.
The KEYN0TE-024 trial17 established a role for the anti-PD-1 antibody pembrolizumab versus chemotherapy as first-line treatment in patients with NSCLC with a PD-L1 expression level of 50% or more as determined by means of the Dako 22C3 PD-L1 test in a prospectively designed trial. The median progression-free survival was 10.3 months in the pembrolizumab group and 6.0 months in the chemotherapy group. The response rate was 45% in the pembrolizumab group and 28% in the chemotherapy group.
Analyses comparing treatment efficacy in patients with a PD-L1 expression level of 50% or more were not prespecified in CheckMate 026, and the two groups had an imbalance in the number of patients (88 vs. 126), thereby limiting the conclusions that can be drawn in this subgroup. By contrast, the KEYN0TE-024 trial prospectively assessed the activity of pembrolizumab versus chemotherapy in patients who had advanced NSCLC with a PD-L1 expression level of at least 50% and who had not received chemo-therapy previously.17 Other differences between the trials have been outlined in a recent review article.18 Examples include the different assays to assess PD-L1 tumor expression, the criteria related to previous radiotherapy and glucocorticoid use during the trials, and imbalances between groups in the characteristics of the patients (e.g., sex in CheckMate 026 and the lower percentage of patients who had never smoked in the immunotherapy group in KEYNOTE-024 [3%] than in Check-Mate 026 [11%]).17,18 Although the precise reasons for the divergent outcomes of the KEYNOTE-024 trial and the CheckMate 026 trial remain unclear and cannot be attributed to a single factor, the differences outlined above may be contributing factors.
In an exploratory, hypothesis-generating analysis, among patients with a high tumor-mutation burden, nivolumab was associated with a higher response rate than chemotherapy (47% vs. 28%) and with a longer median progression-free survival (9.7 vs. 5.8 months). No between-group difference was noted with regard to overall survival in the subgroup of patients with a high tumor-mutation burden, which may be explained in part by the high rate of subsequent nivolumab use (68% of patients) in the chemotherapy group. Nevertheless, the subgroup of patients with a high tumor-mutation burden in the nivolumab group had notable overall survival (median, >18 months; overall survival rate at 1 year, 64%). The level of tumor-mutation burden and the level of tumor PD-L1 expression did not appear to be associated; however, information about the tumor-mutation burden in patients with a PD-L1 expression level of less than 1% was not available, because such patients were not enrolled in this trial. These data are consistent with previous reports suggesting no association between tumor-mutation burden and PD-L1 expression in patients treated with pembrolizumab and only a weak association between tumor-mutation burden and PD-L1 expression in those treated with atezolizumab.19,20 Patients with both a high tumor-mutation burden and a PD-L1 expression level of 50% or more may have a greater likelihood of re sponse to nivolumab than those with only one or neither of these factors. Overall, the current findings are consistent with the hypothesis that immunotherapy may have enhanced activity in patients with a high tumor-mutation burden.14 However, because this was an exploratory analysis that was not prespecified, the data are hypothesis-generating and require further prospective validation.
In conclusion, nivolumab monotherapy did not result in longer progression-free survival than platinum-based chemotherapy as first-line treatment for stage IV or recurrent NSCLC in a broad population of patients with a PD-L1 expression level of 5% or more. Overall survival with single-agent nivolumab was similar to overall survival with platinum doublet chemotherapy. Nivolumab had a favorable safety profile as compared with chemotherapy, and no new safety signals were observed.
Supplementary Material
Acknowledgments
Supported by Bristol-Myers Squibb, ONO Pharmaceutical, a Cancer Center Support Grant (CA016672, to M.D. Anderson Cancer Center [Dr. Blumenschein]) from the National Institutes of Health, and a Hollings Cancer Center K12 Paul Calabresi Career Development Grant (K12 CA157688, to Dr. Wrangle).
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the patients and their families, as well as the participating teams, for making this trial possible; the staff of Dako for collaborative development of the PD-L1 IHC 28–8 pharmDx assay; Peter Szabo, M.D., Ph.D., and Danielle Greenawalt, Ph.D., for contributions to experimental design, downstream analysis, and interpretation of the data regarding the tumor-mutation burden; Judith Bushong, A.S., B.B.M., for serving as the protocol manager; and Roland Tacke, Ph.D., of Evidence Scientific Solutions, for medical writing and editorial assistance with an earlier version of the manuscript.
APPENDIX
The authors’ full names and academic degrees are as follows: David P. Carbone, M.D., Ph.D., Martin Reck, M.D., Ph.D., Luis Paz-Ares, M.D., Benjamin Creelan, M.D., Leora Horn, M.D., Martin Steins, M.D., Ph.D., Enriqueta Felip, M.D., Michel M. van den Heuvel, M.D., Tudor-Eliade Ciuleanu, M.D., Firas Badin, M.D., Neal Ready, M.D., T. Jeroen N. Hiltermann, M.D., Suresh Nair, M.D., Rosalyn Juergens, M.D., Ph.D., Solange Peters, M.D., Ph.D., Elisa Minenza, M.D., John M. Wrangle, M.D., Delvys Rodriguez-Abreu, M.D., Hossein Borghaei, D.O., George R. Blumenschein, Jr., M.D., Liza C. Villaruz, M.D., Libor Havel, M.D., Jana Krejci, M.D., Jesus Corral Jaime, M.D., Han Chang, Ph.D., William J. Geese, Ph.D., Prabhu Bhagavatheeswaran, Ph.D., Allen C. Chen, M.D., and Mark A. Socinski, M.D.
The authors’ affiliations are as follows: the Ohio State University Comprehensive Cancer Center, Columbus (D.P.C.); LungenClinic Grosshansdorf, Airway Research Center North, German Center for Lung Research, Grosshansdorf (M.R.), and Thoraxklinik, Heidelberg University Hospital, Heidelberg (M.S.) — both in Germany; Hospital Universitario Doce de Octubre, Centro Nacional de Investigaciones Oncologicas and Universidad Complutense, Madrid (L.P.-A., J.C.J.), Vall d’Hebron University Hospital, Barcelona (E.F.), and Hospital Universitario Insular de Gran Canaria, Las Palmas (D.R.-A.) — all in Spain; H. Lee Moffitt Cancer Center, Tampa, FL (B.C.); Vanderbilt University Medical Center, Nashville (L. Horn); Antoni van Leeuwenhoek Ziekenhuis, Amsterdam (M.M.H.), and University of Groningen, Universitair Medisch Centrum Groningen, Groningen (T.J.N.H.) — both in the Netherlands; Prof. Dr. Ion Chiricuta Institute of Oncology and University of Medicine and Pharmacy Iuliu Hatieganu, Cluj-Napoca, Romania (T.-E.C.); Baptist Health Lexington, Lexington, KY (F.B.); Duke University, Durham, NC (N.R.); Lehigh Valley Health Network, Allentown (S.N.), Fox Chase Cancer Center, Philadelphia (H.B.), and University of Pittsburgh Medical Center Cancer Center, Pittsburgh (L.C.V., M.A.S.) — all in Pennsylvania; Juravinski Cancer Centre, Hamilton, ON, Canada (R.J.); Oncology Department, Lausanne University Hospital, Lausanne, Switzerland (S.P.); Santa Maria Hospital, Terni, Italy (E.M.); Hollings Cancer Center, Charleston, SC (J.M.W.); Department of Thoracic-Head and Neck Medical Oncology, University of Texas M.D. Anderson Cancer Center, Houston (G.R.B.); Klinika Pneumologie a Hrudni Chirurgie, Nemocnice Na Bulovce, Prague, Czech Republic (L. Havel, J.K.); and Bristol-Myers Squibb, Princeton, NJ (H.C., W.J.G., P.B., A.C.C.).
Footnotes
A complete list of the CheckMate 026 investigators is provided in the Supplementary Appendix, available at NEJM.org.
REFERENCES
- 1.Ettinger DS, Wood DE, Akerley W, et al. NCCN guidelines insights: non-small cell lung cancer, version 4.2016. J Natl Compr Canc Netw 2016;14:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masters GA, Temin S, Azzoli CG, et al. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2015;33:3488–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543–51. [DOI] [PubMed] [Google Scholar]
- 4.Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 2012;30: 2055–62. [DOI] [PubMed] [Google Scholar]
- 5.Patel JD, Socinski MA, Garon EB, et al. PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Clin Oncol 2013; 31:4349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paz-Ares L, Mezger J, Ciuleanu TE, et al. Necitumumab plus pemetrexed and cisplatin as first-line therapy in patients with stage IV non-squamous non-small-cell lung cancer (INSPIRE): an open-label, randomised, controlled phase 3 study. Lancet Oncol 2015;16:328–37. [DOI] [PubMed] [Google Scholar]
- 7.Thatcher N, Hirsch FR, Luft AV, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol 2015;16:763–74. [DOI] [PubMed] [Google Scholar]
- 8.Zinner RG, Obasaju CK, Spigel DR, et al. PRONOUNCE: randomized, open-label, phase III study of first-line pemetrexed + carboplatin followed by maintenance pemetrexed versus paclitaxel + carboplatin + bevacizumab followed by maintenance bevacizumab in patients with advanced nonsquamous non-small-cell lung cancer. J Thorac Oncol 2015;10:134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barlesi F, Steins M, Horn L, et al. Long-term outcomes with nivolumab (Nivo) vs docetaxel (Doc) in patients (Pts) with advanced (Adv) NSCLC: CheckMate 017 and CheckMate 057 2-y update. Ann Oncol 2016;27:Suppl 6:215PD abstract. [Google Scholar]
- 12.Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2016;34:2980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gettinger S, Shepherd FA, Antonia SJ, et al. First-line nivolumab (anti-PD-1; BMS-936558, ONO-4538) monotherapy in advanced NSCLC: safety, efficacy, and cor relation of outcomes with PD-L1 status. Presented at the American Society of Clinical Oncology Annual Meeting, Chicago, June 3–7, 2014. (poster). [Google Scholar]
- 14.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology: mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 16.Wakelee HA, Wang W, Schiller JH, et al. Survival differences by sex for patients with advanced non-small cell lung cancer on Eastern Cooperative Oncology Group trial 1594. J Thorac Oncol 2006;1:441–6. [PubMed] [Google Scholar]
- 17.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375: 1823–33. [DOI] [PubMed] [Google Scholar]
- 18.Remon J, Besse B, Soria JC. Successes and failures: what did we learn from recent first-line treatment immunotherapy trials in non-small cell lung cancer? BMC Med 2017;15:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hellmann M Mutation burden, neo-antigens, and response to T cell checkpoint blockade. Presented at the 14th International Congress on Targeted Anticancer Therapies, Washington, DC, March 21–23, 2016. [Google Scholar]
- 20.Kowanetz M, Zou W, Shames D, et al. Tumor mutation burden (TMB) is associated with improved efficacy of atezolizumab in 1L and 2L+ NSCLC patients. J Thorac Oncol 2017;12:Suppl:S321 abstract. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.