Ripples in EEG recordings are a promising biomarker for epilepsy but are difficult to detect and their relationship to seizure risk remains unknown. Using computational methods, Kramer et al. show that ripples co-occurring with epileptiform discharges predict seizure risk better than spikes alone in childhood epilepsy.
Keywords: high frequency oscillations, HFO, BECTS, EEG biomarker, high density EEG
Abstract
In the past decade, brief bursts of fast oscillations in the ripple range have been identified in the scalp EEG as a promising non-invasive biomarker for epilepsy. However, investigation and clinical application of this biomarker have been limited because standard approaches to identify these brief, low amplitude events are difficult, time consuming, and subjective. Recent studies have demonstrated that ripples co-occurring with epileptiform discharges (‘spike ripple events’) are easier to detect than ripples alone and have greater pathological significance. Here, we used objective techniques to quantify spike ripples and test whether this biomarker predicts seizure risk in childhood epilepsy. We evaluated spike ripples in scalp EEG recordings from a prospective cohort of children with a self-limited epilepsy syndrome, benign epilepsy with centrotemporal spikes, and healthy control children. We compared the rate of spike ripples between children with epilepsy and healthy controls, and between children with epilepsy during periods of active disease (active, within 1 year of seizure) and after a period of sustained seizure-freedom (seizure-free, >1 year without seizure), using semi-automated and automated detection techniques. Spike ripple rate was higher in subjects with active epilepsy compared to healthy controls (P = 0.0018) or subjects with epilepsy who were seizure-free ON or OFF medication (P = 0.0018). Among epilepsy subjects with spike ripples, each month seizure-free decreased the odds of a spike ripple by a factor of 0.66 [95% confidence interval (0.47, 0.91), P = 0.021]. Comparing the diagnostic accuracy of the presence of at least one spike ripple versus a classic spike event to identify group, we found comparable sensitivity and negative predictive value, but greater specificity and positive predictive value of spike ripples compared to spikes (P = 0.016 and P = 0.006, respectively). We found qualitatively consistent results using a fully automated spike ripple detector, including comparison with an automated spike detector. We conclude that scalp spike ripple events identify disease and track with seizure risk in this epilepsy population, using both semi-automated and fully automated detection methods, and that this biomarker outperforms analysis of spikes alone in categorizing seizure risk. These data provide evidence that spike ripples are a specific non-invasive biomarker for seizure risk in benign epilepsy with centrotemporal spikes and support future work to evaluate the utility of this biomarker to guide medication trials and tapers in these children and predict seizure risk in other at-risk populations.
Introduction
In the past decade, brief bursts of high frequency oscillations (80–600 Hz) have been identified as a promising biomarker for epilepsy that track disease with higher fidelity than classic interictal spikes (Worrell and Gotman, 2011; Frauscher et al., 2017). Originally described in invasive intracranial recordings (Worrell et al., 2004), several recent studies have now identified short bursts of ripple range activity (80–200 Hz) in the scalp EEG in patients with epilepsy from a variety of aetiologies (Kobayashi et al., 2004, 2010a; Inoue et al., 2008; Andrade-Valenca et al., 2011, 2012; van Klink et al., 2016b; Chu et al., 2017), raising interest in this biomarker as a potential non-invasive measure of epileptogenicity. Consistent with observations from intracranial EEG recordings (Zijlmans et al., 2009), early work investigating scalp-recorded ripples has shown that these events decrease with medication in infantile spasms (Kobayashi et al., 2015) and track with disease severity, where more frequent ripples were observed in epilepsy patients with more frequent seizures (van Klink et al., 2016b). In addition, ripple-based classification methods identified patients with epilepsy with more specificity than those based on spikes (van Klink et al., 2016b).
Despite the enormous potential clinical utility of scalp-recorded ripples, translation of this biomarker to clinical practice has been limited (Frauscher et al., 2017). Two factors impeding clinical testing and application of ripples include the difficulty of detecting these low amplitude, brief events in noisy brain recordings, and the time-consuming and subjective process of manual detection and verification of ripple events. Recent advances in ripple detection include the development of automated algorithms (Blanco et al., 2010; Zelmann et al., 2010; Dümpelmann et al., 2012; von Ellenrieder et al., 2012; Malinowska et al., 2015; Gliske et al., 2016; Charupanit and Lopour, 2017; Chu et al., 2017), and the observation that nearly half of ripples co-occur with interictal epileptiform discharges (or ‘spikes’), termed ‘spike ripple’ events (Urrestarazu et al., 2007; Jacobs et al., 2009; von Ellenrieder et al., 2012; van Klink et al., 2016a). Spike ripple events are easier to detect than ripples alone, and may have greater pathological significance. Ripples co-occurring with a spike are more closely related to the seizure onset zone than ripples without a spike (Roehri et al., 2018) and are more likely to represent a pathological event than healthy physiology (Blanco et al., 2011; van Klink et al., 2016b; Chu et al., 2017).
Here we sought to objectively test scalp spike ripple events as a non-invasive biomarker for seizure risk. To do so, we analysed a population of patients with the most common childhood focal epilepsy syndrome, benign epilepsy with centrotemporal spikes (BECTS), and healthy control children. We chose this patient population for two reasons. First, despite extensive clinical experience with this disease, there are no clinical predictors available to determine an individual child’s risk of subsequent seizure. One-third of children will have only a single seizure, while others will have recurrent seizures over several years (Bouma et al., 1997). A non-invasive biomarker to isolate which children are at ongoing risk of seizure would improve treatment decisions and prevent the consequences of over- or under-medication during critical years of cognitive and psychosocial development in this large cohort of children. Second, because epilepsy in BECTS spontaneously remits, this patient population provides an ideal cohort to test the ability of a biomarker to track seizure risk over the course of resolving disease.
We hypothesized that the spike ripple rate would be higher in BECTS children during the active phase of epilepsy compared to healthy control children and children with resolving or controlled epilepsy and that the presence of spike ripples would predict seizure risk better than classically identified spikes alone. To test these hypotheses, we quantified spike ripple events from EEG recordings in a cohort of children with BECTS at different stages of the disease and healthy control children, using semi-automated and fully automated detection techniques. We then compared the characteristics and diagnostic accuracy of spike ripple events to manually and automatically detected spikes in these children.
Materials and methods
Subject recruitment
All children aged 4–15 years who received a clinical diagnosis of BECTS by a child neurologist following 1989 ILAE criteria (Commission on Classification and Terminology of the International League Against Epilepsy, 1989) were eligible for this prospective study. Candidate BECTS subjects without both a history of focal motor or generalized seizures and an EEG showing sleep activated centrotemporal spikes were excluded (Fisher et al., 2014). Healthy control school-aged subjects without a history of seizure or known neurological disorder were also recruited. BECTS and healthy control subjects with a history of autism spectrum disorder, intellectual disability, or other unrelated neurological disease were excluded. Children with attention disorders and mild learning difficulties were included, as these profiles are consistent with known BECTS comorbidities (Wickens et al., 2017). Twenty-seven children with BECTS and 17 healthy control children were enrolled. Of those enrolled, five children with BECTS and four healthy control children did not fall asleep during EEG recording and were excluded from this analysis. One further child with BECTS was excluded because of poor EEG recording quality (continuous artefact contaminating all electrodes). In total, 21 children with BECTS (aged 4.9–16.8 years, 17 males) and 13 healthy control subjects (aged 8.7–14.3 years, five males) were included in this study. Two children with BECTS returned after a minimum of 12 months for repeat evaluations.
Clinical data on each subject including age, time from most recent seizure, and medication use were collected from chart review and updated on the day of EEG recording. Detailed clinical data on each subject are provided in Table 1. This research received prior approval by the Massachusetts General Hospital and Boston University institutional review boards and informed consent was obtained from each subject and guardian.
Table 1.
Subject characteristics
| Patient | Age, years | Gender | Group | Medications | Electrodes |
|---|---|---|---|---|---|
| 1 | 4.9 | M | Active | LCM | T3, C4 |
| 2 | 13.7 | F | Remission | None | T3, C4 |
| 3 | 11.8 | M | Seizure-free | LEV | C5, C4 |
| 4 | 14.7 | M | Active | None | C5, T4 |
| 4 Visit 2 | 16.8 | M | Remission | None | C3, C4 |
| 5 | 14.9 | M | Remission | None | C3, C4 |
| 6 | 13.3 | M | Seizure-free | LEV | C3, C4 |
| 7 | 9.1 | F | Active | LEV, LTG | C3, C4 |
| 8 | 9.8 | M | Active | OXC | CP5, C4 |
| 9 | 8 | M | Remission | None | CP5, C4 |
| 10 | 11 | F | Active | None | C5, C4 |
| 10 Visit 2 | 12 | F | Remission | None | CP3, C4 |
| 11 | 8.6 | F | Seizure-free | LEV, LTG | C3, C4 |
| 12 | 9 | F | Healthy | - | C3, C4 |
| 13 | 9 | M | Active | None | C5, FC6 |
| 14 | 12.9 | F | Healthy | - | C3, C4 |
| 15 | 12.2 | F | Healthy | - | C3, C4 |
| 16 | 11.5 | M | Remission | None | C3, C2 |
| 17 | 12.8 | M | Seizure-free | LEV | C3, C4 |
| 18 | 10.5 | F | Active | LEV | C3, F6 |
| 19 | 10.4 | M | Seizure-free | LEV | C3, C2 |
| 20 | 11.9 | M | Seizure-free | LEV | C1, C4 |
| 21 | 11.6 | M | Remission | None | C3, C6 |
| 22 | 9.9 | M | Active | None | CP5, C4 |
| 23 | 14.2 | F | Healthy | - | C3, C4 |
| 24 | 11.3 | M | Active | None | C5, C4 |
| 25 | 9.4 | F | Healthy | - | C3, C4 |
| 26 | 13.6 | F | Healthy | - | C3, C6 |
| 27 | 9.4 | M | Healthy | - | C3, C4 |
| 28 | 14.3 | F | Healthy | - | C3, C4 |
| 29 | 13.4 | F | Healthy | - | C5, C4 |
| 30 | 14.6 | M | Active | LCM | C5, C4 |
| 31 | 8.7 | M | Healthy | - | C3, C4 |
| 32 | 10.9 | M | Healthy | - | C3, C4 |
| 33 | 11.8 | M | Healthy | - | C3, C4 |
| 34 | 11.5 | M | Healthy | - | C3, C4 |
LCM = lacosamide; LEV = levetiracetam; LTG = lamotrigine; OXC = oxcarbazepine.
Subjects were grouped as belonging to one of three categories of seizure risk: BECTS with active disease (here, defined as having had a seizure within the last 12 months, n = 10), BECTS seizure-free (here, defined as seizure-free for at least 12 months, n = 13), and healthy controls (n = 13). Of the 10 active BECTS patients, five were not treated with anticonvulsant drugs (three because of combined parent and provider preference, one because of parent preference, and one because of side effects from a medication trial). Here, we chose to use 1 year of seizure freedom among BECTS subjects to signify a low risk of seizure recurrence because the vast majority of children with BECTS who are seizure-free for 1 year have a sustained remission (Berg et al., 2004). As children who are seizure-free for longer are less likely to have a subsequent seizure (Berg et al., 2001; Sillanpaa et al., 2017), we also evaluated the relationship between spike ripple rate and duration seizure-free as a continuous variable (see below).
EEG acquisition and preparation
All subjects arrived to EEG recording sessions after instructions for sleep restriction (recommended maximum of 4 h of sleep) the previous night. Sleep deprivation prior to an EEG is standard protocol in order to increase the likelihood of capturing sleep in the recording. To optimize spatial and temporal resolution of the EEG signal (Chu et al., 2017), EEG were recorded with a 70-channel cap based on the 10–10 electrode placement system at a 2035 Hz sampling rate (Easycap, Vectorview, Elekta-Neuromag) with additional electrodes placed at T1 and T2 locations. EEG data were visually inspected by a board-certified neurophysiologist (C.J.C.) and channels with significant artefact were excluded from analysis. To minimize the impact of movement and muscle artefacts, and improve sensitivity and consistency of spike measures (Tenney et al., 2016), non-REM sleep was selected for analysis and all available data per subject were used. A median of 780 s of data were used for analysis (range 214–2840 s, mean 1030 s). We note that there was no difference in duration of EEG recording between BECTS and healthy control groups (two-tailed t-test, P = 0.117). Data were referenced to the average reference.
Semi-automated and automated detection of spike ripple events
As patients with BECTS typically have bilaterally independent spikes (Commission on Classification and Terminology of the International League Against Epilepsy, 1989), one channel was selected from each hemisphere to represent each spike population for analysis. If spikes were present, then the channel in which the spike amplitude was maximal was selected. If no spikes were observed, the C3 and C4 electrodes were selected, as these electrodes are most commonly involved in this focal epilepsy syndrome (Koutroumanidis et al., 2017). In the case of artefact contamination, the closest adjacent artefact-free channel was selected. Channel selections are listed in Table 1. The detection of spike ripple events followed a previously described procedure (Chu et al., 2017) using the software available at https://github.com/Mark-Kramer/Spike-Ripple-Detector-Method. To summarize, candidate events that contain a high frequency oscillation (100–300 Hz) approximately sinusoidal in shape, with at least three cycles, that co-occur with a large amplitude discharge were automatically identified, and then validated through visual inspection (Chu et al., 2017). We report for each subject the rate (detections/min) of both the automatically detected and the validated spike ripple events. We note that epileptiform discharges are typically abundant (present at a rate >0.1 Hz) during non-REM sleep in BECTS (Commission on Classification and Terminology of the International League Against Epilepsy, 1989) and there was no relationship between duration of EEG recording and the rate of detected or validated spike ripple events (linear regression, P = 0.29 for predictor of rate in both cases).
Validation of the candidate events requires the subjective judgement of ripple quality by a reviewer, which may introduce potential confounds in the resulting analysis. To assess the inter-rater agreement of each validation, we applied the following procedure. First, we developed a custom procedure to choose at random (without replacement) a subject and hemisphere, and visualized all candidate spike ripple events from this subject and hemisphere for validation. We performed this random selection of a subject, and visual inspection of this subject’s candidate events, until all 36 EEGs were analysed (n = 1534 candidate events total). We designed this procedure to replicate a realistic scenario in which a clinical EEG would be reviewed. Two reviewers with varied experience (C.J.C. and M.A.K.) performed this entire procedure and classified each candidate spike ripple event, blinded to subject and hemisphere and the other reviewer’s classifications.
Manual and automated detection of spike events
For the manual detection of spikes, each sleep epoch was reviewed in 10 s increments by an experienced board certified paediatric neurophysiologist (C.J.C.) in bipolar, average, and nasion-referential montages. All epileptiform discharges were manually marked according to standard clinical criteria (Commission on Classification and Terminology of the International League Against Epilepsy, 1989; Niedermeyer and Lopes da Silva, 2005).
For automated spike detection, we apply one of the most popular automated spike detection methods in common use: the Persyst 13 spike detector (Persyst Development Corporation, San Diego). This commercial software has been shown to be non-inferior to human experts in two separate and large studies: one involving >38 000 manually marked spikes by four neurology board-certified practicing clinicians (Scheuer et al., 2017) and the other involving 5474 manually marked individual spikes by three senior EEG technologists (Joshi et al., 2018). Despite these encouraging results, this automated spike detector does not yet perform well enough to replace human experts (Westover et al., 2017), but nonetheless provides a reproducible approach to detect spikes with similar performance to humans. In our analysis, we applied the Persyst 13 algorithm (as outlined in Scheuer et al., 2017) to the same patients and intervals of data analysed with the semi-automated spike ripple detector. To complement our focus on a single active channel for spike ripple detections, for each patient, we selected the 10–20 channel with the largest number of spike detections reported by Persyst 13 for continued analysis of spike events.
Analysis of the spatial profiles of spike ripples and spikes
To assess the spatial profile of spike ripples and spikes, an experienced electroencephalographer (C.J.C.) first identified through visual inspection of the EEG data the channel with the maximal spike amplitude for each patient; we label this the primary channel. Four channels immediately adjacent to the primary channel—in the anterior, posterier, left and right directions—were then identified; we label these the secondary channels. Finally, four channels immediately adjacent to the secondary channels—again in the anterior, posterior, left and right directions—were identified; we label these the tertiary channels. For each patient with active BECTS (n = 10), we determined for each channel: (i) the rate of candidate spike ripple events; and (ii) the average spike amplitude. To compute the latter, we first identified all validated spike ripples at the primary channel. We then determined the difference between the maximum and minimum voltage in a ±100 ms interval surrounding the time of the validated spike ripple at the primary, secondary, and tertiary channels. We chose the duration of ±100 ms because the typical duration of a spike is <200 ms. We interpret the difference between the maximum and minimum voltage within this interval as an approximation of the spike amplitude. We average these spike amplitudes across all spike ripple occurences to calculate the average spike amplitude for each channel.
Upon computing the results for each patient’s four secondary (tertiary) channels, we then average these results across the secondary (tertiary) channels. Then, to compare (i) the spike ripple rate; and (ii) the average spike amplitude across patients, we normalize the results for each patient by dividing the results of the primary, secondary, and tertiary channels by the value in the primary channels.
To compare the normalized spike ripple rate to the normalized spike amplitude at the secondary or tertiary channels, we implement a bootstrap procedure (Kramer and Eden, 2016). To do so, we define as the statistic the difference between the median normalized spike ripple rate and the median normalized spike amplitude. To test the null hypothesis of no difference between these two median quantities, we sample with replacement from the combined set of 20 normalized values from all patients with active BECTS (n = 10) to generate a surrogate set of 10 normalized spike ripple rates, and a surrogate set of 10 normalized spike amplitudes. We generate these surrogate data 10 000 times, and for each instance we compute the test statistic. We then compare the observed value of the statistic to the distribution of surrogate values, and define as a P-value the proportion of surrogate statistics less than the observed statistic.
Statistical analysis
To mitigate the impact of false positive results following from the multiple testing problem, we tested three a priori hypotheses: (1) that the mean spike ripple rate is higher in children with active BECTS compared to healthy control children and children with BECTS who are seizure-free; (2) that the spike ripple rate decreases with duration seizure-free in patients with BECTS; and (3) that the presence of spike ripples would have greater diagnostic accuracy than the presence of manually identified spikes alone to classify groups based on seizure risk.
For hypothesis (1), we applied a one-way ANOVA with groups corresponding to the three patient populations. Upon finding a significant effect, we performed group comparisons of the spike ripple rates using one-tailed t-tests, following the protected least significant difference method (Kass et al., 2014).
For hypothesis (2), we examined the relationship between the spike ripple rate and duration seizure-free for the validated spike ripple detections. To do so, we constructed a generalized linear model, choosing a binomial distribution for the response variable, defined for each patient as the spike ripple rate (e.g. the number of validated spike ripples per the data length in seconds). The model contained a single predictor—the duration seizure-free—and used the logistic link function. We tested the hypothesis that duration seizure-free is a significant predictor of the spike ripple rate using all BECTS patients. We fit the models and performed statistical tests of the predictor’s significance using the function fitglm in MATLAB. To assess model goodness-of-fit, we compared the single predictor model to a constant model by computing the Akaike information criterion (AIC) and a chi-square test for nested models; we found in all cases that the single predictor model reduced the AIC and provided a statistically significant improvement compared to the constant model.
For hypothesis (3), we first developed a logistic regression model to examine whether the presence of a spike ripple is predictive of active BECTS. For this model, we computed the impact of a non-zero spike ripple rate on the odds of an active BECTS classification. To rule out the potential confounding impact of medication, we explored the impact of medication status on spike ripple rate using a two-tailed t-test.
We then classified each patient as having active BECTS in two ways: (i) the presence of at least one validated spike ripple; or (ii) the presence of at least one manually detected spike. To compare these two classification schemes, we computed the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) and tested for differences using the exact binomial test (Zhou et al., 2011) and the weighted generalized score statistic (Kosinski, 2013) using the R-package DTComPair (Stock and Hielscher, 2015). We also provide the performance of manual spikes on all tests used in hypotheses (1) and (2) for comparison to spike ripples.
We note that for hypothesis (3), the PPV, NPV, sensitivity, and specificity of spike ripples and spikes as detected using manual classification are calculated based on the presence or absence of at least one spike ripple or spike event, respectively. We chose this approach because, for both spikes and spike ripples, experts have excellent consensus on the presence or absence of an event in a subject’s recording (e.g. kappa of 0.83 for the presence of epileptiform discharges, see Stroink et al., 2006; kappa of 0.73 for the presence of spike ripples, as reported below). However, inter-rater agreement at the level of an individual spike or spike ripple (and consequently spike ripple rate or spike rate) is not as reliable, estimated to be 13–18% for spikes (Webber et al., 1993; Scheuer et al., 2017) and 78% (kappa 0.55) for individual spike ripples, as reported here. Thus, based on these observations, the optimal classification threshold based on rate will vary by reviewer, and the results may be misleading and would not be replicable. We also note that although inter-observer agreement is poor for spikes and spike ripples, there is good consistency in any individual reader’s style across subjects (Webber et al., 1993; Chu et al., 2017). We therefore use manually identified spike and spike ripple rates to characterize the difference in rates between groups and the relationship between event rates and duration seizure-free for hypotheses (1) and (2).
We confirmed the objectivity of the results for hypotheses (1–3) by repeating all analyses on the automatically detected candidate spike ripples and spikes. Here, for patient classification, we compared the performance of the automated spike ripple detector with the automated spike detector. To do so, we first computed a threshold corresponding to the optimal operating point in the receiver operator characteristic (ROC) curve for each automated method. We then thresholded the automated spike ripple and spike rates such that patients with rates above (below) the threshold were classified as active (not active). As these automated techniques require no subjective decisions, we expect that creating an ROC curve and selecting the optimum rate upon which to compute PPV, NPV, sensitivity, and specificity will be reproducible.
To correct for multiple comparisons, we implemented a procedure to control the false detection rate proposed by Benjamini and Hochberg (1995) with a false discovery rate level of q = 0.05. The exact P-values, effect sizes, and 95% confidence intervals (CI) are reported. All values are significant unless noted otherwise.
Data availability
Raw data were generated at Massachusetts General Hospital and the Athinoula A. Martinos Center for Biomedical Imaging. Derived data supporting the findings of this study are available from the corresponding author on request. Software for the detection of spike ripple events is available at https://github.com/Mark-Kramer/Spike-Ripple-Detector-Method.
Results
Spike ripples are identified with good inter-rater agreement
We applied a semi-automated method to detect spike ripple events; candidate spike ripple events detected by the method were subsequently validated by visual inspection blinded to subject group. When assessed at the level of individual spike ripple events, we found good inter-rater agreement between two reviewers; the observed proportional agreement was 0.78 and the kappa statistic was 0.55, consistent with studies requiring manual marking of spike ripple events (van Klink et al., 2016b). We also determined for each reviewer whether each patient’s EEG possessed a validated spike ripple event, or not. Comparing this patient-level classification (n = 35 EEGs with candidate spike ripple detections), we found improved agreement between the two reviewers; the observed proportional agreement was 0.89 and the kappa statistic was 0.73. These results suggest that the bias introduced by the subjective visual inspection of the candidate spike ripple events is low, and that the repeatability of the procedure is good at the level of an individual spike ripple event. At the level of patient classification based on the cumulative classification of events detected over the duration of the EEG recording (here, ∼15 min of NREM sleep per subject), repeatability was substantial. Representative examples of agreed positive, agreed negative, and disagreed candidate spike ripple events are shown in Fig. 1.
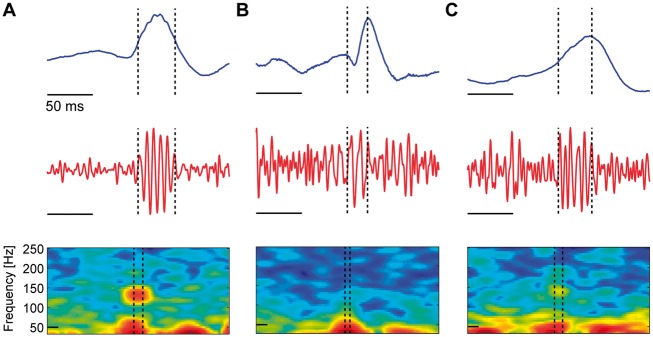
Figure 1.
Three example candidate spike ripple events. Each subfigure displays the unfiltered EEG data (blue), the bandpass filtered EEG data (100–300 Hz, red), and the spectrogram of the EEG data in an interval surrounding the spike ripple event (dashed vertical lines) with power (in decibels) shown in colour. Both reviewers classified the candidate spike ripple events (A) as a true spike ripple, (B) as a false spike ripple, or (C) the reviewers disagreed on the classification. In A, a distinct high frequency ‘spectral island’ is visually evident in the spectrogram, and a ripple is visually evident on the spike in the unfiltered data.
As the detector was designed to identify spike ripple events with low sensitivity and high specificity (Chu et al., 2017), for the analysis that follows, we classified the 22% of events for which the reviewers disagreed as validated spike ripple events. As two channels were evaluated per EEG, for each EEG, we then selected for further analysis the channel with the largest number of validated spike ripple events. In this manner, our results reflect the lowest threshold of validated spike ripple events between the two combined reviewers and the largest number of detections for each subject. In what follows, we also repeat the analysis with candidate spike ripple events without classification, and find qualitatively consistent results.
Spike ripples are more spatially restricted than spikes
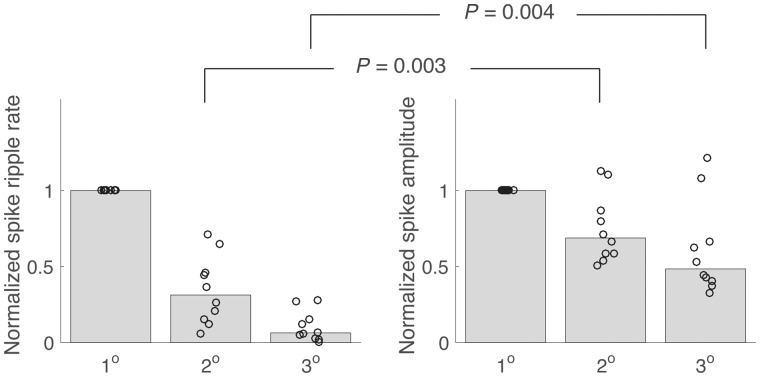
Here we analyse spike ripples that occur at the channel in which the spike amplitude is maximal in the left and right hemispheres. The spatial focus imposed by choosing a single electrode from each hemisphere is motivated by prior observations showing ripples are more spatially focal than spikes (van Klink et al., 2016a). To test this prior result in the scalp EEG data collected here, we applied the semi-automated spike ripple detector to secondary channels immediately adjacent to the channel of maximal spike amplitude (the primary channel), and tertiary channels adjacent to the secondary channels. We found that the candidate spike ripple rate decreases dramatically with distance; the median rate at secondary channels is 31% of that observed at the primary channel, and 6% at the tertiary channels (Fig. 2). In comparison, the median spike amplitude (see ‘Materials and methods’ section) at the secondary channels is 69% of that observed at the primary channels, and 48% at the tertiary channels; the difference between the median spike amplitude and median spike ripple rate is significant (P = 0.003 and P = 0.004, for the secondary and tertiary channels, respectively, bootstrap resampling procedure, see ‘Materials and methods’ section). We conclude that, while both spike amplitude and spike ripple rate decrease with distance from a spatial focus, the spike ripple rate decreases significantly more rapidly. This result, consistent with previous observations (van Klink et al., 2016a), motivates the characterization of spike ripple events using the channel of maximal spike amplitude.
Figure 2.
Spike ripples are more spatially discrete than spikes. The normalized spike ripple rate and the spike amplitude at the primary channel (1°), secondary channels (2°), and tertiary channels (3°). Each circle indicates one patient, and the bar height indicates the median across patients. As the distance from the primary channel increases, both measures decrease, although the decrease is significantly faster for the spike ripple rate.
Spike ripple rate is higher in active disease
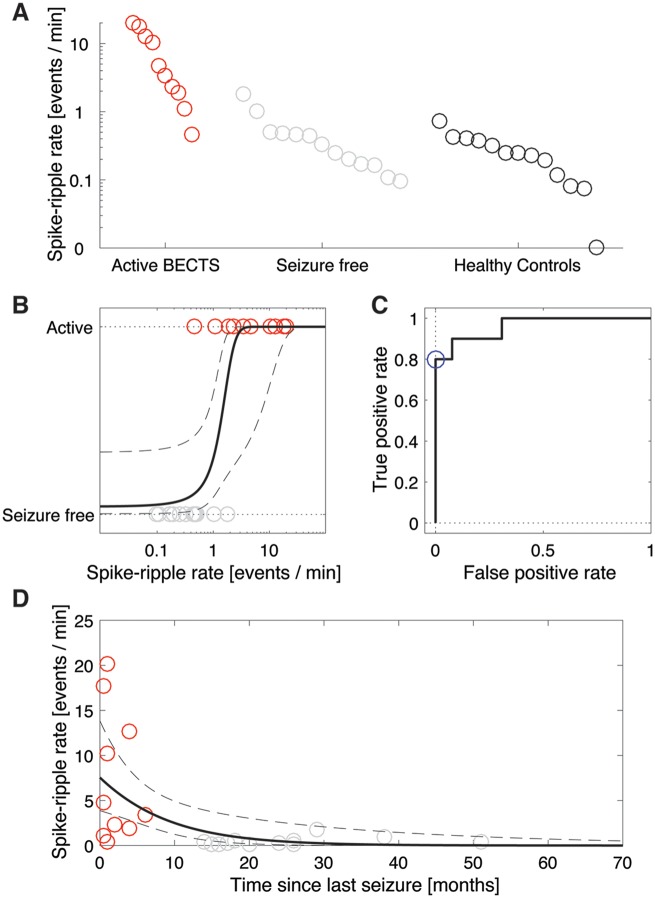
We found a significant difference in the mean spike ripple rate between the three groups of active BECTS, seizure-free, and healthy control subjects (one-way ANOVA, P = 0.00094). To investigate this difference further, we then compared the mean spike ripple rates between the patients with active BECTS and the other groups. We found that the spike ripple rates were significantly higher in patients with active BECTS (n = 10) compared to healthy controls (n = 13, P = 0.0018), and compared to BECTS patients who were seizure-free (n = 13, P = 0.0018, Fig. 3A).
Figure 3.
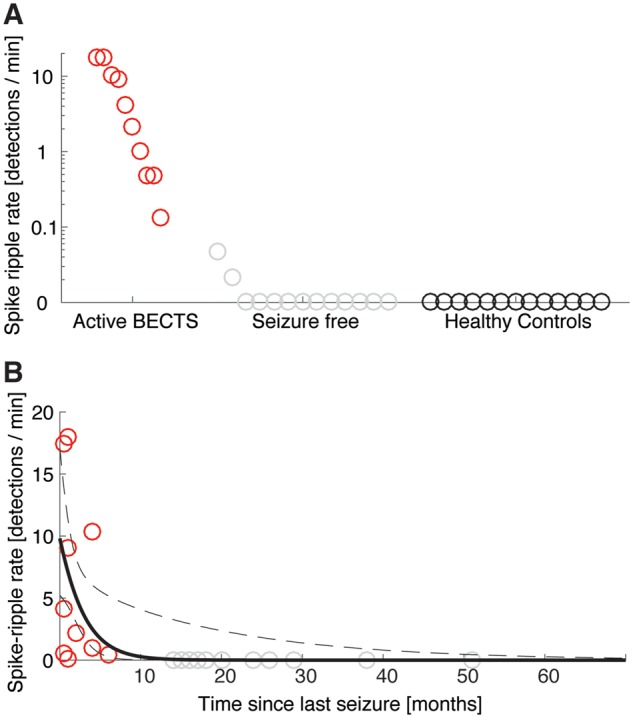
The spike ripple rate is significantly higher in patients with active BECTS, and decreases with each seizure-free month. (A) Each circle indicates the spike ripple rate (detections/min) for a subject, which are divided into three categories. All patients with active BECTS (red circles) have non-zero spike ripple rates, whereas no healthy controls and only two seizure-free patients with BECTS have non-zero spike ripple rates. (B) The spike ripple rate versus time since last seizure for all patients with BECTS. Red (grey) circles indicate patients with active (seizure-free) BECTS. The rate decreases with time since last seizure (black curve indicates model fit, mean in solid, 95% CI in dashed lines).
Among patients with active BECTS, anticonvulsant drug treatment status did not impact spike ripple rate (n = 5 ON medication, n = 5 OFF medication, P = 0.48). We found a similar result for BECTS patients without recent seizure (n = 6 ON medication, n = 7 OFF medication, P = 0.22). Thus, spike ripple rate was decreased in patients taking medication, only if they were also seizure-free.
Spike ripple rate decreases with duration seizure-free
To examine the relationship between spike ripple rate and disease course, we modelled the spike ripple rate as a function of the time since a patient’s last seizure for all patients with BECTS. Visual inspection revealed an inverse relationship (Fig. 3B). To characterize this relationship, we constructed a generalized linear model, see ‘Statistical analysis’ section. Among all BECTS children, we found that each month seizure-free significantly decreased the odds of spike ripple by a factor of 0.69 (95% CI: 0.51–0.93, P = 0.024; Fig. 3B).
We conclude from the generalized linear model that a significant inverse relationship exists between the spike ripple rate and time since a patient’s last seizure; the longer duration seizure-free, the lower the spike ripple rate.
Spike ripples predict seizure risk
To determine whether the presence of a spike ripple increases the odds of active disease, we constructed a logistic regression model with outcome disease state (active or free) and binary predictor of spike ripple rate (non-zero or not). The bounds of the 95% Bayesian confidence region for the effect of this predictor included odds ≥1.65, thus, we conclude that the presence of spike ripples increases the odds of active disease by at least 65%.
Spike ripples have improved diagnostic accuracy compared to spikes
To compare the classification performance of spike ripples and spikes, we computed four measures: the sensitivity, specificity, PPV, and NPV. We computed all four measures for patients with active disease and those who were seizure-free (Table 2). When comparing the presence of at least one manually identified event, we detected no difference in sensitivity or NPV between spikes and spike ripples (P = 1.0); however, spike ripples were more specific than spikes as a diagnostic biomarker of active disease (P = 0.016) and had a better PPV compared to spikes (P = 0.007).
Table 2.
Spike rate and spike ripple rate diagnostic characteristics
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| Spike ripplesa (95% CI) | 1 (1,1) | 0.85 (0.65,1) | 0.83 (0.62,1) | 1 (1,1) |
| Spikesa (95% CI) | 1 (1,1) | 0.31 (0.057, 0.56) | 0.53 (0.27,0.75) | 1 (1,1) |
| Automated spike ripplesb (95% CI) | 0.8 (0.55,1) | 1 (1,1) | 1 (1,1) | 0.87 (0.70,1) |
| Automated spikesb (95% CI) | 1 (1,1) | 0.77 (0.54,0.998) | 0.77 (0.54,0.998) | 1 (1,1) |
aBased on presence or absence of event.
bBased on optimal threshold using ROC curve.
Consistent with the spike ripple results, we found that the spike rate is higher in patients with active BECTS compared to seizure-free patients (P = 0.00052) and healthy control subjects (P = 3.4 × 10−10; Fig. 4A). Among BECTS subjects, the presence of a spike increases the odds of active disease. Computing a 95% Bayesian confidence region for the effect of this predictor, we found that the presence of spikes increased the odds of active disease by at least 1.15 (the 95% confidence region included odds ≥1.15). We conclude that the presence of spike events increases the odds of active disease by at least 15%. We found no relationship between spike rate and the time since last seizure (P = 0.13; Fig. 4B).
Figure 4.
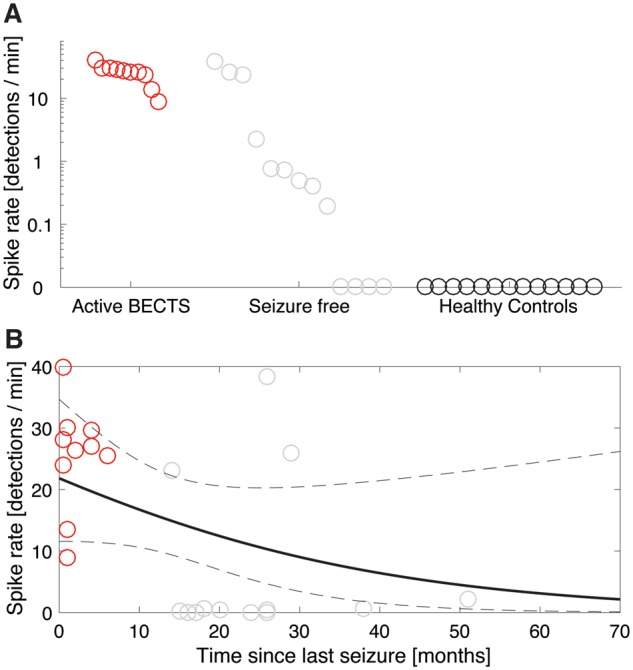
Manually detected spikes performance. (A) The spike rate is higher in patients with active BECTS, though most seizure-free subjects have a non-zero spike rate. Each circle indicates the spike rate (events/min) for the three patient categories. (B) No relationship between the spike rate and time since last seizure is detected. The black curve indicates a generalized linear model fit with mean (solid) and 95% CIs (dashed). In both figures, red (grey) circles indicate patients with active BECTS (seizure-free BECTS).
Automatic detection of spike ripples produces consistent result
Nearly all methods to detect ripples in human data require the subjective classification by an expert reviewer. There are many disadvantages to manual classification, including the possible introduction of bias, the difficulty of standardizing the results of different reviewers, and the time required to perform the classification. To reduce the impact of manual classification, we repeated the analysis above using all candidate spike ripple events, without validation. In this way, the analysis was fully automated, and avoided any subjective classification of events.
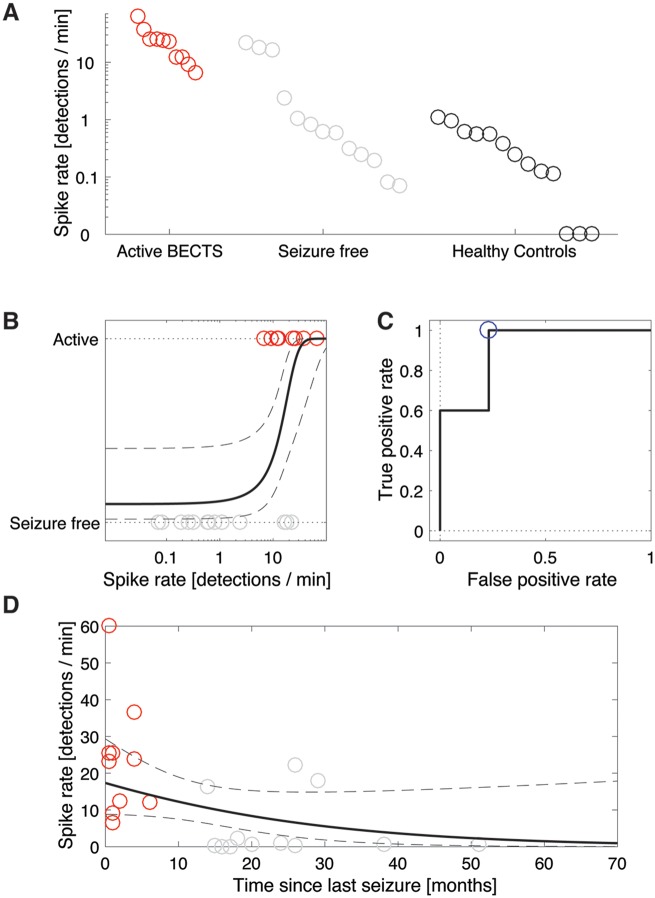
Using the candidate spike ripple events, we found that most patients’ EEGs (n = 35 of 36) had a non-zero spike ripple rate, including the healthy controls (Fig. 5A). We found a significant difference in the mean spike ripple rate between groups (one-way ANOVA, P = 0.00035), where the spike ripple rate was significantly higher in patients with active BECTS (n = 10, 7.5 ± 2.3 events/min) compared to healthy controls (n = 13, 0.27 ± 0.05 events/min, P = 0.0008), and compared to patients who were seizure-free (n = 13, 0.46 ± 0.13 events/min, P = 0.0011).
Figure 5.
Analysis of fully automated detection of spike ripple events produces consistent results. (A) The spike ripple rate is higher in patients with active BECTS. Each circle indicates the spike ripple rate (events/min) for the three patient categories. (B) Patients separated into those with active BECTS (red circles) and seizure-free (grey circles). A logistic regression model (black curves, mean in solid, 95% CIs in dashed) indicates a significant increase in the odds of active BECTS with increasing spike ripple rate. (C) From the ROC curve (black), the optimal operating point (blue) is used for classification. (D) The spike ripple rate decreases with time since last seizure. The black curve indicates a generalized linear model fit with mean (solid) and 95% CIs (dashed).
Constructing a standard logistic regression model of disease state (active or seizure-free) with a single predictor of automated spike ripple rate, we found that spike ripple rate was a significant predictor (P = 0.032) of active BECTS; an increase in spike ripple rate by 0.1 events/min increased the odds of active BECTS by 26% (95% CI: 1.9%–55%, Fig. 5B).
Medication status did not impact spike ripple rate among patients with active BECTS (n = 5 ON medication, n = 5 OFF medication, P = 0.35), nor among BECTS patients without recent seizure (n = 6 ON medication, n = 7 OFF medication, P = 0.29).
All patients with BECTS (n = 23) had a non-zero spike ripple rate, which decreased with time since last seizure (Fig. 5D). Estimating the relationship between spike ripple rate and the time since last seizure for these patients, we found that each unit increase in month significantly decreased the odds of candidate spike ripple by a factor of 0.89 (95% CI: 0.82–0.96, P = 0.0082). We again conclude that the longer the time since a patient’s last seizure, the lower the spike ripple rate.
In conclusion, fully automated detection of candidate spike ripple events qualitatively reproduced the results of the validated spike ripple events.
Automatically detected spike ripples have improved diagnostic accuracy compared to automatically detected spikes
To compare the classification of patients with the two automated methods, we used the optimal operating point in the ROC curve as the threshold for classification of spike ripples (Fig. 5C) and spikes (Fig. 6C). We found that spike ripples had higher specificity and PPV than spikes, although these differences were not statistically significant (Table 2; P = 0.25 and P = 0.11, respectively).
Figure 6.
Fully automated spike detection performance. (A) The spike rate is higher in patients with active BECTS. (B) A logistic regression model (black curves, mean in solid, 95% CIs in dashed) indicates a significant increase in the odds of active BECTS with increasing spike rate. (C) From the ROC curve (black), the optimal operating point (blue) is used for classification. (D) No relationship between the spike rate and time since last seizure is detected. The black curve indicates a generalized linear model fit with mean (solid) and 95% CIs (dashed). In all figures, red (grey) circles indicate patients with active BECTS (seizure-free BECTS).
Similar to the automated spike ripple method, we found that the automated spike rate is significantly higher in patients with active BECTS compared to seizure-free patients (P = 0.00094) and healthy control subjects (P = 3.1 × 10−5; Fig. 6A). For the automated spike detector, the odds of active disease increased significantly with spike rate (P = 0.010; Fig. 6B). While the automated detection of spike ripples decreased significantly with time from last seizure (P = 0.0082; Fig. 5D), we found no such relationship for the automated detection of spikes (P = 0.096; Fig. 6D).
Discussion
Epilepsy is a common neurological disorder, affecting 1 in 26 Americans in their lifetime (Institute of Medicine, 2012), yet diagnosis and management of this disease remains empiric, requiring a trial-and-error approach to determine if and when seizures occur. A reliable, non-invasive biomarker for seizure risk could improve stratification of those potentially at risk from a variety of insults such as trauma, infection, or genetic predisposition (Engel, 2011). Here, we used a unique population of patients with a self-limited epilepsy syndrome to test a proposed non-invasive biomarker for epileptogenicity, spike ripples. We found that spike ripples can be reliably identified in a subject’s scalp EEG using both expert and automated techniques, that these events track with seizure risk in BECTS, and that validated spike ripples improve diagnostic accuracy of seizure risk compared to manually identified spikes.
Growing evidence has highlighted spike ripples as a promising new biomarker of epilepsy in non-invasive EEG studies, with continuing work to better characterize and understand these signals (Frauscher et al., 2017). On scalp recordings, only a small percentage (3–22%) of spikes co-occur with ripples (Andrade-Valenca et al., 2011; Melani et al., 2013; Chu et al., 2017), though approximately half of ripples co-occur with spikes (Andrade-Valenca et al., 2011; Melani et al., 2013). The onset of ripples in spike ripple events typically precedes the spike peak and ripples are more spatially restricted than spikes (van Klink et al., 2016a), suggesting that this coupled activity may provide higher specificity for the epileptogenic process. Our work contributes to these growing observations and helps validate the reliability, utility, and relevance of this biomarker in non-invasive EEG recordings. Previously, we have shown good intra-rater reliability in identifying spike ripple events using a semi-automated technique (Chu et al., 2017). Here, we show good inter-rater reliability, as well as good performance in an unsupervised, fully-automated setting.
We note that, in developing the spike ripple detector, we must balance the trade-off between sensitivity and specificity (Chu et al., 2017). An overly sensitive detector would require visual inspection of a high number of candidate ripple events, making the method less feasible in practice. An overly specific detector would result in missed detections of active BECTS patients. We have shown previously that the sensitivity of the detector to capture spike ripple events is 62% compared to manual review (Chu et al., 2017). As spike ripple rate decreases with duration seizure-free in BECTS patients, and is low but non-zero in some healthy controls, missed detections in a patient with a very low spike ripple rate may have low clinical significance. Here, the detector successfully identified all subjects with active BECTS. By changing parameters in the spike ripple detector (e.g. the envelope threshold; see Chu et al., 2017), we may increase the sensitivity while reducing the specificity. Developing a more sensitive measure, while maintaining the practical utility of the method, would require additional development, for example an automated, machine learning procedure to classify the spectrogram images (Fig. 1). The results reported here were based on the selection of the channel with maximal spike amplitude, or in the case of automated spike detection, the channel with the greatest number of spike detections. Inclusion of all channels to derive a measure of the total burden of spike ripple events or spikes may improve the sensitivity of the method. However, the inclusion of non-informative channels may increase the variability in detection estimates, and thus reduce classification performance. We also note that intracranial studies have suggested that ripple rate varies with respect to brain structure and brain pathologies, such that they may provide a better biomarker of epileptogenicity in mesiotemporal regions than the occipital lobe (von Ellenrieder et al., 2016), and in focal cortical dysplasia than polymicrogyria (Ferrari Mainho et al., 2015), for example. As we focused here on stereotyped spike populations in a single electroclinical syndrome, the results may not generalize to other populations. Future work is required to explore the regional variability and generalizability of spike ripple behaviour to disease in scalp EEG.
Our findings have direct implications for children with BECTS. Although no class I or II evidence is available to guide treatment choice or duration in this disease (Glauser et al., 2006; Tan et al., 2014), most practitioners favour prolonged treatment with anticonvulsant medications until at least 1–2 years after the last clinical seizure (Bourgeois, 2000). Non-treatment or premature taper may result in seizures, injury, and even death (Doumlele et al., 2017); but chronic anticonvulsant drug exposure is also not benign, as the most common medications prescribed for BECTS are known to cause attentional deficits, aggression, hostility, nervousness, and somnolence in 30–70% of exposed children (Perry et al., 2008; Oguni, 2011; Masur et al., 2013; Halma et al., 2014; Mellish et al., 2015). Among BECTS trials in particular, somnolence, psychomotor slowing, dizziness and worsened performance on cognitive testing after treatment was started have been reported with a variety of anticonvulsant drugs (Coppola et al., 2007; Kang et al., 2007; Wirrell et al., 2008; Andrade et al., 2009). Unnecessary cognitive side effects are particularly problematic in light of an emerging recognition that BECTS is associated with subtle but pervasive cognitive difficulties (Wickens et al., 2017). Current methods to estimate remission and attempt medication withdrawal result in seizure relapse in 39% of patients (Bouma et al., 1997). In clinical practice, maintaining treatment in the cohort of BECTS patients with spike ripples could reduce the number of patients that have a breakthrough seizure due to premature taper. Likewise, patients without detected spike ripples may be appropriate for medication taper. Given the complementary performances of the automated and semi-automated approaches, we suggest that practitioners familiar with spike ripple identification implement both methods, while those unfamiliar with the visual classification of spike ripple events could solely use the fully automated approach. We detected no statistical differences between the automated spike ripple and spike methods in the classification of patients with active and inactive BECTS (Table 2). Therefore, a practitioner unfamiliar with spike ripple detection may instead consider automated spike detection to classify BECTS patients. However, we note that we may not have had the power to detect a true difference in performance between automated spike ripple and automated spike detection methods. Additional studies in a larger patient cohort are required to establish whether spike rate alone could provide an equally reliable classification tool for seizure risk in BECTS.
Spikes provide an unreliable estimate of seizure risk as they persist in children with BECTS for years after their last seizure (Bouma et al., 1997; Kobayashi et al., 2010b). Previous pioneering work suggested a relationship between spike ripple events and seizure risk in childhood epilepsy (Kobayashi et al., 2010b); however, subsequent studies raised concerns about the methodology used (van Klink et al., 2016b). Here, we expand on and validate this prior work using both semi-automated and automated detection techniques. Similar to the exponential relationship observed between seizure recurrence risk and duration of time seizure-free (Sillanpaa et al., 2017), we find that the rate of spike ripple events also decays with duration of time seizure-free, suggesting that this biomarker corresponds to epileptogenicity and remission from the disease state. Further, we show that validated spike ripples outperform manually identified spikes as a biomarker of active disease. Additional analysis from a large patient cohort is required to determine whether automated detection of spike ripples significantly improves upon disease classification compared to automated detection of spikes alone. As we used duration seizure-free as a surrogate for seizure risk in this cross-sectional study, a prospective longitudinal study is needed to directly test this biomarker to predict future seizures and sustained remission in BECTS.
We note that in our population, medication treatment alone did not impact spike ripple frequency. Rather, spike ripples decreased in children who were seizure-free compared to children with active seizures, regardless of medication status. Thus, in a child ON medication without spike ripples, this biomarker may not distinguish medication efficacy from true remission. If the former, the spike ripples and seizures may recur with medication taper. This finding raises the potential utility of EEG spike ripple events as an early biomarker for anticonvulsant medication efficacy in BECTS and the need to explore this biomarker in other epilepsy syndromes. A biomarker that tracks with medication efficacy could mitigate the need to rely on breakthrough seizures to guide medication adjustments in this syndrome. Furthermore, quantification of spike ripples prior to medication taper, and at mid-taper, could minimize the chance of recrudescence. Such a biomarker could enable rapid drug screening and bypass the need for prolonged observation and requirement for symptomatic seizures in clinical trials.
Our current study was designed to optimize the yield of biomarker discovery by evaluating a disease population that consistently progresses from active to remission states. This focus on a homogeneous population may have facilitated detection and differentiation of spike ripple features obfuscated in a more heterogeneous population. However, the focus on a single disease population is also a limitation. Future work to understand the pathophysiological mechanisms underlying spike ripples and the relevance of this biomarker across varied populations remains to be done (Jiruska et al., 2017). As new computational tools and improved recording techniques provide novel opportunities for discovery, non-invasive quantitative biomarkers promise to significantly improve patient care.
Acknowledgements
The authors would like to thank Erin Ross and Wenting Xie for assistance with data collection and all of the subjects and families that contributed their time for this project.
Glossary
Abbreviations
- BECTS
benign epilepsy with centrotemporal spikes
- NPV
negative predictive value
- PPV
positive predictive value
Funding
C.J.C. is supported by NINDS K23-NS092923. M.A.K. is supported by NSF #1451384.
Competing interests
The authors report no competing interests.
References
- Andrade-Valenca LP, Dubeau F, Mari F, Zelmann R, Gotman J. Interictal scalp fast oscillations as a marker of the seizure onset zone. Neurology 2011; 77: 524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Valenca L, Mari F, Jacobs J, Zijlmans M, Olivier A, Gotman J et al. Interictal high frequency oscillations (HFOs) in patients with focal epilepsy and normal MRI. Clin Neurophysiol 2012; 123: 100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade R, Garcia-Espinosa A, Machado-Rojas A, Garcia-Gonzalez ME, Trapaga-Quincoses O, Morales-Chacon LM. A prospective, open, controlled and randomised study of clobazam versus carbamazepine in patients with frequent episodes of rolandic epilepsy. Rev Neurol 2009; 49: 581–6. [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 1995; 57: 289–300. [Google Scholar]
- Berg AT, Lin J, Ebrahimi N, Testa FM, Levy SR, Shinnar S. Modeling remission and relapse in pediatric epilepsy: application of a Markov process. Epilepsy Res 2004; 60: 31–40. [DOI] [PubMed] [Google Scholar]
- Berg AT, Rychlik K. The course of childhood-onset epilepsy over the first two decades: a prospective, longitudinal study. Epilepsia 2015; 56: 40–8. [DOI] [PubMed] [Google Scholar]
- Berg AT, Shinnar S, Levy SR, Testa FM, Smith-Rapaport S, Beckerman B et al. Two-year remission and subsequent relapse in children with newly diagnosed epilepsy. Epilepsia 2001; 42: 1553–62. [DOI] [PubMed] [Google Scholar]
- Blanco JA, Stead M, Krieger A, Stacey W, Maus D, Marsh E et al. Data mining neocortical high-frequency oscillations in epilepsy and controls. Brain 2011; 134: 2948–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco JA, Stead M, Krieger A, Viventi J, Marsh WR, Lee KH et al. Unsupervised classification of high-frequency oscillations in human neocortical epilepsy and control patients. J Neurophysiol 2010; 104: 2900–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma PA, Bovenkerk AC, Westendorp RG, Brouwer OF. The course of benign partial epilepsy of childhood with centrotemporal IEDs: a meta-analysis. Neurology 1997; 48: 430–9 [DOI] [PubMed] [Google Scholar]
- Bourgeois BF. Drug treatment in benign focal epilepsies of childhood. Epilepsia 2000; 41: 1057–8. [DOI] [PubMed] [Google Scholar]
- Charupanit K, Lopour BA. A simple statistical method for the automatic detection of ripples in human intracranial EEG. Brain Topogr 2017; 127: 1–15. [DOI] [PubMed] [Google Scholar]
- Chu CJ, Chan A, Song D, Staley KJ, Stufflebeam SM, Kramer MA et al. A semi-automated method for rapid detection of ripple events on interictal voltage discharges in the scalp electroencephalogram. J Neurosci Methods 2017; 277: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 1989; 30: 389–99. [DOI] [PubMed] [Google Scholar]
- Coppola G, Franzoni E, Verrotti A, Garone C, Sarajlija J, Operto FF et al. Levetiracetam or oxcarbazepine as monotherapy in newly diagnosed benign epilepsy of childhood with centrotemporal spikes (BECTS): an open-label, parallel group trial. Brain Dev 2007; 29: 281–4. [DOI] [PubMed] [Google Scholar]
- Doumlele K, Friedman D, Buchhalter J, Donner EJ, Louik J, Devinsky O. Sudden unexpected death in epilepsy among patients with benign childhood epilepsy with centrotemporal spikes. JAMA Neurol 2017; 74: 645–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dümpelmann M, Jacobs J, Kerber K, Schulze-Bonhage A. Automatic 80–250 Hz ‘ripple’ high frequency oscillation detection in invasive subdural grid and strip recordings in epilepsy by a radial basis function neural network. Clin Neurophysiol 2012; 123: 1721–31. [DOI] [PubMed] [Google Scholar]
- Engel J. Biomarkers in epilepsy: introduction. Biomark Med 2011; 5: 537–44. [DOI] [PubMed] [Google Scholar]
- Ferrari Marinho T, Perucca P, Mok K, Olivier A, Hall J, Dubeau F, Gotman J. Pathologic substrates of focal epilepsy influence the generation of high‐frequency oscillations. Epilepsia 2015; 56: 592–8. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014; 55: 475–82. [DOI] [PubMed] [Google Scholar]
- Frauscher B, Bartolomei F, Kobayashi K, Cimbalnik J, van’t Klooster MA, Rampp S et al. High‐frequency oscillations: the state of clinical research. Epilepsia 2017; 58: 1316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser T, Ben-Menachem E, Bourgeois B, Cnaan A, Chadwick D, Guerreiro C et al. ILAE treatment guidelines: evidence-based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia 2006; 47: 1094–120. [DOI] [PubMed] [Google Scholar]
- Gliske SV, Irwin ZT, Davis KA, Sahaya K, Chestek C, Stacey WC. Universal automated high frequency oscillation detector for real-time, long term EEG. Clin Neurophysiol 2016; 127: 1057–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford JJ. Computerized epileptiform transient detection in the scalp electroencephalogram: obstacles to progress and the example of computerized ECG interpretation. Clin Neurophysiol 2009; 120: 1909–15. [DOI] [PubMed] [Google Scholar]
- Halma E, de Louw AJ, Klinkenberg S, Aldenkamp AP, IJff DM, Majoie M. Behavioral side-effects of levetiracetam in children with epilepsy: a systematic review. Seizure 2014; 23: 685–91. [DOI] [PubMed] [Google Scholar]
- Inoue T, Kobayashi K, Oka M, Yoshinaga H, Ohtsuka Y. Spectral characteristics of EEG gamma rhythms associated with epileptic spasms. Brain Dev 2008; 30: 321–8. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Epilepsy Across the Spectrum: Promoting Health and Understanding. 2012. http://nationalacademies.org/HMD/Reports/2012/Epilepsy-Across-the-Spectrum.aspx (24 July 2018, date last accessed). [DOI] [PMC free article] [PubMed]
- Jacobs J, Zelmann R, Jirsch J, Chander R, Dubeau CE, Gotman J. High frequency oscillations (80–500 Hz) in the preictal period in patients with focal seizures. Epilepsia 2009; 50: 1780–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiruska P, Alvarado-Rojas C, Schevon CA, Staba R, Stacey W, Wendling F et al. Update on the mechanisms and roles of high-frequency oscillations in seizures and epileptic disorders. Epilepsia 2017; 58: 1330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi CN, Chapman KE, Bear JJ, Wilson SB, Walleigh DJ, Scheuer ML. Semiautomated spike detection software persyst 13 is noninferior to human readers when calculating the spike-wave index in electrical status epilepticus in sleep. J Clin Neurophysiol 2018; 35: 370–4. [DOI] [PubMed] [Google Scholar]
- Kang H-C, Eun BL, Lee CW, Moon HK, Kim JS, Kim DW et al. The effects on cognitive function and behavioral problems of topiramate compared to carbamazepine as monotherapy for children with benign rolandic epilepsy. Epilepsia 2007; 48: 1716–23. [DOI] [PubMed] [Google Scholar]
- Kass RE, Eden UT, Brown EN. Analysis neural data. London: Springer Press; 2014. [Google Scholar]
- Kobayashi K, Akiyama T, Oka M, Endoh F, Yoshinaga H. A storm of fast (40–150 Hz) oscillations during hypsarrhythmia in West syndrome. Ann Neurol 2015; 77: 58–67. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Oka M, Akiyama T, Inoue T, Abiru K, Ogino T et al. Very fast rhythmic activity on scalp EEG associated with epileptic spasms. Epilepsia 2004; 45: 488–96. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Watanabe Y, Inoue T, Oka M, Yoshinaga H, Ohtsuka Y. Scalp-recorded high-frequency oscillations in childhood sleep-induced electrical status epilepticus. Epilepsia 2010a; 51: 2190–4 [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Yoshinaga H, Toda Y, Inoue T, Oka M, Ohtsuka Y. High-frequency oscillations in idiopathic partial epilepsy of childhood. Epilepsia 2010b; 52: 1812–9. [DOI] [PubMed] [Google Scholar]
- Koutroumanidis M, Arzimanoglou A, Caraballo R, Goyal S, Kaminska A, Laoprasert P et al. The role of EEG in the diagnosis and classification of the epilepsy syndromes: a tool for clinical practice by the ILAE Neurophysiology Task Force (Part 2). Epileptic Disord 2017; 19: 385–437. [DOI] [PubMed] [Google Scholar]
- Kosinski AS. A weighted generalized score statistic for comparison of predictive values of diagnostic tests. Stat Med 2013; 32: 964–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MA, Eden UT. Case studies in neural data analysis: a guide for the practicing neuroscientist. Cambridge, MA: MIT Press; 2016. [Google Scholar]
- Malinowska U, Bergey GK, Harezlak J, Jouny CC. Identification of seizure onset zone and preictal state based on characteristics of high frequency oscillations. Clin Neurophysiol 2015; 126: 1505–13. [DOI] [PubMed] [Google Scholar]
- Masur D, Shinnar S, Cnaan A, Shinnar RC, Clark P, Wang J et al. Pretreatment cognitive deficits and treatment effects on attention in childhood absence epilepsy. Neurology 2013; 81: 1572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melani F, Zelmann R, Dubeau F, Gotman J. Occurrence of scalp-fast oscillations among patients with different spiking rate and their role as epileptogenicity marker. Epilepsy Res 2013; 106: 345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellish LC, Dunkley C, Ferrie CD, Pal DK. Antiepileptic drug treatment of rolandic epilepsy and Panayiotopoulos syndrome: clinical practice survey and clinical trial feasibility. Arch Dis Child 2015; 100: 62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedermeyer E, Lopes da Silva F. Electroencephalography: basic principles, clinical applications, and related fields. 5th edn Philadelphia, PA: Lippincott Williams and Wilkins; 2005. [Google Scholar]
- Oguni H. Treatment of benign focal epilepsies in children: when and who should be treated. Brain Dev 2011; 33: 207–12. [DOI] [PubMed] [Google Scholar]
- Perry S, Holt P, Benetar M. Levetiracetam versus carbamazepine monotherapy for partial epilepsy in children under 16 years of age. J Child Neurol 2008; 23: 515–19. [DOI] [PubMed] [Google Scholar]
- Roehri N, Pizzo F, Lagarde S, Lambert I, Nica A, McGonigal A et al. High-frequency oscillations are not better biomarkers of epileptogenic tissue than spikes. Ann Neurol 2018; 83: 84–97. [DOI] [PubMed] [Google Scholar]
- Scheuer ML, Bagic A, Wilson SB. Spike detection: Inter-reader agreement and a statistical Turing test on a large data set. Clin Neurophysiol 2017; 128: 243–50. [DOI] [PubMed] [Google Scholar]
- Sillanpaa M, Schmidt D, Saarinen MM, Shinnar S. Remission in epilepsy: how long is enough? Epilepsia 2017; 58: 901–6. [DOI] [PubMed] [Google Scholar]
- Stock C, Hielscher T. DTComPair: comparison of binary diagnostic tests in a paired study design. R package version 1.0.3.2014. http://CRAN.R-project.org/package=DTComPair [Google Scholar]
- Stroink H, Schimsheimer RJ, de Weerd AW, Geerts AT, Arts WF, Peeters EA. Interobserver reliability of visual interpretation of electroencephalograms in children with newly diagnosed seizures. Dev Med Child Neurol 2006; 48: 374–7. [DOI] [PubMed] [Google Scholar]
- Tan HJ, Singh J, Gupta R, de Goede C. Comparison of antiepileptic drugs, no treatment, or placebo for children with benign epilepsy with centro temporal spikes. Cochrane Database Syst Rev 2014:CD006779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenney JR, Glauser T, Altaye M, Szaflarski JP, Spencer C, Morita D et al. Longitudinal stability of interictal spikes in benign epilepsy with centrotemporal spikes. Epilepsia 2016; 57: 805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrestarazu E, Chander R, Dubeau F, Gotman J. Interictal high-frequency oscillations (100–500 Hz) in the intracerebral EEG of epileptic patients. Brain J Neurol 2007; 130: 2354–66. [DOI] [PubMed] [Google Scholar]
- van Klink N, Frauscher B, Ziljmans M, Gotman J. Relationships between interictal epileptic spikes and ripples in surface EEG. Clin Neurophys 2016a; 127: 143–9. [DOI] [PubMed] [Google Scholar]
- van Klink NE, van’t Klooster MA, Leijten FS, Jacobs J, Braun DP, Zijlmans M. Ripples on rolandic spikes: a marker of epilepsy severity. Epilepsia 2016b; 57: 1179–89. [DOI] [PubMed] [Google Scholar]
- von Ellenrieder N, Andrade-Valença LP, Dubeau F, Gotman J. Automatic detection of fast oscillations (40–200 Hz) in scalp EEG recordings. Clin Neurophysiol 2012; 123: 670–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ellenrieder N, Frauscher B, Dubeau F, Gotman J. Interaction with slow waves during sleep improves discrimination of physiological and pathological high frequency oscillations (80–500 Hz). Epilepsia 2016; 57: 869–78. [DOI] [PubMed] [Google Scholar]
- Webber WRS, Litt B, Lesser RP, Fisher RS, Bankman I. Automatic EEG spike detection: what should the computer imitate? Electroencephalogr Clin Neurophysiol 1993; 87: 364–73. [DOI] [PubMed] [Google Scholar]
- Westover MB, Halford JJ, Bianchi MT. What it should mean for an algorithm to pass a statistical Turing test for detection of epileptiform discharges. Clin Neurophysiol 2017; 128: 1406–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens S, Bowden SC, D’Souza W. Cognitive functioning in children with self-limited epilepsy with centrotemporal spikes: a systematic review and meta-analysis. Epilepsia 2017; 58: 1673–85. [DOI] [PubMed] [Google Scholar]
- Wirrell E, Sherman EMS, Vanmastrigt R, Hamiwka L. Deterioration in cognitive function in children with benign epilepsy of childhood with central temporal spikes treated with sulthiame. J Child Neurol 2008; 23: 14–21. [DOI] [PubMed] [Google Scholar]
- Wilson SB, Emerson R. Spike detection: a review and comparison of algorithms. Clin Neurophysiol 2002; 113: 1873–81. [DOI] [PubMed] [Google Scholar]
- Worrell G, Gotman J. High-frequency oscillations and other electrophysiological biomarkers of epilepsy: clinical studies. Biomark Med 2011; 5: 557–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain 2004; 127: 1496–506. [DOI] [PubMed] [Google Scholar]
- Zelmann R, Mari F, Jacobs J, Zijlmans M, Chander R, Gotman J. Automatic detector of High Frequency Oscillations for human recordings with macroelectrodes. Conf Proc IEEE Eng Med Biol Soc 2010; 2010: 2329–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Obuchowski N, McClish D. Statistical methods in diagnostic medicine. Wiley series in probability and statistics. 2nd edn Hoboken, NJ: John Wiley & Sons; 2011. [Google Scholar]
- Zijlmans M, Jacobs J, Zelmann R, Dubeau F, Gotman J. High-frequency oscillations mirror disease activity in patients with epilepsy. Neurology 2009; 72: 979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data were generated at Massachusetts General Hospital and the Athinoula A. Martinos Center for Biomedical Imaging. Derived data supporting the findings of this study are available from the corresponding author on request. Software for the detection of spike ripple events is available at https://github.com/Mark-Kramer/Spike-Ripple-Detector-Method.