Using an international data set of 441 patients with AQP4-IgG positive NMOSD, and a collective history of almost 2000 attacks, Palace et al. apply mathematical modelling to predict likelihood of relapse and disability at different time points. Such estimates will help clinicians when counselling patients and aid drug trial design.
Keywords: neuromyelitis optica, aquaporin-4, outcome prediction, disability
Abstract
Pathogenic antibodies targeting the aquaporin-4 water channel on astrocytes are associated with relapsing inflammatory neuromyelitis optica spectrum disorders. The clinical phenotype is characterized by recurrent episodes of optic neuritis, longitudinally extensive transverse myelitis, area postrema attacks and less common brainstem and cerebral events. Patients often develop major residual disability from these attacks, so early diagnosis and initiation of attackpreventing medications is important. Accurate prediction of relapse would assist physicians in counselling patients, planning treatment and designing clinical trials. We used a large multicentre dataset of 441 patients from the UK, USA, Japan and Martinique who collectively experienced 1976 attacks, and applied sophisticated mathematical modelling to predict likelihood of relapse and disability at different time points. We found that Japanese patients had a lower risk of subsequent attacks except for brainstem and cerebral events, with an overall relative relapse risk of 0.681 (P = 0.001) compared to Caucasians and African patients, who had a higher likelihood of cerebral attacks, with a relative relapse risk of 3.309 (P = 0.009) compared to Caucasians. Female patients had a higher chance of relapse than male patients (P = 0.009), and patients with younger age of onset were more likely to have optic neuritis relapses (P < 0.001). Immunosuppressant drugs reduced and multiple sclerosis disease-modifying agents increased the likelihood of relapse (P < 0.001). Patients with optic neuritis at onset were more likely to develop blindness (P < 0.001), and those with older age of onset were more likely to develop ambulatory disability. Only 25% of long-term disability was related to initial onset attack, indicating the importance of early attack prevention. With respect to selection of patients for clinical trial design, there would be no gain in power by selecting recent onset patients and only a small gain by selecting patients with recent high disease activity. We provide risk estimates of relapse and disability for patients diagnosed and treated with immunosuppressive treatments over the subsequent 2, 3, 5 and 10 years according to type of attack at onset or the first 2-year course, ethnicity, sex and onset age. This study supports significant effects of onset age, onset phenotype and ethnicity on neuromyelitis optica spectrum disorders outcomes. Our results suggest that powering clinical treatment trials based upon relapse activity in the preceding 2 years may offer little benefit in the way of attack risk yet severely hamper clinical trial success.
Introduction
The neuromyelitis optica spectrum disorders (NMOSD) are autoimmune, inflammatory disorders of the CNS with a predilection for the optic nerves and spinal cord and are distinct from multiple sclerosis (Wingerchuk et al., 2007). The majority of patients have antibodies to aquaporin-4 (AQP4) water channels, which are situated predominantly on astrocyte foot processes; hence AQP4-IgG-positive NMOSD is now recognized as an autoimmune astrocytopathy with secondary demyelination (Lennon et al., 2004, 2005). The AQP4-IgG seronegative group (considered seronegative NMOSD) likely represents a heterogeneous group of both monophasic and relapsing inflammatory CNS disorders that include post-infectious inflammation and conditions caused by unidentified antibodies (Wingerchuk et al., 2015). Recently some patients in this group have been reported to be positive for antibodies targeting myelin oligodendrocyte glycoprotein (MOG) and these patients have a primary demyelinating disorder (Waters et al., 2015; Jarius et al., 2016; Peschl et al., 2017).
There are important differences between those who are seropositive for AQP4-IgG and MOG-IgG. AQP4-IgG-positive NMOSD in contrast to MOG-antibody-associated disorders (MOGAD) is far more common in females (Quek et al., 2012), has a non-Caucasian ethnic bias, commonly co-associates with other autoantibodies and diseases, is relapsing if untreated and is associated with significant morbidity and mortality from relapse-related disability (Kitley et al., 2014b; Flanagan et al., 2016; Jurynczyk et al., 2017; Cobo-Calvo et al., 2018; Jitprapaikulsan et al., 2018a, b). Additionally, current immunosuppressive treatments (ISTs), such as prednisolone, azathioprine, mycophenolate mofetil and rituximab, may not suppress the disease adequately. Thus there is a growing interest in developing new treatments and currently there are three immune-modulatory drugs being tested in international multicentre phase 4 randomized controlled trials: inebilizumab (anti-CD19 monoclonal antibody targeting B cells), eculizumab (anti-C5 monoclonal antibody targeting complement) and two utilizing satralizumab (anti-IL-6R monoclonal antibody targeting T and B cell activation, Th17 differentiation, and plasmablast survival). These studies enrol exclusively or primarily patients with AQP4 antibodies.
Because the outcomes across patients with AQP4-IgG-positive NMOSD are heterogeneous and because data that permit power calculations for clinical trials are sparse (Weinshenker et al., 2015), we combined datasets from five centres with detailed prospective data collection systems, across four countries with varied ethnicities. We developed a joint modelling framework to understand the factors that influence relapses and disability and predict future attacks and disability events.
Materials and methods
Patient cohort
An international database was created by merging prospectively collected datasets from five neuromyelitis optica (NMO) specialized centres: Oxford and Liverpool (UK), Mayo Clinic (USA), Sendai (Japan) and Martinique. Information collected included sex, ethnicity, onset attack type (optic neuritis, transverse myelitis, brainstem attack, cerebral, and mixed), age at onset, Expanded Disability Status Scale (EDSS) scores and visual acuity and chronic immunomodulatory treatment. All data were anonymized and satisfied the local ethics requirements.
Analysis
We represented each type of recurrent NMO attacks as a counting process with an intensity (rate) function dependent on baseline covariates and treatment histories, as well as unobserved random effects. The model is an extension of the well known multiplicative intensity model of Andersen and Gill (1982) to accommodate multiple types of recurrent events and to account for patient heterogeneity or clustering of recurrent attacks. There is a shared random effect that characterizes the patient’s overall propensity for recurrent attacks, as well as a type-specific random effect that characterizes the patient’s propensity for each type of recurrent attacks. The values of the random effects vary among patients, representing the patient characteristics that are not captured by the measured covariates. The mean of the random effect in the population is set to zero. A patient with a positive value of the random effect tends to have more attacks than an average patient, whereas a patient with a negative value of the random effect tends to have fewer attacks than an average patient. The variance of the random effect reflects the degree of heterogeneity, with a larger value indicating greater heterogeneity.
We formulated the effects of baseline covariates and treatment histories on the hazard function of a disability event through a proportional hazards model (Cox, 1972) in which the shared random effect from the model for recurrent attacks enters as an additional covariate. The regression coefficient for the shared random effect captures the dependence of disability on recurrent attacks. This joint modelling approach allows us to assess the effects of baseline covariates and time-dependent covariates (e.g. treatments) on the rate of recurrence for each type of NMO attack and on the risk of occurrence for each type of disability while accounting for the patient heterogeneity that is not accounted for by the measured covariates. It also allows us to predict future attacks and disability events using not only the baseline characteristics and treatment histories but also the event histories. This model uses a time-dependent analysis which allows for any variation in follow-up times across the different covariate subgroups. The mathematical formulation, estimation procedure, and prediction algorithm are detailed in the Supplementary material.
For the baseline covariates we included ethnicity, sex, age at disease onset, and baseline attack type. We classified patients according to five major ethnicity groups: Caucasian, African (includes all those of African descent), Hispanic, Japanese, and non-Japanese Asian. We combined Hispanic and unknown ethnicity with Caucasian, which serves as the reference. We divided patients into three age groups according to the tertiles: ≤35 years, 35–48 years, and >48 years, with the last tertile as the reference. Such tertiles were used as a balanced distribution yields more stable estimates. The clinical and demographic characteristics of each tertile are shown in Supplementary Table 1. For the baseline attack, we combined brainstem and cerebral and set transverse myelitis as the reference.
There were two time-dependent covariates: ISTs were combined into one group (chronic prednisolone/prednisone, azathioprine, mycophenolate, rituximab, methotrexate or any combination) and were allocated the value of 0 before initiation of the IST treatment and the value of 1 afterwards; licensed multiple sclerosis disease-modifying treatments were combined as one group (multiple sclerosis disease-modifying treatment: mainly interferon beta and glatiramer acetate; anti-CD20 monoclonals were not included in this group) and were allocated the value of 1 between the starting and stopping dates and the value of 0 otherwise. No patients were treated with natalizumab or ocrelizumab. The treatment comparisons pertain to IST versus no treatment and multiple sclerosis disease-modifying treatments versus no treatment.
We combined some types of events in order to increase power and stability. Specifically, we combined unilateral and bilateral optic neuritis, classified unknown attack types (13 of 1976 events, 0.7%) as transverse myelitis attacks, which were the most common type, used the composite endpoint of one-eye blindness and two-eye blindness and the composite endpoint of EDSS 8.0 and death.
The results are based on the joint model with four types of NMO attacks (i.e. optic neuritis, transverse myelitis, brainstem, and cerebral) and the disability events of blindness and EDSS 6.0, except for the results on EDSS 8.0/death, which are based on a second joint model with EDSS 6.0 replaced by EDSS 8.0/death, and for the results on all relapses, which combine the four types of NMO attacks into a single sequence of recurrent events. If a patient had a mix of optic neuritis and transverse myelitis, then he/she would contribute to both types of events in the analysis.
Data availability
The data that support the findings of this study are available from the corresponding authors, upon request.
Results
Clinical and demographic characteristics of the international NMOSD attack database
A total of 441 AQP4-IgG-positive NMOSD patients from the five sites were included (Table 1). There were 396 females and 45 males. The age of onset ranged from 2.7 to 82.7 years, with a median of 40.8 years. Over a median disease duration of 7.1 years (range: 0.3–46.6), 1976 attacks were documented. Supplementary Table 2 shows the frequencies and types of attacks and disability outcomes during the disease course according to baseline characteristics, such as attack type, site and ethnicity.
Table 1.
Demographics of AQP4-IgG-positive NMOSD patients
| Total | Japan | Oxford | Liverpool | Martinique | Mayo | |
|---|---|---|---|---|---|---|
| Total patients | 441 | 63 | 77 | 74 | 56 | 171 |
| Ethnicity | ||||||
| Caucasian | 210 | 0 | 50 | 54 | 1 | 105 |
| African | 115 | 0 | 17 | 11 | 54 | 33 |
| Asian | 100 | 63 | 10 | 7 | 1 | 19 |
| Hispanic | 11 | 0 | 0 | 0 | 0 | 11 |
| Mixed/unknown | 5 | 0 | 0 | 2 | 0 | 3 |
| Sex | ||||||
| Male | 45 | 3 | 9 | 12 | 3 | 18 |
| Female | 396 | 60 | 68 | 62 | 53 | 153 |
| Age at onset | ||||||
| Mean (standard deviation, SD) | 41.2 (15.4) | 43.1 (14) | 39.3 (18.3) | 41.5 (15.6) | 38.1 (15.8) | 42.3 (14.1) |
| Duration of follow-up from onset | ||||||
| Median, years | 7.1 | 7.2 | 6.3 | 6.8 | 7.0 | 8.2 |
| (range) | (0.3–46.6) | (0.3–44.2) | (0.4–34.4) | (0.5–38.4) | (0.5–37.1) | (0.3–46.6) |
Effects of age, sex, ethnicity and treatment on likelihood of relapse
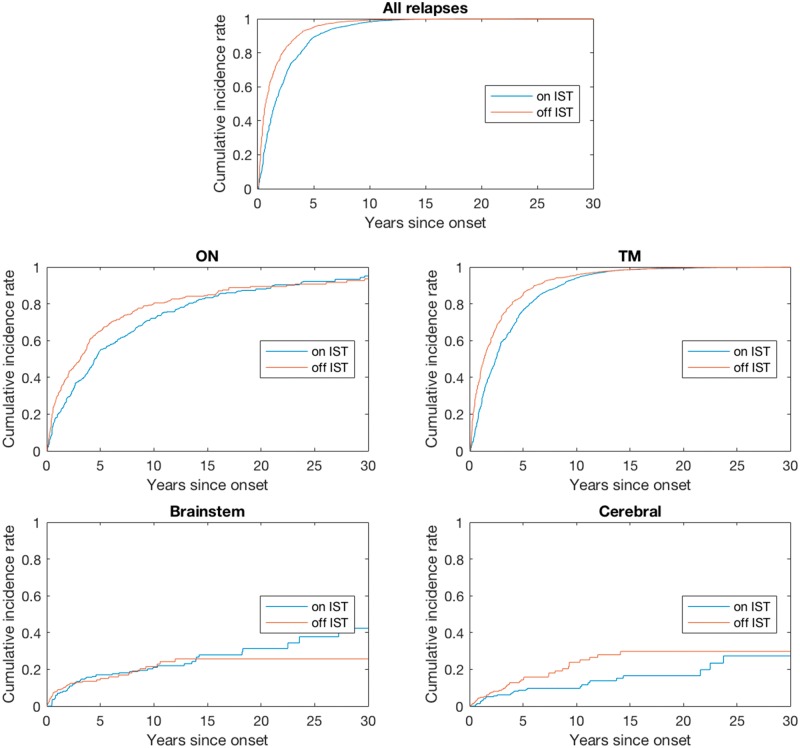
The estimation results for the effects of covariates on recurrent NMO attacks after disease onset (in terms of rate ratio) are summarized in Table 2. Japanese patients had the lowest risk of relapses overall but the highest brainstem attack risk, and African patients had the highest cerebral attack risk. Compared to Caucasian patients (the reference group in the analysis), Japanese patients had lower risk of recurrent attacks (P = 0.001), particularly transverse myelitis attacks (P = 0.001) and optic neuritis attacks (P = 0.026), and African patients had much higher risk for cerebral attacks (P = 0.009). Female patients had higher recurrence rates for transverse myelitis attacks (P < 0.001) and overall relapses (P = 0.009) than male patients. Patients with younger age of onset were more likely to have optic neuritis relapses (P < 0.001) than older patients. In general, the onset attack type was positively associated with relapse of the same attack phenotype. ISTs reduced the likelihood of all relapses by 33% (P < 0.001), with greater effects on optic neuritis, transverse myelitis and cerebral attacks (Fig. 1), whereas multiple sclerosis disease-modifying treatments increased the risk of relapse. Supplementary Fig. 1 shows that the risk of relapse decreases over time, most dramatically after 10 years.
Table 2.
Estimation of the effects of covariates on the rates of recurrence for attacks
| Optic neuritis | Transverse myelitis | Brainstem | Cerebral | All | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Covariate | Rate ratio | P-value | Rate ratio | P-value | Rate ratio | P-value | Rate ratio | P-value | Rate ratio | P-value |
| Site ethnicity | ||||||||||
| African | 0.968 (0.188) | 0.865 | 0.963 (0.120) | 0.761 | 0.813 (0.310) | 0.588 | 3.309 (1.517) | 0.009 | 1.003 (0.099) | 0.974 |
| Japanese | 0.587 (0.141) | 0.026 | 0.588 (0.092) | 0.001 | 1.651 (0.575) | 0.150 | 1.719 (0.921) | 0.312 | 0.681 (0.076) | 0.001 |
| Non-Japanese Asian | 1.124 (0.292) | 0.653 | 0.921 (0.178) | 0.669 | 0.617 (0.402) | 0.458 | 0.717 (0.631) | 0.706 | 1.010 (0.138) | 0.940 |
| USA Caucasian and others | 1 | – | 1 | – | 1 | – | 1 | – | 1 | – |
| Sex | ||||||||||
| Female | 0.903 (0.145) | 0.524 | 1.501 (0.167) | <0.001 | 0.860 (0.316) | 0.681 | 1.596 (0.711) | 0.294 | 1.209 (0.087) | 0.009 |
| Male | 1 | – | 1 | – | 1 | – | 1 | – | 1 | – |
| Age, years | ||||||||||
| ≤35 | 2.078 (0.393) | <0.001 | 0.872 (0.114) | 0.295 | 1.767 (0.643) | 0.118 | 0.823 (0.414) | 0.698 | 1.090 (0.104) | 0.365 |
| 35–48 | 1.468 (0.296) | 0.057 | 0.790 (0.105) | 0.075 | 1.942 (0.762) | 0.091 | 1.507 (0.765) | 0.419 | 0.930 (0.090) | 0.456 |
| >48 | 1 | – | 1 | – | 1 | – | 1 | – | 1 | – |
| Baseline attack | ||||||||||
| Optic neuritis | 1.608 (0.270) | 0.005 | 0.836 (0.103) | 0.146 | 0.838 (0.306) | 0.627 | 1.145 (0.509) | 0.761 | 1.026 (0.096) | 0.783 |
| Brainstem/cerebral | 1.686 (0.498) | 0.077 | 1.084 (0.216) | 0.685 | 3.903 (1.636) | 0.001 | 2.929 (1.518) | 0.038 | 1.287 (0.197) | 0.098 |
| Mixed | 0.992 (0.293) | 0.978 | 0.893 (0.139) | 0.470 | 1.719 (0.750) | 0.215 | 2.284 (1.180) | 0.110 | 0.936 (0.114) | 0.584 |
| Transverse myelitis | 1 | – | 1 | – | 1 | – | 1 | – | 1 | – |
| Treatment | ||||||||||
| IST | 0.662 (0.064) | <0.001 | 0.611 (0.042) | <0.001 | 0.900 (0.220) | 0.665 | 0.486 (0.172) | 0.042 | 0.668 (0.028) | <0.001 |
| MS-DMT | 1.325 (0.242) | 0.124 | 1.382 (0.142) | 0.002 | 0.672 (0.377) | 0.479 | 1.941 (0.852) | 0.131 | 1.383 (0.107) | <0.001 |
| No treatment | 1 | – | 1 | – | 1 | – | 1 | – | 1 | – |
Standard errors are shown in parentheses.
MS-DMT = multiple sclerosis disease-modifying treatment.
Figure 1.
Nelson-Aalen estimates of the cumulative incidence rates for patients on versus off IST. ON = optic neuritis; TM = transverse myelitis.
Effects of age, sex and ethnicity on likelihood of developing disability or blindness
The estimation results for the effects of covariates on the likelihood of developing disability (EDSS ≥ 6) or blindness (in terms of hazard ratio) are summarized in Table 3. African patients were more likely to develop blindness and Japanese patients least likely to reach EDSS 8.0/death; both differences were significant compared to the reference Caucasian group. Females had higher risk for all types of disability events. Patients with a younger age of onset were not only more likely to develop recurrent optic neuritis but also had a higher likelihood of developing blindness. They had a lower risk of developing EDSS 6.0 and EDSS 8.0 or death. In contrast, patients in the oldest age of onset group had a significantly higher likelihood of having ambulatory disability (need for cane or wheelchair) compared with those in the two younger age groups. Patients with optic neuritis or mixed onset attacks were more likely to develop blindness (P < 0.001). Although ISTs were associated with a lower likelihood and multiple sclerosis disease-modifying agents were associated with a higher likelihood of blindness and EDSS 6.0, neither reached statistical significance. In contrast, both ISTs and multiple sclerosis disease-modifying agents were associated with a higher likelihood of reaching EDSS ≥ 8 although there were fewer events.
Table 3.
Estimation of the effects of covariates on the occurrence of disability events
| Blindness | EDSS 6.0 | EDSS 8.0/death | ||||
|---|---|---|---|---|---|---|
| Covariate | Hazard ratio | P-value | Hazard ratio | P-value | Hazard ratio | P-value |
| Site ethnicity | ||||||
| African | 1.724 (0.248) | <0.001 | 1.059 (0.280) | 0.828 | 0.876 (0.306) | 0.704 |
| Japanese | 0.853 (0.160) | 0.395 | 0.799 (0.274) | 0.514 | 0.297 (0.154) | 0.020 |
| Non-Japanese Asian | 1.375 (0.270) | 0.105 | 0.829 (0.316) | 0.622 | 0.628 (0.347) | 0.400 |
| USA Caucasian and others | 1 | – | 1 | – | 1 | – |
| Sex | ||||||
| Female | 1.633 (0.258) | 0.002 | 1.522 (0.290) | 0.027 | 1.508 (0.349) | 0.076 |
| Male | 1 | – | 1 | – | 1 | – |
| Age, years | ||||||
| ≤35 | 1.490 (0.229) | 0.009 | 0.332 (0.090) | <0.001 | 0.339 (0.119) | 0.002 |
| 35–48 | 1.284 (0.211) | 0.128 | 0.522 (0.135) | 0.012 | 0.270 (0.109) | 0.001 |
| >48 | 1 | – | 1 | – | 1 | – |
| Baseline attack | ||||||
| Optic neuritis | 3.810 (0.561) | <0.001 | 0.622 (0.162) | 0.068 | 0.832 (0.275) | 0.578 |
| Brainstem/cerebral | 1.253 (0.446) | 0.527 | 0.726 (0.270) | 0.389 | 0.663 (0.359) | 0.448 |
| Mixed | 2.153 (0.420) | <0.001 | 0.829 (0.246) | 0.526 | 0.801 (0.321) | 0.580 |
| Transverse myelitis | 1 | – | 1 | – | 1 | – |
| Treatment | ||||||
| IST | 0.735 (0.126) | 0.072 | 0.708 (0.141) | 0.082 | 1.647 (0.409) | 0.045 |
| MS-DMT | 1.498 (0.399) | 0.129 | 1.511 (0.502) | 0.214 | 1.865 (0.862) | 0.177 |
| No treatment | 1 | – | 1 | – | 1 | – |
Standard errors are shown in parentheses.
MS-DMT = multiple sclerosis disease-modifying treatment.
The estimation results for the random effects are presented in Supplementary Table 3. The variances of the shared and type-specific random effects are all quite large, indicating strong patient heterogeneity (due to unobserved confounders) in recurrent NMO attacks, especially for optic neuritis and transverse myelitis attacks. In addition, recurrent NMO attacks substantially increase the risks for all types of disability. Specifically, the coefficients of the shared random effect for blindness, EDSS 6.0, and EDSS 8.0/death are all estimated at ∼1.5, indicating that a half unit change in the shared random effect for recurrent attacks would double the risk of each type of disability event.
Relationship between onset attack and significant long-term disability
Only 25% of patients who experienced EDSS ≥ 6 reached that disability milestone due to the onset attack. Only 17% of patients experiencing EDSS 8.0 reached that disability milestone due to the onset attack. For patients developing blindness in one or both eyes, 41% and 21%, respectively, reached that level of disability due to the onset attack. Thus, most patients require multiple attacks in order to acquire significant disability.
Predicting risk of relapse and disability based on age, sex and historical attack frequency and phenotype
Our model can be used to predict the outcomes of individual patients according to their characteristics. For example, Supplementary Fig. 2 displays the estimated cumulative incidence functions of any relapse and an optic neuritis attack after Year 2 for a Japanese female patient who was diagnosed with a transverse myelitis attack at age 40 and did not receive multiple sclerosis disease-modifying treatment. The IST treatment reduces the incidence of any relapse and optic neuritis attack, while having two optic neuritis attacks in the first 2 years substantially increases the incidence of future NMO attacks, especially optic neuritis attacks. As a second example, Supplementary Fig. 3 displays the estimated cumulative incidence functions of blindness and EDSS 6.0 after Year 2 for a Caucasian male patient who was diagnosed at age 30, started IST at disease onset, and did not receive multiple sclerosis disease-modifying agents. If such a patient was diagnosed with an optic neuritis attack as opposed to a transverse myelitis attack at disease onset, then his risk of blindness is increased by nearly 3-fold and his risk of EDSS 6.0 is reduced by nearly 40%. Having two new optic neuritis attacks in the first 2 years increases both the risks of blindness and EDSS 6.0 considerably.
Outcome prediction tables: a helpful tool for clinicians and patients.
Tables 4–6 provide estimates for the risks of recurrent attacks and disability events over time for patients who were treated (i.e. with immunosuppressants from onset and not multiple sclerosis disease-modifying treatments). We considered 54 combinations of baseline characteristics: ethnicity (Japanese, Caucasian, African), sex, age group (<35, 35–48, >48), and onset attack type (optic neuritis, transverse myelitis, brainstem). Table 4 provides risk estimates over the first 2 years. Table 5 and Supplementary Table 4 provide risk estimates at Year 2 and over the subsequent 2, 3, 5 and 10 years for the patients who have not reached the disability endpoints at Year 2. Table 5 and Supplementary Table 4 pertain to patients without any relapse within 2 years after the onset attack. Table 6 and Supplementary Table 5 provide risk estimates at Year 2 over the subsequent 2, 3, 5 and 10 years for the patients who have not reached the disability endpoints at Year 2. Table 6 and Supplementary Table 5 pertain to patients who had one relapse in the first 2 years after the onset attack. These prediction tables can be used in the clinic setting to inform physicians and patients on the choice of initial treatment.
Table 4.
Likelihood (%) of developing attacks and disability by Year 2 for patients on IST from onset
| Attack onset | Risk within first 2 years after onset | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age, years | Type | All attacks | ON | TM | BS | EDSS | Blindness | ||
| 6.0 | ≥8.0 | ||||||||
| Japanese female | |||||||||
| ≤35 | ON | 51.6 | 26.5 | 26.2 | 11.4 | 5.7 | 1.9 | 25.8 | |
| TM | 50.1 | 18.4 | 30.1 | 13.3 | 8.9 | 2.3 | 7.9 | ||
| BS | 68.7 | 27.5 | 31.9 | 38.0 | 6.6 | 1.5 | 9.8 | ||
| 36–48 | ON | 48.0 | 20.4 | 24.2 | 12.4 | 8.8 | 1.5 | 22.8 | |
| TM | 47.4 | 13.8 | 27.9 | 14.4 | 13.5 | 1.8 | 6.9 | ||
| BS | 67.8 | 21.1 | 29.7 | 40.3 | 10.1 | 1.2 | 8.5 | ||
| >48 | ON | 44.3 | 14.9 | 29.1 | 6.8 | 15.7 | 5.5 | 18.5 | |
| TM | 45.1 | 9.8 | 33.3 | 8.0 | 23.3 | 6.6 | 5.4 | ||
| BS | 60.0 | 15.5 | 35.3 | 25.4 | 17.9 | 4.4 | 6.7 | ||
| Japanese male | |||||||||
| ≤35 | ON | 48.9 | 28.6 | 18.7 | 13.0 | 3.8 | 1.3 | 17.1 | |
| TM | 46.2 | 19.9 | 21.8 | 15.2 | 6.0 | 1.5 | 5.0 | ||
| BS | 67.4 | 29.6 | 23.2 | 41.8 | 4.5 | 1.0 | 6.2 | ||
| 36–48 | ON | 44.8 | 22.1 | 17.2 | 14.1 | 5.9 | 1.0 | 15.0 | |
| TM | 43.3 | 15.0 | 20.0 | 16.4 | 9.2 | 1.2 | 4.3 | ||
| BS | 66.3 | 22.9 | 21.4 | 44.3 | 6.9 | 0.8 | 5.4 | ||
| >48 | ON | 39.2 | 16.2 | 21.0 | 7.8 | 10.8 | 3.7 | 12.0 | |
| TM | 38.8 | 10.8 | 24.3 | 9.2 | 16.5 | 4.4 | 3.4 | ||
| BS | 56.1 | 16.8 | 26.0 | 28.4 | 12.4 | 3.0 | 4.2 | ||
| Caucasian female | |||||||||
| ≤35 | ON | 63.6 | 38.3 | 38.8 | 7.2 | 7.1 | 6.2 | 29.2 | |
| TM | 61.5 | 27.7 | 43.7 | 8.5 | 11.0 | 7.4 | 9.2 | ||
| BS | 74.3 | 39.4 | 46.1 | 26.7 | 8.2 | 5.0 | 11.3 | ||
| 36–48 | ON | 58.4 | 30.3 | 36.2 | 7.9 | 10.8 | 5.0 | 26.0 | |
| TM | 57.2 | 21.3 | 41.0 | 9.3 | 16.4 | 6.0 | 8.0 | ||
| BS | 71.4 | 31.4 | 43.2 | 28.7 | 12.4 | 4.0 | 9.9 | ||
| >48 | ON | 56.9 | 22.9 | 42.6 | 4.2 | 19.0 | 16.8 | 21.2 | |
| TM | 57.5 | 15.6 | 47.7 | 5.0 | 27.8 | 19.7 | 6.3 | ||
| BS | 67.7 | 23.7 | 50.1 | 17.0 | 21.6 | 13.8 | 7.8 | ||
| Caucasian male | |||||||||
| ≤35 | ON | 60.0 | 40.8 | 28.8 | 8.3 | 4.8 | 4.2 | 19.6 | |
| TM | 56.2 | 29.8 | 33.0 | 9.8 | 7.5 | 5.0 | 5.8 | ||
| BS | 71.6 | 42.0 | 35.0 | 29.9 | 5.5 | 3.4 | 7.2 | ||
| 36–48 | ON | 54.2 | 32.6 | 26.7 | 9.1 | 7.3 | 3.4 | 17.3 | |
| TM | 51.5 | 23.1 | 30.7 | 10.6 | 11.3 | 4.0 | 5.0 | ||
| BS | 68.3 | 33.6 | 32.6 | 32.0 | 8.4 | 2.7 | 6.2 | ||
| >48 | ON | 50.5 | 24.7 | 32.0 | 4.9 | 13.2 | 11.7 | 13.8 | |
| TM | 49.6 | 17.0 | 36.4 | 5.8 | 19.9 | 13.8 | 3.9 | ||
| BS | 62.0 | 25.6 | 38.6 | 19.3 | 15.2 | 9.5 | 4.9 | ||
| African female | |||||||||
| ≤35 | ON | 63.2 | 37.5 | 37.8 | 6.0 | 7.5 | 5.5 | 43.2 | |
| TM | 60.9 | 27.0 | 42.7 | 7.0 | 11.6 | 6.6 | 15.1 | ||
| BS | 74.0 | 38.6 | 45.0 | 22.8 | 8.6 | 4.4 | 18.4 | ||
| 36–48 | ON | 59.0 | 29.7 | 35.3 | 6.5 | 11.3 | 4.4 | 39.0 | |
| TM | 57.5 | 20.8 | 40.0 | 7.7 | 17.2 | 5.3 | 13.2 | ||
| BS | 72.3 | 30.7 | 42.2 | 24.6 | 13.0 | 3.6 | 16.1 | ||
| >48 | ON | 57.1 | 22.3 | 41.5 | 3.5 | 19.9 | 15.0 | 32.6 | |
| TM | 57.4 | 15.2 | 46.6 | 4.1 | 29.0 | 17.6 | 10.5 | ||
| BS | 68.3 | 23.1 | 49.0 | 14.3 | 22.6 | 12.2 | 12.9 | ||
| African male | |||||||||
| ≤35 | ON | 59.3 | 40.0 | 28.0 | 6.9 | 5.0 | 3.7 | 30.4 | |
| TM | 55.3 | 29.1 | 32.1 | 8.1 | 7.9 | 4.4 | 9.7 | ||
| BS | 70.6 | 41.2 | 34.0 | 25.6 | 5.8 | 3.0 | 11.9 | ||
| 36–48 | ON | 54.1 | 31.8 | 25.9 | 7.5 | 7.7 | 3.0 | 27.1 | |
| TM | 51.1 | 22.5 | 29.8 | 8.8 | 11.9 | 3.6 | 8.4 | ||
| BS | 68.1 | 32.9 | 31.7 | 27.5 | 8.9 | 2.4 | 10.4 | ||
| >48 | ON | 50.3 | 24.1 | 31.1 | 4.0 | 13.9 | 10.3 | 22.2 | |
| TM | 49.1 | 16.5 | 35.5 | 4.8 | 20.9 | 12.2 | 6.7 | ||
| BS | 61.9 | 25.0 | 37.6 | 16.3 | 15.9 | 8.4 | 8.2 | ||
BS = brainstem; ON = optic neuritis; TM = transverse myelitis.
Table 5.
Likelihood (%) of developing attacks and disability over the next few years for patients who are on IST from disease onset and who have not reached the endpoint or experienced any attack 2 years after disease onset
| Attack onset | Risk over next 2 years | Risk over next 5 years | Type | Risk over next 2 years | Risk over next 5 years | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, years | Type | All | ON | TM | BS | EDSS | Blind | All | ON | TM | BS | EDSS | Blind | All | ON | TM | BS | EDSS | Blind | All | ON | TM | BS | EDSS | Blind | |||||
| 6.0 | ≥8.0 | 6.0 | ≥8.0 | 6.0 | ≥8.0 | 6.0 | ≥8.0 | |||||||||||||||||||||||
| Japanese female | Caucasian male | |||||||||||||||||||||||||||||
| ≤35 | ON | 33.5 | 14.4 | 18.8 | 3.6 | 2.6 | 0.9 | 8.4 | 57.8 | 27.5 | 38.2 | 6.1 | 5.7 | 2.0 | 16.9 | ON | 39.6 | 22.1 | 20.7 | 2.6 | 2.1 | 2.0 | 6.1 | 65.2 | 39.7 | 41.2 | 4.4 | 4.7 | 4.2 | 12.5 |
| TM | 33.9 | 10.4 | 22.4 | 4.4 | 4.5 | 1.2 | 2.5 | 58.5 | 20.5 | 43.9 | 7.3 | 9.6 | 2.6 | 5.2 | TM | 38.7 | 16.5 | 24.4 | 3.2 | 3.6 | 2.5 | 1.7 | 64.5 | 31.0 | 47.0 | 5.4 | 7.8 | 5.4 | 3.7 | |
| BS | 43.1 | 14.6 | 22.5 | 12.5 | 2.9 | 0.7 | 2.8 | 67.9 | 27.9 | 44.2 | 19.9 | 6.3 | 1.5 | 5.8 | BS | 47.0 | 22.4 | 24.7 | 9.6 | 2.4 | 1.5 | 2.0 | 72.5 | 40.1 | 47.4 | 15.5 | 5.2 | 3.3 | 4.2 | |
| 36–48 | ON | 31.0 | 11.2 | 17.5 | 4.0 | 4.2 | 0.8 | 7.4 | 54.2 | 22.0 | 36.0 | 6.7 | 8.9 | 1.6 | 15.0 | ON | 35.8 | 17.8 | 19.4 | 2.9 | 3.4 | 1.7 | 5.4 | 60.7 | 33.1 | 39.1 | 4.9 | 7.4 | 3.6 | 11.1 |
| TM | 31.5 | 7.8 | 20.7 | 4.8 | 6.9 | 1.0 | 2.1 | 55.1 | 15.8 | 41.3 | 7.9 | 14.4 | 2.1 | 4.5 | TM | 35.2 | 12.9 | 22.8 | 3.5 | 5.7 | 2.1 | 1.5 | 60.3 | 25.0 | 44.5 | 5.9 | 12.0 | 4.5 | 3.2 | |
| BS | 41.5 | 11.2 | 20.9 | 13.4 | 4.5 | 0.6 | 2.4 | 65.7 | 22.1 | 41.5 | 21.2 | 9.7 | 1.2 | 5.0 | BS | 43.9 | 18.0 | 23.1 | 10.4 | 3.8 | 1.3 | 1.7 | 69.1 | 33.3 | 45.0 | 16.7 | 8.1 | 2.7 | 3.7 | |
| >48 | ON | 30.2 | 8.2 | 21.2 | 2.2 | 7.8 | 2.8 | 5.9 | 54.0 | 16.6 | 42.1 | 3.7 | 16.1 | 6.0 | 12.0 | ON | 34.9 | 13.6 | 23.4 | 1.6 | 6.5 | 5.9 | 4.3 | 60.3 | 26.2 | 45.5 | 2.7 | 13.6 | 12.3 | 8.9 |
| TM | 31.2 | 5.5 | 24.5 | 2.6 | 12.1 | 3.5 | 1.6 | 55.6 | 11.4 | 47.1 | 4.3 | 24.2 | 7.4 | 3.4 | TM | 35.0 | 9.4 | 26.8 | 1.9 | 10.2 | 7.3 | 1.2 | 60.7 | 18.8 | 50.6 | 3.2 | 20.7 | 14.9 | 2.5 | |
| BS | 38.9 | 8.4 | 25.3 | 8.2 | 8.6 | 2.2 | 1.9 | 63.9 | 16.8 | 48.4 | 13.3 | 17.7 | 4.6 | 4.0 | BS | 41.8 | 13.8 | 27.8 | 6.2 | 7.2 | 4.7 | 1.4 | 67.8 | 26.5 | 52.0 | 10.1 | 15.0 | 9.8 | 2.9 | |
| Japanese male | African female | |||||||||||||||||||||||||||||
| ≤35 | ON | 31.0 | 16.0 | 13.9 | 4.3 | 1.9 | 0.6 | 5.6 | 53.8 | 30.1 | 29.4 | 7.2 | 4.1 | 1.4 | 11.4 | ON | 41.7 | 19.1 | 25.6 | 1.7 | 2.9 | 2.3 | 14.1 | 68.0 | 35.2 | 48.9 | 2.9 | 6.4 | 4.9 | 27.3 |
| TM | 30.1 | 11.5 | 16.6 | 5.2 | 3.1 | 0.8 | 1.6 | 52.9 | 22.5 | 34.3 | 8.5 | 6.8 | 1.8 | 3.4 | TM | 42.7 | 14.5 | 30.7 | 2.2 | 5.3 | 3.1 | 4.5 | 69.3 | 27.6 | 56.0 | 3.7 | 11.2 | 6.6 | 9.3 | |
| BS | 40.4 | 16.0 | 16.6 | 14.2 | 2.0 | 0.5 | 1.8 | 64.1 | 30.1 | 34.3 | 22.3 | 4.4 | 1.0 | 3.7 | BS | 50.4 | 19.9 | 31.1 | 7.0 | 3.5 | 1.9 | 5.1 | 76.2 | 36.5 | 56.6 | 11.4 | 7.6 | 4.1 | 10.5 | |
| 36–48 | ON | 28.2 | 12.5 | 12.9 | 4.7 | 3.0 | 0.5 | 4.9 | 49.8 | 24.2 | 27.6 | 7.8 | 6.4 | 1.1 | 10.1 | ON | 38.9 | 15.3 | 24.1 | 1.9 | 4.7 | 1.9 | 12.7 | 64.7 | 29.0 | 46.7 | 3.2 | 10.1 | 4.1 | 24.7 |
| TM | 27.7 | 8.7 | 15.3 | 5.6 | 4.9 | 0.7 | 1.4 | 49.3 | 17.5 | 32.0 | 9.2 | 10.3 | 1.4 | 2.9 | TM | 39.9 | 11.2 | 28.7 | 2.4 | 8.2 | 2.6 | 3.9 | 66.1 | 21.9 | 53.3 | 4.0 | 16.9 | 5.5 | 8.1 | |
| BS | 38.6 | 12.4 | 15.3 | 15.1 | 3.1 | 0.4 | 1.5 | 61.5 | 24.1 | 32.0 | 23.7 | 6.8 | 0.8 | 3.2 | BS | 48.4 | 15.8 | 29.2 | 7.6 | 5.5 | 1.6 | 4.4 | 73.9 | 29.8 | 54.0 | 12.3 | 11.6 | 3.3 | 9.2 | |
| >48 | ON | 26.2 | 9.3 | 15.9 | 2.6 | 5.6 | 2.0 | 3.9 | 48.0 | 18.6 | 33.0 | 4.4 | 11.9 | 4.2 | 8.1 | ON | 38.9 | 11.5 | 28.8 | 1.0 | 8.8 | 6.9 | 10.1 | 65.3 | 22.5 | 53.5 | 1.7 | 18.2 | 14.2 | 20.2 |
| TM | 26.4 | 6.3 | 18.5 | 3.1 | 8.9 | 2.5 | 1.1 | 48.5 | 12.9 | 37.5 | 5.2 | 18.2 | 5.2 | 2.2 | TM | 40.4 | 8.0 | 33.1 | 1.3 | 14.2 | 8.8 | 2.9 | 67.2 | 16.2 | 59.3 | 2.1 | 27.8 | 17.8 | 6.1 | |
| BS | 34.7 | 9.4 | 19.0 | 9.5 | 6.1 | 1.5 | 1.2 | 58.2 | 18.7 | 38.4 | 15.3 | 12.9 | 3.2 | 2.6 | BS | 47.2 | 11.9 | 34.3 | 4.3 | 10.1 | 5.6 | 3.5 | 73.6 | 23.2 | 60.7 | 7.1 | 20.6 | 11.7 | 7.3 | |
| Caucasian female | African male | |||||||||||||||||||||||||||||
| ≤35 | ON | 42.9 | 20.1 | 27.1 | 2.2 | 3.0 | 2.8 | 9.1 | 69.4 | 36.7 | 51.1 | 3.7 | 6.5 | 5.9 | 18.2 | ON | 38.5 | 21.3 | 19.6 | 2.1 | 2.2 | 1.7 | 9.8 | 63.9 | 38.5 | 39.5 | 3.6 | 4.7 | 3.6 | 19.5 |
| TM | 43.4 | 15.0 | 31.8 | 2.7 | 5.1 | 3.6 | 2.7 | 70.1 | 28.4 | 57.4 | 4.5 | 10.9 | 7.6 | 5.6 | TM | 37.9 | 16.1 | 23.6 | 2.6 | 3.8 | 2.2 | 3.0 | 63.6 | 30.3 | 45.8 | 4.4 | 8.2 | 4.7 | 6.2 | |
| BS | 50.7 | 20.6 | 32.2 | 8.3 | 3.4 | 2.2 | 3.1 | 76.6 | 37.4 | 58.1 | 13.5 | 7.4 | 4.7 | 6.4 | BS | 46.5 | 21.8 | 23.9 | 8.1 | 2.5 | 1.3 | 3.4 | 71.9 | 39.3 | 46.2 | 13.2 | 5.4 | 2.9 | 7.0 | |
| 36–48 | ON | 39.4 | 16.1 | 25.6 | 2.4 | 4.8 | 2.3 | 8.1 | 65.5 | 30.3 | 48.9 | 4.1 | 10.2 | 5.0 | 16.3 | ON | 35.3 | 17.1 | 18.4 | 2.4 | 3.5 | 1.4 | 8.7 | 59.9 | 32.0 | 37.5 | 3.9 | 7.5 | 3.0 | 17.5 |
| TM | 40.0 | 11.6 | 29.8 | 3.0 | 7.9 | 3.0 | 2.3 | 66.4 | 22.7 | 54.8 | 4.9 | 16.4 | 6.3 | 4.9 | TM | 34.9 | 12.5 | 22.0 | 2.9 | 5.9 | 1.8 | 2.6 | 59.8 | 24.3 | 43.3 | 4.8 | 12.5 | 3.9 | 5.4 | |
| BS | 47.8 | 16.4 | 30.3 | 9.0 | 5.4 | 1.8 | 2.7 | 73.7 | 30.8 | 55.5 | 14.6 | 11.4 | 3.9 | 5.6 | BS | 44.1 | 17.5 | 22.3 | 8.8 | 3.9 | 1.1 | 2.9 | 69.1 | 32.5 | 43.8 | 14.2 | 8.4 | 2.4 | 6.1 | |
| >48 | ON | 39.6 | 12.1 | 30.3 | 1.3 | 8.8 | 8.1 | 6.3 | 66.3 | 23.6 | 55.5 | 2.2 | 18.1 | 16.6 | 13.0 | ON | 34.2 | 13.1 | 22.4 | 1.3 | 6.6 | 5.1 | 7.0 | 59.3 | 25.2 | 43.9 | 2.1 | 13.8 | 10.6 | 14.2 |
| TM | 40.8 | 8.3 | 34.3 | 1.5 | 13.7 | 10.0 | 1.7 | 67.8 | 16.7 | 60.7 | 2.6 | 27.0 | 20.1 | 3.7 | TM | 34.5 | 9.1 | 26.0 | 1.5 | 10.6 | 6.4 | 2.0 | 60.0 | 18.3 | 49.4 | 2.6 | 21.5 | 13.2 | 4.1 | |
| BS | 46.9 | 12.3 | 35.5 | 5.2 | 9.9 | 6.5 | 2.1 | 73.6 | 24.0 | 62.2 | 8.6 | 20.1 | 13.4 | 4.4 | BS | 41.8 | 13.4 | 26.9 | 5.2 | 7.5 | 4.1 | 2.3 | 67.6 | 25.8 | 50.7 | 8.5 | 15.5 | 8.6 | 4.9 | |
See Supplementary Table 4 for full dataset.
BS = brainstem; ON = optic neuritis; TM = transverse myelitis.
Table 6.
Likelihood (%) of developing attacks and disability over the next 5 years for patients who are on IST from disease onset and who have not reached the endpoint but experienced one attack within 2 years since disease onset
| Ethnicity and sex | Japanese female | Japanese male | Caucasian female | Caucasian male | African female | African male | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Attack | Risk over next 5 years | Risk over next 5 years | Risk over next 5 years | Risk over next 5 years | Risk over next 5 years | Risk over next 5 years | ||||||||||||||
| Onset age, years | Onset type | 2nd type | All | EDSS 6.0 | Blind | All | EDSS 6.0 | Blind | All | EDSS 6.0 | Blind | All | EDSS 6.0 | Blind | All | EDSS 6.0 | Blind | All | EDSS 6.0 | Blind |
| ≤35 | ON | ON | 69.0 | 7.1 | 20.1 | 66.4 | 5.1 | 13.8 | 79.1 | 7.9 | 21.2 | 76.4 | 5.7 | 14.7 | 77.7 | 7.7 | 31.4 | 75.2 | 5.7 | 22.7 |
| TM | ON | 68.6 | 12.1 | 6.4 | 64.4 | 8.6 | 4.2 | 78.9 | 13.4 | 6.8 | 74.9 | 9.7 | 4.5 | 78.2 | 13.8 | 11.1 | 74.1 | 10.2 | 7.5 | |
| BS | ON | 77.1 | 7.9 | 7.0 | 74.4 | 5.5 | 4.5 | 84.4 | 9.0 | 7.6 | 81.7 | 6.4 | 5.0 | 84.0 | 9.2 | 12.4 | 81.2 | 6.6 | 8.3 | |
| ON | TM | 66.9 | 7.3 | 20.6 | 62.5 | 5.3 | 14.2 | 77.7 | 8.1 | 21.8 | 73.5 | 6.0 | 15.3 | 76.2 | 7.9 | 32.0 | 72.2 | 6.0 | 23.5 | |
| TM | TM | 68.4 | 12.2 | 6.5 | 62.6 | 8.8 | 4.3 | 78.9 | 13.6 | 6.9 | 73.6 | 10.0 | 4.6 | 78.1 | 14.0 | 11.3 | 72.8 | 10.4 | 7.7 | |
| BS | TM | 76.1 | 8.1 | 7.2 | 72.1 | 5.7 | 4.7 | 83.8 | 9.2 | 7.8 | 80.0 | 6.6 | 5.2 | 83.4 | 9.5 | 12.7 | 79.4 | 6.9 | 8.6 | |
| ON | BS | 64.2 | 7.3 | 20.6 | 60.7 | 5.3 | 14.1 | 74.6 | 8.3 | 22.0 | 70.9 | 6.1 | 15.4 | 73.0 | 8.1 | 32.4 | 69.4 | 6.1 | 23.6 | |
| TM | BS | 65.3 | 12.2 | 6.5 | 60.4 | 8.7 | 4.2 | 75.5 | 13.9 | 7.0 | 70.6 | 10.1 | 4.7 | 74.7 | 14.3 | 11.5 | 69.6 | 10.5 | 7.8 | |
| BS | BS | 74.9 | 7.8 | 7.0 | 71.9 | 5.4 | 4.5 | 81.8 | 9.2 | 7.8 | 78.4 | 6.5 | 5.1 | 81.3 | 9.5 | 12.7 | 77.8 | 6.8 | 8.5 | |
| 36–48 | ON | ON | 65.2 | 11.1 | 18.1 | 62.0 | 8.1 | 12.3 | 75.4 | 12.5 | 19.3 | 72.2 | 9.2 | 13.3 | 74.5 | 12.2 | 28.7 | 71.4 | 9.2 | 20.7 |
| TM | ON | 64.8 | 18.0 | 5.5 | 60.3 | 13.2 | 3.6 | 75.3 | 20.2 | 6.0 | 70.8 | 15.0 | 4.0 | 74.9 | 20.7 | 9.8 | 70.2 | 15.5 | 6.6 | |
| BS | ON | 74.6 | 12.0 | 6.1 | 71.6 | 8.5 | 4.0 | 81.8 | 13.9 | 6.8 | 78.6 | 10.0 | 4.4 | 81.8 | 14.2 | 11.0 | 78.5 | 10.4 | 7.4 | |
| ON | TM | 63.6 | 11.3 | 18.4 | 58.7 | 8.3 | 12.7 | 74.4 | 12.8 | 19.7 | 69.6 | 9.5 | 13.7 | 73.3 | 12.5 | 29.2 | 68.6 | 9.5 | 21.3 | |
| TM | TM | 65.2 | 18.1 | 5.6 | 59.0 | 13.4 | 3.7 | 75.8 | 20.3 | 6.0 | 69.9 | 15.3 | 4.0 | 75.3 | 20.8 | 9.8 | 69.3 | 15.8 | 6.7 | |
| BS | TM | 74.0 | 12.2 | 6.2 | 69.7 | 8.8 | 4.1 | 81.4 | 14.1 | 6.9 | 77.1 | 10.3 | 4.6 | 81.5 | 14.4 | 11.2 | 77.0 | 10.7 | 7.6 | |
| ON | BS | 61.0 | 11.3 | 18.3 | 57.1 | 8.2 | 12.5 | 71.1 | 12.9 | 19.8 | 66.8 | 9.6 | 13.8 | 70.0 | 12.6 | 29.5 | 65.8 | 9.6 | 21.4 | |
| TM | BS | 62.2 | 18.1 | 5.6 | 57.1 | 13.2 | 3.6 | 72.2 | 20.6 | 6.1 | 66.7 | 15.3 | 4.1 | 71.7 | 21.1 | 10.0 | 66.1 | 15.9 | 6.8 | |
| BS | BS | 73.1 | 11.8 | 6.0 | 69.8 | 8.3 | 3.9 | 79.4 | 14.0 | 6.8 | 75.7 | 10.1 | 4.5 | 79.4 | 14.3 | 11.1 | 75.5 | 10.5 | 7.5 | |
| >48 | ON | ON | 63.9 | 20.0 | 14.6 | 59.3 | 15.0 | 10.0 | 75.2 | 22.0 | 15.5 | 70.8 | 16.7 | 10.7 | 74.2 | 22.0 | 23.8 | 69.9 | 17.0 | 17.0 |
| TM | ON | 64.1 | 29.8 | 4.2 | 58.2 | 22.8 | 2.8 | 75.5 | 32.5 | 4.5 | 70.0 | 25.4 | 3.0 | 74.9 | 33.4 | 7.4 | 69.3 | 26.3 | 5.1 | |
| BS | ON | 72.3 | 21.8 | 4.9 | 68.0 | 16.1 | 3.3 | 81.0 | 24.3 | 5.3 | 76.9 | 18.3 | 3.6 | 80.9 | 24.9 | 8.8 | 76.6 | 19.0 | 6.0 | |
| ON | TM | 64.2 | 20.1 | 14.7 | 57.9 | 15.2 | 10.2 | 75.6 | 22.2 | 15.6 | 69.9 | 17.1 | 10.9 | 74.5 | 22.1 | 23.9 | 68.8 | 17.3 | 17.3 | |
| TM | TM | 66.1 | 29.7 | 4.2 | 58.9 | 22.9 | 2.8 | 77.2 | 32.4 | 4.5 | 70.8 | 25.5 | 3.1 | 76.6 | 33.3 | 7.4 | 70.1 | 26.4 | 5.1 | |
| BS | TM | 73.1 | 21.9 | 5.0 | 67.5 | 16.3 | 3.3 | 81.8 | 24.4 | 5.4 | 76.6 | 18.6 | 3.6 | 81.6 | 25.0 | 8.8 | 76.3 | 19.3 | 6.0 | |
| ON | BS | 60.2 | 20.3 | 14.8 | 54.7 | 15.2 | 10.2 | 71.5 | 22.6 | 15.9 | 66.0 | 17.3 | 11.1 | 70.4 | 22.5 | 24.3 | 64.9 | 17.5 | 17.5 | |
| TM | BS | 61.9 | 29.9 | 4.2 | 55.4 | 22.9 | 2.8 | 72.9 | 33.1 | 4.6 | 66.6 | 25.9 | 3.1 | 72.3 | 34.0 | 7.6 | 65.8 | 26.8 | 5.2 | |
| BS | BS | 70.8 | 21.6 | 4.9 | 66.0 | 15.9 | 3.2 | 78.9 | 24.6 | 5.4 | 74.0 | 18.6 | 3.6 | 78.7 | 25.2 | 8.9 | 73.6 | 19.3 | 6.1 | |
See Supplementary Table 5 for the full dataset.
BS = brainstem; ON = optic neuritis; TM = transverse myelitis.
Optimization of patient recruitment for clinical trials
Our data can be used to more accurately power future clinical trials. It is noteworthy that because disability is solely relapse-related in NMO and because such disability may be severe (and in contrast to multiple sclerosis), primary outcome measures in phase 3 NMO clinical trials to date, have been time to first relapse only (for ethical reasons) as a surrogate for disability. Thus we estimated the risk of relapsing over a 1- and 2-year period to aid trial design. Because there are limited restrictions that can ethically be applied to clinical trial eligibility (e.g. sex and ethnicity criteria would not be acceptable inclusion criteria), we selected baseline characteristics such as prior relapse activity and disease duration to calculate risk of relapse over the subsequent 1 and 2 years. Table 7 shows the estimated proportion of patients who relapse over a 1-year and 2-year period, dependent on the disease duration category and the number of NMO attacks in the preceding 2 years for those on IST because we assumed all diagnosed AQP4-IgG-positive patients will be on IST. A patient with disease duration of <5 years and with at least three attacks in the past 2 years has 73% chance of relapse within the next 2 years (representing 2.7% of the NMO population), whereas any patient (no restriction) has a 54% chance of relapse in 2 years (representing 100% of the population). Such information is useful for powering future clinical trials. The percentage of total AQP4-IgG-positive NMOSD patients who would satisfy these criteria is also included in the table because there will be a trade-off between selecting a rare group of highly active patients versus a broader range of less active patients; the former is favourable for statistical powering purposes whereas the latter favours ease of recruitment and a broader licensing indication. The loss of study eligible patients would be more deleterious to an effective study design than loading the study with highly active patients. For example, there appears no overall advantage in selecting recent onset patients or patients with higher disease activity in the preceding 2 years (used as a criteria for the currently ongoing three randomized control trials) because the increase in disease activity is modest and the loss of eligible patients large so that the entry criteria could be broadened.
Table 7.
Identification of NMOSD patients for drug trials: risk of relapse at 1 and 2 years based on numbers of attacks in preceding 2 years
| No. of attacks in the past 2 years | Disease duration | % of patients that relapse in 1 year | % of patients that relapse in 2 years | Average % patients fulfilling criteria over timea |
|---|---|---|---|---|
| At least 3 | Any | 46.60% | 67.90% | 6.6% |
| <5 years | 52.00% | 73.30% | 2.7% | |
| ≥5 years | 44.90% | 66.30% | 3.9% | |
| At least 2 | Any | 42.60% | 63.70% | 17.6% |
| <5 years | 46.90% | 68.00% | 5.0% | |
| ≥5 years | 40.20% | 62.30% | 12.6% | |
| At least 1 | Any | 38.70% | 59.20% | 40.7% |
| <5 years | 41.10% | 62.50% | 7.7% | |
| ≥5 years | 36.6% | 58.20% | 33.0% | |
| Any | Any | 34.50% | 54.20% | 100% |
| <5 years | 37.90% | 57.50% | 14.8% | |
| ≥5 years | 33.40% | 53.10% | 85.2% |
aOn 1 January 2013.
Discussion
This study has highlighted several important issues, including the effects of ethnicity, sex, onset age, treatment and onset attack phenotype on relapse and disability risks. Using these risk factors we have been able to produce a new useful prognostic tool allowing prediction of the likely outcome in individual patients according to their baseline features and at 2 years depending on their early disease course. Second, we have produced data on the relapse risk over 1 and 2 years based upon disease activity in the prior 2 years as a tool to power future clinical treatment trials, and we have shown that the activity in the prior 2 years has only a modest effect on the subsequent 2 year activity. Third, we have used a model that removes the usual before and after treatment biases, and our results still support the effectiveness of IST and negative effect of multiple sclerosis drugs in AQP4-IgG-positive NMOSD patients. Finally, we have shown that the disability is often due to relapses and not the onset attack, highlighting the importance of starting treatment soon after the index clinical event.
Several previous studies have reported risk factors for relapse and disability using univariate and multivariate regression analysis (Weinshenker et al., 2006; Collongues et al., 2010a, b, 2014; Jiao et al., 2013; Kim et al., 2013). The strength of the analysis model in this study is that it takes into account the timing of the event and allows time-dependent treatment effects to be used. This will for example remove the positive bias due to onset attacks (which occur off treatment) being more severe, independent of treatment. Additionally we can combine the different factors in individual patients to produce individual patient predictions of risk. Importantly in contrast to other models, we can account for the correlation of recurrent attacks and disability events accounting for their dependence explicitly and use the histories of certain events to predict the developments of other events.
Acknowledging the difference in analysis methods our findings are generally in line with other studies of AQP4-IgG-positive NMOSD patients. Kitley et al. (2012), in a smaller population of AQP4-IgG seropositive patients, noted better outcomes in Japanese patients than Caucasians, and although there was no difference in time to first relapse amongst different ethnic groups, there was a lower relapse rate in Japanese than in Caucasians and Afro-Caribbeans. Afro-Caribbeans had the greatest risk of visual disability than the other groups. There were fewer brain attacks in Caucasians than other groups. Additionally, young onset patients were more likely to develop visual disability and older onset patients to develop motor disability and those with optic neuritis onset attacks were more likely to develop visual disability. Initiating IST before the first relapse was associated with longer time to relapse. However, the study had less power and did not adjust for interactions between race, age and onset phenotype, did not factor in time-dependent treatment effects nor incorporate the effects of relapses and disability over time.
Long et al. (2017) reported in a cohort of 292 Chinese AQP4-IgG-positive patients an earlier time to relapse in those presenting with non-optic neuritis non-transverse myelitis attacks although the relapse rates were eventually similar to those presenting with optic neuritis or transverse myelitis (Long et al., 2017). This non-optic neuritis non-transverse myelitis onset group had lower EDSS scores at follow-up. However, these outcomes were not adjusted for other baseline differences such as the younger age of onset and varied follow-up times. Table 2 from our study shows that patients with cerebral or brainstem onset attacks had the highest relapse risk, and Table 3 shows that this group had non-significant lower risks of visual disability, a similar risk to optic neuritis but lower risk than transverse myelitis to reaching EDSS 6.0, and lower risk of EDSS ≥ 8.0.
Seok et al. (2017) noted in Korean AQP4-IgG-positive patients that those with late onset compared to those with early onset disease had a lower risk of relapse (although not time to relapse) and subsequent risk of non-transverse myelitis attacks, a lower risk of visual disability and a trend to a higher risk of EDSS 6.0; however, differences between the onset phenotypes, follow-up times, and use of multiple sclerosis drugs were not adjusted for (Seok et al., 2017). The authors noted their older onset patients appeared to have lower EDSS scores than the Caucasian patients from Kitley et al. (2012).
One important advantage of our analysis model is its ability to predict the risk of future outcomes in individual patients at any time point and account for the number of events (relapses and disability events) already experienced. This model requires a large dataset and the confidence of the prediction will depend not only on the size of the dataset but also on the diversity of the population (age, sex, ethnicity etc.). We have included two illustrative scenarios: one from disease onset, which requires the diagnosis of AQP4-IgG-positive NMOSD to have been made and assumes long-term IST since disease onset, and one at 2 years depending on whether the patients have had no relapse or one relapse over this period and have not reached the disability endpoint of interest (because 0–1 relapse is the most common relapse frequency over the first 2 years). Ideally this model should be set up as an online tool and continuously updated with more patient data, and individual risks for all clinical scenarios could be estimated. A similar tool for multiple sclerosis was developed but not established because of lack of long-term resources (Daumer et al., 2007).
We also provided useful information to power future clinical trials. In contrast to multiple sclerosis, NMO disability is primarily relapse acquired and because the relapses can be severely disabling, the primary outcome in NMO trials has been time to relapse. The current phase 3 clinical trials have focused on recruiting active patients with recent and often multiple relapses. There have been challenges in recruiting partly due to the rarity of the condition and the smaller subpopulation that meet these criteria. However, it has been assumed that activity in the last 2 years has a large effect on the relapse risk going forward. Our data suggest that randomizing all patients (assuming on IST) would produce a reasonable number of relapses within 1 and 2 years and allow a much greater pool of patients to recruit from. Restricting recruitment to very active disease in order to optimize the likelihood of on-study attacks significantly reduces the pool of eligible patients prolonging the recruitment period or increasing the number of centres or both. Our data indicate that such patient selection criteria only moderately increase relapse risk. Additionally, the drug license may be limited to patients who meet the study entry criteria and thus expanding the eligibility could broaden access to treatment. Thirty-four per cent of the total cohort of IST-treated patients relapsed within 1 year from a single time point and this appears surprisingly high. A previous letter (Kitley et al., 2014a, b) noted 25% of all patients from a single time point (on and off IST) relapsed within 7 months and 50% within 19 months (Kitley et al., 2014a). From onset of IST, 50% relapsed within 23 months when early relapses from initiation were included. Neither of these outcomes is directly comparable to our category of patients but these data support our figures although we have used a more practically relevant outcome for clinical trial recruitment i.e. taking all already on IST.
The lack of randomized controlled trials to support the use of IST has been used to advocate the use of placebo-controlled trials in neuromyelitis optica. Cree (2015) noted the biases of using before (historical) and after (post-initiation of IST) comparisons of relapse rates, such as regression towards the mean (Cree, 2015). Additionally the natural history of reduction of relapses over time we have demonstrated would add to this bias. Although not randomized controlled data, our analysis removes these biases and shows a positive effect of IST in all relapse and disability outcomes except for EDSS 8.0/death. There appeared a negative effect of IST on this latter outcome and this is out of keeping with the other IST effects in our cohort and would be at odds with the literature, thus we think this is likely to be a random effect due to the smaller numbers for this outcome.
The purpose of this study was not to compare efficacy of different immunosuppressive medications as attack preventive therapies in NMOSD. Most but not all previous observational studies suggest that rituximab is more effective than azathioprine (Mealy et al., 2014; Jeong et al., 2016; Stellmann et al., 2017). Furthermore, a recent randomized controlled trial also showed superiority of rituximab over azathioprine at relapse prevention (Nikoo et al., 2017). Our non-randomized allocation of treatments would not have added better evidence to the literature and would have reduced our ability to see the influences of other factors on outcome. Lumping all ISTs together does not include a bias because it is standard practice in all centres to advise all AQP4-IgG-positive patients to take ISTs. Additionally if we split the groups into more subgroups it reduces the power to see the effects of relevant and unbiased covariates. For example, there were only 20 patients who started with rituximab as the first line treatment, and among them, only six experienced disability events. We, therefore, did not analyse separately all the different IST or multiple sclerosis disease-modifying treatments for relative efficacy.
We have also demonstrated a negative time-dependent effect of multiple sclerosis disease-modifying treatments, which supports previous reports of increase in relapses and case reports/series of clinical worsening (Papeix et al., 2007; Shimizu et al., 2010; Uzawa et al., 2010; Barnett et al., 2012; Kleiter et al., 2012; Min et al., 2012). We have also demonstrated that 75% of EDSS 6.0 outcomes and 79% of bilateral visual disability outcomes occur subsequent to the onset attack demonstrating the potential for reducing long-term disability. Thus, our study strengthens the evidence for early IST in NMO. On the other hand, given that 41% were blind in one eye after incident optic neuritis and 17% remained wheelchair-bound or worse after incident transverse myelitis, the development of regenerative and reparative strategies in the future warrants emphasis.
Our study has several weaknesses and strengths. Firstly, although all centres have prospectively collected databases, the analysis was not preplanned and some data points were occasionally missing. Some retrospectively collected relapses were included particularly from the early phases of the disease before the diagnosis was made and this relied on patient reporting. The cases in this study may not be representative of disease course and disability in the community (population-based). All centres except Matinique (which is closest to population-based cohort with 31 prevalent cases from 2011) receive referrals from other centres so the referral bias is likely to be similar among centres. It would not be possible to perform a population-based cohort study as the numbers of NMOSD patients in such populations are too small to allow such a mathematical analysis. There are only five patients in Olmsted County with AQP4-IgG positive NMOSD. It is possible than some may have been included in the Mayo cohort, but given the small number, we doubt this would have any significant effect.
Additionally, biases will exist due to patients lost to follow-up although most centres will follow-up patients because they are on long-term immunosuppression. This bias may lead to loss of some patients with milder disease and may explain the variability in mortality rates depending on the completeness of data obtained. However, these data represent the outcomes within NMO specialist centres across different countries that allow for different treatment and follow-up practices making it more relevant to heterogeneous populations of NMO.
Our study supports onset age, onset phenotype and ethnic influences on outcome in NMO and we offer a prognostic tool for individual patients. In addition, we have provided data for powering clinical treatment trials based upon the relapse activity in the preceding 2 years and suggest recruitment of ‘all comers’ would be a reasonable approach. Finally, we have provided additional evidence for the use of IST, especially at an early stage of disease.
Supplementary Material
Acknowledgements
We would like to thank/acknowledge Amy Pace of Alexion Pharmaceuticals for statistical input/review. Alexion Pharmaceuticals provided a courtesy medical review of the manuscript. We thank Mary Curtis, Valerie Peterson, and Sara Vinje for technical assistance.
Glossary
Abbreviations
- EDSS
Expanded Disability Status Scale
- IST
immunosuppressive treatment
- NMOSD
neuromyelitis optica spectrum disorders
Funding
This study was funded by Alexion Pharmaceuticals and Mayo Clinic’s Centre MS and Autoimmune Neurology.
Competing interests
The authors report no competing interests.
Web resources
https://clinicaltrials.gov/ct2/show/NCT01892345
https://clinicaltrials.gov/ct2/show/NCT02003144
https://clinicaltrials.gov/ct2/show/NCT02028884
References
- Barnett MH, Prineas JW, Buckland ME, Parratt JD, Pollard JD. Massive astrocyte destruction in neuromyelitis optica despite natalizumab therapy. Mult Scler 2012; 18: 108–12. [DOI] [PubMed] [Google Scholar]
- Cobo-Calvo A, Ruiz A, Maillart E, Audoin B, Zephir H, Bourre B et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: the MOGADOR study. Neurology 2018; 90: e1858–69. [DOI] [PubMed] [Google Scholar]
- Collongues N, Marignier R, Jacob A, Leite MI, Siva A, Paul F et al. Characterization of neuromyelitis optica and neuromyelitis optica spectrum disorder patients with a late onset. Mult Scler 2014; 20: 1086–94. [DOI] [PubMed] [Google Scholar]
- Collongues N, Marignier R, Zephir H, Papeix C, Blanc F, Ritleng C et al. Neuromyelitis optica in France: a multicenter study of 125 patients. Neurology 2010a; 74: 736–42. [DOI] [PubMed] [Google Scholar]
- Collongues N, Marignier R, Zephir H, Papeix C, Fontaine B, Blanc F et al. Long-term follow-up of neuromyelitis optica with a pediatric onset. Neurology 2010b; 75: 1084–8. [DOI] [PubMed] [Google Scholar]
- Cree BA. Placebo controlled trials in neuromyelitis optica are needed and ethical. Mult Scler Relat Disord 2015; 4: 536–45. [DOI] [PubMed] [Google Scholar]
- Daumer M, Neuhaus A, Lederer C, Scholz M, Wolinsky JS, Heiderhoff M. Prognosis of the individual course of disease–steps in developing a decision support tool for Multiple Sclerosis. BMC Med Inform Decis Making 2007; 7: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan EP, Cabre P, Weinshenker BG, St Sauver J, Jacobson DJ, Majed M et al. Epidemiology of aquaporin-4 autoimmunity and neuromyelitis optica spectrum. Ann Neurol 2016; 79: 775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarius S, Kleiter I, Ruprecht K, Asgari N, Pitarokoili K, Borisow N et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 3: Brainstem involvement - frequency, presentation and outcome. J Neuroinflamm 2016; 13: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong IH, Park B, Kim SH, Hyun JW, Joo J, Kim HJ. Comparative analysis of treatment outcomes in patients with neuromyelitis optica spectrum disorder using multifaceted endpoints. Mult Scler 2016; 22: 329–39. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Fryer JP, Lennon VA, Jenkins SM, Quek AM, Smith CY et al. Updated estimate of AQP4-IgG serostatus and disability outcome in neuromyelitis optica. Neurology 2013; 81: 1197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitprapaikulsan J, Chen JJ, Flanagan EP, Tobin WO, Fryer JP, Weinshenker BG et al. Aquaporin-4 and myelin oligodendrocyte glycoprotein autoantibody status predict outcome of recurrent optic neuritis. Ophthalmology 2018a; 125: 1628–37. [DOI] [PubMed] [Google Scholar]
- Jitprapaikulsan J, Chiriboga ASL, Flanagan EP, Fryer JP, McKeon A, Weinshenker BG et al. Novel glial targets and recurrent longitudinally extensive transverse myelitis. JAMA Neurol 2018b; 75: 892–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurynczyk M, Messina S, Woodhall MR, Raza N, Everett R, Roca-Fernandez A et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain 2017; 140: 3128–38. [DOI] [PubMed] [Google Scholar]
- Kim SM, Park J, Kim SH, Park SY, Kim JY, Sung JJ et al. Factors associated with the time to next attack in neuromyelitis optica: accelerated failure time models with random effects. PLoS One 2013; 8: e82325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitley J, Leite MI, Elsone L, Jacob A, Palace J. Time to next relapse as a primary endpoint in neuromyelitis optica clinical trials. J Neurol Neurosurg Psychiatry 2014a; 85: 589–90. [DOI] [PubMed] [Google Scholar]
- Kitley J, Leite MI, Nakashima I, Waters P, McNeillis B, Brown R et al. Prognostic factors and disease course in aquaporin-4 antibody-positive patients with neuromyelitis optica spectrum disorder from the United Kingdom and Japan. Brain 2012; 135 (Pt 6): 1834–49. [DOI] [PubMed] [Google Scholar]
- Kitley J, Waters P, Woodhall M, Leite MI, Murchison A, George J et al. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol 2014b; 71: 276–83. [DOI] [PubMed] [Google Scholar]
- Kleiter I, Hellwig K, Berthele A, Kumpfel T, Linker RA, Hartung HP et al. Failure of natalizumab to prevent relapses in neuromyelitis optica. Arch Neurol 2012; 69: 239–45. [DOI] [PubMed] [Google Scholar]
- Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 2005; 202: 473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004; 364: 2106–12. [DOI] [PubMed] [Google Scholar]
- Long Y, Liang J, Wu L, Lin S, Gao C, Chen X et al. Different Phenotypes at Onset in Neuromyelitis Optica Spectrum Disorder Patients with Aquaporin-4 Autoimmunity. Front Neurol 2017; 8: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mealy MA, Wingerchuk DM, Palace J, Greenberg BM, Levy M. Comparison of relapse and treatment failure rates among patients with neuromyelitis optica: multicenter study of treatment efficacy. JAMA Neurol 2014; 71: 324–30. [DOI] [PubMed] [Google Scholar]
- Min JH, Kim BJ, Lee KH. Development of extensive brain lesions following fingolimod (FTY720) treatment in a patient with neuromyelitis optica spectrum disorder. Mult Scler 2012; 18: 113–5. [DOI] [PubMed] [Google Scholar]
- Nikoo Z, Badihian S, Shaygannejad V, Asgari N, Ashtari F. Comparison of the efficacy of azathioprine and rituximab in neuromyelitis optica spectrum disorder: a randomized clinical trial. J Neurol 2017; 264: 2003–9. [DOI] [PubMed] [Google Scholar]
- Papeix C, Vidal JS, de Seze J, Pierrot-Deseilligny C, Tourbah A, Stankoff B et al. Immunosuppressive therapy is more effective than interferon in neuromyelitis optica. Mult Scler 2007; 13: 256–9. [DOI] [PubMed] [Google Scholar]
- Peschl P, Schanda K, Zeka B, Given K, Bohm D, Ruprecht K et al. Human antibodies against the myelin oligodendrocyte glycoprotein can cause complement-dependent demyelination. J Neuroinflammation 2017; 14: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quek AM, McKeon A, Lennon VA, Mandrekar JN, Iorio R, Jiao Y et al. Effects of age and sex on aquaporin-4 autoimmunity. Arch Neurol 2012; 69: 1039–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok JM, Cho HJ, Ahn SW, Cho EB, Park MS, Joo IS et al. Clinical characteristics of late-onset neuromyelitis optica spectrum disorder: A multicenter retrospective study in Korea. Mult Scler 2017; 23: 1748–56. [DOI] [PubMed] [Google Scholar]
- Shimizu J, Hatanaka Y, Hasegawa M, Iwata A, Sugimoto I, Date H et al. IFNbeta-1b may severely exacerbate Japanese optic-spinal MS in neuromyelitis optica spectrum. Neurology 2010; 75: 1423–7. [DOI] [PubMed] [Google Scholar]
- Stellmann JP, Krumbholz M, Friede T, Gahlen A, Borisow N, Fischer K et al. Immunotherapies in neuromyelitis optica spectrum disorder: efficacy and predictors of response. J Neurol Neurosurg Psychiatry 2017; 88: 639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzawa A, Mori M, Hayakawa S, Masuda S, Kuwabara S. Different responses to interferon beta-1b treatment in patients with neuromyelitis optica and multiple sclerosis. Eur J Neurol 2010; 17: 672–6. [DOI] [PubMed] [Google Scholar]
- Waters P, Woodhall M, O’Connor KC, Reindl M, Lang B, Sato DK et al. MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol Neuroinflamm 2015; 2: e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker BG, Barron G, Behne JM, Bennett JL, Chin PS, Cree BA et al. Challenges and opportunities in designing clinical trials for neuromyelitis optica. Neurology 2015; 84: 1805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker BG, Wingerchuk DM, Vukusic S, Linbo L, Pittock SJ, Lucchinetti CF et al. Neuromyelitis optica IgG predicts relapse after longitudinally extensive transverse myelitis. Ann Neurol 2006; 59: 566–9. [DOI] [PubMed] [Google Scholar]
- Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015; 85: 177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol 2007; 6: 805–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors, upon request.