Abstract
Background
Hypertension (high blood pressure) in pregnancy carries a high risk of maternal morbidity and mortality. Although antihypertensive drugs are commonly used, they have adverse effects on mothers and fetuses. Guided imagery is a non‐pharmacological technique that has the potential to lower blood pressure among pregnant women with hypertension. Guided imagery is a mind‐body therapy that involves the visualisation of various mental images to facilitate relaxation and reduction in blood pressure.
Objectives
To determine the effect of guided imagery as a non‐pharmacological treatment of hypertension in pregnancy and its influence on perinatal outcomes.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register, and two trials registers (October 2018). We also searched relevant conference proceedings and journals, and scanned the reference lists of retrieved studies.
Selection criteria
We included randomised controlled trials (RCTs). We would have included RCTs using a cluster‐randomised design, but none were identified. We excluded quasi‐RCTs and cross‐over trials.
We sought intervention studies of various guided imagery techniques performed during pregnancy in comparison with no intervention or other non‐pharmacological treatments for hypertension (e.g. quiet rest, music therapy, aromatherapy, relaxation therapy, acupuncture, acupressure, massage, device‐guided slow breathing, hypnosis, physical exercise, and yoga).
Data collection and analysis
Three review authors independently assessed the trials for inclusion, extracted data, and assessed risk of bias for the included studies. We checked extracted data for accuracy, and resolved differences in assessments by discussion. We assessed the certainty of the evidence using the GRADE approach.
Main results
We included two small trials (involving a total of 99 pregnant women) that compared guided imagery with quiet rest. The trials were conducted in Canada and the USA. We assessed both trials as at high risk of performance bias, and low risk of attrition bias; one trial was at low risk for selection, detection, and reporting bias, while the other was at unclear risk for the same domains.
We could not perform a meta‐analysis because the two included studies reported different outcomes, and the frequency of the intervention was slightly different between the two studies. One study performed guided imagery for 15 minutes at least twice daily for four weeks, or until the baby was born (whichever came first). In the other study, the intervention included guided imagery, self‐monitoring of blood pressure, and thermal biofeedback‐assisted relaxation training for four total hours; the participants were instructed to practice the procedures twice daily and complete at least three relief relaxation breaks each day. The control groups were similar ‐ one was quiet rest, and the other was quiet rest as bed rest.
None of our primary outcomes were reported in the included trials: severe hypertension (either systolic blood pressure of 160 mmHg or higher, or diastolic blood pressure of 110 mmHg or higher); severe pre‐eclampsia, or perinatal death (stillbirths plus deaths in the first week of life). Only one of the secondary outcomes was measured.
Low‐certainty evidence from one trial (69 women) suggests that guided imagery may make little or no difference in the use of antihypertensive drugs (risk ratio 1.27, 95% confidence interval 0.72 to 2.22).
Authors' conclusions
There is insufficient evidence to inform practice about the use of guided imagery for hypertension in pregnancy.
The available evidence for this review topic is sparse, and the effect of guided imagery for treating hypertension during pregnancy (compared with quiet rest) remains unclear. There was low‐certainty evidence that guided imagery made little or no difference to the use of antihypertensive drugs, downgraded because of imprecision.
The two included trials did not report on any of the primary outcomes of this review. We did not identify any trials comparing guided imagery with no intervention, or with another non‐pharmacological method for hypertension.
Large and well‐designed RCTs are needed to identify the effects of guided imagery on hypertension during pregnancy and on other relevant outcomes associated with short‐term and long‐term maternal and neonatal health. Trials could also consider utilisation and costs of health service.
Plain language summary
Guided imagery for treating hypertension in pregnancy
What is the issue?
Some women have long‐term high blood pressure, or hypertension, whereas approximately 10% of pregnant women develop high blood pressure as a complication of pregnancy. Guided imagery is a mind‐body therapy that involves the visualisation of various mental images to facilitate relaxation and reduction in blood pressure. It can be performed by oneself, one‐to‐one, or in groups with an instructor using audio or scripts.
Why is this important?
High blood pressure during pregnancy is associated with an increased risk of the mother developing pre‐eclampsia with proteinuria, eclampsia with seizures and liver and blood disorders, and kidney failure. The baby of a pregnant woman with high blood pressure is more likely to be born too soon, be too small, and may need neonatal intensive care. High blood pressure drugs are recommended for women with severe high blood pressure and pre‐eclampsia because of the risk of life‐threatening complications, but such drugs can have adverse effects for the mother (including headache, decreased mental alertness, and exercise intolerance). Such drugs can also cross the placenta and may affect the unborn baby, and are not generally recommended for pregnant women with mild to moderate high blood pressure, which is when other ways of managing blood pressure are sought.
Guided imagery is a non‐pharmacological technique that could potentially lower blood pressure among pregnant women with hypertension and improve pregnancy outcomes for the mother and her baby.
What evidence did we find?
We searched for evidence (October 2018) and found two trials (involving 99 women) conducted in Canada and the USA. Both trials compared guided imagery with quiet rest. There were no trials comparing guided imagery with no intervention, or other with another non‐pharmacological method for hypertension.
The two included studies reported different outcomes and the Intervention frequency was slightly different between the two studies. One study performed guided imagery for 15 minutes at least twice daily for four weeks or until the baby was born (whichever came first). The other study involved guided imagery, self‐monitoring of blood pressure, and thermal biofeedback‐assisted relaxation training for a total of four hours; the women were instructed to practice the procedures twice daily and complete at least three relaxation breaks each day. The control groups between the two studies were similar ‐ one used quiet rest and the other used quiet rest as bed rest.
Neither trial reported data for our main outcomes of interest: severe hypertension, severe pre‐eclampsia, or death of the baby during birth or within the first week of life. The trials provided data for only one of our secondary outcomes of interest.
Low‐certainty evidence from the one trial (69 women) suggests that, compared with quiet rest, guided imagery may make little or no difference in the use of antihypertensive drugs.
What does this mean?
We included two small trials comparing guided imagery with quiet rest. We did not identify any trials comparing guided imagery with no intervention, or another non‐pharmacological treatment for hypertension.
The available evidence for this review is sparse and the effect of guided imagery for treating hypertension during pregnancy (compared with quiet rest) remains unclear.
The included trials did not report on any of the main outcomes in this review and only provided low‐certainty evidence on the uncertain effect on the use of antihypertensive drugs.
There is insufficient evidence to inform practice about using guided imagery for hypertension in pregnancy.
Large and well‐designed studies are needed to identify the effects of guided imagery on hypertension during pregnancy and on other relevant outcomes associated with the short‐term and long‐term health of mothers and their babies. The trials should also consider the use and costs of health services.
Summary of findings
Summary of findings for the main comparison. Guided imagery compared to quiet rest for treating hypertension in pregnancy.
| Guided imagery compared to quiet rest for treating hypertension in pregnancy | ||||||
| Population: pregnant women (between 30 and 36 weeks’ gestation) with elevated blood pressure Setting: university medical centre, US Navy hospital, local obstetricians in USA, hospitals in Atlantic Canada Intervention: guided imagery Comparison: quiet rest | ||||||
| Outcomes | Anticipated absolute effects*(95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with quiet rest | Risk with guided imagery | |||||
| Severe hypertension | See comment | None of the trials reported this outcome. | ||||
| Severe pre‐eclampsia | See comment | None of the trials reported this outcome. | ||||
| Perinatal death (stillbirths plus deaths in the first week of life) | See comment | None of the trials reported this outcome. | ||||
| Antihypertensive drug use | 371 per 1000 | 472 per 1000 (267 to 825) | RR 1.27 (95% CI 0.72 to 2.22) | 69 (1 RCT) |
⊕⊕⊝⊝ low a | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High certainty. Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty. We are very uncertain about the estimate. | ||||||
aDowngraded (‐2) for very serious imprecision ‐ small trial and wide confidence intervals
Background
Description of the condition
Hypertension (high blood pressure) in pregnancy carries a high risk of maternal morbidity and mortality. Approximately 10% of pregnancies are complicated by hypertension (Duley 2009a; Steegers 2010). According to the National High Blood Pressure Education Program of the National Heart, Lung, and Blood Institute in the US, there are four types of hypertensive disorders of pregnancy: chronic hypertension, pre‐eclampsia/eclampsia, pre‐eclampsia superimposed on chronic hypertension, and gestational hypertension (NHBPEP 2000).
Chronic hypertension presents pre‐pregnancy or before 20 weeks' gestation, and complicates 3% of pregnancies. Approximately 20% to 30% of women with chronic hypertension are prone to developing superimposed pre‐eclampsia (Yoder 2009). Pre‐eclampsia is defined as hypertension complicated by proteinuria (protein in the urine (Duley 2009a; NHBPEP 2000)). The condition is a syndrome of high blood pressure occurring after 20 weeks' gestation with the presentation of proteinuria (Lowe 2009; NHBPEP 2000). Gestational hypertension is transient hypertension, appearing after mid‐pregnancy, which is confirmed by a return to normal blood pressure postpartum, and no proteinuria (NHBPEP 2000).
Definition of hypertension
Hypertension in pregnancy is defined as systolic blood pressure of at least 140 mmHg or diastolic blood pressure of at least 90 mmHg, or both. Any rise in blood pressure should be confirmed by a second measurement, ideally at least four hours later. Because of cardiovascular changes, automated blood pressure monitors systematically underestimate blood pressure in pregnancy and pre‐eclampsia. If used, they should be calibrated regularly against a mercury sphygmomanometer (Shennan 2003). The debate over which auscultatory sound to use for assessment of diastolic blood pressure, muffling (Korotkoff phase IV) or disappearance (Korotkoff phase V), has been resolved, and Korotkoff V is now recommended as more reliable (Brown 2001).
Definition of proteinuria
Proteinuria during pregnancy is defined as 300 mg protein, or more, in 24 hours (Brown 2001). In a single midstream urine sample, this usually correlates with 30 mg/dL, 1+ or more on a dipstick, or a spot urine protein/creatinine ratio of at least 30 mg/mmol.
Categories of hypertensive disorders of pregnancy
Four main categories of hypertensive disorders are now widely agreed upon as follows.
(1) Pregnancy‐induced hypertension (PIH) or gestational hypertension (GH)
This is hypertension detected for the first time during the second half of pregnancy (after 20 weeks' gestation) in the absence of proteinuria. It resolves within three months after birth.
(2) Pre‐eclampsia/eclampsia (PE)
Pre‐eclampsia is defined as hypertension and proteinuria detected for the first time in the second half of pregnancy (after 20 weeks' gestation). Eclampsia is the occurrence of seizures in a woman with pre‐eclampsia.
There is no widely accepted definition of severe pre‐eclampsia. Nevertheless, the following are widely regarded as features of severe disease: severe hypertension (blood pressure at least 160 mmHg systolic, or 110 mmHg diastolic), severe proteinuria (usually at least 3 g (range 2 g to 5 g) protein in 24 hours, or 3+ on a dipstick), reduced urinary volume (less than 500 mL in 24 hours), neurological disturbances, such as headache, visual disturbances, and exaggerated tendon reflexes, upper abdominal pain, pulmonary oedema (fluid in the lungs), impaired liver function tests, high serum creatinine, low platelets, intrauterine growth restriction, or reduced liquor (amniotic fluid) volume (Brown 2000; NHBPEP 2000).
(3) Chronic hypertension
This is hypertension known to be present before pregnancy, or detected before 20 weeks' gestation. It is labelled essential hypertension if there is no underlying cause, and secondary hypertension if there is an underlying cause, such as renal, cardiac, or endocrine disease. Chronic hypertension may present for the first time as gestational hypertension. Hence, gestational hypertension that does not resolve after birth should be reclassified as chronic hypertension.
(4) Pre‐eclampsia superimposed on chronic hypertension
Women with chronic hypertension may then develop pre‐eclampsia. This is diagnosed when there is a new onset of proteinuria, or sudden worsening of either hypertension or proteinuria, or development of other signs and symptoms of pre‐eclampsia after 20 weeks' gestation.
Prevalence of hypertensive disorders of pregnancy
The Global Burden of Diseases, Injuries, and Risk Factors Study 2016 provides a comprehensive assessment of the prevalence and incidence for maternal hypertensive disorders of pregnancy (HDP (GBD 2016)). In 195 countries, the prevalence of HDP was 4.4 million (95% uncertainty interval (UI) 2.9 million to 6.1 million), and the incidence was 20.8 million (95% UI 18.2 million to 23.2 million (GBD 2016)). A review of epidemiological studies on maternal HDP showed that the prevalence of HDP ranged from 5.2% to 8.2%, PIH from 4.1% to 19.4%, GH from 1.8% to 4.4%, and PE from 0.2% to 9.2% (Umesawa 2016).
Severe adverse outcomes
Severe adverse maternal outcomes of pre‐eclampsia/eclampsia include maternal death, seizures (eclampsia), placental abruption, haemolysis, elevated liver enzymes, low platelets (HELLP) syndrome, renal failure, increased frequency of caesarean section, and preterm delivery (Heard 2004; MacKay 2001; Mustafa 2012). Pre‐eclampsia/eclampsia also causes adverse neonatal outcomes, including respiratory difficulties, increased frequency of neonatal intensive care unit admission, and infants who are born small‐for‐gestational age (Hauth 2000; Mustafa 2012).
In hypertensive pregnancies, these conditions carry higher risks of neonatal and maternal morbidity and mortality than in normotensive pregnancies (Christine 2011; Khan 2006). According to the World Health Organization Multicountry Survey on Maternal and Newborn Health (WHOMCS), a cross‐sectional analysis implemented in health facilities in 29 countries from Africa, Asia, Latin America, and the Middle East — women with severe maternal outcomes (SMO; i.e. maternal death or maternal near miss) had a higher rate of pre‐eclampsia than did women without SMO (16.3% with SMO versus 2.2% without SMO) and eclampsia (9.6% with SMO versus 0.3% without SMO). The survey showed that a high coverage of essential interventions (e.g. magnesium sulphate for eclampsia) did not result in reduced maternal mortality in the targeted healthcare facilities (Souza 2013).
Antihypertensive therapy is recommended for women with pre‐eclampsia with severe hypertension who are at risk of life‐threatening complications (ACOG 2013).
However, it may be desirable for mothers to take less antihypertensive drugs to avoid side‐effects and potential fetal risks. First‐line antihypertensive drugs during pregnancy include hydralazine, methyldopa, nifedipine, and labetalol (Moodley 2011; Mustafa 2012); however, all antihypertensive drugs cross the placenta and may affect the fetus. Furthermore, antihypertensive drugs have adverse side‐effects for mothers, including headache, decreased mental alertness, impaired sleep, depression, exercise intolerance, and they can also result in elevated liver function tests (ACOG 2013; Mustafa 2012).
Antihypertensive medications are not recommended for pregnant women with mild to moderate hypertension (ACOG 2013). There is insufficient evidence to conclude whether antihypertensive medications for mild to moderate hypertension during pregnancy are effective, because no data from well‐designed randomised controlled trials are available to mandate the safe use of antihypertensive drugs (Abalos 2014).
In addition, angiotensin receptor blockers (ARBs) used to control high blood pressure raise the risk of side‐effects in the fetus, including renal agenesis (failure of the kidney to develop during embryonic growth) and fetotoxicity (Martin 2005).
Alternative approaches to lower blood pressure that are free from side‐effects could be beneficial to pregnant women.
We included definitions of hypertension in pregnancy from the Cochrane Pregnancy and Childbirth Group's generic protocol for treating pre‐eclampsia and its consequences (Duley 2009b).
Prevention and treatment of hypertension in pregnancy
Pharmacological treatment
Antihypertensive therapy (beta‐blockers, calcium channel blockers) (Abalos 2014; Duley 2013)
Diuretic drugs (Churchill 2007)
Anticoagulants (Duley 2007)
Antiplatelet agents (Duley 2007)
Nitric oxide (Meher 2007)
Progesterone (Meher 2006)
Non‐pharmacological treatment
There is insufficient evidence to make reliable conclusions about the usefulness of the following non‐pharmacological treatments of hypertensive disorders during pregnancy.
Healthy diet, Dietary Approaches to Stop Hypertension (DASH) diet (Asemi 2013)
Weight management (Thangaratinam 2012)
Dietary salt restriction, altering salt intake (Duley 1999; Duley 2005)
Physical exercise (Meher 2006a)
Rest or advice to reduce physical activity for normotensive women (Meher 2006b)
Stress management (Jallo 2008)
Guided imagery (Moffatt 2006)
Acupuncture (Betts 2003)
Nutritional supplementation
Calcium supplementation (Hofmeyr 2010)
Magnesium supplements (Makrides 2001)
Antioxidants (Roberts 2010)
Zinc supplement (Mahomed 2007)
Description of the intervention
Guided imagery includes the generation of various mental images (Astin 2003), and is a technique of visualisation that aims to facilitate relaxation (Daake 1989). These visualisations induce a mental representation of reality that is a quasi‐real psychophysiological process with a specific desired goal of a physical or psychological outcome in one's mind without an actual external stimulus (Astin 2003;Jallo 2008). As a cognitive intervention, the generated images cause responses of the following senses: vision, hearing, taste, smell, touch, and body balance, and likewise in the physiological and psychological responses that occur under the actual stimulus presentation (Jallo 2008; Naparstek 1994). Guided imagery creates the interaction between body and mind, and the evoked image leads to a state of relaxation and a targeted condition, such as relief of pain (Park 2012).
The session of guided imagery is performed either by oneself, or one‐to‐one or in groups with an instructor using audio or scripts throughout the visualisation process (Daake 1989). Prerecorded compact discs (CDs) and booklets are often provided to participants to facilitate self‐practice (Gedde‐Dahl 2012; Sharpe 2007). The intervention is non‐pharmacological, and is one of several mind‐body therapies (MBTs) that are defined as "interventions that employ various methods to engage the mind's capacity to affect bodily function and symptoms" (NCCIH 2012), including relaxation, meditation, hypnosis, biofeedback, cognitive behavioural therapy, and psycho‐educational approaches other than guided imagery (Astin 2003). Considerable cross‐over exists between the various MBTs, such as imagery, meditation, and relaxation (Astin 2003). A previous study reported that guided imagery had a positive effect on pregnancy‐health‐related outcomes, such as pregnancy prolongation, psychological well‐being, and limiting an increase in blood pressure (Chuang 2012; Moffatt 2010; Urech 2010).
Types of guided imagery techniques
Energetic imagery (Jallo 2008; Naparstek 1994)
Feeling state or pleasant imagery (Jallo 2008; Naparstek 1994)
End state imagery (Naparstek 1994)
Cellular imagery (Naparstek 1994)
Physiological imagery (Naparstek 1994)
Metaphoric imagery (Naparstek 1994)
Psychological imagery (Naparstek 1994)
Relaxation images, exercise (Jallo 2008; Naparstek 1994)
How the intervention might work
Guided imagery encompasses relaxation or visualisation techniques, or both, in which the individual imagines desirable physical responses in order to reduce psychological stress and to attain a calm state of mind (Astin 2003). Although previous studies have suggested that beneficial effects of guided imagery, such as relaxation response and relief of anxiety, have been found to reduce blood pressure (Crowther 1983; Kwekkeboom 1998), the physiological mechanism of guided imagery in the cardiovascular system during pregnancy has not yet been clarified.
Pregnancy induces dramatic physiological changes in the cardiovascular system. Increases in blood volume, heart rate, stroke volume, and cardiac output occur, while systemic vascular resistance and arterial blood pressure decreases (de Weerth 2005; Fu 2009; Mustafa 2012). Several studies have shown that maternal psychological stress can increase arterial blood pressure (Teixeria 1999; Vianna 2011). Although the biological pathway associated with psychological stress and elevated blood pressure is less clear, possible pathophysiological mechanisms may play a role in activating the hypothalamic‐pituitary‐adrenal (HPA) axis and the sympathetic–adrenal–medullary (SAM) system that produces glucocorticoid hormone and catecholamine responses to psychological stimuli (Johnson 1992; Rozanski 1999). The function of the HPA axis is associated with increases in the levels of plasma glucocorticoid hormones, such as cortisol, under the control of the adrenocorticotropic hormone (ACTH). The secretion of ACTH is regulated by corticotropin‐releasing hormone (CRH), which is secreted from the hypothalamus, and is associated with the regulation of a normal response to stress (Johnson 1992). In pregnancy, placental CRH stimulates maternal pituitary ACTH secretion, which leads to increased cortisol levels as gestation progresses, and results in maternal physiological hypercortisolism (de Weerth 2005; Mastorakos 2003). Even though glucocorticoid hormones physiologically increase by the end of gestation, in normal pregnancies, blood pressure is controlled by the homeostatic function of the neuroendocrine systems (de Weerth 2005; DiPietro 2005; Johnson 1992). Patients with hypertensive disorders of pregnancy show higher levels of mean arterial pressure compared to those with normal pregnancy (Gaillard 2011) The activation of the sympathetic nervous system is observed in both chronic hypertension and gestational hypertension compared with normal pregnancy (Grassi 1998; Greenwood 2003).
Enhanced psychological stress and negative emotional stress in pregnancy also cause changes in the activity of the HPA axis and SAM system (de Weerth 2005; Urech 2010). Conversely, mind‐body therapies, such as relaxation or imagery techniques, or both, may reduce stress responses and negative emotional feelings along with modulating sympathetic and parasympathetic activity that results in cardiovascular system changes, including a reduction in blood pressure, or the heart rate, or both (Moffatt 2010; Urech 2010). A previous Cochrane Review suggested that mind‐body interventions during pregnancy might prevent or treat women's anxiety (Marc 2011).
Why it is important to do this review
Guided imagery is a simple, non‐invasive, and safe technique, which has the potential to lower blood pressure among pregnant women with hypertension, and may reduce maternal, fetal, and infant morbidity and mortality in hypertension in pregnancy (Jallo 2013; Mannix 1999). However, the effectiveness of guided imagery for treating hypertension in pregnancy has not been systematically reviewed.
Objectives
The objective of this systematic review was to determine the effect of guided imagery as a non‐pharmacological treatment of hypertension in pregnancy and its influence on perinatal outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs); quasi‐RCTs or cross‐over trials were not eligible for inclusion. Cluster‐RCTs were eligible, but we did not identify any. Studies presented only as abstracts, with sufficient information, were also eligible, but we did not identify any.
Types of participants
Pregnant women with hypertension, defined as systolic blood pressure greater than or equal to 140 mmHg, or diastolic blood pressure greater than or equal to 90 mmHg, or both. We included women who were both undergoing drug therapy, and those who were not.
Types of interventions
Various guided imagery techniques performed during pregnancy compared with:
any other non‐pharmacological methods for hypertension (e.g. quiet rest, music therapy, aromatherapy, relaxation therapy, acupuncture, acupressure, massage, device‐guided slow breathing, hypnosis, physical exercise, or yoga);
no intervention.
Types of outcome measures
Primary outcomes
Maternal
Severe hypertension: defined as either systolic blood pressure greater than or equal to 160 mmHg, or diastolic blood pressure greater than or equal to 110 mmHg (other definitions by trialists were included)
Severe pre‐eclampsia (pre‐eclampsia with severe hypertension, severe proteinuria (usually at least 3 g (range 2 g to 5 g) protein in 24 hours, or 3+ on a dip‐stick), reduced urinary volume (less than 500 mL in 24 hours), neurological disturbances, such as headache, visual disturbances, and exaggerated tendon reflexes, upper abdominal pain, pulmonary oedema (fluid in the lungs), impaired liver function tests, high serum creatinine, low platelets (platelet count less than 100,000/mL), intrauterine growth restriction, or reduced liquor volume (Brown 2000; NHBPEP 2000)
Neonatal
1. Perinatal death (stillbirths plus deaths in the first week of life)
Secondary outcomes
Maternal health outcomes
Maternal death (during pregnancy, childbirth, or up to 42 days after end of pregnancy)
Pre‐eclampsia: defined as new onset proteinuria (greater than or equal to 1+ or greater than or equal to 300 mg/24 hours) after 20 weeks' gestation in pregnant women with hypertension
Maternal blood pressure during pregnancy: mean arterial pressure (MAP), systolic and diastolic blood pressure (after 20 weeks of gestation)
Severe maternal morbidity: including eclampsia (seizures in a woman with pre‐eclampsia), renal failure (serum creatinine concentration greater than 1.1 mg/dL or a doubling of the serum creatinine concentration in the absence of other renal disease), liver failure, disseminated intravascular coagulation, cerebrovascular accident (stroke), pulmonary oedema, and HELLP syndrome (haemolysis, elevated liver enzymes, low platelets)
Mode of delivery (e.g. spontaneous vaginal, forceps, vacuum extraction, or caesarean section)
Placental abruption
Antihypertensive drug use (oral antihypertensive, intravenous antihypertensive)
Use of intravenous magnesium sulphate
Side‐effects or adverse events: any side‐effects or adverse events related to the intervention, intervention stopped due to side‐effects
Use of hospital resources: visit to daycare unit, antenatal hospital admission, intensive care (admission to intensive care unit, length of stay), ventilation, dialysis
Postnatal depression
Breastfeeding, at discharge and up to one year after birth
Women's experiences and views of interventions: childbirth experience, physical and psychological trauma, mother‐infant interaction and attachment
Number of women who discontinued treatment
Quality of life
Neonatal health outcomes
Small‐for‐gestational age: defined as growth below the third centile, or lowest centile reported
Preterm delivery: defined as birth before 37 completed weeks' gestation
Neontal death (death in the first 28 days after birth)
Infant death (death in the first year of life)
Death before discharge from hospital, or in a special care nursery for more than seven days
Severe neonatal morbidity: including respiratory distress syndrome, sepsis, necrotising enterocolitis, retinopathy of prematurity, and intraventricular haemorrhage
Apgar score of less than seven at five minutes
Use of hospital resources: admission to special care nursery, length of stay, endotracheal intubation, use of mechanical ventilation
Long‐term growth and development: blindness, deafness, seizures, poor growth, neurodevelopmental delay, and cerebral palsy
Side‐effects associated with the intervention
Economic outcomes
Costs to health service resources: short‐term or long‐term care for both mother and baby
Costs to the woman, her family, and society
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth's Trials Register by contacting their Information Specialist (31 October 2018).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register, including the detailed search strategies for the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist, and contains trials identified from:
monthly searches of CENTRAL;
weekly searches of MEDLINE Ovid;
weekly searches of Embase Ovid;
monthly searches of CINAHL EBSCO;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals, plus monthly BioMed Central email alerts.
Search results are screened by two people, and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned, and ongoing trial reports (31 October 2018). See Appendix 1 for search methods used.
Searching other resources
a) We handsearched relevant journals; Hypertension in Pregnancy (1999 to 2017) and Pregnancy Hypertension (2011 to 2017).
b) We searched conference proceedings of national and international conferences related to guided imagery interventions; The Association for Music and Imagery (2017) and The European Association of Music and Imagery (2014 to 2017).
c) We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors (M Haruna (MH), M Matsuzaki (MM)) independently assessed all the potential studies we identified as a result of the search strategy for inclusion. We resolved any disagreement through discussion, or we consulted a third review author (E Ota (EO)).
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors (MH, MM) independently extracted the data using the agreed form. We resolved discrepancies through discussion, or we consulted a third author (EO). We entered data into Review Manager 5 software and checked for accuracy (RevMan 2014).
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (MH, MM) independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion, or by involving a third assessor (EO).
(1) Random sequence generation (assessing for possible selection bias)
For each included study, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (assessing for possible selection bias)
For each included study, we described the method used to conceal allocation to interventions prior to assignment, and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (assessing for possible performance bias)
For each included study, we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high, or unclear risk of bias for participants;
low, high, or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (assessing for possible detection bias)
For each included study, we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high, or unclear risk of bias.
(4) Incomplete outcome data (assessing for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each included study, and for each outcome or class of outcomes, we described the completeness of data, including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups, or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses that we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (assessing for reporting bias)
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review had been reported);
high risk of bias (where not all the study's prespecified outcomes had been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely, and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (assessing for bias due to problems not covered by (1) to (5) above)
For each included study, we described any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there was risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias, and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessing the certainty of the evidence
For this review, we used the GRADE approach to assess the certainty of the body of evidence relating to the following outcomes for our comparison of guided imagery compared with quiet rest (Guyatt 2008; Schünemann 2009).
Severe hypertension
Severe pre‐eclampsia
Perinatal death (stillbirths plus deaths in the first week of life)
Antihypertensive drug use (oral antihypertensive, intravenous antihypertensive)
We used GRADEpro GDT to import data from Review Manager 5, in order to create 'Summary of findings' tables (GRADEpro GDT; RevMan 2014). We produced a summary of the intervention effect and a measure of certainty for each of the above outcomes using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence for each outcome. The evidence can be downgraded from high certainty by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We used the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
Both included studies were individual RCTs, and we did not identify any cluster‐randomised trials for inclusion in this review. In future updates, if we identify relevant cluster‐randomised trials, we will include them in the analyses along with individually randomised trials. We will adjust their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions, using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population. If we use ICCs from other sources, we will report this, and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. We will also acknowledge heterogeneity in the randomisation unit, and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
We planed to exclude cross‐over trials, as the design is unsuitable for this intervention due to the 'period effect', and the effect of the first intervention may also extend into the second period.
Studies with multiple arms
We analysed only the relevant arms that compared guided imagery and the control group, excluding an intervention group without guided imagery.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, we will explore the impact of including studies with high levels of missing data (over 10% of participants with missing outcomes) in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we conducted analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I², and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either Tau² was greater than zero, or there was a low P value (less than 0.10) for the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using Review Manager 5 software (RevMan 2014). We used a fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect, i.e. where trials were examining the same intervention, and the trials' populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects, and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. If we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We did not carry out our planned subgroup analysis because of the absence of data for any of our primary outcomes.
In future updates, if we identify substantial heterogeneity in primary outcomes, we will investigate it using subgroup analyses. We will consider whether an overall summary is meaningful, and if it is, used random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses.
1. Types of hypertensive disorders
Chronic versus gestational hypertension or pre‐eclampsia
Early onset hypertension, which occurs before 32 gestational weeks versus late onset hypertension, which occurs after 32 gestational weeks
2. Characteristics of guided imagery
Onset of intervention (early, before 32 weeks versus later, after 32 weeks of gestation)
Number of sessions (e.g. less than three times versus more than three times)
If sufficient data are included in future updates, we will assess subgroup differences using interaction tests available within Review Manager 5 (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test, I², value.
Sensitivity analysis
In future updates, if there is evidence of significant heterogeneity, we will carry out a sensitivity analysis to explore the effects of fixed‐effect or random‐effects analyses for maternal primary outcomes with statistical heterogeneity, and the effects of any assumptions made, such as the value of the ICC used for cluster‐randomised trials. We will perform sensitivity analyses to explore the effects of trial certainty for the primary outcomes before and after exclusion of the trials with high or unclear risk of bias for sequence generation, allocation concealment, or for incomplete outcome data.
Results
Description of studies
Results of the search
See: Figure 1.
1.
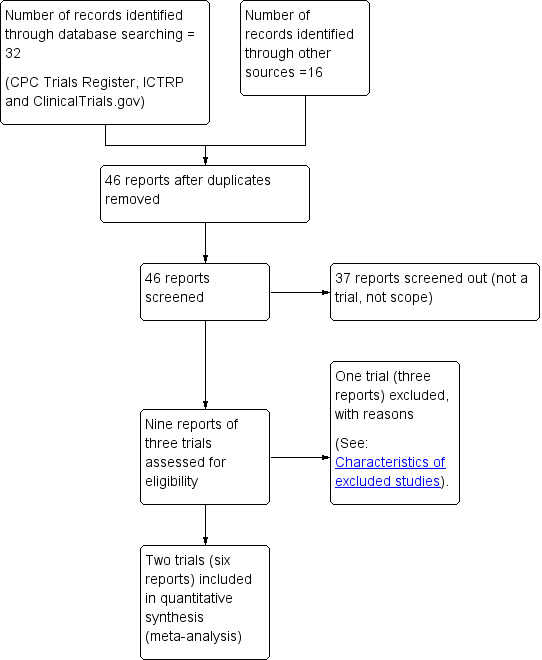
Study flow diagram
The search of Cochrane Pregnancy and Chilldbirth's Register of trials identified nine reports of three trials (Moffatt 2006; Somers 1989; Urech 2009). Of these trials, we included two (Moffatt 2006; Somers 1989), and excluded one (Urech 2009). The additional search identified 16 records, which were either duplicates, or not relevant.
Included studies
Methods
We included two individual randomised controlled trials that met our inclusion criteria (Moffatt 2006; Somers 1989; Characteristics of included studies).
Settings and trial date
One of the two trials was conducted with women enrolled at two Canadian hospitals; the trial date was between September 2004 and December 2006 (Moffatt 2006). In the other trial, participants were recruited from a large university medical centre, a major US Navy hospital, and in three instances, from local obstetricians; the trial date was not clear (Somers 1989).
Participants
Moffatt 2006: the trial included 69 pregnant women with hypertension, who were at < 37 weeks’ gestation, with at least two prenatal diastolic blood pressure readings ≥ 90 mmHg, had clinical investigation for hypertension, and had hearing acuity to hear verbal instructions. Women were excluded if diastolic blood pressure was > 110 mmHg, systolic blood pressure > 170 mmHg, and if they had significant medical conditions.
Somers 1989: the trial included 45 pregnant women who were between 30 and 36 weeks’ gestation. The eligibility criteria were as follows: mean arterial pressure (MAP) ≥ 95 mmHg; diastolic blood pressure ≥ 90 mmHg (or an increase of 15 mmHg during the course of gestation); systolic blood pressure increase of 30 mmHg during the course of gestation. Women were excluded if they had a history of essential hypertension or other blood pressure‐related disorders.
Interventions and comparisons
Two studies compared guided imagery to a control group (quiet rest).
Moffatt 2006: 96 women were randomised into two groups. The intervention group received “a standardised 15 minutes guided imagery with headphones at least twice daily (for 4 weeks, or until childbirth, whichever came first), with or without an audio CD”, whereas the control group received “a standardised verbal introduction to quiet rest and written instructions”, and was asked to “engage in quiet rest periods for 15 minutes at least twice daily (for 4 weeks, or until childbirth, whichever came first) without external stimuli, such as reading, watching television, listening to music, or engaging in conversation”.
Somers 1989: 45 women were randomised into three groups: bed rest alone (as equal to quiet rest; N = 15), compliance enhancement training (N = 15), and biobehavioural intervention (guided imagery; N = 15). We excluded the group of compliance enhancement training (N = 15) from this review. The guided imagery intervention group received a procedure for a total of four hours that involved visual imagery training, thermal biofeedback‐assisted relaxation training, and self‐monitoring of blood pressure, in addition to the procedures for the control group. The control group (N = 15) was prescribed bed rest (quiet rest) and careful obstetrical monitoring, which is standard obstetrical care for mild pregnancy‐induced hypertension (PIH).
Outcomes
Moffatt 2006: primary outcome was change in mean arterial pressure (MAP). Secondary and other outcomes were change in average daytime ambulatory systolic blood pressure or diastolic blood pressure and heart rate, proportions of women who received antihypertensive medication, anxiety, time from randomisation to delivery, relationships between blood pressure changes and frequency of guided imagery use, means and standard deviations of daytime MAP after each week, compliance levels, and participant satisfactions.
Somers 1989: primary outcome was MAP ((systolic pressure − diastolic pressure)/3 + diastolic pressure), at the last prenatal visit prior to hospital admission for delivery, and compliance data.
Sources of trial funding
Moffatt 2006: “The research was funded through: an Association of Women’s Health, Obstetric and Neonatal Nurses (AWHONN) Canada/Johnson & Johnson Canada Award, Ottawa, Ontario; the Atlantic Region of the Association of Canadian Schools of Nursing, Antigonish, Nova Scotia; the Nursing Research Fund, Dalhousie University, Halifax, Nova Scotia; the IWK Health Centre, Halifax, Nova Scotia; and the Canadian Nurses Foundation Nursing Care Partnership, Ottawa, Ontario. During the time of this study, Faith Wight Moffatt also received student funding from: AWHONN, Washington, DC; the Nova Scotia Health Research Foundation, Halifax, Nova Scotia; the University of Toronto, Toronto, Ontario; and the Canadian Institutes of Health Research Strategic Training Initiative in Research in Reproductive Health Sciences, Ottawa, Ontario, Canada.”
Somers 1989: information regarding sources of trial funding was not provided in the trial report.
Trial authors' declarations of interest
The trial authors' declarations of interest were not provided in the two trial reports.
Excluded studies
One study was excluded from the review because the participants of the study were only healthy pregnant women; women with pregnancy‐induced hypertension (PIH), or pre‐eclampsia, or both were not included (Urech 2009; Characteristics of excluded studies).
Risk of bias in included studies
2.
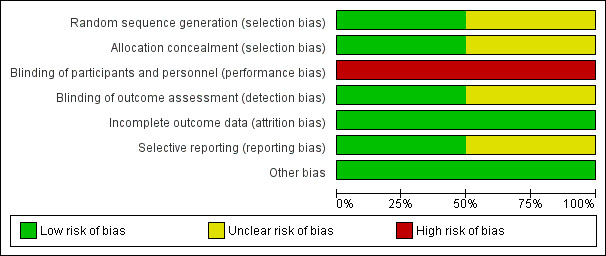
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
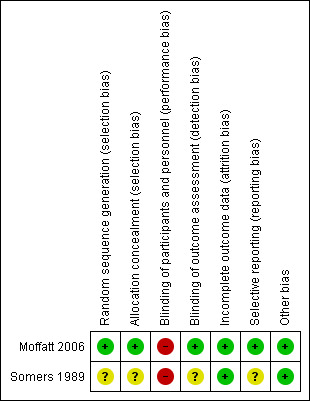
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Sequence generation
Moffatt 2006 had a low risk of bias with adequate allocation, based on a random‐number generator using a centralised computer. Somers 1989 had an unclear risk of bias as no relevant details were available.
Allocation concealment
Moffatt 2006 had a low risk of bias using central allocation, whereas Somers 1989 had an unclear risk of bias, as no information for allocation concealment was available.
Blinding
Blinding of participants and personnel
Both trials had a high risk of bias. The interventions were known to participants and personnel, which possibly affected the outcomes.
Blinding of outcome assessment
Moffatt 2006 had a low risk of bias, as the outcome assessors were not informed of the group allocation or ambulatory blood pressure data. Somers 1989 had an unclear risk of bias, as no information on the outcome assessors' blinding status was available.
Incomplete outcome data
Both trials had a low risk of bias. In the Moffatt 2006 trial, 31/34 (91%) participants in the guided imagery group and 29/35 (83%) participants in the quiet rest group completed the study. In the Somers 1989 trial, 15/20 (75%) participants in the intervention group and 15/15 (100%) participants in the quiet rest (bed rest alone) group were followed up.
Selective reporting
Moffatt 2006 had a low risk of selective reporting bias, as its protocol was registered in ClinicalTrials.gov (NCT00303173). All outcomes in the registry were reported in the study. No protocol was available for Somers 1989; thus, it had an unclear risk of bias.
Other potential sources of bias
For other potential sources of bias, the two trials included in this review had a low risk of bias.
Effects of interventions
See: Table 1
We did not identify any trials looking at any of our other planned comparisons.
The evidence in this review is based on two small studies comparing guided imagery versus quiet rest, including bed rest alone. No meta‐analysis was possible because the two included studies reported different outcomes.
The Intervention frequency was slightly different between the two studies. Moffatt 2006 performed guided imagery for 15 minutes at least twice daily for four weeks or until the baby was born (whichever came first). Somers 1989 performed guided imagery, which included guided imagery, self‐monitoring of blood pressure, and thermal biofeedback‐assisted relaxation training for four total hours; the participants were instructed to practice the procedures twice daily and complete at least three relief relaxation breaks each day. The control groups were similar between the two studies: Moffatt 2006 had a quiet rest group and Somers 1989 also had a quiet rest (as bed rest) group. The settings for the studies were the US (Somers 1989) and Canada (Moffatt 2006).
Guided imagery compared to quiet rest for treating hypertension during pregnancy
Primary outcomes (maternal)
Severe hypertension
Neither of the two included studies measured this outcome.
Severe pre‐eclampsia
Neither of the two included studies measured this outcome.
Primary outcomes (neonatal)
Perinatal death (stillbirths plus deaths in the first week of life)
Neither of the two included studies measured this outcome.
Secondary outcomes (maternal)
Antihypertensive drug use (oral antihypertensive, intravenous antihypertensive)
One trial reported data on antihypertensive drug use (Moffatt 2006), There is low‐certainty evidence that compared with quiet rest, guided imagery may make little or no difference in the use of antihypertensive drugs (risk ratio (RR) 1.27, 95% confidence interval (CI) 0.72 to 2.22; one study, 69 women; Analysis 1.1).
1.1. Analysis.
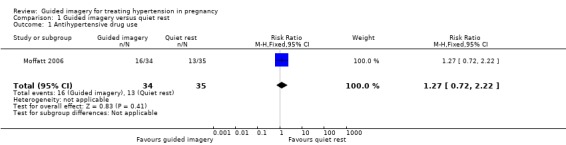
Comparison 1 Guided imagery versus quiet rest, Outcome 1 Antihypertensive drug use.
Other maternal health outcomes
Neither of the two included studies measured these outcomes.
Maternal death (during pregnancy, childbirth, or up to 42 days after end of pregnancy)
Pre‐eclampsia
Maternal blood pressure during pregnancy
Severe maternal morbidity
Mode of delivery
Placental abruption
Use of intravenous magnesium sulphate
Side‐effects or adverse events
Use of hospital resources
Postnatal depression
Breastfeeding
Women's experiences and views of interventions
Number of women who discontinued treatment
Quality of life
Secondary outcomes (neonatal)
Neither of the two included studies measured these outcomes.
Small‐for‐gestational age
Preterm delivery
Neontal death
Infant death
Death before discharge from hospital or in a special care nursery for more than seven days
Severe neonatal morbidity
Apgar score of less than seven at five minutes
Use of hospital resources
Long‐term growth and development
Side‐effects associated with the intervention
Secondary outcomes (economic)
Neither of the two included studies measured these outcomes.
Costs to health service resources
Costs to the woman, her family, and society
Discussion
Summary of main results
The available evidence on using guided imagery for treating hypertension in pregnancy is sparse. We found two small trials (involving a total of 99 pregnant women) for this review. The trials compared guided imagery with quiet rest (Moffatt 2006; Somers 1989).
The included studies did not measure or report on any of this review's primary outcomes of severe hypertension; severe pre‐eclampsia, or perinatal death (stillbirths plus deaths in the first week of life). Similarly, the included trials failed to measure or report on almost all of this review's secondary outcomes.
Low‐certainty evidence from one small trial, (involving 69 women) suggests that compared to quiet rest, guided imagery may make little or no difference in the use of antihypertensive drugs.
Overall completeness and applicability of evidence
We have included all of the known evidence from RCTs but the available evidence is sparse and insufficient to address the objectives of this review. We included two small trials which recruited a total of just 99 pregnant women with hypertension during pregnancy (one study included women with PIH). The trials were conducted some time ago (studies were published in 1989 and 2006) and took place in high‐income countries (USA and Canada). The studies compared the use of guided imagery with a control group. There were differences between the studies in terms of the intervention frequency but the control groups were similar. Neither study reported any of this review's important outcomes ‐ with the exception of one trial which reported antihypertensive drug use (one of this review's secondary outcomes). We did not identify any trials comparing guided imagery with no intervention, or with another non‐pharmacological method for hypertension.
Certainy of the evidence
The overall risk of bias of the two included studies ranged from low to high. We considered one trial to be at low risk of bias across all domains, except performance bias, which we assessed at high risk of bias. The other trial was only at low risk for other bias and attrition bias, and was at high risk of performance bias, and unclear risk for the other domains (selection bias and detection bias).
We assessed the certainty of the evidence using GRADE for our comparison of guided imagery with the quiet rest (Table 1). We assessed the evidence for antihypertensive drug use as low certainty; we downgraded two levels based on imprecision (one small trial, wide confidence interval).
Potential biases in the review process
The review process was conducted according to the Cochrane Handbook for Systematic Reviews of Interventions to minimise potential bias. We performed a comprehensive search, and two authors independently conducted the screening, data extraction, and bias risk assessment.
Agreements and disagreements with other studies or reviews
There are very few clinical trials in which guided imagery has been used to reduce high blood pressure in pregnancy. We included only randomised controlled trials (RCTs), excluding quasi‐RCTs or cross‐over trials. There are just two small included trials included in our review (and outcome data for just one of our review's secondary outcomes). Consequently, there is insufficient evidence for a meaningful comparison with other studies or reviews. To our knowledge, there are no other systematic reviews looking at RCTs on guided imagery for treating hypertension in pregnancy.
The excluded study (Urech 2009) reported that guided imagery (active versus passive relaxation) significantly improved self‐reported relaxation and was associated with a decrease in heart rate in healthy pregnant women; however, systolic blood pressure and diastolic blood pressure were not significantly changed by guided imagery. Given that the population were healthy pregnant women, it is unclear whether the results may generalise to pregnant women with hypertension.
A randomised study (Tang 2009) in older adults, showed that guided relaxation or listening to Mozart, significantly reduced systolic and diastolic blood pressure but that the effect was greater with guided relaxation.
Authors' conclusions
Implications for practice.
There is insufficient evidence to inform practice about the use of guided imagery for hypertension in pregnancy.
The available evidence for this review topic is sparse, and the effect of guided imagery for treating hypertension during pregnancy (compared with quiet rest) remains unclear. There was low‐certainty evidence that guided imagery made little or no difference to the use of antihypertensive drugs, downgraded because of imprecision.
The two included trials did not report on any of the primary outcomes of this review. We did not identify any trials comparing guided imagery with no intervention, or with another non‐pharmacological method for hypertension.
Implications for research.
Large and well‐designed randomised controlled trials are needed to evaluate the effects of guided imagery on hypertension during pregnancy, and on other relevant short‐term and long‐term outcomes associated with maternal and neonatal health. Trials could also consider utilisation and costs of health service.
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team) and the Group's Statistical Adviser. The authors are grateful to the following peer reviewers for their time and comments: Professor Caroline Smith, Misty Pratt.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. ClinicalTrials.gov and ICTRP search methods
ClinicalTrials.gov (advanced search)
Types of study: Interventional
Condition: pregnancy; hypertension
Other terms: visualization; imagery (each term was run separately)
ICTRP
imagery AND pregnancy
visualisation AND pregnancy
visualization AND pregnancy
Data and analyses
Comparison 1. Guided imagery versus quiet rest.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Antihypertensive drug use | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.72, 2.22] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Moffatt 2006.
| Methods | A pilot individual RCT | |
| Participants | 69 pregnant women with hypertension (at least 37 weeks' gestation) in 2 Canadian hospitals were enrolled, based on the following criteria: at least 2 prenatal diastolic blood pressure readings of ≥ 90 mmHg, has had clinical investigation for hypertension, with adequate hearing acuity (for verbal and audiotaped instructions), and planning to give birth at 1 of the study site hospitals. Women were excluded if diastolic blood pressure was > 110 mmHg, systolic blood pressure was > 170 mmHg, or they had significant medical conditions. |
|
| Interventions | Guided imagery (N = 34): the intervention group received "a standardized 15 minutes guided imagery with headphones at least twice daily (for 4 weeks, or until childbirth, whichever came first), with or without an audio CD". Quiet rest (N = 35): the control group received "a standardized verbal introduction to quiet rest and written instructions" and was asked to "engage in quiet rest periods for 15 minutes at least twice daily (for 4 weeks, or until childbirth, whichever came first) without external stimuli, such as reading, watching television, listening to music, or engaging in conversation". |
|
| Outcomes | Primary outcome: change in mean arterial blood pressure. Secondary and other outcomes: change in average daytime ambulatory systolic blood pressure or diastolic blood pressure and heart rate, proportions of women who received antihypertensive medication, anxiety, time from randomisation to delivery, relationships between blood pressure changes and frequency of guided imagery use, means and standard deviations of daytime MAP after each week, compliance levels, and participant satisfactions. |
|
| Notes | Sources of trial funding: "The research was funded through: an Association of Women's Health, Obstetric and Neonatal Nurses (AWHONN) Canada/Johnson & Johnson Canada Award, Ottawa, Ontario; the Atlantic Region of the Association of Canadian Schools of Nursing, Antigonish, Nova Scotia; the Nursing Research Fund, Dalhousie University, Halifax, Nova Scotia; the IWK Health Centre, Halifax, Nova Scotia; and the Canadian Nurses Foundation Nursing Care Partnership, Ottawa, Ontario. During the time of this study, Faith Wight Moffatt also received student funding from: AWHONN, Washington, DC; the Nova Scotia Health Research Foundation, Halifax, Nova Scotia; the University of Toronto, Toronto, Ontario; and the Canadian Institutes of Health Research Strategic Training Initiative in Research in Reproductive Health Sciences, Ottawa, Ontario, Canada." Trial dates were from September 2004 to December 2006. Trial authors' declarations of interest were not provided in the trial report. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer random number generator by centralised computer "the centralized computer randomisation service" ,,, "Blocked, using random block sizes of six and eight". |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel was not possible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were not informed of group allocation or ambulatory blood pressure data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Guided imagery group 31/34 (91%), quiet rest 29/35 (83%); the number of dropouts with reasons, and balanced |
| Selective reporting (reporting bias) | Low risk | The trial registered in ClinicalTrials.gov, NCT00303173. All the outcomes reported in the registry reported in the papers. |
| Other bias | Low risk | No sources of other bias identified. |
Somers 1989.
| Methods | An individual RCT | |
| Participants | Women were required to be between 30 and 36 weeks’ gestation and were recruited from “a large university medical centre, a major US Navy hospital, and in three instances, from local obstetricians.” The eligible criteria were as follows: MAP ≥ 95 mmHg; diastolic blood pressure ≥ 90 mmHg (or an increase of 15 mmHg during the course of gestation), and systolic blood pressure increase of 30 mmHg during the course of gestation. Women were excluded if they had a history of essential hypertension or other blood pressure–related disorders. |
|
| Interventions | Each of the 45 participants was randomly allocated to 1 of the 3 groups: biobehavioural intervention (guided imagery (N = 15)), bed rest alone (quiet rest (N = 15)), or compliance enhancement training (N = 15). The group of compliance enhancement training (N = 15) was excluded in this review. Guided imagery (N = 15): the intervention group (biobehavioural) received a procedure for a total of 4 hours, involved visual imagery training, thermal biofeedback–assisted relaxation training, and self‐monitoring of blood pressure, in addition to procedures of the quiet rest (bed rest alone) and compliance enhancement training groups. Quiet rest (N = 15): the control group (bed rest alone) was prescribed bed rest and careful obstetrical monitoring, which is standard obstetrical care for mild PIH. |
|
| Outcomes | MAP ((systolic pressure ‐ diastolic pressure)/3 + diastolic pressure) at the last prenatal visit prior to hospital admission for delivery, and compliance data | |
| Notes | The following information was not provided in the trial report.
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomly assigned" in the methods but no details available. |
| Allocation concealment (selection bias) | Unclear risk | No details described in the text |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The interventions were evident to participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention about outcome assessors blinding status. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In all, 50 women were recruited, 45 (90%) of which were followed up in each group. "The 5 dropouts were evenly distributed among treatment groups and did not differ in blood pressure readings or demographic variables from those women who completed the study." |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. |
| Other bias | Low risk | No sources of other bias identified |
MAP: mean arterial pressure RCT: randomised controlled trial PIH: pregnancy‐induced hypertension
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Urech 2009 | This study aimed to compare the immediate effects of 2 active and 1 passive 10‐minute relaxation technique on perceived and physiological indicators of relaxation. The study population was out of our scope and included only healthy women; women with pregnancy‐induced hypertension, pre‐eclampsia, or both, were excluded. |
Differences between protocol and review
The are some differences between this review and the published protocol for this review (Haruna 2014).
We added a search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports.
We updated our methods to include the use of GRADE to assess the certainty of the evidence, and presented the results in a 'Summary of findings' table.
The methods in the protocol could not be applied because of a lack of all primary and many secondary outcome data.
Contributions of authors
Megumi Haruna (MH), Masayo Matsuzaki (MM), and Erika Ota (EO) drafted the review, with support from Mie Shiraishi (MS), Nobutsugu Hanada (NH), and Rintaro Mori (RM).
MM, MS, NH, EO, and RM provided comments. All authors read and approved the final manuscript.
Declarations of interest
Megumi Haruna: none known.
Masayo Matsuzaki: none known.
Erika Ota: none known.
Mie Shiraishi: none known.
Nobutsugu Hanada: none known.
Rintaro Mori: none known.
New
References
References to studies included in this review
Moffatt 2006 {published data only}
- Moffatt FW, Hodnett E, Esplen MJ, Watt‐Watson J. Effects of guided imagery on blood pressure in pregnant women with hypertension: a pilot randomized controlled trial. Birth 2010;37(4):296‐306. [DOI] [PubMed] [Google Scholar]
- Moffatt FW, Hodnett E, Esplen MJ, Watt‐Watson J. Guided imagery relaxation effects on blood pressure in pregnant women with hypertension: results of a preliminary RCT. Hypertension in Pregnancy 2008;27(4):514. [Google Scholar]
- Moffatt WF. A randomized controlled trial of the effects of guided imagery on blood pressure in hypertensive pregnant women [thesis]. Toronto: University of Toronto, 2008. [Google Scholar]
- Moffatt WF, Hodnett E, Esplen MJ, Watt‐Watson J. Satisfaction and experiences of pregnant hypertensive women participating in a feasibility study of guided imagery effects on blood pressure. Pregnancy Hypertension 2012;2(3):290. [DOI] [PubMed] [Google Scholar]
- NCT00303173. The relaxation and blood pressure in pregnancy (REBIP) study. clinicaltrials.gov/ct2/show/NCT00303173 (first received 15 March 2006).
Somers 1989 {published data only}
- Somers PJ, Gevirtz RN, Jasin SE, Chin HG. The efficacy of biobehavioral and compliance interventions in the adjunctive treatment of mild pregnancy‐induced hypertension. Biofeedback & Self Regulation 1989;14:309‐18. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Urech 2009 {published data only}
- Fink NS, Urech C, Isabel F, Meyer A, Hoesli I, Bitzer J, et al. Fetal response to abbreviated relaxation techniques. A randomized controlled study. Early Human Development 2011;87:121‐7. [DOI] [PubMed] [Google Scholar]
- Urech C, Alder J, Bitzer J, Hosli I. The effect of relaxation exercises on psychological wellbeing during pregnancy [Entspannungs‐Ubungen wahrend der Schwangerschaft: Der Einfluss auf das psychobiologische Wohlbefinden]. Geburtshilfe und Frauenheilkunde 2009;69:163. [Google Scholar]
- Urech C, Fink NS, Wilhelm FH, Bitzer J, Alder J. Effects of relaxation on psychobiological wellbeing during pregnancy: a randomized controlled trial. Psychoneuroendocrinology 2010;35:1348‐55. [DOI] [PubMed] [Google Scholar]
Additional references
Abalos 2014
- Abalos E, Duley L, Steyn DW. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD002252.pub3] [DOI] [PubMed] [Google Scholar]
ACOG 2013
- American College of Obstetricians and Gynecologists. Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. American College of Obstetricians and Gynecologists, 2013. [DOI] [PubMed] [Google Scholar]
Asemi 2013
- Asemi Z, Tabassi Z, Samimi M, Fahiminejad T, Esmaillzadeh A. Favourable effects of the Dietary Approaches to Stop Hypertension diet on glucose tolerance and lipid profiles in gestational diabetes: a randomised clinical trial. British Journal of Nutrition 2013;109(11):2024‐30. [DOI: 10.1017/S0007114512004242] [DOI] [PubMed] [Google Scholar]
Astin 2003
- Astin JA, Shapiro SL, Eisenberg DM, Forys KL. Mind‐body medicine: state of the science, implications for practice. Journal of the American Board of Family Practice 2003;16(2):131‐47. [DOI] [PubMed] [Google Scholar]
Betts 2003
- Betts D. The use of acupuncture in pregnancy induced hypertension. Journal of Chinese Medicine 2003;71:9‐13. [Google Scholar]
Brown 2000
- Brown MA, Hague WM, Higgins J, Lowe S, McCowan L, Oats J, et al. The detection, investigation and management of hypertension in pregnancy: full consensus statement. Australian & New Zealand Journal of Obstetrics & Gynaecology 2000;40(2):139‐55. [PUBMED: 10925900] [DOI] [PubMed] [Google Scholar]
Brown 2001
- Brown MA, Lindheimer MD, Swiet M, Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertension in Pregnancy 2001;20(1):IX–XIV. [DOI] [PubMed] [Google Scholar]
Christine 2011
- Roberts CL, Ford JB, Algert CS, Antonsen S, Chalmers J, Cnattingius S, et al. Population‐based trends in pregnancy hypertension and pre‐eclampsia: an international comparative study. BMJ Open 2011;1(1):e000101. [DOI: 10.1136/bmjopen-2011-000101] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chuang 2012
- Chuang LL, Lin LC, Cheng PJ, Chen CH, Wu SC, Chang CL. The effectiveness of a relaxation training program for women with preterm labour on pregnancy outcomes: a controlled clinical trial. International Journal of Nursing Studies 2012;49(3):257‐64. [DOI] [PubMed] [Google Scholar]
Churchill 2007
- Churchill D, Beevers GDD, Meher S, Rhodes C. Diuretics for preventing pre‐eclampsia. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD004451.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crowther 1983
- Crowther JH. Stress management training and relaxation imagery in the treatment of essential hypertension. Journal of Behavioral Medicine 1983;6(2):169‐87. [DOI] [PubMed] [Google Scholar]
Daake 1989
- Daake DR, Gueldner SH. Imagery instruction and the control of postsurgical pain. Applied Nursing Research 1989;2(3):114‐20. [DOI] [PubMed] [Google Scholar]
de Weerth 2005
- Weerth C, Buitelaar JK. Physiological stress reactivity in human pregnancy ‐ a review. Neuroscience and Biobehavioral Reviews 2005;29(2):295‐312. [DOI] [PubMed] [Google Scholar]
DiPietro 2005
- DiPietro JA, Costigan KA, Gurewitsch ED. Maternal psychophysiological change during the second half of gestation. Biological Psychology 2005;69(1):23‐38. [DOI] [PubMed] [Google Scholar]
Duley 1999
- Duley L, Henderson‐Smart DJ. Reduced salt intake compared to normal dietary salt, or high intake, in pregnancy. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD001687] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duley 2005
- Duley L, Henderson‐Smart D, Meher S. Altered dietary salt for preventing pre‐eclampsia, and its complications. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005548] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duley 2007
- Duley L, Henderson‐Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre‐eclampsia and its complications. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD004659.pub2] [DOI] [PubMed] [Google Scholar]
Duley 2009a
- Duley L. The global impact of pre‐eclampsia and eclampsia. Seminars in Perinatology 2009;33(3):130‐7. [DOI] [PubMed] [Google Scholar]
Duley 2009b
- Duley L, Henderson‐Smart DJ, Walker GJA. Interventions for treating pre‐eclampsia and its consequences: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007756] [DOI] [Google Scholar]
Duley 2013
- Duley L, Meher S, Jones L. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD001449.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fu 2009
- Fu Q, Levine BD. Autonomic circulatory control during pregnancy in humans. Seminars in Reproductive Medicine 2009;27(4):330‐7. [DOI: 10.1055/s-0029-1225261] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaillard 2011
- Gaillard R, Bakker R, Willemsen SP, Hofman A, Steegers EA, Jaddoe VW. Blood pressure tracking during pregnancy and the risk of gestational hypertensive disorders: the Generation R Study. European Heart Journal 2011;32(24):3088‐97. [DOI: 10.1093/eurheartj/ehr275] [DOI] [PubMed] [Google Scholar]
GBD 2016
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990‐2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390(10100):1211‐59. [DOI: 10.1016/S0140-6736(17)32154-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gedde‐Dahl 2012
- Gedde‐Dahl M, Fors EA. Impact of self‐administered relaxation and guided imagery techniques during final trimester and birth. Complementary Therapies in Clinical Practice 2012;18(1):60‐5. [DOI: 10.1016/j.ctcp.2011.08.008] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro.GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Grassi 1998
- Grassi G. Role of the sympathetic nervous system in human hypertension. Journal of Hypertension 1998;16:1979‐87. [DOI] [PubMed] [Google Scholar]
Greenwood 2003
- Greenwood JP, Scott EM, Walker JJ, Stoker JB, Mary DA. The magnitude of sympathetic hyperactivity in pregnancy‐induced hypertension and preeclampsia. American Journal of Hypertension 2003;16(3):194‐9. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ. GRADE Working Group. Rating quality of evidence and strength of recommendations: What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hauth 2000
- Hauth JC, Ewell MG, Levine RJ, Esterlitz JR, Sibai B, Curet LB, et al. Pregnancy outcomes in healthy nulliparas who developed hypertension. Calcium for Preeclampsia Prevention Study Group. Obstetrics & Gynecology 2000;95(1):24‐8. [DOI] [PubMed] [Google Scholar]
Heard 2004
- Heard AR, Dekker GA, Chan A, Jacobs DJ, Vreeburg SA, Priest KR. Hypertension during pregnancy in South Australia, part 1: pregnancy outcomes. Australian & New Zealand Journal of Obstetrics & Gynaecology 2004;44(5):404‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hofmeyr 2010
- Hofmeyr GJ, Lawrie TA, Atallah AN, Duley L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD001059.pub3] [DOI] [PubMed] [Google Scholar]
Jallo 2008
- Jallo N, Bourguignon C, Taylor AG, Utz SW. Stress management during pregnancy: designing and evaluating a mind‐body intervention. Family & Community Health 2008;31(3):190‐203. [DOI] [PubMed] [Google Scholar]
Jallo 2013
- Jallo N, Cozens R, Smith MW, Simpson RI. Effects of a guided imagery intervention on stress in hospitalized pregnant women: a pilot study. Holistic Nursing Practice 2013;27(3):129‐39. [DOI] [PubMed] [Google Scholar]
Johnson 1992
- Johnson EO, Kamilaris TC, Chrousos GP, Gold PW. Mechanisms of stress: a dynamic overview of hormonal and behavioral homeostasis. Neuroscience and Biobehavioral Reviews 1992;16(2):115‐30. [DOI] [PubMed] [Google Scholar]
Khan 2006
- Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367(9516):1066–74. [DOI] [PubMed] [Google Scholar]
Kwekkeboom 1998
- Kwekkeboom K, Huseby‐Moore K, Ward S. Imaging ability and effective use of guided imagery. Research in Nursing & Health 1998;21(3):189‐98. [DOI] [PubMed] [Google Scholar]
Lowe 2009
- Lowe SA, Brown MA, Dekker GA, Gatt S, McLintock CK, McMahon LP, et al. Guidelines for the management of hypertensive disorders of pregnancy 2008. Australian & New Zealand Journal of Obstetrics & Gynaecology 2009;49(3):242‐6. [DOI: 10.1111/j.1479-828X.2009.01003.x] [DOI] [PubMed] [Google Scholar]
MacKay 2001
- MacKay AP, Berg CJ, Atrash HK. Pregnancy‐related mortality from preeclampsia and eclampsia. Obstetrics and Gynecology 2001;97(4):533‐8. [DOI] [PubMed] [Google Scholar]
Mahomed 2007
- Mahomed K, Bhutta Z, Middleton P. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD000230.pub3] [DOI] [PubMed] [Google Scholar]
Makrides 2001
- Makrides M, Crowther CA. Magnesium supplementation in pregnancy. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD000937] [DOI] [PubMed] [Google Scholar]
Mannix 1999
- Mannix LK, Chandurkar RS, Rybicki LA, Tusek DL, Solomon GD. Effect of guided imagery on quality of life for patients with chronic tension‐type headache. Headache 1999;39(5):326‐34. [DOI] [PubMed] [Google Scholar]
Marc 2011
- Marc I, Toureche N, Ernst E, Hodnett ED, Blanchet C, Dodin S, et al. Mind‐body interventions during pregnancy for preventing or treating women's anxiety. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD007559.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2005
- Martin JN Jr, Thigpen BD, Moore RC, Rose CH, Cushman J, May W. Stroke and severe preeclampsia and eclampsia: a paradigm shift focusing on systolic blood pressure. Obstetrics and Gynecology 2005;105(2):248‐54. [DOI] [PubMed] [Google Scholar]
Mastorakos 2003
- Mastorakos G, Ilias I. Maternal and fetal hypothalamic‐pituitary‐adrenal axes during pregnancy and postpartum. Annals of the New York Academy of Sciences 2003;997:136‐49. [DOI] [PubMed] [Google Scholar]
Meher 2006
- Meher S, Duley L. Progesterone for preventing pre‐eclampsia and its complications. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD006175] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meher 2006a
- Meher S, Duley L. Exercise or other physical activity for preventing pre‐eclampsia and its complications. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD005942] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meher 2006b
- Meher S, Duley L. Rest during pregnancy for preventing pre‐eclampsia and its complications in women with normal blood pressure. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD005939] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meher 2007
- Meher S, Duley L. Nitric oxide for preventing pre‐eclampsia and its complications. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD006490] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moffatt 2010
- Moffatt FW, Hodnett E, Esplen MJ, Watt‐Watson J. Effects of guided imagery on blood pressure in pregnant women with hypertension: a pilot randomized controlled trial. Birth 2010;37(4):296‐306. [DOI: 10.1111/j.1523-536X.2010.00424.x] [DOI] [PubMed] [Google Scholar]
Moodley 2011
- Moodley J. Potentially increasing rates of hypertension in women of childbearing age and during pregnancy – be prepared!. Cardiovascular Journal of Africa 2011;22(6):330‐4. [DOI: 10.5830/CVJA-2010-074] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mustafa 2012
- Mustafa R, Ahmed S, Gupta A, Venuto RC. A comprehensive review of hypertension in pregnancy. Journal of Pregnancy 2012;2012:105918. [DOI: 10.1155/2012/105918] [DOI] [PMC free article] [PubMed] [Google Scholar]
Naparstek 1994
- Naparstek B. Staying Well with Guided Imagery. 1st Edition. New York: Warner Books, 1995. [Google Scholar]
NCCIH 2012
- The National Center for Complementary and Integrative Health. Mind and Body Research ‐ Information for Researchers (Updated October 2012). nccam.nih.gov/grants/mindbody?nav=gsa (accessed 7 October 2013).
NHBPEP 2000
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Report of the National High Blood Pressure Education Program Working Group on high blood pressure in pregnancy. American Journal of Obstetrics and Gynecology 2000;183(1):S1‐S22. [PubMed] [Google Scholar]
Park 2012
- Park C. Mind‐body CAM interventions: current status and considerations for integration into clinical health psychology. Journal of Clinical Psychology 2013;69(1):45‐63. [DOI: 10.1002/jclp.21910] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2010
- Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, et al. Vitamins C and E to prevent complications of pregnancy‐associated hypertension. New England Journal of Medicine 2010;362(14):1282‐91. [DOI: 10.1056/NEJMoa0908056] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rozanski 1999
- Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation 1999;99(16):2192‐217. [DOI] [PubMed] [Google Scholar]
Schünemann 2009
- Schünemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [DOI] [PubMed] [Google Scholar]
Sharpe 2007
- Sharpe PA, Williams HG, Granner ML, Hussey JR. A randomised study of the effects of massage therapy compared to guided relaxation on well‐being and stress perception among older adults. Complementary Therapies in Medicine 2007;15(3):157‐63. [DOI] [PubMed] [Google Scholar]
Shennan 2003
- Shennan A, Waugh J. The measurement of blood pressure and proteinuria in pregnancy. In: Critchley H, MacLean A, Poston L, Walker J editor(s). Pre‐eclampsia. London: RCOG Press, 2003:305–24. [Google Scholar]
Souza 2013
- Souza JP, Gülmezoglu AM, Vogel J, Carroli G, Lumbiganon P, Qureshi Z, et al. Moving beyond essential interventions for reduction of maternal mortality (the WHO Multicountry Survey on Maternal and Newborn Health): a cross‐sectional study. Lancet 2013;381(9879):1747‐55. [DOI] [PubMed] [Google Scholar]
Steegers 2010
- Steegers EA, Dadelszen P, Duvekot JJ, Pijnenborg R. Pre‐eclampsia. Lancet 2010;376(9741):631‐44. [DOI] [PubMed] [Google Scholar]
Tang 2009
- Tang HY, Harms V, Speck SM, Vezeau T, Jesurum JT. Effects of audio relaxation programs for blood pressure reduction in older adults. European Journal of Cardiovascular Nursing 2009;8(5):329‐36. [DOI] [PubMed] [Google Scholar]
Teixeria 1999
- Teixeira JM, Fisk NM, Glover V. Association between maternal anxiety in pregnancy and increased uterine artery resistance index: cohort based study. BMJ 1999;318(7177):153‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thangaratinam 2012
- Thangaratinam S, Rogozińska E, Jolly K, Glinkowski S, Duda W, Borowiack E, et al. Interventions to reduce or prevent obesity in pregnant women: a systematic review. Health Technology Assessment 2012;16(31):iii‐iv, 1‐191. [DOI: 10.3310/hta16310] [DOI] [PMC free article] [PubMed] [Google Scholar]
Umesawa 2016
- Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertension Research 2017;40(3):213‐20. [DOI: 10.1038/hr.2016.126.] [DOI] [PubMed] [Google Scholar]
Urech 2010
- Urech C, Fink NS, Hoesli I, Wilhelm FH, Bitzer J, Alder J. Effects of relaxation on psychobiological wellbeing during pregnancy: a randomized controlled trial. Psychoneuroendocrinology 2010;35(9):1348‐55. [DOI: 10.1016/j.psyneuen.2010.03.008] [DOI] [PubMed] [Google Scholar]
Vianna 2011
- Vianna P, Bauer ME, Dornfeld D, Chies JA. Distress conditions during pregnancy may lead to pre‐eclampsia by increasing cortisol levels and altering lymphocyte sensitivity to glucocorticoids. Medical Hypotheses 2011;77(2):188‐91. [DOI: 10.1016/j.mehy.2011.04.007] [DOI] [PubMed] [Google Scholar]
Yoder 2009
- Yoder SR, Thornburg LL, Bisognano JD. Hypertension in pregnancy and women of childbearing age. American Journal of Medicine 2009;122(10):890‐5. [DOI: 10.1016/j.amjmed.2009.03.036] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Haruna 2014
- Haruna M, Matsuzaki M, Ota E, Shiraishi M, Hanada N, Mori R. Guided imagery for treating hypertension in pregnancy. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD011337] [DOI] [PMC free article] [PubMed] [Google Scholar]


