Abstract
Background
Breast cancer is the most frequently occurring malignancy and the second cause of death for cancer in women. Cancer prevention agents (CPAs) are a promising approach to reduce the burden of breast cancer. Currently, two main types of CPAs are available: selective estrogen receptor modulators (SERMs, such as tamoxifen and raloxifene) and aromatase inhibitors (AIs, such as exemestane and anastrozole).
Objectives
To assess the efficacy and acceptability of single CPAs for the prevention of primary breast cancer, in unaffected women, at an above‐average risk of developing breast cancer.
Using a network meta‐analysis, to rank single CPAs, based on their efficacy and acceptability (an endpoint that is defined as the inverse of CPA‐related toxicity).
Search methods
We searched the Cochrane Breast Cancer Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, World Health Organization's International Clinical Trials Registry Platform (WHO ICTRP), and ClinicalTrials.gov on 17 August 2018. We handsearched reference lists to identify additional relevant studies.
Selection criteria
We included randomized controlled trials (RCTs) that enrolled women without a personal history of breast cancer but with an above‐average risk of developing a tumor. Women had to be treated with a CPA and followed up to record the occurrence of breast cancer and adverse events.
Data collection and analysis
Two review authors independently extracted data and conducted risk of bias assessments of the included studies, and assessed the certainty of the evidence using GRADE. Outcome data included incidence of breast carcinoma (both invasive and in situ carcinoma) and adverse events (both overall and severe toxicity). We performed a conventional meta‐analysis (for direct comparisons of a single CPA with placebo or a different CPA) and network meta‐analysis (for indirect comparisons).
Main results
We included six studies enrolling 50,927 women randomized to receive one CPA (SERMs: tamoxifen or raloxifene, or AIs: exemestane or anastrozole) or placebo. Three studies compared tamoxifen and placebo, two studies compared AIs (exemestane or anastrozole) versus placebo, and one study compared tamoxifen versus raloxifene. The risk of bias was low for all RCTs.
For the tamoxifen versus placebo comparison, tamoxifen likely resulted in a lower risk of developing breast cancer compared to placebo (risk ratio (RR) 0.68, 95% confidence interval (CI) 0.62 to 0.76; 3 studies, 22,832 women; moderate‐certainty evidence). In terms of adverse events, tamoxifen likely increased the risk of severe toxicity compared to placebo (RR 1.28, 95% CI 1.12 to 1.47; 2 studies, 20,361 women; moderate‐certainty evidence). In particular, women randomized to receive tamoxifen experienced a higher incidence of both endometrial carcinoma (RR 2.26, 95% CI 1.52 to 3.38; high‐certainty evidence) and thromboembolism (RR 2.10, 95% CI 1.14 to 3.89; high‐certainty evidence) compared to women who received placebo.
For the AIs versus placebo comparison, AIs (exemestane or anastrozole) reduced the risk of breast cancer by 53% (RR 0.47, 95% CI 0.35 to 0.63; 2 studies, 8424 women; high‐certainty evidence). In terms of adverse events, AIs increased the risk of severe toxicity by 18% (RR 1.18, 95% CI 1.09 to 1.28; 2 studies, 8352 women; high‐certainty evidence). These differences were sustained especially by endocrine (e.g. hot flashes), gastrointestinal (e.g. diarrhea), and musculoskeletal (e.g. arthralgia) adverse events, while there were no differences in endometrial cancer or thromboembolism rates between AIs and placebo.
For the tamoxifen versus raloxifene comparison, raloxifene probably performed worse than tamoxifen in terms of breast cancer incidence reduction (RR 1.25, 95% CI 1.09 to 1.43; 1 study, 19,490 women; moderate‐certainty evidence), but its use was associated with lower toxicity rates (RR 0.87, 95% CI 0.80 to 0.95; 1 study, 19,490 women; moderate‐certainty evidence), particularly relating to incidence of endometrial cancer and thromboembolism.
An indirect comparison of treatment effects allowed us to compare the SERMs and AIs in this review. In terms of efficacy, AIs (exemestane or anastrozole) may have reduced breast cancer incidence slightly compared to tamoxifen (RR 0.67, 95% CI 0.46 to 0.98; 5 RCTs, 31,256 women); however, the certainty of evidence was low. A lack of model convergence did not allow us to analyze toxicity data.
Authors' conclusions
For women with an above‐average risk of developing breast cancer, CPAs can reduce the incidence of this disease. AIs appear to be more effective than SERMs (tamoxifen) in reducing the risk of developing breast cancer. AIs are not associated with an increased risk of endometrial cancer and thromboembolic events. However, long‐term data on toxicities from tamoxifen are available while the follow‐up toxicity data on unaffected women taking AIs is relatively short. Additional data from direct comparisons are needed to fully address the issues of breast cancer prevention by risk‐reducing medications, with special regards to acceptability (i.e. the benefit/harm ratio).
Keywords: Female, Humans, Aromatase Inhibitors, Aromatase Inhibitors/therapeutic use, Breast Neoplasms, Breast Neoplasms/drug therapy, Network Meta‐Analysis, Randomized Controlled Trials as Topic, Selective Estrogen Receptor Modulators, Selective Estrogen Receptor Modulators/therapeutic use
Plain language summary
Medicines to prevent breast cancer in women at above‐average risk of developing breast cancer
What is the issue?
Breast cancer is the most frequent type of cancer and the second cause of death by cancer in women. Therefore, any strategy which can reduce its burden is eagerly awaited. Cancer prevention agents (CPAs) are medicines that might fulfil this need. Currently, two main types of CPAs are available to combat breast cancer: selective estrogen receptor modulators (SERMs, such as tamoxifen and raloxifene) and aromatase inhibitors (such as exemestane and anastrozole). Women without personal history of breast cancer, but with above‐average risk of developing this disease (that is, with a lifetime risk greater than 17%) represent the usual target population for CPAs.
Review question
This Cochrane Review aimed to summarize the evidence on the efficacy and toxicity of CPAs for the prevention of primary breast cancer.
Key messages
CPAs can reduce the incidence of breast cancer although at the cost of some toxicity. Aromatase inhibitors may be more effective than SERMs in reducing the risk of developing breast cancer. Aromatase inhibitors are not associated with the increased severe toxicity (i.e. cancer of the lining of the womb (endometrial cancer)) and thromboembolic events (blood clots)) that characterize the use of tamoxifen (although the lack of long‐term data on aromatase inhibitors in unaffected women do not allow us to draw definitive conclusions). Additional data are needed to fully address the issues of breast cancer prevention by risk‐reducing medicines, with emphasis on collecting information on side effects.
What was studied in the review?
The review authors found six studies enrolling 50,927 women to receive one CPA or placebo (a pretend treatment, e.g. a sugar pill). Three studies involving 23,013 women compared tamoxifen and placebo, two studies involving 8424 women compared aromatase inhibitors (exemestane or anastrozole) and placebo, and one study involving 19,490 women that compared tamoxifen and raloxifene.
What are the main results of the review?
Based on the three studies that compared tamoxifen to placebo, tamoxifen probably reduced the risk of developing breast cancer by 32% compared to placebo. However, tamoxifen was associated with a 28% increased risk of severe side effects compared to placebo based on two studies involving 20,361 women. In particular, women taking tamoxifen experienced higher incidence of endometrial cancer and thromboembolism than women having no medicine.
For women who received either an aromatase inhibitor (exemestane or anastrozole) or placebo, aromatase inhibitors reduced the risk of breast cancer by 53% compared to placebo. Data from two studies involving 8352 women indicated that aromatase inhibitors increased the risk of severe side effects by 18% compared to placebo. These differences were sustained especially by endocrine (hormonal; e.g. hot flashes), gastrointestinal (e.g. diarrhea), and musculoskeletal (e.g. joint pain) side effects, whereas there were no differences in either endometrial cancer or thromboembolism rates.
For women who received either tamoxifen or raloxifene, raloxifene probably performed worse than tamoxifen in terms of breast cancer incidence reduction, but its use was associated with a 13% reduction of toxicity rates especially endometrial cancer and thromboembolism.
A specialized method named a 'network meta‐analysis' allowed us to compare medications never directly compared to each other in a study. Based on this network meta‐analysis, aromatase inhibitors may have led to a 23% additional risk reduction of developing breast cancer compared to tamoxifen. However, the reliability of the evidence was low meaning that further research is likely to have an impact on our confidence in this result. This analysis could not be performed on toxicity data.
How up‐to‐date is this review?
The review authors searched for studies that had been published up to 17 August 2018.
Summary of findings
Summary of findings for the main comparison. Tamoxifen versus placebo.
| Tamoxifen compared with placebo for primary breast cancer prevention | ||||||
|
Patient or population: women with above‐average risk of developing breast cancer Settings: prevention Intervention: tamoxifen Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Tamoxifen | |||||
|
Overall breast cancer incidence Follow‐up: 6–16 years |
72 per 1000 | 49 per 1000 ** (44 to 54) |
RR 0.68 (0.62 to 0.76) |
22,832 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | — |
|
Severe toxicity Follow‐up: 6–16 years |
35 per 1000 | 46 per 1000 (39 to 51) |
RR 1.28 (1.12 to 1.47) |
20,361 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | — |
| *The basis for the assumed risk (control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ** Number needed to treat: 43 CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aDowngraded one level for inconsistency.
Summary of findings 2. Aromatase inhibitors versus placebo.
| Aromatase inhibitors compared with placebo for primary breast cancer prevention | ||||||
|
Patient or population: women with above‐average risk of developing breast cancer Settings: prevention Intervention: aromatase inhibitors (anastrozole and exemestane) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Aromatase inhibitors | |||||
|
Overall breast cancer incidence Follow‐up 3–5 years |
31 per 1000 | 14 per 1000 ** (11 to 19) |
RR 0.47 (0.35 to 0.63) |
8424 (2 RCTs) | ⊕⊕⊕⊕ High | — |
|
Severe toxicity Follow‐up: 3–5 years |
214 per 1000 | 253 per 1000 (233 to 274) |
RR 1.18 (1.09 to 1.28) |
8352 (2 RCTs) | ⊕⊕⊕⊕ High | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ** Number needed to treat: 59 CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
Summary of findings 3. Aromatase inhibitors versus tamoxifen (indirect comparison)a.
| Aromatase inhibitors compared with tamoxifen for primary breast cancer prevention (based on network meta‐analysis findings) | ||||||
|
Patient or population: women with above‐average risk of developing breast cancer Settings: prevention Intervention: aromatase inhibitors Comparison: tamoxifen | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Tamoxifen | Aromatase inhibitors | |||||
|
Overall breast cancer incidence Follow‐up: 3–16 years |
49 per 1000 | 33 per 1000 ** (23 to 48) |
RR 0.67 (0.46 to 0.98) |
31,256 (5 RCTs) | ⊕⊕⊝⊝ Lowb | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ** Number needed to treat: 62 CI: confidence interval; RCTs: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aThe findings are from indirect comparison (network meta‐analysis) of two RCTs comparing aromatase inhibitors with placebo and three RCTs comparing tamoxifen with placebo. bDowngraded two levels for partial lack of transitivity and inconsistency.
Background
Description of the condition
In women, breast cancer is the first tumor type by incidence, and the second cause of death by cancer (Torre 2015). The social burden of breast cancer in terms of mortality, morbidity, psychological stress, and economic cost is huge: accordingly, many efforts are being made to effectively and safely prevent this disease (De la Cruz 2014; Lebovic 2010).
Thus far, the vast majority of favorable results in terms of reducing the disease burden have been yielded by means of secondary prevention programs, which are based on the early detection of breast cancer (primarily by screening mammography) (Fuller 2015; Onega 2014), an approach that has been the subject of some criticism (Pace 2014; Stout 2014).
Several approaches have been advocated for primary prevention (any intervention that can decrease disease incidence, i.e. the occurrence of new cases of breast cancer), with the types of intervention depending upon the risk level of the target population. The lifetime risk of developing breast cancer (as per internationally established guidelines, such as those from the National Institute for health and Care Excellence (NICE 2017)), can be calculated by means of dedicated risk assessment tools (e.g. the Gail model; Gail 2015) based on a set of risk factors such as age, race, age at the time of first menstrual period, age at the time of first live birth of a child, and number of first‐degree relatives with breast cancer). Family history of breast/ovarian cancer is used to assess personal risk of developing breast cancer, and some women have greater than 30% lifetime risk based on family history. Those at high risk (greater than 30%) may be offered screening breast magnetic resonance imaging, in addition to mammography.
Women at highest risk are those who carry germline pathogenic mutations in BRCA1 (personal risks estimated as 57%, 95% confidence Interval (CI) 47% to 66%, as per NICE 2017) or BRCA2 (personal risk 49%, 95% CI 40% to 57%). These women may consider risk‐reducing surgery with bilateral mastectomies, which reduce the risk of developing breast cancer by up to 90%. As these women are at high risk of developing ovarian cancer (10% to 60% risk), risk‐reducing bilateral salpingo‐oophorectomy is strongly recommended, and, when performed around age 40 years, reduces the risk of developing breast cancer by 50% (Lostumbo 2010; Tuttle 2010), although this belief has been the focus of controversies (Heemskerk‐Gerritsen 2015; Kotsopoulos 2016).
Primary prevention has also been suggested for women considered to be at above‐average risk of breast cancer (lifetime risk between 17% and 30%, as per NICE 2017).
For women with an above‐average risk, pharmacological prevention (also known as risk‐reducing medication, chemoprevention, or preventive therapy) has been proposed to reduce the risk of breast cancer (Advani 2014; Colditz 2014; Sestak 2015). Of note, the compounds used for chemoprevention are not to be confused with cytotoxic chemotherapeutic agents, which are effective for adjuvant therapy, but have no role in prevention of breast cancer. Many chemoprevention compounds have been tested in clinical trials, and have shown risk‐reducing properties against a variety of tumors (including breast cancer). Currently, some of these compounds (e.g. tamoxifen and raloxifene, which are approved for the treatment of early and advanced breast cancer) are recommended for breast cancer primary prevention in clinical practice (Carlson 2009; Chlebowski 2002; Colditz 2014; Stubert 2014).
Finally, lifestyle changes (e.g. physical exercise, healthy diet, and smoking/alcohol cessation; Gonçalves 2014; Rossi 2014) are recommended independently of the risk level, also in light of their positive effects on the risk of other illnesses.
Description of the intervention
Cancer prevention agents (CPAs) are a broad group of compounds sharing an anticancer activity, proven experimentally or clinically (or both), against one or more tumor types (Landis‐Piwowar 2014; Serrano 2015). With regards to breast cancer pharmacological prevention (Chlebowski 2014; Cuzick 2011; Den Hollander 2013; Gabriel 2012), the CPA class most tested is that of selective estrogen receptor modulators (SERMs) (Cuzick 2013; Lazzeroni 2013; Visvanathan 2013), due to the hormone dependence of the majority of breast cancers (Williams 2014). Other CPAs have been investigated, such as other antiestrogen drugs (e.g. aromatase inhibitors; Behan 2015; Litton 2012; Olin 2014),; micronutrients (e.g. vitamins; Giammanco 2015; Lazzeroni 2011); and drugs already approved for the treatment of different diseases (e.g. antiosteoporosis agents; Chlebowski 2012; Liu 2012), anticholesterol agents (e.g. Ahern 2014; Santa‐Maria 2013), non‐steroidal anti‐inflammatory drugs (NSAIDs) (Bosetti 2012; Jacobo‐Herrera 2014; Thorat 2013), and antidiabetes agents (Gandini 2014; Stine 2014), for which experimental and (although not always conclusive) epidemiological evidence suggests anticancer activity. Of note, both SERMs and aromatase inhibitors are currently approved for breast cancer treatment both in the adjuvant setting (i.e. postoperatively in women with early disease) and in advanced/metastatic setting.
Overall, CPAs are a group of compounds that differ, often radically, from the chemical and pharmacological viewpoint. There are different CPA classes (e.g. SERMs, aromatase inhibitors, vitamins, NSAIDs, etc.), each one with different anticancer mechanisms, that are sometimes not fully elucidated. The SERMs and aromatase inhibitors, whose activity against breast cancer has been clearly demonstrated in women with both early and metastatic disease (Freedman 2015; Schiavon 2013), act by interfering with the estrogen receptor pathway, which is known to play a key role in breast carcinogenesis.
SERMs act on the estrogen receptor, behaving as both agonists (i.e. by stimulating receptor activity) and antagonists (i.e. by inhibiting receptor activity), depending on the target tissue. For instance, one of the most widely investigated SERMs, tamoxifen, acts as an agonist in bone and uterine tissues, while it acts as an antagonist in breast tissue. Overall, this tissue specificity determines most of the beneficial effects (e.g. antibreast cancer activity) as well as toxic effects (e.g. endometrial cancer‐promoting activity) (Nazarali 2014). In contrast, the more‐recently developed SERMs (e.g. raloxifene, lasofoxifene) act as agonists mainly in bone tissue, and antagonists in both breast and uterine tissues (Mirkin 2015). As their mechanism of action implies an interaction with the estrogen receptor, SERMs activity is much higher (about 10‐fold) against tumors expressing the estrogen receptor or progesterone receptor (so‐called hormone‐positive breast cancer) (or both), and is not effective in tumors that do not express these receptors (estrogen receptor‐negative and progesterone receptor‐negative breast cancer).
Aromatase inhibitors (e.g. anastrozole, letrozole, exemestane) inhibit estrogen synthesis and are only active in postmenopausal women, when the main source of estrogen is the peripheral tissues (whereas the main source of estrogen is the ovaries in premenopausal women). In the peripheral tissues, androgens are converted into estrogens by the aromatase enzyme, which is the target of aromatase inhibitors. Therefore, aromatase inhibitors are used exclusively in postmenopausal women for either the prevention of primary breast cancer (women who never had a primary breast cancer, but who are at an above‐average risk of developing it), or as adjuvant therapy for prevention of breast cancer recurrence, or for control of metastatic hormone‐positive breast cancer (Campagnoli 2013; Chumsri 2015).
For other CPAs, the evidence in the field of breast cancer management is much less extensive. NSAIDs (such as aspirin), which have an established role in colorectal cancer primary prevention (Rothwell 2012; Sostres 2014), block the cyclo‐oxygenase pathway, which is involved in tumor development. For micronutrients (such as vitamin D), a wealth of experimental and epidemiological evidence has been gathered that supports their anticancer potential (Feldman 2014), although their efficacy has yet to be clearly demonstrated in clinical trials. For antiosteoporosis agents, anticholesterol agents, and antidiabetes agents, the anticancer mechanism has only been partially elucidated, and some epidemiological evidence has suggested a potential anticancer effect in primary prevention (Gronich 2013). Finally, for other potential CPAs, only some preclinical evidence of anticancer activity has been reported (Romagnolo 2014; Wang 2015), without any epidemiological data being available on cancer risk reduction.
How the intervention might work
The rationale for using CPAs is to reduce the risk of breast cancer development (and thus to reduce breast cancer incidence, that is, the occurrence of new cases of this disease). The performance of the CPA depends upon two main aspects.
The first aspect is the risk level of the target population. In fact, the absolute risk reduction (ARR = EER – CER, where EER is experimental event rate and CER is control event rate) is higher as the disease risk increases, although the relative risk reduction (RRR = [EER – CER]/CER) is constant across risk levels. An important corollary is that it can be very difficult to demonstrate the efficacy of any type of prevention strategy in a low‐risk population due to the low incidence of the disease, which would require a huge number of participants to be enrolled in order to yield adequate statistical power. Therefore, in order to prove the efficacy of a given CPA, trials usually enroll women at an above‐average risk of breast cancer.
The second aspect is CPA‐related toxicity. Since CPAs are administered to averagely healthy women, they should be characterized by a low (or very low) occurrence of adverse effects. The lower the toxicity rate (i.e. the higher the acceptability), the broader the population that might benefit from the preventive effect of a given CPA. If the CPA had no toxicity, it could be recommended to all women, independently of their risk of developing breast cancer. Since this 'ideal' type of CPA does not yet exist, chemoprevention is currently recommended only to women at an above‐average risk of breast cancer. Risk assessment tools (such as the Gail score) have been devised to identify such a target population (Cummings 2009; Gail 2015; Howell 2014; Layeequr 2009), in which the risk of toxic effects associated with CPAs must be overwhelmed by the benefit in terms of breast cancer prevention.
The risk of toxicity associated with CPAs is the main cause of the relatively low rate of uptake of risk‐reducing agents in breast cancer (Crew 2015; Smith 2016). However, other aspects have been suggested to impact on the uptake of CPAs, such as the impact of others' experience on beliefs about the CPA, the CPA perceived as a 'cancer drug', and daily reminder of cancer risk (Donnelly 2014).
Why it is important to do this review
Chemoprevention for breast cancer is a debated topic (Euhus 2015; Ropka 2010; Serrano 2015; Wuttke 2015). International guidelines such as the American Society of Clinical Oncology (ASCO) (Visvanathan 2013), the National Comprehensive Cancer Network (NCCN) (Gradishar 2015), the US Preventive Services Task Force (Moyer 2013), and the National Institute for Health and Care Excellence (NICE) (NICE 2017) guidelines, recommend offering some CPAs (i.e. tamoxifen and aromatase inhibitors) for women at an above‐average risk of breast cancer (Alés‐Martínez 2015).
Randomized controlled trials (RCTs) and meta‐analyses of single CPAs (or a class of CPAs) support the efficacy of chemoprevention in women at higher than average risk for developing primary (no previous breast cancer) breast cancer (Cuzick 2013; Nelson 2013; Vogel 2015). However, the uptake of CPAs is low, for reasons including the low, but non‐negligible rate of adverse effects associated with CPAs (Crew 2015; Holmberg 2015; Nichols 2014). Therefore, the balance between their benefits and harms is still debated.
For this review, we systematically assessed the RCT‐based evidence for CPAs as potentially useful against primary breast cancer. This provides women, physicians, healthcare agencies, and policy‐makers with objective information to make evidence‐based decisions regarding the use of CPAs as a breast cancer risk‐reducing strategy.
Objectives
To assess the efficacy and acceptability of single‐agent CPAs for the prevention of primary breast cancer, in unaffected women, at an above‐average risk of developing breast cancer.
Using a network meta‐analysis, to rank single CPAs, based on their efficacy and acceptability (an endpoint that is defined as the inverse of CPA‐related toxicity).
Methods
Criteria for considering studies for this review
Types of studies
RCTs examining the performance of any CPA in women at an above‐average risk of sporadic primary breast carcinoma.
We applied no language restrictions. We included RCTs in which allocation to treatment was not adequately concealed (or the concealment was unclear); however, we considered trial methodological quality in both the analysis and discussion. In the light of the preventive nature of the treatment (risk‐reducing therapy), we expected that most trials would compare a given CPA with placebo (or observation). We excluded quasi‐RCTs (i.e. trials in which treatment allocation depended upon predictable methods, such as date of birth or alternation).
Types of participants
Women with no personal history of breast cancer, but with above‐average risk of developing breast cancer (i.e. with a lifetime risk greater than 17% but lower than 30%) represented the target population.
We excluded trials where all participants were women at high risk (i.e. with a lifetime risk greater than 30%) since robust evidence exists for the use of CPAs (e.g. tamoxifen and aromatase inhibitors) in this group (Advani 2014; Freedman 2015; Gradishar 2015; Tuttle 2010; Yeo 2014). In contrast, the use of CPAs for women at above‐average risk of developing breast cancer is still controversial. We included trials of women at above‐average risk (between 10% and 17%) and high risk (greater than 30%), provided the majority of participants were likely to be at above‐average risk of breast cancer.
Types of interventions
-
Intervention: any CPA with proven anticancer properties based on experimental or clinical evidence (epidemiological, i.e. observational, or trial‐based) (or both) gathered for breast cancer or any other type of cancer.
Selective estrogen receptor modulators (SERMs): tamoxifen, raloxifene, lasofoxifene.
Aromatase inhibitors: anastrozole, letrozole, exemestane.
Vitamin D, aspirin, metformin, statins (pravastatin, simvastatin), bisphosphonate (zoledronate, alendronate).
Comparator: placebo (or observation) or any other CPA.
Types of outcome measures
We utilized study‐level data for the following outcomes.
Primary outcomes
We assessed each CPA in terms of:
Overall breast cancer incidence (in situ and invasive carcinoma); and
Severe toxicity (i.e. grade 3 and 4 toxicities according to the National Cancer Institute (NCI) Common Toxicity Criteria or equivalent) .
Secondary outcomes
We assessed each CPA in terms of:
Invasive breast cancer incidence (excluding in situ carcinoma, as opposed to the primary outcome which included both in situ and invasive carcinomas); and
Overall toxicity (any grade toxicity).
Search methods for identification of studies
Electronic searches
We searched the following databases on 17 August 2018.
The Cochrane Breast Cancer Group's (CBCG's) Specialised Register. Details of the search strategies used by the Group for the identification of studies and the procedure used to code references are outlined on the Group's website. We extracted and considered for inclusion in the review, trials with the key words "breast cancer, breast carcinoma, chemoprevention, preventive therapy, primary prevention, risk, randomized controlled trial, tamoxifen, raloxifene, lasofoxifene, selective estrogen receptor modulator, SERM, aromatase inhibitor, anastrozole, letrozole, exemestane, vitamin D, aspirin, metformin, statin, pravastatin, simvastatin, bisphosphonate, zoledronate, alendronate".
Cochrane Central Register of Controlled Trials (CENTRAL) (Appendix 1).
MEDLINE (via OvidSP; Appendix 2). Results of this search were limited to specific years (i.e. 2012 to August 2018) due to the currency of the CBCG's Specialised Register.
Embase (via OvidSP; Appendix 3). Results of this search were limited to specific years (i.e. 2014 to August 2018) due to the currency of Embase records in CENTRAL.
The World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal for all prospectively registered and ongoing trials (www.apps.who.int/trialsearch/Default.aspx; Appendix 4).
ClinicalTrials.gov (www.clinicaltrials.gov; Appendix 5).
We applied no language restrictions for any of the searches.
Searching other resources
Bibliographic searching
We tried to identify additional studies from reference lists of identified relevant studies or reviews. We obtained a copy of the full article for each reference reporting a potentially eligible study.
Data collection and analysis
Selection of studies
We used the search strategy to identify titles and abstracts of relevant studies. Two review authors (SM, SP) independently screened these titles and abstracts, and they discarded non‐relevant or duplicate publications. For studies where the classification of risk was unclear, these were discussed with the third author (AG). We screened studies and reviews that might have included information on potentially relevant studies. We retrieved the full text of potentially relevant publications in order to fully assess study eligibility. We applied no language restrictions. For articles written in languages other than Italian, English, French, and Spanish (the languages the authors know), we were prepared to organize translations. Any disagreement was resolved by iteration, discussion, and consensus with the third review author (AG).
Data extraction and management
Two review authors (SM, SP) independently extracted data using dedicated data extraction forms (i.e.in‐house MS‐Excel spreadsheets that were previously developed and used by the authors for other systematic reviews).
According to the population, intervention, comparison, outcomes, study design (PICOS) list, we extracted the following descriptive data for each study.
Population: mean age; menopausal status; breast cancer risk.
Intervention: CPA; dosage; administration route; duration of administration.
Comparison: as above (unless placebo or no treatment).
Study design: inclusion criteria; primary endpoint; duration of follow‐up; number of participants; date of publication; number of centers; sequence generation; allocation concealment; blinding of outcome assessors; attrition; selective outcome reporting.
Most (if not all) of these data could also be used as potential effect modifiers to investigate both between‐study heterogeneity and inconsistency (and thus, to verify the key assumption of transitivity for network meta‐analysis, see below for more details).
Additionally, we extracted outcome data (risk ratios (RR) for both treatment efficacy and treatment toxicity) from each study.
We (or others) were prepared to translate studies reported in non‐English language journals before assessment. Where more than one publication of one study existed, we used the publication with the most complete and updated data in the analysis; however, if relevant outcomes were only published in earlier versions, we used these data as well. Any disagreement was resolved by iteration, discussion, and consensus with the third review author (AG).
Assessment of risk of bias in included studies
Two review authors (SM, SP) independently assessed the risk of bias in included studies using Cochrane's 'Risk of bias' assessment tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The tool is composed of seven domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other sources of bias (e.g. per‐protocol analysis instead of intention‐to‐treat analysis). Based on the results obtained with this tool, we classified the included studies into one of the following categories: low, high, or unclear risk of bias. Any disagreement was resolved by iteration, discussion, and consensus with the third review author (AG).
Measures of treatment effect
Only dichotomous outcomes were expected, that is, efficacy (ratio of disease incidence in the intervention and comparator arms) and toxicity (ratio of disease rate in the intervention and comparator arms). Therefore, we expressed results as RRs with their 95% CIs. As for efficacy, an RR greater than 1.0 favors the intervention (as compared to comparator); when toxicity is considered, an RR greater than 1.0 favors the comparator.
Direct comparisons of treatment effects (CPA versus placebo/observation or other CPA)
We first carried out a standard pair‐wise meta‐analysis of results from studies comparing the same interventions using a fixed‐effect model; a random‐effects model (and a non‐iterative method of moments estimator as per DerSimonian 1986) can be used in case of between‐study heterogeneity (I2 statistic greater than 50%), but the limited number of studies available made the estimation of between‐study heterogeneity unreliable. Therefore, the fixed‐effect model was always used.
Assessment of heterogeneity within treatment comparisons
We evaluated between‐study heterogeneity within each treatment comparison using the I2 statistic (Higgins 2003). In the presence of statistical (I2 statistic greater than 50%) or clinical heterogeneity, or both, we investigated the potential sources of heterogeneity (provided that necessary data were available; see below for more details).
Indirect and mixed comparisons of treatment effects
Network meta‐analysis is the proposed method to summarize information from a network of studies addressing the same question, but testing different interventions (Caldwell 2014; Cipriani 2013; Salanti 2008; Salanti 2011).
This method allows for indirect treatment comparisons (i.e. comparisons not previously addressed directly), and improves estimate precision for comparisons with few data (as it combines both direct and indirect evidence). Moreover, network meta‐analysis enables investigators to rank treatments based on both efficacy and safety (a measure inversely proportional to toxicity). The aim of adopting this sophisticated statistical method was to compare the two main drug classes used in breast cancer risk reduction (that is, SERMs and aromatase inhibitors) because no studies have ever directly compared these two drug classes.
Assessment of transitivity across treatment comparisons
We evaluated the main assumption underlying network meta‐analysis, which is called transitivity. If one has information about comparisons AB and AC, then network meta‐analysis can derive information regarding the BC comparison based on the transitivity equation AB – AC = BC (Higgins 2012; Veroniki 2013; White 2012). We expect that the transitivity assumption will hold assuming that:
the common treatment (i.e. placebo or observation), used to compare different CPAs indirectly, is similar when it appears in different studies;
all pair‐wise comparisons do not differ substantially with respect to the distribution of effect modifiers (e.g. the design and study characteristics of a given CPA versus placebo studies are similar to those of studies comparing placebo to another CPA); and
participants, could in principle, be randomized to any of the treatments being compared in the network.
We first evaluated the assumption of transitivity by comparing the clinical and methodological characteristics of sets of studies grouped by treatment comparisons. To this aim, we took into consideration the following potential effect modifiers (if data were available): menopausal status, mean age, breast cancer risk score (mean), drug schedule (dose, frequency, duration of treatment), risk of bias category, duration of follow‐up, number of participants, and date of publication.
Assessment of statistical inconsistency
Lack of transitivity can manifest as inconsistency between direct and indirect estimates (loop inconsistency) or between estimates deriving from different study designs (design inconsistency) (Higgins 2012). Statistical methods can be employed to evaluate consistency in a network. We used the following methods (based on the type of studies and network that will result after the identification of the eligible studies).
Loop‐specific approach: when at least three treatments are compared to each other in a network forming a closed path, indirect evidence can be contrasted to direct evidence and their difference defines the so‐called inconsistency factor. To determine whether the inconsistency factor is different from zero, the Bucher method can be used (looking at the 95% CI and a loop‐specific z‐test) (Bucher 1997). This analysis can be extended to all closed loops, assuming a loop‐specific heterogeneity. This approach can identify loops with large inconsistency factors, but cannot determine the consistency of the whole network. Therefore, caution must be used while interpreting the findings derived from this method. Moreover, since loop inconsistency cannot occur in a multi‐arm trial (i.e. a trial with more than two arms), this method cannot be applied when multi‐arm trials are present: thus, a different method must be adopted to unveil inconsistency (see 'design‐by‐treatment interaction model' below).
Design‐by‐treatment interaction model: this has been proposed as a more comprehensive method to identify inconsistency (Higgins 2012), which encompasses both loop and design inconsistencies, and can be applied in the presence of both two‐arm and multi‐arm studies. While the above‐mentioned loop inconsistency approach aims at assessing whether direct and indirect evidence are consistent with each other, design inconsistency addresses the issue of whether a particular study design is associated with different effect sizes for a given treatment comparison (it can be envisaged as a multivariable meta‐regression where design is a study‐level covariate). As an example, this approach tests whether the relative effectiveness of treatment A versus B is different when estimated in studies with different designs, such as AB and ABC. Importantly, the design‐by‐treatment interaction model provides a global test for network inconsistency.
In case of significant inconsistency, its possible sources can be investigated by using multivariable random‐effects meta‐regression. In particular, the distribution of prespecified clinical and methodological features (used as study‐level covariates) that might represent potential sources of inconsistency can be investigated, such as menopausal status, mean age, breast cancer risk score (mean), drug schedule (dose, frequency, duration of treatment), risk of bias category, duration of follow‐up, number of participants, and date of publication.
Unit of analysis issues
There were no unit of analysis issues in the included studies (all studies adopted a simple parallel‐group design). Should we find such issues in updates of this review, we will deal with them as recommended by Cochrane guidelines (Higgins 2011).
Dealing with missing data
We performed an intention‐to‐treat analysis and recorded numbers lost to follow‐up and withdrawals (attrition rates). In the review update, should we find missing data in studies, we will contact the investigators of these studies in order to obtain those data.
Assessment of heterogeneity
We quantified between‐study heterogeneity within each direct comparison using the I2 statistic (Higgins 2003). In particular, we considered heterogeneity to be high for I2 values greater than 50%. Potential sources of heterogeneity (study‐level effect modifiers) such as menopausal status, mean age, breast cancer risk score (mean), drug schedule (dose, frequency, duration of treatment), risk of bias category, duration of follow‐up, number of participants, and date of publication could not be investigated due to the paucity of studies available. This analysis will be taken into consideration in the future should new data be available.
In order to fully describe heterogeneity in network meta‐analysis, we described both the magnitude of heterogeneity within treatment comparisons and the magnitude of the heterogeneity variance assumed common across treatment comparisons. In relation to the common heterogeneity variance across treatment comparisons (also known as Tau2), biostatisticians suggest referring to empirical distributions of heterogeneity values typically found in meta‐analyses (in particular, they consider heterogeneity to be high for a Tau2 value larger than the 75th quartile: Rhodes 2015; Salanti 2014; Turner 2012). Given that the interpretation of this variance is not straightforward, biostatisticians suggest presenting network meta‐analysis summary effects together with their predictive intervals (i.e. an interval where the estimate of a potential future study is expected to be) in order to facilitate the understanding of the results in the light of the heterogeneity magnitude (Chaimani 2013), and we followed this guidance when conducting this review.
Assessment of reporting biases
In this review, we were unable to assess small‐study effects (which includes publication bias) by using funnel plots because there were fewer than 10 studies (Higgins 2011). However, in review updates, we will assess funnel plot asymmetry by means of visual inspection and formally testing by means of Egger's test should we find a sufficient number of studies.
Data synthesis
We presented results for both treatment efficacy and toxicity as risk ratios (RRs) and their 95% confidence intervals (CIs). For standard pair‐wise meta‐analysis (using the fixed‐effect model), we used Review Manager 5 software (Review Manager 2014).
We conducted a network meta‐analysis using the Stata software 11.2/SE (StataCorp 2009); in particular, we used the mvmeta routine (Chaimani 2013; White 2011), that fits the multivariate random‐effects meta‐analysis model (using restricted maximum likelihood) as suggested by White 2011. We presented results from the network meta‐analysis as summary effect sizes (RRs) along with their 95% CIs and predictive intervals. We assumed a common heterogeneity variance, that is, a single heterogeneity variance for the entire network, pertaining to every one of the direct comparisons (this assumption simplifies the analysis and allows for heterogeneity to be incorporated for direct comparisons with only one trial available).
We used the GRADE approach to assess the certainty of the evidence (Guyatt 2011). Briefly, certainty of evidence is graded into four levels: high, moderate, low, and very low certainty. Evidence from RCTs is considered high certainty; however, the certainty can be downgraded by one (serious concern) or two levels (very serious concern) for the following reasons: risk of bias, inconsistency (unexplained heterogeneity, inconsistency of results), indirectness (indirect population, intervention, control, outcomes), and imprecision (wide CIs, CIs crossing the null value (i.e. the results are compatible with both favorable and harmful effects)).
'Summary of findings' table
We included 'Summary of findings' tables (including the number needed to treat, that is, the number of women to be administered the risk reducing medication in order to avoid one case of breast cancer, which is calculated as 1/absolute risk reduction). The baseline risk was considered the mean event rate in the non‐treatment (placebo or observation) arms of the included studies.
Subgroup analysis and investigation of heterogeneity
Neither subgroup analysis nor meta‐regression were feasible, as data were not permissive (i.e. too few studies were available). Should new studies be available in the future, we will between‐study heterogeneity by means of the following.
-
Subgroup analysis: using the following grouping variables:
menopausal status (pre‐ versus postmenopause); and
estrogen receptor status (positive versus negative).
Meta‐regression: to assess an association between duration of both follow‐up and treatment, and treatment efficacy.
Sensitivity analysis
We did not perform sensitivity analysis. However, should additional studies be available in the future, we will evaluate the robustness of the results by excluding studies with high risk of bias, studies not having breast cancer incidence as their primary endpoint, or studies using observation (no treatment) as a comparator.
Results
Description of studies
Results of the search
The literature search led to the identification of 2717 records (see PRISMA flowchart: Figure 1). After the exclusion of duplicates, we screened the title and abstracts of 2381 records, which led to the identification of 41 potentially eligible records. We excluded 35 records after full‐text review. The six remaining records corresponded to six studies that fulfilled the inclusion criteria and thus, were included in the qualitative (six studies) and quantitative (five studies) analyses.
1.

Study flow diagram.
Included studies
The six included studies were published between 2005 and 2015 (Cuzick 2014; Cuzick 2015; Fisher 2005; Goss 2011; Powles 2007; Vogel 2010; see Characteristics of included studies table).
Overall, 50,927 women were enrolled with the mean number of women enrolled per study being 8491 (range 2494 to 19,490). Participants were:
postmenopausal women at higher risk of breast cancer (Cuzick 2014; Goss 2011; Vogel 2010);
women deemed to be at an increased risk of developing breast cancer based on a family history of breast cancer or abnormal benign breast disease (Cuzick 2015);
women either 60 years of age or older or between 35 and 59 years of age with a five‐year predicted risk for breast cancer of at least 1.66% (Fisher 2005); or
women with a family history of breast cancer (Powles 2007).
The six studies (all designed as two‐arm RCTs) investigated four agents: two SERMs (i.e. tamoxifen: Cuzick 2015; Fisher 2005; Powles 2007; Vogel 2010, and raloxifene: Vogel 2010), and two aromatase inhibitors (i.e. anastrozole: Cuzick 2014, and exemestane: Goss 2011). Five studies compared the risk‐reducing medication with placebo (Cuzick 2014; Cuzick 2015; Fisher 2005; Goss 2011; Powles 2007), whereas one compared the efficacy of two SERMs (tamoxifen and raloxifene; Vogel 2010).
Excluded studies
We excluded 35 studies after evaluating the corresponding full‐text articles. The two main reasons for exclusion were data duplication and an inadequate definition of the population risk of breast cancer (for further details, see Characteristics of excluded studies table).
Risk of bias in included studies
Overall, the risk of bias was low as the quality of all included studies was high (see Figure 2). In particular, there was no high risk of bias, and two studies had an unclear risk of bias. The risk of performance bias was unclear in two studies because they were unblinded (Fisher 2005; Vogel 2010). In one study, the risk of attrition bias was unclear due to loss to follow‐up (1.3% of the originally randomized population) (Vogel 2010).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The risk of selection bias was low in all six studies, both in terms of random sequence generation and allocation concealment.
Blinding
The risk of performance bias was generally low, although two studies designed as double blind were unblinded some time after the enrolment was ended (Fisher 2005; Vogel 2010). The impact of this unblinding on findings was unclear, since the results before and after unblinding appeared similar.
The risk of detection bias was low in all studies as all outcomes were considered to be objective outcome measures.
Incomplete outcome data
The risk of attrition bias was low in five out of six studies. In one study, 19,490 women out of the originally randomized 19,747 women participated in the study and the main reason for the attrition was loss at follow‐up (i.e. data were available for 98.7% of initially randomized participants) (Vogel 2010). It is unclear if this loss may have caused any bias when these number of women lost at follow‐up were evenly distributed between the two study arms.
Selective reporting
The risk of reporting bis was low in all included studies because all studies reported the outcomes as specified in the methods section of the trial publications.
Other potential sources of bias
There were no other types of bias detected.
Effects of interventions
See: Table 1; Table 2; Table 3
For each of the four outcomes, data were available for the following comparisons: tamoxifen versus placebo (three studies), anastrozole versus placebo (one study), exemestane versus placebo (one study), and tamoxifen versus raloxifene (one study). Besides comparisons of single risk‐reducing medications (i.e. tamoxifen, raloxifene, anastrozole, and exemestane), we also assessed the efficacy and toxicity of aromatase inhibitors (anastrozole and exemestane) as a single drug class. See Figure 3.
3.

Network plot: the figure represents the available direct comparisons among breast cancer risk‐reducing agents. Each study is represented by a black line. SERM: selective estrogen receptor modulator.
Due to the stringent inclusion criteria (especially in terms of risk of breast cancer), the included studies were highly homogeneous in terms of the type of population. The only difference across the population was the menopausal status of enrolled women. Tamoxifen can be administered both in pre‐ and postmenopausal women, whereas aromatase inhibitors are given to postmenopausal women. Accordingly, studies testing tamoxifen enrolled both pre‐ and postmenopausal women, and studies investigating aromatase inhibitors only enrolled postmenopausal women. This difference was taken into consideration when performing meta‐analyses and interpreting the findings.
Due to the low number of studies available, we used the fixed‐effect model for standard pair‐wise meta‐analysis, due to the intrinsic limit in the quantification of the between‐study heterogeneity measure (Tau2). Moreover, the scarcity of studies did not allow us to investigate the presence of publication bias by formally testing funnel plot asymmetry or to use meta‐regression to investigate sources of between‐study heterogeneity.
Since the two aromatase inhibitors (anastrozole and exemestane) share the same mechanism of action and were associated with very similar efficacy findings, we decided to pool their results. This choice should also favor more robust results of the network meta‐analysis, where the evidence on tamoxifen was supported by three studies, while the evidence regarding the two aromatase inhibitors was supported by one study for each aromatase inhibitor.
Primary outcomes
Overall breast cancer incidence
Tamoxifen versus placebo
Based on data from three studies involving 22,832 women, we found that the use of tamoxifen probably reduced the risk of breast cancer (invasive and in situ carcinoma) by 32% compared to placebo (RR 0.68, 95% CI 0.62 to 0.76; Analysis 1.1; Cuzick 2015; Fisher 2005; Powles 2007). The certainty of evidence was moderate due to remarkable inconsistency (I2 = 62%). We could not identify the source of such between‐study heterogeneity. In particular, meta‐regression suggested that neither a woman's age nor duration of follow‐up had an impact on the outcome, although the limited number of available studies warranted caution when interpreting these findings.
1.1. Analysis.
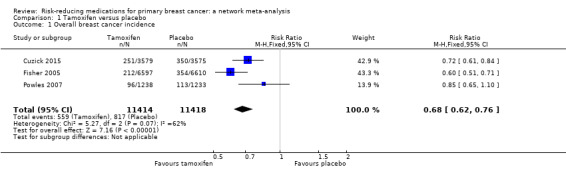
Comparison 1 Tamoxifen versus placebo, Outcome 1 Overall breast cancer incidence.
Aromatase inhibitors versus placebo
In two studies, anastrozole and exemestane reduced the risk of breast cancer when compared to placebo (anastrozole: RR 0.48, 95% CI 0.33 to 0.69; Cuzick 2014; exemestane: RR 0.45, 95% CI 0.27 to 0.77; Goss 2011). Pooling the data from the two studies involving 8424 women, we found that the use of aromatase inhibitors reduced the risk of breast cancer by 53% compared to placebo (RR 0.47, 95% CI 0.35 to 0.63; Analysis 2.1). The certainty of the evidence was high.
2.1. Analysis.
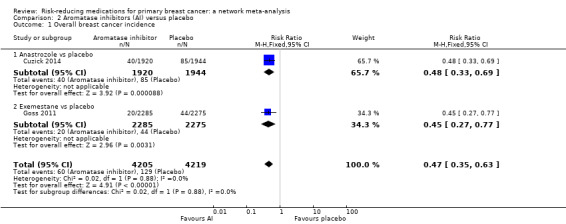
Comparison 2 Aromatase inhibitors (AI) versus placebo, Outcome 1 Overall breast cancer incidence.
Tamoxifen versus raloxifene
In the one study, involving 19,490 women, that compared two SERMs, raloxifene performed worse than tamoxifen in terms of breast cancer incidence reduction (RR 1.25, 95% CI 1.09 to 1.43; moderate‐certainty evidence; Analysis 3.1; Vogel 2010).
3.1. Analysis.
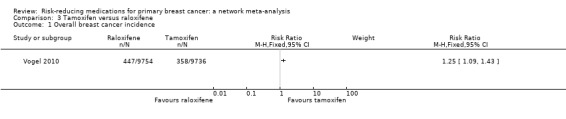
Comparison 3 Tamoxifen versus raloxifene, Outcome 1 Overall breast cancer incidence.
Severe toxicity
Tamoxifen versus placebo
Two out of three studies comparing tamoxifen to placebo provided data on grade 3 to 4 adverse events (Cuzick 2015; Fisher 2005). Based on data from two studies involving 20,361 women, tamoxifen probably increased the risk of severe toxicity compared to placebo (RR 1.28, 95% CI 1.12 to 1.47; Analysis 1.2). The certainty of the evidence was moderate due to inconsistency (I2 = 72%). We could not identify the source of such between‐study heterogeneity.
1.2. Analysis.
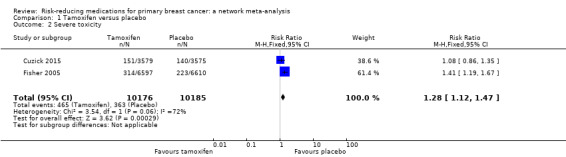
Comparison 1 Tamoxifen versus placebo, Outcome 2 Severe toxicity.
In particular, women taking tamoxifen experienced a higher incidence of both endometrial carcinoma and thromboembolism in all studies (Table 4). Pooling the data from all three studies, tamoxifen significantly increased the risk of both endometrial cancer (RR 2.26, 95% CI 1.52 to 3.38; I2 = 44%; high‐certainty evidence) and thromboembolism (RR 2.10, 95% CI 1.14 to 3.89; no heterogeneity; high‐certainty evidence). In contrast, in one of the two studies reporting bone fracture (Fisher 2005; Powles 2007), women in the tamoxifen arm showed a lower rate of bone fractures compared to women in the placebo arm (0.2% with tamoxifen versus 0.3% with placebo; RR 0.67, 95% CI 0.51 to 0.92; Fisher 2005).
1. Adverse events: tamoxifen versus placebo.
| Fisher 2005 | |||
| Adverse event | Tamoxifena | Placeboa | RR (95% CI) |
| Endometrial cancer | 2.24 | 0.68 | 3.28 (1.87 to 6.03)b |
| Myocardial infarction | 2.79 | 2.70 | 1.03 (0.79 to 1.36) |
| Stroke | 1.75 | 1.23 | 1.42 (0.97 to 2.08) |
| Thromboembolism | 0.69 | 0.32 | 2.15 (1.08 to 4.51)b |
| Bone fractures | 1.97 | 2.88 | 0.68 (0.51 to 0.92)b |
| Total deaths | 3.08 | 2.80 | 1.10 (0.85 to 1.43) |
| Powles 2007 | |||
| Adverse event | Tamoxifena | Placeboa | RR (95% CI) |
| Endometrial cancer | 10.50 | 4.05 | 2.59 (0.86 to 9.30) |
| Myocardial infarction | 8.07 | 9.73 | 0.83 (0.32 to 2.10) |
| Stroke | 5.65 | 7.29 | 0.77 (0.24 to 2.34) |
| Thromboembolism | 6.46 | 2.43 | 2.65 (0.63 to 15.5) |
| Bone fracture | 15.34 | 17.84 | 0.86 (0.44 to 1.67) |
| Cataract | 7.26 | 0.81 | 8.96 (1.24 to 393)b |
| Hot flashes | 483.04 | 319.54 | 1.51 (1.29 to 1.76)b |
| Arthritis | 54.12 | 46.23 | 1.17 (0.80 to 1.71) |
| Constipation/diarrhea | 33.92 | 36.49 | 0.93 (0.59 to 1.45) |
| Total deaths | 43.62 | 43.79 | 0.99 (0.66 to 1.49) |
| Cuzick 2015 | |||
| Adverse event | Tamoxifena | Placeboa | RR (95% CI) |
| Endometrial cancer | 8.10 | 5.59 | 1.45 (0.79 to 2.71) |
| Non‐breast cancer | 98.07 | 88.11 | 1.12 (0.94 to 1.31) |
| Cardiac deaths | 3.35 | 3.91 | 0.85 (0.36 to 1.99) |
| Cerebrovascular deaths | 2.79 | 3.35 | 0.83 (0.32 to 2.10) |
| Thromboembolic deaths | 1.12 | 0.84 | 1.33 (0.22 to 9.09) |
| Total deaths | 50.85 | 46.43 | 1.09 (0.88 to 1.36) |
aRates per 1000 participants of adverse events reported in three RCTs comparing tamoxifen with placebo. bStatistically significant difference. CI: confidence interval; RR: risk ratio.
Aromatase inhibitors versus placebo
In single studies, both anastrozole and exemestane increased the rate of severe toxicity when compared to placebo (anastrozole: RR 1.14, 95% CI 1.02 to 1.28; Cuzick 2014; exemestane: RR 1.22, 95% CI 1.10 to 1.36; Goss 2011). Pooling the data from the two studies involving 8352 women, aromatase inhibitors increased the risk of severe toxicity by 18% compared to placebo (RR 1.18, 95% CI 1.09 to 1.28; Analysis 2.2; high‐certainty evidence).
2.2. Analysis.
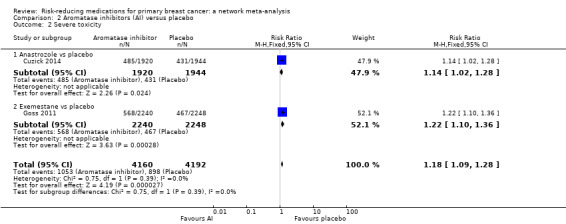
Comparison 2 Aromatase inhibitors (AI) versus placebo, Outcome 2 Severe toxicity.
These differences in adverse events were sustained particularly for endocrine (e.g. hot flashes), gastrointestinal (e.g. diarrhea), and musculoskeletal (e.g. arthralgia) adverse events. No significant difference in bone fracture incidence was observed between women in the treatment arms (ranging from 9% to 6.7% in the two studies) compared to those in the placebo arms (ranging from 8% to 6.4% in the two studies). Moreover, single studies suggested that this class of CPAs do not increase the risk of either endometrial cancer or thromboembolism (Table 5).
2. Adverse events: aromatase inhibitors versus placebo.
| Goss 2011 | |||
| Adverse event | Exemestanea | Placeboa | RR (95% CI) |
| Non‐breast cancer | 19.19 | 16.90 | 1.13 (0.71 to 1.81) |
| Cardiovascular events | 47.32 | 49.3 | 0.96 (0.72 to 1.27) |
| Bone fracture | 66.51 | 63.61 | 1.04 (0.82 to 1.33) |
| Hot flashes (grade 3–4) | 29.91 | 19.12 | 1.56 (1.04 to 2.36)b |
| Arthritis (grade 3–4) | 14.28 | 7.56 | 1.89 (1.01 to 3.63)b |
| Diarrhea (grade 3–4) | 4.01 | 0.44 | 9.03 (1.24 to 395)b |
| Any (grade 3–4) | 253.57 | 207.74 | 1.22 (1.06 to 1.40)b |
| Cuzick 2014 | |||
| Adverse event | Anastrozolea | Placeboa | RR (95% CI) |
| Endometrial cancer | 1.56 | 2.57 | 0.61 (0.09 to 3.12) |
| Non‐breast cancer | 20.83 | 36.01 | 0.58 (0.38 to 0.87)b |
| Other cardiovascularc | 5.73 | 7.71 | 0.74 (0.31 to 1.73) |
| Thromboembolism | 9.89 | 8.74 | 1.13 (0.59 to 2.17) |
| Bone fractures | 85.41 | 76.64 | 1.11 (0.90 to 1.38) |
| Cataract | 46.87 | 48.86 | 0.96 (0.72 to 1.27) |
| Hot flashes | 567.71 | 494.34 | 1.15 (1.08 to 1.22)b |
| Arthritis | 506.25 | 459.87 | 1.10 (1.03 to 1.18)b |
| Total deaths | 9.37 | 8.74 | 1.07 (0.52 to 2.22) |
aRates per 1000 participants of adverse events reported in two randomized controlled trials comparing two aromatase inhibitors (exemestane and anastrozole) with placebo. bStatistically significant difference. cMyocardial infarction, heart failure or cerebrovascular accident. CI: confidence interval; RR: risk ratio.
Tamoxifen versus raloxifene
In the one study comparing two SERMs involving 19,490 women, raloxifene performed better than tamoxifen in terms of grade 3 to 4 adverse events (RR 0.87, 95% CI 0.80 to 0.95; Analysis 3.2; Vogel 2010). Of note, this difference was mainly due to a higher incidence of both endometrial cancer (RR 1.76, 95% CI 1.15 to 2.71) and thromboembolism (RR 1.31, 95% CI 1.06 to 1.63) in women taking tamoxifen (Table 6).
3.2. Analysis.
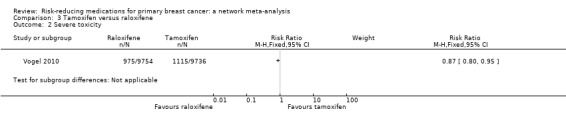
Comparison 3 Tamoxifen versus raloxifene, Outcome 2 Severe toxicity.
3. Adverse events: tamoxifen versus raloxifene.
| Vogel 2010 | |||
| Adverse event | Tamoxifena | Raloxifenea | RR (95% CI) |
| Endometrial cancer | 2.25 | 1.23 | 1.76 (1.15 to 2.71)b |
| Cardiovascular deaths | 4.31 | 4.30 | 1.00 (0.64 to 1.57) |
| Thromboembolism | 3.30 | 2.47 | 1.31 (1.06 to 1.63)b |
| Cataracts | 14.58 | 11.69 | 1.22 (1.10 to 1.37)b |
| Total deaths | 3.81 | 3.22 | 1.17 (0.96 to 1.42) |
aRates per 1000 participants of adverse events reported in an RCT comparing Tamoxifen with Raloxifene. bStatistically significant difference. CI: confidence interval; RR: risk ratio.
Secondary outcomes
Invasive breast cancer incidence
Tamoxifen versus placebo
Based on data from three studies involving 22,832 women, tamoxifen probably reduced the risk of invasive breast carcinoma by 31% compared to placebo (RR 0.69, 95% CI 0.61 to 0.77; Analysis 1.3; Cuzick 2015; Fisher 2005; Powles 2007). The certainty of evidence was moderate due to remarkable inconsistency (I2 = 53%). We could not identify the source of such between‐study heterogeneity. In particular, meta‐regression suggested that neither a woman's age nor duration of follow‐up had an impact on the outcome, although the limited number of available studies warrants caution when interpreting these findings.
1.3. Analysis.
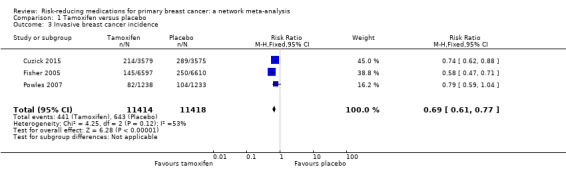
Comparison 1 Tamoxifen versus placebo, Outcome 3 Invasive breast cancer incidence.
Aromatase inhibitors versus placebo
Based on single studies, both anastrozole and exemestane reduced the risk of breast cancer when compared to placebo (anastrozole: RR 0.51, 95% CI 0.33 to 0.77; Cuzick 2014; exemestane: RR 0.34, 95% CI 0.17 to 0.68; Goss 2011). Pooling the data from the two studies involving 8424 women, aromatase inhibitors reduced the risk of breast cancer by 55% compared to placebo (RR 0.45, 95% CI 0.32 to 0.64; Analysis 2.3; high‐certainty evidence).
2.3. Analysis.
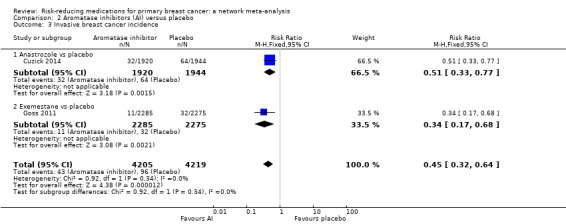
Comparison 2 Aromatase inhibitors (AI) versus placebo, Outcome 3 Invasive breast cancer incidence.
Tamoxifen versus raloxifene
In the one study comparing two SERMs involving 19,490 women, raloxifene performed worse than tamoxifen in terms of breast cancer incidence reduction (RR 1.25, 95% CI 1.06 to 1.48; moderate‐certainty evidence; Analysis 3.3; Vogel 2010).
3.3. Analysis.

Comparison 3 Tamoxifen versus raloxifene, Outcome 3 Invasive breast cancer incidence.
Overall toxicity
Tamoxifen versus placebo
Only one out of three studies comparing tamoxifen to placebo reported data on adverse events of any grade (Powles 2007). In this study, where the authors reported the total number of events (including multiple events for the same participant) and not the number of participants experiencing at least one adverse event, tamoxifen was associated with an 18% increase of overall toxicity compared to placebo (3675 events with tamoxifen versus 3120 events with placebo). In particular, women taking tamoxifen experienced a higher incidence of endometrial carcinoma, thromboembolism, vasomotor symptoms (e.g. hot flashes), and cataract occurrence (Table 4). Of note, there was a higher risk of cataract with tamoxifen compared to both placebo (Powles 2007) and raloxifene (Vogel 2010).
Aromatase inhibitors versus placebo
In the one study comparing anastrozole to placebo, the overall toxicity rate was equal in the anastrozole (89%) and placebo groups (89%) (RR 1.00, 95% CI 0.98 to 1.03; Cuzick 2014). There was a slight increase in overall toxicity in the one study comparing exemestane (88%) to placebo (85%) (RR 1.04, 95% CI 1.01 to 1.06; Goss 2011), a difference mainly due to endocrine (e.g. hot flashes), gastrointestinal (e.g. diarrhea), and musculoskeletal (e.g. arthralgia) adverse events (Table 5).
When combining the data from the two aromatase inhibitors studies involving 8352 women, aromatase inhibitors increased the risk of overall toxicity by 2% compared to placebo with high between‐study heterogeneity (RR 1.02, 95% CI 1.00 to 1.04; I2 = 72%; Analysis 2.4; very low‐certainty evidence).
2.4. Analysis.

Comparison 2 Aromatase inhibitors (AI) versus placebo, Outcome 4 Overall toxicity.
Tamoxifen versus raloxifene
The one study comparing two SERMs reported no data on overall toxicity (Vogel 2010).
Network meta‐analysis (indirect comparisons)
An indirect comparison of treatment effects allowed us to compare two drug classes (i.e. SERMs and aromatase inhibitors), although no study ever directly compared these two types of agents. Of note, statistical inconsistency of the network could not be assessed due to the shape of the network itself (star‐shaped network, which made it technically impossible to assess either loop or design inconsistency). The transitivity assumption was met in terms of trial design (placebo as the common control arm; study populations were similar in terms of breast cancer risk). However, the evidence generated by this type of meta‐analysis was downgraded as participants could not have been randomized to any of the two drug classes due to the restriction of aromatase inhibitors to postmenopausal women (as opposed to SERMs, which can be administered also to premenopausal women).
In terms of efficacy, network meta‐analysis showed that aromatase inhibitors may have reduced the risk of overall breast cancer (invasive and in situ) incidence compared to tamoxifen (RR 0.67, 95% CI 0.46 to 0.98). However, due to the above‐mentioned partial lack of transitivity and considering the non‐negligible between‐study heterogeneity (95% prediction interval 0.27 to 1.68), the certainty of evidence was downgraded to low. See Table 3.
The lack of model convergence (likely due to the low event incidence combined with the availability of only two studies per drug class) did not allow us to analyze toxicity data.
Discussion
Summary of main results
Our analysis supported the view that CPAs (tamoxifen, anastrozole, and exemestane, three drugs currently approved for the treatment of women with early or metastatic breast cancer) significantly reduced the incidence of breast cancer in unaffected women at above average to high risk of developing breast cancer, as summarized in Table 1 (tamoxifen versus placebo) and Table 2 (aromatase inhibitors versus placebo). Interestingly, this reduction in incidence was found for both invasive breast carcinoma and for a combined endpoint assessing in situ disease (ductal carcinoma in situ) and invasive breast carcinoma.
Our findings confirm the results of individual studies, strengthening the evidence that CPAs reduce the risk of developing both invasive and in situ breast cancer. Moreover, using the network meta‐analysis methodology (which allows indirect comparisons even in the absence of studies directly comparing two treatments), we found evidence suggesting that aromatase inhibitors are more effective than tamoxifen as CPAs (Table 3). Aromatase inhibitors further reduced the risk of disease by one third compared to tamoxifen, although the certainty of evidence was low due to indirectness of the assessment and inconsistency.
When compared to placebo, CPAs were associated with an increased risk of some adverse events. In particular, tamoxifen was associated with a significant increase of endometrial cancer and thromboembolism when compared to placebo. For the comparison between aromatase inhibitors and placebo, the data did not allow us to draw definitive conclusions because the two available RCTs used different drugs and reported toxicity in a different manner; however, the findings of single trials suggested that this class of CPAs was not associated with increased risk of either endometrial cancer or thromboembolism.
Finally, the new‐generation SERM agent (raloxifene) showed a better toxicity profile compared to first‐in‐class tamoxifen because of the reduced incidence of endometrial cancer and thromboembolism; however, this advantage was counterbalanced by an inferior therapeutic effect (although this evidence was based on a single trial involving postmenopausal women only). Unfortunately, available data did not enable us to investigate the difference in toxicity between SERMs and aromatase inhibitors by means of indirect comparison (network meta‐analysis). Of note, data on SERMs were on average more mature (with one study reaching a median follow‐up of 16 years; Cuzick 2015) than those available for aromatase inhibitors (median follow‐up approximately between three and five years). Therefore, any long‐term adverse effects of aromatase inhibitors in the primary prevention setting (e.g. on bone health) may require much longer follow‐up in order to be identified (and their extent quantified) and thus, to be comparable with findings with SERMs.
Overall completeness and applicability of evidence
The available evidence only partially addresses the objectives of this review. In fact, although we can state that investigated CPAs reduced the risk of breast cancer in the target population (women without a personal history of breast cancer but with an above‐average risk of breast cancer), there is partial lack of toxicity data for a complete comparison between different drugs. In fact, not all studies reported separately the information on severe toxicity (grading of toxicity), which allowed us to perform direct comparisons including only some of the available studies and did not enable us to perform indirect comparisons using a network meta‐analysis. Moreover, the drug comparison in terms of efficacy was limited to one RCT directly comparing two SERMs (tamoxifen versus raloxifene) and an indirect comparative analysis between SERMs and aromatase inhibitors (through network meta‐analysis).
Therefore, present data support the efficacy of CPAs to reduce the risk of developing breast cancer. Guidelines such as NICE 2017 (UK) and eviQ (Australia) do recommend discussing the option of CPAs with women at both high risk and above‐average risk of developing breast cancer. However, research has shown uptake is low with concerns regarding toxicity of therapy. Data are needed to fully address the issues in this field of preventive medicine, with special regard to the balance between benefit and adverse events.
Quality of the evidence
Current evidence hinges upon six studies enrolling overall 50,950 women. All studies were well conducted, with the risk of bias being low. For the tamoxifen versus placebo comparison, the certainty of evidence for both efficacy and toxicity was moderate with the reason for downgrading being between‐study inconsistency.
For the aromatase inhibitors versus placebo comparison, the certainty of evidence was high for both efficacy and toxicity.
Finally, the certainty of the evidence supporting the superiority of aromatase inhibitors over SERMs was low due to limitations in the network meta‐analysis, as there was inconsistency and partial lack of transitivity assumption (which is intrinsic for any indirect comparison such as that carried out in this case by means of network meta‐analysis).
Potential biases in the review process
The authors believe there were no potential biases in the review process.
Agreements and disagreements with other studies or reviews
In one individual participant‐data meta‐analysis of RCTs testing SERMs as breast cancer risk‐reducing medications, the investigators reported findings that are largely in agreement with ours in terms of both efficacy and toxicity (Cuzick 2013).
In another systematic review and meta‐analysis dedicated to the role of CPAs against breast cancer (Mocellin 2015), the authors considered a different population, that is, any woman independent of the baseline risk of breast cancer: this led to the inclusion of more studies and more types of CPAs as compared to the present work. However, there were similar findings in terms of efficacy of CPAs included in the present review (tamoxifen, exemestane, and anastrozole).
Authors' conclusions
Implications for practice.
Current evidence supports the use of cancer prevention agents (CPAs) such as selective estrogen receptor modulators (SERMs) and aromatase inhibitors in terms of efficacy. There is less serious toxicity with raloxifene compared to tamoxifen for postmenopausal women. Aromatase inhibitors do not have the serious potential toxicity of endometrial cancer or deep‐vein thrombosis. Long‐term data on toxicities from unaffected women taking tamoxifen are available while the follow‐up data for unaffected women taking aromatase inhibitors are short. Aromatase inhibitors appear to be more effective at reducing the incidence of breast cancer for above‐ average and high‐risk women. More data about the severity of less‐serious adverse effects such as hot flashes, arthralgias, and bone fractures would assist in determining the balance of benefit and harm. This supports recommendations in existing guidelines (NICE 2017).
Implications for research.
CPAs reduce the risk of breast cancer in this patient population (compared to placebo). They may cause toxicity which is mostly reversible on cessation of therapy, but uptake of CPAs is low. Therefore, we believe that three main lines of research are needed in this field of medicine.
The direct comparison of different CPAs to identify the CPA with the best efficacy and lowest toxicity; in fact, although network meta‐analysis suggested that aromatase inhibitors perform better than tamoxifen in terms of efficacy, no such (indirect) comparison was feasible for toxicity, which requires direct comparison in randomized and blinded clinical trials.
Research into drug acceptability and toxicity (i.e. the toxicity issue in the field of cancer prevention), which appears to be the main barrier to the routine implementation of this class of compounds (Crew 2015).
Investigation regarding the reasons (so called 'barriers') why women often refuse to take drugs aimed at reducing disease occurrence and the ways these obstacles can be overcome.
Acknowledgements
We would also like to acknowledge and thank our peer reviewers: Alessandra Gennari (clinical editor), an external clinical reviewer, a consumer reviewer, and a statistical reviewer who wish to remain anonymous during the preparation of this review.
Appendices
Appendix 1. CENTRAL
#1 MeSH descriptor: [Breast Neoplasms] explode all trees #2 breast near cancer* #3 breast near neoplasm* #4 breast near carcinoma* #5 breast near tumour* #6 breast near tumor* #7 #1 or #2 or #3 or #4 or #5 or #6 #8 MeSH descriptor: [Anticarcinogenic Agents] explode all trees #9 MeSH descriptor: [Chemoprevention] explode all trees #10 MeSH descriptor: [Selective Estrogen Receptor Modulators] explode all trees #11 Selective Estrogen Receptor Modulator* or SERM #12 MeSH descriptor: [Tamoxifen] explode all trees #13 MeSH descriptor: [Raloxifene] explode all trees #14 (tamoxifen or raloxifene or lasofoxifene) #15 MeSH descriptor: [Aromatase Inhibitors] explode all trees #16 aromatase inhibitor* #17 exemestane or letrozole or anastrozole or arimidex or femara #18 MeSH descriptor: [Vitamin D] explode all trees #19 vitamin D #20 MeSH descriptor: [Aspirin] explode all trees #21 acetylsalicylic acid or aspirin #22 MeSH descriptor: [Metformin] explode all trees #23 MeSH descriptor: [Hydroxymethylglutaryl‐CoA Reductase Inhibitors] explode all trees #24 MeSH descriptor: [Simvastatin] explode all trees #25 MeSH descriptor: [Pravastatin] explode all trees #26 MeSH descriptor: [Lovastatin] explode all trees #27 statin or tenivastatin or simvastatin or rosuvastatin or pravastatin or pitavastatin or mevinolin or glenvastatin or fluindostatin or dalvastatin or crilvastatin or compactin or cerivastatin or bervastatin or atorvastatin #28 MeSH descriptor: [Diphosphonates] explode all trees #29 biphosphonate* or diphosphonate* or diphosphanate* #30 MeSH descriptor: [Etidronic Acid] explode all trees #31 MeSH descriptor: [Clodronic Acid] explode all trees #32 MeSH descriptor: [Alendronate] explode all trees #33 etidronate* or clodronate* or pamidronate* or alendronate* or risedronate* or tiludronate* or ibandronate* or zoledronate* or incadronate* or olpadronate* or neridronate* #34 MeSH descriptor: [RANK Ligand] explode all trees #35 RANK ligand inhibitor #36 denosumab #37 prolia #38 Xgeva #39 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 #40 MeSH descriptor: [Chemoprevention] explode all trees #41 chemoprevent* or chemoprophyla* #42 prevent* or prophyla* #43 MeSH descriptor: [Risk Reduction Behavior] explode all trees #44 "risk reduc*" #45 risk near reduc* #46 #40 or $41 or #42 or #43 or #44 or #45 #47 #7 and #39 and #46
Appendix 2. MEDLINE (via OvidSP)
| 1 | randomized controlled trial.pt. |
| 2 | controlled clinical trial.pt. |
| 3 | randomized.ab. |
| 4 | placebo.ab. |
| 5 | Clinical Trials as Topic/ |
| 6 | randomly.ab. |
| 7 | trial.ti. |
| 8 | (crossover or cross‐over).tw. |
| 9 | Pragmatic Clinical Trials as Topic/ |
| 10 | pragmatic clinical trial.pt. |
| 11 | or/1‐10 |
| 12 | exp Breast Neoplasms/ |
| 13 | (breast adj6 cancer$).tw. |
| 14 | (breast adj6 neoplasm$).tw. |
| 15 | (breast adj6 carcinoma$).tw. |
| 16 | (breast adj6 tumo?r$).tw. |
| 17 | or/12‐16 |
| 18 | exp Anticarcinogenic Agents/ |
| 19 | exp Selective Estrogen Receptor Modulators/ |
| 20 | selective estrogen receptor modulator.tw. |
| 21 | exp Tamoxifen/ |
| 22 | exp Raloxifene/ |
| 23 | (Tamoxifen or Raloxifene or lasofoxifene).tw. |
| 24 | exp Aromatase Inhibitors/ |
| 25 | aromatase inhibitor*.tw. |
| 26 | (exemestane or anastrozole or arimidex or femara or aromasin).tw. |
| 27 | exp Vitamin D/ |
| 28 | vitamin D.tw. |
| 29 | exp Aspirin/ |
| 30 | (aspirin or acetylsalicylic acid).tw. |
| 31 | exp Metformin/ |
| 32 | metformin.tw. |
| 33 | exp Hydroxymethylglutaryl‐CoA Reductase Inhibitors/ |
| 34 | ((Hydroxymethylglutaryl‐CoA or hydroxymethylglutaryl coenzyme a) and reductase inhibitor).tw. |
| 35 | exp Simvastatin/ |
| 36 | exp Pravastatin/ |
| 37 | (statin or simvastatin or tenivastatin or rosuvastatin or pravastatin or mevinolin or glenvastatin or fluindostatin or dalvastatin or crilvastatin or compactin or cerivastatin or bervastatin or atorvastatin).tw. |
| 38 | exp Diphosphonates/ |
| 39 | (biphosphonate$ or bisphosphanate$ or diphosphonate$ or diphosphanate$).tw. |
| 40 | exp Etidronic Acid/ |
| 41 | exp Clodronic Acid/ |
| 42 | exp Alendronate/ |
| 43 | (etidronate$ or clodronate$ or pamidronate$ or alendronate or risedronate$ or tiludronate$ or ibandronate$ or zoledronate$ or incadronate$ or olpadronate$ or neridronate$).tw. |
| 44 | RANK Ligand/ |
| 45 | (RANK ligand or denosumab or prolia or Xgeva).tw. |
| 46 | or/18‐45 |
| 47 | 11 and 17 and 46 |
| 48 | Animals/ not Humans/ |
| 49 | 47 not 48 |
| 50 | exp chemoprevention/ |
| 51 | (chemoprevent$ or chemoprophyla$).tw. |
| 52 | (prevent$ or prophyla$).tw. |
| 53 | exp Risk Reduction Behavior/ |
| 54 | risk reduc$.tw. |
| 55 | (risk adj6 reduc$).tw. |
| 56 | or/51‐55 |
| 57 | 49 and 56 |
Appendix 3. Embase (via OvidSP)
| 1 | Randomized controlled trial/ |
| 2 | Controlled clinical study/ |
| 3 | Random$.ti,ab. |
| 4 | randomization/ |
| 5 | intermethod comparison/ |
| 6 | placebo.ti,ab. |
| 7 | (compare or compared or comparison).ti. |
| 8 | ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. |
| 9 | (open adj label).ti,ab. |
| 10 | ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. |
| 11 | double blind procedure/ |
| 12 | parallel group$1.ti,ab. |
| 13 | (crossover or cross over).ti,ab. |
| 14 | ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. |
| 15 | (assigned or allocated).ti,ab. |
| 16 | (controlled adj7 (study or design or trial)).ti,ab. |
| 17 | (volunteer or volunteers).ti,ab. |
| 18 | human experiment/ |
| 19 | trial.ti. |
| 20 | or/1‐19 |
| 21 | exp breast/ |
| 22 | exp breast disease/ |
| 23 | (21 or 22) and exp neoplasm/ |
| 24 | exp breast tumor/ |
| 25 | exp breast cancer/ |
| 26 | exp breast carcinoma/ |
| 27 | (breast$ adj5 (neoplas$ or cancer$ or carcin$ or tumo$ or metasta$ or malig$)).ti,ab. |
| 28 | 23 or 24 or 25 or 26 or 27 |
| 29 | exp tamoxifen/ |
| 30 | exp raloxifene/ |
| 31 | exp lasofoxifene/ |
| 32 | exp selective estrogen receptor modulator/ |
| 33 | exp aromatase inhibitors/ |
| 34 | exp exemestane/ |
| 35 | exp letrozole/ |
| 36 | exp anastrazole/ |
| 37 | exp arimidex/ |
| 38 | exp femara/ |
| 39 | exp aromasin/ |
| 40 | (Tamoxifen or Raloxifene or lasofoxifene).tw. |
| 41 | (selective estrogen receptor modulator or SERM).tw. |
| 42 | (aromatase inhibitors or exemestane or letrozole or anastrazole or arimidex or femara or aromasin).tw. |
| 43 | exp vitamin D/ |
| 44 | exp acetylsalicylic acid/ |
| 45 | exp Metformin/ |
| 46 | (vitamin d or acetylsalicylic acid or aspirin or metformin).tw. |
| 47 | exp hydroxymethylglutaryl coenzyme A reductase inhibitor/ |
| 48 | exp tenivastatin/ |
| 49 | exp simvastatin/ |
| 50 | exp rosuvastatin/ |
| 51 | exp pravastatin/ |
| 52 | exp pitavastatin/ |
| 53 | exp mevinolin/ |
| 54 | exp glenvastatin/ |
| 55 | exp fluindostatin/ |
| 56 | exp dalvastatin/ |
| 57 | exp crilvastatin/ |
| 58 | exp compactin/ |
| 59 | exp cerivastatin/ |
| 60 | exp bervastatin/ |
| 61 | exp atorvastatin/ |
| 62 | (hydroxymethylglutaryl coenzyme a reductase inhibitor or statin or tenivastatin or simvastatin or rosuvastatin or pravastatin or pitavastatin or mevinolin or glenvastatin or fluindostatin or dalvastatin or crilvastatin or compactin or cerivastatin or bervastatin or atorvastatin).tw. |
| 63 | exp bisphosphonic acid derivative/ |
| 64 | exp etidronic acid/ |
| 65 | exp clodronic acid/ |
| 66 | exp pamidronic acid/ |
| 67 | exp alendronic acid/ |
| 68 | exp risedronic acid/ |
| 69 | exp tiludronic acid/ |
| 70 | exp ibandronic acid/ |
| 71 | exp zoledronic acid/ |
| 72 | exp incadronic acid/ |
| 73 | exp olpadronic acid/ |
| 74 | (bisphosph?nate* or biphosph?nate* or diphosph?nate* or etidron* or clodron* or pamidron* or alendron* or risedron* or tiludron* or ibandron* or zoledron* or incadron* or olpadron* or rank ligand or (rank and ligand) or denosumab or xgeva or prolia).tw. |
| 75 | or/29‐74 |
| 76 | exp chemoprophylaxis/ |
| 77 | (chemoprevent$ or chemoprophyla$).tw. |
| 78 | exp risk reduction/ |
| 79 | (prevent$ or prophyla$).tw. |
| 80 | risk reduc$.tw. |
| 81 | (risk adj6 reduc$).tw. |
| 82 | exp prevention/ |
| 83 | or/76‐82 |
| 84 | 20 and 28 and 75 and 83 |
| 85 | 1 or 2 |
| 86 | limit 85 to yr="1902 ‐ 2015" |
| 87 | 84 not (84 and 86) |
Appendix 4. WHO ICTRP
Basic searches:
1. breast cancer AND chemoprevention
2. breast cancer AND anticarcinogenic agent
3. breast cancer AND primary prevention
4. breast cancer AND cancer prevention
5. breast cancer AND carcinopreventive agent
6. breast cancer AND pharmacological prevention
7. breast cancer AND risk reducing agent
Advanced searches:
1. Title: 1. prevention OR prophylaxis OR prophylactic OR risk reducing OR risk reduction
Condition: breast cancer OR breast neoplasm
Intervention: tamoxifen OR raloxifene OR lasofoxifene OR selective estrogen receptor modulator OR SERM OR vitamin D OR aspirin OR acetylsalicylic acid OR metformin
Recruitment status: ALL
2. Title: prevention OR preventing OR preventive
Condition: breast cancer OR breast neoplasm
Intervention: aromatase inhibitors OR exemestane OR letrozole OR anastrazole OR arimidex OR femara OR aromasin
Recruitment status: ALL
3. Title: 1. prevention OR prophylaxis OR prophylactic OR risk reducing OR risk reduction
Condition: breast cancer OR breast neoplasm
Intervention: CoA reductase inhibitor OR statin
Recruitment status: ALL
4. Title: 1. prevention OR prophylaxis OR prophylactic OR risk reducing OR risk reduction
Condition: breast cancer OR breast neoplasm
Intervention: Denosumab OR Prolia OR Xgeva OR RANK ligand
Recruitment status: ALL
5. Title: 1. prevention OR prophylaxis OR prophylactic OR risk reducing OR risk reduction
Condition: breast cancer OR breast neoplasm
Intervention: Bisphosphonates OR Diphosphonates OR Zoledronate OR clodronate OR Alendronate OR Ibandronate OR Pamidronate OR Risedronate OR Tiludronate OR Incadronate OR Olpadronate OR Neridronate
Recruitment status: ALL
Appendix 5. Clinicaltrials.gov
Basic searches:
1. breast cancer AND chemoprevention
2. breast cancer AND anticarcinogenic agent
3. breast cancer AND risk reducing agent
Advanced searches:
1. Search terms: prevention OR prophylaxis OR prophylactic OR risk reducing OR risk reduction
Condition: breast cancer OR breast neoplasm
Intervention: tamoxifen OR raloxifene OR lasofoxifene OR selective estrogen receptor modulator OR SERM OR vitamin D or aspirin OR acetylsalicylic acid OR metformin
Recruitment: All studies
Study results: All studies
Study type: All studies
Gender: All studies
2. Search terms: prevention OR prophylaxis OR prophylactic OR risk reducing OR risk reduction
Condition: breast cancer OR breast neoplasm
Intervention: aromatase inhibitors OR exemestane OR letrozole OR anastrazole OR arimidex OR femara OR aromasin OR coenzyme a reductase inhibitor OR statin
Recruitment: All studies
Study results: All studies
Study type: All studies
Gender: All studies
3. Search terms: prevention OR prophylaxis OR prophylactic OR risk reducing OR risk reduction
Condition: breast cancer OR breast neoplasm
Intervention: Bisphosphonates OR Diphosphonates OR Denosumab OR Prolia OR Xgeva OR "RANK ligand"
Recruitment: All studies
Study results: All studies
Study type: All studies
Gender: All studies
4. Condition: breast cancer OR breast neoplasm
Intervention: Zoledronate OR "Zoledronic acid" OR clodronate OR "Clodronic acid" OR "Etidronic acid" OR Alendronate OR Ibandronate OR Pamidronate OR Risedronate OR Tiludronate OR Incadronate Olpadronate OR Neridronate
Recruitment: All studies
Study results: All studies
Study type: All studies
Gender: All studies
Data and analyses
Comparison 1. Tamoxifen versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall breast cancer incidence | 3 | 22832 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.62, 0.76] |
| 2 Severe toxicity | 2 | 20361 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.12, 1.47] |
| 3 Invasive breast cancer incidence | 3 | 22832 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.61, 0.77] |
Comparison 2. Aromatase inhibitors (AI) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall breast cancer incidence | 2 | 8424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.35, 0.63] |
| 1.1 Anastrozole vs placebo | 1 | 3864 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.33, 0.69] |
| 1.2 Exemestane vs placebo | 1 | 4560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.27, 0.77] |
| 2 Severe toxicity | 2 | 8352 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.09, 1.28] |
| 2.1 Anastrozole vs placebo | 1 | 3864 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.02, 1.28] |
| 2.2 Exemestane vs placebo | 1 | 4488 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.10, 1.36] |
| 3 Invasive breast cancer incidence | 2 | 8424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.32, 0.64] |
| 3.1 Anastrozole vs placebo | 1 | 3864 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.33, 0.77] |
| 3.2 Exemestane vs placebo | 1 | 4560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.17, 0.68] |
| 4 Overall toxicity | 2 | 8352 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [1.00, 1.04] |
| 4.1 Anastrozole vs placebo | 1 | 3864 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.98, 1.03] |
| 4.2 Exemestane vs placebo | 1 | 4488 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [1.01, 1.06] |
Comparison 3. Tamoxifen versus raloxifene.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall breast cancer incidence | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Severe toxicity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Invasive breast cancer incidence | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cuzick 2014.
| Methods | Multicenter, international, 2‐arm double‐blind RCT Role of anastrozole in preventing primary breast cancer Accrual time: February 2003 to January 2012 Mean follow‐up: 5 years |
|
| Participants | 3864 women in total 1920 women in anastrozole group 1944 women in placebo group Mean age (years): 59.5 Eligibility criteria: postmenopausal women at higher risk of breast cancer. In particular, women were deemed to be postmenopausal when they were ages ≥ 60 years; had had a bilateral oophorectomy; were ages < 60 years, but had a uterus and had had amenorrhea for ≥ 12 months; or were ages < 60 years, had no uterus, and had a concentration of follicle stimulating hormone > 30 IU/L. Entry criteria were designed to include women ages 45–60 years who had a relative risk of breast cancer that was at least 2 times higher than in the general population, those ages 60–70 years who had a risk that was ≥ 1.5 times higher, and those ages 40–44 years who had a risk that was 4 times higher. To be eligible, women had to meet ≥ 1 of the criteria. Exclusion criteria: premenopausal status; any previous diagnosis of breast cancer (except for estrogen receptor‐positive ductal carcinoma in situ diagnosed < 6 months previously and treated by mastectomy); any invasive cancer in the previous 5 years (except for non‐melanoma skin cancer or cervical cancer); present or previous use of SERMs for > 6 months (unless as part of IBIS‐I and treatment was completed ≥ 5 years before study entry); intention to continue hormone replacement therapy; prophylactic mastectomy; evidence of severe osteoporosis (T score < –4 or > 2 vertebral fractures); life expectancy < 10 years; psychologically unfit women or women with history of gluten or lactose intolerance. |
|
| Interventions | Group 1: anastrozole 1 mg/day for 5 years Group 2: placebo for 5 years |
|
| Outcomes | Primary outcome: breast cancer (invasive and in situ) incidence Secondary outcomes: estrogen receptor‐positive breast cancer, breast cancer mortality, other cancers, cardiovascular disease, fractures, adverse events, and deaths not due to breast cancer |
|
| Notes | Trial acronym: IBIS‐II | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible women were randomly assigned (1:1) by central computer allocation to either anastrozole or matching placebo. Randomisation was stratified by country and was done with randomly chosen randomisation blocks (size six, eight, or ten) to maintain balance." |
| Allocation concealment (selection bias) | Low risk | Quote: "Eligible women were randomly assigned (1:1) by central computer allocation to either anastrozole or matching placebo. Randomisation was stratified by country and was done with randomly chosen randomisation blocks (size six, eight, or ten) to maintain balance." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All IBIS‐II personnel, participants, and clinicians were masked to treatment allocation; only the trial statistician had access to unblinded data." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All IBIS‐II personnel, participants, and clinicians were masked to treatment allocation; only the trial statistician had access to unblinded data." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13 non‐eligible participants out of 3864 participants (0.33%), which should not have led to any attrition bias. |
| Selective reporting (reporting bias) | Low risk | No deviations from protocol (ISRCTN31488319). |
Cuzick 2015.
| Methods | Multicenter, international, 2‐arm double‐blind RCT Role of tamoxifen in preventing primary breast cancer Recruitment: April 1992 to March 2001 Median follow‐up: 16 years |
|
| Participants | 7154 women in total 3579 women in tamoxifen group 3575 women in placebo group Mean age (years): 49.9 Multicenter international trial (UK leading) Eligibility criteria: deemed at an increased risk of developing breast cancer based on a family history of breast cancer or abnormal benign breast disease; risk factors for breast cancer indicating at least a 2‐fold increased risk for the disease in women ages 45–70 years, whereas this risk needed to be higher than 2‐fold for women < 45 years of age. Women were defined as postmenopausal if they had 12 consecutive months of amenorrhea or had an oophorectomy. Menopausal hormone therapy use was allowed during the trial. Exclusion criteria: history of any invasive cancer (excluding skin cancer), DVT, pulmonary embolism, or who wanted to become pregnant |
|
| Interventions | Group 1: tamoxifen 20 mg/day for 5 years Group 2: placebo for 5 years |
|
| Outcomes | Primary endpoint: occurrence of any type of breast cancer (including ductal carcinoma in situ) Secondary endpoints: occurrence of invasive estrogen receptor‐positive breast cancer, all‐cause mortality, and adverse events |
|
| Notes | Trial acronym: IBIS‐I | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Balanced block randomization done centrally by the IBIS study group (with no external input) by telephone or fax. |
| Allocation concealment (selection bias) | Low risk | Balanced block randomization done centrally by the IBIS study group (with no external input) by telephone or fax. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All IBIS‐I personnel, participants, and clinicians were masked to treatment allocation and only the IBIS‐I trial statistician had access to unmasked data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7169 women were initially enrolled into the trial and randomly assigned to the 2 treatment groups. 15 (0.21%) women were subsequently found to be ineligible because of previous breast cancer diagnosis (9 of those assigned to placebo and 6 assigned to tamoxifen), leaving 7154 women in the trial. Therefore, attrition bias appeared unlikely. |
| Selective reporting (reporting bias) | Low risk | No deviations from protocol (ISRCTN91879928). |
Fisher 2005.
| Methods | 2‐arm RCT (designed as double blind and then was unblinded in 1998 when first results were reported and participants subsequently chose whether to remain on their allocated treatment or cross‐over) Role of tamoxifen in preventing primary breast cancer Recruitment: 1992–1997 Mean follow‐up: 74 months (6.16 years) |
|
| Participants | 13,388 women in total 6681 women in tamoxifen group 6707 women in placebo group Multicenter, USA Eligibility criteria: either ages ≥ 60 years or ages 35–59 years with a 5‐year predicted risk for breast cancer of ≥ 1.66%; or history of LCIS or atypical hyperplasia. Within 180 days before randomization, women had to have undergone a mammogram that was determined to be negative for breast cancer. Exclusion criteria: received estrogen or progesterone replacement therapy, oral contraceptives, or androgens within 3 months of randomization; history of DVT or pulmonary embolism. Mean age (years): 90% between 40 and 60:
|
|
| Interventions | Group 1: tamoxifen 20 mg/day for 5 years Group 2: placebo for 5 years |
|
| Outcomes | Primary outcome: breast cancer (invasive and in situ) incidence Secondary outcomes: adverse effects |
|
| Notes | Trial acronym: NSABP‐P1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization of participants was performed centrally in a double‐blind fashion by the NSABP Biostatistical Center. |
| Allocation concealment (selection bias) | Low risk | Details unreported, but this bias was unlikely to have occurred. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study was unblinded in 1998 when first results were reported and participants subsequently chose whether to remain on their allocated treatment or cross‐over. It was unclear whether or not this unblinding could have had any impact on final results. Quote: "Despite the potential bias caused by the unblinding of the P‐1 trial, the magnitudes of all beneficial and undesirable treatment effects of tamoxifen were similar to those initially reported, with notable reductions in breast cancer and increased risks of thromboembolic events and endometrial cancer." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Details unreported, but this bias was unlikely to have occurred. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13,388 randomized, 180 without follow‐up (1.34%), which made attrition bias unlikely. |
| Selective reporting (reporting bias) | Low risk | No deviations from protocol (protocol available in previous publication PMID 9747868; Fisher 1998). |
Goss 2011.
| Methods | Multicenter, international, 2‐arm, double‐blind RCT Role of exemestane in preventing primary breast cancer Recruitment: September 2004 to March 2010 Median follow‐up: 35 months (3.12 years) |
|
| Participants | 4560 women in total 2285 women in exemestane group 2275 women in placebo group Eligibility criteria: women ages ≥ 35 years; postmenopausal (ages > 50 years with no spontaneous menses for ≥ 12 months; or ≤ 50 years either with no spontaneous menses (amenorrheic) within 12 months of randomization (e.g. spontaneous or secondary to hysterectomy) and a follicle‐stimulating hormone level within the postmenopausal range or with prior bilateral oophorectomy)). In addition, women had ≥ 1 of the following risk factors: ages ≥ 60 years; Gail risk score > 1.66%; prior atypical ductal or lobular hyperplasia or LCIS on breast biopsy or prior ductal carcinoma in situ treated with mastectomy. Exclusion criteria: premenopausal, had prior invasive breast cancer or prior ductal carcinoma in situ treated with lumpectomy; known carriers of the BRCA1 or BRCA2 gene; or history of other malignancies. Median age (years): 62.5 |
|
| Interventions | Group 1: exemestane 25 mg/day (event‐driven duration: treatment was continued until event or maximum 5 years) Group 2: placebo |
|
| Outcomes | Primary outcome: incidence of invasive breast cancer Secondary endpoints: combined incidence of invasive and non‐invasive (ductal carcinoma in situ) breast cancer; incidence of receptor‐negative invasive breast cancer; incidence of combined atypical ductal hyperplasia, atypical lobular hyperplasia, and LCIS; number of clinical breast biopsies; clinical fractures; adverse cardiovascular events, including myocardial infarction or coronary heart disease that resulted in death; overall incidence of other cancers; adverse‐effect profile and safety; and health‐related and menopause‐specific quality of life |
|
| Notes | Trial acronym: NCIC CTG MAP.3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Dynamic minimization algorithm‐based randomization. |
| Allocation concealment (selection bias) | Low risk | Details unreported, but this bias was unlikely to have occurred. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind design. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Details unreported, but this bias was unlikely to have occurred. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After randomization, 15 women (6 taking exemestane and 9 taking placebo) were considered to be ineligible to continue with the study (0.33%), but are included in the primary intention‐to‐treat analysis as randomly assigned. It is unlikely this led to any bias. |
| Selective reporting (reporting bias) | Low risk | No deviations from protocol (NCT00083174). |
Powles 2007.
| Methods | Single‐center, 2‐arm, double‐blind RCT Role of tamoxifen in preventing primary breast cancer Recruitment: October 1986 to April 1996 Median follow‐up: 13 years and 2 months |
|
| Participants | 2494 women in total 1238 women in tamoxifen group 1233 women in placebo group Eligibility criteria: ≥ 1 first‐degree relative who was < 50 years when diagnosed with breast cancer, 1 first‐degree relative with bilateral breast cancer, or 1 first‐degree relative with breast cancer who was diagnosed at any age plus at least 1 other affected first‐ or second‐degree relative with breast cancer. Women with a history of a benign breast biopsy who had a first‐degree relative with breast cancer were also eligible. Exclusion criteria: history of any cancer, DVT, or pulmonary embolism; risk of pregnancy; or who using oral contraceptives. Median age (years): 47 |
|
| Interventions | Group 1: tamoxifen 20 mg/day for 8 years Group 2: placebo for 8 years |
|
| Outcomes | Primary outcome: breast cancer (invasive and in situ) incidence Secondary outcome: estrogen receptor‐positive breast cancer |
|
| Notes | 11 women in this study were reassigned to a treatment group in error. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | As per protocol (ISRCTN07027313). |
| Allocation concealment (selection bias) | Low risk | Details unreported, but this bias was unlikely to have occurred. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind design. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Participants, clinicians, and data‐processing staff have remained blinded to the treatment options throughout follow‐up." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2494 women enrolled, 23 women resulting non‐eligible (0.92%), which made attrition bias unlikely. |
| Selective reporting (reporting bias) | Low risk | No deviations from protocol (ISRCTN07027313). |
Vogel 2010.
| Methods | Multicenter, 2‐arm RCT in USA (mainly) and Canada (this trial was designed as double‐blind, but in April 2006 it was unblinded). Role of tamoxifen vs raloxifene in preventing primary breast cancer Recruitment: July 1999 to November 2004 Median follow‐up: 81 months (6.75 years) |
|
| Participants | 19,490 women in total 9736 women in tamoxifen group 9754 women in raloxifene group Eligibility criteria: postmenopausal women, ages ≥ 35 years, with 5‐year predicted breast cancer risk ≥ 1.66% were eligible for STAR. Risk determination based on Gail model, as modified and applied in the Breast Cancer Prevention Trial (BCPT P‐1). Participants also met the following criteria: not taking either tamoxifen or raloxifene, hormone therapy, oral contraceptives, or androgens for ≥ 3 months before randomization; not currently taking warfarin or cholestyramine; no history of stroke, transient ischemic attack, pulmonary embolism, or DVT; no atrial fibrillation, uncontrolled diabetes, or uncontrolled hypertension; no psychiatric condition that would interfere with adherence; performance status that would not restrict normal activity; and no history of previous malignancy except basal cell or squamous cell carcinoma of the skin, carcinoma in situ of the cervix, or LCIS of the breast. Age (years):
|
|
| Interventions | Group 1: tamoxifen 20 mg/day Group 2: raloxifene 60 mg/day |
|
| Outcomes | Primary endpoint: invasive breast cancer Secondary endpoints: endometrial cancer, in situ breast cancer, cardiovascular disease, stroke, pulmonary embolism, DVT, transient ischemic attack, osteoporotic fracture, cataracts, death, and quality of life; data on all other invasive cancers were collected prospectively. |
|
| Notes | Data were from the update of the original trial (Vogel 2006), which was initially scheduled to last until December 2005; the trial was unblinded in April 2006. Trial acronym: STAR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was accomplished using a biased‐coin minimization algorithm." |
| Allocation concealment (selection bias) | Low risk | The reported biased‐coin minimization algorithm should have guaranteed correct allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This trial (which started enrolling in 1999 and finished the enrolment in 2004) was designed and conducted as a double‐blind study. However, in April 2006, the trial was unblinded: it was unclear whether this unblinding affected the results, which were based on a cutoff date of 31 March 2009. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Details unreported, but this bias was unlikely to have occurred. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Of the originally randomized 19,747 women, 19,490 participated in the STAR follow‐up described here." Comment: due to loss to follow‐up (i.e. data were available for 98.7% of initially randomized participants: it was unclear if this loss may have caused any bias, also considering that these lost participants were evenly distributed between the 2 study arms). |
| Selective reporting (reporting bias) | Low risk | All outcomes planned to be investigated were reported. |
DVT: deep‐vein thrombosis; IBIS: International Breast Cancer Intervention Study; LCIS: lobular carcinoma in situ; NSABP‐P: National Surgical Adjuvant Breast and Bowel Project, Prevention; RCT: randomized controlled trial; SERM: selective estrogen receptor modulator; STAR: Study of Tamoxifen And Raloxifene.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ALLHAT 2002 | Investigated the protective effect of pravastatin. Breast cancer risk of men and women with risk factors for coronary heart disease was undefined. Main outcome: coronary heart disease (trial acronym: ALLHAT). |
| Archer 2009 | RCT investigating protective effect of bazedoxifene. Breast cancer risk of osteoporotic postmenopausal women was undefined. Main outcome: osteoporosis. |
| Avenell 2012 | RCT investigating protective effect of raloxifene. Breast cancer risk of postmenopausal women was undefined (trial acronym: RECORD). |
| Barrett‐Connor 2006 | RCT investigating protective effect of raloxifene. Breast cancer risk of postmenopausal women was undefined (trial acronym: RUTH). |
| Bolland 2011 | RCT investigating protective effect of calcium and vitamin D. Breast cancer risk of postmenopausal women was undefined (trial acronym: WHI) |
| Chlebowski 2008 | Duplicate of Prentice 2013. |
| Cook 2013 | RCT investigating the protective effect of aspirin. Breast cancer risk of healthy women ages ≥ 45 years was undefined. Main outcome: cardiovascular diseases (trial acronym: WHS). |
| Cummings 2008 | RCT investigating the protective effect of tibolone. Postmenopausal women were selected based on bone mineral density and not on breast cancer risk. Main outcome: bone fracture. |
| Cuzick 2002 | First report of Cuzick 2002, which was used in this review as the source of data from that trial. |
| DeCensi 2009 | RCT investigating the protective effect of tamoxifen and fenretinide. Most of the population of premenopausal women had breast cancer (77%). |
| DeCensi 2013 | RCT investigating the protective effect of tamoxifen. Postmenopausal women were taking hormone replacement therapy by design (trial acronym: HOT). |
| Downs 1998 | RCT investigating the protective effect of lovastatin. Breast cancer risk of men and women without cardiovascular disease was undefined. Main outcome: cardiovascular diseases (trial acronym: AFCAPS/TexCAPS). |
| Erdmann 2014 | RCT investigating the protective effect of pioglitazone. Breast cancer risk of men and women with diabetes was undefined. Main outcome: diabetes and cardiovascular diseases (trial acronym: PROactive). |
| Fisher 1998 | First report of Fisher 2005, which was used in this review as the source of data from that trial. |
| Grady 2008 | Duplicate of Barrett‐Connor 2006. |
| Hercberg 2004 | RCT investigating the protective effect of vitamin C, vitamin A, and vitamin E. Breast cancer risk of men and women was undefined. Main outcome: cardiovascular diseases and cancer (trials acronym: SUVIMAX). |
| Home 2009 | Duplicate of Home 2010. |
| Home 2010 | RCT investigating the protective effect of rosiglitazone, glibenclamide, and metformin. Breast cancer risk of men and women with diabetes was undefined. Main outcome: diabetes and cardiovascular diseases (trials acronym: ADOPT and RECORD). |
| HPSCG 2002 | RCT investigating the protective effect of simvastatin. Breast cancer risk of men and women at high risk of cardiovascular diseases was undefined. Main outcome: cardiovascular diseases. |
| Hue 2014 | RCTs investigating the protective effect of alendronate and zoledronate. Breast cancer risk of postmenopausal women was undefined. Main outcome: bone fracture (trials acronym: FIT and HORIZON‐PTF). |
| LaCroix 2010 | RCT investigating the protective effect of lasofoxifene. Breast cancer risk of postmenopausal women with osteoporosis was undefined. Main outcomes: bone fracture and cancer incidence (trial acronym: PEARL). |
| Lappe 2007 | RCT investigating the protective effect of calcium and vitamin D. Breast cancer risk of healthy postmenopausal women was undefined. Main outcome: bone fracture. |
| Lee 1999 | RCT investigating the protective effect of vitamin A. Breast cancer risk of healthy women ages ≥ 45 years was undefined. Main outcomes: cardiovascular diseases and cancer (trial acronym: WHS). |
| Lee 2005 | RCT investigating the protective effect of vitamin E. Breast cancer risk of healthy women ages ≥ 45 years was undefined. Main outcomes: cardiovascular diseases and cancer (trial acronym: WHS). |
| LIPID 1998 | RCT investigating the protective effect of pravastatin. Breast cancer risk of men and women with cardiovascular disease was undefined. Main outcome: cardiovascular diseases (trial acronym: LIPID) |
| López 2016 | RCT investigating the protective effect of 2 regimens of letrozole. Primary endpoint was suppression in serum estradiol levels at the end of letrozole intervention. Secondary endpoints included changes in serum estrone, testosterone, C‐telopeptide (marker of bone resorption), lipid profile, and quality of life following treatment. |
| Martino 2004 | RCT investigating the protective effect of raloxifene. Breast cancer risk of postmenopausal women with osteoporosis was undefined. Main outcome: bone fracture (trial acronym: MORE‐CORE). |
| Powles 2012 | RCT investigating the protective effect of arzoxifene. Breast cancer risk of postmenopausal women with osteoporosis was undefined. Main outcome: bone fracture. |
| Prentice 2013 | RCT investigating the protective effect of calcium and vitamin D. Breast cancer risk of postmenopausal women was undefined (trial acronym: WHI). |
| Sacks 1996 | RCT investigating the protective effect of pravastatin. Breast cancer risk of men and women with myocardial infarction was undefined. Main outcome: cardiovascular disease. |
| Shepherd 2002 | RCT investigating the protective effect of pravastatin. Breast cancer risk of men and women ages ≥ 70 years at high risk of cardiovascular disease was undefined. Main outcome: cardiovascular disease (trial acronym: PROSPER). |
| Strandberg 2004 | RCT investigating the protective effect of simvastatin. Breast cancer risk of men and women at high risk of cardiovascular disease was undefined. Main outcome: cardiovascular disease. |
| Trivedi 2003 | RCT investigating the protective effect of vitamin D. Breast cancer risk of healthy men and women ages ≥ 65 years was undefined. Main outcome: bone fracture. |
| Veronesi 2007 | RCT investigating the protective effect of tamoxifen. Breast cancer risk of healthy women who underwent hysterectomy was undefined. |
| Vogel 2006 | First report of Vogel 2010, which was used in this review as the source of data from that trial (trial acronym: STAR). |
ADOPT: A Diabetes Outcome Progression Trial; AFCAPS/TexCAPS: Air Force/Texas Coronary Atherosclerosis Prevention Study; ALLHAT: Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial; FIT: Fracture Intervention Trial; HOT: Hypertension Optimal Treatment Study; HORIZON‐PTF: Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly Pivotal Fracture Trial; LIPID: Long‐Term Intervention with Pravastatin in Ischaemic Disease; MORE‐CORE: Multiple Outcomes of Raloxifene Evaluation‐Continuing Outcomes Relevant to Evista; PEARL: Postmenopausal Evaluation and Risk‐reduction With Lasofoxifene; PROactive: Prospective Pioglitazone Clinical Trial In Macrovascular Events; PROSPER: Prospective Study Of Pravastatin In The Elderly At Risk; RCT: randomized controlled trial; RECORD: Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes; RUTH: Raloxifene Use for The Heart; STAR: Study of Tamoxifen And Raloxifene; SUVIMAX: Supplémentation en Vitamines et Minéraux Antioxydants; WHI: Women's Health Initiative; WHS: Women's Health Study.
Differences between protocol and review
We slightly amended the description of the primary and secondary outcomes in the protocol to align with all other sections of the review. That is, we amended: (a) 'efficacy: relative risk of incidence breast cancer (in situ and invasive carcinoma)' to 'overall breast cancer incidence', (b) 'toxicity: relative risk of severe adverse effects (i.e. grade 3 and 4 toxicities)' to 'severe toxicity (i.e. grade 3 and 4 toxicities), (c) 'efficacy: invasive breast cancer only' to 'invasive breast cancer incidence (excluding in situ carcinoma)' and (d) 'toxicity: overall toxicity (any grade toxicity)' to 'overall toxicity (any grade toxicity)'.
Contributions of authors
Draft the protocol: SM, AG, SP.
Study selection: SM, AG, SP.
Extract data from studies: SM, SP.
Enter data into Review Manager 5: SM, SP.
Carry‐out the analysis: SM.
Interpret the analysis: SM.
Draft the final review: SM, AG, SP.
Disagreement resolution: SM, AG, SP (by iteration, discussion, and consensus).
Update the review: SM, SP.
Declarations of interest
SM: none. AG: no competing interest associated with funding of travel to attend a national and international educational meeting or to provide expertise regarding Cancer Genetic counselling in Australia. SP: none.
New
References
References to studies included in this review
Cuzick 2014 {published data only}
- Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S, et al. Anastrozole for prevention of breast cancer in high‐risk postmenopausal women (IBIS‐II): an international, double‐blind, randomised placebo‐controlled trial. Lancet 2014;383(9922):1041‐8. [DOI] [PubMed] [Google Scholar]
Cuzick 2015 {published data only}
- Cuzick J, Sestak I, Cawthorn S, Hamed H, Holli K, Howell A, et al. Tamoxifen for prevention of breast cancer: extended long‐term follow‐up of the IBIS‐I breast cancer prevention trial. Lancet Oncology 2015;16(1):67‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 2005 {published data only}
- Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P‐1 study. Journal of the National Cancer Institute 2005;97(22):1652‐62. [DOI] [PubMed] [Google Scholar]
Goss 2011 {published data only}
- Goss PE, Ingle JN, Alés‐Martínez JE, Cheung AM, Chlebowski RT, Wactawski‐Wende J, et al. Exemestane for breast‐cancer prevention in postmenopausal women. New England Journal of Medicine 2011;364:2381‐91. [DOI] [PubMed] [Google Scholar]
Powles 2007 {published data only}
- Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty‐year follow‐up of the Royal Marsden randomized, double‐blinded tamoxifen breast cancer prevention trial. Journal of the National Cancer Institute 2007;99(4):283‐90. [DOI] [PubMed] [Google Scholar]
Vogel 2010 {published data only}
- Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P‐2 Trial: Preventing breast cancer. Cancer Prevention Research 2010;3(6):696‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
ALLHAT 2002 {published data only}
- ALLHAT Trial Investigators. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT‐LLT). JAMA 2002;288(23):2998‐3007. [DOI] [PubMed] [Google Scholar]
Archer 2009 {published data only}
- Archer DF, Pinkerton JV, Utian WH, Menegoci JC, Villiers TJ, Yuen CK, et al. Bazedoxifene, a selective estrogen receptor modulator: effects on the endometrium, ovaries, and breast from a randomized controlled trial in osteoporotic postmenopausal women. Menopause 2009;16(6):1109‐15. [DOI] [PubMed] [Google Scholar]
Avenell 2012 {published data only}
- Avenell A, MacLennan GS, Jenkinson DJ, McPherson GC, McDonald AM, Pant PR, et al. Long‐term follow‐up for mortality and cancer in a randomized placebo‐controlled trial of vitamin D(3)and/or calcium (RECORD trial). Journal of Clinical Endocrinology and Metabolism 2012;97(2):614‐22. [DOI] [PubMed] [Google Scholar]
Barrett‐Connor 2006 {published data only}
- Barrett‐Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. New England Journal of Medicine 2006;355(2):125‐37. [DOI] [PubMed] [Google Scholar]
Bolland 2011 {published data only}
- Bolland MJ, Grey A, Gamble GD, Reid IR. Calcium and vitamin D supplements and health outcomes: a reanalysis of the Women's Health Initiative (WHI) limited‐access data set. American Journal of Clinical Nutrition 2011;94(4):1144‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chlebowski 2008 {published data only}
- Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski‐Wend J, Rohan T, et al. Calcium plus vitamin D supplementation and the risk of breast cancer. Journal of the National Cancer Institute 2008;100(22):1581‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cook 2013 {published data only}
- Cook NR, Lee IM, Zhang SM, Moorthy MV, Buring JE. Alternate‐day, low‐dose aspirin and cancer risk: long‐term observational follow‐up of a randomized trial. Annals of Internal Medicine 2013;159(2):77‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cummings 2008 {published data only}
- Cummings SR, Ettinger B, Delmas PD, Kenemans P, Stathopoulos V, Verweij P, et al. The effects of tibolone in older postmenopausal women. New England Journal of Medicine 2008;359(7):697‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cuzick 2002 {published data only}
- Cuzick J, Forbes J, Edwards R, Baum M, Cawthorn S, Coates A, et al. First results from the International Breast Cancer Intervention Study (IBIS‐I): a randomised prevention trial. Lancet 2002;360(9336):817‐24. [DOI] [PubMed] [Google Scholar]
DeCensi 2009 {published data only}
- DeCensi A, Robertson C, Guerrieri‐Gonzaga A, Serrano D, Cazzaniga M, Mora S, et al. Randomized double‐blind 2 x 2 trial of low‐dose tamoxifen and fenretinide for breast cancer prevention in high‐risk premenopausal women. Journal of Clinical Oncology 2009;27(23):3749‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
DeCensi 2013 {published data only}
- DeCensi A, Bonanni B, Maisonneuve P, Serrano D, Omodei U, Varricchio C, et al. A phase‐III prevention trial of low‐dose tamoxifen in postmenopausal hormone replacement therapy users: the HOT study. Annals of Oncology 2013;24(11):2753‐60. [DOI] [PubMed] [Google Scholar]
Downs 1998 {published data only}
- Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998;279(20):1615‐22. [DOI] [PubMed] [Google Scholar]
Erdmann 2014 {published data only}
- Erdmann E, Song E, Spanheimer R, Troostenburg de Bruyn AR, Perez A. Observational follow‐up of the PROactive study: a 6‐year update. Diabetes, Obesity and Metabolism 2014;16(1):63‐74. [DOI] [PubMed] [Google Scholar]
Fisher 1998 {published data only}
- Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P‐1 study. Journal of the National Cancer Institute 1998;90(18):1371‐88. [DOI] [PubMed] [Google Scholar]
Grady 2008 {published data only}
- Grady D, Cauley JA, Geiger MJ, Kornitzer M, Mosca L, Collins P, et al. Reduced incidence of invasive breast cancer with raloxifene among women at increased coronary risk. Journal of the National Cancer Institute 2008;100(12):854‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hercberg 2004 {published data only}
- Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, et al. The SU.VI.MAX Study: a randomized, placebo‐controlled trial of the health effects of antioxidant vitamins and minerals. Archives of Internal Medicine 2004;164(21):2335‐42. [DOI] [PubMed] [Google Scholar]
Home 2009 {published data only}
- Home PD, Pocock SJ, Beck‐Nielsen H, Curtis PS, Gomis R, Hanefeld M, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open‐label trial. Lancet 2009;373(9681):2125‐35. [DOI] [PubMed] [Google Scholar]
Home 2010 {published data only}
- Home PD, Kahn SE, Jones NP, Noronha D, Beck‐Nielsen H, Viberti G. Experience of malignancies with oral glucose‐lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 2010;53(9):1838‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
HPSCG 2002 {published data only}
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet 2002;360(9326):7‐22. [DOI] [PubMed] [Google Scholar]
Hue 2014 {published data only}
- Hue TF, Cummings SR, Cauley JA, Bauer DC, Ensrud KE, Barrett‐Connor E, et al. Effect of bisphosphonate use on risk of postmenopausal breast cancer: results from the randomized clinical trials of alendronate and zoledronic acid. JAMA Internal Medicine 2014;174(10):1550‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
LaCroix 2010 {published data only}
- LaCroix AZ, Powles T, Osborne CK, Wolter K, Thompson DD, Allred DC, et al. Breast cancer incidence in the randomized PEARL trial of lasofoxifene in postmenopausal osteoporotic women. Journal of the National Cancer Institute 2010;102(22):1706‐15. [DOI] [PubMed] [Google Scholar]
Lappe 2007 {published data only}
- Lappe JM, Travers‐Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. American Journal of Clinical Nutrition 2007;85(6):1586‐91. [DOI] [PubMed] [Google Scholar]
Lee 1999 {published data only}
- Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH. Beta‐carotene supplementation and incidence of cancer and cardiovascular disease: the Women’s Health Study. Journal of the National Cancer Institute 1999;91(24):2102‐6. [DOI] [PubMed] [Google Scholar]
Lee 2005 {published data only}
- Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA 2005;294(1):56‐65. [DOI] [PubMed] [Google Scholar]
LIPID 1998 {published data only}
- LIPID Investigators. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long‐Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. New England Journal of Medicine 1998;339:1349‐57. [DOI] [PubMed] [Google Scholar]
López 2016 {published data only}
- López AM, Pruthi S, Boughey JC, Perloff M, Hsu CH, Lang JE, et al. Double‐blind, randomized trial of alternative letrozole dosing regimens in postmenopausal women with increased breast cancer risk. Cancer Prevention Research 2016;9(2):142‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martino 2004 {published data only}
- Martino S, Cauley JA, Barrett‐Connor E, Powles TJ, Mershon J, Disch D, et al. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. Journal of the National Cancer Institute 2004;96(23):1751‐61. [DOI] [PubMed] [Google Scholar]
Powles 2012 {published data only}
- Powles TJ, Diem SJ, Fabian CJ, Neven P, Wickerham DL, Cox DA, et al. Breast cancer incidence in postmenopausal women with osteoporosis or low bone mass using arzoxifene. Breast Cancer Research and Treatment 2012;134(1):299‐306. [DOI] [PubMed] [Google Scholar]
Prentice 2013 {published data only}
- Prentice RL, Pettinger MB, Jackson RD, Wactawski‐Wende J, Lacroix AZ, Anderson GL, et al. Health risks and benefits from calcium and vitamin D supplementation: Women's Health Initiative clinical trial and cohort study. Osteoporosis International 2013;24(2):567‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sacks 1996 {published data only}
- Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. New England Journal of Medicine 1996;335(14):1001‐9. [DOI] [PubMed] [Google Scholar]
Shepherd 2002 {published data only}
- Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002;360(9346):1623‐30. [DOI] [PubMed] [Google Scholar]
Strandberg 2004 {published data only}
- Strandberg TE, Pyorala K, Cook TJ. Mortality and incidence of cancer during 10‐year follow‐up of the Scandinavian Simvastatin Survival Study (4S). Lancet 2004;364(9436):771‐7. [DOI] [PubMed] [Google Scholar]
Trivedi 2003 {published data only}
- Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 2003;326(7387):469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Veronesi 2007 {published data only}
- Veronesi U, Maisonneuve P, Rotmensz N, Bonanni B, Boyle P, Viale G, et al. Tamoxifen for the prevention of breast cancer: late results of the Italian Randomized Tamoxifen Prevention Trial among women with hysterectomy. Journal of the National Cancer Institute 2007;99(9):727‐37. [DOI] [PubMed] [Google Scholar]
Vogel 2006 {published data only}
- Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P‐2 trial. JAMA 2006;295(23):2727‐41. [DOI] [PubMed] [Google Scholar]
Additional references
Advani 2014
- Advani P, Moreno‐Aspitia A. Current strategies for the prevention of breast cancer. Breast Cancer 2014;6:59‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahern 2014
- Ahern TP, Lash TL, Damkier P, Christiansen PM, Cronin‐Fenton DP. Statins and breast cancer prognosis: evidence and opportunities. Lancet Oncology 2014;15(10):e461‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alés‐Martínez 2015
- Alés‐Martínez JE, Ruiz A, Chacón JI, Lluch Hernández A, Ramos M, Córdoba O, et al. Preventive treatments for breast cancer: recent developments. Clinical & Translational Oncology 2015;17(4):257‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Behan 2015
- Behan LA, Amir E, Casper RF. Aromatase inhibitors for prevention of breast cancer in postmenopausal women: a narrative review. Menopause 2015;22(3):342‐50. [DOI] [PubMed] [Google Scholar]
Bosetti 2012
- Bosetti C, Rosato V, Gallus S, Cuzick J, Vecchia C. Aspirin and cancer risk: a quantitative review to 2011. Annals of Oncology 2012;23(6):1403‐15. [DOI] [PubMed] [Google Scholar]
Bucher 1997
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. [DOI] [PubMed] [Google Scholar]
Caldwell 2014
- Caldwell DM. An overview of conducting systematic reviews with network meta‐analysis. Systematic Reviews 2014;3:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campagnoli 2013
- Campagnoli C, Pasanisi P, Castellano I, Abbà C, Brucato T, Berrino F. Postmenopausal breast cancer, androgens, and aromatase inhibitors. Breast Cancer Research and Treatment 2013;139(1):1‐11. [DOI] [PubMed] [Google Scholar]
Carlson 2009
- Carlson RW, Allred DC, Anderson BO, Burstein HJ, Carter WB, Edge SB, et al. Breast cancer. Clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network 2009;7(2):122‐92. [DOI] [PubMed] [Google Scholar]
Chaimani 2013
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta‐analysis in STATA. PloS One 2013;8:e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chlebowski 2002
- Chlebowski RT, Col N, Winer EP, Collyar DE, Cummings SR, Vogel VG 3rd, et al. American Society of Clinical Oncology technology assessment of pharmacologic interventions for breast cancer risk reduction including tamoxifen, raloxifene, and aromatase inhibition. Journal of Clinical Oncology 2002;20(15):3328‐43. [DOI] [PubMed] [Google Scholar]
Chlebowski 2012
- Chlebowski RT, Col N. Bisphosphonates and breast cancer prevention. Anti‐Cancer Agents in Medicinal Chemistry 2012;12(2):144‐50. [DOI] [PubMed] [Google Scholar]
Chlebowski 2014
- Chlebowski RT. Current concepts in breast cancer chemoprevention. Polskie Archiwum Medycyny Wewnetrznej 2014;124(4):191‐9. [DOI] [PubMed] [Google Scholar]
Chumsri 2015
- Chumsri S. Clinical utilities of aromatase inhibitors in breast cancer. International Journal of Women's Health 2015;7:493‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cipriani 2013
- Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta‐analysis. Annals of Internal Medicine 2013;159(2):130‐7. [DOI] [PubMed] [Google Scholar]
Colditz 2014
- Colditz GA, Bohlke K. Priorities for the primary prevention of breast cancer. CA: A Cancer Journal for Clinicians 2014;64(3):186‐94. [DOI] [PubMed] [Google Scholar]
Crew 2015
- Crew KD. Addressing barriers to uptake of breast cancer chemoprevention for patients and providers. American Society of Clinical Oncology Education Book 2015;35:e50‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cummings 2009
- Cummings SR, Tice JA, Bauer S, Browner WS, Cuzick J, Ziv E, et al. Prevention of breast cancer in postmenopausal women: approaches to estimating and reducing risk. Journal of the National Cancer Institute 2009;101:384‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cuzick 2011
- Cuzick J, DeCensi A, Arun B, Brown PH, Castiglione M, Dunn B, et al. Preventive therapy for breast cancer: a consensus statement. Lancet Oncology 2011;12(5):496‐503. [DOI] [PubMed] [Google Scholar]
Cuzick 2013
- Cuzick J, Sestak I, Bonanni B, Costantino JP, Cummings S, DeCensi A, et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta‐analysis of individual participant data. Lancet 2013;381(9880):1827‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
De la Cruz 2014
- Cruz MS, Sarfaty M, Wender RC. An update on breast cancer screening and prevention. Primary Care 2014;41(2):283‐306. [DOI] [PubMed] [Google Scholar]
Den Hollander 2013
- Hollander P, Savage MI, Brown PH. Targeted therapy for breast cancer prevention. Frontiers in Oncology 2013;3(250):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Donnelly 2014
- Donnelly LS, Evans DG, Wiseman J, Fox J, Greenhalgh R, Affen J, et al. Uptake of tamoxifen in consecutive premenopausal women under surveillance in a high‐risk breast cancer clinic. British Journal of Cancer 2014;110(7):1681‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Euhus 2015
- Euhus DM, Diaz J. Breast cancer prevention. Breast Journal 2015;21(1):76‐81. [DOI] [PubMed] [Google Scholar]
Feldman 2014
- Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nature Reviews Cancer 2014;14(5):342‐57. [DOI] [PubMed] [Google Scholar]
Freedman 2015
- Freedman OC, Fletcher GG, Gandhi S, Mates M, Dent SF, Trudeau ME, et al. Adjuvant endocrine therapy for early breast cancer: a systematic review of the evidence for the 2014 Cancer Care Ontario systemic therapy guideline. Current Oncology 2015;22(Suppl 1):S95‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fuller 2015
- Fuller MS, Lee CI, Elmore JG. Breast cancer screening: an evidence‐based update. Medical Clinics of North America 2015;99(3):451‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gabriel 2012
- Gabriel EM, Jatoi I. Breast cancer chemoprevention. Expert Review of Anticancer Therapy 2012;12(2):223‐8. [DOI] [PubMed] [Google Scholar]
Gail 2015
- Gail MH. Twenty‐five years of breast cancer risk models and their applications. Journal of the National Cancer Institute 2015;107(5):pii: djv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gandini 2014
- Gandini S, Puntoni M, Heckman‐Stoddard BM, Dunn BK, Ford L, DeCensi A, et al. Metformin and cancer risk and mortality: a systematic review and meta‐analysis taking into account biases and confounders. Cancer Prevention Research 2014;7(9):867‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giammanco 2015
- Giammanco M, Majo D, Guardia M, Aiello S, Crescimannno M, Flandina C, et al. Vitamin D in cancer chemoprevention. Pharmaceutical Biology 2015;53(10):1399‐434. [DOI] [PubMed] [Google Scholar]
Gonçalves 2014
- Gonçalves AK, Dantas Florencio GL, Maisonnette de Atayde Silva MJ, Cobucci RN, Giraldo PC, Cote NM. Effects of physical activity on breast cancer prevention: a systematic review. Journal of Physical Activity & Health 2014;11(2):445‐54. [DOI] [PubMed] [Google Scholar]
Gradishar 2015
- Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. Breast cancer version 2.2015. Journal of the National Comprehensive Cancer Network 2015;13(4):448‐75. [DOI] [PubMed] [Google Scholar]
Gronich 2013
- Gronich N, Rennert G. Beyond aspirin‐cancer prevention with statins, metformin and bisphosphonates. Nature Reviews Clinical Oncology 2013;10(11):625‐42. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64(4):380‐2. [DOI] [PubMed] [Google Scholar]
Heemskerk‐Gerritsen 2015
- Heemskerk‐Gerritsen BA, Seynaeve C, Asperen CJ, Ausems MG, Collée JM, Doorn HC, et al. Breast cancer risk after salpingo‐oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. Journal of the National Cancer Institute 2015;107(5):pii: djv033. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2012
- Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta‐analysis: concepts and models for multi‐arm studies. Research Synthesis Methods 2012;3(2):98‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holmberg 2015
- Holmberg C. Decision making in the context of breast cancer chemoprevention: patient perceptions and the meaning of risk. American Society of Clinical Oncology Educational Book 2015;35:e59‐64. [DOI] [PubMed] [Google Scholar]
Howell 2014
- Howell A, Anderson AS, Clarke RB, Duffy SW, Evans DG, Garcia‐Closas M, et al. Risk determination and prevention of breast cancer. Breast Cancer Research 2014;16(5):446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobo‐Herrera 2014
- Jacobo‐Herrera NJ, Pérez‐Plasencia C, Camacho‐Zavala E, González GF, Urrutia EL, García‐Castillo V, et al. Clinical evidence of the relationship between aspirin and breast cancer risk (review). Oncology Reports 2014;32(2):451‐61. [DOI] [PubMed] [Google Scholar]
Kotsopoulos 2016
- Kotsopoulos J, Huzarski T, Gronwald J, Singer CF, Moller P, Lynch HT, et al. Bilateral oophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers. Journal of the National Cancer Institute 2016;109(1):pii: djw177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Landis‐Piwowar 2014
- Landis‐Piwowar KR, Iyer NR. Cancer chemoprevention: current state of the art. Cancer Growth Metastasis 2014;7:19‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Layeequr 2009
- Layeequr Rahman R, Crawford S. Chemoprevention Indication Score: a user‐friendly tool for prevention of breast cancer – pilot analysis. Breast 2009;18(5):289‐93. [DOI] [PubMed] [Google Scholar]
Lazzeroni 2011
- Lazzeroni M, Gandini S, Puntoni M, Bonanni B, Gennari A, DeCensi A. The science behind vitamins and natural compounds for breast cancer prevention. Getting the most prevention out of it. Breast 2011;20(Suppl 3):S36‐41. [DOI] [PubMed] [Google Scholar]
Lazzeroni 2013
- Lazzeroni M, DeCensi A. Breast cancer prevention by antihormones and other drugs: where do we stand?. Hematology/Oncology Clinics of North America 2013;27(4):657‐72. [DOI] [PubMed] [Google Scholar]
Lebovic 2010
- Lebovic GS, Hollingsworth A, Feig SA. Risk assessment, screening and prevention of breast cancer: a look at cost‐effectiveness. Breast 2010;19(4):260‐7. [DOI] [PubMed] [Google Scholar]
Litton 2012
- Litton JK, Arun BK, Brown PH, Hortobagyi GN. Aromatase inhibitors and breast cancer prevention. Expert Opinion on Pharmacotherapy 2012;13(3):325‐31. [DOI] [PubMed] [Google Scholar]
Liu 2012
- Liu Y, Zhao S, Chen W, Hu F, Zhu L, Zhang Q, et al. Bisphosphonate use and the risk of breast cancer: a meta‐analysis of published literature. Clinical Breast Cancer 2012;12(4):276‐81. [DOI] [PubMed] [Google Scholar]
Lostumbo 2010
- Lostumbo L, Carbine NE, Wallace J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD002748.pub3] [DOI] [PubMed] [Google Scholar]
Mirkin 2015
- Mirkin S, Pickar JH. Selective estrogen receptor modulators (SERMs): a review of clinical data. Maturitas 2015;80(1):52‐7. [DOI] [PubMed] [Google Scholar]
Mocellin 2015
- Mocellin S, Pilati P, Briarava M, Nitti D. Breast cancer chemoprevention: a network meta‐analysis of randomized controlled trials. Journal of the National Cancer Institute 2015;108(2):pii: djv318. [DOI] [PubMed] [Google Scholar]
Moyer 2013
- Moyer VA, U.S. Preventive Services Task Force. Medications to decrease the risk for breast cancer in women: recommendations from the U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine 2013;159(10):698‐708. [DOI] [PubMed] [Google Scholar]
Nazarali 2014
- Nazarali SA, Narod SA. Tamoxifen for women at high risk of breast cancer. Breast Cancer 2014;6:29‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nelson 2013
- Nelson HD, Smith ME, Griffin JC, Fu R. Use of medications to reduce risk for primary breast cancer: a systematic review for the U.S. Preventive Services Task Force. Annals of Internal Medicine 2013;158(8):604‐14. [DOI] [PubMed] [Google Scholar]
NICE 2017
- National Institute for health Care Excellence (NICE). Breast cancer risk category. https://www.nice.org.uk/guidance/cg164/chapter/recommendations#breast‐cancer‐risk‐category 2017.
Nichols 2014
- Nichols HB, DeRoo LA, Scharf DR, Sandler DP. Risk‐benefit profiles of women using tamoxifen for chemoprevention. Journal of the National Cancer Institute 2014;107(1):354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olin 2014
- Olin JL, Pierre M. Aromatase inhibitors in breast cancer prevention. Annals of Pharmacotherapy 2014;48(12):1605‐10. [DOI] [PubMed] [Google Scholar]
Onega 2014
- Onega T, Beaber EF, Sprague BL, Barlow WE, Haas JS, Tosteson AN, et al. Breast cancer screening in an era of personalized regimens: a conceptual model and National Cancer Institute initiative for risk‐based and preference‐based approaches at a population level. Cancer 2014;120(19):2955‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pace 2014
- Pace LE, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA 2014;311(13):1327‐35. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhodes 2015
- Rhodes KM, Turner RM, Higgins JP. Predictive distributions were developed for the extent of heterogeneity in meta‐analyses of continuous outcome data. Journal of Clinical Epidemiology 2015;68(1):52‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Romagnolo 2014
- Romagnolo AP, Romagnolo DF, Selmin OI. BRCA1 as target for breast cancer prevention and therapy. Anti‐Cancer Agents in Medicinal Chemistry 2014;15(1):4‐14. [DOI] [PubMed] [Google Scholar]
Ropka 2010
- Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta‐analysis. Journal of Clinical Oncology 2010;28(18):3090‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossi 2014
- Rossi RE, Pericleous M, Mandair D, Whyand T, Caplin ME. The role of dietary factors in prevention and progression of breast cancer. Anticancer Research 2014;34(12):6861‐75. [PubMed] [Google Scholar]
Rothwell 2012
- Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet 2012;379(9826):1591‐601. [DOI] [PubMed] [Google Scholar]
Salanti 2008
- Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Statistical Methods in Medical Research 2008;17(3):279‐301. [DOI] [PubMed] [Google Scholar]
Salanti 2011
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163‐71. [DOI] [PubMed] [Google Scholar]
Salanti 2014
- Salanti G, Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta‐analysis. Plos One 2014;9(7):e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Santa‐Maria 2013
- Santa‐Maria CA, Stearns V. Statins and breast cancer: future directions in chemoprevention. Current Breast Cancer Reports 2013;5(3):161‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schiavon 2013
- Schiavon G, Smith IE. Endocrine therapy for advanced/metastatic breast cancer. Hematology/Oncology Clinics of North America 2013;27(4):715‐36. [DOI] [PubMed] [Google Scholar]
Serrano 2015
- Serrano D, Lazzeroni M, Bonanni B. Cancer chemoprevention: much has been done, but there is still much to do. State of the art and possible new approaches. Molecular Oncology 2015;9(5):1008‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sestak 2015
- Sestak I, Cuzick J. Update on breast cancer risk prediction and prevention. Current Opinion in Obstetrics & Gynecology 2015;27(1):92‐7. [DOI] [PubMed] [Google Scholar]
Smith 2016
- Smith SG, Sestak I, Forster A, Partridge A, Side L, Wolf MS, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta‐analysis. Annals of Oncology 2016;27(4):575‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sostres 2014
- Sostres C, Gargallo CJ, Lanas A. Aspirin, cyclooxygenase inhibition and colorectal cancer. World Journal of Gastrointestinal Pharmacology and Therapeutics 2014;5(1):40‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
StataCorp 2009 [Computer program]
- StataCorp LP. Stata Statistical Software. Version Release 11. College Station (TX): StataCorp LP, 2009.
Stine 2014
- Stine JE, Bae‐Jump V. Metformin and gynecologic cancers. Obstetrics & Gynecological Survey 2014;69(8):477‐89. [DOI] [PubMed] [Google Scholar]
Stout 2014
- Stout NK, Lee SJ, Schechter CB, Kerlikowske K, Alagoz O, Berry D, et al. Benefits, harms, and costs for breast cancer screening after US implementation of digital mammography. Journal of the National Cancer Institute 2014;106(6):dju092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stubert 2014
- Stubert J, Dieterich M, Gerber B. Medical prevention of breast cancer. Breast Care 2014;9(6):391‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorat 2013
- Thorat MA, Cuzick J. Role of aspirin in cancer prevention. Current Oncology Reports 2013;15(6):533‐40. [DOI] [PubMed] [Google Scholar]
Torre 2015
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians 2015;65(2):87‐108. [DOI] [PubMed] [Google Scholar]
Turner 2012
- Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta‐analysis, using empirical data from the Cochrane Database of Systematic Reviews. International Journal of Epidemiology 2012;41(3):818‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tuttle 2010
- Tuttle TM, Abbott A, Arrington A, Rueth N. The increasing use of prophylactic mastectomy in the prevention of breast cancer. Current Oncology Reports 2010;12(1):16‐21. [DOI] [PubMed] [Google Scholar]
Veroniki 2013
- Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G. Evaluation of inconsistency in networks of interventions. International Journal of Epidemiology 2013;42(1):332‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Visvanathan 2013
- Visvanathan K, Hurley P, Bantug E, Brown P, Col NF, Cuzick J, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. Journal of Clinical Oncology 2013;31(23):2942‐62. [DOI] [PubMed] [Google Scholar]
Vogel 2015
- Vogel VG. Ongoing data from the breast cancer prevention trials: opportunity for breast cancer risk reduction. BMC Medicine 2015;13:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2015
- Wang W, Nag SA, Zhang R. Targeting the NFκB signaling pathways for breast cancer prevention and therapy. Current Medicinal Chemistry 2015;22(2):264‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2011
- White IR. Multivariate random‐effects meta‐regression: updates to mvmeta. Stata Journal 2011;11(2):255‐70. [Google Scholar]
White 2012
- White IR, Barrett JK, Jackson D, Higgins JPT. Consistency and inconsistency in network meta‐analysis: model estimation using multivariate meta‐regression. Research Synthesis Methods 2012;3(2):111‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2014
- Williams N, Harris LN. The renaissance of endocrine therapy in breast cancer. Current Opinion in Obstetrics & Gynecology 2014;26(1):41‐7. [DOI] [PubMed] [Google Scholar]
Wuttke 2015
- Wuttke M, Phillips KA. Clinical management of women at high risk of breast cancer. Current Opinion in Obstetrics & Gynecology 2015;27(1):6‐13. [DOI] [PubMed] [Google Scholar]
Yeo 2014
- Yeo B, Turner NC, Jones A. An update on the medical management of breast cancer. BMJ 2014;348:g3608. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Mocellin 2016
- Mocellin S, Goodwin A, Pasquali S. Risk‐reducing medication for primary breast cancer: a network meta‐analysis. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD012191] [DOI] [PMC free article] [PubMed] [Google Scholar]


