Abstract
Background
Active management of the third stage of labour reduces the risk of postpartum blood loss (postpartum haemorrhage (PPH)), and is defined as administration of a prophylactic uterotonic, early umbilical cord clamping and controlled cord traction to facilitate placental delivery. The choice of uterotonic varies across the globe and may have an impact on maternal outcomes. This is an update of a review first published in 2001 and last updated in 2013.
Objectives
To determine the effectiveness of prophylactic oxytocin to prevent PPH and other adverse maternal outcomes in the third stage of labour.
Search methods
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, WHO International Clinical Trials Registry Platform (ICTRP) (6 March 2019) and reference lists of retrieved studies.
Selection criteria
Randomised, quasi‐ or cluster‐randomised trials including women undergoing vaginal delivery who received prophylactic oxytocin during management of the third stage of labour. Primary outcomes were blood loss 500 mL or more after delivery, need for additional uterotonics, and maternal all‐cause mortality.
Data collection and analysis
Two review authors independently assessed trials for inclusion, extracted data, and assessed trial quality. Data were checked for accuracy. We assessed the quality of the evidence using the GRADE approach.
Main results
This review includes 24 trials, with 23 trials involving 10,018 women contributing data. Due to many trials assessed at high risk of bias, evidence grade ranged from very low to moderate quality.
Prophylactic oxytocin versus no uterotonics or placebo (nine trials) Prophylactic oxytocin compared with no uterotonics or placebo may reduce the risk of blood loss of 500 mL after delivery (average risk ratio (RR) 0.51, 95% confidence interval (C) 0.37 to 0.72; 4162 women; 6 studies; Tau² = 0.10, I² = 75%; low‐quality evidence), and blood loss 1000 mL after delivery (RR 0.59, 95% CI 0.42 to 0.83; 4123 women; 5 studies; low‐quality evidence). Prophylactic oxytocin probably reduces the need for additional uterotonics (average RR 0.54, 95% CI 0.36 to 0.80; 3135 women; 4 studies; Tau² = 0.07, I² = 44%; moderate‐quality evidence). There may be no difference in the risk of needing a blood transfusion in women receiving oxytocin compared to no uterotonics or placebo (RR 0.88, 95% CI 0.44 to 1.78; 3081 women; 3 studies; low‐quality evidence). Oxytocin may be associated with an increased risk of a third stage greater than 30 minutes (RR 2.55, 95% CI 0.88 to 7.44; 1947 women; 1 study; moderate‐quality evidence), however the confidence interval is wide and includes 1.0, indicating that there may be little or no difference. Prophylactic oxytocin versus ergot alkaloids (15 trials)
It is uncertain whether oxytocin reduces the likelihood of blood loss 500 mL (average RR 0.84, 95% CI 0.56 to 1.25; 3082 women; 10 studies; Tau² = 0.14, I² = 49%; very low‐quality evidence) or the need for additional uterotonics compared to ergot alkaloids (average RR 0.89, 95% CI 0.43 to 1.81; 2178 women; 8 studies; Tau² = 0.76, I² = 79%; very low‐quality evidence), because the quality of this evidence is very low. The quality of evidence was very low for blood loss of 1000 mL (RR 1.13, 95% CI 0.63 to 2.01; 1577 women; 3 studies; very low‐quality evidence), and need for blood transfusion (average RR 1.37, 95% CI 0.34 to 5.51; 1578 women; 7 studies; Tau² = 1.34, I² = 45%; very low‐quality evidence), making benefit of oxytocin over ergot alkaloids uncertain. Oxytocin probably increases the risk of a prolonged third stage greater than 30 minutes (RR 4.69, 95% CI 1.63 to 13.45; 450 women; 2 studies; moderate‐quality evidence), although it is uncertain if this translates into increased risk of manual placental removal (average RR 1.10, 95% CI 0.39 to 3.10; 3127 women; 8 studies; Tau² = 1.07, I² = 76%; very low‐quality evidence). Oxytocin may make little or no difference to risk of diastolic blood pressure > 100 mm Hg (average RR 0.28, 95% CI 0.04 to 2.05; 960 women; 3 studies; Tau² = 1.23, I² = 50%; low‐quality evidence), and is probably associated with a lower risk of vomiting (RR 0.09, 95% CI 0.05 to 0.14; 1991 women; 7 studies; moderate‐quality evidence), although the impact of oxytocin on headaches is uncertain (average RR 0.19, 95% CI 0.03 to 1.02; 1543 women; 5 studies; Tau² = 2.54, I² = 72%; very low‐quality evidence).
Prophylactic oxytocin‐ergometrine versus ergot alkaloids (four trials) Oxytocin‐ergometrine may slightly reduce the risk of blood loss greater than 500 mL after delivery compared to ergot alkaloids (RR 0.44, 95% CI 0.20 to 0.94; 1168 women; 3 studies; low‐quality evidence), based on outcomes from quasi‐randomised trials with a high risk of bias. There were no maternal deaths reported in either treatment group in the one trial that reported this outcome (RR not estimable; 1 trial, 807 women; moderate‐quality evidence). Need for additional uterotonics was not reported.
No subgroup differences were observed between active or expectant management, or different routes or doses of oxytocin for any of our comparisons.
Authors' conclusions
Prophylactic oxytocin compared with no uterotonics may reduce blood loss and the need for additional uterotonics. The effect of oxytocin compared to ergot alkaloids is uncertain with regards to blood loss, need for additional uterotonics, and blood transfusion. Oxytocin may increase the risk of a prolonged third stage compared to ergot alkaloids, although whether this translates into increased risk of manual placental removal is uncertain. This potential risk must be weighed against the possible increased risk of side effects associated with ergot alkaloids. Oxytocin‐ergometrine may reduce blood loss compared to ergot alkaloids, however the certainty of this conclusion is low. More high‐quality trials are needed to assess optimal dosing and route of oxytocin administration, with inclusion of important outcomes such as maternal mortality, shock, and transfer to a higher level of care. A network meta‐analysis of uterotonics for PPH prevention plans to address issues around optimal dosing and routes of oxytocin and other uterotonics.
Plain language summary
Oxytocin to prevent excessive blood loss for women during the third stage of labour
What is the issue?
Active management of the third stage of labour (AMTSL) has been shown to decrease the risk of excessive blood loss after delivery. This management strategy has been defined as administration of a medication to increase uterine tone and contractions, early umbilical cord clamping and gentle cord traction to facilitate placental delivery. While AMTSL has become standard practice in many countries and institutions, execution of the individual components varies. Oxytocin is a uterotonic medication that promotes increased uterine tone and contractions, and is commonly administered immediately following delivery of the infant's shoulder as part of AMTSL. This review considers the efficacy and safety of oxytocin prophylaxis in the third stage of labour compared with no uterotonics, a placebo, ergot alkaloids, and in combination with ergometrine compared with ergot alkaloids.
Why is this important?
Postpartum haemorrhage is one of the most prevalent causes of maternal morbidity and mortality worldwide, therefore, determining the most effective preventative strategies is crucial.
What evidence did we find?
We searched for evidence in March 2019 and identified six trials that met the inclusion criteria for the review. Outcomes from an additional 1100 women from these six trials was combined with those from the previous version of this review for a total of 10,018 women (23 trials). Of note, two previously included trials were excluded from this current review due to methodological concerns. The majority of trials contributing information to this review were found to be at high risk of bias. The quality of evidence ranged from very low to moderate, and for most outcomes was assessed as low to very low quality.
Our results showed that compared to no uterotonics or placebo, oxytocin may reduce the risk of blood loss (quality of evidence: low) and the need for additional uterotonics (quality of evidence: moderate). The effect of oxytocin compared with ergot alkaloids is uncertain with regards to blood loss (quality of evidence: very low), need for additional uterotonics (quality of evidence: very low), and need for blood transfusion (quality of evidence: very low), but may increase the risk of a third stage greater than 30 minutes (quality of evidence: moderate). Whether or not this translates into increased risk of needing a manual placental removal is uncertain (quality of evidence: very low). This potential risk of retained placenta must be weighed against a possible increased risk of side effects with ergot alkaloids, including diastolic hypertension (quality of evidence: low), vomiting (quality of evidence: very low), and headaches (quality of evidence: very low). While the combination of oxytocin and ergometrine may slightly reduce the risk of blood loss compared to ergot alkaloids (quality of evidence: low), the certainty of this conclusion is low given the poor quality of contributing studies.
What does this mean?
Oxytocin may reduce blood loss and the need for additional uterotonics when given prophylactically in the third stage of labour, and therefore could be considered as a component of AMTSL. The side‐effect profile may be more favourable than ergot alkaloids, which must be weighed against a possible increased risk of third stage greater than 30 minutes and unclear benefit of oxytocin or ergot alkaloids with regards to blood loss. More placebo‐controlled, randomised, double‐blinded trials are needed to improve the quality of data used to compare oxytocin versus ergot alkaloids. Future studies should aim to include important outcomes such as maternal mortality, shock, transfer to a higher level of care, serious side effects, and other patient‐centred outcomes. A large complex review analysing all available data from different uterotonic medications (network meta‐analysis) will help to inform future choice of uterotonic and the best route and dose of administration.
Summary of findings
Background
Description of the condition
The third stage of labour is defined as the time from the birth of the baby to expulsion of the placenta. Following delivery of the baby, the uterine muscle contracts, resulting in gradual placental separation and expulsion as well as contraction of uterine muscle around maternal vessels within the placental bed. Activation of the maternal coagulation system occurs in tandem. The degree of blood loss following delivery is most directly related to how quickly and efficiently these processes occur. Postpartum haemorrhage (PPH) has commonly been defined by the World Health Organization (WHO) and other expert authorities as blood loss of 500 mL or more from the genital tract within 24 hours of birth (Borovac‐Pinheiro 2018; WHO 2018). However, there is currently no single definition for PPH that has been agreed upon internationally, and multiple guidelines and definitions exist (Borovac‐Pinheiro 2018). A significant issue complicating the use of blood loss thresholds to define PPH is the challenge surrounding quantification of blood loss. Visual estimation has historically been standard practice, but has been shown to lead to underestimation of large volume blood loss by up to 30% to 50%, and overestimation of small volume blood loss regardless of level of training (Dildy 2004; Hogan 2010; Stafford 2008). Gravimetric methods of quantification, such as calibrated drapes and weighing of pads, may be more accurate and are being increasingly adopted by obstetric centres (Al Kadri 2011; Toledo 2007). The impact of any amount of blood loss for an individual woman may be modified by her overall health status, underlying medical conditions, haemodynamic status and access to healthcare resources. Women experiencing excessive blood loss after delivery are at increased risk of significant complications including the need for blood transfusion, hysterectomy and loss of fertility, prolonged hospitalisation, transfer to the intensive care unit, shock, and multi‐organ failure.
Description of the intervention
Techniques to prevent PPH may target any aspect of the third stage of labour. A recent review determined that active management of the third stage of labour (AMTSL), defined as prophylactic administration of a uterotonic, early umbilical cord clamping and controlled cord traction, decreases the risk of blood loss greater than 1000 mL (Begley 2019), although the evidence was found to be of very low quality. While AMTSL is recommended by many organisations and has become standard practice in most obstetric centres, performance of individual components varies. Based on recent evaluations of individual components of the AMTSL, the WHO regards controlled cord traction as optional, routine early cord clamping as generally contraindicated, and uterotonics as the main intervention that should be offered to all women in the third stage of labour (WHO 2012). The WHO and other authorities currently consider oxytocin to be the uterotonic of choice (WHO 2018). Of note, a recent network meta‐analysis examined the effectiveness and side effects of multiple uterotonic agents (Gallos 2018). They concluded that all uterotonics were effective for preventing PPH, but that ergometrine‐oxytocin and misoprostol plus oxytocin might be more efficacious than oxytocin alone, at the expense of a greater risk of side effects. They also concluded that carbetocin may be more effective than oxytocin without an increase in side effects.
Oxytocin is a naturally occurring uterotonic, and first became available for use in 1953 (Du Vigneaud 1953). Oxytocin binds to receptors within the myometrium to facilitate frequent and prolonged uterine contractions within minutes, with a short half‐life of two to four minutes. It may be administered intravenously (IV, typically diluted in an infusion) or intramuscularly (IM). When given in high volumes it may result in an anti‐diuretic effect leading to hyponatraemia, headache, vomiting, drowsiness or convulsions. It can be stored at room temperature for a limited period of time, although long‐term storage is recommended in the dark at four to eight degrees Celsius. Ergometrine was discovered as the uterotonic component of ergot in 1932 (Moir 1932). It became popular for routine management of PPH in the early 1950s (Moir 1955). Methylergometrine and ergometrine are the most commonly used ergot alkaloids. They bind to adrenergic myometrial receptors and increase uterine tone, leading to frequent and then sustained uterine contractions. The onset of action is rapid, within two to 10 minutes, with a half‐life of approximately three hours. They are most commonly administered by the IM or oral route, but can be given IV. Side effects include hypertension, nausea and emesis, and other side effects related to vasoconstriction of vascular smooth muscle. To prevent rapid deterioration, formulations need to be stored in the dark at four to eight degrees Celsius; ergot alkaloids are more unstable at room temperature and with light exposure than oxytocin. A combination of ergometrine and oxytocin (Syntometrine) was synthesised in 1963 (Embrey 1963). Syntometrine is comprised of oxytocin 5 international units (IU) and ergometrine 0.5 mg, and is typically given IM although can be given IV. The pharmacologic properties reflect those of its individual components. It is available in some countries, although not currently available in the USA.
How the intervention might work
Uterotonic drugs increase the tone of the uterine muscles, resulting in uterine contractions. These contractions produce shearing forces that aid in placental separation, and also result in myometrial contraction around the involuting placental bed. After placental separation, expulsion is assisted by continued contractions. By enhancing these mechanisms, uterotonics facilitate rapid placental delivery and contribute to minimising blood loss in the third stage.
Why it is important to do this review
Haemorrhage during childbirth is the leading cause of maternal mortality worldwide, accounting for approximately 25% of maternal deaths, or over half a million women in mostly low‐ and middle‐income countries (Say 2014). PPH accounts for a greater proportion of maternal deaths in countries with a low sociodemographic index (46%) compared to countries with a high sociodemographic index (9%), reflecting a combination of issues including variations in quality and access to care, and population risk factors (Kassebaum 2016; Say 2014). Continuing efforts to determine the most effective PPH prevention strategies are necessary to improve maternal health worldwide. The last version of this Cochrane Review of the prophylactic use of oxytocin for preventing PPH was in 2013. This updated review is needed to inform updated guidelines from the WHO on prevention of PPH, and adds to existing Cochrane systematic reviews examining the use of various medications as prophylaxis in the third stage of labour (Gallos 2018, Liabsuetrakul 2018; Novikova 2015; Tunçalp 2012).
Objectives
To determine the effectiveness of prophylactic oxytocin to prevent postpartum haemorrhage (PPH) and other adverse maternal outcomes in the third stage of labour.
Methods
Criteria for considering studies for this review
Types of studies
All randomised, cluster‐ or quasi‐randomised controlled trials comparing prophylactic oxytocin with another uterotonic (ergot alkaloids) or no uterotonic/placebo for the management of the third stage of labour were considered for inclusion. Studies reported as abstracts have not been included if there was insufficient information for data extraction and 'Risk of bias' assessment.
Types of participants
All trials including pregnant women anticipating a vaginal delivery were considered. Studies where participants received the prophylactic uterotonic after delivery of the placenta were excluded.
Types of interventions
The purpose of this review is to compare three interventions:
use of prophylactic oxytocin at any dose for the third stage of labour versus no uterotonics or placebo;
use of prophylactic oxytocin at any dose for the third stage of labour versus ergot alkaloids;
use of prophylactic oxytocin‐ergometrine (Synometrine) versus ergot alkaloids.
The current review concentrates on oxytocin given by injection into a maternal vein (IV) or muscle (IM). Other uterotonic agents administered to the mother by IV or IM are addressed in Gallos 2018 (all uterotonics) and Su 2012 (carbetocin, oxytocin and oxytocin‐ergometrine). The role of prophylactic prostaglandins or ergot alkaloids and uterotonics given through the umbilical vein, for the treatment of blood loss or retained placenta, are the subjects of other reviews and were not included here (Liabsuetrakul 2018; Mori 2012; Tunçalp 2012). Similarly, endogenous oxytocin (nipple stimulation) is not included in this review.
Types of outcome measures
Outcomes noted with an asterisk are core prevention of PPH outcomes. Outcomes in bold text are the main outcomes that were assessed using the GRADE approach.
Primary outcomes
Blood loss 500 mL or more after delivery*
Need for additional uterotonics*
Maternal all‐cause mortality
Secondary outcomes
Blood loss 1000 mL or more after delivery*
Blood transfusion
Third stage greater than 30 minutes
Diastolic blood pressure > 100 mm Hg between delivery of the baby and discharge from the labour ward
Mean blood loss (mL)
Maternal haemoglobin (Hb) < 7 g/dL 24 to 48 hours postpartum
Mean length of third stage (minutes)
Manual removal of the placenta
Vomiting between delivery of the baby and discharge from the labour ward*
Headache between delivery of the baby and discharge from the labour ward*
Shock*
Transfer to a higher level of care*
Mortality from causes other than bleeding
Maternal satisfaction with therapy*
Quality of life*
Breastfeeding
Search methods for identification of studies
The following search methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (6 March 2019). The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, the Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (6 March 2019) using the search methods described in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeWesthoff 2013.
For this update, the following methods were used for assessing the reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy. When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
We assessed the quality of evidence using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence. Where data were available, we planned to use GRADE to assess the overall quality of the evidence for our main comparisons.
Prophylactic oxytocin at any dose for the third stage of labour versus placebo.
Prophylactic oxytocin at any dose for the third stage of labour versus ergot alkaloids.
Prophylactic oxytocin‐ergometrine versus ergot alkaloids.
We assessed the following outcomes.
Blood loss 500 mL or more after delivery
Need for additional uterotonics
Maternal all‐cause mortality
Blood loss 1000 mL or more after delivery
Blood transfusion
Third stage greater than 30 minutes
Diastolic blood pressure > 100 mm Hg between delivery of the baby and discharge from the labour ward
Grade outcomes are included in our review 'Summary of Findings' tables: Table 1; Table 2; Table 3. We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was be produced using the GRADE approach as outlined in the GRADE handbook. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Summary of findings for the main comparison. Oxytocin compared to no uterotonics or placebo for the third stage of labour to prevent postpartum haemorrhage.
| Oxytocin compared to no uterotonics or placebo for the third stage of labour to prevent postpartum haemorrhage | ||||||
| Patient or population: women in the third stage of labour Setting: hospital labour wards and home births in France, Germany, the Netherlands, Sweden, South Africa, Tunisia, and the UK Intervention: oxytocin Comparison: no uterotonics or placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no uterotonics | Risk with oxytocin | |||||
| Blood loss 500 mL or more after delivery | Study population | RR 0.51 (0.37 to 0.72) | 4162 (6 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 239 per 1000 | 122 per 1000 (89 to 172) | |||||
| Need for additional uterotonics | Study population | RR 0.54 (0.36 to 0.80) | 3135 (4 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | ||
| 114 per 1000 | 62 per 1000 (41 to 91) | |||||
| Maternal all‐cause mortality | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in any of the included studies. |
| Blood loss 1000 mL or more after delivery | Study population | RR 0.59 (0.42 to 0.83) | 4123 (5 RCTs) | ⊕⊕⊝⊝ LOW 1 4 | ||
| 48 per 1000 | 29 per 1000 (20 to 41) | |||||
| Blood transfusion | Study population | RR 0.88 (0.44 to 1.78) | 3081 (3 RCTs) | ⊕⊕⊝⊝ LOW 5 6 | ||
| 12 per 1000 | 10 per 1000 (5 to 21) | |||||
| Third stage greater than 30 minutes | Study population | RR 2.55 (0.88 to 7.44) | 1947 (1 RCT) | ⊕⊕⊕⊝ MODERATE 4 | ||
| 6 per 1000 | 16 per 1000 (5 to 45) | |||||
| Diastolic blood pressure > 100 mm Hg between delivery of the baby and discharge from the labour ward | Study population | ‐ | ‐ | ‐ | This outcome was not reported in any of the included studies. | |
| ‐ | ‐ | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Lack of blinding in the majority of trials raises concern for biased outcome assessment as most trials utilised visual estimation of blood loss (risk of bias ‐1)
2 Large variations in effect and non‐overlapping 95% confidence intervals; I2 = 75% indicating substantial heterogeneity (inconsistency ‐1)
3 Lack of participant blinding in some trials may bias decisions to administer additional uterotonics (risk of bias ‐1)
4 Few events and wide 95% confidence intervals (imprecision ‐1)
5 Lack of personnel blinding may bias decisions to administer blood transfusions (risk of bias ‐1)
6 Wide 95% confidence intervals including line of no effect (imprecision ‐1)
Summary of findings 2. Oxytocin compared to ergot alkaloids for the third stage of labour to prevent postpartum haemorrhage.
| Oxytocin compared to ergot alkaloids for the third stage of labour to prevent postpartum haemorrhage | ||||||
| Patient or population: women in the third stage of labour Setting: hospital labour wards and home births in the Netherlands, Sweden, South Africa, Japan, Singapore, India, Nepal, Tunisia, Nigeria, New Zealand, the UK and the USA Intervention: oxytocin Comparison: ergot alkaloids | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with ergot alkaloids | Risk with oxytocin | |||||
| Blood loss 500 mL or more after delivery | Study population | RR 0.84 (0.56 to 1.25) | 3082 (10 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 116 per 1000 | 97 per 1000 (65 to 145) | |||||
| Need for additional uterotonics | Study population | RR 0.89 (0.43 to 1.81) | 2178 (8 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 3 4 | ||
| 94 per 1000 | 84 per 1000 (40 to 170) | |||||
| Maternal all‐cause mortality | Study population | ‐ | ‐ | ‐ | This outcome was not reported in any of the included studies. | |
| ‐ | ‐ | |||||
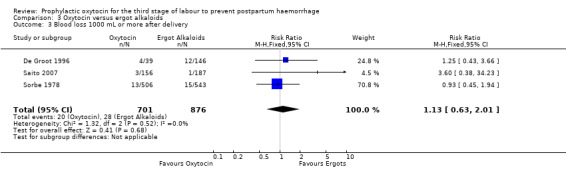
| Blood loss 1000 mL or more after delivery | Study population | RR 1.13 (0.63 to 2.01) | 1577 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 32 per 1000 | 36 per 1000 (20 to 64) | |||||
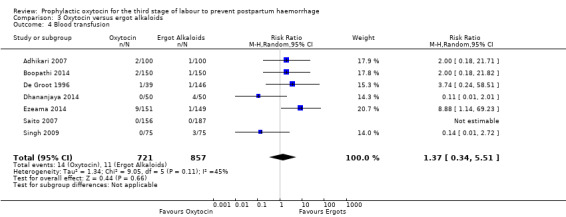
| Blood transfusion | Study population | RR 1.37 (0.34 to 5.51) | 1578 (7 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 5 6 | ||
| 13 per 1000 | 18 per 1000 (4 to 71) | |||||
| Third stage > 30 minutes | Study population | RR 4.69 (1.63 to 13.45) | 450 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 7 | ||
| 18 per 1000 | 84 per 1000 (29 to 240) | |||||
| Diastolic blood pressure > 100 mm Hg between delivery of the baby and discharge from the labour ward | Study population | RR 0.28 (0.04 to 2.05) | 960 (3 RCTs) | ⊕⊕⊝⊝ LOW 6 8 | ||
| 44 per 1000 | 12 per 1000 (2 to 90) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Studies with serious methodological limitations in multiple domains (risk of bias ‐2)
2 Wide 95% confidence intervals including line of no effect (imprecision ‐1)
3 Studies with serious methodological limitations (risk of bias ‐1)
4 Large variations in effect and non‐overlapping 95% confidence intervals; I2 = 79% indicating substantial heterogeneity (inconsistency ‐1)
5 Large variations in effect; I2 = 45% indicating substantial heterogeneity (inconsistency ‐1)
6 Wide 95% confidence intervals including line of no effect, and few events (imprecision ‐1)
7 Wide 95% confidence interval and few events, including one study with no events (imprecision ‐1)
8 Unclear allocation concealment in majority of studies (risk of bias ‐1)
Summary of findings 3. Oxytocin + ergometrine compared to ergot alkaloids for the third stage of labour to prevent postpartum haemorrhage.
| Oxytocin + ergometrine compared to ergot alkaloids for the third stage of labour to prevent postpartum haemorrhage | ||||||
| Patient or population: women in the third stage of labour Setting: hospital labour wards in Sweden, Singapore, Tunisia, and the UK Intervention: oxytocin + ergometrine Comparison: ergot alkaloids | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with ergot alkaloids | Risk with oxytocin + ergometrine | |||||
| Blood loss 500 mL or more after delivery | Study population | RR 0.44 (0.20 to 0.94) | 1168 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 37 per 1000 | 16 per 1000 (7 to 34) | |||||
| Need for additional uterotonics | Study population | ‐ | ‐ | ‐ | This outcome was not reported in any of the included studies. | |
| ‐ | ‐ | |||||
| Maternal all‐cause mortality | Study population | not estimable | 807 (1 RCT) | ⊕⊕⊕⊝ MODERATE 3 | It was not possible to obtain effect estimates as there were no events reported. | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Blood loss 1000 mL or more after delivery | Study population | ‐ | ‐ | ‐ | This outcome was not reported in any of the included studies. | |
| ‐ | ‐ | |||||
| Blood transfusion | Study population | ‐ | ‐ | ‐ | This outcome was not reported in any of the included studies. | |
| ‐ | ‐ | |||||
| Third stage > 30 minutes | Study population | ‐ | ‐ | ‐ | This outcome was not reported in any of the included studies. | |
| ‐ | ‐ | |||||
| Diastolic blood pressure > 100 mm Hg between delivery of the baby and discharge from the labour ward | Study population | ‐ | ‐ | ‐ | This outcome was not reported in any of the included studies. | |
| ‐ | ‐ | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Lack of blinding of estimated outcome assessment, and concern regarding randomisation methods (risk of bias ‐1)
2 Few events and wide 95% confidence intervals (imprecision ‐1)
3 No events reported for this outcome (imprecision ‐1)
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. In future, if necessary, we plan to use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
There were no cluster‐randomised or cross‐over trials included in this review. Three of the included studies had more than two treatment groups (De Groot 1996; Ilancheran 1990; Vaughan Williams 1974). For these studies, we included each pair‐wise comparison separately, but divided shared groups approximately equally amongst the comparisons according to the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (section 16.5.4).
Dealing with missing data
For included studies, levels of attrition were noted. In future updates, if more eligible studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis. For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 40% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
Meta‐analyses containing 10 or more studies were investigated using funnel plots to assess reporting bias. Funnel plot asymmetry was assessed visually. If asymmetry was suggested by a visual assessment, we considered exploratory analyses to investigate it where appropriate.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. Where we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, used random‐effects analysis to produce it.
We carried out the following subgroup analyses.
Management of the third stage: use of oxytocin with or without active management of the third stage of labour (AMTSL)
Route of administration: oxytocin given IV versus IM.
Dose of administration: oxytocin at a dose of at least 10 IU versus less than 10 IU
The following outcomes were used in subgroup analyses.
Blood loss 500 mL or more after delivery
Need for additional uterotonics
Maternal all‐cause mortality
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of risk of bias assessed by concealment of allocation, high attrition rates, or both, with studies at high risk of bias for these domains being temporarily excluded from the analyses in order to assess whether this makes any difference to the overall result.
Results
Description of studies
Results of the search
See Figure 1. The updated search in March 2019 search retrieved 27 trial reports. Of the 27 trials, six met the inclusion criteria for the review, 10 were excluded (12 reports), four are awaiting further classification (five reports), and two trials are ongoing. We added two new reports to already excluded studies. We also reassessed and excluded two studies that were previously included because we assessed that they were not randomised. This updated review includes six new randomised trials, for a total of 23 trials included in the meta‐analyses.
1.
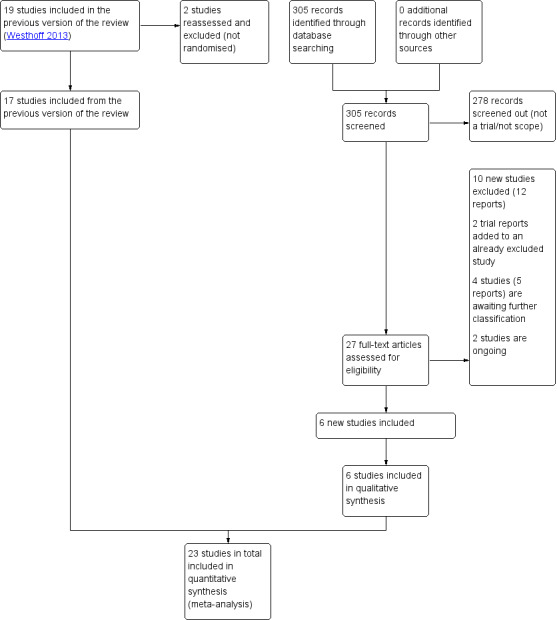
Study flow diagram
Included studies
Methods and setting
This review includes 24 trials, with a total of 10,018 women participating in the 23 included randomised studies comparing oxytocin versus no uterotonics or placebo, oxytocin versus ergot alkaloids, or oxytocin and ergometrine versus ergot alkaloids. Seven of the 23 included trials were deemed to be quasi‐randomised (Adhikari 2007; Boopathi 2014; Dhananjaya 2014; Francis 1965; Pierre 1992; Saito 2007; Sorbe 1978). This review includes trials from low‐, middle‐, and high‐income countries. Nearly all births were attended by midwives or physicians in birth centres or hospitals, although one trial included women who had a home birth attended by an independent midwife (De Groot 1996). Trials were conducted in Egypt and South Africa (Abdel‐Aleem 2010), Nepal (Adhikari 2007), India (Boopathi 2014; Dhananjaya 2014; Modi 2014; Singh 2009), Tunisia (Jerbi 2007), and Nigeria (Ezeama 2014; Jago 2007; Orji 2008). The remainder of the trials were conducted in high‐income countries including France (Pierre 1992), Germany (Bader 2000), Japan (Saito 2007), the Netherlands (De Groot 1996; Poeschmann 1991), New Zealand (Moodie 1976), Singapore (Ilancheran 1990), Sweden (Nordstrom 1997; Sorbe 1978), the UK (Bonham 1963; Francis 1965; Vaughan Williams 1974), and the USA (McGinty 1956).
Dates of study and sources of trial funding
The trial reports spanned 1956 to 2014. Dates of study were reported for most trials except in five trials where the dates were not explicitly stated (Ilancheran 1990; McGinty 1956; Moodie 1976; Singh 2009; Vaughan Williams 1974). Sources of funding included the County Council and County Health Authority Research and Development Foundation in the County of Jamtland, Sweden (Nordstrom 1997). Methergine was provided by Sandoz Pharmaceuticals (McGinty 1956), and Sulprostone provided by Schering‐Plough B.V. (Poeschmann 1991). Please see the table Characteristics of included studies for further details.
Participants
All participants in this study delivered vaginally. Instrumental deliveries were exclusion criteria in many studies (Adhikari 2007; Bader 2000; Bonham 1963; Boopathi 2014; De Groot 1996; Francis 1965; Jago 2007; Singh 2009), but not explicitly stated as exclusion criteria in the remaining studies. One study included only women with forceps or vacuum delivery (Moodie 1976). Full‐term pregnancies were an inclusion criteria in six studies (Adhikari 2007; Ilancheran 1990; Jerbi 2007; Modi 2014; Poeschmann 1991; Singh 2009), while two studies included women at a gestational age of 28 weeks or greater (Dhananjaya 2014; Ezeama 2014). Gestational age criteria were not explicitly stated in the remainder of the studies.
The majority of studies excluded women with multiple gestations (Adhikari 2007; Bader 2000; Bonham 1963; Boopathi 2014; De Groot 1996; Ezeama 2014; Francis 1965; Jago 2007; Jerbi 2007; Modi 2014; Moodie 1976; Nordstrom 1997; Pierre 1992; Poeschmann 1991; Saito 2007; Singh 2009). Women who received oxytocin during the course of labour were excluded in six studies (Bader 2000; Bonham 1963; De Groot 1996; Francis 1965; Saito 2007; Singh 2009).
Many studies attempted to account for postpartum haemorrhage risk by excluding women with risk factors including grand multiparity (Bonham 1963; Jerbi 2007; Modi 2014; Saito 2007), history of postpartum haemorrhage (Bonham 1963; Dhananjaya 2014; Francis 1965; Jerbi 2007; Orji 2008; Saito 2007), and blood coagulation disorders (Boopathi 2014; Dhananjaya 2014; Modi 2014). Anticoagulation therapy was considered an exclusion criteria in four studies (De Groot 1996; Jago 2007; Jerbi 2007; Saito 2007). Several studies did not explicitly state exclusion criteria but excluded women with complications or factors associated with increased blood loss (Adhikari 2007; Bader 2000; Vaughan Williams 1974). See Characteristics of included studies for details.
Interventions and comparisons
Nine trials compared oxytocin versus no uterotonics (Abdel‐Aleem 2010; Bader 2000; Ilancheran 1990; Jerbi 2007; Pierre 1992; Vaughan Williams 1974 or placebo (De Groot 1996; Nordstrom 1997; Poeschmann 1991). The oxytocin was administered immediately after delivery of either the baby or the anterior shoulder, and we excluded any trials where administration was given after placental delivery. Oxytocin was given IV (Bader 2000; Ilancheran 1990; Jerbi 2007; Nordstrom 1997; Pierre 1992) and IM (Abdel‐Aleem 2010; De Groot 1996; Poeschmann 1991; Vaughan Williams 1974), at doses ranging from 3 to 5 IU (Bader 2000; De Groot 1996; Jerbi 2007; Pierre 1992; Poeschmann 1991; Vaughan Williams 1974) up to 10 IU (Abdel‐Aleem 2010; Nordstrom 1997), with one trial not explicitly stating the medication dosage (Ilancheran 1990). The comparison group varied amongst the trials, with some comparing oxytocin with expectant management (Bader 2000; De Groot 1996; Nordstrom 1997) or administration of a normal saline placebo (Poeschmann 1991), and others comparing oxytocin with active management alone (Abdel‐Aleem 2010; Jerbi 2007; Pierre 1992; Vaughan Williams 1974). Abdel‐Aleem 2010 had three intervention groups where women received oxytocin, oxytocin with uterine massage, or uterine massage as part of active management alone. For our analysis we combined the first two groups into the oxytocin intervention group, which we felt was acceptable because other included trials applied active management including uterine massage to both intervention and placebo groups.
Fifteen trials compared oxytocin with ergot alkaloids (Adhikari 2007; Boopathi 2014; De Groot 1996; Dhananjaya 2014; Ezeama 2014; Ilancheran 1990; Jago 2007; McGinty 1956; Modi 2014; Moodie 1976; Orji 2008; Saito 2007; Singh 2009; Sorbe 1978; Vaughan Williams 1974). Oxytocin was administered by IV in eight of the trials (Ilancheran 1990; Jago 2007; McGinty 1956; Moodie 1976; Orji 2008; Singh 2009; Sorbe 1978; Vaughan Williams 1974), and by IM route in the remaining trials, at either 5 IU (De Groot 1996; McGinty 1956; Moodie 1976; Saito 2007; Singh 2009) or 10 IU doses. Eight studies used ergometrine either orally (De Groot 1996), IV (Ilancheran 1990; McGinty 1956; Moodie 1976; Orji 2008; Sorbe 1978; Vaughan Williams 1974) or IM route (Ezeama 2014; Jago 2007). The remaining studies used methylergometrine by IV (Boopathi 2014; Modi 2014; Singh 2009) or IM route (Adhikari 2007; Dhananjaya 2014; Saito 2007); all at a 0.2 mg dose. Doses of IV or IM ergometrine ranged from 0.2 mg (McGinty 1956; Orji 2008; Sorbe 1978) to 0.5 mg (Ezeama 2014; Jago 2007; Moodie 1976; Vaughan Williams 1974), with one study reporting a 0.4 mg oral dose (De Groot 1996). One study did not report specific doses but described that all medications were given at “standard doses” (Ilancheran 1990). Active management or at least one component of the active management of the third stage of labour (AMTSL) was applied to both treatment groups in the eight studies (Adhikari 2007; Boopathi 2014; Ezeama 2014; Modi 2014; Orji 2008; Saito 2007; Singh 2009; Vaughan Williams 1974). One trial utilised expectant management of the third stage (De Groot 1996), and the remaining six trials did not describe management of the third stage.
There were four trials that compared the effects of oxytocin‐ergometrine versus ergot alkaloids (Bonham 1963; Francis 1965; Ilancheran 1990; Vaughan Williams 1974). Oxytocin‐ergometrine was given by IM route at a dose of 0.5 mg of ergometrine and 5 IU of oxytocin in three studies (Bonham 1963; Francis 1965; Vaughan Williams 1974), although in one the study it was given IV at a “standard dose” (Ilancheran 1990). Ergometrine 0.5 mg was given IM (Bonham 1963; Francis 1965) or IV (Vaughan Williams 1974). In one study, ergometrine was given IV at a “standard dose” (Ilancheran 1990). Two trials described AMTSL (Bonham 1963; Vaughan Williams 1974), while the remaining two trials did not specifically describe third stage management.
One trial, Fugo 1958, met the criteria for inclusion but no data from this trial were used because the protocol called for manual removal of the placenta at 10 minutes after delivery of the infant and we felt that the methodology of this trial had high risk of bias and was not translatable into clinical practice.
Outcomes
A range of outcomes were reported in the included trials. Sixteen trials reported blood loss of 500 mL or more after delivery (Abdel‐Aleem 2010; Bonham 1963; Boopathi 2014; De Groot 1996; Dhananjaya 2014; Ezeama 2014; Francis 1965; Ilancheran 1990; Modi 2014; Nordstrom 1997; Orji 2008; Pierre 1992; Poeschmann 1991; Saito 2007; Singh 2009; Sorbe 1978), and seven trials reported blood loss of 1000 mL or more (Abdel‐Aleem 2010; De Groot 1996; Nordstrom 1997; Pierre 1992; Poeschmann 1991; Saito 2007; Sorbe 1978). Mean blood loss was reported in 14 studies (Bader 2000; Boopathi 2014; De Groot 1996; Dhananjaya 2014; Ezeama 2014; Jago 2007; Modi 2014; Nordstrom 1997; Orji 2008; Poeschmann 1991; Saito 2007; Singh 2009; Sorbe 1978; Vaughan Williams 1974). Blood loss was measured gravimetrically (calibrated drapes or other containers) in four studies (Boopathi 2014; Francis 1965; Modi 2014; Sorbe 1978), and by pad weights in six studies (Bader 2000; Dhananjaya 2014; Ezeama 2014; Orji 2008; Poeschmann 1991; Singh 2009). Three studies used a combination of gravimetric assessment and pad weights (De Groot 1996; Nordstrom 1997; Saito 2007). Three studies described collection of the blood in drapes or bins but did not specifically describe how the blood loss amount was determined (Abdel‐Aleem 2010; Pierre 1992; Vaughan Williams 1974). Bonham 1963 reported that blood loss was estimated by “adding to the measured quantity a figure for loss on linen and swabs used during the perineal repair,” implying a combination of gravimetric and visual estimation. The method of blood loss determination was not specifically described in the remaining studies two (Ilancheran 1990; Jago 2007). Several studies reported surrogate outcomes for significant blood loss including need for a blood transfusion (Abdel‐Aleem 2010; Adhikari 2007; Boopathi 2014; De Groot 1996; Dhananjaya 2014; Ezeama 2014; Nordstrom 1997; Saito 2007; Singh 2009) and maternal haemoglobin (Hb) concentration < 7 g/dL 24 to 48 hours postpartum (Jerbi 2007; Nordstrom 1997). The need for additional uterotonics was examined in 11 studies (Abdel‐Aleem 2010; Adhikari 2007; Boopathi 2014; De Groot 1996; Dhananjaya 2014; Ezeama 2014; Nordstrom 1997; Orji 2008; Poeschmann 1991; Saito 2007; Singh 2009). Many studies reported outcomes related to timing of placental delivery. A third stage of labour greater than 30 minutes was reported in three studies (Abdel‐Aleem 2010; Ezeama 2014; Singh 2009), while others reported the mean length of the third stage (Bader 2000; Boopathi 2014; Dhananjaya 2014; Ezeama 2014; Jerbi 2007; Modi 2014; Orji 2008; Poeschmann 1991; Saito 2007; Singh 2009; Sorbe 1978). The need for manual placental removal was reported in 14 trials (Abdel‐Aleem 2010; Adhikari 2007; Bonham 1963; Boopathi 2014; De Groot 1996; Ezeama 2014; Jerbi 2007; Nordstrom 1997; Orji 2008; Pierre 1992; Poeschmann 1991; Saito 2007; Singh 2009; Sorbe 1978). Many studies examined maternal side effects, including elevated diastolic blood pressure (Ezeama 2014; Jago 2007; McGinty 1956), headache (Adhikari 2007; Dhananjaya 2014; Ezeama 2014; Orji 2008; Saito 2007), and vomiting (Adhikari 2007; Boopathi 2014; Dhananjaya 2014; Ezeama 2014; Moodie 1976; Orji 2008; Saito 2007). We included the important outcomes of maternal all‐cause mortality and mortality from causes other than bleeding in our study. Only Bonham 1963 reported the outcome of maternal mortality, however there were no events in either of the treatment groups. The remainder of the studies did not include maternal mortality as an outcome. Similarly, several other important outcomes were not reported in any of the trials, including incidence of shock, transfer to a higher level of care, maternal satisfaction with treatment, quality of life indices or breastfeeding outcomes.
Trial author's declarations of interest
The authors from five trials reported no declarations of interest (Abdel‐Aleem 2010; Boopathi 2014; Ezeama 2014; Modi 2014; Singh 2009), while this information was not reported in the remaining studies.
Excluded studies
The details of all excluded studies are outlined in the table Characteristics of excluded studies. In this updated version of the review, we excluded seven new studies. Six studies had comparison groups that were not in the scope of this review (Jans 2017; Neri‐Mejia 2016; Nuamsiri 2016; Oguz Orhan 2014; Quibel 2016; Sunil 2016). Sharma 2014 was not a randomised trial. In addition, two studies that were included in the previous version of this review were excluded in this version as we felt that they could not be classified as either randomised or quasi‐randomised studies (Barbaro 1961; Soiva 1964). One trial included a large group of women who were not randomised (data were collected retrospectively) and whose outcome data were inseparable from those that were randomised (Soiva 1964).
Risk of bias in included studies
2.
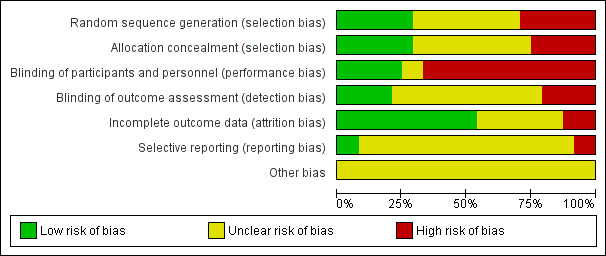
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
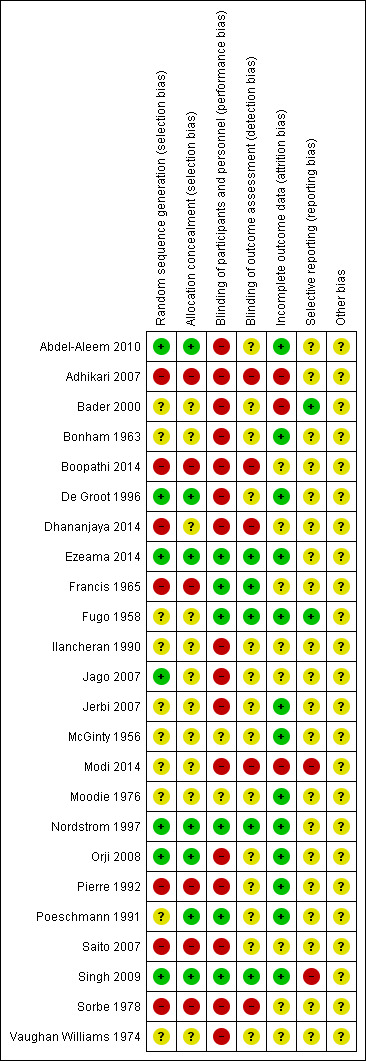
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation and allocation concealment were felt to be adequate and at low risk of bias in six of the 24 included studies (Abdel‐Aleem 2010; De Groot 1996; Ezeama 2014; Nordstrom 1997; Orji 2008; Singh 2009). In one study, we were unable to characterise risk of bias for sequence generation due to lack of description, although allocation concealment methods were adequate (Poeschmann 1991). In another study, random sequence generation was adequate but there was insufficient detail regarding allocation concealment to make a judgement (Jago 2007). We assessed that six of the studies were at high risk of bias in both sequence generation and allocation concealment domains (Adhikari 2007; Boopathi 2014; Francis 1965; Pierre 1992; Saito 2007; Sorbe 1978). All of these studies were assessed to be quasi‐randomised due to the use of randomisation techniques that may have allowed for prediction or anticipation of study group assignment. Similarly, allocation treatment was either not concealed or performed in such a way (alternation or rotation) that could allow for possible identification. Another study was deemed to be at high risk of bias due to quasi‐randomisation, with insufficient information to assess allocation concealment (Dhananjaya 2014). There was not enough information to assess sequence generation or allocation concealment for nine studies (Bader 2000; Bonham 1963; Fugo 1958; Ilancheran 1990; Jerbi 2007; McGinty 1956; Modi 2014; Moodie 1976; Vaughan Williams 1974).
Blinding
Blinding of participants and personnel was assessed to be at high risk of bias in the majority of the included studies (16 of the 23 studies). Trials did not explicitly state a lack of blinding however due to the described methods it was presumed that blinding would not have been possible (different dosages or routes of administration without use of placebos) (Abdel‐Aleem 2010; Adhikari 2007; Bader 2000; Bonham 1963; Boopathi 2014; De Groot 1996; Dhananjaya 2014; Ilancheran 1990; Jago 2007; Jerbi 2007; Modi 2014; Orji 2008; Pierre 1992; Saito 2007; Sorbe 1978; Vaughan Williams 1974). Only six trials were deemed to be at low risk of bias in this domain (Ezeama 2014; Francis 1965; Fugo 1958; Nordstrom 1997; Poeschmann 1991; Singh 2009), with the remaining trials having inadequate description to assess risk of bias. Blinding of outcome assessment was adequate in five studies as blinding of outcome assessors was explicitly described (Ezeama 2014; Francis 1965; Fugo 1958; Nordstrom 1997; Singh 2009). Five studies were judged to be at high risk of bias in this domain due to lack of blinding (Adhikari 2007; Boopathi 2014; Dhananjaya 2014; Modi 2014; Sorbe 1978). There was not adequate detail to assess the remaining studies with regards to detection bias.
Incomplete outcome data
We assessed that all participants who were randomised in the studies were accounted for in outcome data and analysis in 13 trials (Abdel‐Aleem 2010; Bonham 1963; De Groot 1996; Ezeama 2014; Fugo 1958; Jerbi 2007; McGinty 1956; Moodie 1976; Nordstrom 1997; Orji 2008; Pierre 1992; Poeschmann 1991; Singh 2009). Three trials were judged to be at high risk of attrition bias. One study excluded an unknown number of women after randomisation, however the authors did not clarify how many were lost from each group and how this attrition was addressed in the methods (Adhikari 2007). In another study there were 20 patients excluded after randomisation (of 180 enrolled) for various reasons, including need for surgical intervention (forceps or vacuum), unusually high levels of blood loss of unknown origin and placenta delivery times longer than 30 minutes after deliver (Bader 2000). We felt that excluding patients for high blood loss or evidence of retained placenta would place this study at high risk of bias given the nature of the clinical question being addressed. In a third study, the authors did not explicitly state how many patients were included in the analysis (Modi 2014). They reported that women with perineal and cervical lacerations were excluded from the study, however according to their results the majority of their patients received an episiotomy, which would have resulted in exclusion of significant numbers of patients after randomisation.
Selective reporting
Two studies were felt to be at low risk of reporting bias (Bader 2000; Fugo 1958). One study was deemed to be at high risk of selective reporting (Modi 2014), as some adverse outcomes of interest were reported incompletely. The authors report “side effects of various uterotonics” as outcomes, however data regarding vomiting, nausea, shivering, fever, headache and hypertension were incompletely reported for the oxytocin and methylergometrine groups. One other study was also judged to be at high risk for reporting bias (Singh 2009). The authors reported “adverse effects of the drugs” as secondary outcomes, and in the methods describe collection of data regarding postpartum haemoglobin level, however the data are not fully presented. They also report that “the methylergometrine group had the highest incidence of nausea and vomiting” but did not report this data completely. The remaining studies did not have enough detail provided to assess this domain.
Other potential sources of bias
No other potential sources of bias were identified in any of the included trials.
Effects of interventions
See: Table 1; Table 2; Table 3
See: Table 1; Table 2; Table 3.
Thes results are based on 23 studies with a total of 10,018 women.
1) Oxytocin versus no uterotonics or placebo
Primary outcomes
Blood loss greater than 500 mL
Prophylactic oxytocin compared with no uterotonics or placebo may reduce the risk of blood loss of 500 mL or more after delivery (average risk ratio (RR) 0.51, 95% confidence interval (CI) 0.37 to 0.72; 4162 women; 6 studies; Tau² = 0.10, I² = 75%; quality of evidence: low), Analysis 1.1. There were no subgroup differences observed between active and expectant management (Analysis 2.1), intravenous or intramuscular oxytocin (Analysis 2.2) or different doses of oxytocin (Analysis 2.3) for this outcome.
1.1. Analysis.
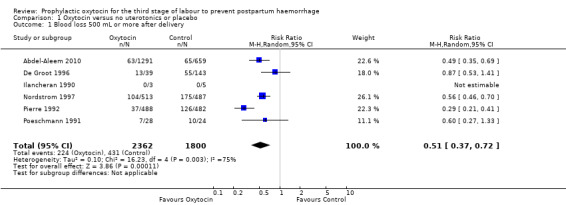
Comparison 1 Oxytocin versus no uterotonics or placebo, Outcome 1 Blood loss 500 mL or more after delivery.
2.1. Analysis.
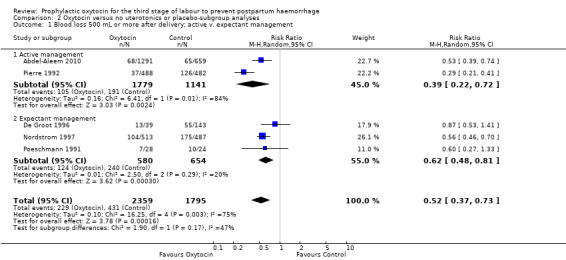
Comparison 2 Oxytocin versus no uterotonics or placebo‐subgroup analyses, Outcome 1 Blood loss 500 mL or more after delivery; active v. expectant management.
2.2. Analysis.
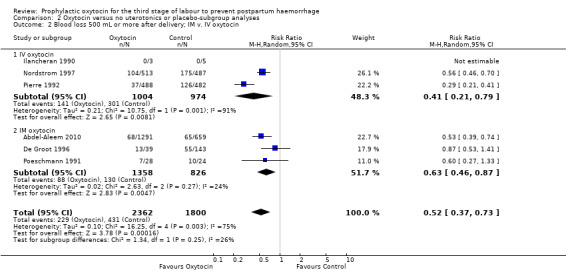
Comparison 2 Oxytocin versus no uterotonics or placebo‐subgroup analyses, Outcome 2 Blood loss 500 mL or more after delivery; IM v. IV oxytocin.
2.3. Analysis.
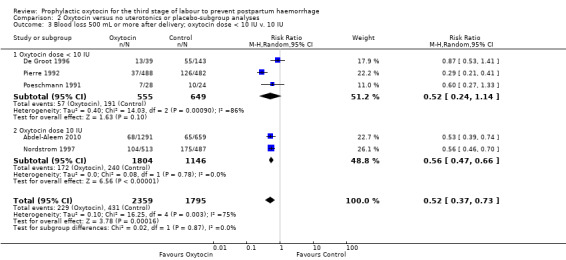
Comparison 2 Oxytocin versus no uterotonics or placebo‐subgroup analyses, Outcome 3 Blood loss 500 mL or more after delivery; oxytocin dose < 10 IU v. 10 IU.
Need for additional uterotonics
Prophylactic oxytocin probably reduces the need for additional uterotonics (average RR 0.54, 95% CI 0.36 to 0.80; 3135 women; 4 studies; Tau² =0.07, I² = 44%; quality of evidence: moderate), Analysis 1.2.There were no subgroup differences observed between active and expectant management (Analysis 2.4), intravenous or intramuscular oxytocin (Analysis 2.5) or different doses of oxytocin (Analysis 2.6) for this outcome.
1.2. Analysis.
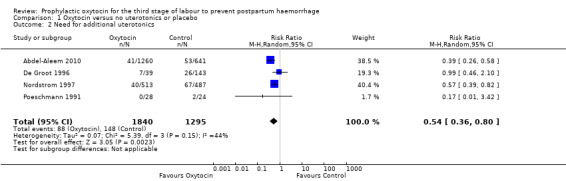
Comparison 1 Oxytocin versus no uterotonics or placebo, Outcome 2 Need for additional uterotonics.
2.4. Analysis.
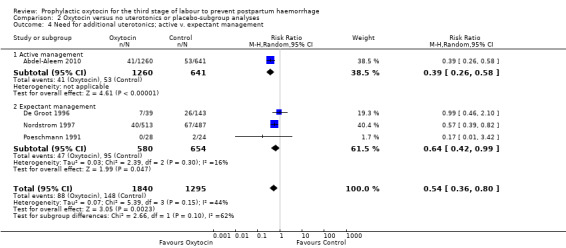
Comparison 2 Oxytocin versus no uterotonics or placebo‐subgroup analyses, Outcome 4 Need for additional uterotonics; active v. expectant management.
2.5. Analysis.
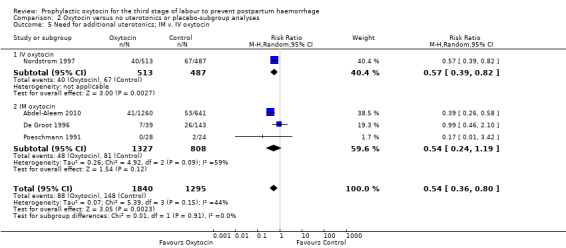
Comparison 2 Oxytocin versus no uterotonics or placebo‐subgroup analyses, Outcome 5 Need for additional uterotonics; IM v. IV oxytocin.
2.6. Analysis.
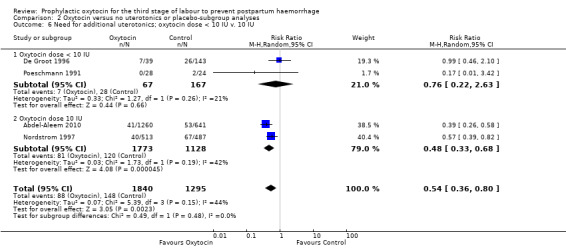
Comparison 2 Oxytocin versus no uterotonics or placebo‐subgroup analyses, Outcome 6 Need for additional uterotonics; oxytocin dose < 10 IU v. 10 IU.
Secondary outcomes
Prophylactic oxytocin compared with no uterotonics or placebo may reduce the risk of blood loss 1000 mL or more after delivery (RR 0.59, 95% CI 0.42 to 0.83; 4123 women; 5 studies; quality of evidence: low),Analysis 1.3. There may be no difference in the risk of needing a blood transfusion in women receiving oxytocin compared to no uterotonics or placebo (RR 0.88, 95% CI 0.44 to 1.78; 3081 women; 3 studies; quality of evidence: low), Analysis 1.4, and there is probably no difference in the risk of developing a postpartum haemoglobin (Hb) < 7 g/dL (RR 0.64, 95% CI 0.18 to 2.26; 1073 women; 2 studies; quality of evidence: moderate), Analysis 1.7. It is unclear if oxytocin is associated with a reduction in mean blood loss (mean blood loss in mL: ‐99.13, 95% CI ‐181.40 to ‐16.85; 1359 women; 5 studies; quality of evidence: very low), Analysis 1.6.
1.3. Analysis.
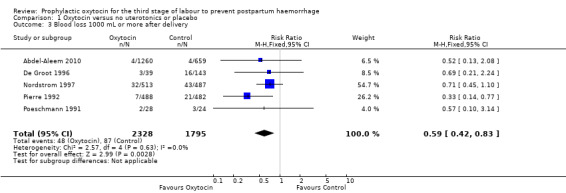
Comparison 1 Oxytocin versus no uterotonics or placebo, Outcome 3 Blood loss 1000 mL or more after delivery.
1.4. Analysis.
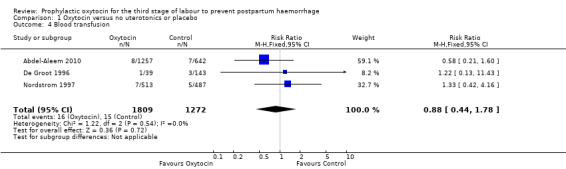
Comparison 1 Oxytocin versus no uterotonics or placebo, Outcome 4 Blood transfusion.
1.7. Analysis.
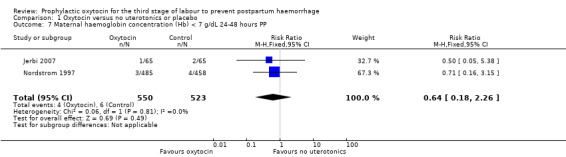
Comparison 1 Oxytocin versus no uterotonics or placebo, Outcome 7 Maternal haemoglobin concentration (Hb) < 7 g/dL 24‐48 hours PP.
1.6. Analysis.
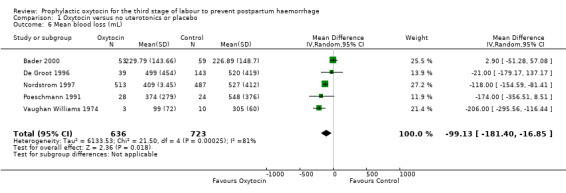
Comparison 1 Oxytocin versus no uterotonics or placebo, Outcome 6 Mean blood loss (mL).
Oxytocin may be associated with an increased risk of a third stage greater than 30 minutes (RR 2.55, 95% CI 0.88 to 7.44; 1947 women; 1 study; quality of evidence: moderate), Analysis 1.5. However the CI is wide and crosses 1.0, which indicates that there may be little or no difference between oxytocin and no uterotonic or placebo. This result is based on a single study of 1947 women, where 20 of 1289 women in the oxytocin group experienced this outcome versus four of 658 women in the no uterotonics group. It is uncertain whether oxytocin affects the mean length of the third stage of labour (mean length of the third stage of labour in minutes: ‐3.61, 95% CI ‐9.06 to 1.83; 294 women; 3 studies; quality of evidence: very low), Analysis 1.8, or the risk of needing manual removal of the placenta (RR 1.27, 95% CI 0.89 to 1.82; 4281 women; 6 studies; quality of evidence: very low), Analysis 1.9, given the very low certainty of this evidence.
1.5. Analysis.
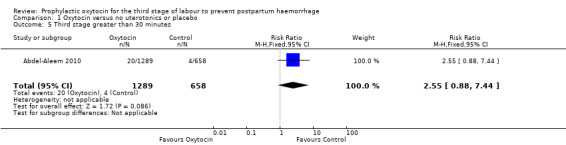
Comparison 1 Oxytocin versus no uterotonics or placebo, Outcome 5 Third stage greater than 30 minutes.
1.8. Analysis.

Comparison 1 Oxytocin versus no uterotonics or placebo, Outcome 8 Mean length of third stage (minutes).
1.9. Analysis.
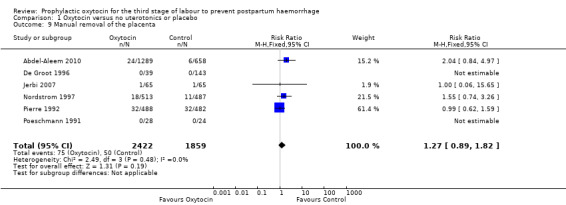
Comparison 1 Oxytocin versus no uterotonics or placebo, Outcome 9 Manual removal of the placenta.
Outcomes not reported
The following pre‐specified outcomes were not reported for this comparison as none of the included trials reported these outcomes: maternal all‐cause mortality, diastolic blood pressure > 100 mg Hg between delivery of the baby and discharge from the labour ward, vomiting between delivery of the baby and discharge from the labour ward, headache between delivery of the baby and discharge from the labour ward, shock, transfer to a higher level of care, mortality from causes other than bleeding, maternal satisfaction with therapy, quality of life, breastfeeding.
2) Oxytocin versus ergot alkaloids
Primary outcomes
Blood loss greater than 500 mL
It is uncertain whether oxytocin reduces the likelihood of blood loss 500 mL or more after delivery (average RR 0.84, 95% CI 0.56 to 1.25; 3082 women; 10 studies; Tau² = 0.14, I² = 49%; quality of evidence: very low),Analysis 3.1, compared to ergot alkaloids, because the certainty of this evidence is very low. There were no subgroup differences observed between active and expectant management (Analysis 4.1), intravenous or intramuscular oxytocin (Analysis 4.2) or different doses of oxytocin (Analysis 4.3) for this outcome.
3.1. Analysis.
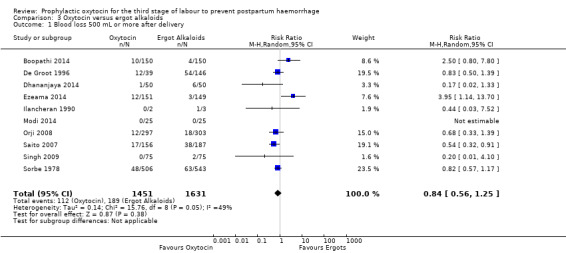
Comparison 3 Oxytocin versus ergot alkaloids, Outcome 1 Blood loss 500 mL or more after delivery.
4.1. Analysis.
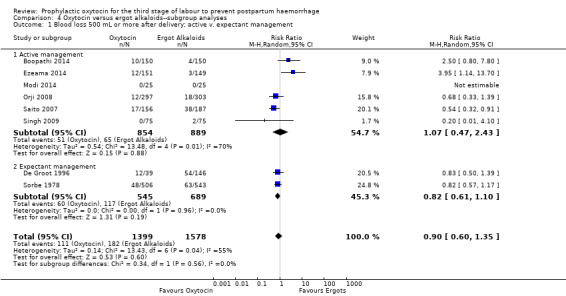
Comparison 4 Oxytocin versus ergot alkaloids‐‐subgroup analyses, Outcome 1 Blood loss 500 mL or more after delivery; active v. expectant management.
4.2. Analysis.
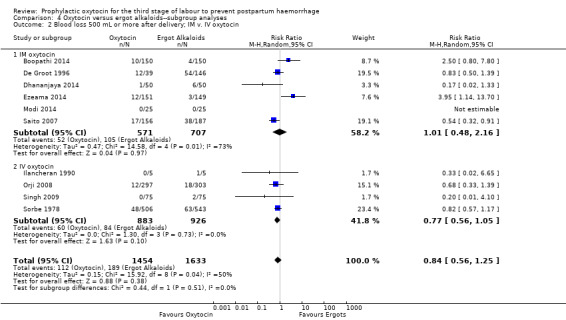
Comparison 4 Oxytocin versus ergot alkaloids‐‐subgroup analyses, Outcome 2 Blood loss 500 mL or more after delivery; IM v. IV oxytocin.
4.3. Analysis.
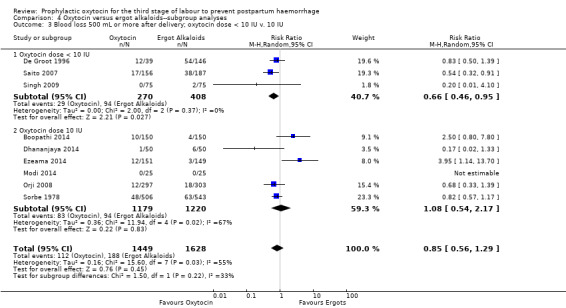
Comparison 4 Oxytocin versus ergot alkaloids‐‐subgroup analyses, Outcome 3 Blood loss 500 mL or more after delivery; oxytocin dose < 10 IU v. 10 IU.
Need for additional uterotonics
It is also unclear whether oxytocin reduces the need for additional uterotonics compared to ergot alkaloids (average RR 0.89, 95% CI 0.43 to 1.81; 2178 women; 8 studies; Tau² = 0.76, I² = 79%; quality of evidence: very low), Analysis 3.2, due to the very low certainty of this evidence. There were no differences observed in any of the prespecified subgroups (Analysis 4.4; Analysis 4.5; Analysis 4.6).
3.2. Analysis.
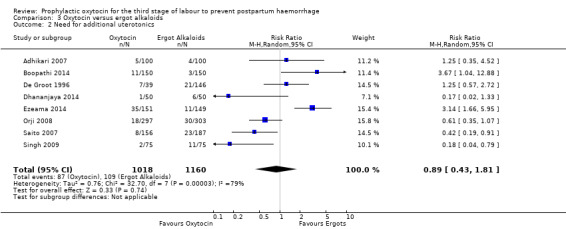
Comparison 3 Oxytocin versus ergot alkaloids, Outcome 2 Need for additional uterotonics.
4.4. Analysis.
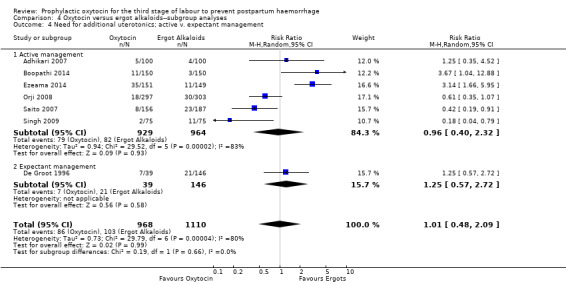
Comparison 4 Oxytocin versus ergot alkaloids‐‐subgroup analyses, Outcome 4 Need for additional uterotonics; active v. expectant management.
4.5. Analysis.
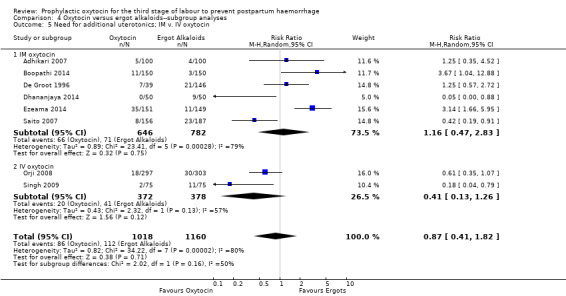
Comparison 4 Oxytocin versus ergot alkaloids‐‐subgroup analyses, Outcome 5 Need for additional uterotonics; IM v. IV oxytocin.
4.6. Analysis.
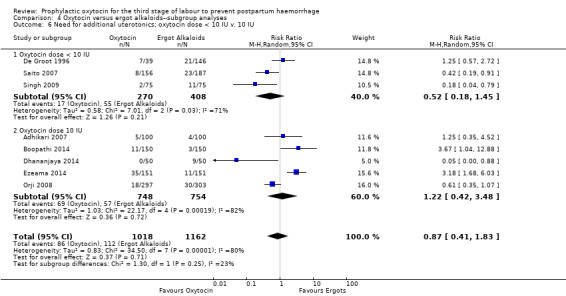
Comparison 4 Oxytocin versus ergot alkaloids‐‐subgroup analyses, Outcome 6 Need for additional uterotonics; oxytocin dose < 10 IU v. 10 IU.
Secondary outcomes
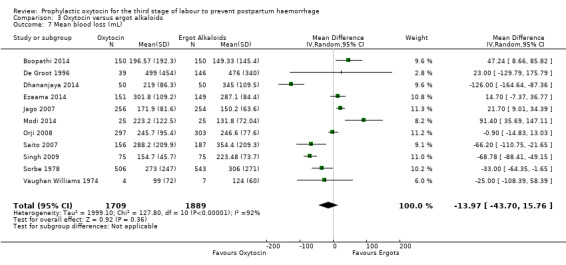
Similarly, the quality of evidence was very low for the outcomes of blood loss of 1000 mL or more after delivery (RR 1.13, 95% CI 0.63 to 2.01; 1577 women; 3 studies; quality of evidence: very low), Analysis 3.3, need for blood transfusion (average RR 1.37, 95% CI 0.34 to 5.51; 1578 women; 7 studies; Tau² = 1.34, I² = 45%; quality of evidence: very low), Analysis 3.4, as well as mean blood loss (mean blood loss in mL: ‐13.97, 95% CI ‐43.70 to 15.76; 3598 women; 11 studies; quality of evidence: very low), Analysis 3.7, making any benefit of oxytocin over ergot alkaloids uncertain.
3.3. Analysis.
Comparison 3 Oxytocin versus ergot alkaloids, Outcome 3 Blood loss 1000 mL or more after delivery.
3.4. Analysis.
Comparison 3 Oxytocin versus ergot alkaloids, Outcome 4 Blood transfusion.
3.7. Analysis.
Comparison 3 Oxytocin versus ergot alkaloids, Outcome 7 Mean blood loss (mL).
Oxytocin probably increases the risk of a third stage greater than 30 minutes compared to ergot alkaloids (RR 4.69, 95% CI 1.63 to 13.45; 450 women; 2 studies; quality of evidence: moderate), Analysis 3.5, although it is uncertain whether or not this translates into an increased risk of manual placental removal (average RR 1.10, 95% CI 0.39 to 3.10; 3127 women; 8 studies; Tau² = 1.07, I² = 76%; quality of evidence: very low), Analysis 3.9. It is unclear whether or not oxytocin affects the mean length of the third stage of labour (mean length of the third stage in minutes: 0.09, 95% CI ‐0.44 to 0.61; 2892 women; 8 studies; quality of evidence: very low), Analysis 3.8.
3.5. Analysis.
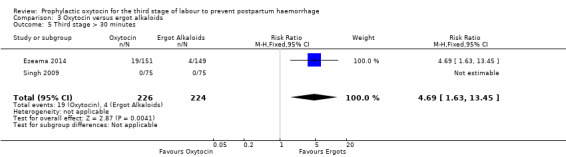
Comparison 3 Oxytocin versus ergot alkaloids, Outcome 5 Third stage > 30 minutes.
3.9. Analysis.

Comparison 3 Oxytocin versus ergot alkaloids, Outcome 9 Manual removal of the placenta.
3.8. Analysis.
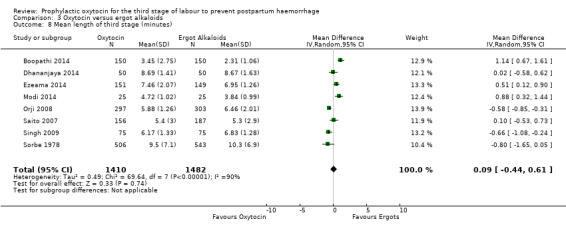
Comparison 3 Oxytocin versus ergot alkaloids, Outcome 8 Mean length of third stage (minutes).
Oxytocin may be associated with a lower risk of diastolic blood pressure > 100 mm Hg after delivery (average RR 0.28, 95% CI 0.04 to 2.05; 960 women; 3 studies; Tau² = 1.23, I² = 50%; quality of evidence: low), Analysis 3.6, although the 95% CI is very wide and indicates that there may possibly be little or no risk reduction. Oxytocin is probably associated with a lower risk of vomiting than ergot alkaloids (RR 0.09, 95% CI 0.05 to 0.14; 1991 women; 7 studies; quality of evidence: moderate), Analysis 3.10. The impact of oxytocin on headaches is uncertain (average RR 0.19, 95% CI 0.03 to 1.02; 1543 women; 5 studies; Tau² = 2.54, I² = 72%; quality of evidence: very low), Analysis 3.11.
3.6. Analysis.
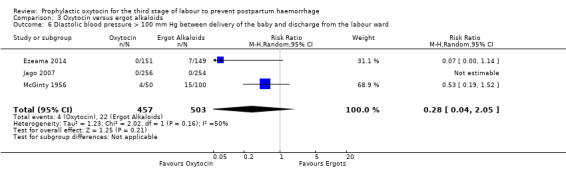
Comparison 3 Oxytocin versus ergot alkaloids, Outcome 6 Diastolic blood pressure > 100 mm Hg between delivery of the baby and discharge from the labour ward.
3.10. Analysis.
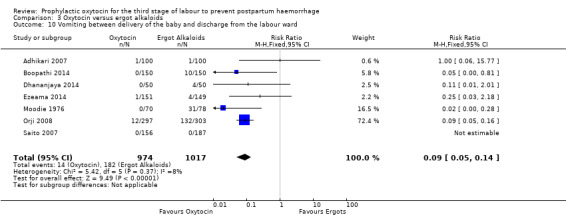
Comparison 3 Oxytocin versus ergot alkaloids, Outcome 10 Vomiting between delivery of the baby and discharge from the labour ward.
3.11. Analysis.
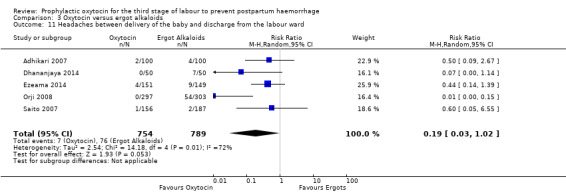
Comparison 3 Oxytocin versus ergot alkaloids, Outcome 11 Headaches between delivery of the baby and discharge from the labour ward.
Outcomes not reported
The following pre‐specified outcomes were not reported for this comparison as none of the included trials reported these outcomes: maternal all‐cause mortality, maternal Hb concentration < 7 g/dL 24 to 48 hours postpartum, shock, transfer to a higher level of care, mortality from causes other than bleeding, maternal satisfaction with therapy, quality of life, breastfeeding.
3) Oxytocin‐ergometrine versus ergot alkaloids
Primary outcomes
Blood loss greater than 500 mL
Oxytocin‐ergometrine may slightly reduce the risk of blood loss greater than 500 mL or more after delivery compared to ergot alkaloids (RR 0.44, 95% CI 0.20 to 0.94; 1168 women; 3 studies; quality of evidence: low), Analysis 5.1. This outcome is based on data from only quasi‐randomised trials deemed to be at high risk of bias.
5.1. Analysis.
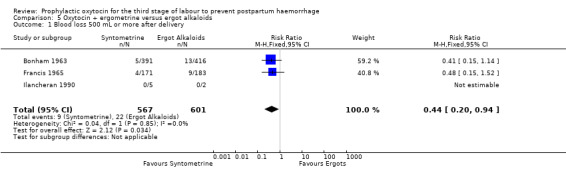
Comparison 5 Oxytocin + ergometrine versus ergot alkaloids, Outcome 1 Blood loss 500 mL or more after delivery.
Maternal all‐cause mortality
One study reported maternal all‐cause mortality as an outcome, however no events in either the oxytocin‐ergometrine group (391 women) or the ergot alkaloid group (416 women) were reported, so an effect could not be estimated.
Secondary outcomes
The effect of oxytocin‐ergometrine compared to ergot alkaloids on mean blood loss is uncertain (mean blood loss in mL: 61.00, 95% CI ‐0.90 to 122.90; 27 women; 1 study; quality of evidence: very low), Analysis 5.3, and there may be no difference in the risk of manual removal of the placenta (RR 1.06, 95% CI 0.31 to 3.65; 807 women; 1 study; quality of evidence: low), Analysis 5.2.
5.3. Analysis.
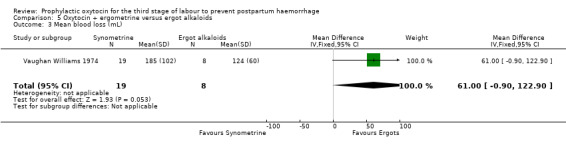
Comparison 5 Oxytocin + ergometrine versus ergot alkaloids, Outcome 3 Mean blood loss (mL).
5.2. Analysis.
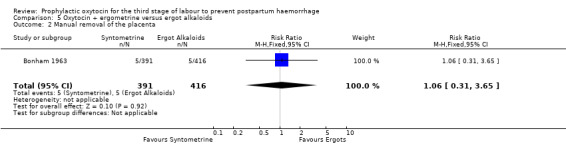
Comparison 5 Oxytocin + ergometrine versus ergot alkaloids, Outcome 2 Manual removal of the placenta.
The need for additional uterotonics, blood loss greater than 1000 mL or more after delivery, blood transfusion, third stage greater than 30 minutes and diastolic hypertension were not outcomes reported in the included studies.
Outcomes not reported
The following pre‐specified outcomes were not reported for this comparison as none of the included trials reported these outcomes: need for additional uterotonics, blood loss 1000 mL or more after delivery, blood transfusion, third stage greater than 30 minutes, diastolic blood pressure > 100 mg Hg between delivery of the baby and discharge from the labour ward, maternal Hb concentration < 7 g/dL 24 to 48 hours postpartum, vomiting between delivery of the baby and discharge from the labour ward, headaches between delivery of the baby and discharge from the labour ward, shock, transfer to a higher level of care, mortality from causes other than bleeding, maternal satisfaction with therapy, quality of life, breastfeeding.
Discussion
Summary of main results
Compared to no uterotonics or placebo, oxytocin may reduce the risk of blood loss greater than 500 mL and greater than 1000 mL (low‐quality evidence), and probably reduces the need for additional uterotonics (moderate‐quality evidence). Oxytocin probably does not affect the risk of developing severe anaemia (haemoglobin (Hb) < 7 g/dL) (moderate‐quality evidence), and may not affect the risk of needing a blood transfusion (low‐quality evidence). The use of oxytocin may be associated with an increased risk of a third stage of labour greater than 30 minutes (moderate‐quality evidence), although whether that translates into greater risk of needing a manual placental removal is uncertain (very low‐quality evidence).
It is unclear whether oxytocin affects the risk of excessive blood loss after delivery compared to ergot alkaloids, as the quality of evidence for outcomes related to blood loss (blood loss greater than 500 mL, blood loss greater than 1000 mL, need for additional uterotonics, need for blood transfusion, mean blood loss) was very low. Oxytocin probably increases the risk of a third stage of labour greater than 30 minutes (moderate‐quality evidence), however it is uncertain whether or not this translates into an increased risk of manual placental removal or a difference in the mean length of the third stage of labour (very low‐quality evidence). This must be weighed against potential side effects, as ergot alkaloids are probably associated with a higher risk of vomiting (moderate‐quality evidence), and may be associated with a higher risk of diastolic hypertension (low‐quality evidence).
The combination of oxytocin and ergometrine compared to ergot alkaloids alone may be associated with a slight reduction in the risk of blood loss greater than 500 mL, based on low‐quality evidence from only quasi‐randomised trials at high risk of bias. The effect on mean blood loss is uncertain (very low‐quality evidence), and there may be no difference in the risk of manual placental removal (low‐quality evidence).
Overall completeness and applicability of evidence
Population
The patient population in the majority of the studies were women in relatively good health, as many studies excluded women with medical co‐morbidities (diabetes, hypertensive disease, renal disease, pre‐existing anaemia). Although many women with risk factors associated with an increased risk of postpartum haemorrhage (PPH) were also excluded (prior PPH, grand multiparity, multiple gestations), generalising these data to those specific patient groups seems reasonable as similar etiologies for PPH are likely to exist in a lower‐risk population. Gestational age criteria were explicitly stated in a minority of the included studies, ranging from inclusion of full‐term patients only and inclusion of patients at 28 weeks' gestation or greater. Women who delivered by caesarean section were not included in the analysis, and many studies also excluded women with an operative vaginal delivery.
Intervention
Oxytocin was given by intramuscular (IM) and intravenous (IV) route at doses ranging from 5 international units (IU) to 10 IU in the included trials, with most trials lacking clarification of whether IV route was administered by bolus or infusion. While this is reflective of practice in some centres, many also choose to use higher doses administered by IV infusion for variable periods of time following delivery. When comparing oxytocin to no uterotonics, active management or some component of active management of the third stage of labour (AMTSL) was applied to the comparator group in approximately half of the studies, but none of the studies applied active management to the oxytocin treatment groups.
Comparators
In this review oxytocin was compared to either no uterotonics or placebo or ergot alkaloids (alone or in combination with ergometrine). As current recommendations from the World Health Organization and multiple obstetric professional organisations recommend the use of active management of the third stage including the use of a uterotonic, most recent studies evaluate the use of oxytocin compared to another uterotonic agent instead of placebo. Ergot alkaloids (methylergometrine or ergometrine) were given in the majority of studies by either IV or IM route and doses ranging from 0.2 mg to 0.5 mg, which reflects typical practice in most centres. Oxytocin‐ergometrine (Syntometrine) was given at the standard dose in the included trials; currently this medication is currently unavailable in the USA. Also of note, approximately half of the studies comparing oxytocin with ergometrine applied active management to all treatment groups, while most of the remaining studies did not explicitly state their practice regarding active management.
Outcomes
Most studies included direct blood loss evaluation with some form of gravimetric measurement described. While most studies did not explicitly describe over what period of time the blood loss was measured, the few that did mention this described measurement of blood loss accumulated within one hour of delivery. Most studies reported blood loss greater than 500 mL, in line with the most commonly utilised definition of PPH, although a third of the studies did specifically report blood loss greater than 1000 mL. Outcomes related to retained placenta were examined in many trials, with most reporting data either for manual placental removal or a third stage greater than 30 minutes. None of the included studies included surrogate outcomes for significant maternal morbidity from haemorrhage such as transfer to higher level of care or shock, and only one reported maternal mortality as an outcome (although there were no events in either group).
Setting
The evidence in this review is based upon trials from a wide range of low‐ to high‐income countries in hospital settings, although one trial included an undisclosed number of women who delivered at home attended by a midwife (De Groot 1996). All studies included data from patients at tertiary medical hospitals, many of them teaching hospitals, although several also included patients recruited from community hospitals.
Summary
Overall, the evidence in this review is directly applicable to healthy women at lower risk of PPH, delivering vaginally in hospital settings at greater than 28 weeks. Extending applicability of these findings with regards to impact on blood loss seems reasonable in women undergoing caesarean delivery, and those with certain risk factors (prior PPH, multiple gestations, grand multiparity). Women with coagulation disorders or other haematological issues (those on anticoagulation) may have different or additional mechanisms underlying postpartum bleeding, and should still receive the usual third stage management although additional therapies may be necessary in the event of PPH depending on the underling disorder. Application of the findings to women delivering outside of a hospital but with access to uterotonic medications is also reasonable given expected similarities in the underlying mechanisms of PPH.
We had planned to assess multiple outcomes for each of our three comparisons, with the goal of evaluating effects of interventions that would reflect a wide range of potential maternal morbidity. In this updated version of the review, we incorporated several additional important outcomes that have been recommended as part of a core outcome set for reporting in PPH prevention studies (Meher 2018). It is important to note that none of the included trials reported many of our pre‐specified outcomes, including maternal mortality (all‐cause and from causes other than bleeding), shock, transfer to a higher level of care, patient‐reported outcomes (maternal satisfaction with therapy and quality of life) and neonatal outcomes (breastfeeding).
Most studies that compared oxytocin versus no uterotonics or with ergot alkaloids included multiple important outcomes and surrogate outcomes aimed at assessing blood loss. Data on complications of delayed placental delivery were more limited for trials comparing oxytocin with no uterotonics, with only one trial reporting outcomes for a third stage greater than 30 minutes (Abdel‐Aleem 2010), although several trials did report on the incidence of manual placental removal (Abdel‐Aleem 2010; De Groot 1996; Jerbi 2007; Nordstrom 1997; Pierre 1992; Poeschmann 1991). In contrast, most of the trials comparing oxytocin with ergot alkaloids reported third stage placental complications including mean length of the third stage and manual placental removal, although only two specifically reported the outcome of third stage greater than 30 minutes (Ezeama 2014; Singh 2009). Potential adverse effects of oxytocin were not reported in trials comparing oxytocin with no uterotonics, possibly secondary to the relatively favourable side‐effect profile of oxytocin when given in smaller limited doses. As ergot alkaloids have been associated with multiple side effects due to vasoconstriction (De Groot 1998; Liabsuetrakul 2018), many studies reported outcomes for side effects including diastolic hypertension, vomiting and headaches.
Overall, evidence on oxytocin‐ergometrine compared to ergot alkaloids was very limited, with only four trials identified that included this comparison (total of 1198 women). Data on a limited number of outcomes were reported, including blood loss greater than 500 mL, mean blood loss and manual removal of the placenta.
Quality of the evidence
We graded the evidence for all outcomes using the GRADE approach, and reported the findings for the outcomes we deemed most important in our 'Summary of findings' tables (Table 1; Table 2; Table 3). See the 'Risk of Bias' summary (Figure 3) for our detailed assessment of risk of bias for each of the included studies.
Evidence for the comparison of oxytocin versus no uterotonics or placebo ranged from very low to moderate quality. For our primary outcomes, the evidence was deemed to be of low to moderate quality due to risk of bias due to lack of blinding, as well as substantial heterogeneity. For secondary outcomes including blood loss greater than 1000 mL and need for blood transfusion, the quality of evidence was downgraded to low due to lack of blinding and wide confidence intervals resulting in imprecision. The evidence for a third stage greater than 30 minutes was based on data from single study and assessed as moderate quality, due to concern over imprecision as confidence intervals were wide and there were very few events. Other evidence evaluating retained placenta (mean length of the third stage and need for manual placental removal) was of very low quality due to serious concerns regarding risk of bias in multiple domains, as well as both substantial heterogeneity and imprecision.
Evidence for the comparison of oxytocin versus ergot alkaloids ranged from very low to moderate quality, with the majority of outcomes assessed as very low to low quality of evidence. Evidence for our primary outcomes of blood loss greater than 500 mL and need for additional uterotonics was downgraded to very low due to serious methodological limitations increasing the risk of bias, wide confidence intervals resulting in imprecision and substantial heterogeneity leading demonstrating inconsistency. Secondary outcomes of blood loss of 1000 mL or more and need for transfusion were also assessed as very low quality for the same reasons. The evidence for risk of third stage greater than 30 minutes was deemed of moderate quality due to lack of precision (there were wide confidence intervals, few events and one of the two studies had no reported events). Evidence for manual placental removal was of very low quality due to serious methodological limitations across multiple domains, wide confidence intervals and substantial heterogeneity. Evidence for various side effects was assessed as low quality for elevated diastolic blood pressure due to concerns regarding heterogeneity and wide confidence intervals, and as moderate quality for vomiting due to risk of bias concerns in reporting trials. Evidence for headaches was of very low quality due to serious methodological concerns, as well as serious imprecision and inconsistency amongst trials. We produced funnel plots for analyses that included 10 or more studies (Analysis 3.1, Figure 4; Analysis 3.7, Figure 5), and neither raised concern for publication bias.
4.
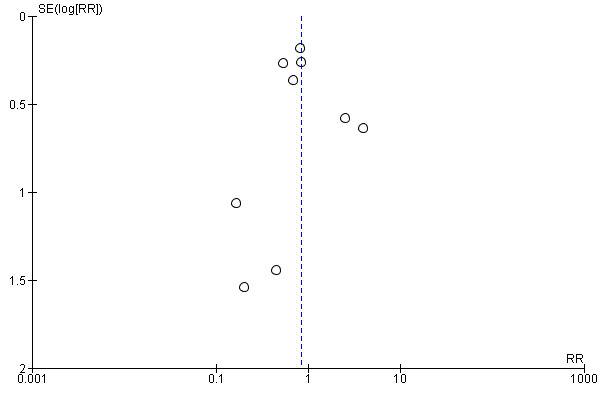
Funnel plot of comparison: 3 Oxytocin versus ergot alkaloids, outcome: 3.1 Blood loss 500 mL or more after delivery.
5.
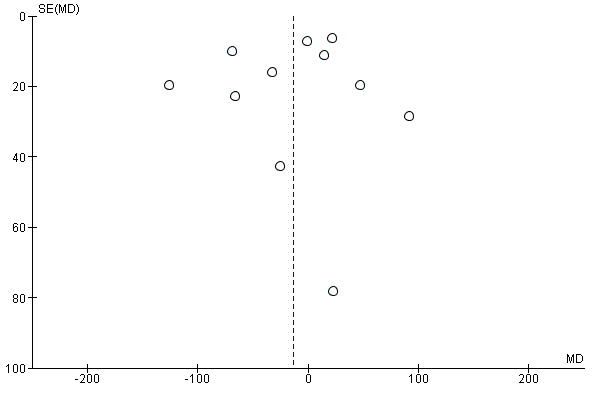
Funnel plot of comparison: 3 Oxytocin versus ergot alkaloids, outcome: 3.7 Mean blood loss (mL).
Evidence for the comparison of oxytocin‐ergometrine versus ergot alkaloids was very limited, but ranged from very low to low quality. For all three outcomes with data reported, there were concerns regarding lack of blinding and randomisation methods increasing the risk of bias, as well as wide confidence intervals combined with few events concerning for imprecision.
Potential biases in the review process
We attempted to minimise the introduction of bias in this review by adhering to the Cochrane methods at all stages of data collection and analysis. Two review authors independently assessed trials for inclusion, extracted data and assessed risk of bias. Any discrepancies were resolved by discussion or by involvement of a third author. GRADE assessment for quality of evidence was assessed by two review authors.
Agreements and disagreements with other studies or reviews
This is the most comprehensive published systematic review specifically comparing oxytocin with no uterotonics or ergot alkaloids for the prevention of PPH. The findings are overall consistent with findings from other related studies that have included oxytocin as a comparison group. One review compared oxytocin with no intervention or standard care in the third stage of labour in out‐of‐hospital settings and found that oxytocin probably decreased the incidence of PPH greater than 500 mL, although evidence for all other outcomes was lacking or the interpretation was limited due to low quality of evidence (Pantoja 2016). A recent network meta‐analysis was undertaken to investigate and rank various uterotonic regimens according to their effectiveness and side‐effect profiles (Gallos 2018). They identified oxytocin‐ergometrine, oxytocin combined with misoprostol, and carbetocin, as being the most effective drugs for reducing postpartum blood loss compared to oxytocin. The regimens associated with the highest risk of side effects compared to oxytocin alone were oxytocin‐ergometrine (increased risk of nausea, vomiting,and diarrhoea), and oxytocin plus misoprostol (increased risk of nausea, vomiting, shivering, fever, and diarrhoea).
Authors' conclusions
Implications for practice.
Prophylactic oxytocin may reduce the risk of blood loss and decrease the need for additional uterotonics, and could be considered as a component of the active management of the third stage of labour (AMTSL). It is unclear whether prophylactic oxytocin reduces postpartum blood loss compared to ergot alkaloids, however it may be associated with fewer side effects. This must be weighed against a possible increased risk of a third stage greater than 30 minutes, although whether or not this translates into greater need for manual placental removal is uncertain given the low quality of the evidence. The combination of oxytocin and ergometrine may slightly reduce the risk of postpartum blood loss compared to ergot alkaloids, however the certainty of this conclusion is low given the lack of randomised trials and imprecision.
Implications for research.
The majority of evidence for outcomes in this review was of low quality, with many trials deemed at high risk of bias due to lack of blinding, concerns regarding allocation concealment, as well as quasi‐randomisation. More high‐quality randomised controlled trials with adequate blinding of participants and use of gravimetric techniques for blood loss measurement, are needed. High‐quality randomised controlled trials examining oxytocin dosing and route of administration, as well as oxytocin in comparison to other available regimens will be important in determining optimal management of the third stage of labour. Additionally, future trials should examine a range of outcomes that include, not only outcomes directly related to blood loss and side effects, but include those outcomes that have been recommended as part of a core outcome set for reporting in postpartum haemorrhage (PPH) prevention studies (Meher 2018). Such outcomes include maternal mortality (all‐cause and from causes other than bleeding), shock, transfer to a higher level of care, patient‐reported outcomes (maternal satisfaction with therapy and quality of life) and neonatal outcomes (breastfeeding). A network meta‐analysis of uterotonics for PPH prevention plans to address issues around optimal dosing and routes of oxytocin and other uterotonics.
Feedback
Pastrana, March 2007
Summary
It is important to take care that the conclusions are based on pre‐specified objectives, as sometimes the study is done and then the objectives decided afterwards.
In this review, there is no discussion of the way different studies determined blood loss, and the limitations of these methods. This is especially true for Pierre 1992. Also, the results should take into account Hoffman 2004, comparing oxytocin with expectant management. In this study, although the mean change in hematocrit was significantly less in the oxytocin group, there was no difference in the incidence of postpartum haemorrhage.
(Summary of comment from Jose Luis Pastrana, March 2007)
Reply
6 July 2011
We agree that there are a lot of limitations to this review, specifically that in the studies included there are differences in the method of delivery of pitocin, definition of the active management of the third stage, and determining accurate blood loss after delivery. However, this review incorporates the only randomised controlled trials that attempt to address this important topic. We agree that a formalized method for determining blood loss is needed as that will further advance our ability to perform useful research in this field.
Please see our conclusion section for a more thorough discussion of these topics.
Contributors
Feedback: Jose Luis Pastrana
Response: Gina Westhoff
What's new
| Date | Event | Description |
|---|---|---|
| 6 March 2019 | New search has been performed | Search updated. Six new trials have been included (Adhikari 2007; Boopathi 2014; Dhananjaya 2014; Ezeama 2014; Modi 2014; Singh 2009) and 10 new studies were excluded (Jans 2017; Neri‐Mejia 2016; Nuamsiri 2016; Oguz Orhan 2014; Quibel 2016; Rouse 2011; Sharma 2014; Stanton 2010; Stanton 2013; Suhrabi 2013). Two studies that were included in the previous version of this review were excluded in this version as we felt that they could not be classified as either randomised or quasi‐randomised studies (Barbaro 1961; Soiva 1964). |
| 6 March 2019 | New citation required but conclusions have not changed | The overall conclusions remain unchanged. Incorporation of new evidence suggests that any benefit of oxytocin over ergot alkaloids is now uncertain with regard to blood loss, and that oxytocin may be associated with an increased risk of a prolonged second stage, with an uncertain effect on manual placental removal. Additionally, there may be a slight reduction in blood loss with oxytocin‐ergometrine compared to ergot alkaloids. |
History
Protocol first published: Issue 4, 1999 Review first published: Issue 4, 2001
| Date | Event | Description |
|---|---|---|
| 24 June 2013 | New citation required and conclusions have changed | There is now evidence to show that prophylactic oxytocin is associated with fewer side effects than ergot alkaloids. A new author has joined the review team and is now the guarantor for the review. |
| 31 May 2013 | New search has been performed | Search updated. Six new trials have been included (Abdel‐Aleem 2010; Jago 2007; Jerbi 2007; Moodie 1976; Orji 2008; Saito 2007) and eight trials excluded (Dickinson 2009; Dommisse 1980; Rouse 2011; Sariganont 1999; Stanton 2012; Tita 2012; Wetta 2011; Vasegh 2005). We also identified one additional report identified for an already excluded trial (Hoffman 2006a). This updated reviews is now comprised of 20 included studies (involving 10,806 women). |
| 6 July 2011 | Feedback has been incorporated | The authors have responded to feedback from Pastrana (March 2007) ‐ seeFeedback 1. |
| 1 October 2009 | Amended | Search updated. Ten reports added to Studies awaiting classification. |
| 20 September 2008 | Amended | Converted to new review format. |
| 1 March 2007 | Feedback has been incorporated | Feedback added from Pastrana, March 2007. |
| 1 December 2004 | New search has been performed | Search updated. We identified 16 new studies; however, none fulfilled the inclusion criteria. |
Acknowledgements
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. ICTRP and ClinicalTrials.gov ‐ search methods
ICTRP
oxytocin AND hemorrhage
oxytocin AND third stage
oxytocin AND labor AND bleeding
oxytocin AND labour AND bleeding
ClinicalTrials.gov
Advanced search
oxytocin | postpartum hemorrhage
oxytocin | third stage
Data and analyses
Comparison 1. Oxytocin versus no uterotonics or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss 500 mL or more after delivery | 6 | 4162 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.37, 0.72] |
| 2 Need for additional uterotonics | 4 | 3135 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.36, 0.80] |
| 3 Blood loss 1000 mL or more after delivery | 5 | 4123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.42, 0.83] |
| 4 Blood transfusion | 3 | 3081 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.44, 1.78] |
| 5 Third stage greater than 30 minutes | 1 | 1947 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [0.88, 7.44] |
| 6 Mean blood loss (mL) | 5 | 1359 | Mean Difference (IV, Random, 95% CI) | ‐99.13 [‐181.40, ‐16.85] |
| 7 Maternal haemoglobin concentration (Hb) < 7 g/dL 24‐48 hours PP | 2 | 1073 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.18, 2.26] |
| 8 Mean length of third stage (minutes) | 3 | 294 | Mean Difference (IV, Random, 95% CI) | ‐3.61 [‐9.06, 1.83] |
| 9 Manual removal of the placenta | 6 | 4281 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.89, 1.82] |
Comparison 2. Oxytocin versus no uterotonics or placebo‐subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss 500 mL or more after delivery; active v. expectant management | 5 | 4154 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.37, 0.73] |
| 1.1 Active management | 2 | 2920 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.22, 0.72] |
| 1.2 Expectant management | 3 | 1234 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.48, 0.81] |
| 2 Blood loss 500 mL or more after delivery; IM v. IV oxytocin | 6 | 4162 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.37, 0.73] |
| 2.1 IV oxytocin | 3 | 1978 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.21, 0.79] |
| 2.2 IM oxytocin | 3 | 2184 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.46, 0.87] |
| 3 Blood loss 500 mL or more after delivery; oxytocin dose < 10 IU v. 10 IU | 5 | 4154 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.37, 0.73] |
| 3.1 Oxytocin dose < 10 IU | 3 | 1204 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.24, 1.14] |
| 3.2 Oxytocin dose 10 IU | 2 | 2950 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.47, 0.66] |
| 4 Need for additional uterotonics; active v. expectant management | 4 | 3135 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.36, 0.80] |
| 4.1 Active management | 1 | 1901 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.26, 0.58] |
| 4.2 Expectant management | 3 | 1234 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.42, 0.99] |
| 5 Need for additional uterotonics; IM v. IV oxytocin | 4 | 3135 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.36, 0.80] |
| 5.1 IV oxytocin | 1 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.39, 0.82] |
| 5.2 IM oxytocin | 3 | 2135 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.24, 1.19] |
| 6 Need for additional uterotonics; oxytocin dose < 10 IU v. 10 IU | 4 | 3135 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.36, 0.80] |
| 6.1 Oxytocin dose < 10 IU | 2 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.22, 2.63] |
| 6.2 Oxytocin dose 10 IU | 2 | 2901 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.33, 0.68] |
Comparison 3. Oxytocin versus ergot alkaloids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss 500 mL or more after delivery | 10 | 3082 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.56, 1.25] |
| 2 Need for additional uterotonics | 8 | 2178 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.43, 1.81] |
| 3 Blood loss 1000 mL or more after delivery | 3 | 1577 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.63, 2.01] |
| 4 Blood transfusion | 7 | 1578 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.34, 5.51] |
| 5 Third stage > 30 minutes | 2 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.69 [1.63, 13.45] |
| 6 Diastolic blood pressure > 100 mm Hg between delivery of the baby and discharge from the labour ward | 3 | 960 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.04, 2.05] |
| 7 Mean blood loss (mL) | 11 | 3598 | Mean Difference (IV, Random, 95% CI) | ‐13.97 [‐43.70, 15.76] |
| 8 Mean length of third stage (minutes) | 8 | 2892 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.44, 0.61] |
| 9 Manual removal of the placenta | 8 | 3127 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.39, 3.10] |
| 10 Vomiting between delivery of the baby and discharge from the labour ward | 7 | 1991 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.05, 0.14] |
| 11 Headaches between delivery of the baby and discharge from the labour ward | 5 | 1543 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.03, 1.02] |
Comparison 4. Oxytocin versus ergot alkaloids‐‐subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss 500 mL or more after delivery; active v. expectant management | 8 | 2977 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.60, 1.35] |
| 1.1 Active management | 6 | 1743 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.47, 2.43] |
| 1.2 Expectant management | 2 | 1234 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.61, 1.10] |
| 2 Blood loss 500 mL or more after delivery; IM v. IV oxytocin | 10 | 3087 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.56, 1.25] |
| 2.1 IM oxytocin | 6 | 1278 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.48, 2.16] |
| 2.2 IV oxytocin | 4 | 1809 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.56, 1.05] |
| 3 Blood loss 500 mL or more after delivery; oxytocin dose < 10 IU v. 10 IU | 9 | 3077 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.56, 1.29] |
| 3.1 Oxytocin dose < 10 IU | 3 | 678 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.46, 0.95] |
| 3.2 Oxytocin dose 10 IU | 6 | 2399 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.54, 2.17] |
| 4 Need for additional uterotonics; active v. expectant management | 7 | 2078 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.48, 2.09] |
| 4.1 Active management | 6 | 1893 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.40, 2.32] |
| 4.2 Expectant management | 1 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.57, 2.72] |
| 5 Need for additional uterotonics; IM v. IV oxytocin | 8 | 2178 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.41, 1.82] |
| 5.1 IM oxytocin | 6 | 1428 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.47, 2.83] |
| 5.2 IV oxytocin | 2 | 750 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.13, 1.26] |
| 6 Need for additional uterotonics; oxytocin dose < 10 IU v. 10 IU | 8 | 2180 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.41, 1.83] |
| 6.1 Oxytocin dose < 10 IU | 3 | 678 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.18, 1.45] |
| 6.2 Oxytocin dose 10 IU | 5 | 1502 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.42, 3.48] |
Comparison 5. Oxytocin + ergometrine versus ergot alkaloids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss 500 mL or more after delivery | 3 | 1168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.20, 0.94] |
| 2 Manual removal of the placenta | 1 | 807 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.31, 3.65] |
| 3 Mean blood loss (mL) | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 61.0 [‐0.90, 122.90] |
| 4 Maternal all‐cause mortality | 1 | 807 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
5.4. Analysis.
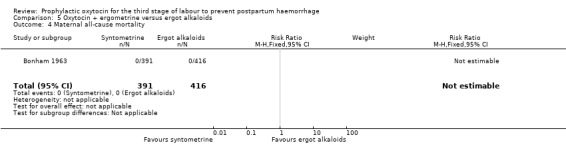
Comparison 5 Oxytocin + ergometrine versus ergot alkaloids, Outcome 4 Maternal all‐cause mortality.
Comparison 6. Oxytocin + ergometrine versus ergot alkaloids‐‐subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss 500 mL or more after delivery; active v. expectant management | 1 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.15, 1.52] |
| 1.1 Active management | 1 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.15, 1.52] |
| 1.2 Expectant management | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Blood loss 500 mL or more after delivery; IM v. IV oxytocin | 3 | 1168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.20, 0.94] |
| 2.1 IM oxytocin | 2 | 1161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.20, 0.94] |
| 2.2 IV oxytocin | 1 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.
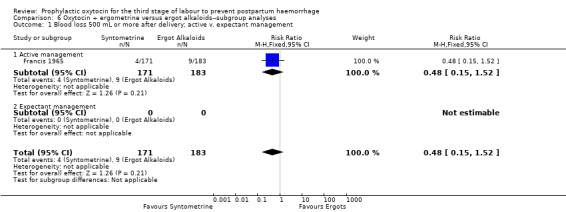
Comparison 6 Oxytocin + ergometrine versus ergot alkaloids‐‐subgroup analyses, Outcome 1 Blood loss 500 mL or more after delivery; active v. expectant management.
6.2. Analysis.
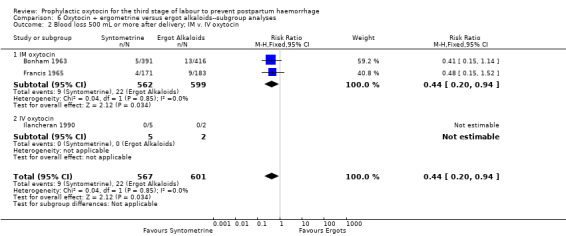
Comparison 6 Oxytocin + ergometrine versus ergot alkaloids‐‐subgroup analyses, Outcome 2 Blood loss 500 mL or more after delivery; IM v. IV oxytocin.
Comparison 7. Oxytocin versus no uterotonics or placebo‐‐subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss 500 mL or more after delivery; active v. expectant management | 5 | 4154 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.37, 0.73] |
| 1.1 Active management | 2 | 2920 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.22, 0.72] |
| 1.2 Expectant management | 3 | 1234 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.48, 0.81] |
| 2 Blood loss 500 mL or more after delivery; IM v. IV oxytocin | 6 | 4162 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.37, 0.73] |
| 2.1 IV oxytocin | 3 | 1978 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.21, 0.79] |
| 2.2 IM oxytocin | 3 | 2184 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.46, 0.87] |
| 3 Blood loss 500 mL or more after delivery; oxytocin dose < 10 IU v. 10 IU | 5 | 4154 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.37, 0.73] |
| 3.1 Oxytocin dose < 10 IU | 3 | 1204 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.24, 1.14] |
| 3.2 Oxytocin dose 10 IU | 2 | 2950 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.47, 0.66] |
| 4 Need for additional uterotonics; active v. expectant management | 4 | 3135 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.36, 0.80] |
| 4.1 Active management | 1 | 1901 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.26, 0.58] |
| 4.2 Expectant management | 3 | 1234 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.42, 0.99] |
| 5 Need for additional uterotonics; IM v. IV oxytocin | 4 | 3135 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.36, 0.80] |
| 5.1 IV oxytocin | 1 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.39, 0.82] |
| 5.2 IM oxytocin | 3 | 2135 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.24, 1.19] |
| 6 Need for additional uterotonics; oxytocin dose < 10 IU v. 10 IU | 4 | 3135 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.36, 0.80] |
| 6.1 Oxytocin dose < 10 IU | 2 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.22, 2.63] |
| 6.2 Oxytocin dose 10 IU | 2 | 2901 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.33, 0.68] |
7.1. Analysis.
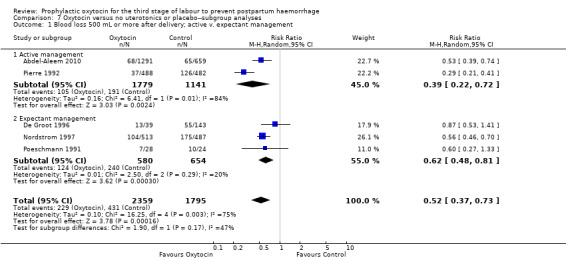
Comparison 7 Oxytocin versus no uterotonics or placebo‐‐subgroup analyses, Outcome 1 Blood loss 500 mL or more after delivery; active v. expectant management.
7.2. Analysis.
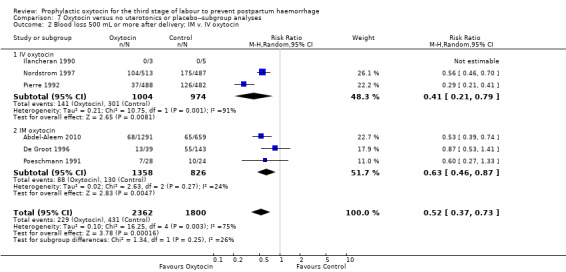
Comparison 7 Oxytocin versus no uterotonics or placebo‐‐subgroup analyses, Outcome 2 Blood loss 500 mL or more after delivery; IM v. IV oxytocin.
7.3. Analysis.
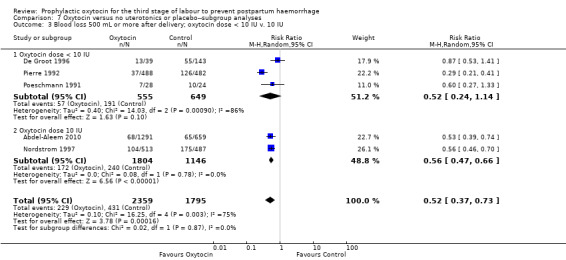
Comparison 7 Oxytocin versus no uterotonics or placebo‐‐subgroup analyses, Outcome 3 Blood loss 500 mL or more after delivery; oxytocin dose < 10 IU v. 10 IU.
7.4. Analysis.
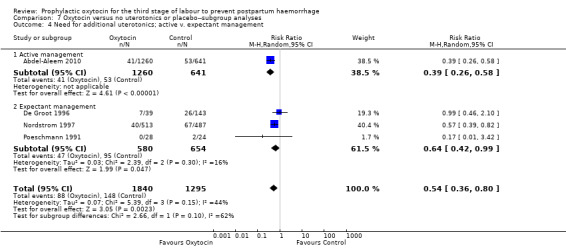
Comparison 7 Oxytocin versus no uterotonics or placebo‐‐subgroup analyses, Outcome 4 Need for additional uterotonics; active v. expectant management.
7.5. Analysis.
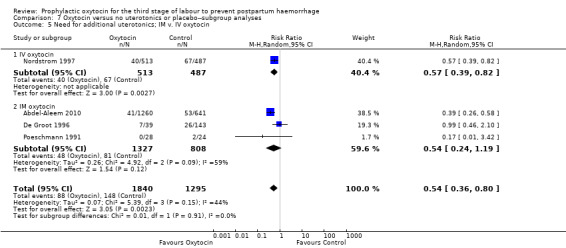
Comparison 7 Oxytocin versus no uterotonics or placebo‐‐subgroup analyses, Outcome 5 Need for additional uterotonics; IM v. IV oxytocin.
7.6. Analysis.
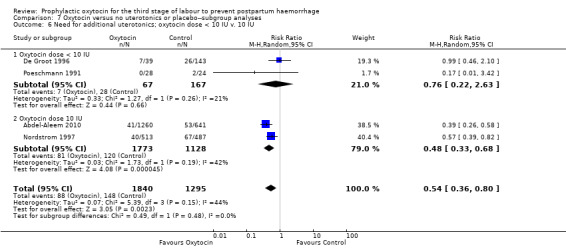
Comparison 7 Oxytocin versus no uterotonics or placebo‐‐subgroup analyses, Outcome 6 Need for additional uterotonics; oxytocin dose < 10 IU v. 10 IU.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdel‐Aleem 2010.
| Methods | Randomised controlled trial. Women were randomly allocated to 1 of 3 groups by selecting the next number in a computer‐generated random number sequence. The allocated group was noted inside opaque sealed envelopes. Not blinded. |
|
| Participants | 1964 pregnant woman who were expected to have a vaginal delivery at Women's Health Center Assiut, Egypt and the Department of Obstetrics and Gynecology, East London Hospital Complex, East London South Africa between September 1, 2006 and February 28, 2009. Women were excluded for medical complications as follows; hypertension, diabetes, previous caesarean section, abdominal wall not thin enough to allow adequate palpation of uterus after delivery. | |
| Interventions | All interventions were given after delivery of the anterior shoulder or after delivery of the neonate. 1) 10 IU IM oxytocin (643 women) 2) Sustained uterine massage shortly after delivery performed by the research midwives; massage was sustained for 30 minutes an involved manual stimulation of the whole surface of the uterus (662 women) 3) Combined management with 10 IU IM oxytocin plus uterine massage (659 women) In all 3 groups active management was performed: the umbilical cord was clamped soon after delivery of the neonate and the placenta was delivered by controlled cord traction when the uterus became contracted. A plastic drape or a low profile plastic bedpan was placed under the mother's buttocks after delivery of the neonate to collect the blood lost within 30 minutes of delivery. For the group that did not initially receive oxytocin, injections of oxytocin were given if blood loss > 500 mL occurred during the 30‐minute collection time. Comparison for review is groups 1 and 3 combined (1302 women) vs group 2 (662 women) |
|
| Outcomes | Blood loss > 300 mL, > 500 mL or > 1000 mL within 30 minutes of delivery, delivery of the placenta within 30 minutes of neonate delivery, use of additional uterotonics or other procedures to manage haemorrhage, Hb level after 12‐24 hours of < 8 g in 100 mL or < 10 g in 100 mL (South Africa only), blood transfusion, MRP or placenta not delivered in 30 minutes, maternal morbidity (including admission to higher level of care), and adverse effects (nausea, vomiting, pain or discomfort). | |
| Notes | Dates of study: September 2006‐February 2009 Funding sources: not reported Declarations of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomly allocated to 1 of 3 groups by selecting the next number in a computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Low risk | The allocated group was noted inside opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding procedures, but given the nature of the treatments it would not have been possible to blind either participants or personnel Assessment of blood loss leading to assessment of need for use of additional uterotonics can be subjective. Uterine massage too can vary amongst providers. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding procedures for outcome assessment. Some outcomes are subjectively assessed and lack of blinding could impact on outcomes, but others are objectively measured and lack of blinding would have little impact. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal loss. Loss similar (n = 3 to 7) in each arm. |
| Selective reporting (reporting bias) | Unclear risk | All appear to be reported that are listed in methods text, however trial protocol not available. |
| Other bias | Unclear risk | Unclear. |
Adhikari 2007.
| Methods | Quasi‐randomised controlled trial. Women were allotted consecutively into Group A or B in the second stage when quote: “delivery imminent.” Participants were not blinded. | |
| Participants | Women presenting for delivery at the Tribhuvan University Teaching Hospital in Nepal over a 1‐year period in 2004. All women with parity < 5, singleton live pregnancy at or above 37 weeks, cephalic, with spontaneous onset of labour and spontaneous vaginal delivery without complicating factors were included. Women with the following were excluded: parity > 4, multiple gestation, < 37 weeks, women with “complicating factors” (not specified). Women were excluded if they had an instrumental or caesarean delivery, precipitous labour or lack of postpartum blood sample. |
|
| Interventions | Immediately after delivery participants received: A. oxytocin 10 IU IM (n = 100) or B. methylergometrine 0.2 mg IM (n = 100) All received early cord clamping, cord traction, and uterine massage. |
|
| Outcomes | Mean decrease in Hb/Hct measurements between admission in labour and 24 hours postpartum, incidence of PPH (defined as peripartum fall in Hct of 10%), need for additional uterotonics, need for exploration and uterine evacuation, blood transfusion, nausea, vomiting, headache, retained placenta (need for manual removal), rise in blood pressure (systolic > 15 mmHg and diastolic > 10 mmHg) | |
| Notes | Dates of study: 2004 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomisation, quote:“women were allotted consecutively into two groups at the second stage of labour…if the first woman was enrolled into Group A then the next would be in Group B and so on.” |
| Allocation concealment (selection bias) | High risk | Alternation of assignment into Group A and B: quote: “women were allotted consecutively into two groups at the second stage of labour…if the first woman was enrolled into Group A then the next would be in Group B and so on.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Women were excluded after randomisation, however the authors do not clarify how many were lost from each group and how this attrition was addressed. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess. |
| Other bias | Unclear risk | Unclear. |
Bader 2000.
| Methods | Randomised controlled trial. Women were randomly allocated. No further information is given aside from confirmation that the allocation was randomised. Not blinded. |
|
| Participants | 180 women in the third stage of labour at the Gynaecological Clinic of the University of Witten/Herdecke, part of the Marienhospital Witten. Primary grounds for exclusion included complicated pregnancies requiring oxytocin stimulation during delivery, multiple pregnancies, weight over 100 kg, uterus myomatosus, previous treatment with oxytocin and conditions tending to increase blood loss. Secondary grounds were the need for surgical intervention (forceps or vacuum) in delivery, unusually high levels of blood loss of unknown origin and placenta delivery times longer than 30 minutes after delivery. |
|
| Interventions | After delivery of the fetus, women were randomly assigned to receive: 1) acupuncture: 2 needles (0.3 x 25 mm) applied 1.5 cm on either side of the navel (point Ni16); 2) oxytocin: 3 units administered intravenously directly after delivery; 3) control: no treatment. After the birth, waterproof bedding was laid down in order to measure blood loss. The time between delivery of the baby and delivery of the placenta was measured in minutes. After delivery of the placenta the waterproof bedding was removed and weighed (to measure blood loss). The Hb levels of each patient were measured on arrival in the delivery room and on leaving the hospital. The midwives involved were advised not to interfere postpartum with the uterus and umbilical cord‐‐expectant management. Comparison for review is group 2 vs group 3. |
|
| Outcomes | Primary outcomes included blood loss and the length of the placental delivery period. The duration of the birth and the delivery period were also recorded. | |
| Notes | Only the oxytocin and control group data are used in the analysis. Dates of study: 1998 to 1999 specified in secondary reference Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described other than quote: "allocation was randomised". |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The treatment was not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were a total of 20 exclusions on various secondary exclusion grounds: 1 in the control group, 12 in the acupuncture group and 7 in the oxytocin group, leaving a total of 160 patients. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | Unclear. |
Bonham 1963.
| Methods | Randomised controlled trial. Selection of drug was made by random numbers. Timing of randomisation not stated. Not blinded. |
|
| Participants | All vaginal deliveries April 1961 to October 1962 in hospital in London, except: multiple pregnancies, previous PPH or manual removal, forceps and breech deliveries must be post‐randomisation exclusions but does not state how many were randomised), parity 4 or more, induction or augmentation with syntocinon. | |
| Interventions | (1) IM 0.5 mg ergometrine + 5 units synthetic oxytocin, given at crowning of the head (n = 391).
(2) IM 0.5 mg ergometrine, given at crowning of the head (n = 416).
[Third group of ergometrine + hyaluronidase not considered for this review.]
Women were also selected in random 2‐week groups to either controlled cord traction (n = 199 ergometrine + oxytocin vs 217 ergometrine alone) or maternal effort/fundal pressure (192 vs 199)‐‐combination of both active and expectant management.
No information about timing of cord clamping/cutting. Blood loss was estimated by adding to the measured quantity a figure for loss on linen and swabs used during the perineal repair. |
|
| Outcomes | Primary PPH (> 568 mL estimated by adding to measured quantity a figure for loss on linen and swabs used for perineal repair); mean blood loss (154 vs 178 mL, SD not given); mean length of third stage (6.3 vs 6.2 minutes, SD not given); prolonged third stage (> 30 minutes); MRP. | |
| Notes | Dates of study: April 1961 – September 1962 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation by numbers; procedure not described in detail. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of the intervention was not possible because differing numbers of ampoules were needed for different trial arms. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition described; no loss of data. |
| Selective reporting (reporting bias) | Unclear risk | Protocol unseen |
| Other bias | Unclear risk | Unclear. |
Boopathi 2014.
| Methods | Quasi‐randomised controlled trial. Women were assigned to groups based on an even or odd inpatient number. | |
| Participants | Women presenting for delivery at a labour ward in India between April 2012 and January 2013. Women presenting with a singleton term cephalic pregnancy with spontaneous labour onset, no contraindications to oxytocin or methylergometrine and no known risk factors for PPH were included. Women with the following were excluded: operative deliveries, multiple pregnancy, fetal demise, Rh alloimmunisation, hypertension, anaemia (Hb < 9 g/dL), heart disease, past history of third stage complications, prior caesarean section, disorders of blood coagulation. |
|
| Interventions | Immediately after delivery participants received: A. oxytocin 10 IU IM (n = 150) or B. methylergometrine 0.2 mg IV (n = 150) All received early cord clamping and intermittent cord traction. Following placental delivery, a conical graduated plastic collection bag was placed below patient and blood loss was measured after 1 hour. |
|
| Outcomes | Incidence of PPH (measured blood loss > 500 mL), pre‐delivery and post‐delivery Hct, need for additional uterotonics, duration of the third stage, measured blood loss by calibrated drapes, blood transfusion, side effects including nausea and vomiting, high blood pressure. | |
| Notes | Dates of study: April 2012 ‐ January 2013 Funding sources: self funded by corresponding author Declarations of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomisation: quote: “eligible women … were assigned to 2 groups at a random of 150 in each group. Women with even inpatient number were allotted to Group 1 and odd inpatient number allotted to Group 2.” |
| Allocation concealment (selection bias) | High risk | Participants assigned to 2 groups based on even or odd inpatient numbers. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Women were excluded from the study following randomisation if there was quote: “profuse bleeding following episiotomy.” Unclear how many women from each group were excluded and how this attrition was addressed. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess. |
| Other bias | Unclear risk | Unclear. |
De Groot 1996.
| Methods | Randomised controlled trial. Hospital pharmacy supplied numbered boxes of tablets and ampoules according to computer‐generated randomisation list. Informed consent asked in early labour. Assigned before delivery of baby's head. Double‐blind for oral ergometrine vs placebo and unblinded for ergometrine and/or placebo vs oxytocin. Randomisation 1:2:2, oxytocin to ergometrine to placebo. Multicentre. |
|
| Participants | 2 university hospitals, a midwifery school and independent midwives attending home births in and around Nijmegen, the Netherlands. Women expecting to deliver in one of these settings, and who did not develop following exclusion criteria: refusal, cardiovascular disease/hypertension, multiple pregnancy, non‐cephalic presentation, polyhydramnios, tocolysis 2 hours prior to delivery, anticoagulant therapy, stillbirth, APH, chemical induction or augmentation (oxytocin, prostaglandins), instrumental/operative delivery (some of these must have been post‐randomisation exclusions), anaemia Hb < 6.8 mmol/L (timing not stated), previous third stage complications. 4 of 371 women were assigned to the study erroneously (3 forceps, 1 augmentation) and were excluded post‐randomisation. Otherwise eligible women wishing a natural childbirth refused to enter the trial (numbers not stated). | |
| Interventions | All 3 interventions given immediately after birth of baby:
(1) IM 5 IU oxytocin (n = 78);
(2) oral 0.4 mg ergometrine (n = 146);
(3) oral placebo (143).
Other third stage management expectant (although no information given about timing of cord clamping/cutting). When mother feels contractions or there are signs of separation, maternal effort encouraged, adopting position to aid gravity. If necessary, flat hand on abdomen to act as brace to aid pushing. Re‐attempt if placenta does not deliver spontaneously. If haemorrhage, administer extra oxytocics and/or controlled cord traction. Blood loss measured gravimetrically‐‐fresh perineal pad under perineum to absorb blood or fluid; gauzes and pads collected until 1 hour after delivery of placenta and weighed. 100 g increase in weight considered equivalent to 100 mL blood. Comparison for review is group 1 vs group 2 and group 1 vs group 3. |
|
| Outcomes | Mean blood loss (mL); PPH (> = 500 mL); severe PPH (> = 1000 mL); length of third stage (11 (range 4‐90), 15 (2‐90), 14 (3‐55) in oxytocin, ergometrine and placebo groups respectively. No information about whether mean or median, and SD not given); blood pressure 15, 30, 45 and 60 minutes after delivery of placenta, in institutional deliveries only (oral ergometrine showed no significant elevation); use of further oxytocics; MRP; transfusion. | |
| Notes | Dates of study: July 1993 – July 1994 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Numbered boxes of tablets and ampoules according to computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | No difference could be detected between boxes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Drugs administered via different routes so blinding not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned in report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition minimal and where outcome data missing, adequate explanation given. |
| Selective reporting (reporting bias) | Unclear risk | Protocol unseen |
| Other bias | Unclear risk | Unclear. |
Dhananjaya 2014.
| Methods | Quasi‐randomised controlled trial. Women were assigned to oxytocin IM or methylergometrine IM by random sampling method alternatively during the third stage of labour. | |
| Participants | Women presenting to the Sri Siddhartha Medical College Hospital between December 2011 and May 2013. Women after 28 weeks' gestation anticipating a vaginal delivery were included. Women with the following were excluded: grand multiparity, rhesus negative, heart disease, diabetes, bleeding disorders, precipitated labour, overdistended uterus, traumatic PPH, PROM or chorioamnionitis, IUD, previous caesarean section or prior uterine scar, inability to obtain informed consent. |
|
| Interventions | Immediately after delivery women received: A. oxytocin 10 IU IM (n = 50) or B. methylergometrine 0.2 mg IM (n = 50) Blood was collected in drapes and pre‐weighed mops following delivery, and blood loss measured by weight. A sample of venous blood before and 24 hours after delivery was obtained for Hb/Hct measurements. |
|
| Outcomes | Measured blood loss (mL), duration of the 3rd stage (minutes), mean percentage fall in Hb and Hct (%), PPH (blood loss > 500 mL), need for additional uterotonics, blood transfusion, nausea, vomiting, headache, diarrhoea | |
| Notes | Dates of study: December 2011 ‐ May 2013. Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Study was quasi‐randomised: quote: “selection of cases were done by systematic random sampling method, assigned to intramuscular oxytocin… or intramuscular methylergometrine… alternatively during third stage” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess. Method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to assess. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess. |
| Other bias | Unclear risk | Unclear. |
Ezeama 2014.
| Methods | Randomised double‐blind controlled trial. Participants were randomised using computer‐generated randomisation numbers. | |
| Participants | Women admitted to the labour and delivery ward at the Nnamdi Azikiwe University Teaching Hospital in Nnewi, Nigeria between September 2011 and May 2012. Women in labour, without an epidural and anticipating a vaginal delivery were included. Women with the following were excluded: anticipating a caesarean delivery, delivery < 28 weeks, multiple gestation, antepartum haemorrhage, hypertensive disorders, severe anaemia, haemoglobinopathy. |
|
| Interventions | Immediately after delivery women received: A. oxytocin 10 IU IM (n = 151) or B. ergometrine 0.5 mg IM (n = 149) All received AMTSL including cord traction and uterine massage. Following drug administration, a fresh pad was placed and volume blood loss assessed after 1 hour by weighing of pads and gauze. |
|
| Outcomes | Primary outcomes: blood loss > 500 mL, occurrence of adverse effects (headache, vomiting, increased diastolic pressure) within 30 minutes of the intervention Secondary: use of additional uterotonics, blood transfusion, evacuation of retained products, manual removal of the placenta, PPH, pre‐ and post‐delivery Hct | |
| Notes | Dates of study: September 2011 ‐ May 2012 Funding sources: not reported Declarations of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using “computer generated randomisation numbers. Quote:” Eligible patients were allocated the “next consecutive randomisation number.” |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed using quote: “computer generated randomisation numbers.” Eligible patients were allocated the “next consecutive randomisation number.” Drug ampoules were “placed in opaque sealed envelopes.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded. Quote: “Person uninvolved with the study prepared the study drugs: 1‐mL ampoules containing either 10 IU oxytocin or 0.5 mg ergometrine. The labels on the ampoules (which were similar in size and color) were removed and the ampoules were placed in opaque sealed envelopes. The study drugs were administered ... by a midwife who was not part of the study.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded. See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess. |
| Other bias | Unclear risk | Unclear. |
Francis 1965.
| Methods | Quasi‐randomised controlled trial. 'Ampoules used in rotation and participants were unselected'. Blinded. |
|
| Participants | 2 maternity hospitals in Liverpool, UK. in 1961. All women expected to deliver except those in whom an abnormal third stage was anticipated (previous PPH, instrumental or breech deliveries, twin pregnancies, APH, severe anaemia, IV oxytocin for induction or augmentation). | |
| Interventions | (1) 1 mL IM ergometrine‐oxytocin (5 IU oxytocin + 0.5 mg per 1 mL ergometrine) after delivery of baby and cord divided, AND 1 mL water after placental delivery (n = 171).
(2) 0.5 mg IM ergometrine after delivery of baby and cord divided, AND 1 mL water after placental delivery (n = 183).
(3) 1 mL IM water after delivery of baby and cord divided, AND 0.5 mg IM ergometrine after placental delivery (n = 167).
The collection of blood commenced with birth of the baby and continued for 1 hour after delivery. Swabs were rung out manually. Blood loss was measured in a graduated jug. When signs of descent became apparent, the placenta delivered with uterine massage and cord traction‐‐active management. Comparison in review is between groups 1 and 2. |
|
| Outcomes | Blood loss (average 4.9, 6.4, 7.0 in groups 1, 2 and 3, respectively ‐ no SD given); for the review, loss of > 20 oz has been taken as PPH; retained placenta (> 20 minutes). | |
| Notes | Dates of study: during 1962 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Treatments were rotated, no random sequence generated. |
| Allocation concealment (selection bias) | High risk | Although intervention administered using identical vials, the rotation method used means that allocation could possibly be foreseen. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Vials were blinded to personnel and participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The authors describe how observers were not blinded in their initial trial and say that this flaw was corrected in the second trial (from which the review draws its data) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of women originally recruited and ‘randomised’ is not stated so it is not possible to know attrition rate. |
| Selective reporting (reporting bias) | Unclear risk | Difficult to assess. Report appears to report outcomes as expected, however protocol unseen. |
| Other bias | Unclear risk | Unclear. |
Fugo 1958.
| Methods | Randomised controlled trial. Numbered identical drug packages administered in rotation. Number meaningless to obstetrician. Blinded. |
|
| Participants | Women delivering in a hospital in Chicago, USA. No details given of inclusion/exclusion criteria, but description of study participants showed that half had labour over 8 hours, and 98% received some anaesthetic agent. |
|
| Interventions | All administered intravenously in 2 mL with anterior shoulder.
(1) 2 IU oxytocin (natural oxytocin) n = 168.
(2) 2 IU syntocinon (synthetic oxytocin) n = 156.
(3) 4 mg ergonovine 149.
(4) 80 mg U3772 (alpha, alpha diphenyl gamma dimethylamino N‐methyl valeramide‐HCl) n = 151.
Blood lost when the placenta separated was collected in a basin containing 200 mL of 4% sodium oxalate solution as an anticoagulant and was measured in a graduated jug. Expectant management of the third stage with MRP at 10 minutes for teaching purposes. Comparison for review is groups 1 and 2 combined vs group 3. |
|
| Outcomes | Method of placental delivery (high % of manual removals for teaching purposes if haemorrhage or undelivered within 10 minutes); length of third stage (not significantly different between groups but data only given for those delivered spontaneously, i.e. within 10 minutes); blood loss with placenta; (1‐hour postpartum average blood loss 50.2 vs 40.8 mL; no SDs given). | |
| Notes | Given the high number of manual placental removals for teaching purposes, the data from this trial were not used due to concern for methodologic bias and lack of clinical translatability of this trial as MRP this early in the third stage is not standard of care. Dates of study: during 1958 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical packages were used, identifiable only by number, which was meaningless to the obstetrician in charge of the case. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Identical packages were used, identifiable only by number, which was meaningless to the obstetrician in charge of the case. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No significant attrition. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | Unclear. |
Ilancheran 1990.
| Methods | Randomised controlled trial. 'Consecutive participants divided equally into 4 subgroups, distribution being done on a random basis'. |
|
| Participants | Women in spontaneous labour between 38 and 42 weeks' gestation with normal vertex deliveries in hospital in Singapore. | |
| Interventions | Control group and 3 groups given IV uterotonic in 'standard' doses with the delivery of the anterior shoulder. A. No oxytocin in third stage (n = 5) B. Oxytocin (n = 5) C. Ergometrine‐oxytocin (n = 5) D. Ergometrine (n = 5) Blood loss estimation technique not described. Other methods to manage third stage of labour not described. Comparisons for this review are: B vs A; B vs D; C vs D. |
|
| Outcomes | Prostaglandin levels 5, 15 and 30 minutes after delivery, and PPH. | |
| Notes | Dates of study: not reported Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding or who prepared the “standard doses” of each drug. Assumed care‐givers knew allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned in report |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear from the report whether any attrition. |
| Selective reporting (reporting bias) | Unclear risk | Appears to report all outcomes pre‐specified in methods, however protocol unseen. |
| Other bias | Unclear risk | Unclear. |
Jago 2007.
| Methods | Randomised controlled trial. Randomisation was performed using a computer‐generated table of random numbers, which were labelled on envelopes containing the drug (ergometrine or oxytocin). |
|
| Participants | 510 consenting normotensive women with singleton pregnancies and no proteinuria at a hospital in Nigeria. Excluded those with history of hypertensive disorders of pregnancy, hypertension, chronic renal disease, endocrine disorders, vascular or cardiac disease, on anticoagulant therapy, having epidural anaesthesia, with allergy to 1 of the drugs under study, and those with intended instrumental/operative delivery. |
|
| Interventions | At delivery of the anterior shoulder: A. oxytocin 10 IU IV (n = 256). B. ergometrine 0.5 mg IM (n = 254). Management of the third stage of labour not otherwise described. Technique for measurement of blood loss not described. |
|
| Outcomes | Elevated blood pressure (> 140/90 mmHg). Estimated blood loss (mL). | |
| Notes | Dates of study: January 2001 – December 2002 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “The numbers were clearly labelled on envelopes containing a particular oxytocic”, however it is unclear whether the envelopes were sealed and opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is not described, but it is unlikely that personnel were blinded as different quantities of each drug were administered. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Report does not include enough information to assess whether there was attrition. |
| Selective reporting (reporting bias) | Unclear risk | Protocol unseen. |
| Other bias | Unclear risk | No data. |
Jerbi 2007.
| Methods | Randomised controlled trial. Not stated. Authors state: "...women were randomly allocated to...". |
|
| Participants | 130 women with singleton pregnancies at term who were expected to deliver vaginally in a hospital in Tunisia. Excluded: placenta previa, APH, non‐cephalic presentation, history of PPH, intrauterine death, parity > 5, caesarean section, uterine fibroids, anticoagulant therapy. |
|
| Interventions | At the time of delivery of the anterior shoulder: A. oxytocin 5 IU IV (n = 65); B. no oxytocin (n = 65). Authors say that the comparison arms are active vs expectant management‐‐active is defined as receiving prophylactic oxytocin. The third stage of labour was managed in the same way for all women: immediate cord clamping and cutting, controlled cord traction and gentle fundal pressure. |
|
| Outcomes | Decrease in Hct, decrease in Hb concentration, duration of the third stage of labour (min), MRP, maternal Hb concentration, postpartum anaemia. Total blood loss was not an outcome. |
|
| Notes | Dates of study: February to March 2005. Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. Authors only state: "...women were randomly allocated to...". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. Authors only state: "...women were randomly allocated to...". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Intervention involves injection, the control did not. There is no suggestion that the control arm received a placebo injection. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned in report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported for the outcomes included in the review. |
| Selective reporting (reporting bias) | Unclear risk | Protocol unseen but outcomes pre‐specified in methods section are reported |
| Other bias | Unclear risk | No data. |
McGinty 1956.
| Methods | Randomised trial. 'Cases picked at random'. Unblinded. |
|
| Participants | All vaginally delivered under pudendal block and demerol/scopolamine, in hospital in the USA. | |
| Interventions | Drug given at birth of anterior shoulder:
A. 1 mL normal saline intravenously (n = 50);
B. 0.2 mg methergine intravenously (n = 50);
C. 0.2 mg ergonovine intravenously (n = 50);
D. oxytocin 5 IU each intravenously and intramuscularly (n = 50).
Comparisons for this review:
D vs B and C. Data not provided for control group so this group was not included in this review. No information about other aspects of third stage management. |
|
| Outcomes | Diastolic and systolic blood pressure 5, 15 and 60 minutes after administration; estimated severe blood loss over 1000 mL mentioned for 1 women in methergine series and 1 in control group (not included in data tables as unlikely to have been systematically recorded). | |
| Notes | Dates of study: not reported Funding sources: quote: “All Methergine used in this study was supplied through the courtesy of Sandoz Pharmaceuticals, New York, New York.” Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Cases picked at random". Randomisation technique not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not explicitly described, although some instances where blinding of personnel breached mentioned which suggests inadequate blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 200 patients recruited, and data for all 200 individuals reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol unseen. |
| Other bias | Unclear risk | Unclear. |
Modi 2014.
| Methods | Randomised controlled trial. Patients were randomised into 4 groups. |
|
| Participants | Women presenting to the Shri Ram Murti Smarak Institute of Medical Sciences, Bareilly, Uttar Pradesh from 2012 to 2013. Women who were gravida < 4 with a singleton pregnancy between 37 and 42 weeks' gestation, cephalic, with no high risk factors presenting for induction or in spontaneous labour, were included. Women with the following were excluded: gestation < 37 weeks or > 42 weeks, fetal demise, fetal growth restriction, hypertension, abruption, placenta previa, multiple pregnancy, grand multipara, malpresentation, chorioamnionitis, known blood coagulation disorder, known allergy to prostaglandins, history of medical disorders including cardiac or renal disease, anaemia with Hb < 8, pulse rate > 100 bpm, blood pressure < 90/60 mm Hg. |
|
| Interventions | Immediately after delivery women received: A. oxytocin 10 IU IM (n = 25) or B. methylergometrine 0.2 mg IV (n = 25) All received controlled cord traction to facilitate placental delivery. Following delivery, calibrated drapes were placed beneath the patient to measure blood loss. There were 2 additional intervention arms in this trial that were not relevant to this review. 1 group received 15‐methyl PGF2‐alpha 125 mcg IM, and the other received misoprostol 600 mcg PR. |
|
| Outcomes | Outcomes: duration of the third stage of labour, measured blood loss in the third stage, decrease in mean Hb levels, post delivery heart rate and blood pressure, side effects of various uterotonics including nausea, vomiting, shivering, fever, hypertension, tachycardia. Other outcomes that were not pre‐specified: # of patients with blood loss > 500 mL, # of patients requiring blood transfusion, use of additional uterotonics. | |
| Notes | Dates of study: 2012 ‐ 2013. Funding sources: none Declarations of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation is unclear. Authors state “patients were randomised into four groups of 25 each” and “patients were distributed in four different groups randomly.” |
| Allocation concealment (selection bias) | Unclear risk | Method of randomisation is unclear. Authors state “patients were randomised into four groups of 25 each” and “patients were distributed in four different groups randomly.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Authors do not explicitly state how many patients were ultimately included in the analysis. They do not mention patient attrition, however they state that women with perineal and cervical lacerations were excluded from the study. According to their demographics table, the majority of patients received an episiotomy, which would have resulted in exclusion of significant numbers of patients after randomisation. |
| Selective reporting (reporting bias) | High risk | Some adverse outcomes of interest are reported incompletely. The authors report “side effects of various uterotonics” as outcomes, however data regarding vomiting, nausea, shivering, fever, headache and hypertension are incompletely reported for the oxytocin and methylergometrine groups. |
| Other bias | Unclear risk | Unclear. |
Moodie 1976.
| Methods | Randomised trial. Not stated, authors state "...the allocation being at random...".. |
|
| Participants | 148 women with instrumental deliveries (143 forceps, 5 vacuum) under epidural anaesthesia in a hospital in New Zealand. Excluded multiple births and breech presentation. |
|
| Interventions | At delivery of the anterior shoulder: A. oxytocin 5 IU IV (n = 70); B. ergometrine 0.5 mg IV (n = 78). No mention of other aspects of the management of the third stage of labour. |
|
| Outcomes | Blood loss (mL). Emetic sequelae (retching or vomiting and nausea). |
|
| Notes | Blood loss was measured in only 54% of women (80/148), so this outcome was not included in this review. Dates of study: not reported Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Authors only state: "...the allocation being at random...". Allocation described as random but sequence generation, and therefore predictability, unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned in report |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned in report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No (for nausea and vomiting). 46% of women excluded from outcome "blood loss", thus this outcome was not included in the review. |
| Selective reporting (reporting bias) | Unclear risk | Protocol unseen. |
| Other bias | Unclear risk | No data. |
Nordstrom 1997.
| Methods | Double‐blind randomised trial. 2 sets of ampoules prepared and numbered according to computer‐generated schedule. Contents unknown to women or caregivers. | |
| Participants | Hospital in Sweden. Singleton cephalic vaginal deliveries. | |
| Interventions | 1 mL IV after delivery of baby of either:
1) 10 IU oxytocin (n = 513)
2) saline (n = 487) Passive (expectant) management of the placenta. Blood loss was calculated by measuring collected blood and adding what was estimated to have been absorbed by surgical cloths and tissues. |
|
| Outcomes | Blood loss; additional uterotonics (methylergometrine), Hb, blood transfusion; manual placental removal. | |
| Notes | Dates of study: 16 December 1993 to 6 October 1994 Funding sources: Quote: “This study was supported by grants from the County Council and County Health Authority Research and Development Foundation in the County of Jämtland, Sweden.” Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 2 sets of ampoules prepared and numbered according to computer‐generated schedule. |
| Allocation concealment (selection bias) | Low risk | No difference in appearance of ampoules, prepared by pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Intervention unknown to women and caregivers. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Intervention unknown to outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No significant attrition. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported but protocol unseen |
| Other bias | Unclear risk | Unclear. |
Orji 2008.
| Methods | Randomised controlled trial. Eligible participants who gave informed consent were randomly allocated to either oxytocin or ergometrine group. Allocation was done by opening a sealed envelope from a pack that had been arranged serially. Not blinded. |
|
| Participants | 600 consenting women in labour with no illnesses or added risk in the active phase at 2 tertiary hospitals in Nigeria. Excluded those with hypertensive disorders of pregnancy, packed cell volume < 30%, history of PPH, haemoglobinopathy, heart disease or caesarean section. |
|
| Interventions | At delivery of the anterior shoulder: A. oxytocin 10 IU IV (n = 297); B. ergometrine 0.25 mg IV (n = 303). In both groups the third stage of labour was managed actively. Blood loss was measured using a pre‐weighed guaze that was weighed again after delivery. |
|
| Outcomes | Primary outcomes: PPH (> 500 mL), severe PPH (> 1000 mL). Secondary outcomes: retained placenta, need for blood transfusion, manual placental removal, estimated blood loss (mL), nausea, vomiting, headaches, elevated blood pressure, need for additional uterotonics. |
|
| Notes | Dates of study: January 2006 to September 2007 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned to previously determined sequence. |
| Allocation concealment (selection bias) | Low risk | Quote: "...sealed envelopes arranged serially...". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned in report assumed not due to different doses of drug being given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned in report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data appear to be reported for all participants. No loss to follow‐up reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol unseen but all outcomes pre‐specified in methods text are reported |
| Other bias | Unclear risk | No data. |
Pierre 1992.
| Methods | Quasi‐randomised trial. Leaflets marked from 1‐1000 alternate allocation quote: "this made possible a control of selection bias at entry by the authors as the order in the trial had the same chronology as the date and time of entry in the labour ward". |
|
| Participants | Women expecting to deliver vaginally in hospital in France. Excluded breech presentations, twins, antepartum haemorrhage, refusal to participate in study. | |
| Interventions | Active management of third stage with (n = 488) and without 5 IU IV oxytocin (n = 488) with the anterior shoulder. Blood loss was estimated by placing a large plastic sheet under the patient's bottom from delivery of the infant until delivery of the placenta. Third stage managed actively. |
|
| Outcomes | Blood loss, length of third stage, manual placental removal, maternal side effects. | |
| Notes | Dates of study: March to October 1987 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised. Numbered leaflets given to all women via their files. Of those who then consented to participation, allocation based on odd/even numbers. |
| Allocation concealment (selection bias) | High risk | Authors claim that clinicians could have no control over order in which patients presented and thus allocation, however this method is not incorruptible. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Intervention not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned in report, although attempt made to accurately and objectively measure blood loss by collecting using a plastic sheet. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up and failure to properly administer interventions similar in both arms. Attrition properly handled and accounted for in report. |
| Selective reporting (reporting bias) | Unclear risk | Protocol unseen. |
| Other bias | Unclear risk | Unclear. |
Poeschmann 1991.
| Methods | Randomised trial. Hospital pharmacy supplied numbered boxes. Allocation of boxes was by order of entry to the labour ward. A nurse not working in the labour room prepared the injection. |
|
| Participants | April 1986 ‐88, 2 hospitals in the Netherlands. Uncomplicated singleton term pregnancies in spontaneous labour with spontaneous vaginal deliveries and Hobel score of less than 10. | |
| Interventions | After birth of baby:
A. IM 5 IU oxytocin (n = 28);
B. 500 micrograms sulprostone;
C. saline (n = 24) Comparison in this review is A vs C. Cord was clamped within 1 minute of birth; otherwise expectant management of the third stage was performed. Blood loss was calculated by measuring the amount of blood and clots collected in the bedpan and by weighing the bloodstained swabs and linen obtained during 1 hour postpartum. |
|
| Outcomes | Blood loss, need for additional uterotonics, length of third stage. | |
| Notes | 77 women were entered into the trial; 3 were excluded because of induction of labour (2) and vacuum extraction (1). Dates of study: April 1986 to April 1988 Funding sources: quote: “Sulprostone was supplied by Schering bv. The Netherlands without charge.” Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A random treatment allocation list was prepared, however method of sequence generation not described. Randomised in blocks of 30. |
| Allocation concealment (selection bias) | Low risk | Allocation was by order of entry to ward, but based on random list. The syringes were prepared elsewhere so the caregivers would not have been able to pre‐empt what treatment was allocated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Nurse not working in labour room prepared the injection. Injection type blinded to participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned in report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes reported. Data for all participants reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol unseen. Only blood loss pre‐specified in methods text. |
| Other bias | Unclear risk | No other obvious signs of bias but trial stopped at 2 years due to organisational issues. |
Saito 2007.
| Methods | Quasi randomised: quote: "...women were allocated to a group in a temporal manner (...) selected weekly or monthly, as determined by each hospital, in alternate shifts". | |
| Participants | 343 consenting women with low risk of PPH at 4 hospitals in Japan Excluded: contraindication for ergometrine, multiple pregnancies, non‐cephalic presentation, uterine fibroids or deformity, placenta previa, history of PPH, parity > 4, previous caesarean section, severe anaemia, pre‐eclampsia, epidural anaesthesia, use of oxytocics, anticoagulation therapy, estimated baby weight < 2000 g or > 4000 g. |
|
| Interventions | Shortly after delivery of the baby: A. oxytocin 5 IU IM (n = 156); B. methylergometrine 0.2 mg IM (n = 187). AMTSL in both groups. immediate cord clamping and cutting, controlled cord traction. Blood loss was calculated objectively by measuring the amount of collected blood and by the weighting of surgical sponges, clothes and drapes by experienced attending midwives who were not involved in the administration of prophylactic oxytocics. |
|
| Outcomes | Blood loss (mL), maternal blood pressure, nausea, vomiting, headache, chest pain, dyspnoea, duration of the third stage (minutes), additional uterotonics, blood transfusion, manual placental removal. | |
| Notes | Dates of study: September 2000 – April 2002 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi random: quote: "...women were allocated to a group in a temporal manner (...) selected weekly or monthly, as determined by each hospital, in alternate shifts.". |
| Allocation concealment (selection bias) | High risk | Inadequate. quote: "...women were allocated to a group in a temporal manner (...) selected weekly or monthly, as determined by each hospital, in alternate shifts." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not blinded, but quote: “Blood loss was calculated objectively by measuring the amount of collected blood and by the weighing of surgical sponges, clothes and drapes by experienced attending midwives who were not involved in the administration of prophylactic oxytocics.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is not possible to know from the study report how many women were originally randomised. |
| Selective reporting (reporting bias) | Unclear risk | Protocol unseen. No outcomes were pre‐specified in methods with the exception of estimated blood loss. |
| Other bias | Unclear risk | Unclear. |
Singh 2009.
| Methods | Double‐blind randomised controlled trial. Computer‐generated randomisation and concealment of treatment group allocations were utilized. |
|
| Participants | Women presenting for delivery at the University College of Medical Sciences, Guru Teg Bahadur Hospital. Women with a healthy singleton pregnancy in spontaneous or induced labour at term were included. Women with the following were excluded: known hypersensitivity or contraindication to prostaglandins, fetal demise, antepartum haemorrhage, multiple pregnancy, malpresentation, cardiac disease, Rhesus‐negative mother, hypertensive disorders, and severe anaemia (Hb < 7 g/dL), and those requiring oxytocin until the second stage of labour. |
|
| Interventions | Immediately after delivery women received: A. oxytocin 5 IU IV (n = 75) or B. methylergometrine 0.2 mg IV (n = 75) All received the allocated drug as well as placebo for the other possible treatment drugs. All received placental cord traction until placental delivery. After infant delivery, a pre‐weighed linen and collection bag were placed beneath the patient and blood loss assessed by weight after 1 hour. Hb and Hct were recorded upon admission and 24 hours after delivery. There were 2 additional intervention arms in this trial that were not relevant to this review. 1 group received misoprostol 400 mcg sublingual, and the other received misoprostol 600 mcg sublingual. |
|
| Outcomes | Primary: blood loss during 3rd and 4th stage of labour Secondary: duration of 3rd stage, need for additional uterotonics, need for blood transfusion, adverse effects of drugs | |
| Notes | Dates of study: unclear Funding sources: not reported Declarations of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using quote: “computer‐generated random numbers.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Drug packets were sealed and coded using a computer‐generated random number chart.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinding of patients and personnel. Authors report "a duty nurse who was not involved in the study opened the allotted packet in a separate room." The patients and investigator were blinded to the packet contents. All patients received the study drug as well as placebo for the other interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinding of patients and personnel. Authors report "a duty nurse who was not involved in the study opened the allotted packet in a separate room." The patients and investigator were blinded to the packet contents. All patients received the study drug as well as placebo for the other interventions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | High risk | Some adverse outcomes of interest are reported incompletely. Authors report “adverse effects of the drugs” as secondary outcomes, and in methods describe collection of data regarding postpartum Hb level,” however the data are not fully presented. They also report that “the methylergometrine group had the highest incidence of nausea and vomiting” but do not report the data completely. |
| Other bias | Unclear risk | Unclear. |
Sorbe 1978.
| Methods | Quasi‐randomised trial. Alternate ‐ odd and even numbers of mothers' hospital records. Not blinded. |
|
| Participants | Hospital in Sweden. | |
| Interventions | Immediately after delivery of the anterior shoulder women received:
A. 10 IU IV oxytocin B. 0.2 mg ergometrine IV Expectant management of the third stage was routine. Blood was collected in a specially designed bedpan which was placed under the buttocks of the women immediately after the delivery of the child. The measurement of the blood loss during the 2‐hour period was then performed with a graduated glass. |
|
| Outcomes | Blood loss, manual placental removal, placental separation time. | |
| Notes | Dates of study: during 1975 to 1976 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation by odd/even hospital record numbers. |
| Allocation concealment (selection bias) | High risk | Allocation would have been open with this method. Assume allocation not concealed given method of randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Does not appear to be blinded. Staff would be aware of treatment. Blinding seems unlikely from description of methods. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assumed staff recorded outcomes. Blinding not mentioned in report. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Denominators not always reported in all tables. Not possible to tell from study report whether all participants reported on. |
| Selective reporting (reporting bias) | Unclear risk | Protocol unseen. |
| Other bias | Unclear risk | Unclear. |
Vaughan Williams 1974.
| Methods | Randomised controlled trial. Quote: "Patients were randomly assigned to one of six treatment groups." No information about blinding or allocation concealment described. |
|
| Participants | 51 women in labour at the Royal Sussex County Hospital, Brighton, who required an IV infusion. Inclusion criteria was no known antenatal complications and expectation to have a spontaneous vaginal delivery. Patients with complications during labour were excluded. Informed consent was obtained. | |
| Interventions | Women were randomly assigned to 1 of 6 treatment groups: 1) no treatment, control; 2) 0.5 mg ergometrine IV with delivery of the anterior shoulder; 3) 0.5 mg ergometrine IV with delivery of the baby; 4) 10 IU oxytocin IV with delivery of the anterior shoulder; 5) ergometrine 0.5 mg plus 5 IU oxytocin IM with delivery of the anterior shoulder; 6) 10 mg diazepam IM in the late first stage of labour followed by ergometrine 0.5 mg plus 5 IU oxytocin IM with delivery of the anterior shoulder. Placenta was delivered actively by controlled cord traction. Blood loss was measured by collection in a kidney dish placed below the perineum after delivery of the infant. Comparisons for this review are group 1 vs 4, groups 2 and 3 vs group 4, and groups 5 and 6 vs group 2 and 3. |
|
| Outcomes | Primary outcomes were mean CVP and blood loss. | |
| Notes | Dates of study: not reported Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to one of six treatment groups." No information about blinding or allocation concealment described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned, but assume no blinding given description of treatment when administering drugs.for each arm (no mention of saline placebo for control). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assumed outcomes were measured by staff as not specified. Method of measuring blood loss not specified – could be subjective. Not mentioned in report. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Denominators not provided in results tables therefore difficult to assess. Not possible to tell if there was attrition from report. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen. Few outcomes reported. CVP only outcome prespecified in text |
| Other bias | Unclear risk | Unclear. |
APH: antepartum haemorrhage AMTSL: active management of the third stage of labour bpm: beats per minute CVP: central venous pressure Hb: haemoglobin Hct: haematocrit IM: intramuscular IU: international units IV: intravenous MRP: manual removal of placenta PPH: postpartum haemorrhage PROM: prelabour rupture of membranes SD: standard deviation vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barbaro 1961 | No randomisation of treatment groups. |
| Boucher 2004 | Comparison of intramuscular carbetocin to a 2‐hour IV oxytocin infusion administered after delivery of the placenta. |
| Dickinson 2009 | Comparison of oxytocin, misoprostol and no additional medication for the third‐stage management after second trimester medical termination. |
| Docherty 1981 | Oxytocin vs ergometrine‐oxytocin (subject of separate review). |
| Dommisse 1980 | No randomisation of treatment groups. |
| Dumoulin 1981 | Oxytocin (different doses) vs ergometrine‐oxytocin (subject of separate review). |
| Friedman 1957 | Likely to be considerable bias after entry to study as 27% of the 1221 were 'deleted from the study' as inadequate observations were obtained. No other reasons given, and no indication of whether these women were missing in similar proportions from the 5 intervention groups. |
| Gerstenfeld 2001 | Comparison of oxytocin with misoprostol (subject of separate review). |
| Hacker 1979 | No randomisation of treatment groups. |
| Hoffman 2006b | Comparison of oxytocin within the context of active vs expectant management (subject of a separate review). |
| Howard 1964 | Oxytocin, methergine or placebo given after delivery of the placenta. |
| Huh 2000 | Excluded as only different timing of administration. |
| Irons 1994 | Comparison of nipple stimulation to ergometrine‐oxytocin which is not a subject of this review. |
| Jackson 2001 | Comparison of oxytocin administered before and after placental delivery so the only difference is timing of administration. |
| Jans 2017 | Comparison of oxytocin to expectant management without placebo. |
| Khan 1997 | Comparison of prophylactic oxytocin within context of active management vs oxytocin after placental delivery within context of expectant management (subject of separate review by Begley et al: Active versus expectant management of third stage of labour ‐ seeBegley 2019). |
| Kundodyiwa 2001 | Comparison of oxytocin with misoprostol (subject of separate review). |
| Lokugamage 2001 | Comparison of oxytocin to misoprostol (subject of separate review) and at caesarean section. |
| Muller 1996 | 5 IU IV oxytocin with crowning of head and Brandt‐Andrews vs expectant. Abstract only, in French and German. No clinical data available from authors. |
| Neri‐Mejia 2016 | Comparison of various oxytocin routes of delivery without a placebo group. |
| Newton 1961 | Oxytocin or placebo given after delivery of the placenta. |
| Nieminen 1963 | No randomisation of treatment groups. |
| Nuamsiri 2016 | Comparison of oxytocin plus ergometrine to oxytocin alone. |
| Oguz Orhan 2014 | Comparison of different routes and timing of oxytocin administration. |
| Parsons 2004 | Comparison of oxytocin with misoprostol (subject of separate review). |
| Porter 1991 | Comparison of different routes of administration of oxytocin. |
| Quibel 2016 | Comparison of oxytocin and misoprostol to oxytocin. |
| Ramirez 2001 | Inadequate information available about randomisation and available only as abstract. |
| Rouse 2011 | Comparison between different doses of oxytocin without placebo or alternate uterotonic. |
| Sariganont 1999 | No randomisation of treatment groups. |
| Schaefer 2004 | Excluded as only difference is timing of administration. |
| Schemmer 2001 | Comparison of oxytocin administered before and after placental delivery so the only difference is timing of administration. |
| Sharma 2014 | Treatment groups were not randomised. |
| Soiva 1964 | Trial includes a large group of women who were not randomised (data were collected retrospectively) and whose outcome data are inseparable from those that were randomised. |
| Soriano 1995 | Compares oxytocin with oxytocin plus ergometrine (subject of separate review). |
| Stanton 2010 | Study withdrawn due to lack of IRB approval. |
| Stanton 2012 | Manuscript published is of study protocol only, data planned to be analysed in 2013. |
| Stanton 2013 | Comparison groups outside scope of this review. |
| Stearn 1963 | Allocation was to 2 different consultants, 1 of whom gave all patients ergometrine‐oxytocin, and the other to give 'normal' cases ergometrine with hyalase and abnormal given IV ergometrine. |
| Suhrabi 2013 | Comparison groups outside scope of this review. |
| Sunil 2016 | Comparison of oxytocin to carboprost. |
| Symes 1984 | Compares oxytocin with oxytocin plus ergometrine (subject of separate review). |
| Tessier 2000 | Excluded as only different routes of administration. |
| Thornton 1988 | Strong likelihood of post‐entry bias as alternate allocation used for 65, but 40 were withdrawn 40 as did not meet inclusion criteria, leaving 10 and 15 in trial comparing oxytocin vs no oxytocin within active management. Primary outcome plasma oxytocin concentration. |
| Tita 2012 | Comparison between different doses of oxytocin without placebo or alternate uterotonic. |
| Vasegh 2005 | Comparison of active vs expectant management of the third stage of labour (subject of a separate review). Study design information not available. |
| Yuen 1995 | Oxytocin vs ergometrine‐oxytocin (subject of separate review). |
IRB: Institutional Review Board IU: international unit IV: intravenous vs: versus
Characteristics of studies awaiting assessment [ordered by study ID]
Draycott 2014.
| Methods | Randomised controlled trial |
| Participants | Female patients age 18 and over undergoing vaginal delivery at > 24 weeks' gestation |
| Interventions | Carbetocin 100 mcg IM versus Syntocinon 10 IU IM versus Syntometrine 500 mcg/5 IU IM given in the third stage of labour |
| Outcomes | Primary: requirement of additional uterotonics within 24 hours of delivery Secondary: estimated blood loss at delivery, transfusion, manual placental removal, need for surgical intervention to manage PPH, maternal hypertension or hypotension, self‐reported quality of life, pain, vomiting, need for anti‐emetic, headache, maternal experience of side effects |
| Notes | Study completed October 2018 |
Narenji 2012.
| Methods | Randomised trial |
| Participants | Female patients age 18‐35 undergoing vaginal delivery between 37‐42 weeks' gestation |
| Interventions | Breast pump stimulation versus oxytocin 30 IU in 1000 mL Ringer's solution infusion administered during the third stage of labour |
| Outcomes | Duration of the third stage of labour, blood loss during the third stage and 24 hours after delivery, Hb and Hct (before and 24 hours after delivery), breastfeeding, pain |
| Notes | Published December 2018 after completion of analysis for this updated review |
Shahbazian 2013.
| Methods | Randomised trial |
| Participants | Pregnant patients with singleton gestations undergoing vaginal delivery |
| Interventions | Misoprostol 400 mcg sublingual versus methylergonovine 0.2 mg IM versus oxytocin 20 IU IV infusion given during the third stage of labour |
| Outcomes | Duration of the third stage of labour, Hb fall 24 hours postpartum, amount of haemorrhage during the third and fourth stage of labour, side effects |
| Notes | Persian language paper pending translation. Abstract available in English but not enough information provided for data extraction. |
Suthutvoravut 2012.
| Methods | Randomised trial |
| Participants | Females age 18‐34 undergoing vaginal delivery at 37‐41 weeks' gestation |
| Interventions | Oxytocin 20 IU infusion versus ergometrine 0.2 mg IV given in the third stage of labour |
| Outcomes | Postpartum blood loss, duration of the third stage, maternal hypertension, maternal heart rate, postpartum haemorrhage, atony, need for additional uterotonic drugs |
| Notes | Trial data became available after completion of analysis for this updated review |
Hb: haemoglobin Hct: haematocrit IM: intramuscular IU: international units IV: intravenous PPH: postpartum haemorrhage
Characteristics of ongoing studies [ordered by study ID]
Hermesch 2014.
| Trial name or title | Postpartum hemorrhage prevention in patients with preeclampsia (PHP3 study) |
| Methods | Blinded, placebo‐controlled randomised controlled trial |
| Participants | Female patients age 13‐45 undergoing vaginal or cesarean delivery at greater than or equal to 20 weeks' gestation with a diagnosis of preeclampsia receiving magnesium sulphate for 24 hours postpartum |
| Interventions | Normal saline placebo versus oxytocin |
| Outcomes | Primary outcome is postpartum Hct collected 24 hours after delivery (or pre‐transfusion). Secondary outcomes include primary PPH (> 500 mL), estimated blood loss at time of delivery, and 2‐ hour postpartum blood loss. |
| Starting date | February 2015 |
| Contact information | Amy Hermesch, MD, amy.hermesch@ucdenver.edu |
| Notes |
Yogev 2014.
| Trial name or title | Management of the third stage of labor |
| Methods | Randomised trial |
| Participants | Female patients 18‐45 at 34‐41 weeks' gestation |
| Interventions | 10 IU IV oxytocin versus 10 IU IM oxytocin versus 10 IU IM oxytocin plus 10 IU IV oxytocin in the third stage of labour |
| Outcomes | Primary outcome is change in Hb concentration during labour. Secondary outcome is CBC on the first and second day after delivery. |
| Starting date | September 2015 |
| Contact information | Yariv Yogev, yarivy@clalit.org.il |
| Notes |
CBC: complete blood count Hb: haemoglobin Hct: haematocrit IM: intramuscular IU: international units IV: intravenous PPH: postpartum haemorrhage
Differences between protocol and review
In this updated version of the review, we have added several new outcomes to reflect those recommended as part of a core outcome set for reporting in postpartum haemorrhage prevention studies (Meher 2018). We have added maternal mortality to the list of primary outcomes. We also changed the previously included secondary outcome of maternal Hb < 9 g/dL to a maternal Hb < 7 g/dL as we felt this was a more specific outcome for significant blood loss. We removed the subgroup analysis examining the effect of quasi‐randomised versus randomised trials, as this was accounted for in our GRADE assessments.
Contributions of authors
For this 2018 update, Jennifer Salati and Sebastian Leathersich independently assessed new trials for inclusion, extracted data and performed risk of bias assessments for the included studies. Myfanwy Williams and Anna Cuthbert assisted in review and data extraction from previously included studies. Jennifer Salati and Myfanwy Williams performed GRADE assessments. Jennifer Salati edited the results and main text of the review based on the updated analysis.
Sources of support
Internal sources
The University of Liverpool, UK.
External sources
Human Reproduction Programme. World Health Organization. Geneva, Switzerland.
Declarations of interest
Jennifer Salati: none known.
Sebastian Leathersich: none known.
Myfanwy Williams: is employed by the University of Liverpool as a Research Associate for Cochrane Pregnancy and Childbirth. Her role is supported by the World Health Organization.
Anna Cuthbert: is employed by the University of Liverpool as a Research Associate for Cochrane Pregnancy and Childbirth. Her role was supported by the World Health Organization.
Jorge Tolosa: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Abdel‐Aleem 2010 {published data only}
- Abdel‐Aleem H, Singata M, Abdel‐Aleem M, Mshweshwe N, Williams X, Hofmeyr GJ. Uterine massage to reduce postpartum hemorrhage after vaginal delivery. International Journal of Gynecology & Obstetrics 2010;111(1):32‐6. [DOI] [PubMed] [Google Scholar]
Adhikari 2007 {published data only}
- Adhikari S, Rana A, Bista KD. Active management of third stage of labour: comparison between prophylactic intramuscular methylergometrine and intramuscular oxytocin. Nepal Journal of Obstetrics and Gynaecology 2007;2(2):24‐8. [Google Scholar]
Bader 2000 {published data only}
- Bader W, Ast S, Hatzmann W. The significance of acupuncture in the third stage of labour. Deutsche Zeitschrift fur Akupunktur 2000;43:264‐8. [Google Scholar]
- Bader W, Ast S, Reinehr J, Hackmann J, Hatzmann W. Oxytocin versus Akupunktur in der Plazentarperiode ‐ eine prospektiv randomisierte Studie [abstract]. Geburtshilfe und Frauenheilkunde 2000;60 Suppl 1:S73. [Google Scholar]
Bonham 1963 {published data only}
- Bonham DG. Intramuscular oxytocics and cord traction in third stage of labour. Brtish Medical Journal 1963;2:1620‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boopathi 2014 {published data only}
- Boopathi A, Nayak SR, Rao A, Rao B. Oxytocin versus methylergometrine in the active management of third stage of labour. Open Journal of Obstetrics and Gynecology 2014;4:666‐71. [Google Scholar]
De Groot 1996 {published data only}
- Groot AN, Roosmalen J, Dongen PW, Borm GF. A placebo‐controlled trial of oral ergometrine to reduce postpartum hemorrhage. Acta Obstetricia et Gynecologica Scandinavica 1996;75:464‐8. [DOI] [PubMed] [Google Scholar]
Dhananjaya 2014 {published data only}
- Dhananjaya BS, Charishma S. Comparative study of efficacy and safety of intramuscular oxytocin with intramuscular methylergometrine in the active management of third stage of labour. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2014;5(3):734‐9. [Google Scholar]
Ezeama 2014 {published data only}
- Ezeama CO, Eleje GU, Ezeama NN, Igwegbe AO, Ikechebelu JI, Ugboaja JO, et al. A comparison of prophylactic intramuscular ergometrine and oxytocin for women in the third stage of labor. International Journal of Gynecology and Obstetrics 2014;124(1):67‐71. [DOI] [PubMed] [Google Scholar]
Francis 1965 {published data only}
- Francis HH, Miller JM, Porteous CR. Clinical trial of an oxytocin‐ergometrine mixture. Australian & New Zealand Journal of Obstetrics and Gynaecology 1965;5:47‐51. [DOI] [PubMed] [Google Scholar]
Fugo 1958 {published data only}
- Fugo NW, Dieckmann WJ. A comparison of oxytocic drugs in the management of the placental stage. American Journal of Obstetrics and Gynecology 1958;76:141‐6. [DOI] [PubMed] [Google Scholar]
Ilancheran 1990 {published data only}
- Ilancheran A, Ratnam SS. Effect of oxytocics on prostaglandin levels in the third stage of labour. Gynecologic and Obstetric Investigation 1990;29:177‐80. [DOI] [PubMed] [Google Scholar]
Jago 2007 {published data only}
- Jago AA, Ezechi OC, Achinge GI, Okunlola MA. Effect of oxytocics on the blood pressure of normotensive Nigerian parturients. Journal of Maternal‐Fetal & Neonatal Medicine 2007;20(9):703‐5. [DOI] [PubMed] [Google Scholar]
Jerbi 2007 {published data only}
- Jerbi M, Hidar S, Elmoueddeb S, Chaieb A, Khairi H. Oxytocin in the third stage of labor. International Journal of Gynecology & Obstetrics 2007;96(3):198‐9. [DOI] [PubMed] [Google Scholar]
McGinty 1956 {published data only}
- McGinty LB. A study of the vasopressor effects of oxytocics when used intravenously in the third stage of labour. Western Journal of Surgery 1956;64:22‐8. [PubMed] [Google Scholar]
Modi 2014 {published data only}
- Modi V, Goel JK, Kashyap A, Arya SB, Kar J, Goel R. Active management of third stage of labor: A comparison of various uterotonic. Journal of South Asian Federation of Obstetrics and Gynaecology ‐ SAFOG 2014;6(3):151‐5. [Google Scholar]
Moodie 1976 {published data only}
- Moodie JE, Moir DD. Ergometrine, oxytocin and extradural analgesia. British Journal of Anaesthesia 1976;48:571‐4. [DOI] [PubMed] [Google Scholar]
Nordstrom 1997 {published data only}
- Nordstrom L, Fogelstam K, Fridman G, Larsson A, Rydhstroem H. Routine oxytocin in the third stage of labour: a placebo controlled randomised trial. British Journal of Obstetrics and Gynaecology 1997;104:781‐6. [DOI] [PubMed] [Google Scholar]
Orji 2008 {published data only}
- Orji E, Agwu F, Loto O, Olaleye O. A randomized comparative study of prophylactic oxytocin versus ergometrine in the third stage of labor. International Journal of Gynecology & Obstetrics 2008;101(2):129‐32. [DOI] [PubMed] [Google Scholar]
Pierre 1992 {published data only}
- Pierre F, Mesnard L, Body G. For a systematic policy of iv oxytocin inducted placenta deliveries in a unit where a fairly active management of third stage of labour is yet applied: results of a controlled trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 1992;43:131‐5. [DOI] [PubMed] [Google Scholar]
Poeschmann 1991 {published data only}
- Poeschmann RP, Doesburg WH, Eskes TK. A randomized comparison of oxytocin, sulprostone and placebo in the management of the third stage of labour. British Journal of Obstetrics and Gynaecology 1991;98:528‐30. [DOI] [PubMed] [Google Scholar]
Saito 2007 {published data only}
- Saito K, Haruki A, Ishikawa H, Takahashi T, Nagase H, Koyama M, et al. Prospective study of intramuscular ergometrine compared with intramuscular oxytocin for prevention of postpartum hemorrhage. Journal of Obstetrics and Gynaecology Research 2007;33(3):254‐8. [DOI] [PubMed] [Google Scholar]
Singh 2009 {published data only}
- Singh G, Radhakrishnan G, Guleria K. Comparison of sublingual misoprostol, intravenous oxytocin, and intravenous methylergometrine in active management of the third stage of labor. International Journal of Gynecology & Obstetrics 2009;107(2):130‐4. [DOI] [PubMed] [Google Scholar]
Sorbe 1978 {published data only}
- Sorbe B. Active pharmacologic management of the third stage of labor. A comparison of oxytocin and ergometrine. Obstetrics & Gynecology 1978;52:694‐7. [PubMed] [Google Scholar]
Vaughan Williams 1974 {published data only}
- Vaughan Williams CA, Johnson A, Ledward R. A comparison of central venous pressure changes in the third stage of labour following oxytocic drugs and diazepam. Journal of Obstetrics and Gynaecology of the British Commonwealth 1974;81:596‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Barbaro 1961 {published data only}
- Barbaro CA, Smith GO. Clinical trial of SE505 ‐ a new oxytocic mixture. Australian and New Zealand Journal of Obstetrics and Gynaecology 1961;1:147‐50. [Google Scholar]
Boucher 2004 {published data only}
- Boucher M, Nimrod C, Tawagi G. Carbetocin IM injection vs oxytocin IV infusion for prevention of postpartum hemorrhage in women at risk following vaginal delivery. American Journal of Obstetrics and Gynecology 2001;185(6 Pt 2):A494. [Google Scholar]
- Boucher M, Nimrod CA, Tawagi GF, Meeker TA, Rennicks, White RE, et al. Comparison of carbetocin and oxytocin for the prevention of postpartum hemorrhage following vaginal delivery:a double‐blind randomized trial. Journal of Obstetrics & Gynaecology Canada: JOGC 2004;26(5):481‐8. [DOI] [PubMed] [Google Scholar]
Dickinson 2009 {published data only}
- Dickinson JE, Doherty DA. Optimization of third‐stage management after second‐trimester medical pregnancy termination. American Journal of Obstetrics and Gynecology 2009;201(3):303.e1‐7. [DOI] [PubMed] [Google Scholar]
Docherty 1981 {published data only}
- Docherty PW, Hooper M. Choice of an oxytocic agent for routine use at delivery. Journal of Obstetrics and Gynaecology 1981;2:60. [Google Scholar]
Dommisse 1980 {published data only}
- Dommisse J. The routine use of oxytocic drugs in the third stage of labour [letter]. South African Medical Journal 1980;46:549. [PubMed] [Google Scholar]
Dumoulin 1981 {published data only}
- Dumoulin JG. A reappraisal of the use of ergometrine. Journal of Obstetrics and Gynaecology 1981;1:178‐81. [Google Scholar]
Friedman 1957 {published data only}
- Friedman EA. Comparative clinical evaluation of postpartum oxytocics. Journal of Obstetrics and Gynecology 1957;73:1306‐13. [DOI] [PubMed] [Google Scholar]
Gerstenfeld 2001 {published data only}
- Gerstenfeld T, Wing D. Rectal misoprostol versus intravenous oxytocin for the prevention of postpartum hemorrhage after vaginal delivery. American Journal of Obstetrics and Gynecology 2001;185:878‐82. [DOI] [PubMed] [Google Scholar]
Hacker 1979 {published data only}
- Hacker NF, Biggs JS. Blood pressure changes when uterine stimulants are used after normal delivery. British Journal of Obstetrics Gynaecology 1979;86:633‐6. [DOI] [PubMed] [Google Scholar]
Hoffman 2006b {published data only}
- Hoffman M, Castagnola D, Naqvi F. A randomized trial of active versus expectant management of the third stage of labor [abstract]. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S107. [Google Scholar]
- Hoffman M, Naqvi F, Sciscione A. A randomized trial of active versus expectant management of the third stage of labor. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S82. [Google Scholar]
Howard 1964 {published data only}
- Howard WF, McFadden PR, Keettel WC. Oxytocic drugs in fourth stage of labor. JAMA 1964;189:411‐3. [DOI] [PubMed] [Google Scholar]
Huh 2000 {published data only}
- Huh W, Chelmow D, Malone FD. A randomized, double‐blinded, placebo controlled trial of oxytocin at the beginning versus the end of the third stage of labor for prevention of postpartum hemorrhage. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S130. [DOI] [PubMed] [Google Scholar]
Irons 1994 {published data only}
- Irons DW, Sriskandabalan P, Bullough CH. A simple alternative to parenteral oxytocics for the third stage of labor. International Journal of Gynecology & Obstetrics 1994;46:15‐8. [DOI] [PubMed] [Google Scholar]
Jackson 2001 {published data only}
- Jackson KJ, Allbert J, Schemmer G, Elliot M, Humphrey A, Taylor J. A randomized controlled trial comparing oxytocin administration before and after placental delivery in the prevention of postpartum hemorrhage. American Journal of Obstetrics and Gynecology 2001;185:873‐7. [DOI] [PubMed] [Google Scholar]
Jans 2017 {published data only}
- Jans S, Herschderfer KC, Diem MT, Aitink M, Rijnders M, Pal‐Bruin K, et al. LENTE study: effectiveness of prophylactic intramuscular oxytocin during third stage of labour amongst low risk women. A randomized controlled trial. 31st International Confederation of Midwives Triennial Congress. Midwives ‐ Making a Difference in the World; 2017 June 18‐22; Toronto, Canada. 2017:Abstract no: P1.063.
Khan 1997 {published data only}
- Khan GQ, John IS, Chan T, Wani S, Doherty T. Controlled cord traction versus minimal intervention techniques in delivery of the placenta: a randomized controlled trial. American Journal of Obstetrics and Gynecology 1997;177(4):770‐4. [DOI] [PubMed] [Google Scholar]
Kundodyiwa 2001 {published data only}
- Kundodyiwa T, Majoko F, Rusakaniko S. Misoprostol versus oxytocin in the third stage of labor. International Journal of Gynecology & Obstetrics 2001;75:235‐41. [DOI] [PubMed] [Google Scholar]
Lokugamage 2001 {published data only}
- Lokugamage A, Paine M, Bassaw‐Balroop K, Sullivan K, El‐Refaey H, Rodeck C. Active management of the third stage at caesarean section: a randomised controlled trial of misoprostol versus syntocinon. Australian and New Zealand Journal of Obstetrics & Gynaecology 2001;41(4):411‐4. [DOI] [PubMed] [Google Scholar]
- Lokugamage AU, Paine M, Bassau‐Balroop H, El‐Refaey K, Sullivan K, Rodek C. Active management of the third stage at caesarean section: misoprostol vs syntocinon. XVI FIGO World Congress of Obstetrics & Gynecology. 2000 Sept 3‐8; Washington DC, USA 2000; Book 2:54.
Muller 1996 {published data only}
- Muller R, Beck G. Active management of the third stage of labour. 19th Swiss Congress of the Swiss Society of Gynecology and Obstetrics; 1996 June; Interlaken, Switzerland. 1996.
Neri‐Mejia 2016 {published data only}
- Neri‐Mejia M, Pedraza‐Aviles AG. Active management of the third stage of labor: three schemes of oxytocin: randomised clinical trial. Ginecologia y Obstetricia De Mexico 2016;84(5):306‐13. [PubMed] [Google Scholar]
Newton 1961 {published data only}
- Newton M, Mosey LM, Egli GE, Gifford WB, Hull CT. Blood loss during and immediately after delivery. Obstetrics & Gynecology 1961;17:9‐18. [PubMed] [Google Scholar]
Nieminen 1963 {published data only}
- Nieminen U, Jarvinen PA. A comparative study of different medical treatments of the third stage of labour. Annales Chirurgiae et Gynaecologiae Fenniae 1963;53:424‐9. [PubMed] [Google Scholar]
Nuamsiri 2016 {published data only}
- Nuamsiri T, Kaewkiattikun K. Prevention of postpartum hemorrhage with oxytocin versus ergometrine plus oxytocin in the third stage of labor. Thai Journal of Obstetrics and Gynaecology 2016;24:97‐103. [Google Scholar]
- Nuamsiri T, TCTR20150820001. Prevention of postpartum hemorrhage with oxytocin versus ergometrine plus oxytocin in the third stage of labor. http://www.clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=1508. (20 May 2015) 2015. [TCTR20150820001]
Oguz Orhan 2014 {published data only}
- Oguz Orhan E, Dilbaz B, Aksakal SE, Altinbas S, Erkaya S. Prospective randomized trial of oxytocin administration for active management of the third stage of labor. International Journal of Gynecology and Obstetrics 2014;127(2):175‐9. [DOI] [PubMed] [Google Scholar]
Parsons 2004 {published data only}
- Parsons S, Ntumy YM, Walley RL, Wilson JB, Crane JM, Matthews K, et al. Rectal misoprostol vs intramuscular oxytocin in the routine management of the third stage of labour. 30th British Congress of Obstetrics and Gynaecology; 2004 July 7‐9; Glasgow, UK 2004;18. 2004.
Porter 1991 {published data only}
- Porter KB, O'Brien WF, Collins MK, Givens P, Knuppel R, Bruskivage L. A randomized comparison of umbilical vein and intravenous oxytocin during the puerperium. Obstetrics & Gynecology 1991;78:254‐6. [PubMed] [Google Scholar]
Quibel 2016 {published data only}
- Quibel T, Ghout I, Goffinet F, Salomon LJ, Fort J, Javoise S, et al. Active management of the third stage of labor with a combination of oxytocin and misoprostol to prevent postpartum hemorrhage: a randomized controlled trial. Obstetrics and Gynecology 2016;128(4):805‐11. [DOI] [PubMed] [Google Scholar]
Ramirez 2001 {published data only}
- Ramirez O, Benito V, Jimenez R, Valido C, Hernandez C, Garcia J. Third stage of labour: active or expectant management? preliminary results. Journal of Perinatal Medicine 2001;Suppl 1(Pt 2):364. [Google Scholar]
Rouse 2011 {published data only}
- Rouse D, Abramovici A, Szychowski J, Seals S, Andrews W, Hauth J, et al. Oxytocin dose‐regimens to prevent uterine atony after vaginal delivery: does treatment efficacy vary by risk status?. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl):S50‐S51. [Google Scholar]
Sariganont 1999 {published data only}
- Sariganont J. Comparative study between syntocinon and methergin in prevention of postpartum hemorrhage. Thai Journal of Obstetrics and Gynaecology 1999;11(4):248. [Google Scholar]
Schaefer 2004 {published data only}
- Schaefer A, Klein L, Wolfe P, Heindricks G, Downs L, Guinn D. Double blind rct of early versus traditional oxytocin management in the third stage to prevent blood loss. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S69. [Google Scholar]
Schemmer 2001 {published data only}
- Schemmer G. A randomized controlled trial comparing prophylactic administration of oxytocin before and after placental delivery in the prevention of postpartum hemorrhage [abstract]. American Journal of Obstetrics and Gynecology 2001;184(1):S20. [DOI] [PubMed] [Google Scholar]
Sharma 2014 {published data only}
- Sharma M, Kaur P, Kaur K, Kaur A, Kaur PK, Kaur MM. A comparative study of oxytocin/misoprostol/methylergometrine for active management of the third stage of labor. Journal of Obstetrics and Gynecology of India 2014;64(3):175‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soiva 1964 {published data only}
- Soiva K, Koistinen O. Clinical experience with simultaneous intramuscular injection of oxytocin and methylergometrine. Annales Chirurgiae et Gynaecologiae 1964;53:173‐86. [PubMed] [Google Scholar]
Soriano 1995 {published data only}
- Soriano D, Dulitzki M, Schiff E, Barkai G, Seldman DS. A randomized prospective trial of oxytocin plus ergometrin vs oxytocin alone for prevention of postpartum hemorrhage. American Journal of Obstetrics and Gynecology 1995;172:361. [Google Scholar]
Stanton 2010 {published data only}
- Stanton C, NCT01108302. Assessing the effectiveness, safety and feasibility of expanding use of oxytocin in uniject™ by auxiliary nurse midwives to prevent postpartum hemorrhage: a community‐based cluster randomized trial in Bagalkot, India. clinicaltrials.gov/show/NCT01108302 (first received 21 April 2010).
Stanton 2012 {published data only}
- Stanton CK, Newton S, Mullany LC, Cofie P, Agyemang CT, Adiibokah E, et al. Impact on postpartum hemorrhage of prophylactic administration of oxytocin 10 IU via Uniject by peripheral health care providers at home births: Design of a community‐based cluster‐randomized trial. BMC Pregnancy and Childbirth 2012;12:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanton 2013 {published data only}
- Stanton C, NCT01108289. The oxytocin initiative: determining the effect of prophylactic administration of oxytocin in uniject™ by a community health officer on postpartum hemorrhage at home births in four districts in. clinicaltrials.gov/ct2/show/record/NCT01108289 (first received 21 April 2010).
- Stanton CK, Newton S, Mullany LC, Cofie P, Tawiah Agyemang C, Adiibokah E, et al. Effect on postpartum hemorrhage of prophylactic oxytocin (10 IU) by injection by community health officers in Ghana: a community‐based, cluster‐randomized trial. PLOS Medicine 2013;10(10):e1001524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stearn 1963 {published data only}
- Stearn RH. Syntometrine in the management of the third stage of labour. Journal of Obstetrics and Gynaecology of the British Commonwealth 1963;70:593‐6. [DOI] [PubMed] [Google Scholar]
Suhrabi 2013 {published data only}
- Suhrabi Z, IRCT201304146790N6. Comparision the effect of dextrose and oxytocine on bleeding rate in 3th stage of labor in women who hospitalized in Shahid Mustafa Khomeini (PBUH) of Ilam city. en.irct.ir/trial/7202 (first received 26 May 2013).
Sunil 2016 {published data only}
- Sunil Kumar KS, Shyam S, Batakurki P. Carboprost versus oxytocin for active management of third stage of labor: a prospective randomized control study. Journal of Obstetrics and Gynaecology of India 2016;66(Suppl 1):S229‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Symes 1984 {published data only}
- Symes JB. A study on the effect of ergometrine on serum prolactin levels following delivery. Journal of Obstetrics and Gynaecology 1984;5:36‐8. [Record 2022] [Google Scholar]
Tessier 2000 {published data only}
- Tessier JL, Davies GAL, Woodman MC, Lipson A. Maternal hemodynamics after oxytocin bolus versus infusion in the third stage of labor. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S128. [DOI] [PubMed] [Google Scholar]
Thornton 1988 {published data only}
- Thornton S, Davison JM, Baylis PH. Plasma oxytocin during third stage of labour: comparison of natural and active management. BMJ 1988;297:167‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tita 2012 {published data only}
- Tita A, NCT00790062. Comparison of the effectiveness of 3 different dose regimens of oxytocin in preventing uterine atony and postpartum hemorrhage during vaginal delivery. https://clinicaltrials.gov/show/NCT00790062 (first received 13 November 2008).
- Tita AT, Szychowski JM, Rouse DJ, Bean CM, Chapman V, Nothern A, et al. Higher‐dose oxytocin and hemorrhage after vaginal delivery: A randomized controlled trial. Obstetrics and Gynecology 2012;119(2 Pt 1):293‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetta L, Szychowski J, Seals S, Mancuso M, Hauth J, Tita A. Risk factors for uterine atony at vaginal delivery: a comprehensive evaluation. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl):S71‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetta LA, Szychowski JM, Seals S, Mancuso MS, Biggio JR, Tita AT. Risk factors for uterine atony/postpartum hemorrhage requiring treatment after vaginal delivery. American Journal of Obstetrics and Gynecology 2013;209(1):51e1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vasegh 2005 {published data only}
- Vasegh FR, Bahiraie A, Mahmoudi M, Salehi L. Comparison of active and physiologic management of third stage of labor. HAYAT: Journal of Tehran Faculty of Nursing & Midwifery 2005;10(23):102. [Google Scholar]
Yuen 1995 {published data only}
- Yuen PM, Chan NST, Yim SF, Chang AM. A randomised double blind comparison of Syntometrine and Syntocinon in the management of the third stage of labour. British Journal of Obstetrics and Gynaecology 1995;102:377‐80. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Draycott 2014 {published data only}
- Draycott T, NCT02216383. Intramuscular oxytocics: a comparison study of intramuscular carbetocin, syntocinon and syntometrine for the third stage of labour following vaginal birth. clinicaltrials.gov/ct2/show/record/NCT02216383 (first received 15 August 2014).
Narenji 2012 {published data only}
- Narenji F. Comparison the effect of intramuscular injection of oxytocin and nipple stimulation on the third stage of delivery length and bleeding. irct.ir/trial/10487 (first received 25 June 2012).
Shahbazian 2013 {published data only}
- Shahbazian N, IRCT201106143756N2. Comparison of the effects of intramuscular methylergonovine, intravascular oxytocin, and sublingual prostaglandin (misoprostol) in active management of the third stage of labor. en.irct.ir/trial/3866 (first received 20 September 2011).
- Shahbazian N, Saadati N, Nezhad, Fathi L. Comparison of the effects of intramuscular methylergonovine, intravascular oxytocin, and sublingual misoprostol in active management of the third stage of labor. Jundishapur Scientific Medical Journal 2013;12(5):559‐605. [Google Scholar]
Suthutvoravut 2012 {published data only}
- Suthutvoravut S, TCTR20120000012. Comparison of postpartum blood loss between intravenous oxytocin and intravenous methylergometrine maleate during third stage of labor. clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=741 (first received 5 August 2012).
References to ongoing studies
Hermesch 2014 {published data only}
- Hermesch A, NCT02221830. Postpartum hemorrhage prevention in patients with preeclampsia (PHP3 Study). clinicaltrials.gov/ct2/show/record/NCT02221830 (first received 20 August 2014). [NCT02221830]
Yogev 2014 {published data only}
- Yogev Y, NCT02319707. Management of the third stage of labor. clinicaltrials.gov/ct2/show/NCT02319707 (first received 18 December 2014).
Additional references
Al Kadri 2011
- Al Kadri HM, Al Anazi BK, Tamim HM. Visual estimation versus gravimetric measurement of postpartum blood loss: a prospective cohort study. Archives of Gynecology and Obstetrics 2011;283(6):1207‐13. [DOI] [PubMed] [Google Scholar]
Begley 2019
- Begley CM, Gyte GML, Devane D, McGuire W, Weeks A, Biesty LM. Active versus expectant management for women in the third stage of labour. Cochrane Database of Systematic Reviews 2019, Issue 2. [DOI: 10.1002/14651858.CD007412.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borovac‐Pinheiro 2018
- Borovac‐Pinheiro A, Pacagnella RC, Cecatti JG, Miller S, Ayadi AM, Souza JP, et al. Postpartum hemorrhage: new insights for definition and diagnosis. American Journal of Obstetrics and Gynecology 2018;219(2):162‐8. [DOI] [PubMed] [Google Scholar]
De Groot 1998
- Groot AN, Dongen PW, Vree TB, Hekster YA, Roosmalen J. Ergot alkaloids. Current status and review of clinical pharmacology and therapeutic use compared with other oxytocics in obstetrics and gynecology. Drugs 1998;56(4):523‐35. [DOI] [PubMed] [Google Scholar]
Dildy 2004
- Dildy GA, Paine AR, George NC, Velasco C. Estimating blood loss: can teaching significantly improve visual estimation?. Obstetrics and Gynecology 2004;104(3):601‐6. [DOI] [PubMed] [Google Scholar]
Du Vigneaud 1953
- Du Vigneaud V, Ressler C, Tippet S. The sequence of amino acids in oxytocin with a proposal for the structure of oxytocin. Journal of Biological Chemistry 1953;205:949. [PubMed] [Google Scholar]
Embrey 1963
- Embrey MP, Barber DT, Scudamore JH. Use of syntometrine in prevention of post partum haemorrhage. British Medical Journal 1963;1:1387‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallos 2018
- Gallos ID, Papadopoulou A, Man R, Athanasopoulos N, Tobias A, Price MJ, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta‐analysis. Cochrane Database of Systematic Reviews 2018, Issue 12. [DOI: 10.1002/14651858.CD011689.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoffman 2006a
- Hoffman M, Castagnola D, Naqvi F. A randomized trial of active versus expectant management of the third stage of labor [abstract]. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S107. [Google Scholar]
Hogan 2010
- Hogan MC, Foreman KJ, Naghavi M, Ahn SY, Wang M, Makela SM, et al. Maternal mortality of 181 countries, 1980‐2008: a systemic analysis of progress towards Millennium Development Goals 5. Lancet 2010;375(9726):1609‐23. [DOI] [PubMed] [Google Scholar]
Kassebaum 2016
- Kassebaum NJ, Barber RM, Bhutta ZA, Dandona L, Gething PW, Hay SI, et al. Global, regional, and national levels of maternal mortality, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388(10053):1775‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liabsuetrakul 2018
- Liabsuetrakul T, Choobun T, Peeyananjarassri K, Islam QM. Prophylactic use of ergot alkaloids in the third stage of labour. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD005456.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meher 2018
- Meher S, Cuthbert A, Kirkham JJ, Williamson P, Abalos E, Aflaifel N, et al. Core outcome sets for prevention and treatment of postpartum hemorrhage: an international Delphi consensus study. British Journal of Obstetrics and Gynaecology 2018;126(1):83‐93. [DOI: 10.1111/1471-0528.15335] [DOI] [PubMed] [Google Scholar]
Moir 1932
- Moir JC. The action of ergot preparation on the puerperal uterus. British Medical Journal 1932;1:1119‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moir 1955
- Moir JC. The history of present day use of ergot. Canadian Medical Association Journal 1955;72:727‐34. [PMC free article] [PubMed] [Google Scholar]
Mori 2012
- Mori R, Nardin JM, Yamamoto N, Carroli G, Weeks A. Umbilical vein injection for the routine management of third stage of labour. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD006176.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Novikova 2015
- Novikova N, Hofmeyr GJ, Cluver C. Tranexamic acid for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD007872.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pantoja 2016
- Pantoja T, Abalos E, Chapman E, Vera C, Serrano VP. Oxytocin for preventing postpartum haemorrhage (PPH) in non‐facility birth settings. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011491.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Say 2014
- Say L, Chou D, Gemmill A, Tuncalp O, Moller A, Daniels J, et al. Global cause of maternal death: a WHO systematic analysis. Lancet 2014;2(6):e323‐e333. [DOI] [PubMed] [Google Scholar]
Stafford 2008
- Stafford I, Dildy GA, Clark SL, Belfort MA. Visually estimated and calculated blood loss in vaginal and cesarean delivery. American Journal of Obstetrics and Gynecology 2008;199(5):519.e1‐7. [DOI] [PubMed] [Google Scholar]
Su 2012
- Su LL, Chong YS, Samuel M. Carbetocin for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD005457.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Toledo 2007
- Toledo P, McCarthy RJ, Hewlett BJ, Fitzgerald PC, Wong CA. The accuracy of blood loss estimation after simulated vaginal delivery. Anesthesia Analgesia 2007;105(6):1736‐40. [DOI] [PubMed] [Google Scholar]
Tunçalp 2012
- Tunçalp Ö, Hofmeyr GJ, Gülmezoglu AM. Prostaglandins for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD000494.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2012
- World Health Organization. WHO recommendations for the prevention and treatment of postpartum haemorrhage. https://apps.who.int/iris/bitstream/handle/10665/75411/9789241548502_eng.pdf;sequence=1. Geneva: World Health Organization, (date accessed 27 August 2018).
WHO 2018
- World Health Organization. WHO recommendations for the prevention of postpartum haemorrhage. https://afro.who.int/sites/default/files/2017‐06/WHO_MPS_0706_engPPH.pdf. Geneva: World Health Organization, (accessed 1 February 2019).
References to other published versions of this review
Cotter 2001
- Cotter AM, Ness A, Tolosa JE. Prophylactic oxytocin for the third stage of labour. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD001808] [DOI] [PubMed] [Google Scholar]
Elbourne 1988
- Elbourne D, Prendiville W, Chalmers I. Choice of oxytocic preparation for routine use in the management of the third stage of labour: an overview of evidence from controlled trials. British Journal of Obstetrics and Gynaecology 1988;95(1):17‐30. [DOI] [PubMed] [Google Scholar]
Prendiville 1988b
- Prendiville W, Elbourne D, Chalmers I. The effect of routine oxytocic administration in the management of the third stage of labour: an overview of the evidence from controlled trials. British Journal of Obstetrics and Gynaecology 1988;95(1):3‐16. [DOI] [PubMed] [Google Scholar]
Prendiville 1989
- Prendiville WJ, Elbourne DR. Care during the third stage of labour. In: Chalmers I, Enkin M, Keirse MJNC editor(s). Effective Care in Pregnancy and Childbirth. Vol. 2, Oxford: Oxford University Press, 1989:1145‐69. [Google Scholar]
Westhoff 2013
- Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD001808.pub2] [DOI] [PubMed] [Google Scholar]


