Abstract
This research paper presents findings of an investigation about antibacterial effect of methanol/water fractions as well as cytotoxic activity of the extracts obtained from Pedicularis wilhelmsiana (Scrophulariaceae) which grows in Azarbaijan/Iran by agar well diffusion method and brine shrimp lethality test successively. Phytochemical study of this plant was determined as well. A combination of solid-phase extraction (SPE), preparative reversed-phase HPLC analysis and spectroscopic means were applied for fractionation, purification, and identification of ingredients respectively. Antimicrobial test demonstrated that 40% and 60% methanol/water fractions were more active than methanolic extract and other SPE fractions. No cytotoxic effect was detected from the extracts of this plant by brine shrimp lethality assay. Phytochemical study of aerial parts of Pedicularis wilhelmsiana (P. wilhelmsiana) afforded two phenylethanoids (verbascoside and martynoside), three iridoids (Aucubin, ipolamiid, 5-hydroxy-8-epi-loganin) and two flavonoids (luteolin, luteolin-7-glucoside) along with mannitol on the basis of spectral evidences (UV, 1H and 13CNMR) as well as comparison with literature data. The findings of this research supported further studies related to antibiotic potential of methanolic extract of P. wilhelmsiana.
Key Words: Pedicularis wilhelmsiana, Scrophulariaceae, Phytochemistry, Antimicrobial
Introduction
Pedicularis genus known as “lousewort” or “wood betony” (Scrophulariaceae), with common Persian name of “sonbol e batlaghi” including 9 species endemic to Iran (1, 2), is one of the most widely used groups of medicinal plants which has demonstrated different therapeutic effects on cardiac problems, exhaustion, spontaneous sweating, and digestion problems. Moreover, hepatoprotective, antitumor, anti-oxidative, antihaemolysis, and antibacterial activities have been reported from different species of this genus (3-5). The most important secondary metabolites which have been reported from Pedicularis genus were iridoids, phenylethnoids, lignan glycosides, flavonoids, alkaloids, and other compounds (6-9). Most researches have only been carried out on Chinese species of Pedicularis genus; however far too little attention has been paid to P. wilhelmsiana Fisch ex. (Sonbol e batlaghi ye Arasbarani) which grows in Azerbaijan/Iran. Previous studies showed that Pedicularis species have been used to some clinical disorders such as cold, cough, and fever (10). So, these traditional applications could be attributed in part to the antimicrobial activity of the plant. Also, in our previous research, antibacterial activity of methanolic extract was demonstrated (11). Therefore, in this study we intended to experimentally evaluate the in-vitro antibacterial effects of P. wilhelmsiana methanol/water fractions, against susceptible species by agar well diffusion method. Since plant products could be a source for antibacterial agents to treat infectious diseases (12, 13), toxicity of these valuable sources plays a vital role whether or not these remedies have the potential to be administered clinically in the future. So, to validate their cytotoxic activity, brine shrimp lethality test were selected. To the best of our knowledge, there was no report on the basis of antibacterial activity of SPE fractions as well as phytochemical study of P. wilhelmsiana, except verbascoside (a widespread phenylethanoid) that was previously isolated from this plant (14). Therefore, this paper will focus on first, antimicrobial activity of methanol/water fractions, second, cytotoxic activity of extracts, and third, phytochemical study of P. wilhelmsiana to find effective phytochemicals responsible to biological activities of this plant.
Experimental
Plant material and extraction
The samples of Pedicularis wilhelmsiana Fisch ex. were collected from Arasbaran region in East Azarbaijan province, Iran in June 2008. A voucher specimen for this collection has been deposited (Tbz-FPh-701) at the Herbarium of Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran. Arial parts of plant were air dried and powdered. Two-hundred grams of powdered sample was extracted by n-hexane (8 h), dichloromethane (10 h) and methanol (8 h) in soxhlet extractor. Solvents were removed by rotary evaporator and resulting extracts were used for mentioned experiments.
Table 1.
Antimicrobial activity of the MeOH extract and SPE fractions of the aerial parts of P. wilhelmsiana by the agar well diffusion assay (n = 3)
|
MIZD ± SD
a
(mm) |
MIC (mg/mL)
|
|||
|---|---|---|---|---|
| MO b | Pa c | Sa d | Se e | Ml f |
| Test samples | ||||
| MeOH extract | 14.3 ± 0.47 (200) | 8.5 ± 0.4 (200) | 15.6 ± 0.6 (200) | 15.6 ± 0.53 (200) |
| SPE fraction 10% | - | - | - | - |
| SPE fraction 20% | 14.3 ± 052 (100) | - | - | - |
| SPE fraction 40% | 10.2 ± 0.41 (100) | 10.3 ± 0.5 (100) | 16.3 ± 0.41 (100) | 15.7 ± 0.27 (100) |
| SPE fraction 60% | 14.4 ± 0.36 (100) | 14.1 ± 0.46 (100) | 23 ± 0.53 (100) | 26.9 ± 46 (100) |
| SPE fraction 80% | 15.1 ± 0.38 (100) | - | 14 ± 0.34 (100) | - |
| SPE fraction 100% | - | - | - | - |
- : No activity at tested concentration.
Data related to MIZD ± SD (mm) of MeOH extract had been reported in our previous study. The reason of including it in the table, iscomparison of SPE fractions data with MeOH extract data.
Mean Inhibition Zone Diameter ± Standard Deviation (applied volume 100 μL);
MO: Microorganisms;
Pa: Pseudomonas aeruginosa;
Sa: Staphylococcus aureus;
Se: Staphylococcus epidermidis;
Ml: Micrococcus luteus;
Separation
Two grams of methanolic (MeOH) extract and sep-pak Vac C18 6 cc column were used for fractionation of MeOH extract. A 20 mL aliquot of the MeOH extract was loaded on to the column. The column was first eluted with water (200 cc) and then with pure methanol. Fractionation was continued by elution of extract with mixtures of water and increasing amount of methanol (200 cc): fraction 1: 10% methanol, fraction 2: 20% methanol, fraction 3: 40% methanol, fraction 4: 60% methanol, fraction 5: 80% methanol, fraction 6: 100% methanol. The eluted fractions were combined and dried by rotary evaporation.
Antimicrobial assay
The antimicrobial activity of tested extract was monitored using agar diffusion method, which is highly recommended method for routine assessment of preliminary antimicrobial screening. Microorganisms were obtained as lyophilized culture from Persian Type Culture Collection (Iran). Microorganisms were as follows: gram negative species, Pseudomonas aeroghinosa (P. aeroghinosa) (ATCC 9027) as well as Gram-positive species namely Staphylococcus epidermidis (S. epidermidis)(ATCC 12228), Staphylococcus aureus (S. aureus) (ATCC 6538), Micrococcus luteus (M. luteus) (ATCC 10240) and a fungus, Candida albicans (ATCC 10231), that were used to evaluate antimicrobial properties of the SPE fractions. Centrifuged pallets of bacteria from 24 h cultures were mixed with distilled water, and the turbidity was corrected by adding sterile distilled water until 0.5 McFarland’s turbidity standard [108 colony forming units (CFUs) per mL] was obtained. Then, these inoculums were used for seeding the Muller Hinton agar (MERCK). Autoclaved Muller Hinton agar medium was allowed to cool up. Then, it was seeded with 10 mL of prepared inoculums (106 CFUs per mL). The mentioned plates were inoculated with a 0.5 McFarland’s standard of selected bacteria. Each petri plate contained 5 wells for test samples, four for solutions of SPE fractions, and one for vehicle control (DMSO). For incubation and analysis, 100 µL of test solution was poured in respective wells. The petri plates were incubated at 37 °C. After 24 h of incubation, the diameter of the clear zones, showing no bacterial growth, around each well (excluding well diameter) was measured with the help of venire calipers. Triplet plates were prepared for each sample (10-12). The samples, which have shown significant antibacterial activity at the mentioned concentration, were further assayed for their Minimum Inhibitory Concentration (MIC). The MIC was defined as the lowest concentration of a fraction which was able to completely inhibit the growth of each bacterial strain. Serial two-fold dilutions of fractions were prepared in broth. The cultures containing only sterile nutrient broth, which did not influence bacterial growth, were used as controls. To each test tube, an equal volume of the adjusted inoculums was added. After incubation at 37 °C for 24 h the MIC was read (13).
Brine shrimp lethality assay
The method was adopted to study the general toxicity of the plant. The brine shrimps (Artemia salina), were purchased from Water Life, Middlesex, UK. The eggs were hatched in a conical shaped vessel, filled with 300 mL artificial seawater, prepared using NaCl (38 g/L) in distilled water. The flasks were kept in a water bath at 29-30 °C under constant aeration by the aid of an air pump and a left on bright light. The nauplii hatched within 48 h. Half mL of n-hexane, dichloromethane and MeOH extracts were separately dissolved in 4.5 mL of constantly aerated brine solution to obtain a concentration of 1 mg/mL. Solutions were serially diluted to obtain seven different concentrations. About 10 nauplii were transferred in to each vial and maintained at room tempreture for 24 h. after 24 h, the number of surviving larvae were counted. End point of this bioassay was defined as the absence of controlled forward motion during 30 sec of observation. Experiments were conducted along with the control. This experiment was carried out twice per each test substance (14-17).
Purification and Isolation
Ten%, 20%, 40%, 60% and 80% MeOH/water fractions were subjected to preparative reverse phase HPLC, conducted on a Knauer HPLC (preparative pump 1800) fitted with Reprosil 100 C18 (250 × 20 mm i.d, particle size 10 µm, Dr. Maisch, Germany) column. The mobile phase consisted of (A) methanol and (B) distilled water. The following mobile phase program was used over 30 min to isolate aucubin (compound 5, 27.2 mg, tr = 21.5 min) and mannitol (compound 8, 18.8 mg, tr = 6.5 min) from the 10% SPE fraction: 10% A, 90% B initially, changed to 20% A in 20 min, maintained for 5 min, changed to 10% A in 2 min and maintained for 5 min.
The following mobile phase program was performed over 50 min to isolate 5-hydroxy-8-epi-loganin (compound 7, 8.3 mg, tr = 22 min), Ipolamide (compound 6, 6.7 mg, tr = 24.5 min) from the 20% SPE fraction: initially 10% A, changed to 60% A in 30 min, maintained for 4 min, changed to 100% A in 1 min, maintained for 6 min, changed to 10% in 2 min, maintained for 7 min. Following gradient elution with total run time of 75 min was carried out to separate verbascoside (compound 3, 8.7 mg, tr = 44 min) from the 40% SPE fraction: 35% A 65% B initially, changed to 45% A in 50 min, maintained for 5 min, changed to 100% A in 2 min, maintained for 7 min, changed to 35% A in 3 min, maintained for 8 min. Following gradient elution with total run time of 45 min was applied to separate luteolin (compound 1, 17.8 mg, tr = 23.5 min) luteolin-7-glucoside (compound 2, 7.1 mg, tr = 10.5 min), martinoside (compound 4, 8.1 mg, tr = 17.5 min) from the 60% SPE fraction: 45% A 55% B initially, changed to 90% A in 20 min, maintained for 5 min, changed to 45% A in 2 min, maintained for 3 min, changed to 100% A in 2 min, maintained for 6 min, changed to 45% A in 2 min and maintained for 5 min. Photodiode array detector (PDA) was used to monitor the chromatogram; HPLC separation was performed at the room temperature. The flow rate and injection volume were 8 mL/min and 1 mL respectively.
The structures of isolated pure compounds were identified by the aid of spectroscopic means (1H, 13C NMR and UV spectrophotometer) and also comparison with literature data of respective compounds (15-19). Chemical shifts were given on δ (ppm) scale with TMS was as the initial standard.
Results
Results of antibacterial test
As it was reported in our previous study, total methanolic extract of P. wilhelmsiana demonstrated inhibitory activity against some strains (P. aeroginosa, S. epidermidis, S. aureus, M. luteus) (11). In the following, to obtain the most potent MeOH/water fraction, the inhibitory activities of SPE fractions were examined against the susceptible strains by agar-well diffusion method. The results were shown in Table 1.
Results of brine shrimp lethality assay
In the present study, with the performed method, no cytotoxic activity was found for n-hexane, dichloromethane and methanolic extracts of P.wilhelmsiana.
Results of phytochemical study
1H, 13C NMR (Bruker-spectrospin at 200 MHz) and UV spectroscopy as well as comparison with literature data (15-19) were used to identify the following structures: luteolin (compound 1), luteolin-7-glucoside (compound 2), verbascoside (compound 3), martinoside (compound 4), aucubin (compound 5), ipolamiid (compound 6), 5-hydroxy-8-epi-loganin (compound 7) and mannitol (compound 8) shown in Figures 1-5.
Figure 1.
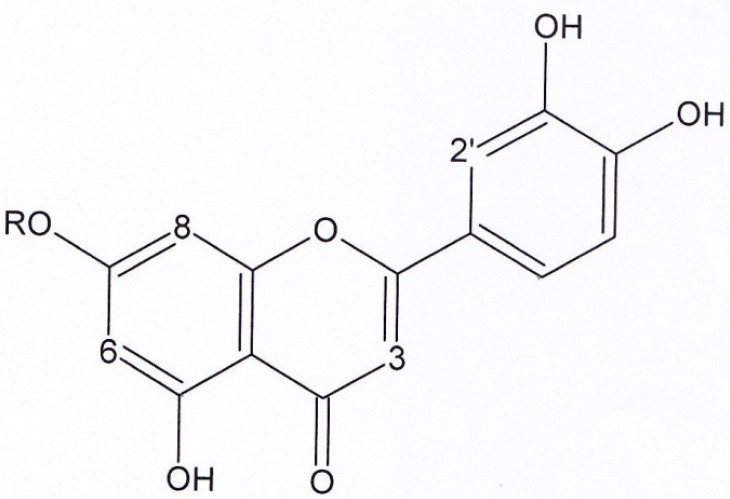
Structures of luteolin (compound 1) and luteolin-7- glucoside (compound 2). Compound 3: R = H. Compound 4: R = Methyl
Figure 5.
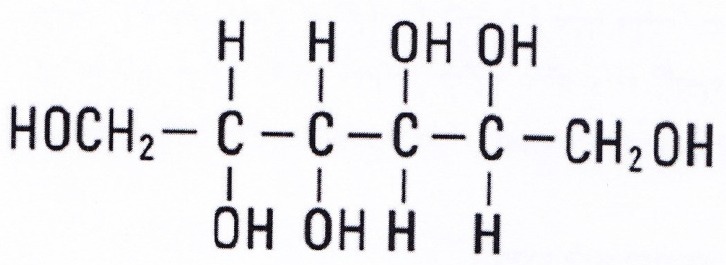
Structure of mannitol (compound 8)
Figure 1, compound 1, luteolin: yellow solid. 1HNMR and 13CNMR (200 MHz, CD3OD). 1HNMR: δ 6.42 (1H, S, H3), δ 6.18 (1H, d, J = 1.8, H6), δ 6.51 (1H, d, J = 1.8, H8), δ 7.37 (1H, s, H2′), δ 7.37 (1H, d, J = 7.8, H5′), δ 6.89 (1H, d, J = 7.8, H6′). 13CNMR: δ 164.63 (C2), δ 102.37 (C3), δ182.39 (C4), δ 161.7 (C5), δ 97.81 (C6), δ 164.85 (C7), δ 93.59 (C8), δ 157.93 (C9), δ 103 (C10), δ122.2 (C1′), δ 112.69 (C2′), δ 145.59 (C3′), δ 149.55 (C4′), δ 115.32 (C5′), δ 118.85 (C6′). UV spectrum, bands ІІ and І respectively (MeOH, λmax, nm): 280, 350.
Figure 1, compound 2, luteolin-7-glucoside: Yellow solid. 1HNMR and 13CNMR (200MHz, CD3OD). 1HNMR: δ6.54 (1H, S, H3), δ6.21 (1H, d, J = 2, H6), δ6.45 (1H, d, J = 2, H8), δ7.37 (1H,S, H2′), δ7.35 (1H, d, J = 8.8, H5′), δ6.91 (1H, d, J = 8.8, H6′), δ4.47 (1H, d, J = 7.6, H1′′), δ3.2-3.8 (1H, unclear pattern due to overlapping, H2′,3′,4′,5′,6′). 13CNMR: δ164.62 (C2), δ102.43 (C3), δ182.39 (C4), 161.77 (C5), δ98.71 (C6), 164.85 (C7), δ93.59 (C8), δ157.93 (C9), δ102.43 (C10), δ122.2 (C2′), δ112.74 (C2′), δ145.59 (C3′), δ149.55 (C4′), δ115.37 (C5′), δ118.88 (C6′), δ98.15 (C1′′), δ72.45 (C2′′), δ76.54 (C3′′), δ70.93 (C4′′), δ76.98 (C5′′), δ62.99 (C6′′). UV spectrum, bands ІІ and І respectively (MeOH, λmax, nm): 280, 350.
Figure 2.
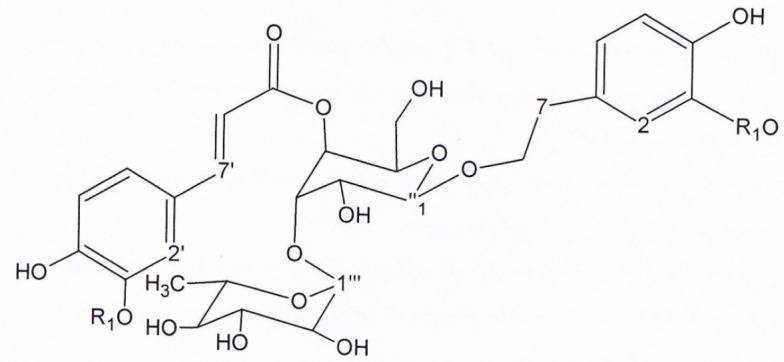
Structures of verbascoside (compound 3) and martinoside (compound 4)
Figure 2, Compound 3, verbascoside: brown solid. 1HNMR and 13CNMR (200MHz, CD3OD). 1HNMR:δ 6.69 (1H, d, J = 1.8, H2), δ6.64 (1H, d, J = 7.8, H5), δ 6.68 (1H,d, J = 7.8, H6), δ 2.79 (3H, t, J = 8, H7), δ 3.8-4.1 (1H, overlapped peak, H8), δ 7.06 (1H, d, J = 1.8, H2′), δ 6.78 (1H, d, J = 7.9, H5′), δ 6.92 (1H, d, J = 7.9, H6′), δ 7.6 (1H, d, J = 15.7, H7′), δ 6.28 (1H, d, J = 15.7, H8′), δ 4.34 (1H, d, J = 8.6, H1′′), δ 3.2-3.8 (1H, signal pattern unclear due to overlapping, H2′′,3′′, 4′′,5′′, 6′′), δ 5.19 (1H, d, J = 1.5, H1′′′), δ 3.2-3.8 (1H, signal pattern unclear due to overlapping, H 2′′′,3′′′, 4′′′, 5′′′), δ 1.08 (1H, d, J = 6, H6′′′). 13CNMR. δ 130 (C1), δ 113.72 (C2), δ144.76 (C3), δ 143.23 (C4), δ 115.00 (C5), δ 121.81 (C6), δ35.15 (C7), δ 72.32 (C8), δ 126.21 (C1′), δ114.86 (C2′), δ145.43 (C3′), δ148.42 (C4′), δ115.61 (C5′), δ121.84 (C6′), δ 146.63 (C7′), δ 115.20(C8′), δ166.86 (C9′), δ101.62 (C1′′), δ 74.73 (C2′′), δ 80.21 (C3′′), δ 69.16 (C4′′), δ74.53 (C5′′), δ60.92 (C6′′), δ 102.74 (C1′′′), δ 70.95 (C2′′′), δ 70.63 (C3′′′), δ 72.36 (C4′′′), δ 69.43 (C5′′′), δ 17.01 (C6′′′). UV spectrum, (MeOH, λmax, nm): 285, 330.
Figure 2, Compound 4, martinoside: pale brown solid 1HNMR and 13CNMR (200MHz, CD3OD). 1HNMR: δ 6.75 (1H, d, J = 1.7, H2), δ 6.82 (1H, d, J = 8, H5), δ6.7 (1H, dd, J = 8,1.7,H6), δ2.83 (3H, t, J = 7,H7), δ 3.8-4.1(1H, overlapped signal, H8), δ3.82 (3H, s, O-CH3), δ7.2 (1H, d, J = 1.5, H2′), δ6.79 (1H, d, J = 7.7, H5′), δ7.09 (1H, dd, J = 8,1.5, H6′), δ7.66 (1H, d, J = 16, H7′), δ6.38 (1H, d, J = 16, H8′), δ3.85 (3H, S, O-CH3), δ4.38 (1H, d, J = 7.8, H1′′), δ3.2-3.8 (1H, unclear pattern due to overlapping, H2′,3′,4′, 5′, 6′), δ5.16 (1H, d, J=1.7, H1′′′), δ3.2-3.8 (1H, unclear pattern due to overlapping, H2′, 3′,4′, 5′), δ1.085 (1H,d, J = 6, H6′′′). 13CNMR: δ 132.93 (C1), δ112.95 (C2), δ147.98 (C3), δ147.37(C4), δ117.19 (C5), δ122.94 (C6), δ35.12 (C7), δ72.35 (C8), δ53.39 (O-CH3), δ127.71 (C1′), δ111.91 (C2′), δ149.41 (C3′), δ150.81 (C4′), δ116.66 (C5′), δ124.46 (C6′), 148.03 (C7′), δ115.19 (C8′), δ166.85 (C9′), δ53.49 (O-CH3), δ101.58 (C1′′), δ76.24 (C2′′), δ81.65 (C3′′), δ70.74 (C4′′), δ76.04 (C5′′), δ62.49 (C6′′), δ102.79 (C1′′′), δ72.42 (C2′′′), δ72.20 (C3′′′), δ73.87 (C4′′′), δ70.31 (C5′′′), δ 17.03 (C6′′′). UV spectrum, (MeOH, λmax, nm): 285, 330.
Figure 3, Compound 5, aucubin: pale brown solid. 1HNMR and 13CNMR (200 MHz, CD3OD). δ 5.69 (1H, S, H1), δ 6.13 (1H, dd, J = 6.1;1.6, H3), δ 4.96 (1H,dd, J = 6.1;3.5, H4), δ 2.61 (1H,m, H5), 4.38 (1H,m, H6), δ 5.11 (1H, d, J = 4.98, H7), δ 2.97 (1H, t, J = 5.6, H9), δ4.09 (1H, d, J = 15.4, H10), δ 4.62 (1H, d, J = 7.9, H1’), δ 3.2-3.8 (1H, signal patterns unclear due to overlapping, H 2’,3’,4’,5’, 6’), 13C NMR (200MHZ, CD3OD): Aglycon moiety: δ 98.24 (C1), δ 146.72 (C3), δ 105.08 (C4), δ 42.17 (C5), δ 80.33 (C6), δ 128.32 (C7), δ 139.41 (C8), δ 46.20 (C9), δ 59.35 (C10), δ 95.12 (C1’), δ 72.64 (C2’), δ 76.06 (C3’), δ 69.42 (C4’), δ 75.52 (C5’), δ 60.49 (C6’).
Figure 3.
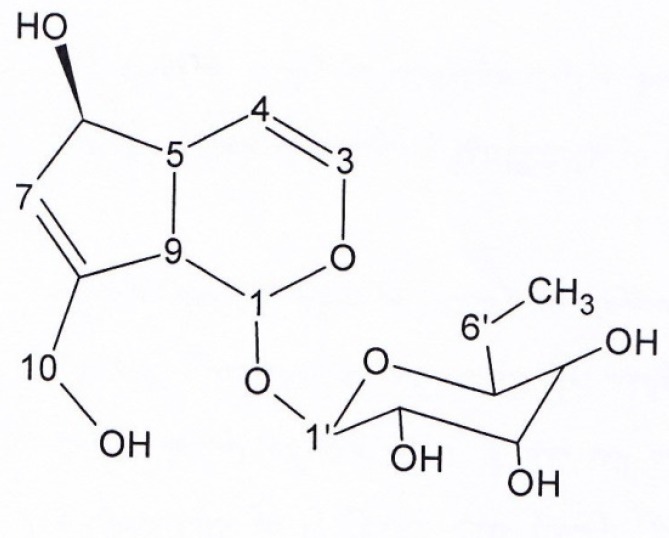
Structure of aucubin (compound 5)
Figure 4, Compound 6, Ipolamid: brown solid. 1HNMR and 13CNMR (200MHz, CD3OD): Aglycon moity δ5.82 (1H,S, H1), δ7.44 (1H,S, H3), δ 2.01-2.31 (1H, m, H6), δ 2.01-2.31 (1H, m, H6), δ 1.92 (1H, m, H7), δ 1.57 (1H, m, H7), δ 2,48 (1H, S, H9), δ 1.15 (1H, S, H10), δ 3.73 (3H, S, OMe), Glucose moiety: δ 4.61 (1H,d, J=8, H1’), δ 3.2-3.8 (1H, signal patterns unclear due to overlapping, H2’, H3’, H4’, H5’, H6’), 13C NMR (200MHZ, CD3OD): δ 92.75 (C1), δ 151.16 (C3), δ 113.79 (C4), δ 70.22 (C5), δ 37.42 (C6), δ 38.46 (C7), δ 76.98 (C8), δ 60.25 (C9), δ 21.81 (C10), δ 166.58 (C11), δ 50.21 (OMe), 98.15 (C1’), δ 72.96 (C2’), δ 76.57 (C3’), δ 70.27 (C4’), δ 76.98 (C5’), δ 61.43 (C6’). UV spectrum, (MeOH, λmax, nm): 215-250.
Figure 4.
Structure of ipolamiid and 5-hydroxy-8-epi-loganin (compounds 6, 7).
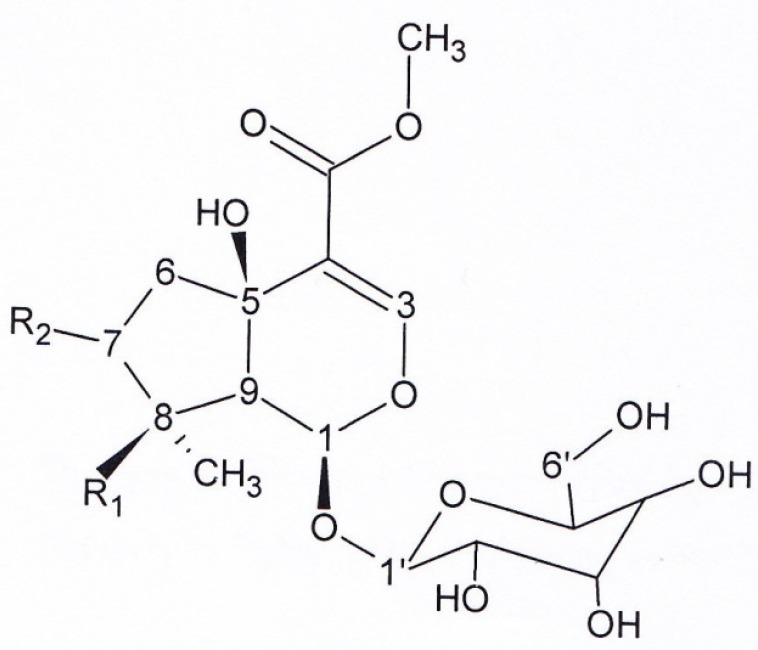
Compound 6: R1 = OH, R2 = H. Compound 7: R1 = H, R2 = OH.
Figure 4, Compound 7, 5-hydroxy-8-epi-loganin: brown solid. 1HNMR and 13CNMR (200 MHz, CD3OD). 1HNMR: Aglycone moiety: δ 5.76 (1H, d, J = 1.52, H1), δ 7.49 (1H, S, H3), δ 2.57 (1H, dd, J = 13.6; 6.5, H6), δ 2.06 (1H, dd, J = 13.6; 6.5, H6), δ 3.2-3.8 (1H, overlapped peak, H7), δ 2.3 (1H, m, H8), δ 2.82 (1H, dd, J = 10.2;1.2, H9), δ 0.95 (3H, d, J = 7.4,H10), δ 3.74 (3H, S, OMe), Glucose moiety: δ 4.64 (1H, d, J=7.7, H1’), δ 3.2-3.8 (1H, signal patterns unclear due to overlapping, H 2’,3’,4’,5’, 6’). 13C NMR: Aglycon moiety: δ 94.23 (C1), δ 152.26 (C3), δ 113.71 (C4), δ 70.21 (C5), δ 46.33 (C6), δ 76.93 (C7), δ 42.05 ( C8), δ 50.43 (C9), δ 12.46 (C10), δ 166.83 (C11), δ 50.67 (O-Me), δ 98.36 (C1’), δ 72.9 (C2’), δ 75.95 (C3’), δ 69.9(C4’), δ 76.45 (C5’), δ 61.3 (C6’). UV spectrum (MeOH, λmax, nm): 215-250.
Figure 5, Compound 8, mannitol: white solid. 1HNMR and 13CNMR (200MHZ, D2O) 1HNMR: δ 3.53-3.6 (2H, dd, overlapped peak, H1), δ 3.47-3.5 (1H, overlapped peak, H2), δ 3.635 (1H, d, J = 8.5). 13CNMR: 63.10 (C1), 69.08 (C2), 70.66 (C3).
Compound 1: R = H
Compound 2: R = glucose
Discussion
Recently, specially over the last 25 years, the utility of natural products as sources of novel medicines has been expanded (20). Moreover, due to their chemical diversity and bioactivity, natural products offer templates for development of novel drugs (21). Pedicularis genus belonging to Scrophulariaceae family, is one of the world’s largest flowering plants, containing around 600-800 species as well as valuable pharmacological effects (22). Our Previous experiments showed antioxidant activity of P. wilhelmsiana MeOH extract and SPE fractions (specially 40% and 60% and in lower potency 80% SPE fractions) along with antibacterial activity of them against gram positive strains (S. ureus and S. epidermidis) as well as gram negative strain (P. areoginosa) which was attributed to probable phenolic compounds existing in MeOH extract (11). Consistent with previous studies, total phenol and flavonoid content of P. wilhelmsiana methanolic extract as well as SPE fractions had been demonstrated (23). So, this study set out with the aim of assessing antibacterial effect of SPE fractions as well as phytochemical study of MeOH extract of this plant to find out possible effective phyto constituents.
As it is apparent in Table 1, 40% and 60% SPE fractions were active against S. aureus, S. epidermidis, M. luteus, and P. aeroginosa. The data of Table 1 reveal that, neither 10% (the most polar SPE fraction) nor 100% (the least polar SPE fraction) indicated any activity against susceptible strains. Twenty% SPE fraction was only active against P. aeroginosa. Forty% SPE fraction indicated more potent inhibitory effect than MeOH extract against susceptible gram positive strains; However, its inhibitory activity was weaker than MeOH extract against P. aeroginosa. Sixty% SPE fraction showed stronger inhibitory activity than MeOH extract against all four susceptible strains; however it was found to be more potent against S. epidermidis and M. luteus. Eighty% SPE fraction indicated inhibitory activity against P. aeroginosa and S. epidermidis. Activity of this SPE fraction against P. aeroginosa was more potent than other fractions. Among the SPE fractions the most prominent inhibitory activity was exhibited against M. luteus by the 60% MeOH/water fraction.
Solid phase extraction, isolation and structural elucidation of compounds of MeOHextract obtained from dried arial parts of P. wilhelmsiana afforded 8 compounds on the basis of spectroscopic studies (NMR analysis and UV spectroscopy) as well as comparison with respective literature data. All spectroscopic findings were in agreement with respective published data. To the best of our knowledge there is no report on the occurrence of Luteolin (compound 1), luteolin-7-glucoside (compound 2), martynoside (compound 4), aucubin (compound 5), ipolamiid (compound 6), 5-hydroxy-8-epi-loganin (compound 7), mannitol (compound 8) in this plant; however, occurrence of verbascoside, a widespread phyto constituent in Pedicularis genus, was previously reported from P. wilhelmsiana (14).
Many plant poly phenols are known to possess antimicrobial properties (24). According to our previous experiments, higher total phenol and flavonoid content of P. wilhelmsiana specially in 40% and 60% SPE fractions could be a good reason for more potent antibacterial activity of these fractions which lead to phytochemical study of P. wilhelmsiana MeOH extract (23). Occurrence of verbascoside, a poly phenolic compound in 40%, also existence of luteolin, luteolin-7-glucoside and martinoside in 60% SPE fractions, which have indicated antibacterial activities (13-15), could be regarded as a good reason for inhibitory effect of mentioned fractions which contain forenamed isolated phyto constituents.
Irridoids, widly distributed in Scrophulariaceae family, with variety of biological activities, also possess antimicrobial properties (25). In the previous study, total phenol content of 20% SPE fraction was demonstrated (23), also in the present study, with the described method, two iridoids were isolated and identified in 20% SPE fraction. Since both groups of phytochemicals (phenolic compounds and iridoids) possess antibacterial activities, further studies are needed to determine which phyto constituents are responsible to antibacterial activity of 20% MeOH fraction against P. aeroginosa. In the previous study, existence of phenolic compounds was indicated in 80% SPE fraction, however in the present study with the described method, no phytochemical compound could be isolated from this fraction. So, further studies are necessary to find out the responsible phytochemical compound to inhibitory activity of 80% SPE fraction against P. aeroginosa as well as S. epidermidis.
In this study another important finding was that, there was not any significant difference between activities of fractions (20%, 40%, 60% and 80%) against P.aeroginosa (Table 1). Further investigations in future would reveal more active ingredients which are responsible for this inhibitory activity. By the means of brine shrimp lethality assay, which is a rapid method to screen general toxicity of the plant, this plant was not indicated to be toxic. So, as a conclusion antioxidant activity, no cytotoxic as well as antibacterial effects of MeOH extract of this plant, supports further studies related to antibiotic potential of P. wilhelmsiana MeOH extract which could be good source of antioxidant reagents.
Acknowledgment
We are grateful to Dr. Asnaashari and Mrs Bamdad for their valuable contributions to this study.
References
- 1.Mozaffarian V. A dictionary of Iranian plant names. 5th ed. Tehran : Farhang Mo′aser; 2007. p. 196. [Google Scholar]
- 2.Mozaffarian V. Identification of medicinal and aromatic plants of Iran. 2nd ed. Tehran: Farhang Mo′aser; 2013. p. 56. [Google Scholar]
- 3.Jiang TF, Ou QY, Shi YP. Separation and determination of phenylpropanoid glycosides from Pedicularis species by capillary electrophoresis. J. Chromatogr. A . 2003;986:163–7. doi: 10.1016/s0021-9673(02)01918-0. [DOI] [PubMed] [Google Scholar]
- 4.Zhang ZX, Xie WD, Jia ZJ. Glycosids from two Pedicularis species. Biochem. Syst. Ecol. 2008;36:467–72. [Google Scholar]
- 5.Li M, He X, Tao R, Cao X. Phytochemistry and pharmacologyof the Genus Pedicularis used in traditional Chinese medicine. Am. J. Chin. Med. 2014;42:1071–95. doi: 10.1142/S0192415X14500670. [DOI] [PubMed] [Google Scholar]
- 6.Akdemir Z, Calis I, Junior P. Iridoids and phenylpropanoid glycosides from Pedicularis nordmanniana. Planta Med. 1991;57:584–5. doi: 10.1055/s-2006-960215. [DOI] [PubMed] [Google Scholar]
- 7.Fujii M, Miyaichi Y, Tomimori T. Flavonoid, phenylethanoid and iridoid constituents of the whole plant of Pedicularis longiflora var. tubiformis. Planta Med. 1995;61:584. doi: 10.1055/s-2006-959387. [DOI] [PubMed] [Google Scholar]
- 8.Liu ZM, Jia ZJ. Phenylpropanoid and iridoid glycosides from Pedicularis striata. Phytochemistry . 1991;30:1341–4. doi: 10.1016/s0031-9422(00)95221-x. [DOI] [PubMed] [Google Scholar]
- 9.Su BN, Zhai JJ, Jia ZJ. New iridoids from Pedicularis artselaeri. J. Asian Nat. Prod. Res. 1998;1:103–9. doi: 10.1080/10286029808039851. [DOI] [PubMed] [Google Scholar]
- 10.Ballabh B, Chaurasia OP. Traditional medicinal plants of cold desert Ladakh--used in treatment of cold, cough and fever. J. Ethnopharmacol. 2007;112:341–9. doi: 10.1016/j.jep.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 11.khodaie L, Delazar A, Lotfipour F, Nazemiyeh H. Antioxidant and antimicrobial activity of Pedicularis sibthorpii Boiss and Pedicularis wilhelmsiana Fisch ex. Adv. Pharm. Bull. 2012;2:89–92. doi: 10.5681/apb.2012.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowan MM. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ríos JL, Recio MC. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005;100:80–4. doi: 10.1016/j.jep.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Eribekyan M, Agababyan É, Arutyunyan L, Mnatsakanyan V. Phenylpropanoid glycosides of Pediculariscondensata P wilhelmsiana and P sibthorpii. Chem. Nat. Compd. 1991;27:639–40. [Google Scholar]
- 15.Albach D, Lib H, Zhao N, Jensenc S. Molecular systematics and phytochemistry of Rehmannia (Scrophulariaceae) Biochem. Syst. Ecol. 2007;35:293–300. [Google Scholar]
- 16.Jensen S, Opitz SW, Gotfredsen C. A new phenylethanoid triglycoside in Veronica beccabunga L. Biochem. Sys. Ecol. 2011;39:193–7. [Google Scholar]
- 17.Calis I, Lahloub M, Rogenmoser E, Sticher O. Isomartynoside, a phenylpropanoid glycoside from Galeopsis pubescens. Phytochemistry . 1984;23:2313–5. [Google Scholar]
- 18.Gafner S, Wolfender J, Nianga M, Hostettmann K. Phenylpropanoid glycosides from Newbouldia leavis roots. Phytochemistry . 1997;44:687–90. doi: 10.1016/s0031-9422(96)00611-5. [DOI] [PubMed] [Google Scholar]
- 19.Dinda B, Debnath S, Banik R. Naturally occurring iridoids and secoiridoids An updated review, Part 4. Chem. Pharm. Bull. 2011;59:803–33. doi: 10.1248/cpb.59.803. [DOI] [PubMed] [Google Scholar]
- 20.Newman D, Cragg G. Natural products as sources of new drugs over the last 25 years. J. Nat Prod. 2007;70:461–77. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 21.Butler M. The role of natural product chemistry in drug Discovery. J. Nat. Prod. 2004;67:2141–53. doi: 10.1021/np040106y. [DOI] [PubMed] [Google Scholar]
- 22.Li H. Evolution in the Flowers of Pedicularis. Evolution. 1951;5:158–64. [Google Scholar]
- 23.Khodaie L, Bamdad S, Delazar A, Nazemiyeh H. Antioxidant, Total Phenol and Flavonoid Contents of Two Pedicularis L Species from Eastern Azerbaijan, Iran. BioImpacts. 2012;2:47–53. doi: 10.5681/bi.2012.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaqueroa MJR, Serravalle LRT, Nadra MCMd, Saad AMSD. Antioxidant capacity and antibacterial activity of phenolic compounds from argentinean herbs infusions. Food Control. 2010;21:779–85. [Google Scholar]
- 25.Saleem M, Nazir M, Ali M, Hussain H, Lee Y, Riaz N. Antimicrobial natural products: an update on future antibiotic drug candidates. Nat. Prod. Rep. 2010;27:238–54. doi: 10.1039/b916096e. [DOI] [PubMed] [Google Scholar]


