Abstract
During the last 20 years, Leishmania (Mundinia) spp. have emerged as new causative agents of human and animal leishmaniases. We provide a historical view of these parasites, from their initial description to their emergence as pathogens, to help avoiding future confusion in species assignation of these newly emerging pathogens.
More than one billion people are at risk of contracting visceral leishmaniasis (VL) or cutaneous leishmaniasis (CL). VL causes 20 000 to 30 000 deaths annually, and CL affects the quality of life of millions, causing disability and permanent scarring after healing (https://www.who.int/leishmaniasis/en/). The first account of human leishmaniasis dates from the Middle Ages, but the discovery of Leishmania parasites as causative agents only occurred in modern times. Until recently, it was thought that the aetiologic agents responsible for leishmaniases belonged almost exclusively to the subgenus Leishmania in the Old and New Worlds and to the subgenus Viannia in the New World. This view is currently challenged [1].
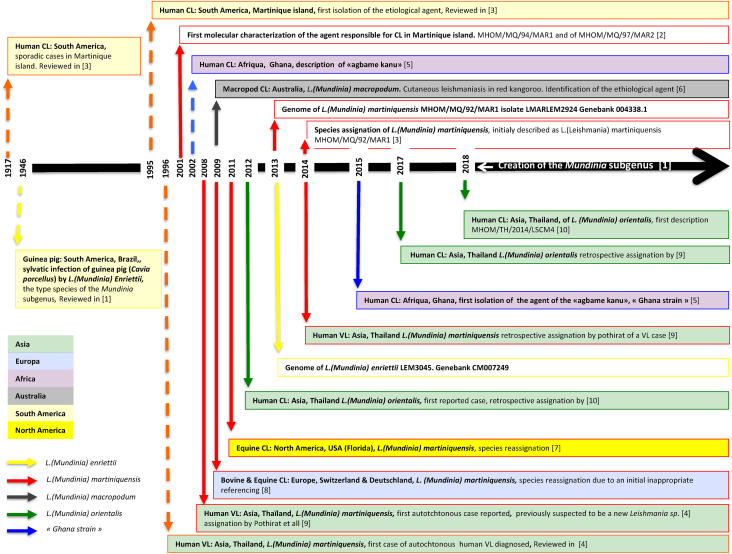
Between 1995 and 2002, human and animal CL and VL caused by noncanonical Leishmania parasites were reported in geographically distant areas (Thailand, Martinique, Switzerland, Australia and Ghana). In Martinique, reports of cutaneous-like infections date back to 1917, but the aetiologic agent was not formally characterized until 2001 [2], [3]. A draft genome of the isolate was released in 2013, and the species, named L. martiniquensis, was included in the Leishmania subgenus [3]. In Thailand, before the description of first autochthonous VL cases in 1999, leishmaniases were only diagnosed in travellers returning from the Middle East. In 2008, a new species was suspected of being the aetiologic agent of VL cases in Thailand [4]. In West Africa, CL cases caused by L. major have been documented. In Ghana, from 2002 to 2003, a total of 8876 possible CL cases were reported, and L. major and another Leishmania sp. were identified [5]. Surprisingly, animal cases (horse, cow and red kangaroo) of CL were detected in North America, Europe and Australia [6], [7], [8]. The enigmatic Leishmania parasite responsible for CL in horses is genetically closed to the suspected new species identified in Thailand. The name ‘Leishmania siamensis’ was used at the time [7]; nevertheless, given the lack of adherence to the international code for zoologic nomenclature, the name ‘Leishmania siamensis’ is considered a nomen nudum. In 2014, clues emerged to incriminate L. martiniquensis in some CL and VL cases reported in Thailand since 1999 [9]. Surprisingly, all these pathogens were genetically related to a parasite, Leishmania enriettii, previously isolated from a guinea pig in Brazil [1], [9]. In 2018, the subgenus Mundinia was created [1]. It encompasses Leishmania species responsible for human and animal diseases previously gathered in the L. enriettii complex, L. (Mundinia) martiniquensis, previously assigned to the subgenus Leishmania [1], and L. (Mundinia) orientalis, a newly described species responsible for CL in Thailand [1], [10]. A schematic timeline the Mundinia subgenus of characterization is provided in Fig. 1.
Fig. 1.
Historical events leading to identification of Leishmania (Mundinia) spp. as newly emerging Leishmania pathogens worldwide. Dashed lines indicate absence of molecular identification of aetiologic agent. In Asia, members of the Mundinia (Shaw, Camargo and Teixeira 2016) subgenus are currently identified only in Thailand. In Africa, they were isolated in Ghana and are referred as Ghana strains. In Europe, presence was reported in Deutschland (Bavaria) and Switzerland; in North and South America, United States (Florida), Brazil (Curitiba, Sao Polo) and Martinique Island. Described species belonging to the Mundinia subgenus are: Leishmania (Mundinia) enriettii (Muniz and Medina 1948), Leishmania (Mundinia) martiniquensis (Desbois, Pratlong and Dedet, 2014), Leishmania (Mundinia) orientalis (Bates and Jariyapan 2018) and Leishmania (Mundinia) macropodum (Barratt, Kaufer and Ellis, 2017), plus parasites responsible for CL in Ghana (Ghana strain). CL, cutaneous leishmaniasis; VL, visceral leishmaniasis.
Strikingly, the last 20 years have seen parasites of the subgenus Mundinia emerging as new human pathogens, causing VL and CL in HIV+ and HIV− patients. These parasites challenge our current knowledge of the worldwide distribution and physiopathology of leishmaniases. To elucidate the underlying factors triggering the worldwide emergence of L. (Mundinia) spp. and to address the future risks for human and animal health, we must investigate the vectors and hosts involved in the transmission cycles of these pathogens.
Conflict of Interest
None declared.
References
- 1.Espinosa O.A., Serrano M.G., Camargo E.P., Teixeira M.M.G., Shaw J.J. An appraisal of the taxonomy and nomenclature of trypanosomatids presently classified as Leishmania and Endotrypanum. Parasitology. 2018;145:430–442. doi: 10.1017/S0031182016002092. [DOI] [PubMed] [Google Scholar]
- 2.Noyes H., Pratlong F., Chance M., Ellis J., Lanotte G., Dedet J.P. A previously unclassified trypanosomatid responsible for human cutaneous lesions in Martinique (French West Indies) is the most divergent member of the genus Leishmania ss. Parasitology. 2002;124:17–24. doi: 10.1017/s0031182001008927. [DOI] [PubMed] [Google Scholar]
- 3.Desbois N., Pratlong F., Quist D., Dedet J.P. Leishmania (Leishmania) martiniquensis n. sp. (Kinetoplastida: Trypanosomatidae), description of the parasite responsible for cutaneous leishmaniasis in Martinique Island (French West Indies) Parasite. 2014;21:12. doi: 10.1051/parasite/2014011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sukmee T., Siripattanapipong S., Mungthin M., Worapong J., Rangsin R., Samung Y. A suspected new species of Leishmania, the causative agent of visceral leishmaniasis in a Thai patient. Int J Parasitol. 2008;38:617–622. doi: 10.1016/j.ijpara.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Kwakye-Nuako G., Mosore M.T., Duplessis C., Bates M.D., Puplampu N., Mensah-Attipoe I. First isolation of a new species of Leishmania responsible for human cutaneous leishmaniasis in Ghana and classification in the Leishmania enriettii complex. Int J Parasitol. 2015;45:679–684. doi: 10.1016/j.ijpara.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Rose K., Curtis J., Baldwin T., Mathis A., Kumar B., Sakthianandeswaren A. Cutaneous leishmaniasis in red kangaroos: isolation and characterisation of the causative organisms. Int J Parasitol. 2004;34:655–664. doi: 10.1016/j.ijpara.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Reuss S.M., Dunbar M.D., Calderwood Mays M.B., Owen J.L., Mallicote M.F. Autochthonous Leishmania siamensis in horse, Florida, USA. Emerg Infect Dis. 2012;18:1545–1547. doi: 10.3201/eid1809.120184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lobsiger L., Müller N., Schweizer T., Frey C.F., Wiederkehr D., Zumkehr B. An autochthonous case of cutaneous bovine leishmaniasis in Switzerland. Vet Parasitol. 2010;169:408–414. doi: 10.1016/j.vetpar.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Pothirat T., Tantiworawit A., Chaiwarith R., Jariyapan N., Wannasan A., Siriyasatien P. First isolation of Leishmania from Northern Thailand: case report, identification as Leishmania martiniquensis and phylogenetic position within the Leishmania enriettii complex. PLoS Negl Trop Dis. 2014;8:e3339. doi: 10.1371/journal.pntd.0003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jariyapan N., Daroontum T., Jaiwong K., Chanmol W., Intakhan N., Sor-Suwan S. Leishmania (Mundinia) orientalis n. sp. (Trypanosomatidae), a parasite from Thailand responsible for localised cutaneous leishmaniasis. Parasit Vectors. 2018;11:351. doi: 10.1186/s13071-018-2908-3. [DOI] [PMC free article] [PubMed] [Google Scholar]